Abstract
Venous malformation (VM) is a kind of congenital vascular anomaly with high recurrence, and screening for VM lacks an efficient, inexpensive and noninvasive approach now. Serum miRNAs with stable structures are expected to become new postoperative and postablative monitoring biomarkers. Thus, we identified a prognostic serum miR-18a-5p and validated its function in VM. Notably, higher expression level of miR-18a-5p was detected in VM patients than in healthy individuals. We found that miR-18a-5p plays a promotive role in human umbilical vein endothelial cells in vitro. In addition, immunohistochemistry (IHC) results showed a distinct increase of vessels in miR-18a-5p mimics group and a decrease of vessels in inhibitors group compared to the control group in a murine VM model. Furthermore, thrombospondin-1 (TSP1), a potential miR-18a-5p-binding protein, was identified via RNA-seq, luciferase reporter and RNA immunoprecipitation (RIP) assays. Moreover, miR-18a-5p regulated the activation of P53 signaling pathway constituents and consequently led to the regulation of proliferation, migration, invasion and angiogenesis. These results provide a strong theoretical basis for further investigations into pathological mechanism of VM and may provide novel and noninvasive biomarker for VM diagnosis and monitoring.
Keywords: Serum, miR-18a-5p, biomarker, venous malformation, thrombospondin-1, P53
Introduction
Venous malformation (VM) is a commonly encountered vascular malformation with an incidence of approximately 1% of the population [1,2]. Deficits in venous network development lead to dysfunctional venous channels [3,4]. These low-flow venous sacs develop at birth and progressively expand along with age; ultimately, these sacs infiltrate and compress normal adjacent tissues [5]. Clinically, symptoms of VM range from small blue localized varicosities to massively swollen lesions [6]. VM may result in not only pain and coagulation abnormalities, but also some potentially life-threatening complications [7] (Figure 1A). There are several treatment techniques available, including sclerotherapy, surgery and laser ablation [8], however, there is currently no standard treatment for VM, and the outcomes of some patients are still not satisfactory, especially for those with massive lesions. Thus, efficient evaluation and screening of VM is still a medical challenge.
Figure 1.
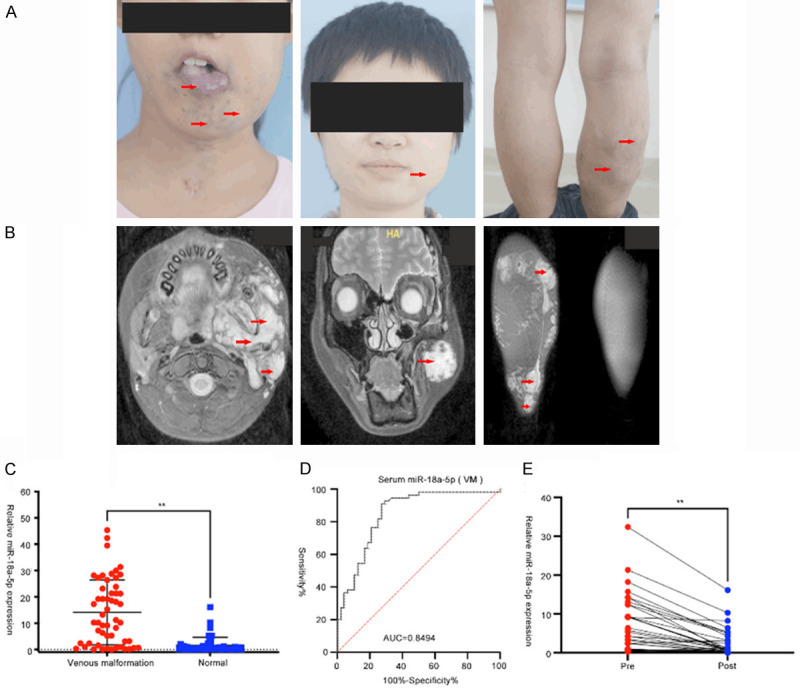
Serum expression of miR-18a-5p in VM patients. A. VM lesions involving the tongue, face and legs, which seriously affected the appearance and function of patients. Red arrows represent the VM lesions. B. The MRI imaging below shows extensive disordered vessels. Red arrows represent the VM lesions. C. A comparison of serum miR-18a-5p levels in VM patients and healthy controls (unpaired t test, **P<0.05). D. The diagnostic accuracy of serum miR-18a-5p for discriminating VM patients and healthy controls. E. Comparison of serum miR-18a-5p in paired pre- and post-operative samples (n = 41). **P<0.05 (paired t test). Data were obtained from three independent repeated experiments. **P<0.05; n.s.: not significant.
MicroRNAs (miRNAs) are 18 to 25 nucleotides non-coding RNAs that regulate numerous pathological processes by binding to target mRNA sequences and inhibiting translation [9,10]. Previous studies showed that miRNAs are relatively stable in serum, which makes it possible to monitor the presence and progression of diseases via miRNA analysis [11-14]. A previous study reported that miR-145 may be involved in the sclerotherapy of VM [15] despite not having a valuable clinical function, so more researches are needed to find promising miRNAs in blood for the evaluation and management of VM. MiR-18a-5p, belonging to the miR-17-92 miRNA cluster, has been reported to participate in brain arteriovenous malformations (BAVMs) [16] and pulmonary arterial hypertension (PAH) [17], indicating that miR-18a-5p may contribute to the process of angiogenesis. Besides, a previous study has reported that miR-18a-5p can function as an effective index of radio-sensitivity in treating lung cancer [18]. Although miR-18a-5p has been characterized regarding its function in many diseases, its role in the pathophysiology of VM has yet to be elucidated.
In this work, we first studied serum samples from venous malformation patients and analyzed the level of angiogenic-related miR-18a-5p, which could potentially act as a serum biomarker for monitoring VM. In addition, we performed some studies to identify the potential target genes of miR-18a-5p and explored the regulatory mechanisms of miR-18a-5p function in venous malformation.
Materials and methods
Clinical samples
No patients received any therapy prior to treatment. We collected 143 serum samples from 102 participants, including 54 patients with VM and 48 healthy controls. The 54 patients were diagnosed with venous malformation by their clinical features and magnetic resonance imaging (MRI). They were recruited at the Ninth People’s Hospital between 2019 and 2020 (Figure 1B). Blood and serum samples from all participants were collected and stored at -80°C. We obtained informed consent from all participants for publication of identified information/images in an online open-access publication. The performance of the study followed the guidelines of the committee and the Declaration of Helsinki. All experiments were performed in accordance with the relevant guidelines and regulations. Our study was approved by the Ethics Review Committee of the Ninth People’s Hospital at the Shanghai Jiao Tong University School of Medicine.
Cell culture and infection
Human umbilical vein endothelial cells (HUVECs) were ordered from ScienCell (USA). The cells underwent mycoplasma testing and short tandem repeat (STR) analyses. Cells were cultured with MEM-α medium (Gibco, USA), including 10% fetal bovine serum (FBS) and 100 mg/ml streptomycin (Gibco, USA) and 1% penicillin). Cells were incubated at 37°C in a humidified atmosphere consisting of 5% CO2. siTSP1, NC siRNA, TSP1 overexpression (OE) vector, NC OE vector, miR-18a-5p mimics, NC mimics, miR-18a-5p inhibitors, NC inhibitors, miR-18a-5p overexpressing (OE)-lentivirus and miR-18a-5p knockdown (KD)-lentivirus were synthesized by Genome Co. (Shanghai, China). In addition, HUVECs were screened by puromycin after lentivirus infection.
Immunohistochemistry (IHC)
IHC was performed on paraformaldehyde-fixed paraffin-embedded sections with antibodies targeting CD31, P53, bax, bcl-2 (Cell Signaling Technology) and TSP1 (Proteintech Group). Visualization was conducted via the streptavidin-peroxidase conjugation method.
Bioinformatic prediction of miR-18a-5p targets
Common genome-wide targets of miR-18a-5p were explored by using online website starBase (http://starbase.sysu.edu.cn/).
Luciferase reporter assay
We transfected pmirGLO, pmirGLO-NC-wt, pmirGLO-TSP1-wt or pmirGLO-TSP1-mut into miR-18a-5p-overexpressing HUVECs by using Lipofectamine 2000 reagent (Invitrogen, USA). The specific experimental steps were described in our previous published articles [19]. The luciferase activity was detected by Dual-Luciferase System (Promega, USA).
HE staining
We fixed matrigel nodules from animals of different group in 4% paraformaldehyde, and after 24 h, we used a gradient series of alcohol for dehydration. The details of the HE assays were described in our previous article [19]. At last, the sections were sequentially dehydrated with a gradient and fluorescence imaging was used to find the potential changes of blood vessels.
Western blotting (WB)
WB was done as described in our previous article [19]. Antibodies (1:1000) specific for TSP1, bax, P53, bcl-2, and GAPDH were bought from Cell Signaling Technology (Boston, MA, USA). Anti-p-TIE2 antibody was purchased from Affinity Biosciences (Cat# AF2424) (1:1000). All original uncropped gels of Western blot assay were shown in Supplementary Figure 1.
Statistical analysis
Data are expressed as the mean ± SD and comparisons between two groups were performed using an independent sample t-test. Data among multiple groups were compared by one-way analysis of variance (ANOVA). Statistical analyses were performed by SPSS 20.0 (SPSS, Chicago, IL, USA) and plotting and graphing were performed by GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). P value <0.05 was considered as statistically significant.
Supplementary methods
RNA extraction and quantitative real-time polymerase chain reaction, cell proliferation assay, transwell assay, wound healing assay, in vitro tube formation assay, RNA immunoprecipitation and establishment of an animal model of VM are described in Supplementary File 1.
Results
Serum level of miR-18a-5p in VM patients
We first conducted a comprehensive comparative analysis of serum miR-18a-5p level between 54 VM patients and 48 healthy donors. Serum expression of miR-18a-5p was dramatically higher in VM patients than in healthy group (Figure 1C). Then, we performed ROC analyses to evaluate the accuracy of miR-18a-5p for predicting VM. We found that serum miR-18a-5p can well discriminate patients with VM and healthy group, and the AUC value is 0.8494 (sensitivity = 90.91%, specificity = 72.92%) (Figure 1D). In addition, we obtained pre- and post-operative serum with 41 successfully managed VM patients, and found that serum miR-18a-5p expressions of these patients were significantly downregulated after treatment (Figure 1E). These results indicated that miR-18a-5p has a potential to be a novel serum biomarker for VM screening. In addition, we evaluated the correlations between patients’ clinical characteristics and miR-18a-5p expression, all data are summarized in Table 1. Sex, age and maximum length of VMs were highly associated with serum level of miR-18a-5p. Female, age >18 and bigger VMs corresponded to higher serum miR-18a-5p level.
Table 1.
Clinicopathological features correlated with serum miR-18a-5p expression in VM patients
| Characteristics | Number of cases (%) | Expression of serum miR-18a-5p | P-value | |
|---|---|---|---|---|
|
| ||||
| High (%) | Low (%) | |||
| Sex | ||||
| Male | 26 (48.1) | 9 (34.6) | 17 (65.4) | 0.0293** |
| Female | 28 (51.9) | 18 (64.3) | 10 (35.7) | |
| Ages, years | ||||
| ≤18 | 27 (50.0) | 9 (33.3) | 18 (66.7) | 0.0143** |
| >18 | 27 (50.0) | 18 (66.7) | 9 (33.3) | |
| Max length, cm | ||||
| ≤2 cm | 23 (42.6) | 3 (13.0) | 20 (87.0) | |
| >2 cm, ≤4 cm | 22 (40.7) | 17 (77.3) | 5 (22.7) | 0.00005** |
| >4 cm | 9 (16.7) | 8 (88.9) | 1 (11.1) | |
| Location | ||||
| Lips | 11 (20.4) | 6 (54.5) | 5 (45.5) | |
| Cheek | 6 (11.1) | 3 (50.0) | 3 (50.0) | |
| Orbital | 3 (5.6) | 2 (66.7) | 1 (33.3) | |
| Tongue | 22 (40.7) | 10 (45.5) | 12 (54.5) | 0.7675 |
| Limb | 4 (7.4) | 2 (50.0) | 2 (50.0) | |
| Body | 2 (3.7) | 1 (50.0) | 1 (50.0) | |
| Other | 6 (11.1) | 3 (50.0) | 3 (50.0) | |
Abbreviations: VM, venous malformation; Max length, maximum length of VM according to the MRI results;
P<0.05.
MiR-18a-5p promoted cell proliferation, invasion, migration and angiogenesis of HUVECs in vitro and in vivo
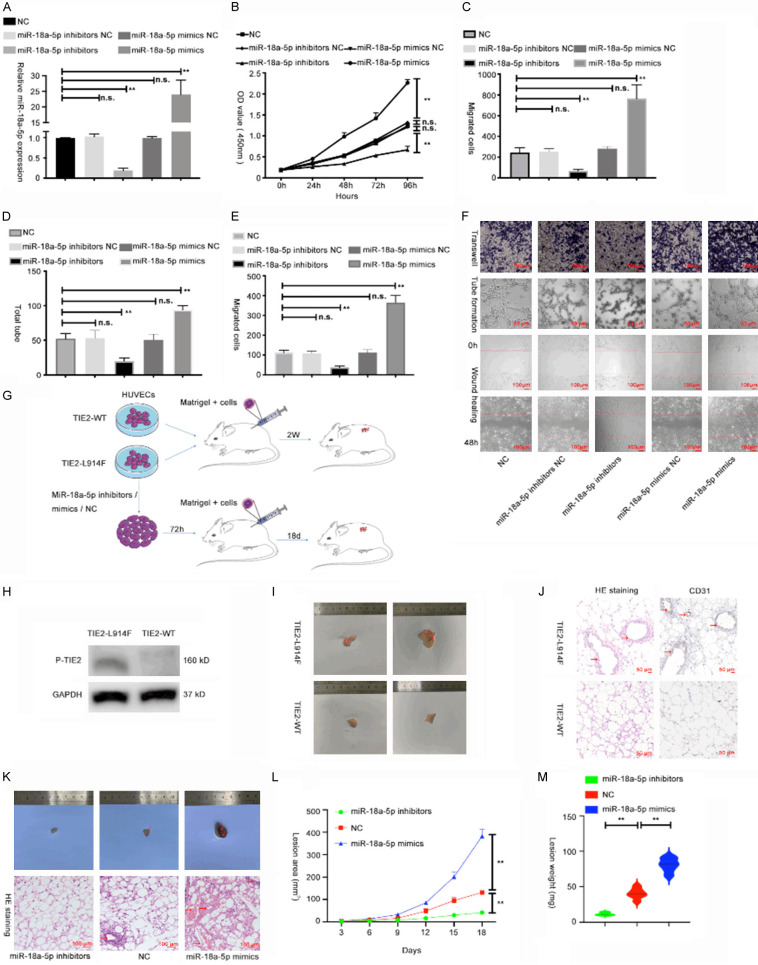
To explore biological function of miR-18a-5p in VM, mimics and inhibitors were separately transfected into HUVECs, and the transfection efficiency was validated via qRT-PCR (Figure 2A). We then applied CCK-8 to check the effect of miR-18a-5p on HUVEC proliferation and observed that increased miR-18a-5p contributed to the proliferation of HUVEC, while decreased miR-18a-5p attenuated HUVEC proliferation (Figure 2B). Then wound healing and transwell assays were used to examine cell invasion and migration. As shown in Figure 2C, 2E and 2F, miR-18a-5p mimics enhanced HUVEC invasion and migration, but these activities were suppressed by miR-18a-5p inhibitors. Besides, tube formation assay (an in vitro measure of angiogenesis) showed that the tube-forming ability of HUVECs was enhanced with miR-18a-5p mimics and suppressed with miR-18a-5p inhibitors (Figure 2D, 2F).
Figure 2.
MiR-18a-5p promoted cell proliferation, invasion, migration and angiogenesis of HUVECs in vitro and in vivo. (A) MiR-18a-5p expression was measured via qRT-PCR in HUVECs transfected with plasmids for 48 h. (B) Cell viability was measured by CCK-8 assay in HUVECs transfected with miR-18a-5p inhibitors, miR-18a-5p mimics or corresponding NCs. (C-F) Results of transwell (C, up F), tube formation (D, middle F), and wound healing assays (E, down F) in HUVECs transfected with miR-18a-5p inhibitors, miR-18a-5p mimics or corresponding NCs. (G) Schematic diagram: establishment and treatment of the VM mouse model. (H) The phosphorylated TIE-2 level was detected by Western blot assay. (I) The lesions were harvested 2 weeks after inoculation. (J) H&E and CD31 staining of the lesions. (K) Lesions and H&E staining at 18 days after inoculation of VM model mice subjected to different treatments. (L) Growth curve of the lesion area shows the speed of VM mass growth. (M) The weight of masses excised from mice in the ectopic expression and vector groups were measured and analyzed. The relative expression fold changes in mRNA expression were calculated by the 2-ΔΔCt method. Data were obtained from three independent repeated experiments. **P<0.05; n.s.: not significant.
Venous malformation mouse model was constructed with previous study results [20] (Figure 2G). First, we constructed the lentivirus owning TIE2-WT-GFP or TIE2-L914F mutant-GFP, then we infected HUVECs with lentivirus. After infecting, we used WB to check the transfection efficiency (Figure 2H). The results showed that the level of phosphorylated TIE2 in HUVECs infected with TIE2-L914F mutant was higher than that of TIE2-WT group. Then, 5×106 HUVECs infecting lentivirus were inoculated into one side of mice in an equal volume of Matrigel subcutaneously, and the masses were resected 2 weeks after inoculation (Figure 2I). The TIE2-L914F mutation group showed masses with more vessel-like formations, and HE staining revealed that the HUVECs infecting TIE2-L914F mutation could cause more vessels (Figure 2J).
To validate whether miR-18a-5p has an effect on angiogenesis in vivo, TIE2-L914F mutant HUVECs were transfected with miR-18a-5p inhibitors, mimics or vehicle for 72 h. Three groups of cells with different treatments were seeded into mice as previously described (Figure 2K). We measured the masses every 3 days and harvested them 18 days after treatment. We observed that miR-18a-5p inhibitors group showed the least vessel formation, and the extent of vessel formation was medium in the vehicle group, whereas miR-18a-5p mimics group showed the most vessels by H&E staining (Figure 2K). Compared to the vehicle group, the miR-18a-5p inhibitors group presented smaller lesion areas and lower total weight of the masses, while the miR-18a-5p mimics group manifested larger lesions and a larger total weight (Figure 2L, 2M). Thus, we hypothesized that miR-18a-5p plays a promotive role in VM progression in vivo and in vitro.
Identification of miR-18a-5p target genes in HUVECs
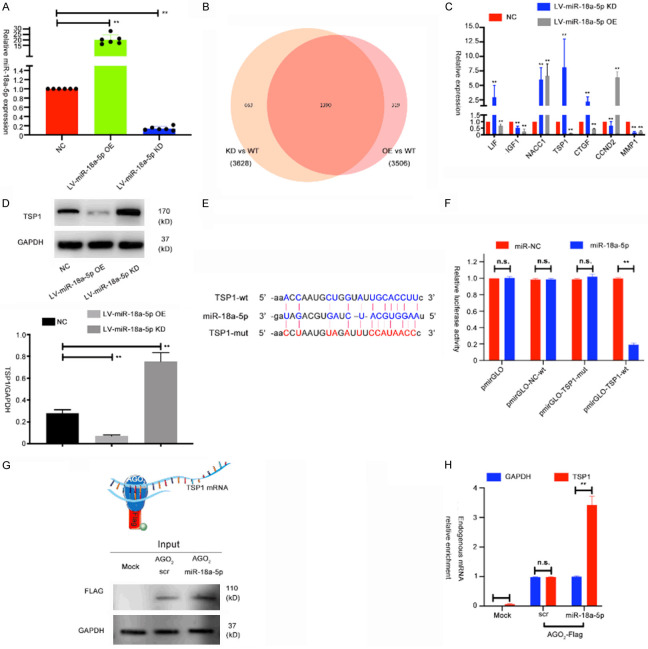
To study the regulatory mechanisms of miR-18a-5p in HUVECs, we constructed stable miR-18a-5p-overexpressing (OE) and miR-18a-5p-knockdown (KD) HUVECs by infecting the cells with lentivirus (Figure 3A). Then, we used RNA-seq to discriminate the differentially expressed genes between the overexpressing group, control group and knockdown group. We used a Venn diagram to show all differentially expressed genes (Figure 3B and Supplementary Figure 2A-D). And in Figure 3C, 7 miR-18a-5p-regulated genes with significant fold changes were chosen to be validated in HUVECs. qRT-PCR was conducted to evaluate the level of indicated genes in HUVECs transfected with either lentivirus-miR-18a-5p OE or KD. Results showed that thrombospondin-1 (TSP1) exhibited the largest change compared with the other 6 genes; thus, we chose TSP1 for further study (Figure 3C). We then did Western blot to validate the expression of TSP1 in miR-18a-5p OE or KD groups (Figure 3D). It is well known that miRNAs mostly bind to the 3’ UTR region of target mRNAs [21]. To determine potential binding sites that interact with miR-18a-5p, we used starBase 3.0, an online public resource that identifies target mRNAs of miRNAs, to identify the binding sites. To verify whether the biological functions of miR-18a-5p are dependent on the binding to the 3’ UTR of TSP1, we constructed a luciferase reporter vector containing the TSP1 3’ UTR with wild-type or mutated miR-18a-5p binding site (Figure 3E). And results showed that, luciferase activity of the TSP1 3’ UTR wild-type reporter was decreased when HUVECs were transfected with the miR-18a-5p mimics but has no significant difference when transfected with scramble control or when miR-18a-5p mimics were co-transfected with the mutant TSP1 3’ UTR reporter (Figure 3F).
Figure 3.
Identification of miR-18a-5p target genes in HUVECs. A. Expression levels of miR-18a-5p in HUVECs transfected with lentivirus either overexpressing miR-18a-5p (OE) or miR-18a-5p knockdown (KD). B. Venn diagram and prediction legends of the target genes regulated by miR-18a-5p in HUVECs. C. Relative expression of putative target genes in HUVECs transfected with NC, miR-18a-5p OE or miR-18a-5p KD. D. Expression levels of TSP1 were measured via Western blot in HUVECs transfected with NC, miR-18a-5p OE or miR-18a-5p KD. E. Potential miR-18a-5p base pairing alignment with TSP1 (blue), as identified by starBase v3.0. A mutation in the putative binding site was also established (red). F. Luciferase activity in HUVECs co-transfected with miR-18a-5p mimics and luciferase reporters containing empty vector, TSP1-wt or TSP1-mutant transcripts. G. Scheme of the RIP assay of AGO2-FLAG pull-down and RT-qPCR analysis of endogenous mRNA enrichment upon miR-18a-5p overexpression. H. QRT-PCR analysis of endogenous TSP1 mRNA upon immunoprecipitation of AGO2-FLAG in one representative experiment of three replicates. Data were obtained from three independent repeated experiments. **P<0.05; n.s.: not significant.
We further analyzed whether miR-18a-5p form the RISC complex with TSP1 by conducting an RIP assay. In brief, FLAG-tagged AGO2 (Argonaute 2) was overexpressed in HUVECs. And then endogenously bound RNAs were pulled down and purified, followed by qRT-PCR analysis. We postulated that if miR-18a-5p can bind TSP1 mRNA, miR-18a-5p overexpression could lead to a raise of TSP1 mRNAs with AGO2 (Figure 3G). Lastly, an enrichment of TSP1 mRNA with AGO2 in cells overexpressing miR-18a-5p was validated (Figure 3H). All these data indicated that miR-18a-5p guides AGO2 proteins to TSP1 mRNA. Thus, we concluded that miR-18a-5p directly regulates TSP1 gene in HUVECs.
TSP1 restored the function of miR-18a-5p in HUVECs
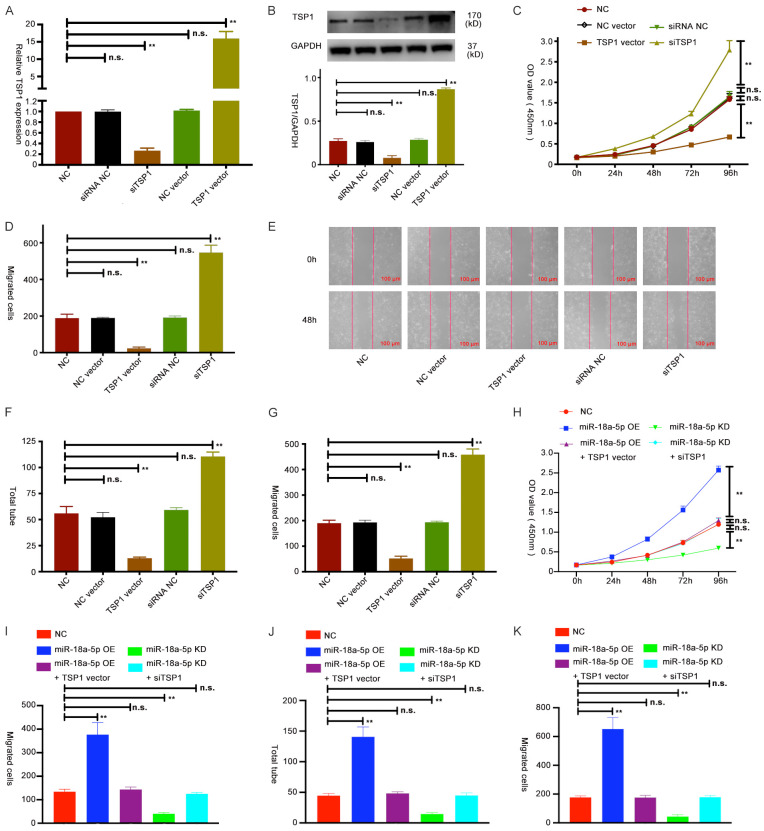
To explore the mechanism of TSP1 in HUVECs, we first transfected TSP1 siRNA and a vector into HUVECs, and then checked their efficiency via qRT-PCR and Western blot assays (Figure 4A, 4B). We then explore the role of TSP1 in HUVECs by performing CCK-8, wound healing, tube formation and transwell assays (Figure 4C-G and Supplementary Figures 2B, 3A). All the assay results showed that TSP1 can inhibit proliferation, invasion, angiogenesis and migration in HUVECs. Next, we performed rescue experiments by downregulating TSP1 expression in miR-18a-5p KD HUVECs and upregulating TSP1 expression in miR-18a-5p OE HUVECs. We found that TSP1 can restore the promotive role of miR-18a-5p in HUVECs (Figure 4H-K and Supplementary Figure 3C-E). These data further support our hypothesis that miR-18a-5p exerts its function by regulating TSP1 expression in HUVECs.
Figure 4.
TSP1 restored the function of miR-18a-5p in HUVECs. (A, B) Expression of TSP1 in HUVECs transfected with siRNA NC, siTSP1, NC vector and TSP1 vector was examined by qRT-PCR (A) and Western blot (B). (C) The viability of HUVECs after transfection with TSP1 vector or siTSP1 was determined using CCK-8 assay. (D, E) A wound healing assay was performed with HUVECs transfected with TSP1 vector or siTSP1. (F, G) Tube formation (F) and transwell assays (G) were conducted in HUVECs with TSP1 vector or siTSP1. (H-K) Growth, invasion, migration and angiogenesis of HUVECs transfected with NC, miR-18a-5p OE, miR-18a-5p KD, miR-18a-5p OE + TSP1 OE vector or miR-18a-5p KD + siTSP1 were evaluated by CCK-8 (H), wound healing (I), tube formation (J) and transwell assays (K). Data were obtained from three independent repeated experiments. **P<0.05; n.s.: not significant.
Verification of the regulatory relationship between miR-18a-5p and the P53 signaling pathway in vitro and in vivo
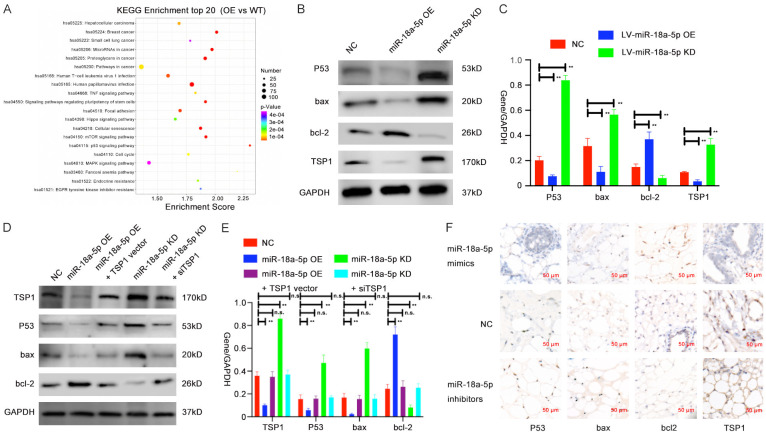
According to the results of GO function and KEGG pathway enrichment analyses (Figure 5A and Supplementary Figure 4A-C), P53 signaling pathway showed the highest enrichment score among all the affected pathways after miR-18a-5p levels changed (Figure 5A and Supplementary Figure 4A). Therefore, we postulated that miR-18a-5p and its target gene TSP1 may predominantly function through the P53 signaling pathway. Then, we assessed the protein expression of genes related to the P53 signaling pathway (P53, bax, bcl-2) after altering the expression of miR-18a-5p in HUVECs via Western blot assay (Figure 5B, 5C). We found that the expressions of P53, bax, and TSP-1were decreased while that of bcl-2 was increased in miR-18a-5p OE HUVECs; in contrast, the opposite results were observed in miR-18a-5p KD HUVECs.
Figure 5.
Verification of the regulatory relationship between miR-18a-5p and the P53 signaling pathway in vitro and in vivo. A. KEGG analysis was performed to identify pathway terms enriched in the dysregulated mRNAs in HUVECS with miR-18a-5p OE vs WT. B, C. Western blot analysis of P53 signaling-related protein levels in lysates from HUVECs transfected with NC, miR-18a-5p OE or miR-18a-5p KD. D, E. Expressions of P53 signaling-related protein in NC-, miR-18a-5p OE-, miR-18a-5p KD-, miR-18a-5p OE + TSP1 OE vector, or miR-18a-5p KD + siTSP1-transfected HUVECs were evaluated via Western blotting. F. Relative expression of TSP1 and P53 signaling-related molecules was shown by immunohistochemistry in each group of VM mice model. Data were obtained from three independent repeated experiments. **P<0.05; n.s.: not significant.
To explore whether the target gene TSP1 participates in the regulation of miR-18a-5p to P53 signaling pathway, we made the same rescue experiments as for previous groups, extracted their protein and assessed these genes’ expression via Western blot assay. We saw that expression of these P53 signaling pathway-related genes did show restored phenomenon when we transfected miR-18a-5p OE HUVECs with TSP1 vector or transfected miR-18a-5p KD HUVECs with siTSP1. Expression of P53, bax and bcl-2 all returned back to the original level in miR-18a-5p OE + TSP1 vector group and miR-18a-5p KD + siTSP1 group compared with the control group. (Figure 5D, 5E).
To validate previous results in vivo, we conducted an IHC assay of the cell-containing Matrigel nodules, and we analyzed the expression of TSP1, P53, bax, bcl-2 in miR-18a-5p mimics, miR-18a-5p inhibitors and control groups. We found that miR-18a-5p mimics group showed higher expression of bcl-2 and lower expression of P53, bax and TSP1, and the miR-18a-5p inhibitors group had lower expression of bcl-2 and higher expression of P53, bax and TSP1 compared with the control group (Figure 5F). Thus, we draw a conclusion that miR-18a-5p can promote VM progression by regulating the TSP1/P53 signaling pathway.
Discussion
It was reported that human serum containing miRNAs or cytokines can play important roles in various devastating diseases and infections [22-24]. There has been increasing interest in recent years in the use of human serum components as biomarkers or therapeutic targets [25,26], particularly in the field of hematopathy. Numerous studies on the role of human serum composition in blood diseases or cancer progression have been published [27-29]. During the developmental process of human diseases, the serum composition varies profoundly. These variations include changes in the quantities and types of various miRNAs, suggesting a potential for human serum to be used for identification, monitoring and treatment of diseases [30,31]. In our investigation, we measured the expression of serum miR-18a-5p derived from VM patients and healthy donors by quantitative real-time PCR. Our results showed that VM patients had a significantly higher serum miR-18a-5p than the healthy donors, and its expression returned to a relatively normal level when the patients’ diseases were successfully controlled. All these results validate that miR-18a-5p could serve as a predictive or monitoring serum biomarker of VM.
According to the most recent studies about the function and regulation of miRNAs in other diseases, lncRNAs and circRNAs can function as competing endogenous RNAs (ceRNAs) by sponging corresponding miRNAs in various psychological and pathological processes. Lots of dysregulated ceRNAs networks have been reported and explored. Thus, another study about the differentially expressed serum exosomal circRNAs and lncRNAs has also been conducted by our team to find the possible molecular mechanism by which miR-18a-5p is up-regulated in VM patients.
Previous studies revealed that miR-18a-5p can participate in many processes of cancers [32,33]. In addition, miR-18a-5p has also been verified to play a vital role in vascular endothelial cell proliferation, migration, invasion, and angiogenesis [34,35]. However, the effect of miR-18a-5p on VM is still poorly understood. Our results validated that miR-18a-5p levels were higher in the serum of VM patients than healthy group. Studies showed that miR-18a-5p overexpression significantly enhanced the proliferation, migration, invasion and angiogenesis of HUVECs in vitro. Besides, downregulation of miR-18a-5p gave rise to contrary results. Previous studies have shown that miRNAs can exert their functions by affecting the expression of their target mRNAs [36]. Thus, to check the predictive binding and explore the potential target genes of miR-18a-5p in HUVECs, mRNA levels in miR-18a-5p knockdown and miR-18a-5p overexpression as well as in control groups were further measured by RNA-seq. Then seven genes showing the largest changes (TSP1, IGF1, CTGF, LIF, CCND2, NACC1 and MMP1) were chosen for further validation, with the TSP1 gene showing the strongest negative correlation with miR-18a-5p as checked by qRT-PCR and Western blot.
Thrombospondins (TSPs) participate in lots of physical and pathological processes, such as cell differentiation, proliferation, vascular hemeostasis and wound healing [37]. Thrombospondin-1 (TSP-1) is one of the thrombospondin family [38], and TSP-1 is mostly synthesized and secreted by platelets and endothelial cells [39]. Numerous studies have reported that TSP1 shows a wide range of biological effects, such as inhibiting angiogenesis, antitumor activity and so on [40]. Taken together, TSP1 might play an important role in the regulation of biological processes. According to our study, the level of TSP1 was directly regulated by miR-18a-5p as indicated by the results of dual-luciferase reporter and RIP assays. Besides, when miR-18a-5p expression changed in HUVECs, ectopic TSP1 expression rescued the promotive role of miR-18a-5p in HUVECs via rescue assays, indicating a latent role of TSP1 in miR-18a-5p regulatory axis in VM.
To explore the detailed mechanism of miR-18a-5p in HUVECs, we performed an RNA-seq assay. By performing KEGG analysis, we saw that P53 signaling pathway was the most important pathway regulated by miR-18a-5p. Previous studies showed that P53 can regulate gene transcription, leading to cell death, DNA injures and changes in angiogenesis [41-45]. More importantly, TSP-1 had been reported to participate in the P53 signaling in several diseases [46-48]. Thus, we decided to validate whether miR-18a-5p function by regulating the P53 signaling pathway to some degree. Remarkably, Western blot analysis of protein level showed that, overexpression of miR-18a-5p downregulated the expression of P53, bax, TSP1 and increased the expression of bcl-2 in HUVECs. By contrast, downregulating miR-18a-5p in HUVECs had the opposite effect on the expression pattern of these genes. Thus, we confirmed that miR-18a-5p can regulate TSP1 expression and further played a significant role in the P53 signaling pathway. Considering the P53 signaling pathway constituents, we also conducted the flow cytometry assay to verify the apoptosis rate of different miR-18a-5p changed groups, but we found that, there was no significant statistical difference between the three groups. Thus we postulated that, TSP1 had the angio-inhibitory signaling downstream of miR-18a-5p that might override other pro-apoptotic signaling. Besides, considering there were also lots of genes regulated by the miR-18a-5p, it may be possible that some molecules among these genes counteracted the apoptotic influence of P53 signaling pathway constituents.
Finally, to verify the function of miR-18a-5p in the vessel formation process in vivo, we established a VM mouse model, and the results suggested that compared to the control, miR-18a-5p inhibitors led to less vessel formation whereas miR-18a-5p mimics caused more vessel formation. We also found that the expression trends of TSP1 and P53 signaling-related proteins were also consistent with the in vitro expression levels in HUVECs by IHC.
To our best knowledge, our study is the first report describing the clinical significance of serum miR-18a-5p in VM. Our results suggest that miR-18a-5p can promote VM progression by suppressing TSP1 and further regulating the P53 signaling pathway (Figure 6). Therefore, serum miR-18a-5p might be a promising therapeutic target and serum biomarker for VM.
Figure 6.
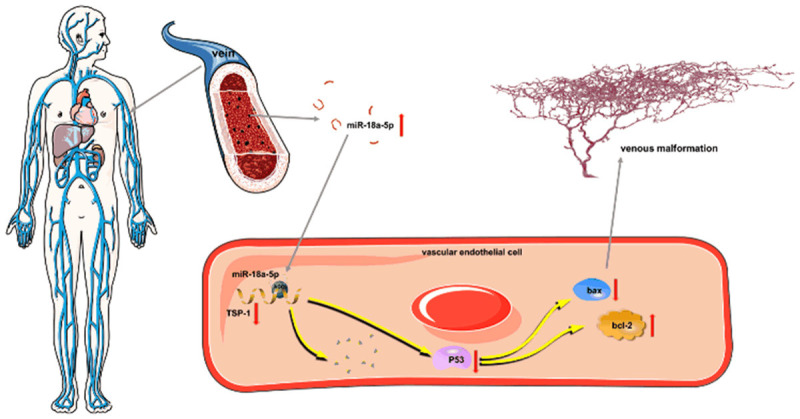
Hypothetical model describing the function of miR-18a-5p in VM. High expression of serum miR-18a-5p downregulates the expression level of TSP-1 in vascular endothelial cells, which participates in the P53 signaling pathway, resulting in VM progression.
Acknowledgements
The authors would like to thank Prof. Wayne F. Yakes for teaching the technique of ethanol embolization and supporting clinical work during these years. We thank all the doctors and nurses in our apartment and the work was done with their kind help. This work was supported by National Natural Science Foundation of China (Grant No.81871458) and Clinical Research Program of Ninth People’s Hospital, Shanghai Jiaotong University, School of Medicine (Grant No. JYLJ201911 and No. JYLJ201801).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Han YY, Sun LM, Yuan SM. Localized intravascular coagulation in venous malformations: a system review. Phlebology. 2021;36:38–42. doi: 10.1177/0268355520946211. [DOI] [PubMed] [Google Scholar]
- 2.Olivieri B, White CL, Restrepo R, McKeon B, Karakas SP, Lee EY. Low-flow vascular malformation pitfalls: from clinical examination to practical imaging evaluation--part 2, venous malformation mimickers. AJR Am J Roentgenol. 2016;206:952–962. doi: 10.2214/AJR.15.15794. [DOI] [PubMed] [Google Scholar]
- 3.Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJ, Mitchel S, Powell J, Prendiville J, Vikkula M, Board I, Scientific C. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203–214. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
- 4.Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, Eklund L, Boon LM, Vikkula M. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41:118–124. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta R, Patel M. Venous malformations. Semin Pediatr Surg. 2014;23:198–202. doi: 10.1053/j.sempedsurg.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AC, Li EY, Chan TC, Wong AC, Chan PC, Poon WW, Fung DH, Yuen HK. Hybrid procedure for orbital venous malformation in the endovascular operation room. Eye (Lond) 2015;29:1069–1075. doi: 10.1038/eye.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, Deneys V, Pocock B, Lequerrec A, Labbe D, Barrellier MT, Vanwijck R, Vikkula M, Boon LM. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144:873–877. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seront E, Vikkula M, Boon LM. Venous malformations of the head and neck. Otolaryngol Clin North Am. 2018;51:173–184. doi: 10.1016/j.otc.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21:22–36. doi: 10.1038/s41568-020-00306-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang MW, Shen YJ, Shi J, Yu JG. MiR-223-3p in cardiovascular diseases: a biomarker and potential therapeutic target. Front Cardiovasc Med. 2020;7:610561. doi: 10.3389/fcvm.2020.610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mens MMJ, Heshmatollah A, Fani L, Ikram MA, Ikram MK, Ghanbari M. Circulatory MicroRNAs as potential biomarkers for stroke risk: the rotterdam study. Stroke. 2021;52:945–953. doi: 10.1161/STROKEAHA.120.031543. [DOI] [PubMed] [Google Scholar]
- 12.Komoll RM, Hu Q, Olarewaju O, von Dohlen L, Yuan Q, Xie Y, Tsay HC, Daon J, Qin R, Manns MP, Sharma AD, Goga A, Ott M, Balakrishnan A. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. 2021;74:122–134. doi: 10.1016/j.jhep.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ, Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, Jia WH, Jie Y, Chen MS, Chen MX, Fang JH, Zeng C, Zhang Y, Guo RP, Wu Y, Lin G, Zheng L, Zhuang SM. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]
- 14.Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, Calatayud D, Nielsen SE, Yilmaz M, Hollander NH, Andersen KK, Johansen JS. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 15.Xia HF, Ren JG, Zhu JY, Yu ZL, Zhang W, Sun YF, Zhao YF, Chen G. Downregulation of miR-145 in venous malformations: Its association with disorganized vessels and sclerotherapy. Eur J Pharm Sci. 2017;100:126–131. doi: 10.1016/j.ejps.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Marin-Ramos NI, Thein TZ, Ghaghada KB, Chen TC, Giannotta SL, Hofman FM. miR-18a inhibits BMP4 and HIF-1alpha normalizing brain arteriovenous malformations. Circ Res. 2020;127:e210–e231. doi: 10.1161/CIRCRESAHA.119.316317. [DOI] [PubMed] [Google Scholar]
- 17.Miao R, Liu W, Qi C, Song Y, Zhang Y, Fu Y, Liu W, Lang Y, Zhang Y, Zhang Z. MiR-18a-5p contributes to enhanced proliferation and migration of PASMCs via targeting Notch2 in pulmonary arterial hypertension. Life Sci. 2020;257:117919. doi: 10.1016/j.lfs.2020.117919. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Wu L, Li D, Xu Y, Zhang L, Niu K, Kong R, Gu J, Xu Z, Chen Z, Sun J. Radiosensitizing effects of miR-18a-5p on lung cancer stem-like cells via downregulating both ATM and HIF-1alpha. Cancer Med. 2018;7:3834–3847. doi: 10.1002/cam4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LM, Su LX, Hu JZ, Wang M, Ju HY, Li X, Han YF, Xia WY, Guo W, Ren GX, Fan XD. Epigenetic regulation of VENTXP1 suppresses tumor proliferation via miR-205-5p/ANKRD2/NF-κB signaling in head and neck squamous cell carcinoma. Cell Death Dis. 2020;11:838. doi: 10.1038/s41419-020-03057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015;125:3491–3504. doi: 10.1172/JCI76004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doxakis E. Cell-free microRNAs in Parkinson’s disease: potential biomarkers that provide new insights into disease pathogenesis. Ageing Res Rev. 2020;58:101023. doi: 10.1016/j.arr.2020.101023. [DOI] [PubMed] [Google Scholar]
- 23.Kiuchi J, Komatsu S, Imamura T, Nishibeppu K, Shoda K, Arita T, Kosuga T, Konishi H, Shiozaki A, Okamoto K, Fujiwara H, Ichikawa D, Otsuji E. Low levels of tumour suppressor miR-655 in plasma contribute to lymphatic progression and poor outcomes in oesophageal squamous cell carcinoma. Mol Cancer. 2019;18:2. doi: 10.1186/s12943-018-0929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori MA, Ludwig RG, Garcia-Martin R, Brandao BB, Kahn CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. 2019;30:656–673. doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willeit P, Skroblin P, Kiechl S, Fernandez-Hernando C, Mayr M. Liver microRNAs: potential mediators and biomarkers for metabolic and cardiovascular disease? Eur Heart J. 2016;37:3260–3266. doi: 10.1093/eurheartj/ehw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Zhu X, Yu Y, Zhu W, Jin L, Zhang X, Li S, Zou P, Xie C, Cui R. Dissecting miRNA signature in colorectal cancer progression and metastasis. Cancer Lett. 2021;501:66–82. doi: 10.1016/j.canlet.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Garg A, Seeliger B, Derda AA, Xiao K, Gietz A, Scherf K, Sonnenschein K, Pink I, Hoeper MM, Welte T, Bauersachs J, David S, Bar C, Thum T. Circulating cardiovascular microRNAs in critically ill COVID-19 patients short title: microRNA signatures in COVID-19. Eur J Heart Fail. 2021;23:468–475. doi: 10.1002/ejhf.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebetrau C, Mollmann H, Dorr O, Szardien S, Troidl C, Willmer M, Voss S, Gaede L, Rixe J, Rolf A, Hamm C, Nef H. Release kinetics of circulating muscle-enriched microRNAs in patients undergoing transcoronary ablation of septal hypertrophy. J Am Coll Cardiol. 2013;62:992–998. doi: 10.1016/j.jacc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Karakas M, Schulte C, Appelbaum S, Ojeda F, Lackner KJ, Munzel T, Schnabel RB, Blankenberg S, Zeller T. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur Heart J. 2017;38:516–523. doi: 10.1093/eurheartj/ehw250. [DOI] [PubMed] [Google Scholar]
- 30.Chiacchiarini M, Trocchianesi S, Besharat ZM, Po A, Ferretti E. Role of tissue and circulating microRNAs and DNA as biomarkers in medullary thyroid cancer. Pharmacol Ther. 2020;219:107708. doi: 10.1016/j.pharmthera.2020.107708. [DOI] [PubMed] [Google Scholar]
- 31.Moccia M, Caratelli V, Cinti S, Pede B, Avitabile C, Saviano M, Imbriani AL, Moscone D, Arduini F. Paper-based electrochemical peptide nucleic acid (PNA) biosensor for detection of miRNA-492: a pancreatic ductal adenocarcinoma biomarker. Biosens Bioelectron. 2020;165:112371. doi: 10.1016/j.bios.2020.112371. [DOI] [PubMed] [Google Scholar]
- 32.Zhao W, Zhao J, Guo X, Feng Y, Zhang B, Tian L. LncRNA MT1JP plays a protective role in intrahepatic cholangiocarcinoma by regulating miR-18a-5p/FBP1 axis. BMC Cancer. 2021;21:142. doi: 10.1186/s12885-021-07838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma B, Randhawa V, Vaiphei K, Gupta V, Dahiya D, Agnihotri N. Expression of miR-18a-5p, miR-144-3p, and miR-663b in colorectal cancer and their association with cholesterol homeostasis. J Steroid Biochem Mol Biol. 2021;208:105822. doi: 10.1016/j.jsbmb.2021.105822. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Chen X. miR-18a-5p promotes proliferation and migration of vascular smooth muscle cells by activating the akt/extracellular regulated protein kinases (ERK) signaling pathway. Med Sci Monit. 2020;26:e924625. doi: 10.12659/MSM.924625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan JT, Li XX, Peng DW, Zhang WM, Qu J, Lu F, D’Amato RJ, Chi ZL. MicroRNA-18a-5p administration suppresses retinal neovascularization by targeting FGF1 and HIF1A. Front Pharmacol. 2020;11:276. doi: 10.3389/fphar.2020.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogg EM, Abplanalp WT, Bischof C, John D, Schulz MH, Krishnan J, Fischer A, Poluzzi C, Schaefer L, Bonauer A, Zeiher AM, Dimmeler S. Analysis of cell type-specific effects of MicroRNA-92a provides novel insights into target regulation and mechanism of action. Circulation. 2018;138:2545–2558. doi: 10.1161/CIRCULATIONAHA.118.034598. [DOI] [PubMed] [Google Scholar]
- 37.Qi C, Lei L, Hu J, Wang G, Liu J, Ou S. Thrombospondin-1 is a prognostic biomarker and is correlated with tumor immune microenvironment in glioblastoma. Oncol Lett. 2021;21:22. doi: 10.3892/ol.2020.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- 39.Sun S, Dong H, Yan T, Li J, Liu B, Shao P, Li J, Liang C. Role of TSP-1 as prognostic marker in various cancers: a systematic review and meta-analysis. BMC Med Genet. 2020;21:139. doi: 10.1186/s12881-020-01073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts DD. Thrombospondins: from structure to therapeutics. Cell Mol Life Sci. 2008;65:669–671. doi: 10.1007/s00018-007-7483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutant p53 aids cancer cells in evading lethal innate immune responses. Cancer Discov. 2021;11:OF14. doi: 10.1158/2159-8290.CD-RW2021-022. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Blayney A, Liu X, Gandy L, Jin W, Yan L, Ha JH, Canning AJ, Connelly M, Yang C, Liu X, Xiao Y, Cosgrove MS, Solmaz SR, Zhang Y, Ban D, Chen J, Loh SN, Wang C. EGCG binds intrinsically disordered N-terminal domain of p53 and disrupts p53-MDM2 interaction. Nat Commun. 2021;12:986. doi: 10.1038/s41467-021-21258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller I, Strozyk E, Schindler S, Beissert S, Oo HZ, Sauter T, Lucarelli P, Raeth S, Hausser A, Al Nakouzi N, Fazli L, Gleave ME, Liu H, Simon HU, Walczak H, Green DR, Bartek J, Daugaard M, Kulms D. Cancer cells employ nuclear caspase-8 to overcome the p53-dependent G2/M checkpoint through cleavage of USP28. Mol Cell. 2020;77:970–984. e977. doi: 10.1016/j.molcel.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 45.Pfaff MJ, Mukhopadhyay S, Hoofnagle M, Chabasse C, Sarkar R. Tumor suppressor protein p53 negatively regulates ischemia-induced angiogenesis and arteriogenesis. J Vasc Surg. 2018;68:222S–233S. e221. doi: 10.1016/j.jvs.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linderholm B, Karlsson E, Klaar S, Lindahl T, Borg AL, Elmberger G, Bergh J. Thrombospondin-1 expression in relation to p53 status and VEGF expression in human breast cancers. Eur J Cancer. 2004;40:2417–2423. doi: 10.1016/j.ejca.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Sundaram P, Hultine S, Smith LM, Dews M, Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV, Thomas-Tikhonenko A. p53-responsive miR-194 inhibits thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer Res. 2011;71:7490–7501. doi: 10.1158/0008-5472.CAN-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y, Jiang Q, Wang Y, Li W, Geng M, Han Z, Chen X. The anti-proliferative effects of oleanolic acid on A7r5 cells-role of UCP2 and downstream FGF-2/p53/TSP-1. Cell Biol Int. 2017;41:1296–1306. doi: 10.1002/cbin.10838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.