Abstract
Megestrol acetate is a common and efficient anticancer progesterone. To explore the activity and the therapeutic mechanisms of megestrol acetate in endometrial cancer, human endometrial cancer cell lines Ishikawa and HHUA overexpressing progesterone receptor A (PR-A) and progesterone receptor B (PR-B) were treated with megestrol acetate. Cell viability, apoptosis, cycle arrest, and senescence, as well as the expressions of p21 and p16, two hallmarks of cellular senescence, were evaluated. Compared with the control, >10 nmol/L megestrol acetate treatment could significantly reduce endometrial cancer cell growth, and induce the irreversible G1 arrest and cell senescence. The expression of cyclin D1 in megestrol acetate treated cells was downregulated, while the expressions of p21 and p16 were upregulated via PR-B isoform. FOXO1 inhibitor AS1842856 could significantly abrogate megestrol acetate-induced cell senescence, suggesting that FOXO1 was involved in megestrol acetate/PR-B axis. These findings may provide a new understanding for the treatment of human endometrial cancer.
Keywords: Endometrial carcinoma, megestrol acetate, progesterone receptor B, senescence, Bcl-2
Impact statement
This study demonstrates that megestrol acetate could induce irreversible G1 arrest and cellular senescence, affect the survival and growth of endometrial cancer cells, thus exhibiting anticancer property in human endometrial cancer. Moreover, megestrol acetate exerts its anticancer roles in human endometrial cancer through PR-B/FOXO1. These findings provide a novel understanding for investigating the molecular mechanism of megestrol acetate in human endometrial cancer treatment.
Introduction
Endometrial carcinoma (EC) is one of the most common malignant cancers among women worldwide, with increasing incidence and mortality rates.1,2 There are two predominant types of EC, Type I and Type II. Type I EC, often arising from atypical hyperplasia/endometrioid intraepithelial neoplasia, accounts for approximately 70% of all patients with endometrial cancer.1,3 Type I ECs are estrogen sensitive, and mostly demonstrate low histological grade (well or moderate differentiation), with a favorable prognosis. 4 Type II EC is more likely to be high grade with a poor prognosis and a high risk of relapse and metastasis, including serous, clear cell histologic type, undifferentiated carcinosarcoma, and other non-endometrioid histology.5–7 Most patients with stage I and II EC will have a favorable prognosis, whereas patients with stage III or IV EC will have a worse likelihood of survival despite advanced development in surgery, radiotherapy, and chemotherapy. Hence, further understanding is needed to improve the therapeutic strategies and clinical prognoses in human EC.
Progestin therapy for the treatment of EC was proposed in 1960s, and numerous retrospective studies have been published examining the roles of hormonal therapy, including nomegestrol acetate, medroxyprogesterone acetate, megestrol acetate, levonorgestrel, cyproterone acetate, hydroxyprogesterone caproate, and other unspecified/miscellaneous progestins.8,9 The majority of published studies reported treatments with either medroxyprogesterone acetate or megestrol acetate, with no consensus on the optimal dosage and duration. Megestrol acetate, a synthetic progestin, belongs to the 17 alpha-hydroxyprogesterone derivates, and is usually used as a short-acting contraceptive or an anticancer drug for the treatment of terminal breast or endometrial cancer. 10 Megestrol acetate administration has been reported to be associated with reduced serum cortisol concentrations in patients with cancer or AIDS.11–13 In older individuals, megestrol acetate administration could affect the secretion of several pituitary hormones and end-organ hormone synthesis. 14 Since the 1971 approval for the palliative treatment of advanced EC, megestrol acetate is frequently used in endometrial cancer patients, mostly in type I endometrial cancer. In gynecologic oncology group (GOG) study #121, high-dose megestrol acetate yields a response rate of 26%. 15 The effect of megestrol acetate on the endometrium has been studied in vivo and in vitro.16–18
The roles of megestrol acetate are mainly mediated by progesterone receptors (PRs) family members, progesterone receptor A (PR-A) and progesterone receptor B (PR-B). 19 Stable expression of PR-A and PR-B is crucial for normal physiological development, whereas dysregulation of PR-A and PR-B can initiate and exacerbate various types of gynecological cancers. 20 In endometriosis and other related diseases, altered expressions of PR-A and PR-B have been reported.21,22 And overexpression of PR-B could suppress cell invasiveness and inhibit EC growth via affecting the expression of matrix metalloproteinases. 23
Senescence is a genetically regulated mechanism that involves in the normal development, and is responsible for the ending of tumor cells after chemotherapy. In endometrial cancer, downregulation of Sushi domain containing 2 (SUSD2), an endometrial mesenchymal stem cell marker, induced senescence and death of endometrial cancer cells. 24 In human breast cancer cells, activation of PR-B by the specific ligand hydroxyprogesterone counteracted the senescence and autophagy. 25 In the present study, we aim to investigate whether megestrol acetate inhibits or suppresses the development of endometrial cancer cells by activating PR-B and, to further explore the protective roles of megestrol acetate in human endometrial cancer.
Materials and methods
Cell culture and treatment
Human endometrial cancer cell lines Ishikawa and HHUA were purchased from ATCC (Manassas, VA, USA), and cultured in the Dulbecco’s Modified Eagles’ medium containing 10% fetal bovine serum (FBS, Invitrogen), 1% penicillin (Gibco), and streptomycin (Invitrogen) solution at 37°C in a 5% CO2 atmosphere.
For megestrol acetate treatment, megestrol acetate (Adooq Bioscience, Shanghai, China) stock solution (10 mmol/L) was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in all experimental solutions was set below 0.25% (v/v). After washing with PBS, cultured cells were treated with different concentrations of megestrol acetate (1, 10, or 100 nmol/L) or the vehicle DMSO, or not (control) and incubated for 96 h. Then, cells were collected for subsequent analysis. For forkhead box protein O1 (FOXO1) inhibition, a selective inhibitor AS1842856 (EMD Millipore) was used. Cells were pretreated with AS1842856 for 1 h prior to the addition of megestrol acetate.
Western blotting
Total proteins were extracted from cultured cells and then separated by SDS-PAGE. After electrophoretic transfer to a polyvinylidene fluoride (PVDF) membrane, the blots were incubated with primary antibodies (rabbit antiprogesterone receptor, cat# ab177930; anti-Bcl-2, cat# ab32124, 1:1000 dilution, Abcam; mouse anti-β-actin, cat# ab20272, 1:3000 dilution, ABclonal) at 4°C overnight. Then, blots were incubated with specific secondary antibodies for 1 h at room temperature. Enhanced chemiluminescence (Amersham Pharmacia, NJ, USA) was performed to visualize the bands.
Cell transfection
PR-A or PR-B stable overexpression cell lines were generated by transfecting cells with 2.0 μg of PR-A or PR-B plasmids using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions, respectively. The small interfering RNA (siRNA) targeting PR-B, as well as negative control siRNA, was synthesized by Takara (Dalian, China). A blast search was performed to ensure the absence of homology in selected sequences with any other sequences. Specific PR-B and negative control siRNA were transfected into cultured cells using Lipofectamine 3000 according to the manufacturer’s instructions, respectively. After 48 h, interference efficiency was detected by Western blotting. Transfected cells were conducted for subsequent experiments.
Cell growth assay
The CCK-8 kit (Bioroot, Shanghai, China) was used to analyze the growth of identical cells following the manufacturer's guidelines. Cells were cultured in 96-well plates (5 × 104 cells/mL) for 12, 24, 48, 72, and 96 h, respectively. Then, 10 μL of CCK-8 solution was added into the wells, followed by incubation for 4 h at 37°C. The absorbance of cells was tested at 450 nm.
Cell cycle analysis
Cell cycle analysis was performed using flow cytometry. In brief, cultured cells (5 × 106 cells/well) were harvested with 500 μL of ice-cold PBS, and fixed with 70% ethanol. Then, cells were re-suspended gently with binding buffer to obtain monodispersed cell suspension, and double stained with annexin V-FITC and propidium iodide (PI) at 37°C for 1 h, followed by analysis using FACS CaliburTM (Becton Dickinson).
Beta-galactosidase assay
Beta-galactosidase assay was performed using the senescence β-galactosidase staining kit (Cell Signaling Technology) according to the manufacturer’s instructions. Briefly, cultured cells were washed with ice-cold PBS and fixed for 15 min at room temperature. Cell suspensions (5 × 106 cells/well) were stained with fresh β-galactosidase staining solution overnight at 37°C. Senescent cells were counted under microscopy, with four random fields for each sample.
Statistical analysis
The data were expressed as mean ± SD. The statistical analysis was conducted using the GraphPad Prism 6 (La Jolla, CA, USA) and SPSS 18.0. Comparisons between two groups were calculated using two independent sample t-tests, and comparisons among multiple groups were calculated using one-way ANOVAs and LSD tests. All experiments were performed independently at least three times.
Results
Megestrol acetate reduces cell growth in human endometrial cancer cells
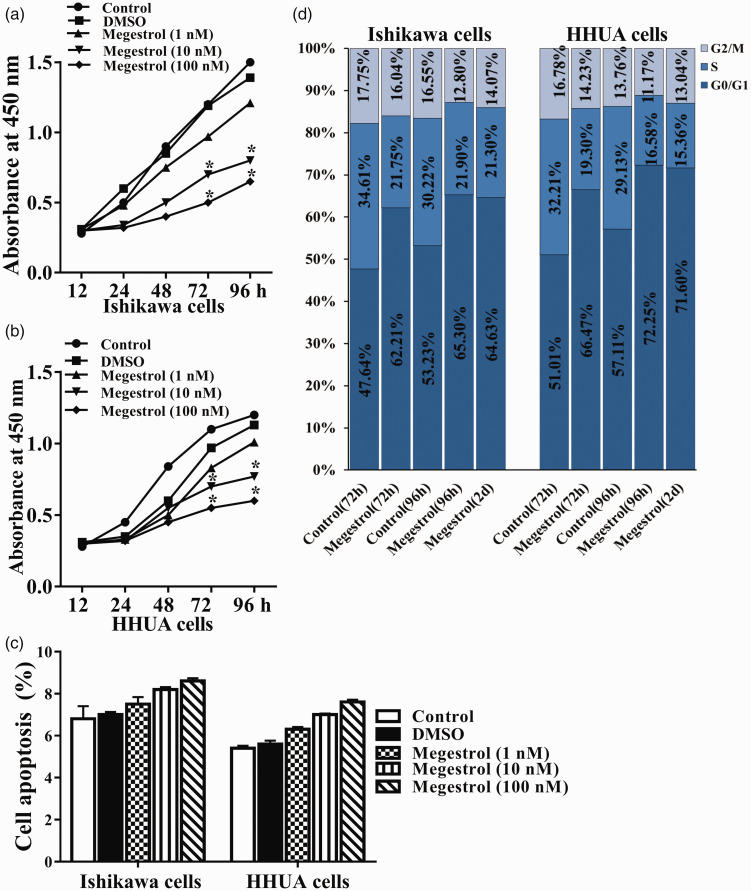
To determine the role of megestrol acetate in endometrial cancer, we firstly investigated the effect of megestrol acetate on the growth of endometrial cancer cells in vitro. Human endometrial cancer cell lines Ishikawa and HHUA were treated with different concentrations of megestrol acetate (1, 10, or 100 nmol/L) for different times, respectively. Compared with untreated control, the growth of Ishikawa cells was significantly reduced in a time-dependent manner upon megestrol acetate treatment (Figure 1(a)). Similar results were found in HHUA cells (Figure 1(b)). To further demonstrate the cellular mechanism of megestrol acetate, cell apoptosis and cell cycle were further evaluated. As shown in Figure 1(c) and Supplementary Figure S1, treatment with different concentrations of megestrol acetate for 96 h slightly increased the apoptosis of Ishikawa and HHUA cells, compared with corresponding untreated control and DMSO group. However, no significant difference was found (P > 0.05). The cell cycle of Ishikawa cells was arrested in G0/G1 phase after 72-h treatment with 100 nmol/L megestrol acetate, compared with the control. G0/G1 arrest was observed throughout 96-h treatment of megestrol acetate (Figure 1(d)). After 96 h-treatment, cells were washed, and re-cultured with fresh medium for another two days. The percentage of cells in G0/G1 phase remained approximately unchanged (Figure 1(d)). Similar results were found in megestrol acetate treated HHUA cells (Figure 1(d)). These results indicate that megestrol acetate has an inhibitory effect on cell growth and induces irreversible cell cycle arrest of human endometrial cancer cells.
Figure 1.
Megestrol acetate reduces cell survival and induces irreversible G1 arrest in human endometrial cancer cells. The human endometrial cancer Ishikawa and HHUA cells were treated without (control) or with different concentrations of megestrol (1, 10, 100 nmol/L) or the vehicle DMSO for different time, respectively. Then the proliferation assay of (a) Ishikawa and (b) HHUA cells were performed (*P < 0.05 vs. control). (c) Cell apoptosis was detected by flow cytometry. (d) Ishikawa and HHUA cells were treated with 10 nmol/L megestrol or the vehicle DMSO (control) for 72 or 96 h, respectively. Then the cell cycles were analyzed (*P < 0.05 vs. control). (A color version of this figure is available in the online journal.)
Megestrol acetate induces senescence in endometrial cancer cells
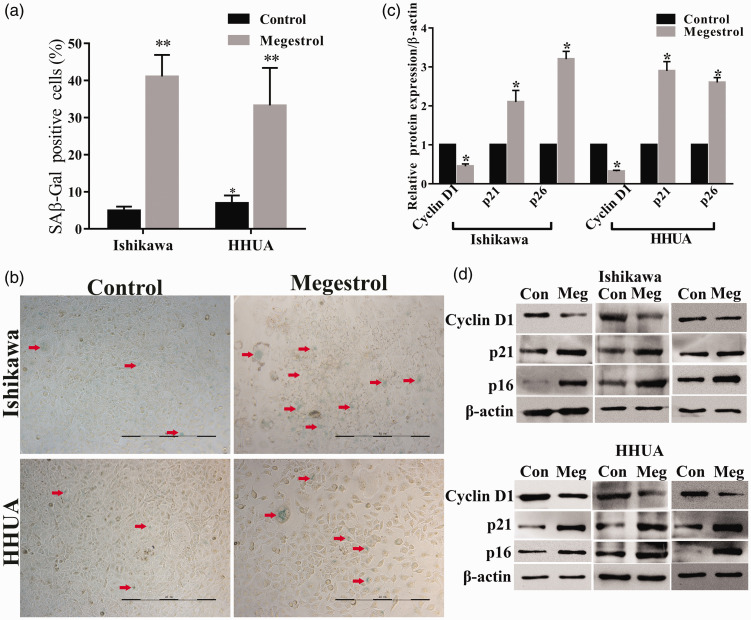
Irreversible cell cycle arrest may result in cell senescence. We next determined the accumulation of endogenous lysosomal β-galactosidase (SAβGal), the common marker of cellular senescence, in megestrol acetate-treated endometrial cancer cells. Megestrol acetate treatment significantly increased the activity of SAβ-Gal in endometrial cancer cells (Figure 2(a) and (b)). It has been reported that increased p21 expression and activity directly induced cellular senescence. Thus, we next analyzed the expression of cyclin D1, a cell-cycle-related protein, as well as p21 and p16, hallmarks of cellular senescence, in endometrial cancer cells. Megestrol acetate downregulated the expression of cyclin D1 in Ishikawa and HHUA cells, whereas expressions of p21 and p16 were increased compared with the control (Figure 2(c) and (d)). These results suggest that megestrol acetate affects cell cycle progression and induces cellular senescence, thus suppressing human endometrial cancer.
Figure 2.
Megestrol acetate induces cell senescence in endometrial cancer cells. Ishikawa and HHUA cells were treated without (control) or with 10 nmol/L megestrol for 96 h. Cell senescence was detected by SAβGal staining and the protein expressions of cyclin D1, p21, and p16 were detected by Western blotting. (a) SAβGal positive cell numbers. (b) SAβGal staining. Senescent cells were SAβGal positive, and were stained with blue. Red arrows: representative senescent cells. (c) Relative expression of cyclin D1, p21, and p16. (d) The expression of cyclin D1, p21, and p16 of three repeated experiments (**P < 0.01 vs. control, *P < 0.05 vs. control). Bar = 200 μm. (A color version of this figure is available in the online journal.)
Megestrol acetate regulates the growth and senescence of endometrial cancer cells through the PR-B isoform
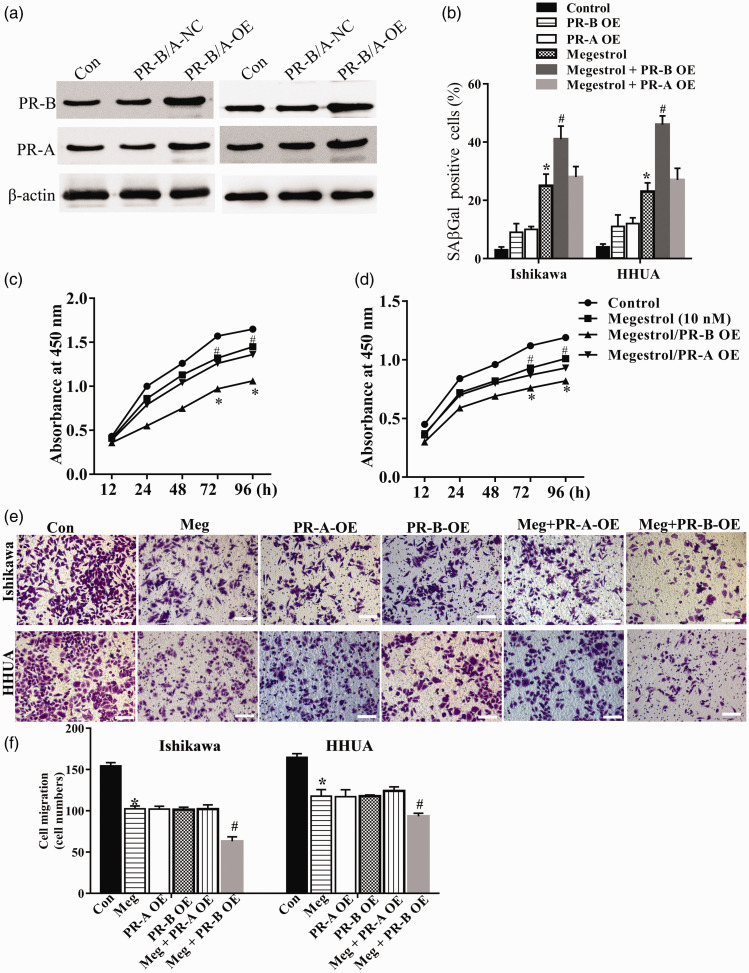
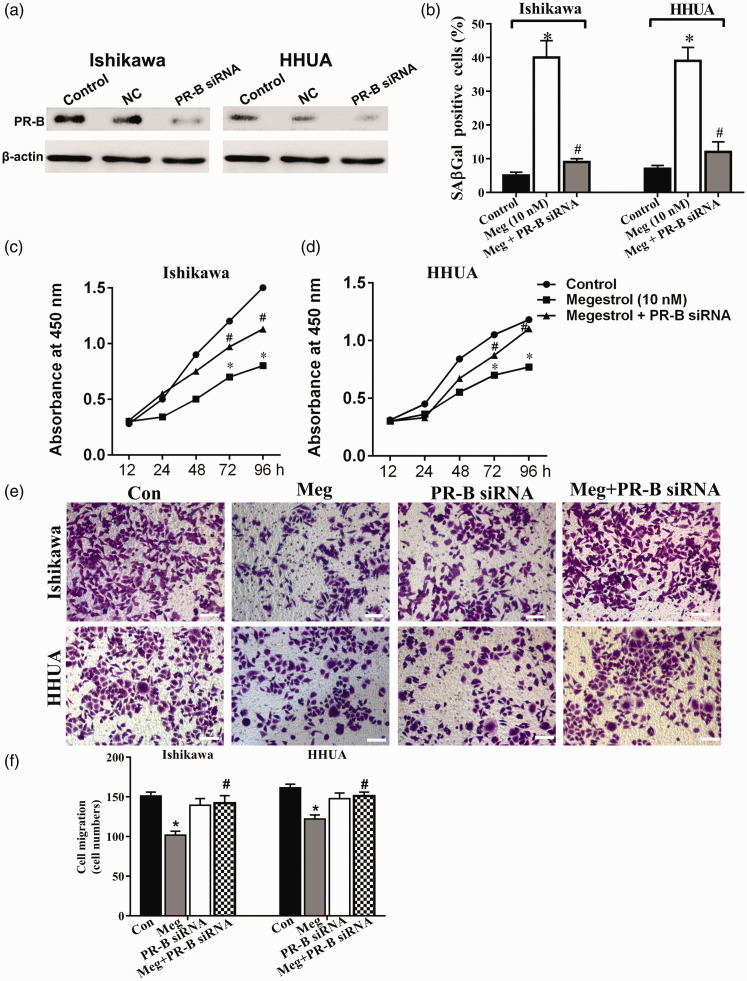
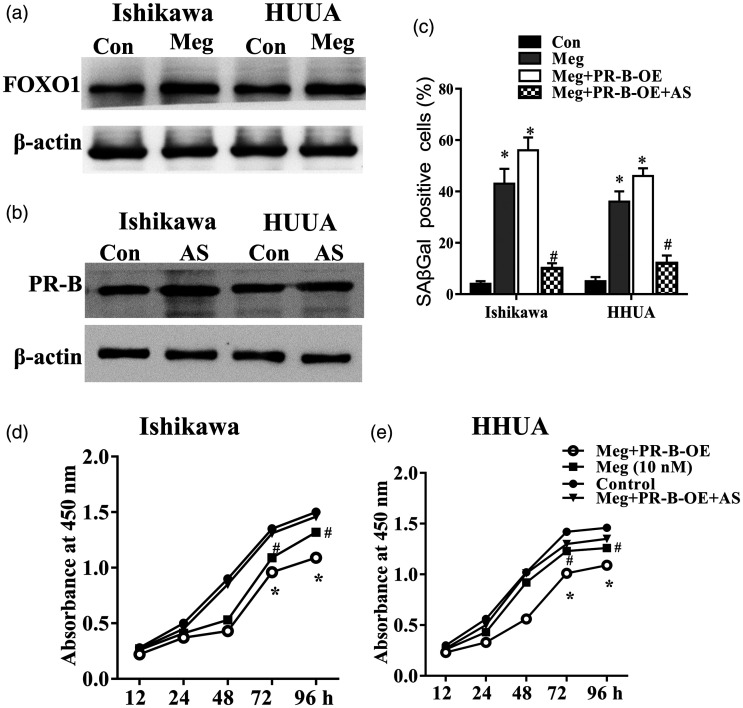
To fully understand whether PR-B or PR-A is involved in the role of megestrol acetate in endometrial cancer cells, we successfully constructed PR-B and PR-A overexpressing Ishikawa and HHUA cells (Figure 3(a)). PR-B overexpression (PR-B-OE) significantly augmented the effects of megestrol acetate on senescence (B), growth (Figure 3(c) and (d)), and cell migration (Figure 3(e) and (f)) of endometrial cancer cells. In contrast, the effect of megestrol acetate treatment on cell growth, senescence, and cell migration in PR-A overexpression (PR-A-OE) cells was slightly enhanced or unchanged. To confirm the involvement of PR-B in megestrol acetate’s effect, a PR-B specific siRNA was further utilized (Figure 4(a)). PR-B knockdown could significantly abolish megestrol acetate-induced cell activity changes, including cellular senescence (Figure 4(b)), cell growth (Figure 4(c) and (d)), and cell migration (Figure 4(e) and (f)). These results indicated that PR-B, not PR-A, was involved in megestrol acetate’s effect on endometrial cancer cells.
Figure 3.
PR-B isoform overexpression enhances the changes of endometrial cancer cells induced by megestrol acetate. (a) The PR-A and PR-B expression in PR-B or PR-A respective plasmids transfected cells were measured by Western blotting. Cell senescence (b), cell proliferation ((c) and (d)), and migration ((e) and (f)) of PR-A and PR-B overexpressed endometrial cancer cells were then analyzed (*P < 0.05 vs. control; #P < 0.05 vs. megestrol). Bar = 50 μm. (A color version of this figure is available in the online journal.)
Figure 4.
Megestrol acetate mediates the decreased survival of endometrial cancer cells through the PR-B isoform. (a) The PR-B expressions in PR-B specific siRNA transfected cells were measured by Western blotting. The human endometrial cancer Ishikawa and HHUA cells were treated with 10 nmol/L megestrol alone or with PR-B specific siRNA, cell senescence was detected (b), cell growth was detected by CCK8 assay ((c) and (d)), and cell migration was detected by transwell ((e) and (f)) (*P < 0.05 vs. control, #P < 0.05 vs. megestrol). Bar = 50 μm. (A color version of this figure is available in the online journal.)
Megestrol acetate/PR-B axis induces senescence of endometrial cancer cells via the FOXO1 pathway
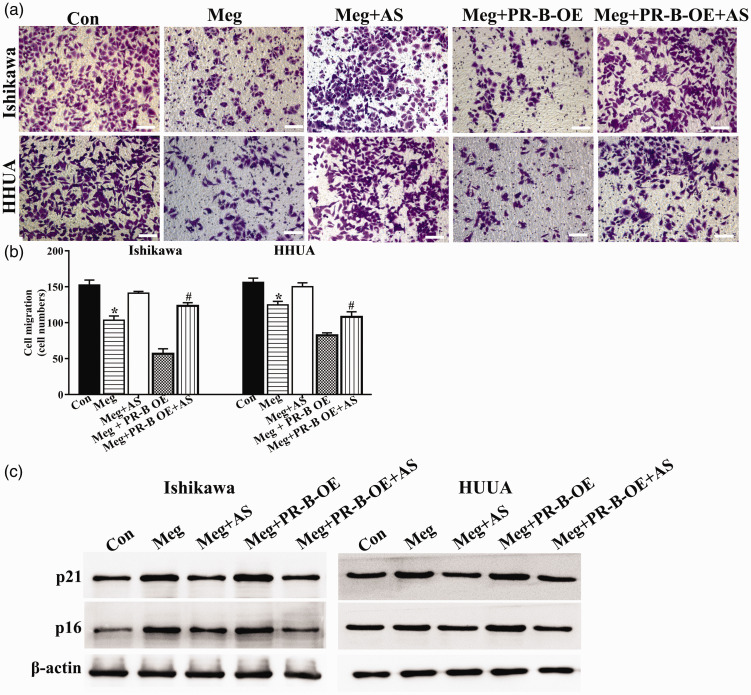
FOXO1 is a targeted gene of PR and a master regulator of cell cycle mediators (p16, p21, and p27). 26 The expression of FOXO1 protein was robustly upregulated in both Ishikawa and HHUA cells upon megestrol acetate treatment (Figure 5(a)). Using a selective small-molecular inhibitor of FOXO1, AS1842856, we found that FOXO1 inhibition alone did not exert induction or inhibition on PR-B expression (Figure 5(b)). However, the megestrol acetate-induced senescence and growth arrest were significantly abrogated upon AS1842856 treatment (Figure 5(c) to (e)). Cell migration inhibited by megestrol acetate in both cells was also alleviated upon AS1842856 treatment (Figure 6(a) and (b)). Similar changing patterns were observed in the expression of p21 and p16 in megestrol acetate-treated endometrial cancer cells (Figure 6(c)). These results indicate that megestrol acetate may induce the senescence of endometrial cancer cells by FOXO1 through PR-B isoform.
Figure 5.
FOXO1 participates in megestrol/PR-B axis-induced senescence. (a) FOXO1 expression was increased in megestrol treated Ishikawa and HHUA cells. Ishikawa and HHUA cells or PR-B overexpressed Ishikawa and HHUA cells were treated with megestrol alone or pretreated with AS1842856, cell senescence was detected by SAβGal staining (b), cell growth was detected by CCK8 assay ((c) and (d)) (*P < 0.05 vs. control; #P < 0.05 vs. megestrol).
Figure 6.
FOXO1 participates in megestrol/PR-B axis-induced cell cycle arrest and megestrol/PR-B axis inhibits migration of endometrial cancer cells. Ishikawa and HHUA cells or PR-B overexpressed Ishikawa and HHUA cells were treated with megestrol alone or pretreated with AS1842856, and cell migration was detected by transwell ((a) and (b)), and the expressions of p21 and p16 were detected by Western blotting (*P < 0.05 vs. control; #P < 0.05 vs. megestrol). Bar = 50 μm. (A color version of this figure is available in the online journal.)
Discussion
In the present study, we demonstrate that megestrol acetate could induce irreversible G1 arrest and cellular senescence, affect the survival and growth of endometrial cancer cells, thus exhibiting anticancer property in human endometrial cancer. Moreover, megestrol acetate exerts its anticancer roles in human endometrial cancer through PR-B/FOXO1. These results provide a novel understanding for the molecular mechanism of megestrol acetate in the treatment human endometrial cancer.
Megestrol is a synthetic progesterone with high efficacy. It has short-acting contraceptive role in the general use by oral or injection manner. Notably, megestrol and other progesterones are widely used in the clinical treatment of various cancers, especially malignant gynecologic carcinoma. In the treatment of advanced breast cancer, megestrol acetate provided effective palliation. In a two-stage phase II trial with postmenopausal women who had hormone-sensitive advanced breast cancer and experienced disease progression on a third-generation non-steroidal aromatase inhibitor, megestrol acetate had demonstrated activity and acceptable tolerability. 27 In a phase II trial with patients with platinum-refractory epithelial ovarian cancer, megestrol acetate showed modest but definite activity. 28 In vitro studies showed that progesterone treatment modulated protein expressions in human endometrial cancer cell lines. Besides their inhibitory effect on cell growth, morphologic changes such as multinucleation, multinucleolation, vacuolation, and extensive Golgi apparatus upon progesterone treatment were also reported.29,30 The clinical safety and efficacy of megestrol application have been identified in multiple randomized, double-blind and placebo-controlled trials.31–33 However, the exact anticancer molecular mechanism involved is not completely known.
As a frequently used drug in endometrial cancer patients, megestrol acetate, administered alone or combined with other drugs such as tamoxifen and metformin, exhibits high efficacy and low side effects.15,34 Despite inhibiting the secretion of progesterone in pituitary gland, it is supposed that other potential mechanisms are also implicated in its anticancer roles. 31 In this study, we investigated the role of megestrol acetate in regulating survival and growth of endometrial cancer cells. Megestrol acetate treatment reduced the proliferation of Ishikawa cells and HHUA cells in vitro, suggesting that megestrol acetate is an anticancer agent in endometrial cancer by directly influencing tumor cell growth.
Senescence is a genetically regulated mechanism in normal development. Disruption of cell cycle progression is an important cytological factor leading to the occurrence and development of tumor.35,36 And induction of tumor cell senescence is also a therapeutic mechanism of various antitumor drugs. Herein, we found that megestrol acetate significantly inhibited cell cycle progression, and induced irreversible G1 arrest and senescence of endometrial cancer cells. Consistently, expression of cyclin D1 was remarkably downregulated, whereas p21 and p16 were increased in megestrol acetate treated endometrial cancer cells. These findings suggest that megestrol acetate suppresses cell cycle progression, which is consistent with a previous report showing that downregulation of SUSD2, endometrial mesenchymal stem cell marker, induces endometrial cancer cells into senescence and death. 24
PRs are the main mediators recognizing megestrol acetate and other progestagens. Decreased expressions of PR isoforms PR-A and PR-B are associated with the initiation and progression of various gynecological cancers.20,37 In breast cancer, loss of PR-B expression is observed, and hydroxyprogesterone (OHPg) can activate PR-B to further drive the autophagy in human breast cancer cells. 38 The altered expression and functional roles of PR also have been found in endometriosis and other related diseases. Overexpressed PR-B inhibits tumor growth by suppressing the invasive activity of EC cells via affecting the expression of matrix metalloproteinases. 23 PR transcriptional activity is commonly linked to the expression of many cell cycle regulators such as cyclin-dependent kinase and p21/p27 families. In this study, we found that PR-B, not PR-A, participated in the anticancer role of megestrol acetate in endometrial cancer. FOXOs participate in cell proliferation, oxidative stress, and apoptosis. In PR-B+ ovarian cancer cells, FOXO1 and p21 are required for progestin-mediated cellular senescence.39,40 In this study, we found that FOXO1 inhibition abrogated the megestrol acetate-induced growth arrest and senescence of endometrial cancer cells, suggesting that the FOXO1 pathway might be involved in the decreased survival of endometrial cancer cells mediated by megestrol acetate/PR-B. From a clinical perspective, FOXO1 might be a potential therapeutic target, and compounds which are capable of regulating FOXO1 activities may be necessary to enhance the efficacy of megestrol acetate in the future treatment of endometrial cancer and other cancers.
In summary, the present study reveals that megestrol acetate can induce the growth arrest and senescence of endometrial cancer cells through PR-B/FOXO1/p21 axis, thus contributing to the suppression of human endometrial cancer. These findings may provide new understanding for treating human endometrial cancer.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211026566 for Megestrol acetate drives endometrial carcinoma cell senescence via interacting with progesterone receptor B/FOXO1 axis by Hong Wang and Huirong Shi in Experimental Biology and Medicine
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript. SHR conceived and designed the study, and reviewed the article. WH performed the experiments and wrote the article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DATA AVAILABILITY: The data that support the findings of this study are available from the corresponding author upon reasonable request.
ORCID iD: Huirong Shi https://orcid.org/0000-0002-6751-2858
Supplemental material: Supplemental material for this article is available online.
References
- 1.Buhtoiarova TN, Brenner CA, Singh M. Endometrial carcinoma. Am J Clin Pathol 2016; 145:8–21 [DOI] [PubMed] [Google Scholar]
- 2.Di CA, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol 2007; 2:57. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983; 15:10–7 [DOI] [PubMed] [Google Scholar]
- 4.Gompel A. Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2020; 69:95–107 [DOI] [PubMed] [Google Scholar]
- 5.Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol 2004; 35:649–62 [DOI] [PubMed] [Google Scholar]
- 6.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology 2013; 62:111–23 [DOI] [PubMed] [Google Scholar]
- 7.Salvesen HB, Akslen LA. Molecular pathogenesis and prognostic factors in endometrial carcinoma. APMIS 2002; 110:673–89 [DOI] [PubMed] [Google Scholar]
- 8.Corzo C, Barrientos Santillan N, Westin SN, Ramirez PT. Updates on conservative management of endometrial cancer. J Minim Invasive Gynecol 2018; 25:308–13 [DOI] [PubMed] [Google Scholar]
- 9.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012; 125:477–82 [DOI] [PubMed] [Google Scholar]
- 10.Hua J, Han J, Wang X, Guo Y, Zhou B. The binary mixtures of megestrol acetate and 17α-ethynylestradiol adversely affect zebrafish reproduction. Environ Pollut 2016; 213:776–84 [DOI] [PubMed] [Google Scholar]
- 11.Leinung MC, Liporace R, Miller CH. Induction of adrenal suppression by megestrol acetate in patients with AIDS. Ann Intern Med 1995; 122:843–5 [DOI] [PubMed] [Google Scholar]
- 12.Loprinzi CL, Jensen MD, Jiang NS, Schaid DJ. Effect of megestrol acetate on the human pituitary-adrenal axis. Mayo Clin Proc 1992; 67:1160–2 [DOI] [PubMed] [Google Scholar]
- 13.McKone EF, Tonelli MR, Aitken ML. Adrenal insufficiency and testicular failure secondary to megestrol acetate therapy in a patient with cystic fibrosis. Pediatr Pulmonol 2002; 34:381–3 [DOI] [PubMed] [Google Scholar]
- 14.Bodenner DL, Medhi M, Evans WJ, Sullivan DH, Liu H, Lambert CP. Effects of megestrol acetate on pituitary function and end-organ hormone secretion: a post hoc analysis of serum samples from a 12-week study in healthy older men. Am J Geriatr Pharmacother 2005; 3:160–7 [DOI] [PubMed] [Google Scholar]
- 15.Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2004; 92:10–4 [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013; 34:130–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellenger CR, Chen JC. Effect of megestrol acetate on the endometrium of the prepubertally ovariectomised kitten. Res Vet Sci 1990; 48:112–8 [PubMed] [Google Scholar]
- 18.Schacter L, Rozencweig M, Canetta R, Kelley S, Nicaise C, Smaldone L. Megestrol acetate: clinical experience. Cancer Treat Rev 1989; 16:49–63 [DOI] [PubMed] [Google Scholar]
- 19.Valadez-Cosmes P, Vazquez-Martinez ER, Cerbon M, Camacho-Arroyo I. Membrane progesterone receptors in reproduction and cancer. Mol Cell Endocrinol 2016; 434:166–75 [DOI] [PubMed] [Google Scholar]
- 20.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor a form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 1993; 7:1244–55 [DOI] [PubMed] [Google Scholar]
- 21.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform a but not B is expressed in endometriosis. J Clin Endocrinol Metab 2000; 85:2897. [DOI] [PubMed] [Google Scholar]
- 22.Wölfler MM, Küppers M, Rath W, Buck VU, Meinhold-Heerlein I, Classen-Linke I. Altered expression of progesterone receptor isoforms a and B in human eutopic endometrium in endometriosis patients. Ann Anat 2016; 206:1–6 [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Mizumoto H, Tanaka R, Satohisa S, Adachi K, Horie M, Kudo R. Overexpressed progesterone receptor form B inhibit invasive activity suppressing matrix metalloproteinases in endometrial carcinoma cells. Cancer Lett 2004; 209:237–43 [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Zeng N, Alowayed N, Singh Y, Cheng A, Lang F, Salker MS. Downregulation of endometrial mesenchymal marker SUSD2 causes cell senescence and cell death in endometrial carcinoma cells. PloS One 2017; 12:e0183681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De AF, Guido C, Santoro M, Giordano F, Donã A, Rizza P, Pellegrino M, Perrotta I, Bonofiglio D, Sisci D. Ligand activated progesterone receptor B drives autophagy-senescence transition through a beclin-1/Bcl-2 dependent mechanism in human breast cancer cells. Oncotarget 2016; 7:57955–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA, Cancer Genome Atlas Research N. Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bines J, Dienstmann R, Obadia RM, Branco LGP, Quintella DC, Castro TM, Camacho PG, Soares FA, Costa MEF. Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: a phase II trial. Ann Oncol 2014; 25:831–6 [DOI] [PubMed] [Google Scholar]
- 28.Wilailak S, Linasmita V, Srisupundit S. Phase II study of high-dose megestrol acetate in platinum-refractory epithelial ovarian cancer. Anticancer Drugs 2001; 12:719–24 [DOI] [PubMed] [Google Scholar]
- 29.Ishiwata I, Udagawa Y, Okumura H, Nozawa S. Effects of progesterone on human endometrial carcinoma cells in vivo and in vitro. J Natl Cancer Inst 1978; 60:947–54 [DOI] [PubMed] [Google Scholar]
- 30.Kimura J. Effect of progesterone on cell division in chemically induced endometrial hyperplasia and adenocarcinoma in mice. Cancer Res 1978; 38:78–82 [PubMed] [Google Scholar]
- 31.Sharifzadeh F, Aminimoghaddam S, Kashanian M, Fazaeli M, Sheikhansari N. A comparison between the effects of metformin and megestrol on simple endometrial hyperplasia. Gynecol Endocrinol 2016; 33:152–5 [DOI] [PubMed] [Google Scholar]
- 32.Myers AP, Filiaci VL, Zhang Y, Pearl M, Behbakht K, Makker V, Hanjani P, Zweizig S, Nd BJ, Downey G. Tumor mutational analysis of GOG248, a phase II study of temsirolimus or temsirolimus and alternating megestrol acetate and tamoxifen for advanced endometrial cancer (EC): an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2016; 141:43–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pautier P, Vergote I, Joly F, Melichar B, Kutarska E, Hall G, Lisyanskaya A, Reed N, Oaknin A, Ostapenko V. A phase 2, randomized, open-label study of irosustat versus megestrol acetate in advanced endometrial cancer. Int J Gynecol Cancer 2016; 27:258. [DOI] [PubMed] [Google Scholar]
- 34.Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, Luo XZ, Zhang HW, Zhu Q, Ma FH, Liu J, Sun L, Yu M, Guan J, Chen XJ. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG 2020; 127:848–57 [DOI] [PubMed] [Google Scholar]
- 35.Yamada H. Suppression of tumorigenicity and induction of senescence on human endometrial carcinoma cell lines by transfer of normal human chromosomes. Hokkaido Igaku Zasshi 1994; 69:1443–54 [PubMed] [Google Scholar]
- 36.Alektiar KM, Venkatraman E, Abu-Rustum N, Barakat RR. Is endometrial carcinoma intrinsically more aggressive in elderly patients? Cancer 2003; 98:2368–77 [DOI] [PubMed] [Google Scholar]
- 37.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 2002; 277:5209–18 [DOI] [PubMed] [Google Scholar]
- 38.De Amicis F, Guido C, Santoro M, Lanzino M, Panza S, Avena P, Panno ML, Perrotta I, Aquila S, Andò S. A novel functional interplay between progesterone receptor-B and PTEN, via AKT, modulates autophagy in breast cancer cells. J Cell Mol Med 2014; 18:2252–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA. Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle 2013; 12:1433–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diep CH, Knutson TP, Lange CA. Active FOXO1 is a key determinant of isoform-specific progesterone receptor transactivation and senescence programming. Mol Cancer Res 2016; 14:141–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211026566 for Megestrol acetate drives endometrial carcinoma cell senescence via interacting with progesterone receptor B/FOXO1 axis by Hong Wang and Huirong Shi in Experimental Biology and Medicine