Abstract
Objective: To explore the impacts of bosentan combined with sildenafil on chronic obstructive pulmonary disease (COPD) patients with pulmonary arterial hypertension (PAH). Methods: From April 2019 to October 2020, 90 COPD patients with PAH diagnosed in our hospital were recruited and divided into groups A and B. The patients in group A (50 cases) were treated with bosentan combined with sildenafil, and the patients in group B (40 cases) were administered bosentan combined with iloprost solution for inhalation. The PAH conditions, the heart rates (HR), the cardiac function, the pulmonary function, the blood gas indexes, the inflammatory factor expressions, the incidences of adverse reactions, the overall response rates (ORR), and the patient satisfaction levels were determined or evaluated. Results: Compared with group B, the patients in group A had better recovered PAH, HR, cardiac function, pulmonary function, and blood gas indexes, lower inflammatory factor expression levels and a lower incidence of adverse reactions, as well as higher ORR and higher satisfaction levels. Conclusion: Bosentan combined with sildenafil can reduce pulmonary artery pressure and promote the recovery of cardiopulmonary function in COPD patients with PAH.
Keywords: Chronic obstructive pulmonary disease, pulmonary arterial hypertension, bosentan, sildenafil
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as the gradual deterioration of pulmonary function and a series of mental and physical complications [1]. An increasingly serious global health issue, this disease has become the third leading cause of death worldwide and the main cause of morbidity [2]. Currently, the disease affects about 10% of the middle-aged and elderly population and 50% of heavy smokers. It is estimated that more than 25% of patients now develop COPD with a cumulative lifetime risk [3,4]. COPD is characterized by chronic bronchitis, chronic airway obstruction, airway remodeling and emphysema, leading to a progressive and irreversible decline of pulmonary function [5]. The release of inflammatory mediators and destructive enzymes caused by inflammation is the main cause of the progressive destruction of pulmonary tissue and pulmonary function in COPD [6]. Pulmonary function changes caused by inflammation can lead to pulmonary arterial hypertension (PAH), which gradually increases pulmonary artery resistance, and leads to right heart failure and death in severe cases if not treated in time [7]. Therefore, the treatment of this disease should focus on eliminating inflammation and restoring the pulmonary structure.
At present, the treatment of COPD combined with pulmonary hypertension is mainly conventional symptomatic treatment, through auxiliary oxygen inhalation, cardiotonic agents, diuretics, and other measures to alleviate the clinical symptoms, but the effect is not ideal [8]. Drug therapy can markedly improve patients’ quality of life and prolong their survival [9]. Glucocorticoids, long-acting muscarinic antagonists (LAMA), and long-acting β2-agonists (LABA) have been shown to have a positive effect on alleviating COPD symptoms and improving pulmonary function [10]. Among them, bosentan is an endothelin receptor antagonist clinically used to treat PAH [11]. And its combination with another drug, sildenafil, a phosphodiesterase 5 inhibitor (PDE5i), is effective for treating PAH [12]. The combination of bosentan and sildenafil can effectively antagonize endothelial dysfunction and restore pulmonary function [13,14]. The combination of bosentan and sildenafil has been shown to improve and control pulmonary hypertension [15]. However, there are few studies on whether the two drugs have an effect on PAH caused by COPD. Accordingly, this study was carried out to investigate whether their combination can relieve the symptoms associated with COPD while improving PAH.
Methods
General data
A total of 90 patients with COPD and PAH diagnosed in the Pingxiang People’s Hospital from April 2019 to October 2020 were recruited as study cohort, and their clinical data were analyzed retrospectively. A comparison of the two groups’ general data showed no significant differences (P>0.05).
Inclusion criteria: all the enrolled patients were diagnosed with COPD and PAH using pulmonary ventilation imaging, pulmonary perfusion imaging, or echocardiography.
Exclusion criteria: patients who did not meet the diagnostic criteria; patients who did not follow the doctor’s advice or who withdrew from the study during the follow-up; patients with severe mental illnesses; patients who refused to cooperate with the study; patients with drug allergies; patients with liver or renal dysfunctions, connective tissue diseases, or chronic bronchitis.
The patients and their families were informed and signed the relevant consents. This study was approved by the hospital ethics committee (SV-713-947).
Methods
After admission, both groups of patients were administered routine symptomatic treatment such as oxygen inhalation, atomization, fluid infusion, anti-infection, cough relief, phlegm resolving, and spasmolysis.
The patients in group A were treated with bosentan (Actelion Pharmaceuticals Ltd., approval number H20110291) combined with sildenafil (Pfizer Pharmaceutical Co., Ltd., SFDA approval number H20020528) in addition to the routine treatment. The bosentan was administered orally twice a day (after breakfast and after dinner), 62.5 mg/time. The dosage was gradually increased to the recommended maintenance dosage after 4 weeks of continuous treatment. The sildenafil was administered orally 50 mg twice a day. The treatment continued for 3 months.
The patients in group B were treated with bosentan (Actelion Pharmaceuticals Ltd., approval number H20110291) combined with iloprost solution for inhalation (Berlimed S.A, approval number J20070001) in addition to the routine treatment. The bosentan was taken orally twice a day (after breakfast and after dinner), 62.5 mg/time. After continuous treatment for 28 days, the dosage was gradually increased to the recommended maintenance dose of 125 mg/time, twice a day. The starting dose of iloprost solution for inhalation was 2.5 g/dose, which was diluted with 20 mL of sterile saline for aerosol inhalation. The dosage was increased to 5.0 g/time according to each patient’s tolerance. The treatment lasted for 3 months.
Measurement indicators
Pulmonary artery pressure and heart rate (HR)
The arterial pressure at admission and at 1 month after treatment and the HR were compared between the two groups. The pulmonary artery pressure levels and the HRs in both groups were measured in real time after each patient’s hospitalization. The pulmonary artery pressure was examined using color Doppler ultrasound (Baden Medical Co., LTD., Nanjing, China, V514371) at a frequency of 1-5 MHZ. According to the specific condition of each patient, each patients was placed in the left or supine position, and the diameter of the pulmonary artery and the size of the attrix were measured from the 3-4 intercostal space adjacent to the sternum. The pulmonary artery pressure was evaluated using a color Doppler spectrum.
Cardiac function
The patients’ cardiac function was compared upon admission and at one month after treatment. The 6-minute walking distances (6MWD) and the right ventricular ejection fraction (RVEF) levels were chosen to evaluate the patients’ cardiac function before and after the treatment. In 6MWD, a longer walking distance indicated a better recovery of cardiac function. The RVEF was measured using color Doppler ultrasound.
Pulmonary function
The two groups’ pulmonary functions were compared upon admission and at one month after the treatment. The measurement indexes included forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and FEV1/FVC.
Blood gas analysis
The patients’ blood gas levels upon admission and at one month after the treatment were analyzed and compared, including arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2).
Inflammatory factors
The interleukin-13 (IL-13), IL-17, and serum C-reactive protein (CRP) protein expression levels in the patients’ blood were determined and compared upon admission and at one month after the treatment. Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Thermo Fisher Scientific (Shanghai, China), with the batch numbers of BMS231-3TEN, BMS2017HS, and KHA0031, respectively.
Incidence of adverse reactions
The incidence of adverse reactions in the two groups during the treatment was statistically analyzed. The related indexes included flushing, headache, dizziness, and nausea.
Overall response rate
The ORR of two groups of patients during the treatment was statistically analyzed. Marked response: clinical symptoms such as cough, wheezing and dyspnea were alleviated, the pulmonary rales were reduced, the cardiac function was improved to grade II or above, and the blood oxygen saturation was over 95%; Effective response: the above symptoms and signs were partially alleviated, the cardiac function was improved to grade III or above, and the blood oxygen saturation was increased by more than 90%; No response: no relief or improvement in the symptoms, the cardiac function, or the blood oxygen saturation was seen.
Treatment satisfaction
The patients’ satisfaction towards the nursing was assessed using a treatment satisfaction questionnaire, and the two groups’ scores were compared. The test contents and evaluation criteria were self-made. The total score was 100 points, with 100-85 points being satisfied, 60-85 being basically satisfied, and less than 60 being dissatisfied.
Statistical methods
SPSS 19.0 (Asia Analytics Formerly SPSS China) was used to statistically analyze the comprehensive data, and the pictures were drawn using GraphPad Prism 7. X2 tests were utilized to compare the count data. The measurement data were represented in the form of (x ± sd) and analyzed using t tests. Paired T tests were used for the intra-group comparisons before and after the treatment. When P<0.05, the difference was statistically significant.
Results
General data
There were no significant differences between the two groups in terms of their general data such as gender, age, body mass index (BMI), NYHAFC grade, hypertension and hyperlipidemia (P>0.05), as shown in Table 1.
Table 1.
General data of the patients in the two groups
| Classification | Group A (n=50) | Group B (n=40) | t/X2 | P |
|---|---|---|---|---|
| Gender | 0.14 | 0.706 | ||
| Male | 27 (54.00) | 20 (50.00) | ||
| Female | 23 (46.00) | 20 (50.00) | ||
| Age (years) | 35.91±6.73 | 35.88±7.49 | 0.02 | 0.984 |
| BMI (kg/m2) | 21.68±2.33 | 21.45±2.42 | 0.46 | 0.649 |
| NYHAFC grade | 0.02 | 0.887 | ||
| II | 28 (56.00) | 23 (57.50) | ||
| III | 22 (44.00) | 17 (42.50) | ||
| Hypertension | 0.56 | 0.453 | ||
| Present | 38 (76.00) | 33 (82.50) | ||
| Absent | 12 (24.00) | 7 (17.50) | ||
| Hyperlipidemia | 0.01 | 0.958 | ||
| Present | 36 (72.00) | 29 (72.50) | ||
| Absent | 14 (28.00) | 11 (27.50) | ||
| Diabetes | 0.01 | 0.922 | ||
| Present | 32 (64.00) | 26 (65.00) | ||
| Absent | 18 (36.00) | 14 (35.00) |
Comparison of the pulmonary artery pressure and HR
We compared the pulmonary artery pressure and HR before and after the treatment between the two groups, and the results showed no significant differences before the treatment. The pulmonary artery pressure and HRs in the two groups after one month of treatment were significantly lower than they were before the treatment, and the pulmonary artery pressure and HR in group A after one month of treatment were significantly lower than they were in group B (P<0.05), as shown in Figure 1.
Figure 1.
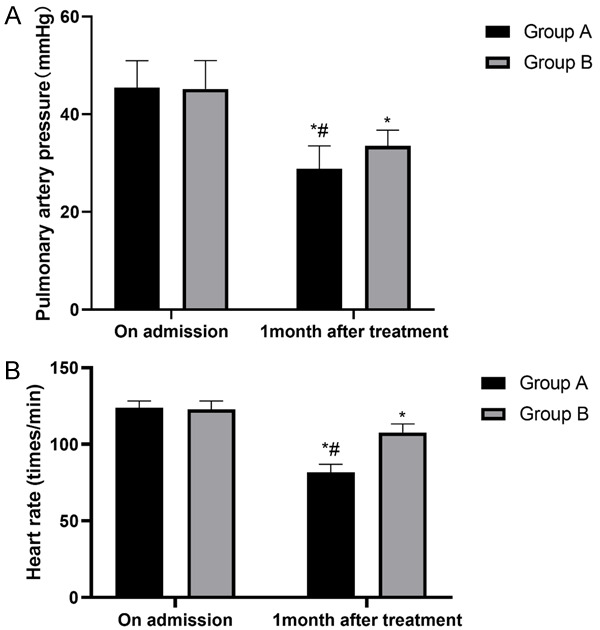
The pulmonary artery pressures and the heart rates in the two groups of patients. A. Pulmonary artery pressure: both groups of patients have significantly changed pulmonary artery pressure, and it was lower in group A than in group B after the treatment (P<0.05). B. Heart rate: both groups of patients have significantly changed heart rates, and they were lower in group A than in group B after the treatment (P<0.05). Notes: *P<0.05 vs. on admission, #P<0.05 vs. group B.
Comparison of the cardiac function
We compared the two groups’ cardiac function indexes (6MWD and RVEF) before and after treatment, and the results revealed no significant differences in the 6MWD or RVEF levels before the treatment. The two groups’ 6MWD and RVEF levels after one month of treatment were significantly higher than they were before the treatment, and the two indexes in group A after one month of treatment were significantly higher than they were in group B (P<0.05), as shown in Figure 2.
Figure 2.
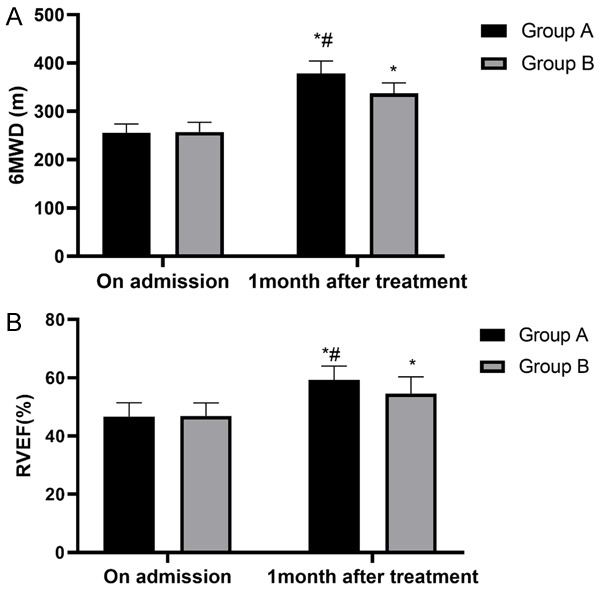
Cardiac function of two groups of patients. A. 6MWD: both groups of patients have significantly changed 6MWD, which was higher in group A than in group B after the treatment (P<0.05). B. RVEF: both groups of patients have significantly changed RVEF, which was higher in group A than in group B after the treatment (P<0.05). Notes: *P<0.05 vs. on admission, #P<0.05 vs. group B.
Comparison of the two groups’ pulmonary function and blood gas indexes before and after the treatment
Before the treatment, there were no differences in the pulmonary function indexes (FEV1, FVC, FEV1/FVC) or the blood gas indexes (PaO2 and PaCO2) between the two groups (all P>0.05), but after the treatment, the pulmonary function and blood gas indexes of the patients in both groups were significantly improved, and group A showed higher levels of FEV1, FVC, FEV1/FVC, and PaO2 and a lower PaCO2 level than group B (all P<0.05), As shown in Figure 3.
Figure 3.
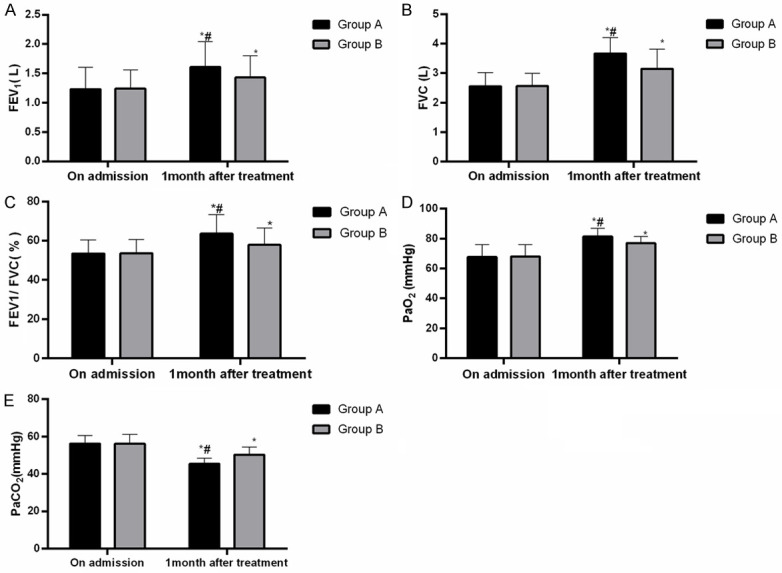
The pulmonary function and blood gas indexes of two groups of patients. A. FEV1: both groups of patients have significantly changed FEV1, which was higher in group A than in group B after the treatment (P<0.05). B. FVC: both groups of patients have significantly changed FVC, which was higher in group A than in group B after the treatment (P<0.05). C. FEV1/FVC: both groups of patients have significantly changed FEV1/FVC, which was higher in group A than in group B after the treatment (P<0.05). D. PaO2: there were significant changes in both groups in PaO2, and PaO2 in group A was higher than that in group B after treatment (P<0.05). E. PaCO2: there were significant changes in both groups in PaCO2, and PaCO2 in group A was lower than that in group B after treatment (P<0.05). Notes: *P<0.05 vs. on admission, #P<0.05 vs. group B.
Comparison of the inflammatory factors
We compared the inflammatory cytokine (IL-13, IL-17 and CRP) levels in the two groups before and after the treatment, and the results showed no significant differences before the treatment. After one month of treatment, the IL-13, IL-17, and CRP levels in the two groups were significantly lower than they were before the treatment and were significantly lower in group A compared with group B (P<0.05), as shown in Figure 4.
Figure 4.
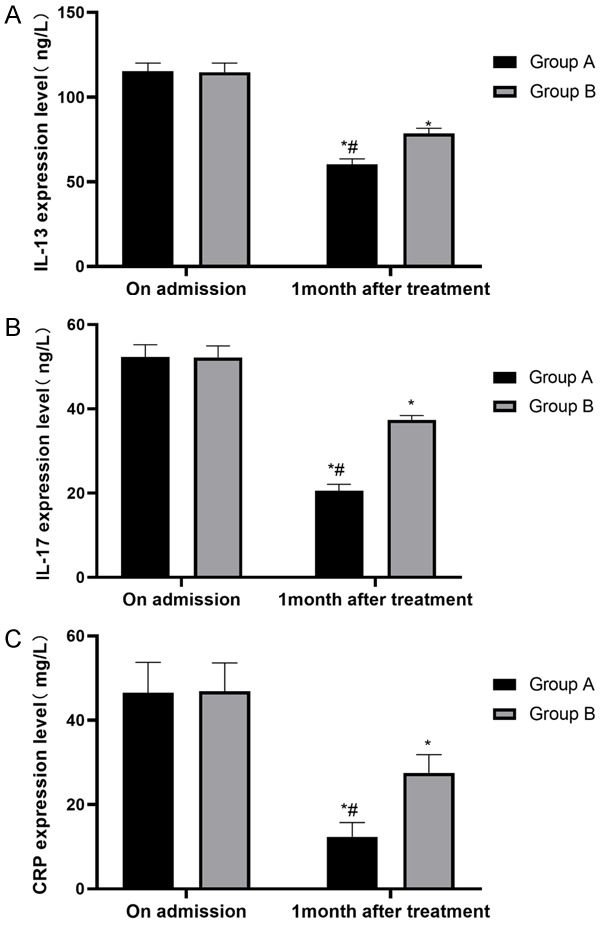
The inflammatory factor levels of the two groups of patients. A. IL-13: both groups of patients have significantly changed IL-13, which was lower in group A than in group B after treatment (P<0.05). B. IL-17: both groups of patients have significantly changed IL-17, which was lower in group A than in group B after treatment (P<0.05). C. CRP: both groups of patients have significantly changed CRP, which is lower in group A than in group B after treatment (P<0.05). Notes: *P<0.05 vs. on admission, #P<0.05 vs. group B.
Comparison of the total adverse reaction rates
We compared the incidences of adverse reactions in the two groups after the treatment, and the results showed that the total incidence of adverse reactions in group A was significantly lower than it was in group B (6% vs. 30%, P<0.05), as shown in Table 2.
Table 2.
The incidences of adverse reactions in two groups
| Classification | Group A (n=50) | Group B (n=40) | X2 | P |
|---|---|---|---|---|
| Flushing | 1 (2.00) | 4 (10.00) | - | - |
| Headache | 0 (0.00) | 1 (2.50) | ||
| Dizziness | 1 (2.00) | 6 (15.00) | - | - |
| Nausea | 1 (2.00) | 1 (2.50) | - | - |
| Adverse reaction rate (%) | 3 (6.00) | 12 (30.00) | 9.22 | 0.002 |
Comparison of the ORR
We compared the two groups’ ORR after the treatment, and the results showed that the ORR of the patients in group A was significantly higher than it was in group B (98% vs. 75%, P<0.05), as shown in Table 3.
Table 3.
The overall response rates in the two groups
| Classification | Group A (n=50) | Group B (n=40) | X2 | P |
|---|---|---|---|---|
| Marked response | 32 (64.00) | 18 (45.00) | - | - |
| Effective response | 17 (34.00) | 12 (30.00) | - | - |
| No response | 1 (2.00) | 10 (20.00) | - | - |
| ORR (%) | 49 (98.00) | 30 (75.00) | 10.96 | <0.001 |
Comparison of the nursing satisfaction levels
We compared the total effective rate of patients in the two groups after treatment, and the results showed that the total effective rate of patients in group A was significantly higher than that in group B (96% vs. 80%, P<0.05), as shown in Table 4.
Table 4.
A comparison of nursing satisfaction levels in the two groups
| Classification | Group A (n=50) | Group B (n=40) | X2 | P |
|---|---|---|---|---|
| Greatly satisfied | 36 (72.00) | 22 (55.00) | - | - |
| Satisfied | 12 (24.00) | 10 (25.00) | - | - |
| Dissatisfied | 2 (4.00) | 8 (20.00) | - | - |
| Satisfaction (%) | 48 (96.00) | 32 (80.00) | 5.76 | 0.016 |
Discussion
COPD is a systemic disease that predisposes to PAH and systemic inflammation, which in turn leads to respiratory distress and increased mortality [16,17]. Treatment methods such as drugs, oxygen therapy and rehabilitation can relieve the symptoms of airflow limitation caused by COPD and PAH and alleviate the breathing difficulties [18]. In this study, we explored the impacts of bosentan in combination with sildenafil on COPD and HAP, specifically from the angles of the inflammatory reactions and the cardiac and pulmonary functions.
In terms of the cardiac and pulmonary functions, the pulmonary artery pressure and HR, the recovery of cardiac function, the pulmonary function and blood gas indexes in group A (bosentan combined with sildenafil therapy) were better than they were in group B (bosentan combined with iloprost solution for inhalation therapy), and the pulmonary artery pressure and HR in group A decreased faster. Iloprost drugs are sometimes used to treat patients with COPD and HAP, and in this study, iloprost solution for inhalation was used in the control group. While maintaining gas exchange, these drugs can effectively lead to pulmonary vasodilation, which has a certain effect on the recovery of pulmonary function [19]. However, with regard to drugs such as iloprost solution for inhalation, its effects on lowering blood pressure are limited, with relatively significant side effects [20]. Sildenafil, as a kind of PDE5i, has been proved to have a good clinical effectiveness in the clinical studies of PAH treatment with fewer associated side effects [21]. By increasing intracellular circulation, it triggers pulmonary artery vasodilation, thereby reducing pulmonary artery pressure, dilating blood vessels, and increasing the blood flow to specific parts of the body [22,23]. Bosentan can reduce pulmonary vascular resistance, improve the hemodynamic indexes, and enhance exercise tolerance and the survival rate of patients [24,25]. The combined treatment of the two drugs can not only remarkably reduce the pulmonary artery blood pressure of patients, but it can also improve their exercise ability and ameliorate their cardiac function and symptoms of dyspnea [26,27]. According to the above and the results of this study, we can see that although bosentan was used in both groups, group A had a better effect on vasodilation because sildenafil was used to reduce the pulmonary artery pressure. The results imply that bosentan combined with sildenafil can strongly reduce the pulmonary artery pressure and improve the pulmonary function. Compared with group B, the patients in group A had a greater decrease in their pulmonary artery pressure, improved blood oxygen, a better HR recovery, and better recovery of cardiac and pulmonary functions. The results show that bosentan combined with sildenafil can better prevent and reduce the incidence of adverse cardiac events compared with bosentan combined with iloprost. Therefore, we conclude that the ORR of the patients in group A is higher. The efficacy and safety of bosentan combined with sildenafil in the treatment of pulmonary hypertension were also reported in the studies of Hoeper [28] and Verlinden [29]. They didn’t use iloprost as a control, so the potential mechanism of bosentan combined with sildenafil for better efficacy remains to be further explored, and there is no evidence of a comparative study of iloprost and sildenafil. But one thing is certain, this combination is related to a lower incidence of complications.
From the previous paragraph, we know that sildenafil and bosentan have fewer side effects than other drugs. According to a study [30], many cytokines are involved in the inflammatory process of COPD, among which IL-13 is involved in the occurrence and development of COPD and is highly expressed in the serum of patients with COPD. The damage of vascular endothelial function caused by PAH can easily lead to the further deterioration of the disease, and the subsequent inflammatory reaction can aggravate the vascular injury. Endothelin receptor antagonists such as bosentan, and PDE5i like sildenafil, can effectively inhibit the inflammatory reaction and reduce the inflammatory factor levels by changing the hemodynamics [31]. However, the results of this study showed that compared with group B, the inflammatory cytokines in group A were lower and the adverse reactions were also fewer. This suggests that the intervention of Bosentan combined with sildenafil can better reduce the inflammatory mediators in patients with fewer adverse reactions after intervention. In a study on pulmonary hypertension, patients treated with the bosentan and sildenafil were found to have higher safety profiles and more stable recoveries of their clinical symptoms [32], which is similar to this study. The total clinical effectiveness rate of the patients in group A after the treatment were significantly higher than it was in group B, indicating that the combination of the two drugs not only had good efficacy, but also was relatively safer.
This study first compared the efficacy of bosentan combined with sildenafil and bosentan combined with iloprost in COPD with PAH, and it may provide a reference for clinical treatment. But there are still some shortcomings to be improved. At first, we did not record the degrees of cooperation and drug acceptance of the patients in detail, which is not conducive to the further improvement of the treatment plan. In addition, due to limited conditions, we did not measure some factors. In our future clinical research, we will take care of the patients’ feelings and further improve the treatment plan. Moreover, we will further improve the our study of the related factors to further analyze the pathological basis of the disease.
To sum up, the administration of bosentan combined with sildenafil in COPD patients with PAH can reduce their pulmonary artery pressure and HR and promote the recovery of their cardiac and pulmonary functions, so it is safer and worthy of clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi: 10.2147/COPD.S161555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sood A, Assad NA, Barnes PJ, Churg A, Gordon SB, Harrod KS, Irshad H, Kurmi OP, Martin WJ 2nd, Meek P, Mortimer K, Noonan CW, Perez-Padilla R, Smith KR, Tesfaigzi Y, Ward T, Balmes J. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur Respir J. 2018;51:1700698. doi: 10.1183/13993003.00698-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544. doi: 10.1016/j.redox.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348. doi: 10.2147/COPD.S176122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, Massion PP, Ware LB, Lee JW, Kononov AV, Lawson WE, Blackwell TS. Secretory IgA deficiency in individual small airways is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med. 2017;195:1010–1021. doi: 10.1164/rccm.201604-0759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montani D, Gunther S, Dorfmuller P, Perros F, Girerd B, Garcia G, Jais X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, Sitbon O. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013;8:97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang P, Yang J, Yang Y, Ding Z. Effect of azithromycin in combination with simvastatin in the treatment of chronic obstructive pulmonary disease complicated by pulmonary arterial hypertension. Pak J Med Sci. 2017;33:260–264. doi: 10.12669/pjms.332.11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ried M, Neu R, Lehle K, Grosser C, Szoke T, Lang G, Hofmann HS, Hoenicka M. Superior vasodilation of human pulmonary vessels by vardenafil compared with tadalafil and sildenafil: additive effects of bosentan. Interact Cardiovasc Thorac Surg. 2017;25:254–259. doi: 10.1093/icvts/ivx108. [DOI] [PubMed] [Google Scholar]
- 10.Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, Kilbride S, Lange P, Lomas DA, Martinez FJ, Singh D, Tabberer M, Wise RA, Pascoe SJ IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues RM, Kollipara L, Chaudhari U, Sachinidis A, Zahedi RP, Sickmann A, Kopp-Schneider A, Jiang X, Keun H, Hengstler J, Oorts M, Annaert P, Hoeben E, Gijbels E, De Kock J, Vanhaecke T, Rogiers V, Vinken M. Omics-based responses induced by bosentan in human hepatoma HepaRG cell cultures. Arch Toxicol. 2018;92:1939–1952. doi: 10.1007/s00204-018-2214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omarjee L, Fontaine C, Mahe G, Jaquinandi V. Improvement of peripheral artery disease with Sildenafil and Bosentan combined therapy in a patient with limited cutaneous systemic sclerosis: a case report. Medicine (Baltimore) 2017;96:e6988. doi: 10.1097/MD.0000000000006988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016;69:177. doi: 10.1016/j.rec.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Li T. Combined methods (formal adjusted indirect comparison, meta-analysis and principal component analysis) comparisons of the safety and efficacy of ambrisentan, bosentan, and sildenafil in the patients with pulmonary arterial hypertension. Front Pharmacol. 2020;11:400. doi: 10.3389/fphar.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatima N, Arshad S, Quddusi AI, Rehman A, Nadeem A, Iqbal I. Comparison of the efficacy of sildenafil alone versus sildenafil plus bosentan in newborns with persistent pulmonary hypertension. J Ayub Med Coll Abbottabad. 2018;30:333–336. [PubMed] [Google Scholar]
- 16.Lim JU, Lee JH, Kim JS, Hwang YI, Kim TH, Lim SY, Yoo KH, Jung KS, Kim YK, Rhee CK. Comparison of World Health Organization and Asia-Pacific body mass index classifications in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2465–2475. doi: 10.2147/COPD.S141295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:669–675. doi: 10.2147/COPD.S130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen KH, Iversen M, Kjaergaard J, Mortensen J, Nielsen-Kudsk JE, Bendstrup E, Videbaek R, Carlsen J. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31:373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Jin YZ, Zhao QH, Jiang R, Wu WH, Gong SG, He J, Liu JM, Jing ZC. Hemodynamic and gas exchange effects of inhaled iloprost in patients with COPD and pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2017;12:3353–3360. doi: 10.2147/COPD.S141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chon MK, Cho KI, Cha KS, Seo JS, Kim DS. Effects of long-term iloprost treatment on right ventricular function in patients with Eisenmenger syndrome. J Cardiol. 2017;69:741–746. doi: 10.1016/j.jjcc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Bermejo J, Yotti R, García-Orta R, Sánchez-Fernández PL, Castaño M, Segovia-Cubero J, Escribano-Subías P, San Román JA, Borrás X, Alonso-Gómez A, Botas J, Crespo-Leiro MG, Velasco S, Bayés-Genís A, López A, Muñoz-Aguilera R, de Teresa E, González-Juanatey JR, Evangelista A, Mombiela T, González-Mansilla A, Elízaga J, Martín-Moreiras J, González-Santos JM, Moreno-Escobar E, Fernández-Avilés F Sildenafil for Improving Outcomes after Valvular Correction (SIOVAC) Investigators. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J. 2018;39:1255–1264. doi: 10.1093/eurheartj/ehx700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins JLG, Law MA, Borasino S, Erwin WC, Cleveland DC, Alten JA. Correction to: routine sildenafil does not improve clinical outcomes after Fontan operation. Pediatr Cardiol. 2018;39:644–645. doi: 10.1007/s00246-018-1816-9. [DOI] [PubMed] [Google Scholar]
- 23.Hill KD, Tunks RD, Barker PC, Benjamin DK Jr, Cohen-Wolkowiez M, Fleming GA, Laughon M, Li JS. Sildenafil exposure and hemodynamic effect after stage II single-ventricle surgery. Pediatr Crit Care Med. 2013;14:593–600. doi: 10.1097/PCC.0b013e31828aa5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaRue SJ, Garcia-Cortes R, Nassif ME, Vader JM, Ray S, Ravichandran A, Rasalingham R, Silvestry SC, Ewald GA, Wang IW, Schilling JD. Treatment of secondary pulmonary hypertension with bosentan after left ventricular assist device implantation. Cardiovasc Ther. 2015;33:50–55. doi: 10.1111/1755-5922.12111. [DOI] [PubMed] [Google Scholar]
- 25.Ye W, Li B, Sheng W, Yao M, Shang L, Gao C. Efficacy of oral bosentan for treatment of congenital heart disease-associated pulmonary arterial hypertension. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1846–1848. [PubMed] [Google Scholar]
- 26.D’Alto M, Romeo E, Argiento P, Sarubbi B, Santoro G, Grimaldi N, Correra A, Scognamiglio G, Russo MG, Calabro R. Bosentan-sildenafil association in patients with congenital heart disease-related pulmonary arterial hypertension and Eisenmenger physiology. Int J Cardiol. 2012;155:378–382. doi: 10.1016/j.ijcard.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 27.Gao C, Liu J, Zhang R, Zhao M, Wu Y. The efficacy of bosentan combined with vardenafil in the treatment of postoperative pulmonary hypertension in children with congenital heart disease: a protocol of randomized controlled trial. Medicine (Baltimore) 2021;100:e23896. doi: 10.1097/MD.0000000000023896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoeper MM, Faulenbach C, Golpon H, Winkler J, Welte T, Niedermeyer J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J. 2004;24:1007–1010. doi: 10.1183/09031936.04.00051104. [DOI] [PubMed] [Google Scholar]
- 29.Verlinden NJ, Benza RL, Raina A. Safety and efficacy of transitioning from the combination of bosentan and sildenafil to alternative therapy in patients with pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020945523. doi: 10.1177/2045894020945523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomme EE, Provoost S, De Smet EG, De Grove KC, Van Eeckhoutte HP, De Volder J, Hansbro PM, Bonato M, Saetta M, Wijnant SR, Verhamme F, Joos GF, Bracke KR, Brusselle GG, Maes T. Quantification and role of innate lymphoid cell subsets in chronic obstructive pulmonary disease. Clin Transl Immunology. 2021;10:e1287. doi: 10.1002/cti2.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Chen S, Du J. Bosentan for treatment of pediatric idiopathic pulmonary arterial hypertension: state-of-the-Art. Front Pediatr. 2019;7:302. doi: 10.3389/fped.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Li T. Inadequate dosage may lead to the recurrence of postoperative pulmonary hypertension in patients with congenital heart disease. Front Pharmacol. 2021;12:660405. doi: 10.3389/fphar.2021.660405. [DOI] [PMC free article] [PubMed] [Google Scholar]


