Abstract
We report the case of a thrombotic occlusion of an extra-anatomical aortic bypass graft performed 37 years ago in a 46-year-old male with Takayasu’s arteritis. He presented with uncontrolled upper body hypertension, claudication pain in both the lower limbs, and kidney dysfunction which returned to normal post-surgery. On table we found a pseudoaneurysm at the proximal anastomosis along with calcification of the capsular tissue surrounding the graft. We attempt to explain the mechanism behind the graft occlusion, along with management of this late surgical complication which has not been described in the existing literature.
Keywords: Cardiovascular, surgery, rheumatology/clinical immunology
Introduction
Takayasu’s arteritis (TA) is a disease of unknown etiology characterized by segmental and patchy granulomatous inflammation of the aorta and its major branches. This inflammation often leads to arterial stenosis, thrombosis, and aneurysms. According to the prognostic classification given by Ishikawa and Maetani, 1 patients with a progressive disease course marked by onset of severe symptoms more than a year from diagnosis, and a major complication such as an aortic aneurysm are classified under stage-3 TA requiring surgery. TA patients with long segment aortic stenosis often need extra-anatomic bypass. 2 TA patients requiring arterial reconstruction show symptomatic improvement and resolution of cardiac failure post-surgical intervention. 3 Long-term complications that can occur any time post-surgery include anastomotic false aneurysms, abdominal aortic aneurysms, renal failure, congestive heart failure, cerebrovascular accident, and graft deterioration. 4 The survival and event-free survival rates at 20 years post-aorto-aortic bypass with a prosthetic graft are 62.3% and 58.4%, respectively. 4 Authors present the graft occlusion case of a TA patient 37 years after extra-anatomic bypass.
Case report
A 46-year-old male with TA, status post an extra-anatomical aortic bypass 37 years ago presented with chief complaints of intractable migraine headaches with edema of face and upper limbs for 6 months, tingling and numbness in arms and legs for 3 months, occasional episodes of epistaxis, and claudication pain in the legs for 1 month. The patient was diagnosed with TA at the age of 9 years using the American College of Rheumatology criteria 5 with the presence of four of the six criteria’s, namely, being: (1) onset at age 9 years, (2) claudication pain of lower limbs, (3) bruits heard over the right subclavian artery and thoracic aorta, and (4) arteriographic evidence of narrowing of the mid-distal thoracic aorta and right subclavian artery. The patient was started on medical treatment with 20 mg oral prednisolone (1 mg/kg/day) and surgical management with extra-anatomical aorto-aortic bypass was performed. Post-surgery the patient’s symptoms resolved, and the dosage of prednisolone treatment was slowly tampered to 1 mg/day. Since the last 37 years, patient has not come for follow-up monitoring for TA.
The patient’s present symptoms started 6 months ago with severe headaches that lasted throughout the day without relief from any medications. During the same time, he also developed swelling in his face and upper limbs and claudication pain in his lower limbs that has caused significant impediment of his day-to-day activities. On physical examination, bruits were heard on auscultation of the abdomen, and his blood pressure was 160/110 mm Hg in the left upper limb, 155/100 in the right upper limb, and 70/30 mm Hg in both the lower limbs. His lab C-reactive protein level and erythrocyte sedimentation rate (ESR) were raised. The patient was started on 50 mg of prednisolone to control the systemic inflammation.
Chest and abdominal X-ray showed calcifications of the native abdominal aorta and extra-anatomical graft. Further imaging with computed tomography (CT) angiogram of aorta revealed severe narrowing of the mid-distal native thoracic aorta and a significant stenosis involving the right subclavian artery origin with distal reformation by collaterals from the vertebral artery. The extra-anatomical graft was occluded for 7.5 cm with a residual patent lumen measuring 3–4 mm in diameter just above the distal anastomosis (Figure 1(a) and (b)). The systolic pressure difference of 90 mm Hg and the diastolic pressure difference of 70 mm Hg between the upper limbs and lower limbs were seen due to obstruction of the extra-anatomical graft. His creatinine level was 2.46 mg/dL with an estimated glomerular filtration rate of 28.47 mL/min/1.73 m3 indicative of renal impairment. The patient with stage-3 TA was diagnosed with calcification and thrombosis of the extra-anatomical aortic bypass graft.
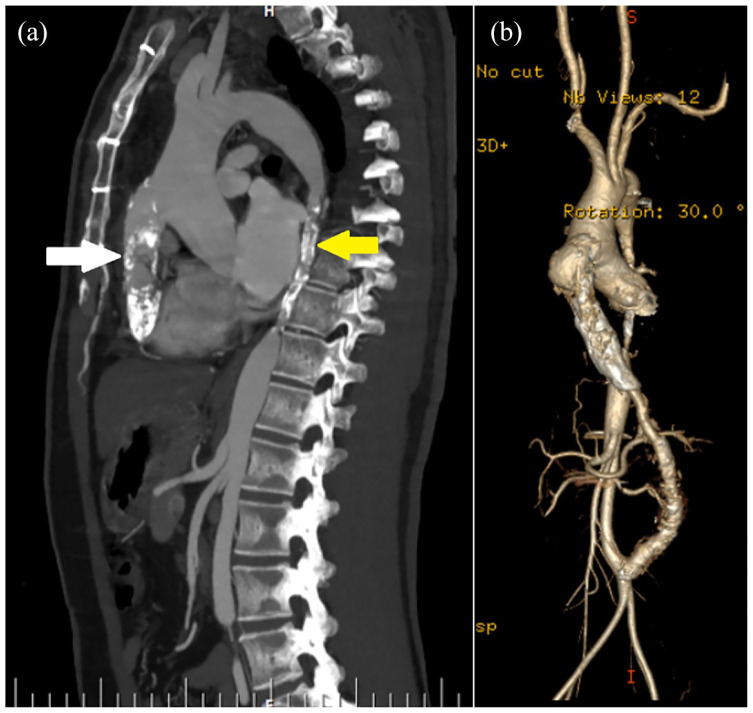
Figure 1.
(a) Pre-operative CT-angiogram sagittal-section showing severe narrowing of the extra-anatomic aortic graft (white arrow) and native aorta (yellow arrow). (b) Pre-operative 3D volume rendered image showing a pseudoaneurysm at the proximal anastomosis of the graft.
Due to severe obstruction and calcific damage to the bypass graft, surgical replacement of the graft was the only feasible option, and therefore, the patient was referred for open surgery. A Redo midline sterno-laparotomy was performed. Intra-operative findings showed compression of the right ventricle by the extra-anatomical graft (Figure 2(a)). There was an anastomotic dehiscence at the proximal end of the graft causing a leak that had thrombosed and blocked the extra-anatomical aortic graft. The proximal and distal end of the previous anastomosis were dissected and looped with a cotton tape; the graft was released from the surrounding adhesions. Systemic heparin was administered, and the ascending aorta was clamped with a side biting clamp. The previous anastomoses were opened, the dehisced margin was excised. Re-anastomosis was performed using a 22-mm Hemashield (Maquet-Getinge, Rastatt, Germany) graft with the proximal anastomosis re-done to the ascending aorta and distal anastomosis to the stump of the native graft above the infrarenal abdominal aorta (Figure 2(b)) using continuous 4-0 polypropylene sutures.
Figure 2.
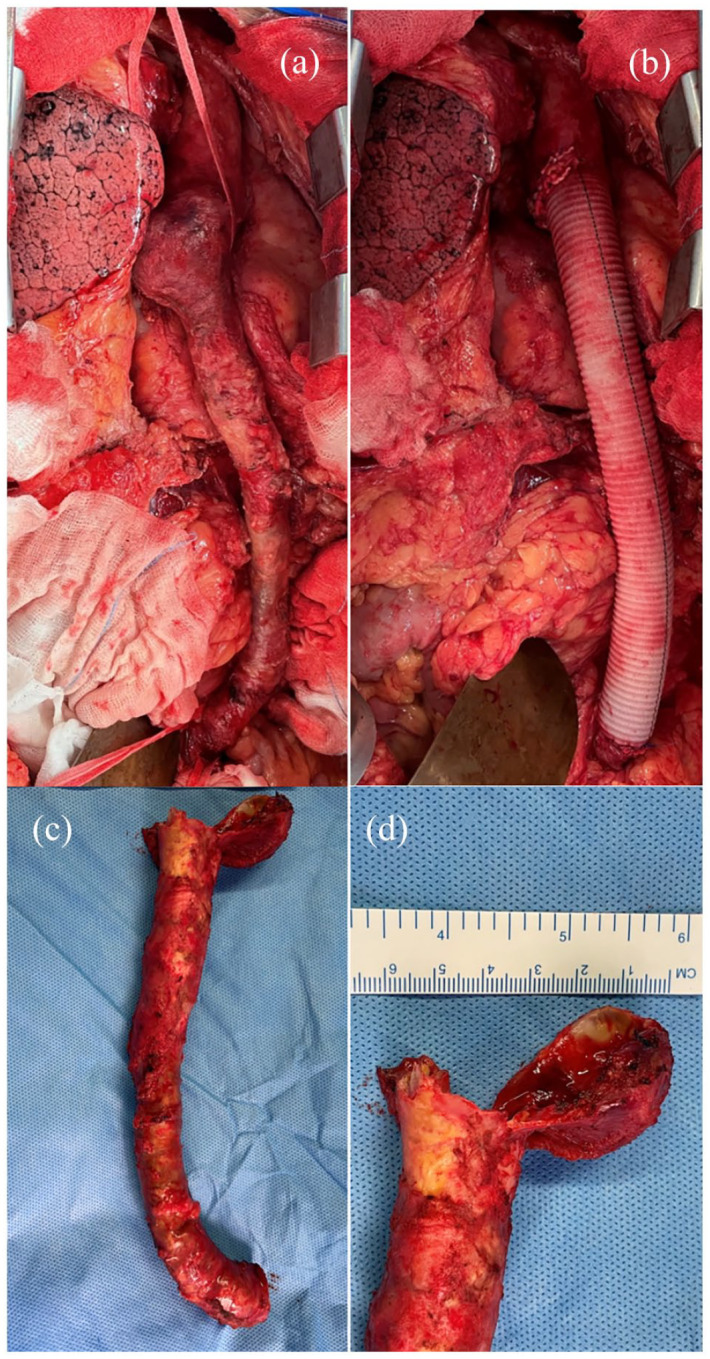
Intra-operative findings. (a) On table extra-anatomic graft showing tubular calcification. (b) Post-operative replacement with a Dacron graft. (c) Excised extra-anatomical graft. (d) Pseudo-aneurysm at the proximal anastomosis measuring 4 cm.
On inspection of the excised graft, there was a large pseudoaneurysm around the proximal end (Figure 2(c) and (d)). The tubular capsule of tissue around the graft was calcified, and the graft was extrinsically compressed by the calcified thrombotic content (Figure 3(a)). Inner surface of extra-anatomical graft showed ulcerations and a lumen filled with thrombosed blood (Figure 3(b)).
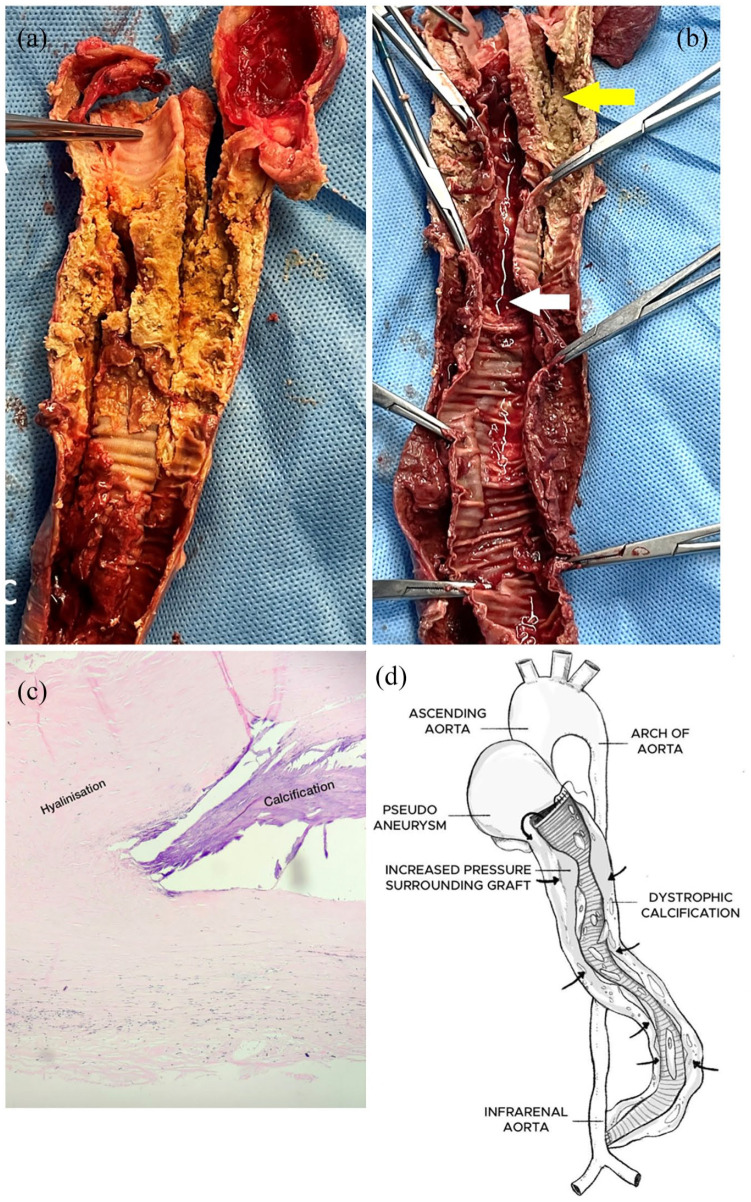
Figure 3.
(a) Cut section of the extra-anatomical graft showing extensive dystrophic calcification and organized thrombus. (b) Inner surface of extra-anatomical graft showing lumen filled with thrombosed blood (white arrow). Outer tubular capsule surrounding graft showing calcification (yellow arrow). (c) Histopathological slide of the tissue surrounding the excised graft showing hyalinization and calcification. (d) Schematic representation of the extra-anatomical graft explaining the mechanism of occlusion. Proximal anastomosis dehiscence leading to the formation of a pseudoaneurysm filled with thrombotic content causing increased pressure in the surrounding tubular capsule of the graft (black arrow) and dystrophic calcification.
Histopathological examination of the graft specimen showed hyalinization of the vessel wall with chunks of calcification (Figure 3(c)). Post-surgery the gradient between the blood pressure in the upper limbs and lower limbs disappeared. On post-operative day 1, serum creatinine decreased to 1.19 mg/dL and glomerular filtration rate increased to 65 mL/min/1.73 m3. The lab ESR level came down to normal range and oral prednisolone medication was slowly tampered to a lower dose. The post-operative course was uneventful, and the patient is continuing to do well 6 months after surgery.
Discussion
Our patient presented to us with long-term post-surgical complications of pseudoaneurysm, calcification, and thrombosis of the extra-anatomical graft. To the best of our knowledge, no case describing calcification of the surrounding tubular capsule of an extra-anatomical graft leading to severe thrombotic obstruction has been reported. Our patient underwent his first operation for stage 3 TA 37 years ago when he was 9 years old. As he grew up, the graft required additional length which might have also contributed to the stress placed on the graph. The likely cause of our patient’s presentation is the proximal anastomosis dehiscence leading to the formation of a pseudoaneurysm filled with thrombotic content causing increased pressure in the surrounding tubular capsule of the graft (Figure 3(d)). The mechanical trauma due to increased pressure along the walls of the tubular capsule enclosing the graft, along with chronic inflammation due to TA and hyper-phosphate calcification from the renal impairment may have contributed to the process of dystrophic calcification as seen surrounding the graft. 6 A retrospective review of TA patients who underwent surgical treatment showed that 17.9% developed anastomotic aneurysms suggesting that these patients require regular yearly follow-ups and monitoring with CT or magnetic resonance imaging (MRI). 7
Five-year survival following extra-anatomical bypass is over 79%, with reduction in the number of antihypertensive medications, and reduction of the inter-ventricular diameter and left ventricular mass index on echocardiography. 8 Our patients extra-anatomical graft lasted for 37 years before requiring re-do grafting due to proximal anastomosis dehiscence. However, to monitor for long-term complications of extra-anatomical aortic grafts, we propose regular yearly follow-ups with chest and abdominal X-rays to monitor for dystrophic calcifications formation at the earliest and ESR levels to monitor for systemic inflammation and overall disease progression. We also recommend that regular imaging with CT-scan or MRI be done in the setting of active disease to monitor for complications such as graft dehiscence, pseudoaneurysm, and calcifications. Evaluation of TA patients imaging techniques like CT or MRI is critical in monitoring stage-3 disease, allowing for early surgical intervention. This case serves as a reminder to the importance of regular follow-up visits, serial imaging scans, and compliance to medical management. This patient presented to us only after the onset of symptoms. It is likely that the patient’s disease course would have been less severe with regular follow-up, medication compliance, patient education, and continued communication with the provider.
Conclusion
Dystrophic calcification and graft thrombosis are potential complications of extra-anatomical aortic bypass grafts. Regular follow-up monitoring with chest and abdominal X-rays for calcific changes and CT or MRI for thrombotic changes is essential for diagnosis and treatment of such complications at an early stage, thus preventing severe signs and symptoms. It is also pivotal to medically manage active stages of TA which has been shown to contribute to disease pathophysiology of extra-anatomical aortic graft complications.
Acknowledgments
The authors acknowledge Mr Aaprampal Singh for providing help with the Medical illustration.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iDs: Sneha Kumar Jayaswal  https://orcid.org/0000-0002-9552-0070
https://orcid.org/0000-0002-9552-0070
Varun Shetty  https://orcid.org/0000-0003-3120-5888
https://orcid.org/0000-0003-3120-5888
References
- 1. Ishikawa K, Maetani S. Long-term outcome for 120 Japanese patients with Takayasu’s disease: clinical and statistical analyses of related prognostic factors. Circulation 1994; 90(4): 1855–1860. [DOI] [PubMed] [Google Scholar]
- 2. Kumar MV, Choudhary SK, Talwar S, et al. Extraanatomic bypass to supraceliac abdominal aorta for complex thoracic aortic obstruction. Ann Thorac Surg 2016; 101(4): 1552–1557. [DOI] [PubMed] [Google Scholar]
- 3. Joh JH, Kim DK, Park KH, et al. Surgical management of Takayasu’s arteritis. Journal of Korean Medical Science 2006; 21(1): 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogino H, Matsuda H, Minatoya K, et al. Overview of late outcome of medical and surgical treatment for Takayasu arteritis. Circulation 2008; 118(25): 2738–2747. [DOI] [PubMed] [Google Scholar]
- 5. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990; 33(8): 1129–1134. [DOI] [PubMed] [Google Scholar]
- 6. Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol 2014; 34(4): 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyata T, Sato O, Koyama H, et al. Long-term survival after surgical treatment of patients with Takayasu’s arteritis. Circulation 2003; 108(12): 1474–1480. [DOI] [PubMed] [Google Scholar]
- 8. Kim YS, Cho YH, Sung K, et al. Clinical outcome of extraanatomic bypass for midaortic syndrome caused by Takayasu arteritis. Ann Thorac Surg 2020; 109(5): 1419–1425. [DOI] [PubMed] [Google Scholar]