Abstract
The ability to efficiently isolate and expand various stem cell populations in vitro is crucial for successful translation of cell-based therapies to the clinical setting. One such heterogeneous population that possesses a remarkable potential for the development of cell-based treatments for a variety of degenerative diseases and disorders is called the Side Population (SP). For many years, investigators have isolated these primitive cells based upon their ability to efflux the fluorophore Hoechst 33342. This attribute enabled separation of SP cells derived from multiple tissue sources from other endogenous cell populations using fluorescence-activated cell sorting (FACS). While all tissue-specific SP fractions appear to contain cells with multi-potent stem cell activity, the therapeutic utility of these cells has yet to be fully realized because of the scarcity of this fraction in vivo. In view of that, we developed a method to expand adult murine bone marrow and skeletal muscle-derived SP cells in vitro. Here, we describe a spinner-flask culture system that supports the growth of SP cells in suspension when they are combined with feeder cells cultured on spherical microcarriers. In this way, their distinguishing biological characteristics can be maintained, attachment-stimulated differentiation is avoided, and therapeutically relevant quantities of SP cells are generated. Modification of the described procedure may permit expansion of the SP from other relevant tissue sources and our method is amenable to establishing compliance with current good manufacturing practices.
Keywords: Side population cells, SP cells, Adult stem cells, Cell expansion, Microcarrier, Skeletal muscle, Bone marrow, Spinner flasks, Suspension culture
1. Introduction
There remains widespread interest in employing Side Population (SP) stem cells isolated from various tissue sources, including skeletal muscle and bone marrow, for cell-based therapies directed at regenerating or repairing damaged tissue. However, there are significant obstacles to overcome before any such treatment would be considered clinically relevant [1-3]. The inability to dependably grow ex vivo cultures of this population continues to represent a fundamental impediment to translating SP cell-based therapeutics to the clinic. A clearly defined and well-controlled means to consistently expand SP cells in culture would enable sufficient cell yields to be generated for further experimental characterization of this fraction and evaluation of these cells as an autologous, regenerative stem cell source in humans.
Thus far, there have been few attempts to culture primary SP cells specifically for in vitro expansion. In general, SP cell cultures produce small, uniform, non-adherent, rounded cells that demonstrate limited ability to proliferate [4-7]. Previously published reports using cultured SP cells utilized various matrices (e.g., methylcellulose or collagen) in combination with supplements to study the differentiation potential of this fraction [8-14]. Although these approaches did promote some proliferation of differentiated hematopoietic lineages, they were never intended to yield sufficient numbers of undifferentiated SP cells for repair of tissues of mesenchymal lineage like the heart or skeletal muscle.
Here, we describe a three-dimensional (3D) coculture bioreactor to grow SP cells from two commonly used tissue sources; namely, skeletal muscle and bone marrow [15]. We reasoned that successful expansion of undifferentiated SP cells would require contributions from other cells normally associated with their in vivo microenvironment or niche. For this reason, we decided to maintain the SP in coculture with “feeder” cells that would presumably provide growth factors and, possibly, other conditions necessary for long-term, in vitro cell survival and proliferation. At the same time, we sought to provide a simple means to separate expanded SP cells from feeder cells. To achieve this, we employed a microcarrier-based suspension culture system. Using this approach, feeder cells were grown as a single layer on the surface of small spheres and maintained in suspension using magnetic impeller-driven spinner flasks placed on a stir plate in a tissue culture incubator [15-17]. Following an equilibration period, SP cells were added to these bioreactor flasks to determine their growth and differentiation status over the course of several passages.
The culture system was designed to allow for effortless separation of feeder cells from suspended SP cells. Conceptually, this separation would be performed by allowing the relatively heavy microcarriers (with feeder cells attached to them) to sink to the bottom of the spinner flasks by simply removing them from the magnetic stir plate. Suspended cells and settled microcarriers would then be collected separately. In practice, we found SP cells often aggregate around the feeder cell-covered microcarriers and they need to be harvested using mild enzymatic and mechanical dissociation techniques [15]. On the other hand, de-cellularized microcarriers can be readily separated from cocultures by filtration. Despite the inconvenience of working with mixed cell populations, we found a large number of expanded SP cells, whether derived from bone marrow or skeletal muscle, retain the ability to efflux Hoechst dye and possess a protein expression profile consistent with freshly isolated, unfractionated SP cells. While some SP cells grown in culture do undergo lineage commitment and differentiation, they can be easily distinguished from undifferentiated cells using flow cytometry and protein expression analyses.
In summary, we have developed a method to grow SP stem cells using a microcarrier-based coculture bioreactor system. This method allows for expansion of this rare cell fraction through multiple passages and collection of bone marrow and skeletal muscle-derived SP cells that retain attributes of freshly isolated cells. We believe this culture system could be modified to support the growth of SP cells from other tissue sources, such as the heart, by simply changing the type of feeder cells to be attached to the microcarriers [18]. In addition, the method described here should stimulate further study of this cell fraction with the goal of streamlining the process of isolating and purifying these cells. Ultimately, we anticipate that this method will establish the therapeutic potential of the SP in regenerating or repairing damaged tissue.
2. Materials
2.1. Disposables
Mice (C57Bl/6 or C57BL/6-Tg[ACTB-EGFP]1Osb/J strains) (Jackson Laboratory).
70 % Ethanol (EtOH).
Hanks Buffered Salt Solution (HBSS).
Razor blades.
Muscle Digestion Buffer: 100 mL Dispase 2, 1 g Collagenase 2, 0.037 g CaCl2 passed through a 0.2 μm syringe filter and stored at −20 °C.
40 μm Cell Strainer.
100 μm Cell Strainer.
1× Ca2+ and Mg2+- free Phosphate Buffered Saline.
1× PBS containing 0.5 % bovine serum albumin (1× PBS + 0.5 % BSA).
Red blood cell lysis buffer: 0.2 % Tris-HCl (pH 7.5), 0.747 % NH4Cl (store at 4 °C).
Hoechst 33342.
Verapamil.
Propidium iodide.
C2C12 myoblast cell line.
150 mm tissue culture plates.
C2C12 expansion medium: High-glucose Dulbecco’s Modified Eagle Medium (DMEM) GlutaMAX with sodium pyruvate and phenol red, 20 % fetal bovine serum (FBS), 1 % penicillin–streptomycin, 1 % Fungizone.
0.05 % trypsin–EDTA.
15 and 50 mL CELLSTAR conical tubes.
Cytodex 1 microcarriers.
Petri dishes.
Sigmacote.
Basic fibroblast growth factor (bFGF).
Mouse bone marrow or skeletal muscle.
1 % Trypsin.
Disposable serological pipettes (1, 5, 10, and 25 mL sizes).
Multi-wipes laboratory wipes.
23 G 1″ needle and 3 cc syringe.
2.2. Equipment
Dissection tools (scissors, scalpels, forceps).
Centrifuge.
Humidified cell culture incubator.
Fluorescence Activated Cell Sorter.
100 mL borosilicate glass spinner flasks with an internal overhead assembly or adjustable hanging bar with curved polytetrafluo-roethylene paddle.
250 mL borosilicate glass spinner flasks with an internal overhead assembly or adjustable hanging bar with curved polytetrafluo-roethylene paddle.
Bellenium 5-position magnetic stirrer or MultiMagStir Genie.
Enviro-Genie incubator.
Autoclave.
SterilGARD III Advance° Laminar Flow Hood.
Portable Pipette-Aid.
3. Methods
3.1. Preparation of Microcarrier Suspension Cultures
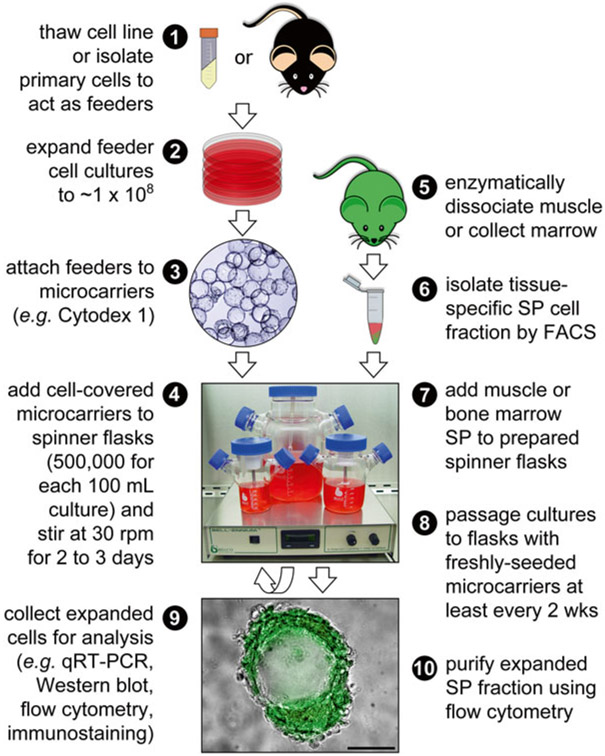
Culture myoblast feeder cells on 150 mm plates in C2C12 expansion medium (Fig. 1), (see Note 1).
Hydrate and sterilize 1 × 106 Cytodex 1 microcarriers using purified water, 70 % ethanol, PBS and C2C12 expansion medium according to the manufacturer’s directions.
Coat two 100 mL borosilicate glass spinner flasks with Sigmacote and sterilize for 20 min in the autoclave on the dry cycle.
Remove the medium from approximately 1 × 108 C2C12 cells by aspiration and rinse the cells twice with 37 °C 1× PBS (see Note 2).
Detach cells from each plate using 3 mL of pre-warmed 0.05 % trypsin–EDTA at 37 °C for 2–5 min.
Add 5 mL expansion medium to each plate to inactivate the trypsin and help detach cells from the culture plate surface.
Collect the detached cells in 50 mL conical tubes and centrifuge at 600 × g.
Remove the supernatant and resuspend cell pellets in a total 30 mL 37 °C C2C12 expansion medium and combine with the prepared microcarriers.
Divide the entire preparation between 6 and 8 100 mm petri dishes and place in a humidified culture incubator with 5 % CO2. Agitate the dishes every 15 min for 2 h by gently shaking the dishes back and forth (see Note 3).
Assemble two sterilized, Sigmacote-treated 100 mL glass spinner flasks in the culture hood, if necessary.
Divide the feeder cell-covered microcarriers equally between the two spinner flasks (i.e., 500,000 in each flask) and fill to 100 mL with 37 °C C2C12 expansion medium.
Loosen the side-arm caps half way to allow for gas transfer in the culture incubator.
Stir the flask contents at 30 rpm using a magnetic stirrer placed in a humidified tissue culture incubator (see Note 4).
Fig. 1.
Schematic overview of the method to expand SP stem cells in suspension. The C2C12 myoblast cell line was used as the feeder cell layer on microcarriers; however, other adherent cell lines or appropriate primary cells could be used. Here, we employed SP cells isolated from mice expressing enhanced green fluorescent protein (EGFP) to allow the expanding SP population to be distinguished from the feeder cells. The procedural order is indicated by numbers and the scale bar = 100 μm
3.2. Isolation of Skeletal Muscle Cells
Euthanize a mouse using CO2-asphyxiation followed by cervical dislocation. Pin down limbs on a silicone dissection pad and soak the fur with 70 % EtOH.
Extract the major hind-limb muscles (quadriceps, gastronemius, tibialis anterior), forelimb muscles (triceps), as well as the paraspinal muscles and place these in a 50 mL conical tube containing 25 mL HBSS (see Note 5).
Transfer all skeletal muscle to a 100 mm petri dish with approximately 2 mL HBSS and using forceps and scalpel, remove as much fat and connective tissue as possible (see Note 6).
Transfer the muscle pieces to a clean petri dish with approximately 2 mL HBSS.
Using two razor blades finely mince the muscle into slurry using sterile gloves.
Add 10 mL of 37 °C muscle digestion buffer.
Place muscle slurry into a 50 mL conical tube with warm digestion buffer.
Digest for approximately 45 min or until tissue fragments are no longer apparent using an Enviro-Genie on the 15:30 rocking setting at 37 °C.
Throughout the digest triturate every 5 min by drawing the liquid up and down with a disposable serological pipette (see Note 7).
Place a 100 μm Cell Strainer over a sterile 50 mL conical tube to filter out connective tissue and undigested fragments from the digestion solution.
Rinse the Cell Strainer with 1× PBS + 0.5 % BSA to collect residual cells trapped in the nylon mesh.
3.3. Isolation of Bone Marrow Cells
Dissect skeletal muscle and other tissue from the region surrounding the femur and tibia in situ.
Remove these bones from the body by cutting as close to the pelvis as possible while avoiding fragmenting the bones.
Carefully and thoroughly clean the bones using a razor blade and laboratory wipes (see Note 8).
Cut at the knee joint to separate the femur and tibia.
Use a 23 G needle and 3 cc syringe to inject 1× PBS + 0.5 % BSA into the marrow cavity of each bone and force the contents out the other end into a clean 15 mL conical tube.
Dilute the bone marrow/PBS + 0.5 % BSA mixture (usually ~3 mL) 1:7 with red blood cell lysis buffer (usually ~21 mL).
Strain the solution through a 100 μm Cell Strainer collecting the wash-through in a 50 mL conical tube.
Rinse the Cell Strainer with 1× PBS + 0.5 % BSA to collect residual cells trapped in the nylon mesh.
3.4. Preparation of Isolated Skeletal Muscle or Bone Marrow Cells for Flow Cytometry
Filter the dissociated skeletal muscle and bone marrow cell suspensions through a 40 μm Cell Strainer and collect flowthrough using a 50 mL conical tube.
Rinse the Cell Strainer with 1× PBS + 0.5 % BSA to collect residual cells trapped in the nylon mesh.
Centrifuge (600 × g) at 4 °C for 10 min and resuspend to 1 × 106 cells/mL in 1× PBS + 0.5 % BSA.
Add 100 mM verapamil to an aliquot of each cell type to provide a negative control for SP cell gating.
Add Hoechst 33342 at a concentration of 5 μg/mL for bone marrow cells and 12.5 μg/mL for skeletal muscle cells (i.e., both the primary cell suspensions and the control aliquots containing verapamil) and stain in the dark for 90 min at 37 °C using a water bath.
Wash all stained cells with 5 volumes of PBS + 0.5 % BSA.
Prior to flow cytometric analysis and sorting, resuspend the cells in PBS + 0.5 % BSA containing 2 μg/mL propidium iodide for 5 min and store on ice (see Note 9).
3.5. Fluorescence Activated Cell Sorting (FACS) of SP Cells
Perform cell sorting using a FACS machine capable of generating an excitation wavelength of 365 nm.
Measure fluorescence emission using a 400 nm long-pass filter for Hoechst 33342 and a 600 nm long-pass filter for propidium iodide.
Set the sort head frequency at 26,000 Hz using the “normal sort” mode.
Set the sheath pressure to 11–12 psi.
Following cytometric analysis of a portion of each sample, set the gate to select for live cells (the fraction emits the least red signal) with the lowest Hoechst dye concentrations (the fraction emits the least blue signal) (see Note 10).
3.6. Inoculation and Maintenance of Suspension Cultures
2–3 days after setting up the microcarrier spinner flasks (steps described in Subheading 3.1), supplement the culture medium with 5 ng/L bFGF and inoculate each flask with freshly isolated SP cells (see Note 11). This culture is referred to as passage 0 (P0).
To replenish medium, remove the flasks from the stir plate and allow the microcarriers and SP cells to settle to the bottom of the flasks (5–10 min) (see Note 12).
Slowly remove 50–70 % of the medium from the top portion of the flask and replace it with fresh pre-warmed medium containing 5 ng/L bFGF (see Note 13).
3.7. Passaging of Suspension Cultures
No more than 2 weeks after SP cell inoculation, collect the contents of each flask by allowing cells and microcarriers to settle on the bottom of the flask (see Note 14).
Remove most of the medium and transfer the cell and microcarrier aggregates to 50 mL conical tubes.
Allow the contents to settle and remove as much medium as possible.
Rinse the cell and microcarrier aggregates twice with 1× PBS.
Incubate the aggregates with about 5 mL 1 % trypsin and agitate using the Enviro-Genie incubator at 37 °C with a 5:10 rocking setting for 10 min.
Stop the trypsin reaction by adding 10 mL FBS.
Remove the microcarriers from the rest of the dispersed cell suspension by passing the mixture through a 100 μM Cell Strainer.
Rinse the Cell Strainer with 1× PBS + 0.5 % BSA to collect residual cells trapped in the nylon mesh.
Centrifuge the filtered solution at 600 × g for 10 min at room temperature.
Remove supernatant and resuspend cell pellets in 25 mL each of expansion medium containing bFGF.
Add these resuspensions to 250 mL spinner flasks each containing 1 × 106 microcarriers prepared and seeded with C2C12 cells as described above (see Notes 15 and 16). These cultures are referred to as passage 1 (P1).
In the next passage, cells are split into 2 × 250 mL spinner flasks (P2) and then 4 × 250 mL spinner flasks for each culture (P3).
4. Notes
We used the C2C12 cell line to develop this method; however, Main Population (MP) cells from bone marrow or skeletal muscle could be used for this purpose. Alternatively, if SP cells were isolated from a different tissue source, an appropriate tissue-specific feeder cell would be desirable.
Usually four to six 50–70 % confluent 150 mm tissue culture dishes provide 1 × 108 C2C12 cells. C2C12 cells should be passaged at no more than 70 % confluence to prevent fusion and differentiation of these cells into multinucleated myotubes.
Petri dishes should be used rather than tissue culture plates as feeder cells will not attach to this plastic. This forces the feeders to adhere to the microcarrier surface.
The stir bar and paddle assembly should rotate at a steady rate and not in an erratic manner. A stable, continuous mixing of the culture minimizes the chance of cells detaching from the microcarrier surface as a result of intermittent exposure to shear forces.
Keep muscles wet with 1× PBS throughout muscle isolation procedure to maintain cell viability.
This step and those immediately following it should be performed in a laminar flow hood.
Avoid bubbling the liquid during trituration and use progressively smaller volume pipettes as the decreasing entrance hole diameter will help break the tissue down.
Meticulous removal of all cells and tissue from the outside of the bones reduces the chance of non-marrow contaminates and permits visualization of the marrow.
It is very important to keep the cells on ice and move quickly to the FACS machine to acquire consistent data as well as viable cells to culture following the sort.
What has been described here is, by definition, the side population or SP. This is a greatly abbreviated version of the steps required to identify and purify this stem cell-containing population. There are numerous publications describing these steps in greater detail [5, 19, 20].
Generally, from a single mouse there will be 10,000–15,000 muscle SP cells and 20,000–50,000 bone marrow SP cells.
It is absolutely crucial that the medium in SP cell suspension/expansion flasks is replenished every 2–3 days.
Small aliquots of the culture (5 mL) can be collected from the side arms of the suspension flasks as needed for enumeration and analysis (Fig. 1).
If cultures are allowed to grow longer than 15 days before passaging, the feeder cells will begin to detach from the microcarriers, which negatively impacts SP expansion.
As the culture continues to expand the cells can continue to be passaged every 2 weeks by dividing the SP cell yield from each flask in ½ and using each ½ to seed a freshly prepared 250 mL spinner flask.
Increasing the spinner-flask size beyond the 250 mL volume (i.e., 1,000 mL) results in a collapse in the culture, likely due to insufficient gas exchange within the culture.
Acknowledgements
Funding was provided by a grant from the National Institutes of Health (HL088206), a Grant-in-Aid from the American Heart Association (12GRNT11910008), the Children’s Hospital Medical Corporation Anesthesia Foundation, a grant from the Children’s Heart Foundation and donations to the Cardiac Conduction Fund, the Ryan Family Fund, and by David Pullman.
References
- 1.Cossu G (2004) Fusion of bone marrow-derived stem cells with striated muscle may not be sufficient to activate muscle genes. J Clin Invest 114:1540–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossu G, Sampaolesi M (2004) New therapies for muscular dystrophy: cautious optimism. Trends Mol Med 10:516–520 [DOI] [PubMed] [Google Scholar]
- 3.Itescu S, Schuster MD, Kocher AA (2003) New directions in strategies using cell therapy for heart disease. J Mol Med 81:288–296 [DOI] [PubMed] [Google Scholar]
- 4.Bachrach E, Li S, Perez AL et al. (2004) Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A 101:3581–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montanaro F, Liadaki K, Schienda J et al. (2004) Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res 298:144–154 [DOI] [PubMed] [Google Scholar]
- 6.Majka SM, Jackson KA, Kienstra KA et al. (2003) Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest 111:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeson AP, Hawke TJ, Graham S et al. (2004) Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells 22: 1305–1320 [DOI] [PubMed] [Google Scholar]
- 8.Hierlihy AM, Seale P, Lobe CG et al. (2002) The post-natal heart contains a myocardial stem cell population. FEBS Lett 530: 239–243 [DOI] [PubMed] [Google Scholar]
- 9.McKinney-Freeman SL, Majka SM, Jackson KA et al. (2003) Altered phenotype and reduced function of muscle-derived hematopoietic stem cells. Exp Hematol 31:806–814 [DOI] [PubMed] [Google Scholar]
- 10.McKinney-Freeman SL, Jackson KA, Camargo FD et al. (2002) Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci U S A 99:1341–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asakura A, Seale P, Girgis-Gabardo A et al. (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamaki T, Akatsuka A, Okada Y et al. (2003) Growth and differentiation potential of main- and side-population cells derived from murine skeletal muscle. Exp Cell Res 291:83–90 [DOI] [PubMed] [Google Scholar]
- 13.Tamaki T, Akatsuka A, Ando K et al. (2002) Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 157:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadin BM, Goodell MA, Hirschi KK (2003) Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood 102:2436–2443 [DOI] [PubMed] [Google Scholar]
- 15.Pacak CA, Eddy MT, Woodhull L et al. (2013) Microcarrier-based expansion of adult murine side population stem cells. PLoS One 8:e55187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis DH, Volkers SA (1979) Use of a new bead microcarrier for the culture of anchorage dependent cells in pseudo suspension. Dev Biol Stand 42:147–151 [PubMed] [Google Scholar]
- 17.van Wezel AL (1967) Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 216:64–65 [DOI] [PubMed] [Google Scholar]
- 18.Sereti KI, Oikonomopoulos A, Unno K et al. (2013) Methods to study the proliferation and differentiation of cardiac side population (CSP) cells. Methods Mol Biol 1036:95–106 [DOI] [PubMed] [Google Scholar]
- 19.Rossi L, Challen GA, Sirin O et al. (2011) Hematopoietic stem cell characterization and isolation. Methods Mol Biol 750:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodell MA, McKinney-Freeman S, Camargo FD (2005) Isolation and characterization of side population cells. Methods Mol Biol 290: 343–352 [DOI] [PubMed] [Google Scholar]