Abstract
The pituitary gland is an unusual site for metastatic spread and has been associated with a poor prognosis. Clinical presentation is variable but can include visual field defects, cranial nerve palsies, anterior pituitary dysfunction and/ or diabetes insipidus. Management options include surgery or radiotherapy, chemotherapy/immunotherapy or a conservative approach. The pituitary should not be overlooked as a site for metastasis in patients with known cancer and can be the first presentation of neoplastic disease in some patients. Given that patients are now living longer with cancer, clinicians should be alert to the varied presentation of pituitary metastasis. We provide a clinical overview of pituitary metastasis with the aid of illustrative clinical cases.
INTRODUCTION
The pituitary gland is a rare site for metastasis, and accounts for less than 1% of clinical intracranial secondary tumours in clinical practice1. Autopsy studies have demonstrated its prevalence between 1-4% in patients with advanced cancer, which suggests that these lesions may go unrecognised2-3. More recently there appears to be an increasing incidence of patients diagnosed with pituitary metastasis (PM) in part due to improvements in neuroimaging, laboratory testing and the fact that patients are now living longer with cancer4-7. This leads us to anticipate that the number of patients developing metastatic involvement of the pituitary gland will rise, creating a greater need for awareness of its presentation among clinicians. Clinical presentation of PM is variable but can include visual field defects, cranial nerve palsies, anterior pituitary dysfunction, diabetes insipidus and compression of adjacent structures8-10. Management options can include surgical decompression, radiotherapy, chemotherapy/immunotherapy or a conservative approach11-13. However, given that metastasis is commonly present in other sites when PM becomes clinically evident, prognosis is often poor. In addition, endocrine dysfunction arising from PM can complicate the clinical picture and has the potential to adversely affect clinical outcomes14-16.
Case 1
A 19-year-old male presented with a one week history of headache, pyrexia and back pain. His medical history included asthma and polycystic kidney disease. Routine investigations revealed raised inflammatory markers, deranged liver function and hypercalcaemia. A CT-CAP scan showed extensive hepatosplenomegaly, multiple lytic skeletal lesions, para-aortic and porta-hepatic lymphadenopathy. Serum LDH was 4888 U/L (135-225). After transfer to the Regional Oncology Centre in Belfast, a bone marrow biopsy confirmed a diagnosis of diffuse large B-cell lymphoma. He underwent chemotherapy with one cycle of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) and two cycles of COPADM (cyclophosphamide, vincristine, prednisolone, doxorubicin and methotrexate) with partial disease response.
Four months later he represented with worsening headache and new left upper seventh cranial nerve palsy with CNS relapse confirmed on MRI and lumbar puncture. He underwent three cycles of R-IE (rituximab, ifosamide and etoposide). MRI brain at that time did not show any focal abnormality. One month later, his symptoms progressed to include nystagmus and ataxia, MRI brain (Figure 2 A+B) demonstrated a 14 x 12 mm mass involving the floor of the 3rd ventricle, hypothalamus, pituitary stalk and pituitary fossa. Pituitary function testing revealed secondary hypothyroidism (fT4 11pmol/l and TSH 0.03mU/l), hyperprolactinaemia (1004mU/l) and hypogonadism (testosterone < 0.2 nmol/l). He developed hyponatraemia (Serum Na 116 mmol/l) without overt polyuria or polydipsia. This was attributed to SIADH and confirmed on biochemical testing, and slowly resolved with the addition of slow sodium, furosemide and fluid restriction. Diabetes insipidus was not present. He was commenced on oral dexamethasone 750 mcg daily. No hormonal replacement was required. Given the extent of his disease with CNS involvement refractory to treatment he received input from palliative care and with cranial radiotherapy and comfort measures, he died 14 months after his initial presentation (3 months after PM was diagnosed).
Figure 2.
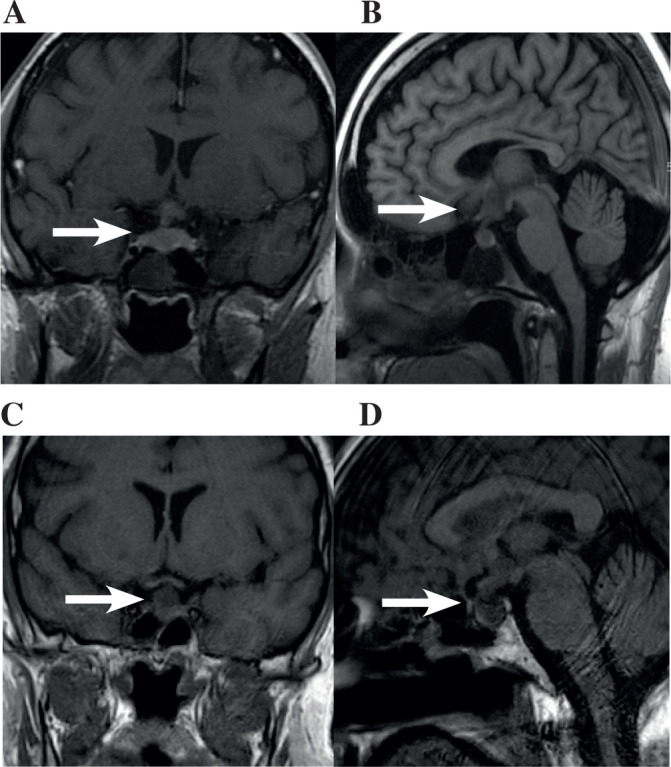
Imaging related to case 1 (A+B) and case 3 (C+D).
(A) Coronal and (B) sagittal MRI confirming malignant infiltration of the hypothalamus, pituitary stalk and pituitary fossa. (C) Coronal and (D) sagittal view demonstrating abnormal soft tissue mass in the pituitary fossa involving the pituitary gland and extending into the pituitary stalk
Case 2
A 53-year-old man presented to his GP with a short history of hip pain and general malaise. Initial pelvic x-ray revealed multiple lytic lesions in both hips and proximal femora. Serum calcium was significantly elevated at 3.9 nmol/l (RR 2.20-2.60). Urinary Bence-Jones protein was positive. He was diagnosed with ISS stage III multiple myeloma with favourable cytogenetics. He was commenced on VTD chemotherapy (bortezomib, thalidomide and dexamethasone) combined with zoledronic acid with the intention to progress to autogenic stem cell transplant. However, despite treatment his kappa free light chains continued to rise and he was instead escalated to lenalidomide and cyclophosphamide with dexamethasone. Treatment was further complicated by a traumatic humeral fracture requiring surgical fixation and multiple chest infections which precluded him from transplant options despite successful harvest.
While on third line treatment with bortezomib and panobinostat he represented with left eye pain, ptosis and double vision. MRI scanning (Figure 1) revealed a large mass arising from within the clivus and extending anteriorly into the sphenoid sinus and superiorly into the pituitary fossa in keeping with a plasmocytoma, he was deemed not suitable for neurosurgical intervention. Endocrine investigations revealed hypogonadism, and the rest of the anterior pituitary function was normal. He had no clinical evidence of diabetes insipidus. Shortly afterward he developed a respiratory tract infection. Palliative care was initiated and he died 19 months after his initial presentation and one month after diagnosis of PM.
Figure 1.
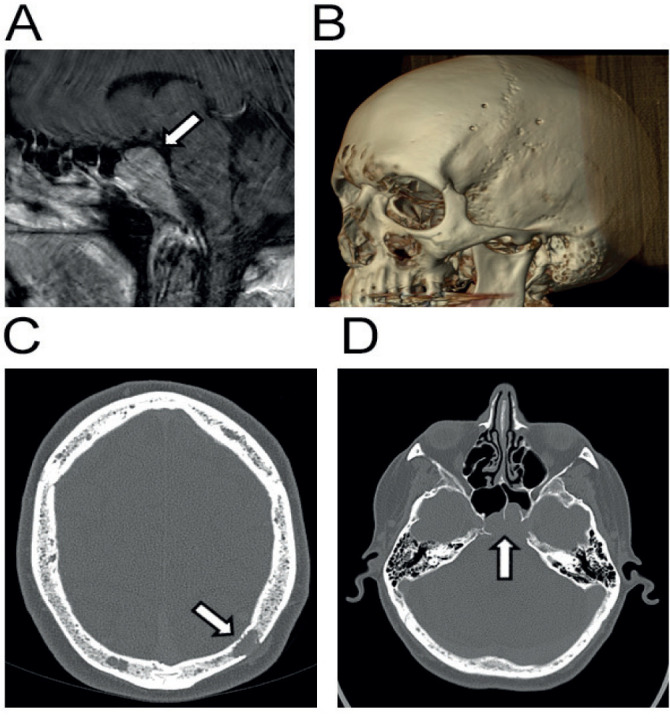
Imaging related to case 2. Sagittal MRI image demonstrating presence of myelomatous mass invading anteriorly from clivus of skull (A). Reconstruction of CT imaging demonstrating multiple lytic lesions of cranial vault characteristic of multiple myeloma (B). Same lytic lesions demonstrated transversely (C). Transverse view of clivus mass invading and compressing the pituitary gland (D).
Case 3
A 41-year-old man presented with a four week history of polyuria (12 litres/24 hours), polydipsia (15 litres/day) and a right sided headache while attending a routine respiratory outpatient appointment for follow up of his asthma. He had a complex medical history including squamous cell carcinoma of the anus treated by excision and mapping biopsy, severe eczema, mitral regurgitation and severe non-atopic asthma requiring multiple ICU admissions and long-term steroids (leading to secondary myopathy and osteoporosis). Investigations revealed a normal serum sodium (141mmol/l) a low urine osmolality (153mOsm/kg) and urine sodium (<20mmol/l). He was admitted for investigation and treatment of clinical diabetes insipidus.
CT scanning of his chest, abdomen and pelvis revealed extensive mediastinal lymphadenopathy, hepatic, pulmonary and para-aortic node involvement. MRI brain revealed an abnormal soft tissue mass in the pituitary fossa (Figure 2 C+D), extending into the pituitary stalk which was thickened, with some slight expansion of the sella. The optic chiasm appeared unremarkable. Hormone profiling revealed panhypopituitarism with secondary hypogonadism (LH 0.4IU/l, testosterone <0.2nmol/l), hypothyroidism (TSH 0.14mU/l, free thyroxine 6.5pmol/l) and hypoadrenalism Cortisol was 154 nmol/l 30 minutes after synacthen (RR > 450). He was commenced on desmopressin and prednisolone.
The midline distribution and raised β-HCG (129.6U/l) suggested a testicular tumour: however testicular ultrasound was normal. Lymph node biopsy revealed a mucin secreting adenocarcinoma of unknown primary, most probably of the upper GI tract. He received palliative chemotherapy with carboplatin but this had to be withdrawn after a single cycle due to poor clinical condition. He developed respiratory failure from a combination of his asthma, pneumonia, tumour obstructed bronchi and lymphangitis carcinomatosis. He received palliative care and died less than one month after his initial presentation.
Case 4
A 73-year-old lady presented with an acute loss of power in both her legs whilst a surgical inpatient for treatment of acute cholecystitis with the development of hyponatraemia (127 mmol/l). Her medical history included retrosternal goitre, heart failure and bilateral total hip replacements. On examination she had absent lower limb reflexes and was found to be in urinary retention. Sensation was intact over her legs and anus, however she was unable to feel the insertion of a urinary catheter. An urgent MRI scan of her spine revealed metastatic infiltration of vertebra T5-8 and L1 causing cord compression at the level of T5/6. On MRI brain imaging an infiltrating pituitary lesion (Figure 3) was centered in the sella. The soft tissue extended into the sphenoid sinuses, the cavernous sinuses and along the dorsum sellae over the posterior cisternal surface of the clivus. The pituitary gland was bulky within an expanded sella. She was commenced on dexamethasone and received emergency radiotherapy to which she responded well. CT CAP did not reveal a primary tumour. Endocrine investigations showed hyperprolactinaemia (1517mU/l) and primary hyperthyroidism (TSH 0.01mU/l and free thyroxine 39.6pmol/l) although the latter was related to her retrosternal goitre rather than any carcinoma. Measurements of serum osmolality (256mOsm/kg) and serum sodium (123mmol/l) were consistent with a diagnosis of SIADH. Her condition worsened with bronchopneumonia following transfer to the oncology centre and she died three weeks after her initial admission.
Figure 3.
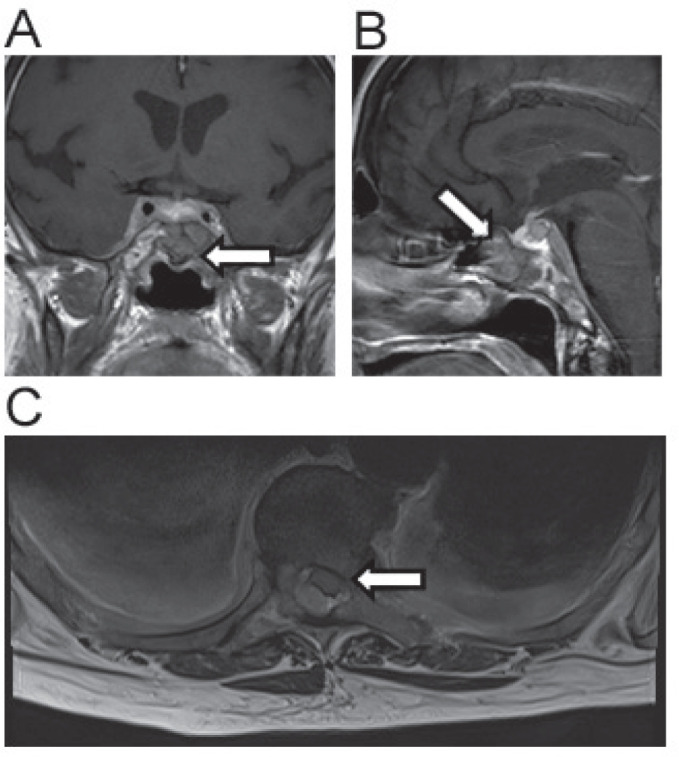
Imaging related to case 4. Coronal MRI image demonstrating compression of pituitary gland by cancer of unknown primary (A). Sagittal view of same lesion (B). Spinal cord compression by a posteriorly situated mass at the level T5/6 (C).
INCIDENCE
Pituitary metastasis occurs most often between the ages of 60 and 70 years and incidence is unaffected by gender4. PM is rare, accounting for only 0.4% of secondary intracranial tumours. It is estimated that of all trans-sphenoidal hypophysectomies performed for cancer only 1% are for metastatic lesions17. A recent analysis of patients with PM reported an incidence of 1.9%, which implies they are often under recognised or fail to present clinically18.
IMAGING
Failure of clinical recognition may be explained by the fact that most pituitary metastasis are asymptomatic. Many are noted incidentally on imaging studies performed for other clinical reasons. Differentiating PM from pituitary adenomas can be difficult. PM on pituitary MRI can appear as dumbbell shaped and can indent the diaphragm sellae. There can be sellar erosion without sellar enlargement and loss of the posterior pituitary bright spot19. As pituitary adenomas can present with increased metabolic uptake, functional imaging with PET-FDG is limited in distinguishing between adenomas and PM.
PATHOLOGY
More than half of all reported cases arise from the breast and lung. Other primary carcinomas known to metastasize to the pituitary in around 3-5% of cases include kidney, prostate and colon. Other rare causes include melanoma, thyroid, pancreas, haematological and from unknown primary cancers20-22.
ENDOCRINE DYSFUNCTION
Cranial diabetes insipidus is the most common presenting complaint of pituitary metastasis and is present in around 50% of PM cases11. Other signs and symptoms include visual disturbance, cranial nerve palsies and headache. Anterior pituitary deficiency has been reported in around 25-45% of cases with central hypothyroidism and hypocortisolism frequently identified.Asin our case series hyperprolactinaemia is also commonly found in around 2/3 of cases, presumably from pituitary stalk interruption23-24. Hyperfunction is more unusual but there are reports of acromegaly and Cushing’s syndrome related to pituitary metastases from excessive GH and ACTH secretion respectively. SIADH is also a rare feature, estimated to occur in 1.5% of cases19. However in cases with apparent hyper function it can be difficult to exclude alternative causes, such as co-existing microscopic adenomas of the pituitary or ectopic secretion.
MECHANISMS OF METASTATIC SPREAD
Several mechanisms of metastatic spread to the pituitary have been proposed. Conventional haematogenous spread can occur in two ways, either through the hypophyseal arteries of the posterior lobe or the hypophyseal portal system of the anterior lobe. The second-degree nature of the portal system may confer a degree of protection to the adenohypophysis by siphoning malignant cells from the rest of the body’s circulation. This explains why most metastases appear in the neurohypophysis. Only 15% of pituitary metastases involve the anterior lobe alone25-26. Alternatively, this could be due to the smaller volume of the posterior lobe, making it more vulnerable to disruption of function than a similarly sized lesion in the anterior lobe and thus more likely to be diagnosed by producing symptoms. Another mechanism is through circulating cerebrospinal fluid, which can act as a vector for malignant cells throughout the central nervous system19. This also contributes to the increased likelihood of posterior lobe involvement as it has more surface area in direct contact with the meninges. Finally, and less commonly, bony metastases to the base of the skull can erupt from the bone’s surface to compress or invade the gland as seen in the MRI of case two.
MANAGEMENT
There is lack of consensus on how best to manage PM due to a paucity of data in addition limited survival of these patients leaves little time for meaningful research to take place and the inherent low incidence makes recruitment for sufficiently powered trials on outcomes difficult. The effects of chemotherapy are largely unknown. Surgical resection of pituitary metastasis has been shown to have beneficial effects through symptom relief but is not associated with increased survival12. Total resection via the trans-sphenoidal approach is technically challenging due to invasion of densely packed adjacent structures, such as the cavernous sinus, and due to high vascularization. Radiotherapy has been shown to have comparable symptomatic relief and may be better tolerated than surgery due to fewer complications and can be the more easily delivered option in the palliative setting27.
PROGNOSIS
The prognosis of patients diagnosed with pituitary metastases is poor, as they invariably occur in the context of advanced disease. Estimated survival is between 6 and 22 months (median of 12.9 months) and no treatment has been shown to have a significant survival advantage1, 8. Younger age, smaller volume lesion and time between initial disease diagnosis and pituitary metastasis presentation have been associated with longer survival1.
SUMMARY
Pituitary metastases are rare and under recognised but not innocuous. While their impact on survival is devastating for a patient’s prognosis there is significant scope for symptom relief in the inevitable palliative setting. Clinicians should have a low threshold of suspicion for pituitary involvement in malignant disease, particularly in patients presenting with features of diabetes insipidus.
Table 1. Patient characteristics at diagnosis.
| Case | Age at diagnosis | Gender | Primary disease | Survival* |
|---|---|---|---|---|
| 1 | 18 | Male | Large B-cell lymphoma | 3 |
| 2 | 53 | Male | Multiple myeloma | 1 |
| 3 | 41 | Male | Unknown primary | 1 |
| 4 | 73 | Female | Unknown primary | <1 |
Survival after diagnosis of PM (in months)
Table 2. Pituitary function at initial presentation.
| Hormone | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| ACTH | ND | ↔ | ND | ↔ |
| Cortisol | ND | ↔ | ↓ | ND |
| FSH | ↓ | ↔ | ↓ | ↓ |
| IGF-1 | ND | ↔ | ↔ | ↔ |
| LH | ND | ↔ | ↓ | ↓ |
| Prolactin | ↑ | ↑ | ↔ | ↑ |
| Testosterone | ↓ | ↓ | ↓ | ND |
| Free Thyroxine | ↓ | ↔ | ↓ | ↑ |
| TSH | ↓ | ↔ | ↓ | ↓ |
Anterior pituitary function measured at time of first recognition of pituitary involvement (↔, within normal range; ↑, beyond upper limit of normal; ↓, below lower limit of normal). ACTH, adrenocorticotrophic hormone; FSH, follicle stimulating hormone; IGF1, insulin-like growth factor; LH, luteinising hormone; TSH, thyroid stimulating hormone), ND-not done.
Footnotes
UMJ is an open access publication of the Ulster Medical Society (http://www.ums.ac.uk).
REFERENCES
- 1.Habu M, Tokimura H, Hirano H, Yasuda S, Nagatomo Y, Iwai Y, et al. Pituitary metastases: current practice in Japan. J Neurosurg. 2015;123(4):998–1007. doi: 10.3171/2014.12.JNS14870. [DOI] [PubMed] [Google Scholar]
- 2.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3(1):74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus. 2004;16(4):E8. [PubMed] [Google Scholar]
- 4.He W, Chen F, Dalm B, Kirby PA, Greenlee JD. Metastatic involvement of the pituitary gland: a systematic review with pooled individual patient data analysis. Pituitary. 2015;18(1):159–68. doi: 10.1007/s11102-014-0552-2. [DOI] [PubMed] [Google Scholar]
- 5.Morita A, Meyer FB, Laws ER. Symptomatic pituitary metastases. J Neurosurg. 1998;89(1):69–73. doi: 10.3171/jns.1998.89.1.0069. [DOI] [PubMed] [Google Scholar]
- 6.Luu ST, Billing K, Crompton JL, Blumbergs P, Lee AW, Chen CS. Clinicopathological correlation in pituitary gland metastasis presenting as anterior visual pathway compression. J Clin Neurosci. 2010;17(6):790–3. doi: 10.1016/j.jocn.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 7.Schubiger O, Haller D. Metastases to the pituitary--hypothalamic axis. An MR study of 7 symptomatic patients. Neuroradiology. 1992;34(2):131–4. doi: 10.1007/BF00588159. [DOI] [PubMed] [Google Scholar]
- 8.Ismail E, Issam L, Hamid M. Pituitary metastasis of rhabdomyosarcoma: a case report and review of the literature. J Med Case Rep. 2014;8:144. doi: 10.1186/1752-1947-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson PB, Robinson AG, Martinez AJ. Metastatic tumor of the pituitary gland. Neurosurgery. 1987;21(6):941–4. doi: 10.1227/00006123-198712000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Zager EL, Hedley-Whyte ET. Metastasis within a pituitary adenoma presenting with bilateral abducens palsies: case report and review of the literature. Neurosurgery. 1987;21(3):383–6. doi: 10.1227/00006123-198709000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Javanbakht A, D’Apuzzo M, Badie B, Salehian B. Pituitary metastasis: a rare condition. Endocr Connect. 2018;7(10):1049–57. doi: 10.1530/EC-18-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoli M, Mazzatenta D, Faustini-Fustini M, Pasquini E, Frank G. Pituitary metastases: role of surgery. World Neurosurg. 2013;79(2):327–30. doi: 10.1016/j.wneu.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Lithgow K, Siqueira I, Senthil L, Chew HS, Chavda SV, Ayuk J, et al. Pituitary metastases: presentation and outcomes from a pituitary center over the last decade. Pituitary. 2020;23(3):258–65. doi: 10.1007/s11102-020-01034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs K. Metastatic cancer of the pituitary gland. Oncology. 1973;27:533–42. doi: 10.1159/000224763. [DOI] [PubMed] [Google Scholar]
- 15.Siqueira PF, Mathez AL, Pedretti DB, Abucham J. Pituitary metastasis of lung neuroendocrine carcinoma: case report and literature review. Arch Endocrinol Metab. 2015;59(6):548–53. doi: 10.1590/2359-3997000000139. [DOI] [PubMed] [Google Scholar]
- 16.Max MB, Deck MD, Rottenberg DA. Pituitary metastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology. 1981;31(8):998–1002. doi: 10.1212/wnl.31.8.998. [DOI] [PubMed] [Google Scholar]
- 17.Chuang YC, Tsai CC. Pituitary metastasis of breast cancer: a case report. J. Gerontol. 2018;12(3):267. [Google Scholar]
- 18.Castle-Kirszbaum M, Goldschlager T, Ho B, Wang YY, King J. Twelve cases of pituitary metastasis: a case series and review of the literature. Pituitary. 2018;21(5):63–473. doi: 10.1007/s11102-018-0899-x. [DOI] [PubMed] [Google Scholar]
- 19.Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89(2):574–80. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 20.Souza Mota J, de Sa Caldas A, de Araujo Cortes Nascimento AGP, Dos Santos Faria M, Pereira Sobral CS. Pituitary metastasis of thyroid carcinoma: a case report. Am J Case Rep. 2018;19:896–902. doi: 10.12659/AJCR.909523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormando M, Puliani G, Barnabei A, Lauretta R, Bianchini M, Chiefari A, et al. A rare case of pituitary melanoma metastasis: a dramatic and prolonged response to dabrafenib-trametinib therapy. Front Endocrinol. 2020;11:471. doi: 10.3389/fendo.2020.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gur C, Lalazar G, Salmon A, Dubiner V, Gross DJ. Metastatic pancreatic neuroendocrine tumor presenting as a pituitary space occupying lesion: a case report. Pituitary. 2008;11(3):293–7. doi: 10.1007/s11102-007-0053-7. [DOI] [PubMed] [Google Scholar]
- 23.Glezer A, Bronstein MD. Prolactinomas. Endocrinol Metab Clin North Am. 2015;44(1):71–8. doi: 10.1016/j.ecl.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Kruse A, Astrup J, Gyldensted C, Cold GE. Hyperprolactinaemia in patients with pituitary adenomas. The pituitary stalk compression syndrome. Br J Neurosurg. 1995;9:453–7. doi: 10.1080/02688699550041089. [DOI] [PubMed] [Google Scholar]
- 25.McCormick PC, Post KD, Kandji AD, Hays AP. Metastatic carcinoma to the pituitary gland. Br J Neurosurg. 1989;3:71–9. doi: 10.3109/02688698909001028. [DOI] [PubMed] [Google Scholar]
- 26.Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the pituitary gland. Cancer. 1975;36(1):216–20. doi: 10.1002/1097-0142(197507)36:1<216::aid-cncr2820360123>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Kano H, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for pituitary metastases. Surg Neurol. 2009;72(3):248–55. doi: 10.1016/j.surneu.2008.06.003. [DOI] [PubMed] [Google Scholar]


