Abstract
With the progression of the COVID-19 pandemic, the classic manifestations of COVID-19 (e.g., persistent fever, dry cough, pneumonia, and acute respiratory distress syndrome in the severe disease) have expanded to include less common complications of the extrapulmonary organs. Recent evidence has shown that COVID-19 patients with concomitant presence of GI symptoms are at higher risk of developing severe disease and have poor clinical outcomes. Recently, multiple SARS-CoV-2-induced acute pancreatitis (AP) cases have been reported. This literature review aims to provide an insight into SARS-CoV-2-directed invasion of the pancreas. We will also review the currently available literature on the clinical effects of SARS-CoV-2, including AP and mild elevation of lipase levels in patients with COVID-19. In addition, we will discuss plausible mechanisms that underly SARS-CoV-2-induced pancreatitis.
Keywords: COVID-19, pancreas, cytokines, SARS-CoV-2, lipase, amylase, pancreatitis
Introduction
In the past year, medical and scientific communities have provided an abundance of valuable insight into the epidemiology, pathogenesis, and clinical spectrum of coronavirus disease 2019 (COVID-19). COVID-19 is caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. SARS-CoV-2 primarily affects the pulmonary system by the cytopathic effects elicited through viral replication and by overwhelming the immune system by cytokine storm [2]. As a result, the classical manifestations of COVID-19 include persistent fever, dry cough, pneumonia, and acute respiratory distress syndrome [1-3]. Nevertheless, SARS-CoV-2 also affects extrapulmonary organs. The viral particles and their receptors are also present in various human cells, including organs of the gastrointestinal (GI) tract, pancreas, cholangiocytes, brain, kidney, placenta, and other organ systems [4]. Extrapulmonary symptoms of COVID-19 are increasingly being identified and reported in the literature. Recent evidence has shown that COVID-19 patients with the simultaneous presence of GI symptoms such as nausea, vomiting, and diarrhea are at higher risk of developing severe disease and have poor clinical outcomes [5].
Recently, multiple SARS-CoV-2-induced acute pancreatitis (AP) cases have been published in the literature [6-8]. Most of these patients had no obvious cause of AP, and the occurrence of AP mostly coincided with a COVID-19 diagnosis [9,10]. The causal relationship between the two is plausible because various viruses are one of AP’s known etiologies [11]. In particular, viruses such as hepatotropic virus (Hepatitis B virus being the most common), Coxsackie virus, cytomegalovirus (CMV), human immunodeficiency virus (HIV), herpes simplex virus (HSV), mumps, varicella-zoster virus, and others have been shown to cause pancreatic inflammation [11]. The mechanism of action differs between these viral agents, varying from direct viral injury of the exocrine and endocrine pancreas to the indirect systematic inflammatory response elicited by the virus [12,13]. Earlier, SARS-associated coronavirus (SARS-CoV) was identified in the pancreas of pigeons suffering from AP [6]. Furthermore, during the 2003 global outbreak of the severe acute respiratory syndrome (SARS) caused by SARS-CoV, the viral nucleoprotein and the RNA polymerase gene fragment were found in the pancreas by using the immunohistochemistry and the in situ hybridization in the tissue of patients that died from the disease [7]. The genome sequences of SARS-CoV and SARS-CoV-2 are 79.6% identical to each other. They also share the same host entry machinery by angiotensin-converting enzyme 2 (ACE2) [14]. Recent clinical studies have shown that COVID-19 patients with hyperlipasemia have poor clinical outcomes [15]. Similarly, AP patients with SARS-CoV-2 infection are found to have a severe form of AP [16]. Thus, pancreatic injury by SARS-CoV-2 has recently received much attention in the medical community [9,10].
This literature review aims to provide an insight into the SARS-CoV-2-directed invasion of the pancreas. We will also review the currently available literature on the clinical effects of SARS-CoV-2, including AP and mild elevation of lipase levels in patients with COVID-19. In addition, we will discuss the plausible mechanisms of SARS-CoV-2-induced pancreatitis.
Differential expression of SARS-COV-2 receptors (ACE-2 and TMPRSS-2) in the pancreas
SARS-CoV-2 entry into the host cells commences with the engagement of its spike protein with the host cell surface receptor, ACE2 (Figure 1A) [17,18]. Upon this association, the spike protein undergoes proteolytic cleavage mediated by the host’s serine protease TMPRSS-2 (Figure 1A) [19]. In concert, these processes promote the endocytosis of the virus and the subsequent release of its genome into the host’s cytoplasm (Figure 1A) [19,20]. Thus, the magnitude of direct cellular infection and the infectivity rate heavily depend on the expression levels and the functionalities of ACE2 and TMPRSS-2.
Figure 1.
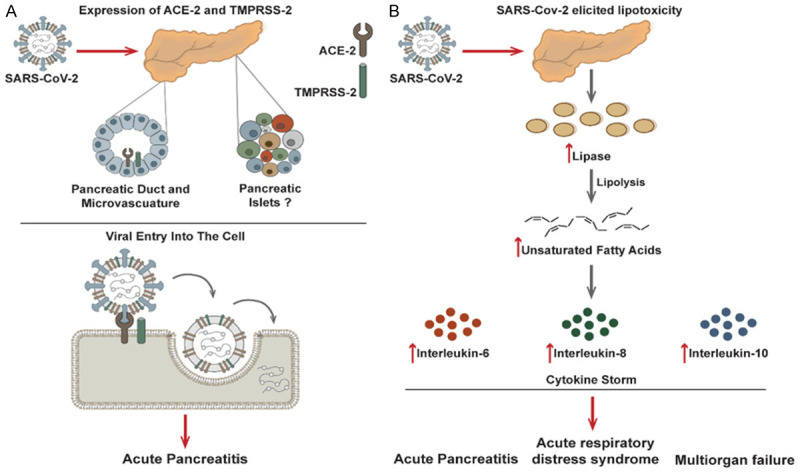
The mechanistic rationale of SARS-CoV-2 elicited acute pancreatitis. A. Differential expression patterns of SARS-CoV-2 receptors in pancreatic cells can allow viral entry and thereby initiate acute pancreatitis or exacerbate an existing one; B. SARS-CoV-2 elicited lipotoxicity can initiate a pro-inflammatory cytokine storm to further aggravate acute pancreatitis, triggering acute respiratory distress syndrome and multiorgan failure.
Based on the transcriptomic and proteomic investigations, ACE2 expression in the human pancreas was comparable to its expression in the lungs [21-23]. Still, it was significantly lower than that found in the small and large intestines [21-23]. The pancreatic ACE2 expression levels were positively correlated with BMI, with a rapid decline after the age of 50 years [24]. Furthermore, pancreatic ACE2 protein levels coincided with the TMPRSS-2 protein levels, thus giving the prospective for the direct entry of the SARS-CoV-2 into the pancreatic cells [23]. In particular, the co-expression of ACE2 and TMPRSS-2 was primarily located in the interlobular pancreatic ducts [21,23-25]. Simultaneously, the ACE2 alone dominated the pancreatic endothelial pericytes [21,23-25].
There is a discrepancy in the literature about the presence of ACE2 in endocrine pancreatic islet cells. In 2010, a higher ACE-2 expression was identified in the endocrine pancreas than in the exocrine pancreas [22]. It was noted that this expression variability could influence the pathogenesis of the coronavirus-associated acute hyperglycemia [22]. These findings were further supported as ACE2 was preferentially expressed in the insulin-producing β-cells of the pancreas than in the α-cells [25]. The authors showed that a distinct ACE2 isoform type (the short ACE2 version) was associated with the β-cells [25]. The ACE2 expression in the β-cell line EndoC-βH1 and in primary human pancreatic islets was also dictated by the pro-inflammatory cytokines, such as interleukin (IL) 1β, tumor necrosis factor (TNF) α, and interferon (IFN) γ [25]. Both α- and β-cells were permissive to SARS-CoV-2 infection by human pancreatic organoids model; however, replicative capability of virus in the pancreas is still unknown [26].
However, these findings stand in contrast to the following scientific investigations [23,24]. By analyzing two existing bulk RNA sequencing data of human α- and β-cells, and four single-cell RNA sequencing of human pancreatic cells low amounts of ACE2 and TMRPSS-2 transcripts were detected, as well as no co-expression of these two receptors in β-cells of the human pancreas [23]. Furthermore, when the pancreas was challenged with SARS-CoV-2, the viral nucleocapsid was found only in the pancreatic ducts and not in the endocrine pancreatic islets [24].
SARS-CoV-2 elicits lipotoxicity/cytokine storm and multiorgan failure
Although SARS-CoV-2 effects on the pancreas remain an active area of investigation, current models propose a link between the release of pancreatic lipase and elevations in unsaturated fatty acids [27,28]. It was hypothesized that intestinal release of pancreatic lipase increased lipolysis and plasma levels of unsaturated fatty acids which could damage mitochondria and cause an increase in pro-inflammatory immune mediators (e.g., IL-6, IL-8, and IL-10). This increase in cytokines can accelerate the disease pathogenesis and lead to life-threatening multiorgan failure and acute respiratory distress syndrome (ARDS) (Figure 2) [16,27,28]. This fits with previous clinical studies which showed a link between cytokine storms in COVID-19 patients and increased morbidity and mortality rates in hospitalized patients. However, additional clinical studies and analysis are needed to further elucidate and confirm this hypothesis.
Figure 2.
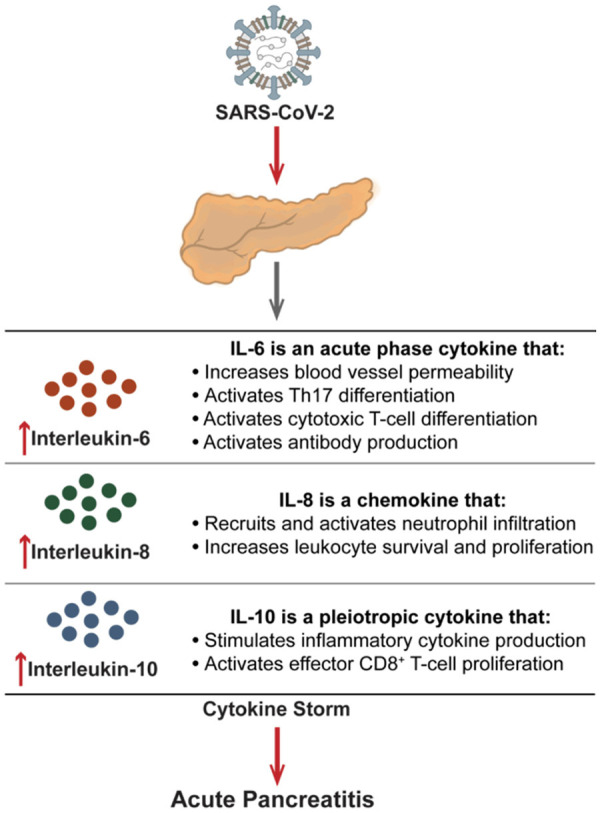
Mechanisms of SARS-CoV-2-elicited pro-inflammatory cytokines (Interleukin-6, interleukin-8, and interleukin-10) that can mediate the development of acute pancreatitis.
Acute pancreatitis (AP) in COVID-19 patients and their prognosis
The occurrence of AP in COVID-19 was reported by many case reports and retrospective studies. AP was diagnosed by following the revised Atlanta classification, which required that the patient needed to exhibit at least two out of three criteria, such as: (i) abdominal pain, (ii) serum amylase or lipase activity at least three times greater than the upper limit of the normal, and (iii) characteristic imaging of pancreatitis [29]. The patients were also diagnosed with COVID-19 by performing PCR on the nasopharyngeal swab [1]. The studies conducted in China, India, and United States reported a low incidence of AP in hospitalized COVID-19 patients [30-32]. Among hospitalized COVID-19 patients, AP was associated with higher morbidity and mortality rates [30]. In contrast, within the cohort of 42 participants, the mortality rate was 42.8% with no significant correlation to the amylase and lipase levels [31]. However, this study reported that 33% of patients exhibited hyperamylasemia, and 24.1% suffered from hyperlipidemias. This investigation, however, had its limitations, because the lipase levels were unavailable for all patients, and the imaging was not performed due to COVID-19-related restrictions at the time. Therefore, the proper AP diagnosis could be missing for all participants.
Furthermore, the manifestation and the outcome of the AP were exacerbated when the patient was diagnosed with COVID-19 [33,34]. In comparison to the SARS-CoV-2 negative patients, the infection elicited increased multiorgan and persistent organ failure, which resulted in higher morbidity and mortality rates [33]. Furthermore, when pancreatitis was accompanied by COVID-19, patients had a longer hospitalization stay and required more help from the mechanical ventilator [34]. The outcome did not depend on the etiology of pancreatitis, and the patients with high alcohol consumption, gallstones, drugs, or hypertriglyceridemia, were taken into account while conducting these investigations [33,34].
Multiple independent case reports have also described the progression of COVID-19 highlighted by the persistent abdominal pain, increased amylase, and lipase levels and imaging changes of pancreas indicative of AP [35-44]. The usual presentation included sharp abdominal pain, nausea, vomiting, and diarrhea around the time of the positive SARS-CoV-2 PCR test and the onset of disease symptoms [35-44]. The biochemical analyses revealed the elevated pancreatic enzyme levels, while the imaging scans showed either normal or atrophic pancreas with tail parenchymal enlargement and surrounding retroperitoneal fat stranding [35,37-44]. Patients were held with nothing per os, intravenous fluids, and analgesics [35-44]. This was followed by the rapid decrease of the amylase and lipase levels. Patients were discharged after the abdominal and pulmonary symptoms were resolved without sequelae [35-44]. The manifestation of COVID-19-associated pancreatitis was unlike the usual disease [44]. Although the disease initially appeared severe, they reported a pancreaticoduodenal inflammation with steatosis [44]. Due to the idiopathic nature of AP in these patients, case reports argued for a differential diagnosis of the COVID-19 [35-37,39-44]. The pancreatitis origin in the observed patient perhaps lay in the COVID-19 medication treatment, as the disease developed after the pulmonary issues were resolved upon therapy with acetaminophen, dexamethasone, ciprofloxacin, pantoprazole, and tocilizumab [38]. However, one case study reported that the AP developed in a patient with only mild respiratory tract symptoms and without any COVID-19 treatments [42].
A handful of case reports documented an AP diagnosis before the manifestation of the upper respiratory problems and the COVID-19 testing. These patients presented to the hospital with persistent GI-related complications, such as abdominal pain, diarrhea, and vomiting [8,45-48]. Upon determining the lipase/amylase levels and performing the appropriate imaging, they were admitted with an idiopathic AP diagnosis [8,45-48]. The patient’s sex, age, and prior underlying medical history were ruled out as contributing factors to the diagnosis. Upon hospitalization, many patients started developing respiratory issues and tested positive for the SARS-CoV-2 with the nasopharyngeal swab PCR [8,45-48]. After this point, the manifestations of both AP and COVID-19 were congruent with each other. The resolution of one concurred with the other, followed by patient discharge without any visible permanent consequences. As pancreatitis manifested prior to the prominent systematic inflammation incited by the SARS-CoV-2 infection, these reports ignite the debate on the ability of the virus to directly affect the pancreas and elicit pancreatitis [8,45-48]. Furthermore, recognizing that idiopathic AP can be one of the early manifestations of COVID-19 is important for physicians so that they can take additional precautions to protect themselves and minimize the spread.
Can lipase be a marker for pancreatic injury secondary to COVID-19?
A common laboratory marker for pancreatic injury is an elevation in lipase levels. The lipase is produced by the pancreatic acinar cells that enzymatically degrade fatty acids for uptake and subsequent metabolism [49]. When the pancreas is injured (e.g., pancreatitis), the increased permeability of acinar cells allows for the release of lipase into the blood. In general, lipase levels increase within three to six hours of injury, peak within 24 hours, and remain elevated for two weeks [49]. Although amylase is also used to detect pancreatic injury, lipase is preferred due to its higher specificity for pancreatic injury [49]. During the COVID-19 pandemic, several reports showed an elevation in lipase in COVID-19 patients with GI symptoms [35,37-44]. Given this association, two retrospective studies examined the incidence and correlation of COVID-19 and pancreatitis to other factors, such as increased intensive care unit (ICU) admissions, intubation rate, leukocytosis, and abnormal liver enzymes [15,50-53].
Of 83 COVID-19 patients in one study, 14 (17%) had elevated lipase levels greater than 3 times the upper limit of normal (ULN). Compared to patients with lipase levels less than 3 times the ULN, elevated lipase levels were associated with increased ICU admissions (92.9% vs. 32.8%), rate of intubation (78.6% vs. 23.5%), leukocytosis (22,000 white blood cells/uL vs. 10,400 white blood cells/uL), and abnormal liver enzymes (AST/ALT) (92.9% vs. 52.9%) [51]. Interestingly, there was a greater male predominance (78.6% vs.38.8%) in COVID-19 patients with elevated lipase levels compared to patients with lipase levels less than 3 times the ULN. A similar retrospective study examined 71 hospitalized COVID-19 patients with lipase enzyme laboratory information from 2 tertiary and 4 community hospitals in Massachusetts [50]. Among the 71 hospitalized COVID-19 patients, 12% had increased serum lipase levels [50]. In contrast, there was no difference in the rate of intubation, ICU hospitalization, gender, or mortality between COVID-19 patients with or without increased serum lipase levels [50,51]. However, this study did not examine whether the patients had increased leukocytosis/white blood cells or liver enzymes in patients with increased serum lipase levels [51,52]. Whether lipase levels correlates with the severity or mortality of AP related to COVID-19 remains an area of active investigation [52].
Pancreatic cancer and COVID-19
The COVID-19 pandemic also affected the chemotherapy regimens of pancreatic cancer patients, and placed them in a high-risk group for developing severe disease. Before the COVID-19 pandemic, only 20% of pancreatic cancers were eligible for surgery [54]. During the initial stages of the COVID-19 pandemic, triaging cancer patients for surgical resections was proposed based on the risk of treatment delay, disease progression, and patient’s age [55,56]. In patients who were unable to proceed to surgery, chemotherapy was used during the interim period before surgical intervention. The long-term outcomes of cancer patients during the COVID-19 pandemic remain to be seen. Recent studies showed that the COVID-19 pandemic adversely impacted the delay of surgery for pancreatic cancer patients and the wait times for the diagnostic imaging. These delays often prevented patients from having their tumors resected [57,58]. As a result, efforts to fast-track pancreatic cancer patients for assessment and treatment were set in place to improve the rates of successful pancreatic cancers resections [59].
Similar to other cancers, pancreatic adenocarcinoma patients have an increased expression of ACE-2 [60]. Further analysis showed that decreased methylation was correlated with increased ACE-2 expression in pancreatic adenocarcinoma [60]. However, ACE-2 expression in pancreatic adenocarcinoma did not affect a patient’s treatment, and it was not relevant to patients’ prognosis [60]. Furthermore, it remains uncertain whether continuing chemotherapy after contracting COVID-19 is safe. A recent case report described the outcome of the pancreatic cancer patient who was infected with COVID-19 seven days after starting his chemotherapy regimen [61]. After successfully recovering and showing a negative COVID-19 test result, he was re-started on his chemotherapy regimen without recurrence of COVID-19 [61]. However, SARS-CoV-2 positive patients undergoing surgical resection of pancreatic cancer require more appropriate supportive care [62].
Future directions
Whether AP is a direct result of COVID-19 remains an area of active investigation. Pre-existing conditions and increased inflammation from SARS-CoV-2 infection may increase the risk of pancreatic injury. However, the administration of monoclonal antibodies or other drugs, such as remdesivir, may also damage gastrointestinal organs leading to AP. There is little guidance on the therapeutic management of AP in COVID-19 patients. Although the revised Atlanta guidelines provides guidance for diagnosing AP, it may not be suitable for diagnosing AP in COVID-19 patients. The remaining uncertainties about several aspects of AP secondary to COVID-19 include the clinical course and the severity of disease, determining the early and late phase of the disease, and the inflammatory responses leading to shock and multisystem organ failure. It is also possible that increased levels of amylase and lipase may not correlate to the extent of pancreatic damage and do not really indicate AP. Without proper intervention, it remains unclear whether the pancreatic function remains permanently diminished or altered from the SARS-CoV-2 virus. Longitudinal studies are needed to assess the long-term effects of SARS-CoV-2 on the pancreas.
Despite several reports, it is unknown whether ethnicity may influence the frequency of AP in hospitalized COVID-19 patients. Minority groups, particularly Latino, American Indian, and African American populations, have higher hospitalization and mortality rates associated with COVID-19 than Caucasian patients [63]. It is believed that a combination of socioeconomic factors and a higher prevalence of pre-existing medical conditions (e.g., diabetes, hypertension, obesity, asthma, and heart disease) increase the minority groups’ risk of contracting COVID-19. Therefore, minority groups may have a higher risk for developing AP from COVID-19 infections based on the increased COVID-19 infection rates in these communities. However, the biological factors that influence which COVID-19 patient will exhibit gastrointestinal and/or respiratory symptoms within each ethnic group remains unknown. Additionally, it is unknown whether differences in the expression of ACE2 and TMPRSS2 may explain why certain patients develop AP within minority groups. Further research into the biological mechanisms for assessing AP risk may provide additional insight into managing pancreatic injury in COVID-19 patients.
Lastly, large-scale studies are needed to assess the frequency of AP in COVID-19 patients. Most cases of AP have been reported in case reports and smaller retrospective studies. The lack of larger sample sizes and longitudinal studies provides a significant source of bias about these studies. Larger clinical studies reporting the ethnicities and drugs administered to these patients may provide insight into clinical management and risk factors for developing AP in COVID-19 patients.
Conclusion
The development of AP secondary to the SARS-CoV-2 virus remains a novel complication of COVID-19. However, the exact mechanism, frequency, and long-term effects of AP related to COVID-19 remain unknown due to a lack of larger retrospective studies [64]. Further preclinical and clinical analysis of autopsies and mouse model studies would provide insight into the pathogenesis of SARS-CoV-2 on the pancreas [64]. Additional studies on ACE2 and TMPRSS2 may improve understanding of the biological mechanisms and risk factors for AP with respect to ethnicity. Beyond AP, it remains to be seen whether pancreatic cancer patients will develop short- or long-term complications from COVID-19. Longitudinal studies of pancreatic cancer patients who contracted SARS-CoV-2 before or after starting chemotherapy may provide additional insight into managing complications within this patient population.
Disclosure of conflict of interest
None.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, Wade RG. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765. doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S, Xin Y, Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz M, Haghbin H, Lee-Smith W, Goyal H, Nawras A, Adler DG. Gastrointestinal predictors of severe COVID-19: systematic review and meta-analysis. Ann Gastroenterol. 2020;33:615–630. doi: 10.20524/aog.2020.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian DH, Zhu GJ, Wu LZ, Hua GX. Isolation and characterization of a coronavirus from pigeons with pancreatitis. Am J Vet Res. 2006;67:1575–1579. doi: 10.2460/ajvr.67.9.1575. [DOI] [PubMed] [Google Scholar]
- 7.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao Y, Lidove O, Mauhin W. First case of acute pancreatitis related to SARS-CoV-2 infection. Br J Surg. 2020;107:e270. doi: 10.1002/bjs.11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A, Kochhar R. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;20:1567–1575. doi: 10.1016/j.pan.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zippi M, Hong W, Traversa G, Maccioni F, De Biase D, Gallo C, Fiorino S. Involvement of the exocrine pancreas during COVID-19 infection and possible pathogenetic hypothesis: a concise review. Infez Med. 2020;28:507–515. [PubMed] [Google Scholar]
- 11.Rawla P, Bandaru SS, Vellipuram AR. Review of infectious etiology of acute pancreatitis. Gastroenterology Res. 2017;10:153–158. doi: 10.14740/gr858w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parenti DM, Steinberg W, Kang P. Infectious causes of acute pancreatitis. Pancreas. 1996;13:356–371. doi: 10.1097/00006676-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kottanattu L, Lava SAG, Helbling R, Simonetti GD, Bianchetti MG, Milani GP. Pancreatitis and cholecystitis in primary acute symptomatic epstein-barr virus infection-systematic review of the literature. J Clin Virol. 2016;82:51–55. doi: 10.1016/j.jcv.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal H, Sachdeva S, Perisetti A, Mann R, Inamdar S, Tharian B. Hyperlipasemia and potential pancreatic injury patterns in COVID-19: a marker of severity or innocent bystander? Gastroenterology. 2021;160:946–948. e942. doi: 10.1053/j.gastro.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandanaboyana S, Moir J, Leeds JS, Oppong K, Kanwar A, Marzouk A, Belgaumkar A, Gupta A, Siriwardena AK, Haque AR, Awan A, Balakrishnan A, Rawashdeh A, Ivanov B, Parmar C, M Halloran C, Caruana C, Borg CM, Gomez D, Damaskos D, Karavias D, Finch G, Ebied H, K Pine J, R A Skipworth J, Milburn J, Latif J, Ratnam Apollos J, El Kafsi J, Windsor JA, Roberts K, Wang K, Ravi K, V Coats M, Hollyman M, Phillips M, Okocha M, Sj Wilson M, A Ameer N, Kumar N, Shah N, Lapolla P, Magee C, Al-Sarireh B, Lunevicius R, Benhmida R, Singhal R, Balachandra S, Demirli Atıcı S, Jaunoo S, Dwerryhouse S, Boyce T, Charalampakis V, Kanakala V, Abbas Z, Nayar M COVID PAN collaborative group. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study. Gut. 2021;70:1061–1069. doi: 10.1136/gutjnl-2020-323364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G, Brissova M, Powers AC. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020;32:1028–1040. e1024. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusmartseva I, Wu W, Syed F, Van Der Heide V, Jorgensen M, Joseph P, Tang X, Candelario-Jalil E, Yang C, Nick H, Harbert JL, Posgai AL, Paulsen JD, Lloyd R, Cechin S, Pugliese A, Campbell-Thompson M, Vander Heide RS, Evans-Molina C, Homann D, Atkinson MA. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32:1041–1051. e1046. doi: 10.1016/j.cmet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P, Mathieu C, Eizirik DL, Sebastiani G, Dotta F. SARS-CoV-2 receptor angiotensin i-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne) 2020;11:596898. doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136. e127. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pezzilli R. Exocrine pancreas involvement in celiac disease: a review. Recent Pat Inflamm Allergy Drug Discov. 2014;8:167–172. doi: 10.2174/1872213x08666141122210738. [DOI] [PubMed] [Google Scholar]
- 28.Hegyi P, Szakács Z, Sahin-Tóth M. Lipotoxicity and cytokine storm in severe acute pancreatitis and COVID-19. Gastroenterology. 2020;159:824–827. doi: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 30.Akarsu C, Karabulut M, Aydin H, Sahbaz NA, Dural AC, Yegul D, Peker KD, Ferahman S, Bulut S, Dönmez T, Asar S, Yasar KK, Adas GT. Association between acute pancreatitis and COVID-19: could pancreatitis be the missing piece of the puzzle about increased mortality rates? J Invest Surg. 2020 doi: 10.1080/08941939.2020.1833263. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Bansal P, Margekar SL, Suman V, Sud R, Meena S, Sharma AK, Islam SY, Gurtoo A, Agrawal A, Pangtey GS, Prakash A. Pancreatic injury in COVID-19 patients. J Assoc Physicians India. 2020;68:58–60. [PubMed] [Google Scholar]
- 32.Funt SA, Cohen SL, Wang JJ, Sanelli PC, Barish MA. Abdominal pelvic CT findings compared between COVID-19 positive and COVID-19 negative patients in the emergency department setting. Abdom Radiol (NY) 2021;46:1498–1505. doi: 10.1007/s00261-020-02796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dirweesh A, Li Y, Trikudanathan G, Mallery JS, Freeman ML, Amateau SK. Clinical outcomes of acute pancreatitis in patients with coronavirus disease 2019. Gastroenterology. 2020;159:1972–1974. doi: 10.1053/j.gastro.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ. Prevalence, risk factors, and outcomes of hospitalized patients with coronavirus disease 2019 presenting as acute pancreatitis. Gastroenterology. 2020;159:2226–2228. e2222. doi: 10.1053/j.gastro.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alves AM, Yvamoto EY, Marzinotto MAN, Teixeira ACS, Carrilho FJ. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz J Infect Dis. 2020;24:561–564. doi: 10.1016/j.bjid.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand ER, Major C, Pickering O, Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107:e182. doi: 10.1002/bjs.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bokhari S, Mahmood F. Case report: novel coronavirus-a potential cause of acute pancreatitis? Am J Trop Med Hyg. 2020;103:1154–1155. doi: 10.4269/ajtmh.20-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brikman S, Denysova V, Menzal H, Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ. 2020;192:E858–E859. doi: 10.1503/cmaj.201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung S, Delgado Fuentes A, Fetterman AD. Recurrent acute pancreatitis in a patient with COVID-19 infection. Am J Case Rep. 2020;21:e927076. doi: 10.12659/AJCR.927076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology. 2020;20:665–667. doi: 10.1016/j.pan.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kataria S, Sharif A, Ur Rehman A, Ahmed Z, Hanan A. COVID-19 induced acute pancreatitis: a case report and literature review. Cureus. 2020;12:e9169. doi: 10.7759/cureus.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazrouei SSA, Saeed GA, Al Helali AA. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15:1601–1603. doi: 10.1016/j.radcr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabice SR, Altshuler PC, Bovet C, Sullivan C, Gagnon AJ. COVID-19 infection presenting as pancreatitis in a pregnant woman: a case report. Case Rep Womens Health. 2020;27:e00228. doi: 10.1016/j.crwh.2020.e00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szatmary P, Arora A, Thomas Raraty MG, Joseph Dunne DF, Baron RD, Halloran CM. Emerging phenotype of severe acute respiratory syndrome-coronavirus 2-associated pancreatitis. Gastroenterology. 2020;159:1551–1554. doi: 10.1053/j.gastro.2020.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20:1026–1027. doi: 10.1016/j.pan.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gadiparthi C, Bassi M, Yegneswaran B, Ho S, Pitchumoni CS. Hyperglycemia, hypertriglyceridemia, and acute pancreatitis in COVID-19 infection: clinical implications. Pancreas. 2020;49:e62–e63. doi: 10.1097/MPA.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP) BMJ Case Rep. 2020;13:e237903. doi: 10.1136/bcr-2020-237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patnaik RNK, Gogia A, Kakar A. Acute pancreatic injury induced by COVID-19. IDCases. 2020;22:e00959. doi: 10.1016/j.idcr.2020.e00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clinical Biochemistry. 2017;50:1275–1280. doi: 10.1016/j.clinbiochem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 50.McNabb-Baltar J, Jin DX, Grover AS, Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Shen L, Chan WW. Lipase elevation in patients with COVID-19. Am J Gastroenterol. 2020;115:1286–1288. doi: 10.14309/ajg.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barlass U, Wiliams B, Dhana K, Adnan D, Khan SR, Mahdavinia M, Bishehsari F. Marked elevation of lipase in COVID-19 disease: a cohort study. Clin Transl Gastroenterol. 2020;11:e00215. doi: 10.14309/ctg.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de-Madaria E, Capurso G. COVID-19 and acute pancreatitis: examining the causality. Nat Rev Gastroenterol Hepatol. 2021;18:3–4. doi: 10.1038/s41575-020-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmed A, Fisher JC, Pochapin MB, Freedman SD, Kothari DJ, Shah PC, Sheth SG. Hyperlipasemia in absence of acute pancreatitis is associated with elevated D-dimer and adverse outcomes in COVID 19 disease. Pancreatology. 2021;21:698–703. doi: 10.1016/j.pan.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 55.Pericleous S, Bhogal RH. Coronavirus disease 2019 pandemic: potential collateral damage on patients with operable pancreatic cancer. Pancreas. 2020;49:e61–e62. doi: 10.1097/MPA.0000000000001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moslim MA, Hall MJ, Meyer JE, Reddy SS. Pancreatic cancer in the era of COVID-19 pandemic: which one is the lesser of two evils? World J Clin Oncol. 2021;12:54–60. doi: 10.5306/wjco.v12.i2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lukács G, Kovács Á, Csanádi M, Moizs M, Repa I, Kaló Z, Vokó Z, Pitter JG. Benefits of timely care in pancreatic cancer: a systematic review to navigate through the contradictory evidence. Cancer Manag Res. 2019;11:9849–9861. doi: 10.2147/CMAR.S221427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanjeevi S, Ivanics T, Lundell L, Kartalis N, Andrén-Sandberg Å, Blomberg J, Del Chiaro M, Ansorge C. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br J Surg. 2016;103:267–275. doi: 10.1002/bjs.10046. [DOI] [PubMed] [Google Scholar]
- 59.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172:756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katopodis P, Anikin V, Randeva HS, Spandidos DA, Chatha K, Kyrou I, Karteris E. Pan-cancer analysis of transmembrane protease serine 2 and cathepsin L that mediate cellular SARS-CoV-2 infection leading to COVID-19. Int J Oncol. 2020;57:533–539. doi: 10.3892/ijo.2020.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai K, Kitamura K, Hirai Y, Nutahara D, Nakamura H, Taira J, Matsue Y, Abe M, Kikuchi M, Itoi T. Successful and safe reinstitution of chemotherapy for pancreatic cancer after COVID-19. Intern Med. 2021;60:231–234. doi: 10.2169/internalmedicine.6294-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bacalbasa N, Diaconu C, Savu C, Savu C, Stiru O, Balescu I. The impact of COVID-19 infection on the postoperative outcomes in pancreatic cancer patients. In Vivo. 2021;35:1307–1311. doi: 10.21873/invivo.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and gender-based differences in COVID-19. Front Public Health. 2020;8:418. doi: 10.3389/fpubh.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juhász MF, Ocskay K, Kiss S, Hegyi P, Párniczky A. Insufficient etiological workup of COVID-19-associated acute pancreatitis: a systematic review. World J Gastroenterol. 2020;26:6270–6278. doi: 10.3748/wjg.v26.i40.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]


