Abstract
Objective: This study investigated and analyzed the pain degree after dental implantation and its influencing factors, and provided a scientific basis for reducing post-surgical pain in patients. Methods: A total of 137 patients who underwent dental implantation between June 2018 to December 2019 were selected as the research subjects. Their pain intensity immediately after surgery, 24 h after surgery, and 72 h after surgery were evaluated respectively by a numerical rating scale (NRS), and the factors that affected the postoperative pain were analyzed by univariate and multivariate logistic regression analysis. Results: The pain intensity of patients at 24 h after dental implantation was more serious than immediately after operation and 72 h after operation (P<0.05). The results of univariate and multivariate logistic regression analysis showed that the duration of surgery and whether analgesic drug was taken postoperatively were used in the regression model (P<0.05), which are independent risk factors for the occurrence of pain 24 h after surgery. Conclusion: The pain degree of most patients after oral implantation is mild, and the most obvious pain reaction is 24 h after operation. The use of postoperative analgesics can effectively relieve the pain of patients, and the long duration of surgery is one of the key factors leading to postoperative pain.
Keywords: Dental implants, postoperative pain, investigation, influencing factors
Introduction
Dentition defect is a common and frequent disease in oral prosthodontics. It may lead to a decrease in masticatory efficiency, affect word pronunciation and the aesthetics, and even cause stomatognathic system issues and overall health issues that result in the decrease of people’s living quality [1,2]. In recent years, with the gradual improvement of dental implantation treatment, it has been chosen by an increasing number of dentists and patients, and has become a conventional treatment in dentition defects [3,4]. However, as implant restoration is a surgical operation, the postoperative pain response has become the main factor for patients to feel anxious and afraid [5,6]. There are currently limited studies on postoperative pain after dental implantation, and people have insufficient understanding of the regularity of postoperative pain, with lots of patients and doctors considering postoperative pain as an inevitable natural phenomenon which can only be endured. The purpose of this study is to investigate and analyze the incidence of postoperative pain in patients with dental implants, explore the possible causes, and provide a basis for reducing postoperative pain response and formulating corresponding pain management plans. The report is as follows.
Materials and methods
Research subjects
A total of 137 patients, who underwent dental implantation in Sanya Central Hospital between June 2018 to December 2019, were selected as research subjects. This research was approved by our hospital ethics committee.
The inclusive and exclusive criteria
Inclusion criteria: (1) Patients’ ≥8 years old; (2) Patients in good general condition and are tolerable to the operation, and had not experienced poorly controlled hypertension, diabetes or heart diseases; (3) Patients with normal spirit and cognition, and are able to understand and cooperate with the investigation and research; (4) Patients have mobile phones and can answer calls in time; and (5) Patients that voluntarily sign the informed consent forms.
Exclusion criteria: (1) Females during pregnancy; (2) Patients with progressive periodontitis; (3) Patients who had taken anti-anxiety, antidepressant, or antipsychotic drugs within 6 months; (4) Patients who diagnosed by clinical and CT examinations with deficiencies of soft and hard oral tissues, and were required to conduct the incremental operations for soft-tissue or bone-tissue ; or (5) Patients who have experienced oral or maxillofacial neuropathic pain within the past 6 months.
Perioperative treatment plan
We conducted detailed inquiries about the patients’ conditions, understood their health status and excluded surgical contraindications before surgery. After surgery, the patients decided whether to take analgesics on their own terms. For voluntary users, they were treated with ibuprofen 400 mg/time, 12 h/time, with a total of two doses. The surgery was performed under local anesthesia, and the surgical incision was designed as a linear incision through the crest of the alveolar ridge in edentulous area. After the patient’s mucoperiosteal flap was opened, we used a ball drill to trim the alveolar ridge, a dental drill for positioning, and a reaming drill to prepare holes step by step. Placed the implant, screwed in the covering screw, and performed intermittent sutures with ordinary sutures.
The patients took oral ornidazole 500 mg/time, 2 times/d; oral cephalosporin 150 mg/time, 2 times/d; and gargled with chlorhexidine gargle 3 times/d for 7 days. The patients were instructed to maintain good oral hygiene after surgery.
Postoperative pain and data collection
The patients were instructed to assess their pain degree immediately after surgery, 24 hours and 72 hours after surgery by a numerical rating scale (NRS). A score of 0 represented no pain, and 10 was severe pain. In particular, 1-3 points corresponded to mild pain, 4-6 points pointed to moderate pain and 7-10 points was severe pain.
We collected and recorded the patients’ information, including gender, age, education degree, smoking history, whether analgesics was taken before surgery, implantation site, number of implants, whether patients experienced implant surgery, the length of surgery, and postoperative ice-compress, etc.
The Modified Dental Anxiety Scale (MDAS) was used to assess the patient’s anxiety degree prior to surgery [7]. The scale consisted of 4 items, and each item included 5 options from light to heavy. The lowest score was 4 points and the highest was 20 points. MDAS ≥11 points revealed the patient had dental anxiety, and a higher score indicated a more obvious level of anxiety.
Statistical analysis
Data processing and analysis were conducted by statistical application SPSS 23.0. The comparison of measurement data was by t-test, and the comparison of enumeration data was by chi-squared test, with P<0.05 considered as statistically significant difference.
Results
Clinical data
The clinical data of the 137 patients selected in this research are shown in Table 1. There are 76 males (55.47%) and 61 females (44.53%); 73 cases (53.28%) aged ≤50 years, and 64 cases (46.72%) with >50 years old. There were 36 patients who (26.28%) had smoking history; 81 cases (59.12%) with a high school education degree and below, and 56 cases (40.88%) with a college degree or above.
Table 1.
The clinical data of the selected 137 research subjects
| Clinical data | Number of cases | The percentage (%) |
|---|---|---|
| Gender | ||
| Male | 76 | 55.47 |
| Female | 61 | 44.53 |
| Age (years old) | ||
| ≤50 | 73 | 53.28 |
| >50 | 64 | 46.72 |
| Smoking history | ||
| YES | 36 | 26.28 |
| NO | 101 | 73.72 |
| Education degree | ||
| Senior high school or below | 81 | 59.12 |
| College degree or above | 56 | 40.88 |
The pain degree at different time points during post-surgery
The average NRS scores of patients immediately after surgery were (0.57±0.18), of which 81.75% had no pain, 12.41% had mild pain, 4.24% had moderate pain, and 0.73% had severe pain. The score of patients at 24 hours postoperatively was (2.16±0.37) points, of which 49.64% had no pain, 35.77% had mild pain, 11.68% had moderate pain, and 2.92% had severe pain. The score of the patients 72 hours after the operation was (0.37±0.20) points, of which 63.50% had no pain, 25.55% had mild pain, 9.49% had moderate pain, and the remaining 1.46% had severe pain. The pain intensity of patients at 24 h after dental implantation was more serious than that immediately after operation and 72 h after operation (P<0.05), and the pain degree at 24 h postoperatively was selected for further analysis (presented in Table 2 and Figure 1 below).
Table 2.
The pain degree distribution of patients at different time points after operation
| Pain degree | Immediately after surgery | 24 h after surgery | 72 h after surgery |
|---|---|---|---|
| Average NRS score (points, x̅±s) | 0.57±0.18* | 2.16±0.37 | 0.37±0.10* |
| Without pain (n, %) | 112 (81.75) | 68 (49.64) | 116 (84.67) |
| Mild pain (n, %) | 17 (12.41) | 49 (35.77) | 21 (15.33) |
| Moderate pain (n, %) | 7 (4.24) | 16 (11.68) | 0 (0.00) |
| Severe pain (n, %) | 1 (0.73) | 4 (2.92) | 0 (0.00) |
P<0.05 indicated the comparison with 24 h after operation.
Figure 1.
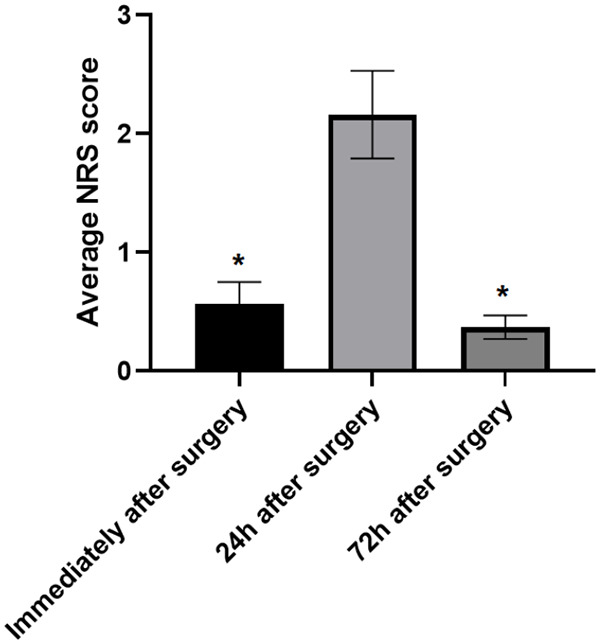
Changes of NRS scores at different time points after operation. Note: *P<0.05 indicated the comparison with 24 h after operation.
Univariate analysis
The results of univariate analysis showed that the factors of whether the patient took analgesics, the number of implants, and the duration of the surgery were connected with the pain of the patient 24 hours postoperatively (P<0.05), while there was insignificant difference of 24 h postoperative pain in different genders, ages, education degree, smoking history, implantation site, experience of implant surgery, postoperative ice-compress or preoperative anxiety (P>0.05), as shown in Table 3.
Table 3.
The univariate analysis of postoperative pain
| Related factors | Case | NRS score 24 h after operation | t | P |
|---|---|---|---|---|
| Gender | ||||
| male | 76 | 2.11±0.42 | 1.781 | 0.077 |
| female | 61 | 2.25±0.50 | ||
| Age | ||||
| ≤50 | 73 | 2.08±0.69 | 1.765 | 0.080 |
| >50 | 64 | 2.29±0.70 | ||
| Degree of education | ||||
| High school and below | 81 | 2.35±0.87 | 1.685 | 0.094 |
| College or above | 56 | 2.11±0.74 | ||
| Smoking history | ||||
| Yes | 36 | 2.22±0.39 | 1.539 | 0.126 |
| No | 101 | 2.09±0.45 | ||
| Number of implants | ||||
| single | 110 | 1.78±0.49 | 7.229 | 0.000 |
| Multiple | 27 | 2.55±0.52 | ||
| Take painkillers after operation | ||||
| Yes | 89 | 1.47±0.30 | 19.564 | 0.000 |
| No | 48 | 2.76±0.47 | ||
| Operation time | ||||
| <1 h | 95 | 1.47±0.43 | 15.160 | 0.000 |
| ≥1 h | 42 | 2.60±0.33 | ||
| Experience of implant surgery | ||||
| Yes | 25 | 2.05±0.66 | 1.759 | 0.081 |
| No | 112 | 2.26±0.51 | ||
| Postoperative ice compress | ||||
| Yes | 103 | 2.10±0.52 | 1.509 | 0.134 |
| No | 34 | 2.26±0.47 | ||
| Preoperative anxiety | ||||
| Yes | 39 | 2.29±0.55 | 1.678 | 0.096 |
| No | 98 | 2.08±0.70 |
Multivariate logistic analysis
A further multivariate logistic analysis was performed on the statistically significant factors in the above-mentioned univariate analysis. The results showed that the factors of duration of surgery and whether analgesic drugs were taken postoperatively were revealed from the regression model (P<0.05), and are the independent risk factors that affecting the occurrence of postoperative pain 24 h after surgery (Table 4).
Table 4.
Multivariate Logistic analysis of postoperative pain
| Important factor | b | S. E | χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Taking painkillers | -1.066 | 0.318 | 11.237 | 0.001 | 0.344 | 0.185~0.642 |
| Operation time | 1.039 | 0.475 | 4.785 | 0.029 | 2.826 | 1.114~7.171 |
Discussion
In recent years, along with the continual development and progress of dental implant techniques, an increasing number of oral physicians and patients choose implant restoration as the first choice for treating the dentition defects [8,9]. The pain response after dental implantation has gradually gained the attention of people. The postoperative pain response is caused by multiple factors, including sensory and emotional experience, and the subjective pain expectations of patients corresponding to dental operations have imposed a huge impact on pain perception [9,10]. Therefore, the analysis of pain degree after dental implantation enables patients to be aware of true pain expectations after implantation, which is conducive to effective nurse-patient communication and to reduce the anxiety of patients and acquire acceptance of them [11-13]. At present, only a few studies have analyzed the pain experience related to implantation surgery, and the research on the influencing factors of postoperative pain in patients is relatively rare. This study investigated and analyzed the pain and the influencing factors in patients after dental implantation, and provided a scientific basis for reducing post-surgical pain of patients.
We analyzed the pain degree of patients immediately after surgery, 24 hours and 72 hours after surgery respectively by NRS. The results showed that the patients’ immediate NRS score was (0.57±0.18) points, the 24 h postoperative NRS score was (2.16±0.37) points, and the postoperative 72 h NRS score was (1.37±0.20) points; the postoperative pain degree after 24 h of surgery was more intensive than immediately after surgery and 72 h after surgery (P<0.05). Most patients were without pain or only mild pain, while a small number of patients had moderate or severe pain after surgery. The postoperative pain reached a peak at 24 h, then gradually decreased and basically disappeared at 72 h, which is consist with the results by other scholars [14,15]. There are studies showing [16,17] that the postoperative pain reaches a high peak at 12 h after surgery. However, considering that the patients resting after surgery, it is difficult to inquire about the pain at that time, this study set the pain investigation point at 24 h after surgery. The postoperative pain of the patients in this study was lighter than which was reported in the literature. This may be because the subjects included in this study were all undergoing conventional implantation without bone augmentation, the duration of pain response was short and the pain degree was low. For surgical operations including soft-tissue or bone-tissue augmentation with larger area of the surgical wound and more obvious postoperative tissue tension, the tissue healing time was longer and the pain more obvious [18-20].
Pain has long been simply regarded as a body feeling. Currently, with further studies, people have gradually realized that postoperative pain is not only an objective sensation caused by tissue damage, but also an unpleasant emotional experience that is influenced by multiple factors [21-23]. The results of univariate and multivariate logistic regression analysis showed that the duration of surgery and whether analgesic drugs were taken postoperatively came out of the regression model (P<0.05), which are the independent risk factors of the occurrence of pain 24 h postoperatively. The results, that the longer a patient has to undergo surgery, the higher their risk of experiencing pain after surgery, was consisted with other scholars [24,25]. This is because the long operation time leads to an increased risk of postoperative infection and more obvious postoperative inflammation, which leads to an increase in pain. Therefore, the medical staff should inform the patients of the time to be taken for the operation. For cases that are complicated or with longer operation time, adequate communication should be conducted with patients to guide them with postoperative pain management. In this study, we chose whether to give patients ibuprofen capsules orally before operation according to their own wishes. Ibuprofen is a non-steroidal drug with effects of anti-inflammatory, analgesia and fever relieve. The drug can inhibit the activity of cyclooxygenase (COX), reduce the synthesis of prostaglandin (PG), and also be widely used to reduce the postoperative pain response of patients with dental diseases [26,27]. The ibuprofen sustained-release capsule used in this study lasted 12 hours and were taken twice. Therefore, for patients with dental implants, were given 2 doses of sustained-release ibuprofen capsules to effectively alleviate their pain 24 h after surgery [28]. However, although the side effects of ibuprofen are limited, practitioners still need to pay attention to it, and the patient should be notified of the possible symptoms that may occur.
Above all, the pain degree of most patients after oral implantation is mild, and the most obvious pain reaction is 24 h after operation. The use of postoperative analgesics postoperatively can effectively relieve the pain of patients, and the long duration of surgery is one of the key factors leading to postoperative pain.
Disclosure of conflict of interest
None.
References
- 1.Heinrich D, Bruland Ø, Guise TA, Suzuki H, Sartor O. Alkaline phosphatase in metastatic castration-resistant prostate cancer: reassessment of an older biomarker. Future Oncol. 2018;14:2543–2556. doi: 10.2217/fon-2018-0087. [DOI] [PubMed] [Google Scholar]
- 2.Pabis A, Kamerlin SC. Promiscuity and electrostatic flexibility in the alkaline phosphatase superfamily. Curr Opin Struct Biol. 2016;37:14–21. doi: 10.1016/j.sbi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Siller AF, Whyte MP. Alkaline phosphatase: discovery and naming of our favorite enzyme. J Bone Miner Res. 2018;33:362–364. doi: 10.1002/jbmr.3225. [DOI] [PubMed] [Google Scholar]
- 4.Kang W, Wang ZH, Liu L, Guo X. Alkaline phosphatase activity in the phosphorus-limited southern Chinese coastal waters. J Environ Sci (China) 2019;86:38–49. doi: 10.1016/j.jes.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Mori K, Janisch F, Parizi MK, Mostafaei H, Lysenko I, Enikeev DV, Kimura S, Egawa S, Shariat SF. Prognostic value of alkaline phosphatase in hormone-sensitive prostate cancer: a systematic review and meta-analysis. Int J Clin Oncol. 2020;25:247–257. doi: 10.1007/s10147-019-01578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brichacek AL, Brown CM. Alkaline phosphatase: a potential biomarker for stroke and implications for treatment. Metab Brain Dis. 2019;34:3–19. doi: 10.1007/s11011-018-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danikowski KM, Cheng T. Alkaline phosphatase activity of staphylococcus aureus grown in biofilm and suspension cultures. Curr Microbiol. 2018;75:1226–1230. doi: 10.1007/s00284-018-1514-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Rader E, Peters-Carr M, Bent RC, Smilowitz JT, Guillemin K, Rader B. Ontogeny of alkaline phosphatase activity in infant intestines and breast milk. BMC Pediatr. 2019;19:2. doi: 10.1186/s12887-018-1379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haji SM, Chipchase A, Fraser WD, Gomez J. Retrospective evaluation of a local protocol used to enhance laboratory savings through minimizing the performance of alkaline phosphatase isoenzyme analysis. Ann Clin Biochem. 2019;56:298–301. doi: 10.1177/0004563218817571. [DOI] [PubMed] [Google Scholar]
- 10.Fodor A, Kenesei É, Szabó JA. Differencial diagnosis of the low alkaline phosphatase activities. Orv Hetil. 2017;158:1003–1007. doi: 10.1556/650.2017.30785. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi ML, Vai S. Alkaline phosphatase replacement therapy. Adv Exp Med Biol. 2019;1148:201–232. doi: 10.1007/978-981-13-7709-9_10. [DOI] [PubMed] [Google Scholar]
- 12.Mei Y, Hu Q, Zhou B, Zhang Y, He M, Xu T, Li F, Kong J. Fluorescence quenching based alkaline phosphatase activity detection. Talanta. 2018;176:52–58. doi: 10.1016/j.talanta.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 13.Hai Z, Li J, Wu J, Xu J, Liang G. Alkaline phosphatase-triggered simultaneous hydrogelation and chemiluminescence. J Am Chem Soc. 2017;139:1041–1044. doi: 10.1021/jacs.6b11041. [DOI] [PubMed] [Google Scholar]
- 14.Nizet A, Cavalier E, Stenvinkel P, Haarhaus M, Magnusson P. Bone alkaline phosphatase: an important biomarker in chronic kidney disease - mineral and bone disorder. Clin Chim Acta. 2020;501:198–206. doi: 10.1016/j.cca.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Zierk J, Arzideh F, Haeckel R, Cario H, Frühwald MC, Groß HJ, Gscheidmeier T, Hoffmann R, Krebs A, Lichtinghagen R, Neumann M, Ruf HG, Steigerwald U, Streichert T, Rascher W, Metzler M, Rauh M. Pediatric reference intervals for alkaline phosphatase. Clin Chem Lab Med. 2017;55:102–110. doi: 10.1515/cclm-2016-0318. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Xu W, Maddera L, Tsuchiya D, Thomas N, Yu CR, Parmely T. Alkaline phosphatase-based chromogenic and fluorescence detection method for BaseScope In Situ hybridization. J Histotechnol. 2019;42:193–201. doi: 10.1080/01478885.2019.1620906. [DOI] [PubMed] [Google Scholar]
- 17.Davidson JA, Urban T, Tong S, Twite M, Woodruff A, Wischmeyer PE, Klawitter J. Alkaline phosphatase, soluble extracellular adenine nucleotides, and adenosine production after infant cardiopulmonary bypass. PLoS One. 2016;11:e0158981. doi: 10.1371/journal.pone.0158981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girault M, Beneyton T, Pekin D, Buisson L, Bichon S, Charbonnier C, Del Amo Y, Baret JC. High-content screening of plankton alkaline phosphatase activity in microfluidics. Anal Chem. 2018;90:4174–4181. doi: 10.1021/acs.analchem.8b00234. [DOI] [PubMed] [Google Scholar]
- 19.Pickkers P, Mehta RL, Murray PT, Joannidis M, Molitoris BA, Kellum JA, Bachler M, Hoste EAJ, Hoiting O, Krell K, Ostermann M, Rozendaal W, Valkonen M, Brealey D, Beishuizen A, Meziani F, Murugan R, de Geus H, Payen D, van den Berg E, Arend J STOP-AKI Investigators. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320:1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo S, Han J, Lee YL, Yoon S, Lee J, Wang SE, Kim TH. Regulation of osteoblasts by alkaline phosphatase in ankylosing spondylitis. Int J Rheum Dis. 2019;22:252–261. doi: 10.1111/1756-185X.13419. [DOI] [PubMed] [Google Scholar]
- 21.Azpiazu D, Gonzalo S, Villa-Bellosta R. Tissue non-specific alkaline phosphatase and vascular calcification: a potential therapeutic target. Curr Cardiol Rev. 2019;15:91–95. doi: 10.2174/1573403X14666181031141226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balbaied T, Moore E. Overview of optical and electrochemical alkaline phosphatase (ALP) biosensors: recent approaches in cells culture techniques. Biosensors (Basel) 2019;9:102. doi: 10.3390/bios9030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuehn F, Adiliaghdam F, Hamarneh SR, Vasan R, Liu E, Liu Y, Ramirez JM, Hoda RS, Munoz AR, Ko FC, Armanini M, Brooks DJ, Bouxsein ML, Demay MB, Hodin RA. Loss of intestinal alkaline phosphatase leads to distinct chronic changes in bone phenotype. J Surg Res. 2018;232:325–331. doi: 10.1016/j.jss.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 24.Grote-Koska D, Klauke R, Brand K, Schumann G. Alkaline phosphatase activity - pH impact on the measurement result. Clin Chem Lab Med. 2017;55:e146–e149. doi: 10.1515/cclm-2016-0771. [DOI] [PubMed] [Google Scholar]
- 25.Brady JJ, McGoldrick D, O’Callaghan K, McNamara F, Mulready KJ, Cullen MR, Denieffe S, Fitzgibbon M. Bone alkaline phosphatase on the IDS-iSYS automated analyser; cross-reactivity with intestinal ALP. Clin Chem Lab Med. 2019;57:e186–e188. doi: 10.1515/cclm-2018-0991. [DOI] [PubMed] [Google Scholar]
- 26.Channar PA, Shah SJ, Hassan S, Nisa ZU, Lecka J, Sévigny J, Bajorath J, Saeed A, Iqbal J. Isonicotinohydrazones as inhibitors of alkaline phosphatase and ecto-5’-nucleotidase. Chem Biol Drug Des. 2017;89:365–370. doi: 10.1111/cbdd.12861. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Zhao J, Bao X, Wang Q, Yang X. Alkaline phosphatase assay based on the chromogenic interaction of diethanolamine with 4-aminophenol. Anal Chem. 2018;90:6339–6345. doi: 10.1021/acs.analchem.8b01371. [DOI] [PubMed] [Google Scholar]
- 28.Lallès JP. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr Rev. 2019;77:710–724. doi: 10.1093/nutrit/nuz015. [DOI] [PubMed] [Google Scholar]


