Abstract
Rapid infectivity of SARS-CoV2 with recent viral variants is posing a challenge in the development of robust therapeutic strategies. On the other hand, microbiota is debated for its involvement in SARS-CoV2 infection with varied opinions. Although ample data about the role of microbiota and probiotics in respiratory viral infections are available, their role in COVID-19 is limited albeit emerging rapidly. The utilization of probiotics for the management of COVID-19 is still under investigation in many clinical trials. Existing information coupled with recent COVID-19 related studies can suggest various ways to use microbiota modulation and probiotics for managing this pandemic. Present article indicates the role of microbiota modulation and probiotics in respiratory infections. In addition, scattered evidence was gathered to understand the potential of microbiota and probiotics in the management of SARS-CoV2. Gut-airway microbiota connection is already apparent in respiratory tract viral infections, including SARS-CoV2. Though few clinical trials are evaluating microbiota and probiotics for COVID-19 management, the safety evaluation must be given more serious consideration because of the possibility of opportunistic infections among COVID-19 patients. Nevertheless, the information about microbiota modulation using probiotics and prebiotics can be helpful to manage this outbreak and this review presents different aspects of this idea.
Keywords: Microbiota, SARS-CoV-2, COVID-2019, probiotics, pandemic, infection
Introduction
The global catastrophe of this century emerged with an incidence of viral pneumonia in late 2019 that eventually led to a global health emergency. The clinical features of this pandemic ranged from asymptomatic to mild and severe infection with its ability to affect lungs, rapid transmission rate, and the possibility of multi-organ involvement among susceptible individuals [1]. Several additional complications have arisen with these typical clinical features after the detection of new viral variants and the emergence of post-COVID complications including secondary infections [2,3]. The etiologic agent of this pandemic was initially named as a novel coronavirus (nCoV-2019), which was later called SARS-CoV-2, causing the disease COVID-2019. COVID-19 caused global public health emergencies leading to unprecedented steps to curb the chances of infection in different countries. Unfortunately, the incidence and mortalities by COVID-2019 are still increasing in many countries with an increasing number of cases. Several treatment guidelines were issued to manage this pandemic, but sometimes treatment efficacy and related events [4] led to shifting on alternative therapeutic regimens and the situation is not truly under control in certain geographic locations. Generally, coronaviruses are known for causing enteric and respiratory infections. Their role in the severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) outbreaks gave them popularity, which is still rising with their role in COVID-19 [5]. These viruses are enveloped, positive-stranded RNA viruses from the Coronaviridae family and primarily involved in zoonotic infections. Human infections are suggested to develop after the evolution of animal strains to infect humans, and coronavirus outbreaks including SARS, MERS, and COVID-2019 are also suggested as zoonotic origin [6]. Early studies predicted that COVID-2019 can be a combination of bat and pangolin coronavirus. These findings were supported by the fact that COVID-2019 showed more than 96% whole-genome sequence identity with bat coronavirus and 90% similarity to Malaysian pangolin coronavirus. However, the specific region of spike (S) protein of pangolin coronavirus is highly similar to SARS-CoV2, possibly leading to its highly efficient host cell attachment and entry through angiotensin-converting enzyme 2 (ACE2) [7-9], though polymorphism in ACE2 and recent viral variants need reconsideration of this whole paradigm [10,11]. Recent studies have highlighted that the continual evolution of SARS-CoV2 is still taking place and creating a challenge of re-infection and diagnosis due to the generation of variants contributing to pandemic waves [12].
The role of microbiota is also discussed with a range of observations for their effects on infection susceptibility, including coronavirus infection. Microbiota represents microbial species coexisting with an individual and could be divided into transient and resident microbiota. The geographical diversity in microbiota is evident and affected by several factors, including environmental condition, natural habitat, nutritional and occupational differences in addition to host factors like host genetics, immune status, hygiene, etc. [13]. The role of microbiota is discussed in several diseases including COVID-2019 with the identification of modulated microbiota among infected patients [14-16]. These modulated bacteria can contribute to the severity of COVID-19 in multiple ways and have been discussed in some studies [17,18]. The presence of ACE2 receptor on intestinal epithelial cells that contributes to SARS-CoV-2 pathogenesis and the subsequent presence of a large number of microbes in the gut leaves several caveats [19]. A recent article has reviewed the role of ACE2 as a key player for determining the disease outcome of COVID-19 through gut microbiota dysbiosis [20]. It has been verified that gut microbiota affects the levels of inflammatory mediators in COVID-19 patients and immune mediated molecules play an important role in clinical presentation of COVID-19 disease [21]. Therefore it is involved in modulating the severity of clinical presentation based on microbiota composition [15]. The data about the role of microbiota in COVID-2019 outbreak is limited but gradually increasing with some studies identifying microbiota modulation among COVID-19 patients from different geographic locations. We summarized information about microbiota diversity and its modulation during various respiratory infections, in addition to recent studies about COVID-19 patients. We gathered these diverse threads to assess the role of microbiota in the COVID-2019 outbreak. Moreover, we also covered the role of probiotics in COVID-19 with clinical trials undergoing to utilize this aspect in management of current pandemic. Probiotics are beneficial bacteria and their role is already known in several diseases [22-24].
Respiratory tract microbiota: composition, modulation and infection
The microbiota of the upper respiratory tract (URT) and lower respiratory tract (LRT) varies considerably and is therefore studied separately. The microbiota of the respiratory tract is affected by several external and internal factors, including age, immunity, disease status, and smoking habits, leading to variations in resident and transient microbiota [25]. The human microbiota is already known for its involvement in modulating susceptibility to respiratory infections [26]. The lower respiratory tract, including lungs, were previously considered sterile, however recent culture-independent microbial identification, reveals several breakthroughs about the respiratory tract microbiota and its association with several diseases. The lung microbiome is known to modify the risk of viral infections and their subsequent severity by affecting the immune response [27]. Several articles about respiratory tract microbiota are already available [28-30]. Therefore our article does not focus on general overview of respiratory tract microbiota. Studies evaluating the composition of respiratory microbiota and its modulation in different clinical conditions are presented in Table 1.
Table 1.
Studies from different geographical locations indicating microbiota composition of the respiratory and its variation during different clinical conditions
| Primary Location | Study | Remark | Ref |
|---|---|---|---|
| Asia | |||
| China | 16S rDNA analysis of 83 (AN), 60 (NP), & 97 (OP) samples of 98 healthy children (≤12 years of age). | Moraxella, Staphylococcus, Corynebacterium, Streptococcus, and Dolosigranulum (AN/NP). | [67] |
| Streptococcus, Prevotella, Neisseria, Veillonella, Rothia, Leptotrichia, and Haemophilus (OP). | |||
| High throughput gene sequencing was used to evaluate BALF samples of normal and IPF patients. | Healthy individuals were showing the dominance of Sutterella, Coprococcus, Parasutterella, Paludibacter and Dorea. | [68] | |
| IPF samples were showing the dominance of Streptococcus (23.0% of total reads), followed by Pseudobutyrivibrio, Anaerorhabdus, Campylobacter, and Blautia. | |||
| Respiratory samples from 171 healthy children and 76 children having pneumonia were analyzed for the presence of microbes. | The Prevotella and Streptococcus were found with healthy subjects, while Staphylococcus spp. and M. pneumoniae were predominant in the patient group. | [69] | |
| Sputum microbiota of tuberculosis patients (25 new, 30 recurrent, 20 with treatment failure) and 20 healthy controls were analyzed through 16s RNA sequencing. | Tuberculosis patients were primarily showing Firmicutes, while Bacteriodetes was found with healthy controls. Tuberculosis patients were showing Streptococcus, Gramulicatella and Pseudomonas, while Catonella and Coprococcus were abundant in healthy controls. | [70] | |
| Sputum and respiratory secretions from 31 tuberculosis (TB) and 24 healthy controls were analyzed by 16S rRNA. | Some species like Actinomyces, Granulicatella, Prevotella, Streptococcus, and Veillonella were abundant in both groups. The Anoxybacillus, Acinetobacter, Abiotrophia, Klebsiella, Pilibacter, Paucisalibacillus, and Rothia were higher in TB patients, while Campylobacter, Fusobacterium, Haemophilus, Neisseria, Porphyromonas, Parvimonas, and TM7_genera_incertae_sedis were lower in TB patients than control. | [71] | |
| Upper respiratory tract microbiota of H1N1 influenza virus-infected patients were analyzed through 16s rDNA analysis. | Proteobacteria was abundant while Actinobacteria, Bacteroidetes, Candidate division TM7, Firmicutes, Fusobacteria and SR1 were reduced in H1N1 patients samples. At genera level, Ochrobactrum, Brevundimonas, Caulobacter, Aquabacterium and Serratia were increased whereas Actinomyces, Acinetobacter, Haemophilus, Neisseria, Prevotella, Porphyromonas, Streptococcus, and Veillonella decreased in H1N1 samples. | [72] | |
| Hong Kong | Sputum microbiota was analyzed by 16s rRNA in 22 TB patients and 14 control. | Controls were showing an abundance of Firmicutes while Proteobacteria and Bacteroidetes were overrepresented in the TB group. | [73] |
| The core TB sputum microbiota was made of Actinomyces, Fusobacterium, Leptotrichia, Prevotella, Streptococcus, and Veillonella while Mogibacterium, Moryella and Oribacterium were also present. Unclassified Lactobacillales was overrepresented in the control group. | |||
| Singapore | Twenty-four genetically related healthy pairs (n=48) were divided into young (≤40 years) and old (≥60 years). The sputum sample of participants was collected and analyzed for microbiota composition using 16s rRNA. | Old subjects group was having an abundance of Firmicutes and relatively less abundance of Proteobacteria. Ageing is linked with augmented Firmicutes and reduced Proteobacteria in the healthy Asian cohort’s airway microbiota. Haemophilus and Lautropia were abundant in young. Firmicutes (Gemella) were related to young group lung function, while Fusobacteria and Leptotrichia were linked to elder’s arterial stiffness. | [74] |
| Korea | Microbiota analysis of 27 BAL fluid samples from patients undergoing diagnostic bronchoscopy using conventional microbiology media and identification through MALDI-TOF MS. In addition, 16S rRNA NGS was performed for comparison. | BAL samples showed the highest culture of Streptococcus spp. and Neisseria spp. Actinomyces and Veillonella spp. were the most common anaerobes. Other bacteria like Prevotella spp., Staphylococcus spp., Clostridium spp., and Bifidobacterium spp. were also present. | [75] |
| Iran | Respiratory microbiota of 3-6 years old healthy children was determined through culture and 16s rRNA. | Actinobacteria (4%), Firmicutes (74%), and Proteobacteria (22%) were most common. At genera level Staphylococci, Streptococci, Enterobacteriaceae spp. and A. baumannii were common. | [76] |
| India | Sputum microbiota of TB was analyzed using 16S rRNA and compared with healthy controls. | In healthy controls, Gammaproteobacteria (22%), Streptococcus (20.5%), Neisseria (16.8%) and Haemophilus (15.4%). Actinobacillus were present. | [77] |
| Firmicutes and Actinobacteria were abundant in TB patient’s samples with the dominance of Streptococcus, Neisseria and Veillonella. | |||
| North America | |||
| Canada | Microbiota of URT among 65 H1N1 influenza patients during the 2009 outbreak was determined by cpn60 universal target amplification and sequencing. | The microbiota was dominated by Actinobacteria, Firmicutes and Proteobacteria including 13-20 species and diversity increasing with age. | [78] |
| USA | 16S rRNA gene was used to identify abundant pathogens in the lungs. Only 12 out of 56 showed positive culture for the pathogen. | The results of 16s rRNA analysis were in concordance with clinically isolated pathogens among 11 positive samples. S. aureus, Pseudomonas aeruginosa, E. coli, Fusobacterium, H. influenza, Klebsiella pneumoniae, Enterococcus, Mycoplasma, Prevotella, Veillonella, Rothia, Neisseria, Streptococcus, Garanulicattella, and Gemella were found with the lung samples. | [79] |
| The URT and LRT microbiota of smokers and non-smokers were analyzed through 16s rRNA among a total of 64 participants. | Generally, the same bacteria were present in the lungs and oral cavity, except Enterobacteriaceae, Haemophilus, Methylobacterium, and Ralstonia spp., those were disproportionate in lungs and oral cavity. Tropheryma was lungs specific. The oral microbiota, but not the lung microbiota varies among smokers and non-smoker. | [80] | |
| Lung microbiota was analyzed through BAL collected from 29 asymptomatic individuals, including 9 never, 14 formers, and 6 current smokers with the use of 16s rRNA. Pulmonary inflammation was also evaluated. | LRT microbiota was showing two groups called as pneumotypes. The first pneumotype was similar to the saline group while the second penumotype was showing a higher abundance of supraglottic-characteristic taxa. The later was called as pneumotypeSCT and consist of Veillonella and Prevotella, this was associated with increased lung inflammation. | [81] | |
| 40 participants included in the BOBCAT asthma-related study were selected, and their airway microbiota was analyzed through 16s rRNA. The microbiota profile was matched with clinical and inflammatory features. | The microbiota dysbiosis was found to be related with the inflammatory process, and microbiota composition varies in mild to severe asthma. Preteobacteria were primarily associated with poor asthma control questionnaire (ACQ) score, while Actinobacteria were related with improved ACQ score. | [82] | |
| Central America | |||
| Nicaragua | Participants were enrolled for household transmission study of Influenza and to understand the role of respiratory microbiota in the susceptibility to influenza virus infection. | The microbiota were divided into oligotypes, Alloprevotella sp. and Prevotella histicola/sp./veroralis/fusca/scopos/melaninogenica were positively involved in influenza virus infection. Bacteroides vulgatus oligotype was negatively associated with infection. | [83] |
| South America | |||
| Colombia | Respiratory samples, including, sputum, OP, and nasal samples were collected from TB patients and healthy controls in order to determine bacterial and fungal diversity. | Only OP samples were showing variability among TB and control group. Bacteroidetes, Fusobacteria, Actinobacteria, Proteobacteria were showing differences in relative abundance. | [84] |
| Europe | |||
| Italy | 165 samples were collected as non-malignant lung tissue from cancer patients, and the microbiota profile was determined. | The Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria were forming core microbiota. At the genera level, Proteobacteria genera: Acinetobacter, Pseudomonas, Ralstonia, and other two unknown genera belonging to Comamonadacea and Oxalobacteraceae were forming core microbiota. | [85] |
| BAL samples from 10 pulmonary sarcoidosis patients and 9 interstitial lung disease patients were collected and analyzed for microbiota analysis using 16s rRNA. | Microbiota was dominated by 4 phyla, and Bacteroidetes was abundant in both groups. | [86] | |
| Netherland | 25 set of samples from CF patients were involved in the study and their NP, OP, and BAL samples were collected and analyzed for microbiota using 16s rRNA. | BAL microbiota was mixed with oral and NP flora. The OP and BAL showed an abundance of commensals like Neisseria, Streptococcus, Rothia, Veillonella, Gemella and Prevotella spp., while NP and BAL samples showed an abundance of pathogen like S. aureus, H. influenzae and Moraxella. Corynebacterium and Dolosigranulum spp. were present with all NP samples. | [87] |
| France | 16s rRNA analysis was performed to analyze the microbiota composition of 225NP samples collected from 177 viral respiratory infected patients and 48 controls. | Symptomatic respiratory infections are linked with decreased alpha diversity, loss of microbiota components, especially Prevotella spp. and increased respiratory pathogens including Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, Dolosigranulum pigrum and Corynebacterium propinquum/pseudodiphtheriticum. | [35] |
| UK | Experimental post influenza challenged throat microbiota was analyzed using 16s rRNA from 52 experimental and 35 healthy controls. | At genera level Streptococcus, Fusobacterium and Prevotella, Neisseria, Haemophilus, and Campylobacter were abundant. Prevotella and Fusobacterium were found to varied between Influenza-Like Illness/Sflu+ and Asymptomatic control groups. | [88] |
| Africa | |||
| Uganda | BAL was collected from 60 HIV infected acute pneumonia patients, and microbiota was analyzed through 16s rRNA. | Rich and diverse microbiota was related to low bacteria burden. Pseudomonas aeruginosa was most prevalent in the group. | [89] |
| Kenya | NP microbiota of 60 children who have received PHiD-CV (n=30) or Hepatitis A vaccine was analyzed through 16s rRNA. | Moraxella catarrhalis, Streptococcus pneumoniae and Corynebacterium spp. were abundant in pre-vaccination NP microbiota, while Streptococcus, Moraxella, and Haemophilus spp. were abundant in post-vaccination NP microbiota. | [90] |
| South Africa, Mozambique and Morocco | Respiratory virus and bacteria were identified in acute lower respiratory infection patients using 16s rRNA. | Streptococcus was the most common bacteria, while human rhinovirus was the most common virus linked with acute lower respiratory tract infections. | [91] |
| Australia | |||
| Australia | NP microbiota was analyzed from persistent wheezing disease due to infection associated with lower airway inflammation patients. | Alloiococcus (11.1%), Corynebacterium (12.1%), Haemophilus (8.6%), Moraxella (40.1%), Streptococcus (13.3%), and Staphylococcus (4.2%) were abundant in microbiota. | [92] |
Abbreviations: AN-anterior nares; NP-Nasopharynx; OP-Oropharynx; URT-upper respiratory; LRT-lower respiratory; ILI/Sflu+-Influenza-like illness.
Gut-airway microbiota interactions and respiratory infections
As per an estimate, respiratory infections such as pneumonia and influenza leads to 3.2 million death annually [31], and COVID-2019 pandemic is also contributing to increase these number by causing significant mortality. Identification and modulation of immune response in the respiratory tract is one of the major challenges for the management of respiratory tract infections, including COVID-2019. The persistence of several viruses in the bat without causing major host damage is also supported by the fact that bats mount rapid antiviral response using interferon without a widespread inflammatory response. This condition is suggested to make bats an ideal incubator for increasing viral virulence without damaging their incubation machinery [32]. The role of microbiota in respiratory infections is proven in several studies (Table 1), but it is complicated due to the mixing of oral microbiota. A recent study involving the identification of oral taxa in lung microbiome revealed that certain oral taxa are linked with lung inflammation. These oral taxa are called pneumotypeSPT and lead to increased neutrophils and lymphocytes in bronchoalveolar lavage [33].
In addition, the gut harbors a maximum number of microbes that display several systemic responses on diverse body parts, including the respiratory system. They can modulate several important mechanisms, like inflammatory regulation, oral tolerance development, and production of some essential short-chain fatty acids (SCFA), which translocate to lungs and modulate inflammatory pathways [31]. This connection between gut and lung is known as the gut-lung axis and plays a significant role in the development of respiratory infections [34]. SARS-CoV2 is mainly known to infect the respiratory system, but its detection in the gastrointestinal tract is well known and lead to certain gastrointestinal abnormalities, including diarrhea, which is correlated with decreased diversity and richness of gut microbiota, delayed SARS-CoV2 clearance and immune regulation [18]. Figure 1 shows different factors influencing the development of respiratory microbiota and the chances of its variability.
Figure 1.
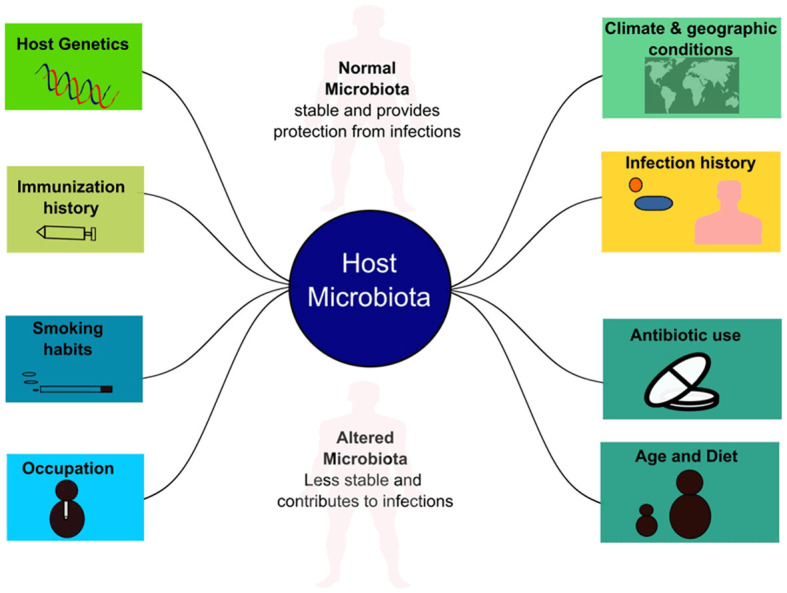
Factors influencing respiratory microbiota and its variability.
Microbiota modulation during respiratory infections
It has been proved in several studies that respiratory microbiota plays a vital role in the development of viral infection. A recent study with nasopharyngeal samples from 177 patients of viral respiratory infections and 48 healthy controls, and subsequent microbiota identification using 16S rRNA revealed a significant difference in microbiota profiling of both groups. This study included respiratory viral infections like influenza A and B, respiratory syncytial virus, metapneumovirus, rhinovirus, etc. Results indicated that several microbial species are absent among the patient group, which forms the core microbiota of healthy individuals. The patients also showed decreased alpha diversity. However, this study also indicated the inter-individual respiratory microbiota variability and stressed on detection of microbiota in a large group of subjects [35].
It has been found that healthy microbiota consists of highly diverse and balanced microbial population. Microbiota alters during the development of respiratory viral infections that promotes colonization by opportunistic pathogens. In contrast, modulation of innate immune mediators under different disease conditions is already involved in regulating secondary infections susceptibility and this aspect must be given serious consideration for COVID-19 [36]. The limitation in analyses of respiratory microbiota arises due to mixing of oral microbiota, and other factors, including antibiotic usage, vaccination, allergies, asthma, and personal habitats, which may contribute to variation in respiratory microbiota profiles.
Certain respiratory infections can also alter the composition of gut microbiota in addition to respiratory microbiota. For example, lung infections by respiratory syncytial and influenza viruses increase Bacteriodetes and reduce the relative abundance of Firmicutes in the gut [37]. In addition, other studies found gut microbiota modulation during respiratory viral infections. It has been found that the influenza virus induces gastroenteritis in patients by systemic effects of respiratory influenza on intestinal microbiota leading to microbiota modulation and subsequent IL-15 production by intestinal epithelial cells. This leads to Th17 cell-dependent inflammation [38]. Moreover, viral infections also lead to faecal metabolome alteration and its shifting towards lipid metabolism. Short-chain fatty acids (SCFA), polyunsaturated fatty acids (PUFA) and sphingolipids are found to be increased in the faecal metabolome of post-RSV infection [39].
The microbiota modulation is also evident with the recent COVID-2019 outbreak. A significant number of COVID-19 patients show gastrointestinal symptoms [40] and the presence of viral genetic material in the faecal samples of COVID-2019 patients indicates its fecal-oral transmission [41]. The impact of gut microbiota on systemic immunity and subsequent respiratory infection risk and inflammation is already explored in a number of studies [42-44]. It is proposed that gut microbiota modulation towards a healthy state can play an adjuvant therapeutic role in the management of COVID-2019 [45]. The information about microbiota modulation during COVID-19 is limited, though emerging. Some studies about coronavirus have found that bacterial ribonuclease known as binase are able to inhibit the replication of MERS-coronavirus and low pathogenic human coronavirus 229E in cell culture studies [46]. However, they obtained this enzyme from Bacillus pumilus B3073, which is normally present in soil and included as normal microbiota of plants, but the presence of several other related bacteria within normal human microbiota raises the scope of debate for their action against SARS-CoV2. It has been identified that commensal immunomodulatory bacteria, such as Bifidobacteria, Faecalibacterium prausnitzii, and Eubacterium rectale are underrepresented in the COVID-19 patient groups even after disease resolution and it is associated with elevated inflammatory markers contributing to disease severity [15]. These all caveats indicate a role of microbiota in COVID-19 susceptibility and its subsequent severity.
SARS-CoV2 and microbiota modulation
The management of COVID-2019 through microbiota modulation is not the main point of debate in case of the current pandemic. Nevertheless, the management of COVID-2019 by microbiota is not completely neglected. Chinese National Health Commission and National Administration of Traditional Chinese medicine recommended the use of probiotics for the treatment of severe COVID-2019 infections during early February 2020. They suggested that probiotics can be used for maintaining healthy intestinal microbiota and subsequent prevention of secondary infections [45,47]. Clinical trials are also undergoing for evaluation of microbiota modulation for the management of COVID-19 [48]. Lung and gut microbiota studies of COVID-2019 patients indicated that the microbiota of infected patients is different from healthy individuals [15,49]. In silico host-pathogen interactions analysis of overrepresented bacteria in the lungs of COVID-19 patients indicate that overexpressed microbial proteins may be involved in viral growth and inflammation [17]. However, the in silico studies need experimental validation and must be seen with its limitation [50], but such computational host-pathogen interaction analysis have revealed several insights about different diseases, including COVID-19 [51-54]. Another study indicated that modulated microbiota of URT among COVID-19 patients is associated with fatality and needs further investigations [55]. It is speculated that microbiota modulation towards healthy microbiota can support gastrointestinal health and may also protect from respiratory infections. The SARS-CoV2 virus uses ACE2 receptor for entry into the host cell, and recent findings reveal that ACE2 has a non-catalytic role in amino acid transfer in the gut. Therefore its expression can be modulated by microbiota. In addition, gut and lung microbiota dysbiosis is associated with several cardiopulmonary diseases, which provide further support for the use of microbiota modulation in the management of the COVID-2019 outbreak [56].
Current status of probiotics in respiratory infections and suggestions for COVID-19
Several experimental evidences are available regarding the prevention of viral and bacterial infections using probiotics. However, the majority of them are based on in vitro or in vivo studies. For example, intranasal inoculation of live or heat-inactivated Lactobacillus plantarum or L. reuteri can protect the mice from lethal infection of Pneumovirus, and this effect lasts for at least 13 weeks [57]. Unfortunately, the use of probiotics is highly specific and needs a separate study for COVID-2019 patients to identify beneficial strains for the prevention of infection. Some commercial probiotic strains are available and proposed to boost up the immune system in case of upper respiratory infections. For example, probiotic strain L. reuteri DSM17938 is known to protect from the respiratory tract and gastrointestinal ailments in children [58,59]. A systemic review of randomized controlled trials regarding the assessment of probiotics’ impact on antibiotic utilization on common acute infections, including respiratory tract infections revealed that probiotics could reduce the risk of common acute infections [60]. The application of probiotics and prebiotics is also known for enhancing immunogenicity in influenza vaccine inoculated adults [61]. The benefits of probiotics and prebiotics in the prevention of respiratory tract infections, including viral infection, are proposed in many studies. Some of these studies are mentioned in Table 2. The possible role of microbiota and probiotics-mediated prevention of respiratory infection is shown in Figure 2, in addition to possible microbiota modulation during SARS-CoV2 infection.
Table 2.
Studies about the use of probiotics in respiratory infections
| Objective of the study | Study Type | Participants | Results | Location of study | Ref. |
|---|---|---|---|---|---|
| Assessment of long term probiotics use on respiratory infection prevention. | Double-Blind, Randomized, Seven months study with placebo control. | 571 healthy children (1-6 years) | Lactobacillus rhamnosus GG can decrease respiratory infections and their severity in children. | Helsinki, Finland | [93] |
| Assessment of Lactobacillus casei Shirota (LcS) in aged subjects with nursing homes stay for reducing respiratory symptoms and enhanced immune response to influenza vaccine. | Double-blind, randomized, placebo-controlled trial. | 737 healthy people (age ≥65 years) in 53 nursing homes | No statistically significant effects of probiotics revealed. | Antwerp, Belgium | [94] |
| Assessment of URTI preventive effects of probiotics (Lactobacillus casei strain Shirota (LcS)) on elder subjects. | double-blinded, randomized, placebo-controlled parallel-group study. | 154 elderly subjects | Probiotics reduce acute upper respiratory infection duration. | Tokyo, Japan | [95] |
| Evaluation of probiotic Lactobacillus casei DN-114001 (fermented product) for prevention of common infectious diseases in elderly. | Multicentric, randomized, double blind and controlled trial. | 1072 participants (median age: 76.0 years) | The probiotic reduces both cumulative and episode duration of URTI and rhinopharyngitis. The probiotic was safe and well tolerated. | 125 centres in France | [96] |
| Evaluation of long term probiotic use of Lactobacillus rhamnosus GG (GG) containing milk on respiratory illness. | randomized, double-blind, placebo-controlled trial. | 523 children (age: 2-6 years) | Probiotic consumption reduces respiratory illness occurrence, but not in total. Future clinical trial required. | Finland | [97] |
| Evaluation of Lactobacillus GG (LGG) for prevention of respiratory and gastrointestinal infections in children attending day care centre. | randomized, double-blind, placebo-controlled trial. | 281 children | LGG reduces URTI risk and lower the number of respiratory symptoms. | Zagreb, Croatia | [98] |
| Assessment of Lactobacillus GG (LGG) in prevention of nosocomial infection of gastrointestinal and respiratory at pediatric hospital. | randomized, double-blind, placebo-controlled trial. | 742 hospitalized children | Reduced risk of respiratory and gastrointestinal infections among hospitalized children. | Zagreb, Croatia | [99] |
| Evaluation of prebiotic (galacto-oligosaccharide and polydextrose mixture, 1:1) and probiotic (Lactobacillus rhamnosus GG, ATCC53103) for reducing risk of virus mediated RTI among preterm infants. | randomized, double-blind, placebo-controlled trial. | 94 preterm infants (gestational age, ≥32 + 0 and ≤36 + 6 weeks; birth weight, >1500 g) | Reduced incidence of RTI through prebiotics and probiotics. | Turku, Finland | [100] |
| Evaluation of coadjuvant ability of oral Lactobacillus fermentum (CECT5716) for influenza vaccine. | randomized, double-blinded, placebo-controlled trial. | 50 participants (31 ♂ and 19 ♀) with intramuscular influenza vaccine | Increase immune response of influenza vaccine through Th1 response and neutralizing antibodies. | Puleva Food S.A. (Granada, Spain) | [101] |
| Evaluation of Bifidobacterium longum SP 07/3, Lactobacillus gasseri PA 16/8, B. bifidum MF 20/5 on the severity and incidence of the common cold. | randomized, double-blind, placebo-controlled. | 479 healthy adults (18-67 years) | Symptoms and duration of common cold reduces with reduced fever days. | Germany | [102] |
| Meta-analysis of clinical evaluation of synbiotics on prevention of RTI. | Meta-analysis of randomized control trials. | 16/62 studies were used for meta-analysis (including >10,000 individuals) | Synbiotic reduces the incidence of RTI and proportion of RTI experience by participants. This can be an alternative stretagy for preventing RTI. | [103] | |
| Effect of probiotics specially Bifidobacterium and Lactobacillus was evaluated on ARTI. | Meta-analysis of randomized controlled trials. | 20 randomized control trials were included | Probiotics use can significantly reduce the number of days of illness, days of absence from daycare/school/work. | [104] | |
| Evaluation of probiotics effects and safety on prevention of URTI in person at risk. | Meta-analysis of randomized controlled trials. | 13 trials were included for analysis | Probiotics are beneficial in URTI. It reduces the duration of an episode of URTI and antibiotic prescription rate. | [105] | |
| Evaluation of possible effects of probiotics on RTI associated expenses in USA primary care settings. | Evaluation of two meta-analysis of probiotic use in RTI including YHEC and Cochrane was performed independently. | Meta-analysis of York Health Economic consortium (YHEC) and Chochrane | Probiotics use in USA can save $1.4 billion cost due to ARTI, including ILI ranging from severe flu to mild cold. It also decreases antibiotic use and duration from the absence of work. | [106] |
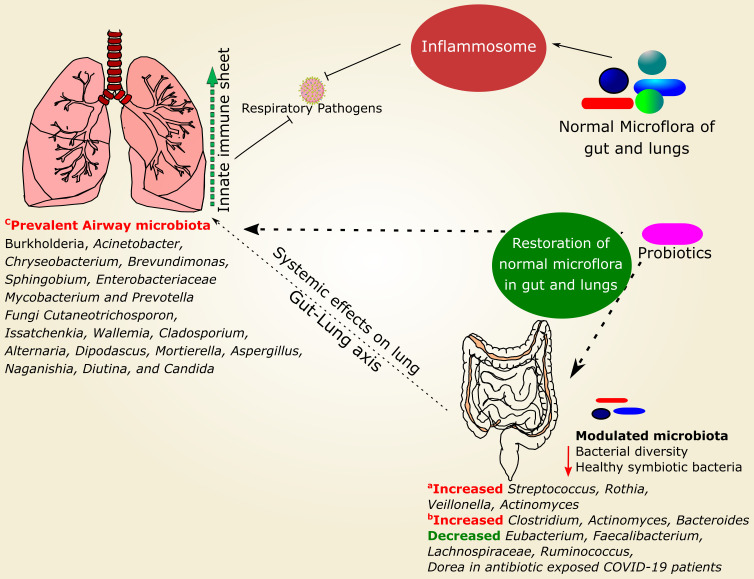
Figure 2.
Role of normal microbiota in the prevention of respiratory infections and possible mechanistic interventions of probiotics. Inflammosome generally elicit antiviral response [107], but SARS-CoV2 engages inflammosome and triggers pyroptosis resulting in increased pro-inflammatory cytokines in human primary monocytes [108]. Microbiota modulation associated with COVID-19 is also indicated separately with representative studies for gut microbiota and respiratory microbiota. a: [109], b: [110], c: [111].
Uses of probiotics is a common strategy to modulate microbiota towards a healthy state, as it has been proven clinically useful in managing several diseases [62]. Due to the recently identified role of modulated microbiota in deciding the fate of COVID-2019 disease, modulation of microbiota to a healthy state is also studied for controlling virus infection or to prevent infection severity. Probiotic bacteria are known to support the immune system and induce an immune response against invading pathogens. They are also known for mediating certain direct and indirect effects on host-microbiota resulting in beneficial physiological changes [63,64]. It is suggested that probiotics and a high fiber diet can manifest anti-inflammatory effects in COVID-19 through targeting the gut-lung microbiota axis [65]. Some clinical trials regarding the study of probiotics for management of COVID-19 are already undergoing and the results may provide future direction for the prevention of this pandemic (Table 3). It is suggested that reduced immune response leads to increased mortality with recent COVID-2019 pandemic [66]. Therefore, the study of probiotics’ application in respiratory infections must be given a serious consideration, especially in the case of the current COVID-2019 outbreak, where the virus is continually challenging healthcare researchers.
Table 3.
Some representative clinical trials evaluating efficacy of microbiota modulation and probiotics in COVID-19
| Country | Study type (Trial number) | Evaluation goals | Intervention/Treatment |
|---|---|---|---|
| Canada | Randomised, single-blinded trial with 23 participants (NCT04458519) | Evaluation of intranasal probiotic administration for reduction of COVID-19 symptoms severity. | Probiorinse (2.4 Billion CFU (Colony-Forming Units) of Lactococcus Lactis W136, (NPN: 80085895)) |
| USA | open-label, randomized, and controlled clinical trial with 100 participants (NCT04540406) | Evaluation of NBT-NM108 as an early treatment by modulating gut microbiota in COVID-19. | NBT-NM108, a novel botanical based fixed combination drug |
| Mexico | Interventional, randomized, single-blind trial with 240 participants (NCT04507867) | Evaluation of nutritional support system (NSS) in COVID-19 patients with co-morbidities and reduction of complications. | NSS with Saccharomyces bourllardii |
| Argentina | Randomized, triple blind trial with 140 participants (NCT04403646) | Evaluation of Tannin specific natural extract for COVID-19 due to their anti-inflammatory, anti-oxidative and intestinal microbiota modulatory activity. | Dry extract of polyphenols (tannins) from quebracho and chestnut |
| Spain | Randomized, open label trial with 41 participants (NCT04390477) | Evaluation of probiotics in COVID-19 patients for improvement of symptoms and reduction of hospital stay. | Probiotic strain with maltodextrin as excipient |
| Spain | Randomized, Quadruple blind trial with 314 participants (NCT04366180) | Evaluation of Lactobacillus coryniformis K8 on the incidence and severity of Covid-19 in health workers exposed to virus. | Lactobacillus K8 per day (3×10^9 cfu/day) |
| Mexico | Randomized, quadruple blind trial with 300 participants (NCT04517422) | Evaluation of efficacy of L. plantarum and P. acidilactici in Adults With SARS-CoV-2. | Combination of Lactobacillus plantarum CECT30292, Lactobacillus plantarum CECT7484, Lactobacillus plantarum CECT7485, and Pediococcus acidilactici CECT7483 |
| Italy | Randomized, single blind trial with 152 participants (NCT04366089) | Evaluation of oxygen ozone and probiotics as an adjuvant strategy for management of COVID-19 and modulation of microbiota. | Oxygen-ozone therapy with dietary supplements SivoMixx (Streptococcus thermophilus DSM322245, Bifidobacterium lactis DSM32246, Bifidobacterium lactis DSM32247, Lactobacillus acidophilus DSM32241, Lactobacillus helveticus DSM32242, Lactobacillus paracasei DSM32243, Lactobacillus plantarum DSM32244, Lactobacillus brevis DSM27961) |
| USA | Randomized, triple blind trial with 182 participants (NCT04399252) | Evaluation of the effect of probiotic Lactobacillus rhamnosus GG (LGG) and COVID-19 on the microbiome in exposed household contacts. | Lactobacillus rhamnosus GG |
Final remarks
COVID-2019 outbreak poses a formidable challenge to the global scientific community. Scientific efforts, worldwide are unraveling several new insights of this viral infection at a rapid pace, though the complete management of this pandemic is still challenging. Earlier studies with other respiratory viral infections confirmed the involvement of microbiota in respiratory infections, which is also verified with COVID-19 through some limited studies. Recent studies that performed microbiota analyses of COVID-19 patients have found a significant difference in microbiota among healthy and SARS-CoV2 infected individuals. Therefore, microbiota balance towards a healthy state using probiotics and prebiotics has been recommended and is currently under investigation in some clinical trials. As microbiota modulation is becoming significant in SARS-CoV2 infection, more studies are required to understand this aspect on mechanistic implications of SARS-CoV2 infection and its subsequent management. Many studies have identified microbiota modulation and probiotics application in respiratory tract viral infections. Such studies can act as a stepping stone in the identification of suitable probiotics strains for the management of current pandemic. The application of probiotics for immune system enhancement and management of COVID-2019 must be given a serious consideration, as we are still struggling to manage this pandemic on a global scale. Unfortunately, the recent trend in the COVID-19 pandemic has also witnessed the development of several opportunistic infections among recovered patients. Virus-mediated immunomodulation and subsequent therapy-associated immunosuppression are believed to be responsible for increased sensitivity to opportunistic infections, but the exact cause is still under investigation and must be investigated separately. This aspect also needs serious safety consideration of probiotic in such population which is already vulnerable to several opportunistic infections. However, such studies may be time-consuming and not practical under the current emergency situation, therefore the available information on probiotics safety in other respiratory viral infections can give an idea about their application in SARS-CoV2 infected groups, and this knowledge could prove to be helpful to design strategies for management of COVID-19. We must be optimistic with the current knowledge of microbiota modulation during respiratory viral infections and its subsequent management through probiotics and prebiotics. Prebiotics are substances used to promote the growth of probiotics, and these substances are under clinical trials for the management of COVID-19. We have compiled several studies about the use of prebiotics in respiratory viral infections, including SARS-CoV2, and such data will be helpful for designing strategies for the application of prebiotics in the management of COVID-19. As mentioned earlier, the existing information about the potential of probiotics and prebiotics in other respiratory viral infections can certainly reduce time and cost on such analyses with respect to COVID-19 and can therefore provide the right direction for such studies. The use of probiotics and prebiotics in SARS-CoV2 can definitely provide suitable adjunctive therapy to control the effects of this pandemic, as the studies based on previous experiences with other respiratory viral infections can increase the chances of success. The studies are already being conducted at a reasonable fast pace and hopefully this information will help to achieve this endeavor for successful management of COVID-19.
Disclosure of conflict of interest
None.
References
- 1.Zhou L, Liu HG. Early detection and disease assessment of patients with novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E003. doi: 10.3760/cma.j.issn.1001-0939.2020.0003. [DOI] [PubMed] [Google Scholar]
- 2.Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52:575–581. doi: 10.1016/j.arcmed.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadikia K, Hashemi SJ, Khodavaisy S, Getso MI, Alijani N, Badali H, Mirhendi H, Salehi M, Tabari A, Mohammadi Ardehali M, Kord M, Roilides E, Rezaie S. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021;64:798–808. doi: 10.1111/myc.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AA, Khan Z. System biological investigations of hydroxychloroquine and azithromycin targets and their implications in QT interval prolongation. Chem Biol Interact. 2020;332:109299. doi: 10.1016/j.cbi.2020.109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habibzadeh P, Stoneman EK. The novel coronavirus: a bird’s eye view. Int J Occup Environ Med. 2020;11:65–71. doi: 10.15171/ijoem.2020.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassanin A. Coronavirus origins: genome analysis suggests two viruses may have combined. The Conversation. 2020. March: https://theconversation.com/coronavirus-origins-genome-analysis-suggests-two-viruses-may-have-combined-134059.
- 8.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KK, Sridhar S, Chiu KH, Hung DL, Li X, Hung IF, Tam AR, Chung TW, Chan JF, Zhang AJ, Cheng VC, Yuen KY. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. 2021;10:507–535. doi: 10.1080/22221751.2021.1898291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh H, Choudhari R, Nema V, Khan AA. ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb Pathog. 2021;150:104621. doi: 10.1016/j.micpath.2020.104621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh D, Yi SV. On the origin and evolution of SARS-CoV-2. Exp Mol Med. 2021;53:537–547. doi: 10.1038/s12276-021-00604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, Chen N, Li A, Lu W, Chan FKL, Chan PKS, Ng SC. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159:1302–1310. e1305. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan AA, Shrivastava A, Khurshid M. Normal to cancer microbiome transformation and its implication in cancer diagnosis. Biochim Biophys Acta. 2012;1826:331–337. doi: 10.1016/j.bbcan.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Khan AA, Khan Z. COVID-2019 infection associated over-expressed Prevotella proteins mediated host-pathogen interactions and their role in coronavirus outbreak. Bioinformatics. 2020;36:4065–4069. doi: 10.1093/bioinformatics/btaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota modulation of the gut-lung axis in COVID-19. Front Immunol. 2021;12:635471. doi: 10.3389/fimmu.2021.635471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhar D, Mohanty A. Gut microbiota and COVID-19-possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viana SD, Nunes S, Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities - role of gut microbiota dysbiosis. Ageing Res Rev. 2020;62:101123. doi: 10.1016/j.arr.2020.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh H, Singh A, Khan AA, Gupta V. Immune mediating molecules and pathogenesis of COVID-19-associated neurological disease. Microb Pathog. 2021;158:105023. doi: 10.1016/j.micpath.2021.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan AA, Nema V, Khan Z. Current status of probiotics for prevention and management of gastrointestinal cancers. Expert Opin Biol Ther. 2021;21:413–422. doi: 10.1080/14712598.2021.1828858. [DOI] [PubMed] [Google Scholar]
- 23.Afzal M, Mazhar SF, Sana S, Naeem M, Rasool MH, Saqalein M, Nisar MA, Rasool M, Bilal M, Khan AA, Khurshid M. Neurological and cognitive significance of probiotics: a holy grail deciding individual personality. Future Microbiol. 2020;15:1059–1074. doi: 10.2217/fmb-2019-0143. [DOI] [PubMed] [Google Scholar]
- 24.Khurshid M, Aslam B, Nisar MA, Akbar R, Rahman H, Khan AA, Rasool MH. Bacterial munch for infants: potential pediatric therapeutic interventions of probiotics. Future Microbiol. 2015;10:1881–1895. doi: 10.2217/fmb.15.102. [DOI] [PubMed] [Google Scholar]
- 25.Kumpitsch C, Koskinen K, Schopf V, Moissl-Eichinger C. The microbiome of the upper respiratory in health and disease. BMC Biol. 2019;17:87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libertucci J, Young VB. The role of the microbiota in infectious diseases. Nat Microbiol. 2019;4:35–45. doi: 10.1038/s41564-018-0278-4. [DOI] [PubMed] [Google Scholar]
- 27.Khatiwada S, Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): possible link and implications. Hum Microb J. 2020;17:100073. doi: 10.1016/j.humic.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell AB, Glanville AR. The human respiratory microbiome: implications and impact. Semin Respir Crit Care Med. 2018;39:199–212. doi: 10.1055/s-0037-1617441. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell AB, Glanville AR. The human respiratory microbiome: the end of the beginning? In: Glanville AR, editor. Essentials in lung transplantation. Cham: Springer International Publishing; 2019. pp. 87–97. [Google Scholar]
- 30.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook CE, Boots M, Chandran K, Dobson AP, Drosten C, Graham AL, Grenfell BT, Müller MA, Ng M, Wang LF, van Leeuwen A. Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence. Elife. 2020;9:e48401. doi: 10.7554/eLife.48401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, Li Y, Shen N, Ghedin E, Morris A, Diaz P, Huang L, Wikoff WR, Ubeda C, Artacho A, Rom WN, Sterman DH, Collman RG, Blaser MJ, Weiden MD. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016;1:16031. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 35.Edouard S, Million M, Bachar D, Dubourg G, Michelle C, Ninove L, Charrel R, Raoult D. The nasopharyngeal microbiota in patients with viral respiratory infections is enriched in bacterial pathogens. Eur J Clin Microbiol Infect Dis. 2018;37:1725–1733. doi: 10.1007/s10096-018-3305-8. [DOI] [PubMed] [Google Scholar]
- 36.Khan AA, Khan Z, Warnakulasuriya S. Cancer-associated toll-like receptor modulation and insinuation in infection susceptibility: association or coincidence? Ann Oncol. 2016;27:984–997. doi: 10.1093/annonc/mdw053. [DOI] [PubMed] [Google Scholar]
- 37.Groves HT, Cuthbertson L, James P, Moffatt MF, Cox MJ, Tregoning JS. Respiratory disease following viral lung infection alters the murine gut microbiota. Front Immunol. 2018;9:182. doi: 10.3389/fimmu.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397–410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS. Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio. 2020;11:e03236–19. doi: 10.1128/mBio.03236-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57–69. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, Wang YY, Xiao GF, Yan B, Shi ZL, Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol. 2020;11:301. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haak BW, Littmann ER, Chaubard JL, Pickard AJ, Fontana E, Adhi F, Gyaltshen Y, Ling L, Morjaria SM, Peled JU, van den Brink MR, Geyer AI, Cross JR, Pamer EG, Taur Y. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131:2978–2986. doi: 10.1182/blood-2018-01-828996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome. 2018;6:9. doi: 10.1186/s40168-017-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao QY, Chen YX, Fang JY. 2019 novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller C, Ulyanova V, Ilinskaya O, Pleschka S, Shah Mahmud R. A novel antiviral strategy against MERS-CoV and HCoV-229E using binase to target viral genome replication. Bionanoscience. 2017;7:294–299. doi: 10.1007/s12668-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Health Committee of the People’s Republic of China NAoTCM. Diagnostic and therapeutic guidance for 2019 novel coronavirus disease (version 5) http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf. National Health Committee Report 2020.
- 48.Haran JP, Pinero JC, Zheng Y, Palma NA, Wingertzahn M. Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study. Trials. 2021;22:245. doi: 10.1186/s13063-021-05157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostafa HH, Fissel JA, Fanelli B, Bergman Y, Gniazdowski V, Dadlani M, Carroll KC, Colwell RR, Simner PJ. Metagenomic next-generation sequencing of nasopharyngeal specimens collected from confirmed and suspect COVID-19 patients. mBio. 2020;11:e01969–20. doi: 10.1128/mBio.01969-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan AA, Khan Z, Kalam MA, Khan AA. Inter-kingdom prediction certainty evaluation of protein subcellular localization tools: microbial pathogenesis approach for deciphering host microbe interaction. Brief Bioinform. 2018;19:12–22. doi: 10.1093/bib/bbw093. [DOI] [PubMed] [Google Scholar]
- 51.Khan AA, Khan Z. Comparative host-pathogen protein-protein interaction analysis of recent coronavirus outbreaks and important host targets identification. Brief Bioinform. 2021;22:1206–1214. doi: 10.1093/bib/bbaa207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan AA, Bano Y. Salmonella enterica subsp. enterica host-pathogen interactions and their implications in gallbladder cancer. Microb Pathog. 2021;157:105011. doi: 10.1016/j.micpath.2021.105011. [DOI] [PubMed] [Google Scholar]
- 53.Khan AA, A Abuderman A, Ashraf MT, Khan Z. Protein-protein interactions of HPV-Chlamydia trachomatis-human and their potential in cervical cancer. Future Microbiol. 2020;15:509–520. doi: 10.2217/fmb-2019-0242. [DOI] [PubMed] [Google Scholar]
- 54.Bose T, Das C, Dutta A, Mahamkali V, Sadhu S, Mande SS. Understanding the role of interactions between host and Mycobacterium tuberculosis under hypoxic condition: an in silico approach. BMC Genomics. 2018;19:555. doi: 10.1186/s12864-018-4947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li M, Ren L, Wang Y, Zhong J, Zhang D, Xiao Y, Yang J, Fan G, Guo L, Shen Z, Liu W, Kang L, Shi L, Li X, Li Q, Li J, Di L, Li H, Wang C, Wang Y, Wang X, Zou X, Rao J, Zhang L, Wang J, Huang Y, Cao B, Wang J. Dynamics of the upper respiratory microbiota and its association with fatality in COVID-19 patients. Available at SSRN: https://ssrn.com/abs=3719095 or PrePrint 2020; 10.21203/rs.3.rs-95239/v1.
- 56.Cole-Jeffrey CT, Liu M, Katovich MJ, Raizada MK, Shenoy V. ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J Cardiovasc Pharmacol. 2015;66:540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, Rosenberg HF. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol. 2011;186:1151–1161. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gutierrez-Castrellon P, Lopez-Velazquez G, Diaz-Garcia L, Jimenez-Gutierrez C, Mancilla-Ramirez J, Estevez-Jimenez J, Parra M. Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial. Pediatrics. 2014;133:e904–909. doi: 10.1542/peds.2013-0652. [DOI] [PubMed] [Google Scholar]
- 59.Tubelius P, Stan V, Zachrisson A. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled study. Environ Health. 2005;4:25. doi: 10.1186/1476-069X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King S, Tancredi D, Lenoir-Wijnkoop I, Gould K, Vann H, Connors G, Sanders ME, Linder JA, Shane AL, Merenstein D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494–499. doi: 10.1093/eurpub/cky185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9:1175. doi: 10.3390/nu9111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan AA, Khurshid M, Khan S, Alshamsan A. Gut microbiota and probiotics: current status and their role in cancer therapeutics. Drug Dev Res. 2013;74:365–375. [Google Scholar]
- 63.Wieers G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy JM, Dequenne I, de Timary P, Cani PD. How probiotics affect the microbiota. Front Cell Infect Microbiol. 2019;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27:496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conte L, Toraldo DM. Targeting the gut-lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther Adv Respir Dis. 2020;14:1753466620937170. doi: 10.1177/1753466620937170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fehr AR, Channappanavar R, Jankevicius G, Fett C, Zhao J, Athmer J, Meyerholz DK, Ahel I, Perlman S. The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. mBio. 2016;7:e01721–16. doi: 10.1128/mBio.01721-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Dai W, Feng X, Zhou Q, Wang H, Yang Y, Li S, Zheng Y. Microbiota composition in upper respiratory tracts of healthy children in Shenzhen, China, differed with respiratory sites and ages. Biomed Res Int. 2018;2018:6515670. doi: 10.1155/2018/6515670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tong X, Su F, Xu X, Xu H, Yang T, Xu Q, Dai H, Huang K, Zou L, Zhang W, Pei S, Xiao F, Li Y, Wang C. Alterations to the lung microbiome in idiopathic pulmonary fibrosis patients. Front Cell Infect Microbiol. 2019;9:149. doi: 10.3389/fcimb.2019.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai W, Wang H, Zhou Q, Li D, Feng X, Yang Z, Wang W, Qiu C, Lu Z, Xu X, Lyu M, Xie G, Li Y, Bao Y, Liu Y, Shen K, Yao K, Feng X, Yang Y, Zhou K, Li S, Zheng Y. An integrated respiratory microbial gene catalogue to better understand the microbial aetiology of Mycoplasma pneumoniae pneumonia. Gigascience. 2019;8:giz093. doi: 10.1093/gigascience/giz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J, Liu W, He L, Huang F, Chen J, Cui P, Shen Y, Zhao J, Wang W, Zhang Y, Zhu M, Zhang W, Zhang Y. Sputum microbiota associated with new, recurrent and treatment failure tuberculosis. PLoS One. 2013;8:e83445. doi: 10.1371/journal.pone.0083445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Z, Zhou Y, Li H, Zhang Y, Zhang S, Tang S, Guo X. Complex sputum microbial composition in patients with pulmonary tuberculosis. BMC Microbiol. 2012;12:276. doi: 10.1186/1471-2180-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Ding J, Xiao Y, Xu B, He W, Yang Y, Yang L, Su M, Hao X, Ma Y. 16S rDNA sequencing analysis of upper respiratory flora in patients with influenza H1N1 virus infection. Frontiers in Laboratory Medicine. 2017;1:16–26. [Google Scholar]
- 73.Cheung MK, Lam WY, Fung WY, Law PT, Au CH, Nong W, Kam KM, Kwan HS, Tsui SK. Sputum microbiota in tuberculosis as revealed by 16S rRNA pyrosequencing. PLoS One. 2013;8:e54574. doi: 10.1371/journal.pone.0054574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SY, Mac Aogáin M, Fam KD, Chia KL, Binte Mohamed Ali NA, Yap MMC, Yap EPH, Chotirmall SH, Lim CL. Airway microbiome composition correlates with lung function and arterial stiffness in an age-dependent manner. PLoS One. 2019;14:e0225636. doi: 10.1371/journal.pone.0225636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sung JY, Hwang Y, Shin MH, Park MS, Lee SH, Yong D, Lee K. Utility of conventional culture and MALDI-TOF MS for identification of microbial communities in bronchoalveolar lavage fluid in comparison with the GS junior next generation sequencing system. Ann Lab Med. 2018;38:110–118. doi: 10.3343/alm.2018.38.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maleki A, Zamirnasta M, Taherikalani M, Pakzad I, Mohammadi J, Krutova M, Kouhsari E, Sadeghifard N. The characterization of bacterial communities of oropharynx microbiota in healthy children by combining culture techniques and sequencing of the 16S rRNA gene. Microb Pathog. 2020;143:104115. doi: 10.1016/j.micpath.2020.104115. [DOI] [PubMed] [Google Scholar]
- 77.Krishna P, Jain A, Bisen PS. Microbiome diversity in the sputum of patients with pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2016;35:1205–10. doi: 10.1007/s10096-016-2654-4. [DOI] [PubMed] [Google Scholar]
- 78.Chaban B, Albert A, Links MG, Gardy J, Tang P, Hill JE. Characterization of the upper respiratory microbiomes of patients with pandemic H1N1 influenza. PLoS One. 2013;8:e69559. doi: 10.1371/journal.pone.0069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kitsios GD, Fitch A, Manatakis DV, Rapport SF, Li K, Qin S, Huwe J, Zhang Y, Doi Y, Evankovich J, Bain W, Lee JS, Methé B, Benos PV, Morris A, McVerry BJ. Respiratory microbiome profiling for etiologic diagnosis of pneumonia in mechanically ventilated patients. Front Microbiol. 2018;9:1413. doi: 10.3389/fmicb.2018.01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, Jablonski K, Kleerup E, Lynch SV, Sodergren E, Twigg H, Young VB, Bassis CM, Venkataraman A, Schmidt TM, Weinstock GM Lung HIV Microbiome Project. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Chen H, Berger KI, Goldring RM, Rom WN, Blaser MJ, Weiden MD. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1:19. doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee KH, Gordon A, Shedden K, Kuan G, Ng S, Balmaseda A, Foxman B. The respiratory microbiome and susceptibility to influenza virus infection. PLoS One. 2019;14:e0207898. doi: 10.1371/journal.pone.0207898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Botero LE, Delgado-Serrano L, Cepeda ML, Bustos JR, Anzola JM, Del Portillo P, Robledo J, Zambrano MM. Respiratory clinical sample selection for microbiota analysis in patients with pulmonary tuberculosis. Microbiome. 2014;2:29. doi: 10.1186/2049-2618-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu G, Gail MH, Consonni D, Carugno M, Humphrys M, Pesatori AC, Caporaso NE, Goedert JJ, Ravel J, Landi MT. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016;17:163. doi: 10.1186/s13059-016-1021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Argenio V, Casaburi G, Precone V, Moccia LG, Postiglione I, Bocchino M, Sanduzzi A. A common microbial signature is present in the lower airways of interstitial lung diseases including sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2018;35:354–362. doi: 10.36141/svdld.v35i4.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prevaes SM, de Steenhuijsen Piters WA, de Winter-de Groot KM, Janssens HM, Tramper-Stranders GA, Chu ML, Tiddens HA, van Westreenen M, van der Ent CK, Sanders EA, Bogaert D. Concordance between upper and lower airway microbiota in infants with cystic fibrosis. Eur Respir J. 2017;49:1602235. doi: 10.1183/13993003.02235-2016. [DOI] [PubMed] [Google Scholar]
- 88.Ramos-Sevillano E, Wade WG, Mann A, Gilbert A, Lambkin-Williams R, Killingley B, Nguyen-Van-Tam JS, Tang CM. The effect of influenza virus on the human oropharyngeal microbiome. Clin Infect Dis. 2018;68:1993–2002. doi: 10.1093/cid/ciy821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwai S, Huang D, Fong S, Jarlsberg LG, Worodria W, Yoo S, Cattamanchi A, Davis JL, Kaswabuli S, Segal M, Huang L, Lynch SV. The lung microbiome of Ugandan HIV-infected pneumonia patients is compositionally and functionally distinct from that of San Franciscan patients. PLoS One. 2014;9:e95726. doi: 10.1371/journal.pone.0095726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feazel LM, Santorico SA, Robertson CE, Bashraheil M, Scott JA, Frank DN, Hammitt LL. Effects of vaccination with 10-valent pneumococcal non-typeable haemophilus influenza protein D conjugate vaccine (PHiD-CV) on the nasopharyngeal microbiome of Kenyan toddlers. PLoS One. 2015;10:e0128064. doi: 10.1371/journal.pone.0128064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Annamalay AA. The role of viruses and bacteria in childhood acute lower respiratory infections in Africa. The University of Western Australia. 2015. PhD Thesis: https://research-repository.uwa.edu.au/en/publications/the-role-of-viruses-and-bacteria-in-childhood-acute-lower-respira.
- 92.Teo SM, Tang HH, Mok D, Judd LM, Watts SC, Pham K, Holt BJ, Kusel M, Serralha M, Troy N, Bochkov YA, Grindle K, Lemanske RF, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. Dynamics of the upper airway microbiome in the pathogenesis of asthma-associated persistent wheeze in preschool children. bioRxiv. 2017:222190. [Google Scholar]
- 93.Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Puyenbroeck K, Hens N, Coenen S, Michiels B, Beunckens C, Molenberghs G, Van Royen P, Verhoeven V. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95:1165–1171. doi: 10.3945/ajcn.111.026831. [DOI] [PubMed] [Google Scholar]
- 95.Fujita R, Iimuro S, Shinozaki T, Sakamaki K, Uemura Y, Takeuchi A, Matsuyama Y, Ohashi Y. Decreased duration of acute upper respiratory infections with daily intake of fermented milk: a multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am J Infect Control. 2013;41:1231–1235. doi: 10.1016/j.ajic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br J Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- 97.Kumpu M, Kekkonen RA, Kautiainen H, Jarvenpaa S, Kristo A, Huovinen P, Pitkaranta A, Korpela R, Hatakka K. Milk containing probiotic Lactobacillus rhamnosus GG and respiratory illness in children: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2012;66:1020–1023. doi: 10.1038/ejcn.2012.62. [DOI] [PubMed] [Google Scholar]
- 98.Hojsak I, Snovak N, Abdovic S, Szajewska H, Misak Z, Kolacek S. Lactobacillus GG in the prevention of gastrointestinal and respiratory infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2010;29:312–316. doi: 10.1016/j.clnu.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 99.Hojsak I, Abdovic S, Szajewska H, Milosevic M, Krznaric Z, Kolacek S. Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory infections. Pediatrics. 2010;125:e1171–1177. doi: 10.1542/peds.2009-2568. [DOI] [PubMed] [Google Scholar]
- 100.Luoto R, Ruuskanen O, Waris M, Kalliomaki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–413. doi: 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, Rodriguez JM, Xaus J. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 102.de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, Ott S, Hampe J, Schreiber S, Heller K, Schrezenmeir J. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24:481–491. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Chan CKY, Tao J, Chan OS, Li HB, Pang H. Preventing respiratory infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:979–988. doi: 10.1093/advances/nmaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41–54. doi: 10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015:CD006895. doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 106.Lenoir-Wijnkoop I, Merenstein D, Korchagina D, Broholm C, Sanders ME, Tancredi D. Probiotics reduce health care cost and societal impact of Flu-like respiratory infections in the USA: an economic modeling study. Front Pharmacol. 2019;10:980. doi: 10.3389/fphar.2019.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao C, Zhao W. NLRP3 inflammasome-a key player in antiviral responses. Front Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias SDSG, Fintelman-Rodrigues N, Sacramento CQ, Mattos M, de Freitas CS, Temerozo JR, Teixeira L, Damaceno Hottz E, Barreto EA, Pão CRR, Palhinha L, Miranda M, Bou-Habib DC, Bozza FA, Bozza PT, Souza TML. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021;7:43. doi: 10.1038/s41420-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. e948. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan J, Li X, Gao Y, Zhou J, Wang S, Huang B, Wu J, Cao Q, Chen Y, Wang Z, Luo D, Zhou T, Li R, Shang Y, Nie X. The lung tissue microbiota features of 20 deceased patients with COVID-19. J Infect. 2020;81:e64–e67. doi: 10.1016/j.jinf.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]