Abstract
Objective: To determine the effect of decompressive craniectomy (DC) on the recovery of neurological function, daily living ability and life quality of patients with intracerebral hemorrhage (ICH) after surgery. Methods: Totally 290 patients with ICH admitted to our hospital from January 2018 to June 2020 were retrospectively enrolled and assigned to two groups according to different surgical methods. Among them, 138 patients who received craniotomy evacuation of hematoma (CEH) only were assigned to a control group (Con group), while the other 152 who received CEH combined with DC to a research group (Res group). The two groups were compared in the total effective rate, hematoma clearance rate, and complication rate. Additionally, the ICP and MMP-9 levels after surgery, National Institutes of Health Stroke Scale (NIHSS), activities of daily living (ADL), Fugl-Meyer Assessment of motor function (FMA), Glasgow outcome scale (GOS), Glasgow coma scale (GCS), and MOS 36-Item Short-Form Health Survey (SF-36) scores before and after surgery were also compared between the two groups. Results: After treatment, the Res group showed a notably higher total effective rate, hematoma clearance rate, and a notably lower complication rate than the Con group. On postoperative day 3 and 7, the Res group showed notably lower ICP than the Con group, and on postoperative day 7, the Res group showed a notably lower MMP-9 level as compared with the Con group. Additionally, 6 months after the surgery, the Res group got notably lower NIHSS scores and higher ADL, GOS, and SF-36 scores as compared with the Con group, and at 1 month after surgery, the Res group got notably higher FMA scores as compared to the Con group. Moreover, on postoperative day 7, the Res group got notably higher GCS scores than the Con group. Conclusion: DC can improve the recovery of neurological function, daily living ability and life quality of patients with ICH after surgery.
Keywords: Decompressive craniectomy, intracerebral hemorrhage, recovery of neurological function, daily living ability, life quality
Introduction
Intracerebral hemorrhage (ICH), also known as hemorrhagic stroke, refers to the non-traumatic parenchymal hemorrhage caused by the rupture of cerebral vessels, accounting for 20-30% of all strokes [1]. With a mortality rate as high as 25-50% in the acute phase, ICH belongs to diseases with high mortality, and only 20% of patients are able to fully take care of themselves at 6 months after onset. Currently, ICH has become the first cause of death [2]. It is commonly caused by hypertension complicated with arteriosclerosis, microaneurysms or microangiomas. Hypertensive intracerebral hemorrhage (HICH) is a common ICH [3], with incidence often related to hyperlipidemia, diabetes mellitus, hypertension, vascular aging, smoking, etc. [4]. Patients with ICH are often suddenly attacked by the disease due to emotional excitement and struggling force exertion. The early mortality of ICH is blindingly high, and most of the survivors suffer sequelae such as dyskinesia, cognitive impairment, and dysphagia [5]. Thus, the disease seriously endangers human health and life quality of patients and brings a heavy burden to society and families [6]. How to lower the mortality of ICH and improve the life quality of patients has always been a hot spot in clinical and medical research [7].
HICH will damage brain nerve cells once it occurs, and hematoma will cause compression on the surrounding brain tissues. Such a situation is likely to bring about secondary brain edema, eventually giving rise to increased intracranial pressure and even brain hernia, thus gravely threatening patients’ life safety [8]. The key to clinical treatment of HICH is to quickly relieve the occupying effect of hematoma and to repair the function of damaged nerve cells [9]. There are two treatments for HICH: surgical treatment based on operation and conservative treatment based on drugs [10]. Patients with mild HICH who have small hematoma and no obvious consciousness disorder are usually given conservative treatment based on drugs, while those with moderate or severe HICH who have larger hemorrhage amount, severe damage to body function, and cerebral hernia or have suffered cerebral hernia are given surgical treatment preferably [11]. Thanks to the continuous improvement in the medical practice level and surgical instruments, there are a growing number of surgical approaches for HICH, mainly including craniotomy evacuation of hematoma (CEH), hematoma puncture and aspiration, and neuroendoscopic evacuation of hematoma [12]. Early clearance of hematoma can improve the neurological function and reduce the secondary brain damage as soon as possible, thus improving the prognosis of patients [13]. However, according to a related study [14], relieving the occupying effect of hematoma does not necessarily guarantee satisfactory surgical results. Due to the existence of bone flap, secondary brain edema can give rise to a severe increase in intracranial pressure and result in cerebral hernia. So, for such patients, bone flap removal should be adopted for decompression. Decompressive craniectomy (DC) can help quickly and effectively reduce intracranial pressure, so it is usually adopted to reduce intracranial pressure in the neurosurgery department [15]. However, the application of DC in patients with ICH is rarely studied [16].
Therefore, this study applied DC in patients with ICH and explored its influence on the recovery of neurological function, daily living ability and life quality of such patients after surgery.
Materials and methods
General materials
A total of 290 patients with ICH admitted to our hospital between January 2018 and June 2020 were enrolled and assigned to two groups according to different surgical methods. Among them, 138 patients who received CEH only were assigned to a control group (Con group), while the other 152 who received CEH combined with DC to a research group (Res group). The Res group consisted of 88 males and 64 females between 45 and 70 years old (58.63±8.52 years on average), while the Con group consisted of 82 males and 56 females between 49 and 72 years old (60.18±9.06 years on average). This is a retrospective study, and the ethics approval number of this study is No202011089.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients meeting the diagnostic criteria of ICH [17]; (2) Patients suffering ICH for the first time; (3) Patients with GCS score ≥5 points; (4) This study was approved by the ethics committee of our hospital, and all participants and their families signed informed consent forms after being informed of the study.
Exclusion criteria: (1) Patients with severe comorbid primary organ diseases or end-stage malignant tumors; (2) Patients with primary cerebrovascular diseases; (3) Patients with coagulation dysfunction; (4) People with cognitive dysfunction, central nervous system diseases or severe peripheral nerve diseases; (5) Patients without complete information or dropped out of the study halfway.
Surgical methods
The Con group: Each patient was given conventional CEH. Specifically, the patient was anaesthetized generally, and then an arc or L-shaped incision in front of the tragus was cut on the patient in a supine position. Subsequently, the hematoma was located based on the results of preoperative imaging examination. According to the hematoma location, the part of skull nearest to the hematoma was marked, and the skull part was drilled with surgical instruments to expand the bone window to 3-5 cm. The dura mater was cut in a cross shape, and the hematoma was evaluated. The presence of hematoma was determined by puncture of the superior temporal gyrus. The cortex was cut by electrocoagulation. Then the brain tissue was separated by the auxiliary action of a brain retractor, and most of the hematoma was removed by an aspirator from the hematoma cavity, and washed repeatedly with normal saline. Afterwards, the bleeding was stopped by electrocoagulation and compression. After it was confirmed that there was no bleeding, an intracranial drainage tube was routinely retained, and the dura mater was sutured, followed by closure of bone flap.
The Res group: Each patient was given DC additionally based on the surgery to the Con group. Specifically, during conventional CEH, a small incision was made in the scalp with the smallest distance from the center of hematoma, and the brain tissue was separated to the hematoma cavity. A drainage tube was retained after hematoma removal, and the skull was drilled down according to the specific condition of the patient. Subsequently, the bone window was expanded to the upper edge of zygomatic arch, and the dura mater was sutured under reduced tension. According to the actual cerebral edema, the bone flap was removed, and the incision was closed step by step after full decompression.
Outcome measures
(1) Total effective rate: The total effective rate was evaluated according to the National Institutes of Health Stroke Scale (NIHSS) score at postoperative 6 months, and the evaluation criteria were as follows: Markedly effective: decrease in the NIHSS score after surgery ≥ 75%; Effective: decrease in the NIHSS score after surgery by 50-75%; Ineffective: decrease in the NIHSS score after surgery < 50%. Total effective rate (%) = (The number of markedly effectively treated patients + the number of effectively treated patients)/the total number of patients ×100%.
(2) Hematoma clearance rate and complication rate: Complications included cerebral hernia, infection, and secondary ICH.
(3) Postoperative ICP: The ICP of each patient on postoperative day 3 and 7 was recorded.
(4) MMP-9 level: Fasting venous blood (5 mL) was sampled from each patient in the morning before surgery and on the 7th day after surgery, and MMP-9 in the samples was quantified with an ELISA assay strictly according to the instructions of human MMP-9 ELISA kit (JK-ELISA-04337, Shanghai Jingkang Bioengineering Co., Ltd., Shanghai, China) [18].
(5) NIHSS score: The NIHSS was adopted to evaluate the neurological deficits of the two groups before surgery and at postoperative 6 months [19]. The total score of NIHSS is 45 points and a higher score indicates more serious neurological deficits.
(6) ADL score: The Barthel index was adopted to evaluate the activities of daily living (ADL) of the two groups before surgery and at postoperative 6 months [20]. The scale covered 10 items: defecating, peeing, grooming, toilet use, eating, moving, activity, dressing, going up and down stairs, and bathing. It has a full score of 100 points, with a score < 20 points for extremely serious functional defects and total dependence in terms of life, a score between 20 and 40 points for great requirement of help in terms of life, a score between 40 and 60 points for requirement of help in terms of life, and a score > 60 points for basic self-care ability. A higher score indicates stronger daily living ability.
(7) Fugl-Meyer Assessment of motor function (FMA) score: The FMA scale was adopted to evaluate patients at postoperative 1 month [21]. With a total score of 100 points, the scale covers upper limb function and lower limb function, and a higher score indicates better function.
(8) Glasgow coma scale (GCS) score: The GCS was used to evaluate the consciousness state of the two groups before surgery and on postoperative day 7 [22]. With a total score of 0-15 points, the scale indicates normal consciousness state by 15 points, mild coma by 12-14 points, moderate coma by 9-11 points and severe coma by a score less than 8 points. A higher score denotes better consciousness state.
(9) Prognostic Glasgow outcome scale (GOS) score: The GOS was adopted to evaluate the prognosis of the two groups at postoperative 6 months [23]. With a total score of 5 points, the scale indicates death by 1 point, persistent vegetative state by 2 points, severe disability and requirement of living care by 3 points, mild disability and ability of working under protection by 4 points, and good recovery and resumption of normal life by 5 points.
(10) MOS 36-Item Short-Form Health Survey (SF-36) score: The SF-36 was used to evaluate the life quality of the two groups at postoperative 6 months. It covers general health, physiological functioning, role physical, bodily pain, vitality, social function, role emotional and mental health [24]. Each item is scored by 0-100 points, and a higher score indicates better life quality.
Statistical analyses
SPSS24.0 (IBM Corp, Armonk, NY, USA) was adopted for statistical analyses and GraphPad Prism 7 for drawing of corresponding figures. Enumeration data, expressed as [n (%)], were compared between groups via the chi-square test. Those with theoretical frequency in the chi-square test less than 5 were analyzed via the continuity correction chi square test. Measurement data, expressed as mean ± standard deviation (x̅ ± sd), were compared between groups via the independent-samples t test, and within groups before and after treatment by the paired t test. P < 0.05 means that the difference is statistically significant.
Results
General materials
There was no significant difference between the two groups in general clinical baseline data such as sex, age, body mass index (BMI), hemorrhage amount, disease type, marriage, place of residence, educational background, smoking history, drinking history, and diabetes mellitus history (all P > 0.05) (Table 1).
Table 1.
Comparison of general data between the two groups [n (%)] (x̅ ± sd)
| Item | Research group (n=152) | Control group (n=138) | t/χ2 value | P-value |
|---|---|---|---|---|
| Sex | 0.069 | 0.792 | ||
| Male | 88 (57.89) | 82 (59.42) | ||
| Female | 64 (42.11) | 56 (40.58) | ||
| Age (Y) | 58.63±8.52 | 60.18±9.06 | 1.501 | 0.134 |
| BMI (kg/m2) | 22.86±3.54 | 23.07±3.25 | 0.524 | 0.600 |
| Hemorrhage amount (mL) | 60.63±20.31 | 62.89±21.76 | 0.914 | 0.361 |
| Disease type | 1.788 | 0.877 | ||
| Subarachnoid hemorrhage | 61 (40.13) | 50 (36.23) | ||
| Intracerebral hemorrhage | 26 (17.10) | 27 (19.57) | ||
| Subdural hematoma | 30 (19.74) | 26 (18.84) | ||
| Cerebral infarction | 2 (1.32) | 1 (0.72) | ||
| Traumatic intracranial hemorrhage | 17 (11.18) | 21 (15.22) | ||
| Rupture of aneurysm | 16 (10.53) | 13 (9.42) | ||
| Marital status | 0.013 | 0.907 | ||
| Married | 107 (70.39) | 98 (71.01) | ||
| Unmarried or widowed | 45 (29.61) | 40 (28.99) | ||
| Place of residence | 0.195 | 0.658 | ||
| Urban area | 92 (60.53) | 80 (57.97) | ||
| Rural area | 60 (39.47) | 58 (42.03) | ||
| Education background | 0.122 | 0.726 | ||
| ≥ senior high school | 63 (41.45) | 60 (43.48) | ||
| < senior high school | 89 (58.55) | 78 (56.52) | ||
| Smoking history | 1.192 | 0.274 | ||
| Yes | 48 (31.58) | 52 (37.68) | ||
| No | 104 (68.42) | 86 (62.32) | ||
| Drinking history | 0.007 | 0.928 | ||
| Yes | 79 (51.97) | 71 (51.45) | ||
| No | 73 (48.03) | 67 (48.55) | ||
| Diabetes mellitus history | 2.162 | 0.141 | ||
| Yes | 64 (42.11) | 70 (50.72) | ||
| No | 88 (57.89) | 68 (49.28) |
Comparison of total effective rates after therapy
After therapy, the Res group showed a notably higher total effective rate than the Con group (95.39% vs. 85.51%, P < 0.05) (Table 2).
Table 2.
Comparison of hematoma clearance rate and complication rate between the two groups after therapy [n (%)]
| Group | Cerebral hernia | Infection | Secondary intracerebral hemorrhage | Complication rate (%) | Hematoma clearance rate (%) |
|---|---|---|---|---|---|
| Research group (n=152) | 2 (1.32) | 5 (3.29) | 2 (1.32) | 9 (5.93) | 142 (93.42) |
| Control group (n=138) | 8 (5.80) | 14 (10.15) | 5 (3.62) | 27 (19.57) | 102 (73.91) |
| χ2 | - | - | - | 12.380 | 20.630 |
| P-value | - | - | - | < 0.001 | < 0.001 |
Hematoma clearance rate and complication rate
After therapy, the Res group showed a notably higher hematoma clearance rate as compared to the Con group (93.42% vs. 73.91%), and also showed a remarkably lower complication rate (5.93% vs. 19.57%, both P < 0.001) (Table 3).
Table 3.
Comparison of total effective rate between the two groups [n (%)]
| Group | Markedly effectively treated patients | Effectively treated patients | Ineffectively treated patients | Total effective rate (%) |
|---|---|---|---|---|
| Research group (n=152) | 102 (67.11) | 43 (28.29) | 7 (4.61) | 145 (95.39) |
| Control group (n=138) | 61 (44.21) | 57 (41.30) | 20 (14.49) | 118 (85.51) |
| χ2 | - | - | - | 8.375 |
| P-value | - | - | - | 0.003 |
Comparison of ICP after surgery
The Res group showed notably lower ICP than the Con group on postoperative day 3 and 7 (Figure 1).
Figure 1.
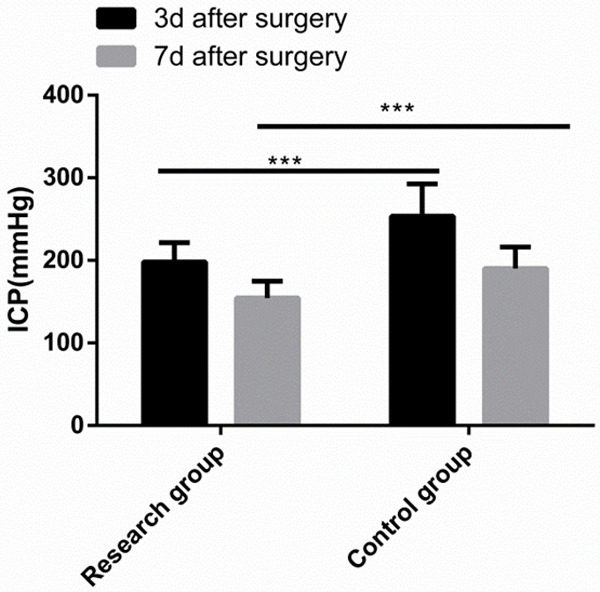
Comparison of ICP after surgery. The research group showed notably lower ICP than the control group at 3 and 7 days after surgery. Note: ***P < 0.001.
Comparison of MMP-9 level
Before surgery, there was no significant difference between the two groups in MMP-9 level (P > 0.05), while at postoperative 1 month, the MMP-9 level in both groups decreased greatly, and the MMP-9 level in the Res group was notably lower than that in the Con group (P < 0.05) (Figure 2).
Figure 2.
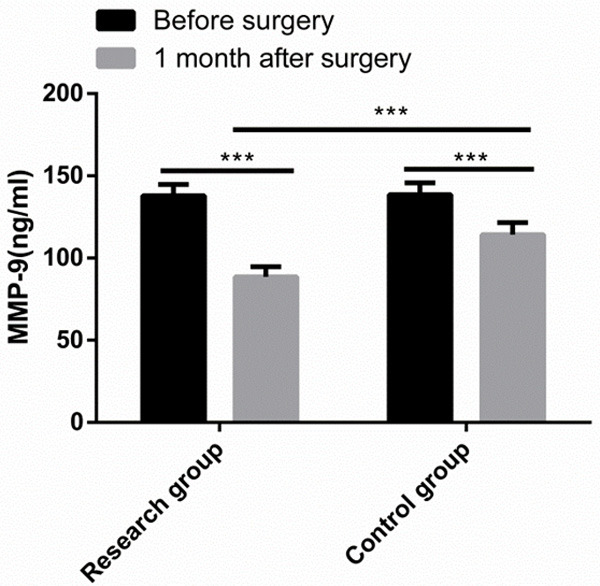
Comparison of MMP-9 level. One month after surgery, the MMP-9 level in both groups decreased greatly, and the MMP-9 level in the research group was notably lower than that in the control group. Note: ***P < 0.001.
Comparison of NIHSS and ADL scores
Before surgery, there were no significant differences between the two groups in NIHSS and ADL scores (both P > 0.05). At postoperative 6 months, the NIHSS scores of both groups decreased greatly, and the NIHSS score of the Res group was notably lower than that of the Con group (P < 0.05). In addition, at postoperative 6 months, the ADL score of both groups increased significantly, and the ADL score of the Res group was significantly higher than that of the Conn group (P < 0.05) (Figure 3).
Figure 3.
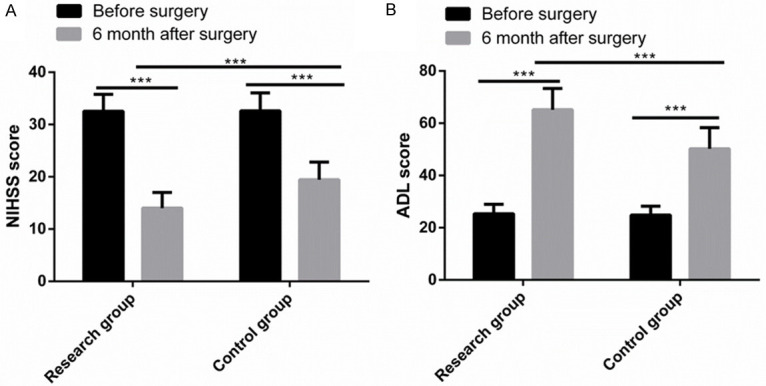
Comparison of NIHSS and ADL scores. A. Six months after surgery, the NIHSS score of both groups decreased greatly, and the NIHSS score of the research group was notably lower than that of the control group. B. Six months after surgery, the ADL score of both groups increased significantly, and the ADL score of the Res group was significantly higher than that of the control group. Note: ***P < 0.001.
Comparison of FMA score
Before surgery, there was no significant difference between the two groups in FMA score (P > 0.05), while 1 month after surgery, the FMA scores of both groups increased greatly, and the FMA score of the Res group was notably higher than that of the Con group (P < 0.05) (Figure 4).
Figure 4.
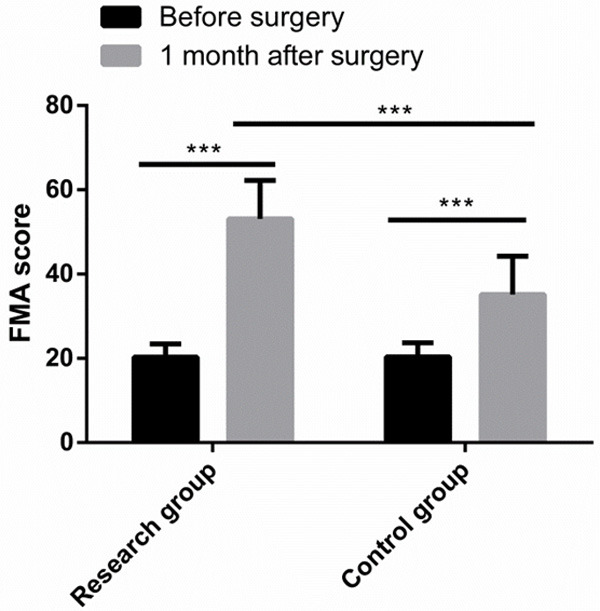
Comparison of FMA score. One month after surgery, the FMA scores of both groups increased greatly, and the FMA score of the research group was notably higher than that of the control group. Note: ***P < 0.001.
Comparison of GCS and GOS scores
Before surgery, there were no significant differences between the two groups in GCS and GOS scores (both P > 0.05). On postoperative day 7, the GCS scores of both groups increased greatly, and the GCS score of the Res group was notably higher than that of the Con group (P < 0.05). In addition, at postoperative 6 months, the GOS score of the Res group was also significantly higher than that of the Con group (P < 0.05) (Figure 5).
Figure 5.
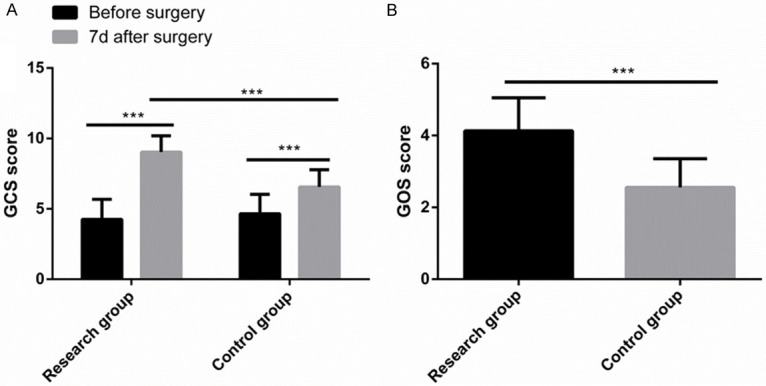
Comparison of GCS and GOS scores. A. Seven days after surgery, the GCS scores of both groups increased greatly, and the GCS score of the research group was notably higher than that of the control group. B. Six months after surgery, the GOS score of the research group was significantly higher than that of the control group. Note: ***P < 0.001.
Comparison of prognostic GOS score
At postoperative 6 months, the Res group had more patients with favorable prognosis as compared to the Con group (P < 0.001) (Table 4).
Table 4.
Comparison of prognostic GOS score between the two groups [n (%)]
| Group | 5 points | 4 points | 3 points | 2 points | 1 point |
|---|---|---|---|---|---|
| Research group (n=152) | 63 (41.45) | 52 (34.21) | 20 (13.16) | 12 (7.89) | 5 (3.29) |
| Control group (n=138) | 20 (14.49) | 15 (10.87) | 49 (35.51) | 36 (26.09) | 18 (13.04) |
| χ2 | - | - | - | - | 73.740 |
| P-value | - | - | - | - | < 0.001 |
Comparison of SF-36 score
At postoperative 6 months, the Res group got notably higher SF-36 scores of life quality than the Con group in general health, physiological functioning, role physical, bodily pain, vitality, social function, role emotional, and mental health (all P < 0.001) (Figure 6).
Figure 6.
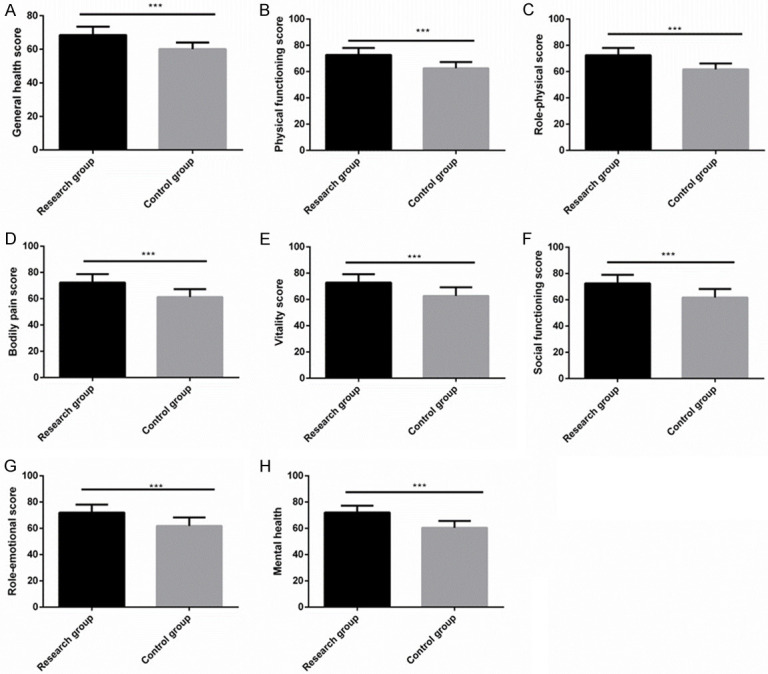
Comparison of SF-36 score. Six months after surgery, the general health (A), physiological functioning (B), role physical (C), bodily pain (D), vitality (E), social function (F), role emotional (G) and mental health (H) scores of the research group were significantly higher than those of the control group. Note: ***P < 0.001.
Discussion
As a cerebrovascular disease with mortality rate up to 50%, ICH is the most common critical disease of the nervous system, and patients are primarily manifested as nausea, headache, and lethargy [25]. As people’s lifestyle and eating habits change, the incidence of ICH is rising and is increasingly common in young people [26]. With high morbidity, disability and mortality, it severely impacts people’s life quality and safety [27]. Timely and effective treatment measures are the key to alleviating patients’ ICH [28]. At the present stage, surgical treatment remains the primary clinical therapy for ICH [29]. Thanks to the continuous updating of medical devices and development of evidence-based medicine, the conventional CEH is found to have certain drawbacks that bring about possible secondary complications [30]. Therefore, it is of profound clinical significance to find a novel safe and effective surgical method for ICH [31]. Our study applied both CEH and DC to patients with ICH and discussed the impact of this combined surgical scheme on the prognosis and life quality of such patients.
A study by Lo et al. [32] has revealed that for patients with spontaneous ICH, DC can significantly improve their survival rate, functional recovery and prognosis. Additionally, study by Yao et al. [33] has uncovered that for patients with ICH, DC can deliver greatly higher operative efficiency, reduce postoperative complications and mortality, and improve the patients’ functional recovery. In our study, patients additionally treated by DC showed a notably higher total effective treatment rate and hematoma clearance rate and a notably lower postoperative complication rate than the Con group. It may be due to the fact that the removal of bone flap reduces the intracranial pressure of patients and releases more space, thus exerting positive effects on the curative effect and complications of patients. The results are similar to those obtained by Lo et al. In our study, on postoperative day 3 and 7, the Res group showed notably lower intracranial pressure compared with the Con group, indicating that DC can strongly relieve the formation of postoperative intracranial hypertension and reduce the occurrence of secondary events. In another study, DC effectively removed hematoma and lowered the intracranial pressure of patients with ICH, which is similar to the results of ours. According to the study by Hou et al. [34], DC notably downregulated MMP-9 in rat models of ICH and thus promoted their recovery of cerebral microcirculation and neural function. Similar to the results obtained by Hou et al., our study revealed that MMP-9 level in the Res group was notably lower than that in the Con group. The results indicate that DC is able to more effectively reduce edema, promote the recovery of cerebral microcirculation and improve blood flow supply. In our study, the Res group got notably lower NIHSS score and higher ADL and FMA scores than the Con group, suggesting the effectiveness of DC in promoting the recovery of neurological function and improving daily living ability and limb movement ability. The situation may be explained by the fact that the decrease of intracranial pressure through skull removal protects patients from hematoma compression and injury. A study by Kamal et al. [35] has pointed out that the application of DC can accelerate the recovery of postoperative neurological function and motor ability of patients with cerebral infarction, which is in line with the results of our research. Moreover, Shahid et al. [36] have revealed the beneficial effect of DC on the recovery of neurological function and improvement of prognostic outcomes among patients with ICH. In our study, the Res group got remarkably higher GCS and GOS scores and had more patients with favorable prognosis than the Con group. The data imply that DC can significantly improve the prognostic outcome of patients, which is similar to research results obtained by Shahid et al. Finally, we evaluated the life quality of all patients at postoperative 6 months, and found that the Res group got notably higher SF-36 scores than the Con group. The results denote that DC can significantly improve the patients’ life quality after surgery.
Our study has verified the benefits of CEH combined with DC on patients and its innovation lies in that we have used the latest decompressive craniectomy for treatment and analyzed its influence on the neurological function and life quality of patients. However, this study still has some limitations. For instance, we have not conducted animal experiments for verification. In addition, it will be better to prolong the postoperative follow up time to determine the long-term effect of this surgical plan on patients, and we should also analyze the risk factors of postoperative recovery, so as to provide more favorable conditions for postoperative recovery of patients. In the future, we will carry out research from the above perspectives.
To sum up, CEH combined with DC can strongly improve the clinical efficacy in patients with ICH, reduce postoperative complications, and promote their recovery of neurological function, daily living ability and movement ability, thus improving their prognosis and life quality.
Acknowledgements
This project was supported by the Longitudinal Provincial and Ministerial-level Scientific Research Projects, National Ministries and Commissions General Scientific Research Projects 12.00 2020-01-01 2021-12-31.
Disclosure of conflict of interest
None.
References
- 1.Xin Y, Shi S, Yuan G, Miao Z, Liu Y, Gu Y. Application of CT imaging in the diagnosis of cerebral hemorrhage and cerebral infarction nerve damage. World Neurosurg. 2020;138:714–722. doi: 10.1016/j.wneu.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, Zhang D, Lv Z, Cui Y, Fei Y, Chang T, Yu M, Lu J, Huang Q, Zhang Y, Xu P, Lan T, Wang J. Optimal intervention time and risk of the activating blood and removing stasis method in acute cerebral hemorrhage patients: a randomized placebo-controlled trial. Medicine (Baltimore) 2021;100:e24214. doi: 10.1097/MD.0000000000024214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BJ, Kwon SU, Park JH, Kim YJ, Hong KS, Wong LKS, Yu S, Hwang YH, Lee JS, Lee J, Rha JH, Heo SH, Ahn SH, Seo WK, Park JM, Lee JH, Kwon JH, Sohn SI, Jung JM, Navarro JC, Kim HY, Kim EG, Kim S, Cha JK, Park MS, Nam HS, Kang DW PICASSO Investigators. Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO trial. Stroke. 2020;51:931–937. doi: 10.1161/STROKEAHA.119.023855. [DOI] [PubMed] [Google Scholar]
- 4.Yuan JH, Chen SH, Fu SS, Ma MY, Li SS, Shi RX, Zhang RY, Yang P, Wu SL, Li Y, Yin SF. Analysis on the gender-specific risk factors of new-onset cerebral hemorrhage. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:725–731. doi: 10.3760/cma.j.issn.0253-3758.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Foster LD, Lobanova I, Huang W, Suarez JI. Intensive blood pressure lowering in patients with moderate to severe grade acute cerebral hemorrhage: post hoc analysis of antihypertensive treatment of acute cerebral hemorrhage (ATACH)-2 trial. Cerebrovasc Dis. 2020;49:244–252. doi: 10.1159/000506358. [DOI] [PubMed] [Google Scholar]
- 6.Keep RF, Andjelkovic AV, Xiang J, Stamatovic SM, Antonetti DA, Hua Y, Xi G. Brain endothelial cell junctions after cerebral hemorrhage: changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab. 2018;38:1255–1275. doi: 10.1177/0271678X18774666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma X, Wang T, Wen J, Wang J, Zeng N, Zou W, Yang Y. Role of Xingnaojing injection in treating acute cerebral hemorrhage: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19648. doi: 10.1097/MD.0000000000019648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S, Du B, Shan A, Shi F, Wang J, Xie M. The risk factors for the postoperative pulmonary infection in patients with hypertensive cerebral hemorrhage: a retrospective analysis. Medicine (Baltimore) 2020;99:e23544. doi: 10.1097/MD.0000000000023544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao Y, Shen Z, Zhu H, Yu Z, Chen X, Lu H, Zhong F, Cheng H. Observation on therapeutic effect of stereotactic soft channel puncture and drainage on hypertensive cerebral hemorrhage. Ann Palliat Med. 2020;9:339–345. doi: 10.21037/apm.2020.03.12. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Chen Y, Wang Z, Qian M. Clinical research of early hyperbaric oxygen therapy on patients with hypertensive cerebral hemorrhage after craniotomy. Turk Neurosurg. 2020;30:361–365. doi: 10.5137/1019-5149.JTN.25044-18.3. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Shao G. Clinical effect of minimally invasive intracranial hematoma in treating hypertensive cerebral hemorrhage. Pak J Med Sci. 2016;32:677–681. doi: 10.12669/pjms.323.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G, Zhang J, Xu B. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy. J Neurosurg. 2018;128:553–559. doi: 10.3171/2016.10.JNS161589. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Yin F, Fu D, Gao X, Lv Z, Li X. Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. BMC Neurol. 2018;18:136. doi: 10.1186/s12883-018-1138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moussa WM, Khedr W. Decompressive craniectomy and expansive duraplasty with evacuation of hypertensive intracerebral hematoma, a randomized controlled trial. Neurosurg Rev. 2017;40:115–127. doi: 10.1007/s10143-016-0743-6. [DOI] [PubMed] [Google Scholar]
- 15.Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. 2019;12:CD003983. doi: 10.1002/14651858.CD003983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celi F, Saal-Zapata G. Decompressive craniectomy for traumatic brain injury: in-hospital mortality-associated factors. J Neurosci Rural Pract. 2020;11:601–608. doi: 10.1055/s-0040-1715998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marti-Fabregas J, Prats-Sanchez L, Martinez-Domeno A, Camps-Renom P, Marin R, Jimenez-Xarrie E, Fuentes B, Dorado L, Purroy F, Arias-Rivas S, Delgado-Mederos R. The H-ATOMIC criteria for the etiologic classification of patients with intracerebral hemorrhage. PLoS One. 2016;11:e0156992. doi: 10.1371/journal.pone.0156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015;110:2.1.1–2.1.23. doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- 19.Eskioglou E, Huchmandzadeh Millotte M, Amiguet M, Michel P. National institutes of health stroke scale zero strokes. Stroke. 2018;49:3057–3059. doi: 10.1161/STROKEAHA.118.022517. [DOI] [PubMed] [Google Scholar]
- 20.Mlinac ME, Feng MC. Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol. 2016;31:506–516. doi: 10.1093/arclin/acw049. [DOI] [PubMed] [Google Scholar]
- 21.Rech KD, Salazar AP, Marchese RR, Schifino G, Cimolin V, Pagnussat AS. Fugl-meyer assessment scores are related with kinematic measures in people with chronic hemiparesis after stroke. J Stroke Cerebrovasc Dis. 2020;29:104463. doi: 10.1016/j.jstrokecerebrovasdis.2019.104463. [DOI] [PubMed] [Google Scholar]
- 22.Enriquez CM, Chisholm KH, Madden LK, Larsen AD, de Longpre T, Stannard D. Glasgow coma scale: generating clinical standards. J Neurosci Nurs. 2019;51:142–146. doi: 10.1097/JNN.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 23.Yamal JM, Hannay HJ, Gopinath S, Aisiku IP, Benoit JS, Robertson CS. Glasgow outcome scale measures and impact on analysis and results of a randomized clinical trial of severe traumatic brain injury. J Neurotrauma. 2019;36:2484–2492. doi: 10.1089/neu.2018.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725. doi: 10.1177/2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA. 2019;321:1295–1303. doi: 10.1001/jama.2019.2413. [DOI] [PubMed] [Google Scholar]
- 26.Cusack TJ, Carhuapoma JR, Ziai WC. Update on the treatment of spontaneous intraparenchymal hemorrhage: medical and interventional management. Curr Treat Options Neurol. 2018;20:1. doi: 10.1007/s11940-018-0486-5. [DOI] [PubMed] [Google Scholar]
- 27.Weimar C, Kleine-Borgmann J. Epidemiology, prognosis and prevention of non-traumatic intracerebral hemorrhage. Curr Pharm Des. 2017;23:2193–2196. doi: 10.2174/1381612822666161027152234. [DOI] [PubMed] [Google Scholar]
- 28.Purrucker JC, Steiner T. Atypical intracerebral hemorrhage-etiology and acute management. Nervenarzt. 2019;90:423–441. doi: 10.1007/s00115-019-0695-5. [DOI] [PubMed] [Google Scholar]
- 29.Di Rienzo A, Colasanti R, Esposito D, Della Costanza M, Carrassi E, Capece M, Aiudi D, Iacoangeli M. Endoscope-assisted microsurgical evacuation versus external ventricular drainage for the treatment of cast intraventricular hemorrhage: results of a comparative series. Neurosurg Rev. 2020;43:695–708. doi: 10.1007/s10143-019-01110-7. [DOI] [PubMed] [Google Scholar]
- 30.Cai Q, Zhang H, Zhao D, Yang Z, Hu K, Wang L, Zhang W, Chen Z, Chen Q. Analysis of three surgical treatments for spontaneous supratentorial intracerebral hemorrhage. Medicine (Baltimore) 2017;96:e8435. doi: 10.1097/MD.0000000000008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu C, Wang N, Chen B, Wang P, Chen H, Liu W, Liu L. Surgical management of moderate basal ganglia intracerebral hemorrhage: comparison of safety and efficacy of endoscopic surgery, minimally invasive puncture and drainage, and craniotomy. World Neurosurg. 2019;122:e995–e1001. doi: 10.1016/j.wneu.2018.10.192. [DOI] [PubMed] [Google Scholar]
- 32.Lo YT, See AAQ, King NKK. Decompressive craniectomy in spontaneous intracerebral hemorrhage: a case-control study. World Neurosurg. 2017;103:815–820. e812. doi: 10.1016/j.wneu.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Yao Z, Ma L, You C, He M. Decompressive craniectomy for spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2018;110:121–128. doi: 10.1016/j.wneu.2017.10.167. [DOI] [PubMed] [Google Scholar]
- 34.Hou Z, Tian R, Han F, Hao S, Wu W, Mao X, Tao X, Lu T, Dong J, Zhen Y, Liu B. Decompressive craniectomy protects against hippocampal edema and behavioral deficits at an early stage of a moderately controlled cortical impact brain injury model in adult male rats. Behav Brain Res. 2018;345:1–8. doi: 10.1016/j.bbr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Kamal Alam B, Bukhari AS, Assad S, Muhammad Siddique P, Ghazanfar H, Niaz MJ, Kundi M, Shah S, Siddiqui M. Functional outcome after decompressive craniectomy in patients with dominant or non-dominant malignant middle cerebral infarcts. Cureus. 2017;9:e997. doi: 10.7759/cureus.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahid AH, Mohanty M, Singla N, Mittal BR, Gupta SK. The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J Neurosurg. 2018;128:229–235. doi: 10.3171/2016.10.JNS16678. [DOI] [PubMed] [Google Scholar]


