Abstract
Objectives: To explore the expression levels and the potential regulatory mechanism of miR-21-5p in LPS-treated H9c2 cells. Methods: The secretions of the inflammatory cytokines induced by LPS in H9c2 cells were evaluated using ELISA. We used RT-RCR and western blot to measure the relative mRNA and protein expression levels in LPS-treated H9c2 cells. CCK-8 and EdU assays showed the viability and proliferation profiles of the H9c2 cells. TUNEL assays demonstrated the apoptotic behaviors of the H9c2 cells, and a luciferase reporter analysis was used to investigate the interactions between miR-21-5p and programmed cell death protein 4 (PDCD4). Results: LPS induced damage to the H9c2 cells by reducing the cell viability and down-regulating miR-21-5p. On the other hand, miR-21-5p overexpression inhibited the LPS-induced inflammatory damage in the H9c2 cells. Moreover, PDCD4 was verified as a downstream target gene of miR-21-5p, and its expression was inhibited by the higher miR-21-5p content. Finally, miR-21-5p inhibited septic processes, and the PDCD4 overexpression rescued the miR-21-5p effect in the LPS-treated H9c2 cells. Conclusion: Our findings suggest that miR-21-5p inhibits the LPS-induced progression of sepsis in H9c2 cells. Additionally, PDCD4 is a downstream target gene of miR-21-5p, and both molecules serve as potential therapeutic targets for heart sepsis patients.
Keywords: miR-21-5p, programmed cell death protein 4, LPS, heart sepsis, H9c2 cells
Introduction
A syndrome of physiologic, pathological, and biochemical abnormalities, sepsis is induced by infection [1]. It is a major public health concern and a leading cause of death in hospitals worldwide [2,3]. Severe sepsis and septic shock lead to organ dysfunctions by elevating oxidative stress and exacerbating inflammation in organs like the brain, heart, kidneys, liver, and lungs [4]. It has been reported that the mortality of sepsis is related to cardiac dysfunction [5,6]. However, the specific regulatory mechanism with the progression of septic cardiac dysfunction remain elusive.
Lipopolysaccharide (LPS) can activate the excessive production of pro-inflammatory mediators, leading to myocardial damage, heart failure, and even death [7]. Since one initial feature of sepsis is acute inflammation, H9c2 cells have been used to mock how cardiomyocytes respond to such a situation by exposing them to LPS [8].
Non-coding RNAs that contain 21 to 24 nucleotides are defined as microRNAs (miRNAs). miRNAs regulate gene expression during translation. They have been studied as regulators in various physiological and inflammatory processes in human diseases [9]. Specifically, three miRNAs (miR-21-5p, miR-125-5p, and miR-18b) have been shown to act as major regulators in immune-inflammatory responses [10-12]. Furthermore, numerous studies have revealed miRNAs as modulators of sepsis-induced damage in inflamed organs [13-15]. Specifically, miR-21-5p suppresses inflammation and cell apoptosis in the injured brain microvascular endothelial barrier, as well as the progression of aged intracerebral hemorrhages [12,16]. In this study, we explored the role miR-21-5p plays in LPS-treated H9c2 cells.
Programmed cell death protein 4 (PDCD4) is a tumor suppressor that is normally down-regulated in various cancers [17,18]. Many studies have found that PDCD4 participates in many cellular functions, especially transcription, translation, and proliferation [19]. Furthermore, miR-21 was found to target the PDCD4 gene at the 3’-UTR region [20], and the inhibition of miR-21 blocks the LPS-induced down-regulation of PDCD4 in periodontitis, suggesting that miR-21 is vital in inflammatory responses and cancers by targeting PDCD4 [21]. Silencing miR-21-5p, a member of the miR-21 family, will improve paclitaxel (PTX) resistance and inhibit PTX-resistant processes in breast cancer cells partly by targeting PDCD4 [22]. Nevertheless, the underlying regulatory mechanisms between miR-21-5p and PDCD4 in septic H9c2 cells remain to be elucidated, which is precisely the topic of this article.
In this study, a model of LPS-induced sepsis in H9c2 cells was established. Subsequently, we measured the expression levels and clarified the regulatory mechanisms of miR-21-5p in septic H9c2. The results show that miR-21-5p promotes cell viability and inhibits apoptosis in LPS-treated H9c2 cells. Our study provides experimental evidence to clarify the molecular mechanisms of sepsis in H9c2 cells, as well as information about potential therapeutic targets against sepsis.
Materials and methods
Cell treatments and transfection
The cardiomyoblast culture medium (H9c2 cells; ATCC, Manassas, VA, USA) was DMEM (Gibco Laboratories, USA) with 10% FBS (Gibco Laboratories, USA) and the antibiotics 1% penicillin and streptomycin (Gibco Laboratories, USA), which were incubated at 5% CO2, 37°C.
The H9c2 cells were respectively co-cultured with a concentration (0.5, 1, 2 μg/mL) of LPS for 24 h (Sigma, Missouri, USA). Cells or cell supernatants were extracted and collected for the analysis.
The miR-21-5p mimics, the miR-21-5p mimic negative controls (NC), the miR-21-5p inhibitors, the miR-21-5p inhibitor negative controls (NC), the pcDNA3.1 vector, and the pcDNA3.1-programmed cell death 4 (PDCD4) were purchased from Shanghai GenePharma Co., Ltd. The cells were seeded in a 6-well plate with a density of 105 cells/ml and pre-incubated for 12 h at 37°C, before being transfected with 50 nM miR-21-5p mimics or miR-21-5p mimics NC, 2 µg pc DNA3.1 or pcDNA3.1-PDCD4, 50 nM miR-21-5p inhibitors or miR-21-5p inhibitors NC with 20 nmol/l Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) then incubated at 37°C with 5% CO2 for 48 h. The cells were harvested 48 h after the transfection for the subsequent experiments. The sequences of the miRNA constructs are shown in Table 1.
Table 1.
The sequences of the miRNA constructs
| miR-21-5p mimics NC | 5’-GUGUAACACGUCUAUACGCCCA-3’ |
| miR-21-5p mimics | 5’-UAGCUUAUCAGACUGAUGUUGA-3’ |
| miR-21-5p inhibitors NC | 5’-UCACAACCUCCUAGAAAGAGU-3’ |
| miR-21-5p inhibitors | 5’-UCAACAUCAGUCUGAUAAGCUA-3’ |
ELISA assay
The supernatants of the LPS-treated H9c2 cells were harvested and the IL-1β, TNF-α, and IL-6 releases were measured ELISA Kits (TaKaRa, Dalian, China).
CCK-8 cell viability assays
The cells were seeded into a 96-well plate and treated with LPS at 37°C and 5% CO2 for 24 h. The cell proliferation was measured CCK-8 Kits (Abcam, Cambridge, UK). The optical density values were measured at 450 nm using an ultraviolet spectrophotometer (Thermo Fisher Scientific, Inc.).
EdU assays
The cells were seeded into a 96-well plate and treated with LPS at 37°C and 5% CO2 for 24 h. The cell proliferation was measured using EdU assays (Solarbio, Beijing, China). The EdU was added for 2 h. After fixation, 1× Apollo was added to stain the cells for 0.5 h without light. Next, we DAPI stained the nuclei for 5 min. Finally, the cells were viewed under an inverted fluorescence microscope (Olympus IX51, Japan).
TUNEL assays
The apoptosis of the LPS-treated cells was assessed using a commercial Cell Death Detection kit (Roche, Germany). The labelled cells were viewed using a fluorescence microscope (Olympus IX51, Japan).
Western blotting
The protein extracted from the H9c2 cells was lysed in an ice-cold RIPA buffer plus PMSF on ice (Beyotime Institute of Biotechnology, Shanghai, China).
The primary antibodies were anti-Bax (Abcam, 1:1,000), anti-Bcl-2 (Abcam, 1:500), anti-pro-cleaved Caspase-3 (Proteintech, 1:500), anti-cleaved Caspase-3 (Proteintech, 1:500), anti-pro-cleaved Caspase-9 (Proteintech, 1:300), anti-cleaved Caspase-9 (Proteintech, 1:300), anti-β-actin (Abcam, 1:1,000), and anti-PDCD4 antibody (Proteintech, 1:1,000).
qRT-PCR assays
The RNA was isolated using TRIzol (Takara, Otsu, Japan) and reversed transcribed into cDNA using Superscript II reverse transcriptase (Thermo Fisher Scientific, Inc.). The conditions for the RT reaction were incubation at 25°C for 10 min, 50°C for 15 min, and heated to 85°C for 5 min to terminate the reaction. A real-time PCR machine (Bio-Rad) was used to conduct the quantitative qPCR (Table 2). The qRT-PCR was subsequently performed using SYBR Green I Super mix (Takara, Otsu, Japan) according to the manufacturer’s protocol. The thermocycling conditions for the RT-qPCR were 95°C for 10 min, followed by 35 cycles at 95°C and at 60°C for 1 min. The RT-qPCR results were analyzed using the 2-ΔΔCT method.
Table 2.
The primers for qPCR
| Rno-miR-21-5pforward | GACAAGCTTGCGGCCGCCCTTTAGGAGCATTATGAGCAT |
| Rno-miR-21-5p reverse | ATCCTCTAGAGTCGACGAAGGTCAAGTAACAGTCATAC |
| Rno-PDCD4 forward | TTGAGCACGGAGATACGAAC |
| Rno-PDCD4 reverse | GTCCCGCAAAGGTCAGAAAG |
| Rno-U6 forward | CTCGCTTCGGCAGCACACATA |
| Rno-U6 reverse | ACGCTTCACGAATTTGCGTGTC |
| Rno-β-actin forward | GTTGGAGCAAACATCCCCCA |
| Rno-β-actin reverse | CGCGACCATCCTCCTCTTAG |
Note: The 2-ΔΔCT relative quantification method was used to quantify the qPCR data. PDCD4: programmed cell death protein 4.
Statistical analysis
All the results are presented as the means ± sd from three independent experiments. The comparisons were determined using unpaired/paired Student’s t tests, and Bonferroni, Tukey, two-way ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
The miR-21-5p expressions in the LPS-treated H9c2 cells
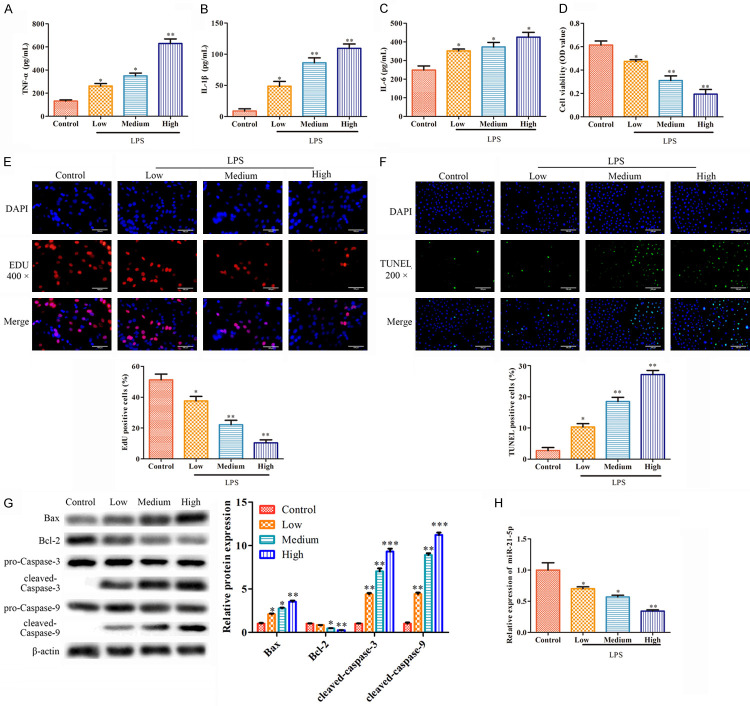
H9c2 cells were co-cultured with low, medium, and high concentrations of LPS for 24 h, respectively. The LPS-induced secretions of the inflammatory cytokines in the H9c2 cells, including TNF-α, IL-1β, and IL-6, were evaluated using ELISA, and the results demonstrated dose-dependent secretions of these cytokines with respect to the LPS concentrations (Figure 1A-C). Moreover, the CCK-8 and EdU assays revealed that the viability and proliferation of the H9c2 cells were decreased (Figure 1D, 1E). Furthermore, the number of TUNEL-positive H9c2 cells increased in a dose-dependent manner concerning the LPS concentrations, indicating the apoptosis of the H9c2 cells is promoted by LPS (Figure 1F).
Figure 1.
The expression of miR-21-5p is down-regulated in LPS-treated H9c2 cells. H9c2 cells were treated with different concentrations of LPS for 24 h, respectively. A-C. The secretions of the cytokines were determined using ELISA. D. The cell viability was measured using CCK-8 assays. E. We used EdU assays to analyze the cell proliferation (400×). F. We used TUNEL assays to analyze the apoptosis (400×). G. We used Western blot to analyze the expression levels of the apoptosis-associated proteins. H. We used qRT-PCR to test the expressions of miR-21-5p. The values are the mean ± sd, *P<0.05, **P<0.01 vs. the control, n=3 per group.
Moreover, the Bcl-2 expressions were found to drop, but the Bax, Cleaved caspase-3, and Cleaved caspase-9 levels were elevated in the LPS-treated H9c2 cells in an LPS-dose-dependent manner (Figure 1G). Notably, the miR-21-5p levels were down-regulated in the H9c2 cells (Figure 1H). Judging from the data, LPS can induce damage in H9c2 cells by reducing the cell viability and promoting apoptosis, and the power of such an induction was positively correlated to the LPS concentration. Moreover, miR-21-5p was downregulated by LPS in H9c2 cells.
Overexpression of miR-21-5p suppresses the inflammatory damage in H9c2 cells
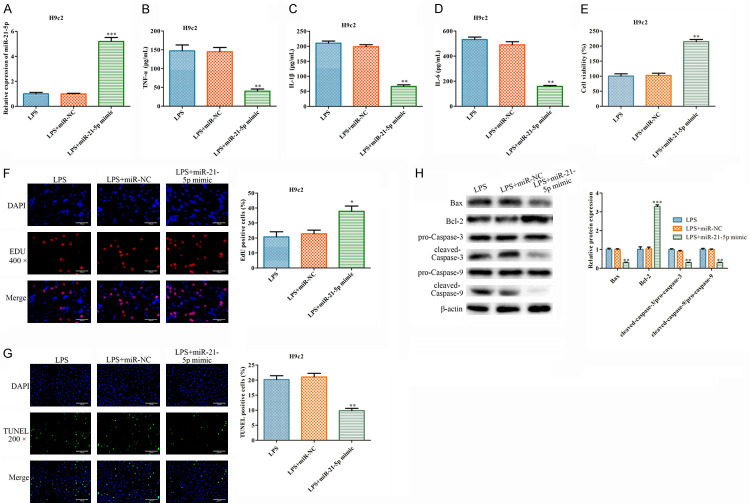
To investigate the functions of miR-21-5p in the LPS-treated H9c2 cells, miR-21-5p mimics and NC mimics were transfected into these cells. The qRT-PCR results indicated that the miR-21-5p mimics can upregulate the miR-21-5p contents in the H9c2 cells (Figure 2A), while downregulating the secretions of TNF-α, IL-1β, and IL-6 (Figure 2B-D). Moreover, the CCK-8 and EdU assays showed that the miR-21-5p mimics can significantly promote the viability and proliferation of the LPS-treated H9c2 cells (Figure 2E, 2F). Then, the proportion of the TUNEL-positive cells dropped in the group transfected with the miR-21-5p mimics (Figure 2G). Moreover, the miR-21-5p mimics elevated the expression level of Bcl-2 and inhibited the expressions of Bax, Cleaved caspase-3, and Cleaved caspase-9 (Figure 2H). All these results confirmed that the overexpression of miR-21-5p can inhibit LPS-induced inflammatory damage in H9c2 cells.
Figure 2.
The overexpression of miR-21-5p inhibits inflammatory injuries in LPS-treated H9c2 cells. miR-NC and miR-21-5p were transfected in the LPS-treated H9c2 cells. A. A qRT-PCR analysis of the expression of miR-21-5p. B-D. The cytokines were measured using ELISA. E. The cell viability was measured using CCK-8 assays. F. EdU assays were used to analyze the cell proliferation (400×). G. TUNEL assays were used to analyze the apoptosis (400×). H. Western blot was used to analyze the expression levels of the apoptosis associated proteins. The values are the mean ± sd, *P<0.05, **P<0.01, ***P<0.001 vs. LPS + miR-NC, n=3 per group.
PDCD4 is a downstream target gene of miR-21-5p
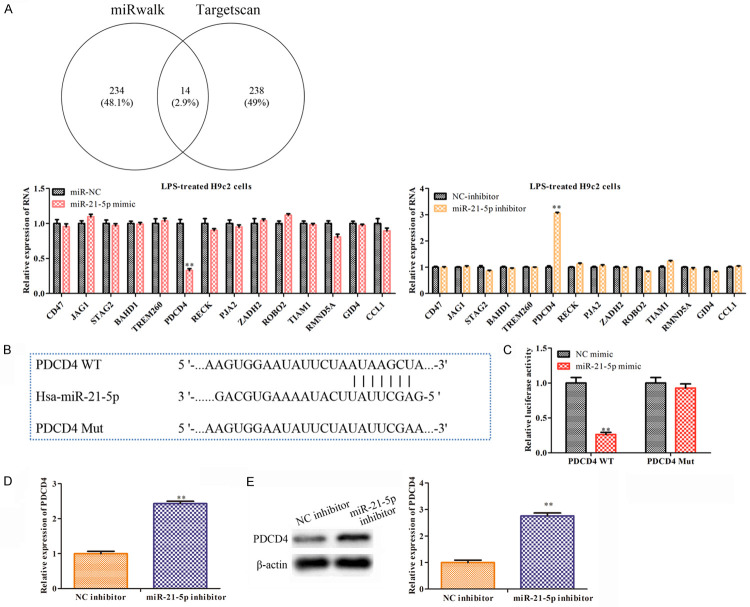
To explore the regulatory mechanisms of miR-21-5p in the LPS-treated H9c2 cells, we used miRwalk and TargetScan to search the target genes and found 14 intersection target genes (Figure 3A). To specify the target gene that interacts with miR-21-5p, the miR-21-5p mimics and the miR-21-5p inhibitors were transfected into the LPS-treated H9c2 cells. Among the 14 target genes, the qRT-PCR analysis found that PDCD4 exhibited an expression pattern that was significantly affected by the mimics and the miR-21-5p inhibitors in the LPS-treated H9c2 cells (Figure 3A). Subsequently, we predicted the potential interaction sites between miR-21-5p and PDCD4 (Figure 3B) and performed a luciferase reporter analysis to verify them. The results demonstrated that the luciferase activity at the 3’UTR region in PDCD4 dropped significantly when the H9c2 cells were co-transfected with the PDCD4-WT and miR-21-5p mimics. However, the luciferase activity of the PDCD4-Mut didn’t change significantly between the groups of the miR-21-5p mimics and the control (Figure 3C). Moreover, the miR-21-5p inhibitors were found to increase the expressions of the PDCD4 mRNA and protein in the H9c2 cells (Figure 3D, 3E). In general, PDCD4 was confirmed to be a downstream target gene of miR-21-5p and its expression is negatively correlated with the miR-21-5p expression.
Figure 3.
PDCD4 is the downstream target gene of miR-21-5p. A. We used miRWalk and TargetScan to search for the related target genes of miR-21-5p. The MiR-NC/miR-21-5p mimics and the NC-inhibitor/miR-21-5p inhibitor were transfected in the LPS-treated H9c2 cells, and we used qRT-PCR to test the levels of the 14 intersection target genes. Values are mean ± sd, **P<0.01 vs. miR-NC or NC-inhibitor, n=3 per group. B. The predicted binding sites of PDCD4 and miR-21-5p. C. Luciferase reporter assays were performed to analyze the interaction of WT or MUT PDCD4 3’-UTR with miR-21-5p. The values are the mean ± sd, **P<0.01 vs. the NC mimics, n=3 per group. D. The NC-inhibitor and the miR-21-5p inhibitor were transfected in the H9c2 cells. The expression of PDCD4 was analyzed using qRT-PCR. E. We used Western blot to determine the expressions of PDCD4. The values are the mean ± sd, **P<0.01 vs. NC-inhibitor, n=3 per group.
Pc-PDCD4 reversed the effects of the miR-21-5p inhibitors in the LPS-treated H9c2 cells
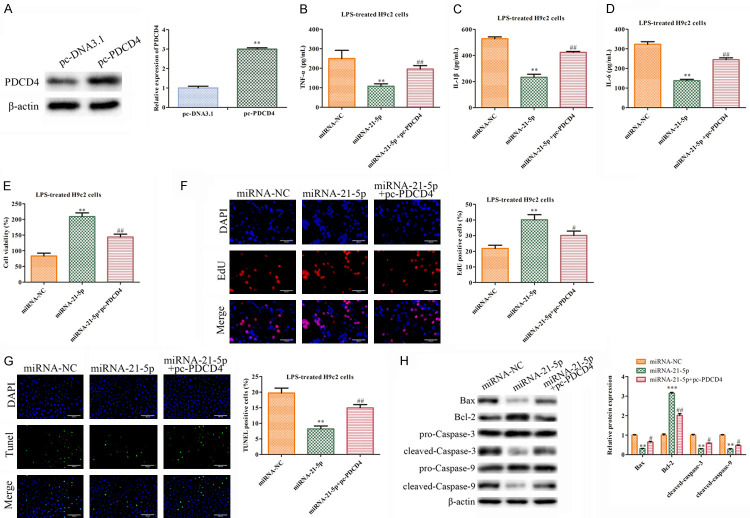
Next, we performed a rescue experiment to investigate the functions of the PDCD4 and miR-21-5p mimics in the LPS-treated H9c2 cells. First, the PDCD4 expressions were up-regulated in the H9c2 cells transfected with pc-PDCD4 compared with the pc-DNA3.1 group (Figure 4A). Then, the pc-PDCD4 was found to reverse the down-regulation of TNF-α, IL-1β, and IL-6 by the overexpression of miR-21-5p (Figure 4B-D). Moreover, the CCK-8 and EdU assays revealed that the PDCD4 up-regulation could reverse the promotive effects of the miR-21-5p mimics on the LPS-treated H9c2 cells (Figure 4E, 4F). Meanwhile, pc-PDCD4 increased the proportion of the TUNEL-positive cells, indicating that the overexpression of PDCD4 aggravated the apoptosis of the H9c2 cells that were treated with LPS and transfected with the miR-21-5p mimics (Figure 4G). Finally, a western blot analysis proved that pc-PDCD4 inhibited the up-regulation of Bcl-2 and reversed the inhibitory effects on the expressions of Bax, Cleaved caspase-3, and Cleaved caspase-9 seen in the miR-21-5p group (Figure 4H). In general, miR-21-5p can inhibit the septic process, and such an effect was rescued by the PDCD4 overexpression of PDCD4 in the LPS-treated H9c2 cells.
Figure 4.
The function of the miR-21-5p inhibitor is reversed by pc-PDCD4 in the LPS-treated H9c2 cells. A. The protein levels of PDCD4 were measured using western blot. The values are the mean ± SD, **P<0.01 vs. pc-DNA3.1. n=3 per group. The LPS-treated H9c2 cells transfected with miR-NC, miR-21-5p mimics or miR-21-5p mimics + pc-PDCD4. B-D. We used ELISA to measure the secretions of cytokines TNF-α, IL-1β, and IL-6. **P<0.01 vs. miRNA-NC group, ##P<0.01 vs. miRNA-21-5p group. E. The cell viability was measured using CCK-8 assays. **P<0.01 vs. the miRNA-NC group, ##P<0.01 vs. the miRNA-21-5p group. F. We used an EdU assay to analyze the cell proliferation (400×). **P<0.01 vs. the miRNA-NC group, #P<0.05 vs. miRNA-21-5p group. G. We used a TUNEL assay to analyze the apoptosis (400×). **P<0.01 vs. the miRNA-NC group, ##P<0.01 vs. miRNA-21-5p group. H. A Western blot analysis of the expression levels of the proteins. The values are the mean ± SD. **P<0.01, ***P<0.001 vs. miRNA-NC group, #P<0.05, ##P<0.01 vs. miRNA-21-5p group. n=3 per group.
Discussion
LPS is an essential component of the outer membrane of gram-negative bacteria [23]. As a molecule responsible for the systemic inflammatory response, it is routinely used as a sepsis model in studies [15,24]. LPS-induced sepsis is defined as a systemic inflammatory response process that is related to many cardiac diseases [25]. Moreover, LPS is involved in various molecular metabolism mechanisms, mitochondrial dysfunction, apoptosis, and inflammation [15]. In this study, LPS was used to induce damage in H9c2 cells and to create an experimental model of sepsis in vitro. Our results indicated that the viability of the H9c2 cells was significantly inhibited by LPS. On the other hand, the proportion of apoptotic cells and the expressions of the apoptosis-related proteins increased when LPS was administered in the cell model. Moreover, LPS contributed to the overexpressions of IL-6, IL-1β, and TNF-α in the H9c2 cells, suggesting that an experimental model of sepsis was established.
MiRNAs are regulatory molecules that are vital in disease diagnosis and treatment, and they usually interact with the target gene at the 3’ untranslated region (3’-UTR) [12,26]. Previous studies have shown that miRNAs participate in regulating cell metabolism and are involved in LPS pathophysiology [21,27,28]. Therefore, miRNAs may become new potential therapeutic targets for LPS-induced sepsis. Specifically, human NLRP3 inflammasomes are found to be inhibited by miR-21 knockdown in septic shock [29]. Moreover, the inhibition of miR-21 suppresses snail expression and prevents LPS-induced podocyte injuries [30].
As a member of the miR-21 family, miR-21-5p has been shown to be involved in many diseases [31,32]. Although scientists have conductive extensive research about the inflammation inhibition of miR-21-5p across lots of diseases, the upstream regulatory mechanisms of miR-21-5p in sepsis have remained almost unknown until now. In this paper, we confirmed that LPS downregulates the expression of miR-21-5p in H9c2 cells while upregulating the concentrations of the inflammatory cytokines and the apoptosis-related proteins. When LPS-treated H9c2 cells are transfected with the miR-21-5p mimics, the expressions of the inflammatory cytokines and the apoptosis-related proteins dropped, and the cell viability increased. Therefore, miR-21-5p is verified to inhibit inflammation in LPS-treated H9c2 cells.
With the bioinformatics analysis, PDCD4 was predicted as one of the potential targets of miR-21-5p. Moreover, PDCD4 has been shown to significantly suppress tumor growth and progression. Moreover, miR-21-5p has been found to play a role in disease development by targeting PDCD4 [20]. In our study, PDCD4 was experimentally confirmed as a target of miR-21-5p, and its expression was found to be negatively correlated with miR-21-5p expression in H9c2 cells. Furthermore, pc-PDCD4 was found to partially antagonize the inhibitory effects on the inflammation of the miR-21-5p mimics in LPS-treated H9c2 cells (Figure 5).
Figure 5.
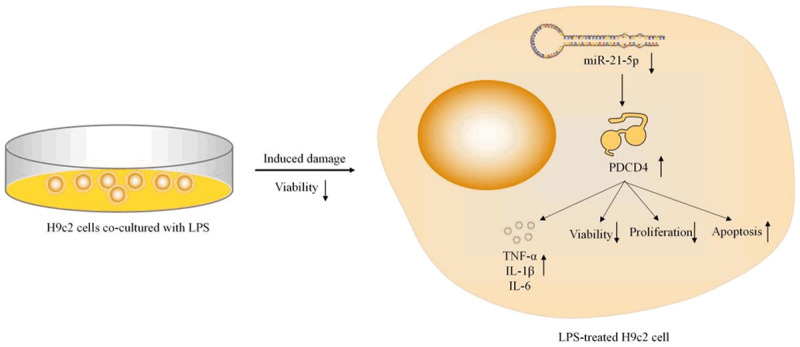
A schematic diagram of relationship between LPS and miR-21-5p. The overexpression of miR-21-5p cannot protect the cells from LPS-induced damage in the LPS-treated H9c2 cells by promoting the viability of the cells, inhibiting the cell apoptosis or the secretions of the inflammatory-related cytokines.
However, there are still some limitations to the present study. The in vivo animal model experiments were lacking. The roles of miR-21-5p and PDCD4 in the sepsis animal model should be further determined. We will further confirm our conclusions by performing animal model experiments in our future studies.
In summary, the overexpression of miR-21-5p can protect cells from LPS-induced damage by promoting cell viability and inhibiting apoptosis and the secretions of the inflammation-related cytokines. Meanwhile, the relationship between miR-21-5p and PDCD4 in LPS-treated H9c2 cells is clarified in vitro, and a novel therapeutic target (PDCD4) for sepsis is proposed. Such findings should be verified further by in vivo studies.
Acknowledgements
This work was supported by the Science and Technology Project of Nantong City (JCZ19116) and the Science and Technology Project of Nantong Municipal Health Commission (MA2020023).
Disclosure of conflict of interest
None.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med. 2013;14:686–693. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 3.Haileselassie B, Mukherjee R, Joshi AU, Napier BA, Massis LM, Ostberg NP, Queliconi BB, Monack D, Bernstein D, Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy. J Mol Cell Cardiol. 2019;130:160–169. doi: 10.1016/j.yjmcc.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar-Hari M, Bertolini G, Brunkhorst FM, Bellomo R, Annane D, Deutschman CS, Singer M. Judging quality of current septic shock definitions and criteria. Crit Care. 2015;19:445. doi: 10.1186/s13054-015-1164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29:500–511. doi: 10.1016/j.jcrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Lai J, Yang L, Ruan G, Chaugai S, Ning Q, Chen C, Wang DW. Trimetazidine prevents macrophage-mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1. Br J Pharmacol. 2016;173:545–561. doi: 10.1111/bph.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Lin C, Wang T, Zhang P, Liu Z, Lu C. Ergosterol attenuates LPS-induced myocardial injury by modulating oxidative stress and apoptosis in rats. Cell Physiol Biochem. 2018;48:583–592. doi: 10.1159/000491887. [DOI] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, Feinberg MW. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Fierro ML, Garza-Veloz I, Gutierrez-Arteaga C, Delgado-Enciso I, Barbosa-Cisneros OY, Flores-Morales V, Hernandez-Delgadillo GP, Rocha-Pizaña MR, Rodriguez-Sanchez IP, Badillo-Almaraz JI, Ortiz-Rodriguez JM, Castañeda-Miranda R, Solis-Sanchez LO, Ortiz-Castro Y. Circulating levels of specific members of chromosome 19 microRNA cluster are associated with preeclampsia development. Arch Gynecol Obstet. 2018;297:365–371. doi: 10.1007/s00404-017-4611-6. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang Y, Li D, Wang H, Wan Z, Luo Q, Zhong Y, Yin M, Qing Z, Li Z, Bao B, Chen Z, Yin X, Zhu LQ. MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell. 2019;18:e13022. doi: 10.1111/acel.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funahashi Y, Kato N, Masuda T, Nishio F, Kitai H, Ishimoto T, Kosugi T, Tsuboi N, Matsuda N, Maruyama S, Kadomatsu K. miR-146a targeted to splenic macrophages prevents sepsis-induced multiple organ injury. Lab Invest. 2019;99:1130–1142. doi: 10.1038/s41374-019-0190-4. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Xu K, Sun Y, Tian T, Shen W, Sun F, Yuan W, Wu H, Chen G, Yuan L, Zhang W, Lu M, Lei M. MiR-215-5p inhibits the inflammation injury in septic H9c2 by regulating ILF3 and LRRFIP1. Int Immunopharmacol. 2020;78:106000. doi: 10.1016/j.intimp.2019.106000. [DOI] [PubMed] [Google Scholar]
- 15.An R, Feng J, Xi C, Xu J, Sun L. miR-146a attenuates sepsis-induced myocardial dysfunction by suppressing IRAK1 and TRAF6 via targeting ErbB4 expression. Oxid Med Cell Longev. 2018;2018:7163057. doi: 10.1155/2018/7163057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge X, Huang S, Gao H, Han Z, Chen F, Zhang S, Wang Z, Kang C, Jiang R, Yue S, Lei P, Zhang J. miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res. 2016;1650:31–40. doi: 10.1016/j.brainres.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Manirujjaman M, Ozaki I, Murata Y, Guo J, Xia J, Nishioka K, Perveen R, Takahashi H, Anzai K, Matsuhashi S. Degradation of the tumor suppressor PDCD4 is impaired by the suppression of p62/SQSTM1 and autophagy. Cells. 2020;9:218. doi: 10.3390/cells9010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehler O, Singh P, Haas A, Ulrich D, Müller JP, Ohnheiser J, Klempnauer KH. An evolutionarily conserved interaction of tumor suppressor protein Pdcd4 with the poly(A)-binding protein contributes to translation suppression by Pdcd4. Nucleic Acids Res. 2014;42:11107–11118. doi: 10.1093/nar/gku800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M, Zhu N, Hao F, Song Y, Wang Z, Ni Y, Ding L. The regulatory role of non-coding RNAs on programmed cell death four in inflammation and cancer. Front Oncol. 2019;9:919. doi: 10.3389/fonc.2019.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, Su L, Duan X, Chen X, Hays A, Upadhyayula S, Shivde J, Wang H, Li Y, Huang D, Liang S. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol Immunol. 2018;101:608–614. doi: 10.1016/j.molimm.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao L, Wu YQ, Zhang SP. MiR-21-5p enhances the progression and paclitaxel resistance in drug-resistant breast cancer cell lines by targeting PDCD4. Neoplasma. 2019;66:746–755. doi: 10.4149/neo_2018_181207N930. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Liu J, You X, Kong P, Song Y, Cao L, Yang S, Wang W, Fu Q, Ma Z. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci Lett. 2016;613:60–65. doi: 10.1016/j.neulet.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Liu P, Feng X, Ma C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med. 2017;21:3178–3189. doi: 10.1111/jcmm.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyu Y, Jiang X, Dai W. The roles of a novel inflammatory neopterin in subjects with coronary atherosclerotic heart disease. Int Immunopharmacol. 2015;24:169–172. doi: 10.1016/j.intimp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Hu YL, Wang H, Huang Q, Wang G, Zhang HB. MicroRNA-23a-3p promotes the perihematomal edema formation after intracerebral hemorrhage via ZO-1. Eur Rev Med Pharmacol Sci. 2018;22:2809–2816. doi: 10.26355/eurrev_201805_14980. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Wang Y, Gao H, Wang B. MicroRNA-30a-3p overexpression improves sepsis-induced cell apoptosis in vitro and in vivo via the PTEN/PI3K/AKT signaling pathway. Exp Ther Med. 2018;15:2081–2087. doi: 10.3892/etm.2017.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Li X, Zou M, He J, Han Y, Wu D, Yang H, Wu J. miR-135a inhibition protects A549 cells from LPS-induced apoptosis by targeting Bcl-2. Biochem Biophys Res Commun. 2014;452:951–957. doi: 10.1016/j.bbrc.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang Z, Li Y, Yang G, Zhou D, Yang H, Zhang L, Zhang Q, Gu C, Yang J, Da Y, Yao Z, Duo S, Zhang R. miR-21 promotes NLRP3 inflammasome activation to mediate pyroptosis and endotoxic shock. Cell Death Dis. 2019;10:461. doi: 10.1038/s41419-019-1713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Wang J, Zhang Z, Miao H. Decreased miR-128 and increased miR-21 synergistically cause podocyte injury in sepsis. J Nephrol. 2017;30:543–550. doi: 10.1007/s40620-017-0405-y. [DOI] [PubMed] [Google Scholar]
- 31.Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1–9. doi: 10.1016/j.atherosclerosis.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Paiva RM, Zauli DAG, Neto BS, Brum IS. Urinary microRNAs expression in prostate cancer diagnosis: a systematic review. Clin Transl Oncol. 2020;22:2061–2073. doi: 10.1007/s12094-020-02349-z. [DOI] [PubMed] [Google Scholar]