Abstract
Objective: To analyze the value of contrast-enhanced ultrasound (CEUS) versus shear wave elastography (SWE) in differentiating benign and malignant superficial lymph node lesions. Methods: In this retrospective study, a total of 140 superficial lymph nodes from 140 patients pathologically confirmed to have an enlargement of their superficial lymph nodes were examined using CEUS and SWE. The results and diagnostic efficacy were analyzed. Results: Among the 67 benign lymph nodes, there were 38 cases of type I, 17 of type II, and 12 of types III and IV. Among the 73 malignant lymph nodes, there were 53 cases of type III, 11 of type IV, and 9 of types I and II. Among the patients with lymph nodes <1 cm, there were 20, 4, 8, and 5 cases of types I, II, III, and IV, respectively. Among the patients with 1-2 cm lymph nodes, there were 15, 10, 26 and 7 cases of types I, II, III, and IV, respectively. There were 6, 10, 27, and 2 cases of types I, II, III, and IV in the >2 cm lymph nodes, respectively. The accuracy, sensitivity, and specificity of CEUS in the diagnosis of malignant lymph nodes were 85.00%, 87.67%, and 82.09%, respectively, and those of SWE were 89.29%, 80.82%, and 98.51%, respectively. SWE showed higher specificity than CEUS (P<0.05). SWE showed mean shear wave velocity (SWV) values of (2.11±0.41) m/s for the benign lymph nodes and (3.22±0.79) m/s for the malignant lymph nodes (P<0.05). The receiver operating characteristic (ROC) curves of the SWV values for the benign and malignant lymph nodes showed AUC=0.9948. Conclusion: Both CEUS and SWE are valuable in the differentiation of benign and malignant lymph node lesions, but SWE has a higher specificity. The SWV value of SWE is superior in the differentiation of benign and malignant lymph nodes. The combination of the two methods can achieve a higher accuracy.
Keywords: Superficial lymph nodes, ultrasonography, shear wave elastography, differentiation, benign, malignant
Introduction
The lymphatic system of the body is made up of lymphatic capillaries, a reticular network, and interconnected lymph nodes [1]. Lymph nodes are crucial peripheral immune organs that play important roles in filtering the lymphatic fluid, producing immune cells, and in the immune response [2]. Lymph nodes are kidney-shaped and bean-shaped, and range in size from 1 to 3 mm, depending on their age and location [3]. The superficial lymph nodes in this study refer to the group of lymph nodes no more than 3 cm under the skin, mainly distributed in the inguinal region, the proximal limbs, both axillae, and the head and neck [4].
Bacterial or viral infections, tumor metastases, hematologic diseases, immune factors, and lymphomas may lead to the enlargement of superficial lymph nodes. There are different treatment schemes for lymph node lesions induced by different causes, and the benign and malignant lesions of the enlarged lymph nodes should be accurately identified [5]. The benign lymph node lesions include bacterial lymphadenitis, reactive lymphadenopathy, and lymphatic tuberculosis, and the malignant lymph node lesions include lymphoma and metastatic lymph nodes [6]. A diagnostic tool that can accurately measure and summarize the image features is vital for the early diagnosis and treatment of lymph node lesions.
Ultrasound, computerized tomography (CT), and magnetic resonance imaging (MRI) have their own advantages in the diagnosis of lymph node lesions, but they also have some shortcomings [7]. With the advances in technology and equipment, contrast-enhanced ultrasound (CEUS) has been widely used clinically, exhibiting good performance in the diagnosis of many diseases [8]. CEUS can gather blood perfusion data and accurately demonstrate the blood supply pattern and microvascular distribution in the lymph nodes, so it has an outstanding diagnostic value [9]. Shear wave elastography (SWE) is a new diagnostic technique that can qualitatively and quantitatively display the softness and hardness of tissue and organ lesions [10]. To investigate the diagnostic value of CEUS and SWE in superficial lymph node lesions, 140 superficial lymph nodes from patients with enlarged superficial lymph nodes were selected for the analysis in this study.
Materials and methods
Data
In this retrospective study, a total of 140 patients with a pathologically confirmed enlargement of the superficial lymph nodes were enrolled in this study, with a total of 140 superficial lymph nodes. One lymph node with the highest degree of suspicion in each case was examined using CEUS and SWE.
Inclusion criteria: all the pathological findings of the lymph nodes were confirmed by ultrasound-guided puncture biopsy or surgical specimens, and all the patients understood the study procedures and signed the consent form. Exclusion criteria: patients with no confirmed results; patients with contraindications to puncture or surgery, patients comorbid with other diseases that may affect the study accuracy; and the patients who did not agree to participate in the study were excluded.
This study was approved by the Ethics Committee of the First People’s Hospital of Fuyang Hangzhou (No. ChiCTR1800012684).
Methods
The CEUS examination was performed using a color Doppler ultrasound diagnostic instrument (Philips, IU22). The frequency of the L9-3 linear array ultrasound probe transducer was set to 3-9 MHz. Sono Vue was selected as the contrast agent for the microbubble suspension, and 2.5 mL of the prepared suspension was injected through a superficial elbow vein, and then 5 mL of normal saline was injected. The lymph nodes were first routinely examined (Figure 1) for their sizes, borders, echotextures, hila, and vascular patterns. The lymph nodes were differentiated into groups of <1 cm, 1 to 2 cm, and >2 cm according to their maximum diameters. The blood perfusion characteristics of the lymph nodes on the maximum diameter section were observed for 2-3 min under contrast mode.
Figure 1.
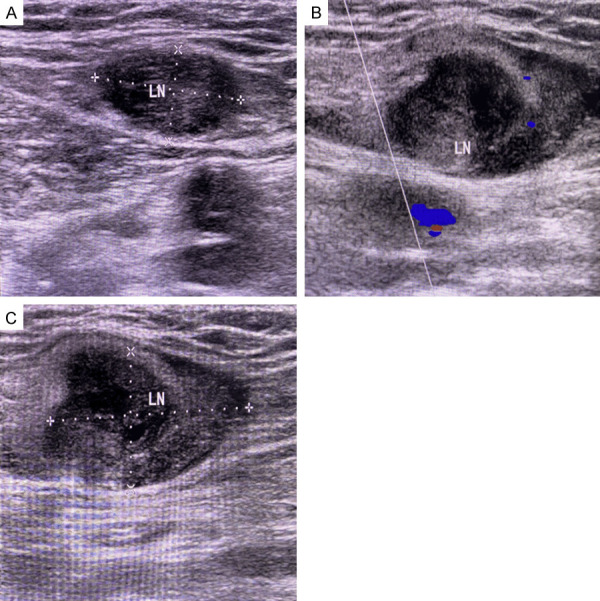
Gray-scale ultrasound images of lymph nodes. A: A 2D sonogram of a normal lymph node, with a normal morphology and distinct cortical and medullary structures; B, C: 2D ultrasound sonograms of metastatic lymph nodes with abnormal morphology (distorted aspect ratio), poorly defined cortical and medullary structures, and a peripheral pattern.
The SWE was performed using a color Doppler ultrasound diagnostic instrument (Siemens, Acuson S2000). A 9L4 line array probe set at 4-9 MHz was selected. The patients were instructed to hold their breath during the examination, and the lymph nodes were examined qualitatively and quantitatively using virtual touch tissue imaging (VTI) and virtual touch tissue quantification (VTQ). The sampling frames were placed to avoid the necrotic areas and the calcified foci in the lymph nodes as much as possible according to the imaging results. VTQ measurements were done for each lymph node, and the shear wave velocities (SWV) were accurately recorded.
Outcome measurement
Pathological analysis: The obtained lymph node biopsy tissue specimens were fixed with 4% neutral formaldehyde and embedded in paraffin, with a section thickness of 4-5 μm. The histopathological morphology was observed under an optical microscope after staining with acid fast, Periodic-acid Schiff (PAS) and hexamine silver. Both the acid-fast and PAS staining kits were purchased from Baso Diagnostics Inc. Zhuhai, and the procedures were carried out according to the kits’ instructions. The hexamine silver staining was performed using a Roche automatic immunohistochemical staining device.
The criteria for the diagnosis of malignant lymph nodes using 2-dimensional ultrasound were as follows: length/short diameter ≤2, an unclear boundary between the cortex and outer medulla; a displaced or absent fatty hila, an inhomogeneous internal echo, and liquefaction and calcification. The color Doppler flow patterns of the lymph nodes included a peripheral pattern, a hilar pattern, or a mixed pattern. Malignant lymph node criteria [11]: (1) A mixed or peripheral pattern, along with ≥1 malignancy index on 2-dimensional ultrasound; (2) A hilar pattern, along with >2 malignant indicators on 2D ultrasound.
CEUS manifestations of the lymph nodes [12]: Type I: Uniformly hyper-enhancing type with significant and uniform perfusions of the lymph nodes. Type II: Inhomogeneous enhancing or ring-shape enhancing with regular nonperfused areas and a significant overall enhancement of the lymph nodes. However, single or several regular morphologically well-defined non-perfused areas could be seen inside them. Type III: Unevenly enhanced with irregular perfusion-deficient areas and perfusion-deficient areas within the lymph nodes, including focal hypoperfusion, nonperfusion, and irregular morphology and perfusion-deficient areas with unclear boundaries. Type IV: The weakly enhancing type, with weakly uniform or heterogeneous perfusions of the lymph nodes. Types I and II were identified as benign lymph nodes, and types III and IV were identified as malignant lymph nodes.
Statistical analysis
The statistical analysis was performed using SPSS 23.0. The count data were expressed as [n (%)] and examined using X2 tests. The measurement data were expressed as (x̅±s) and examined using t tests. The figures were produced using GraphPad Prism 8. The ROC curves were plotted using GraphPad Prism 8. P<0.05 was considered statistically significant.
Results
General patient information
Among the 140 patients enrolled in this study, 55 were females and 85 were males. Of the 140 lymph nodes, 67 lymph nodes were pathologically benign and 73 lymph nodes were malignant. Among the benign lymph nodes, there were 7 with histiocytic necrotizing lymphadenitis, 12 with nonspecific lymphadenitis, 22 were tuberculous lymph nodes, and 26 were reactive lymph nodes. Among the malignant lymph nodes, there were 5 lymphomas and 68 metastatic lymph nodes. According to the maximum diameters, there were 37 lymph nodes of <1 cm (21 benign lymph nodes and 16 malignant lymph nodes), 58 lymph nodes of 1 to 2 cm (28 benign lymph nodes and 30 malignant lymph nodes), and 45 lymph nodes >2 cm (18 benign lymph nodes and 27 malignant lymph nodes).
CEUS results
Among the 67 benign lymph nodes, 38 were type I, 17 were type II, and 12 were types III and IV. Among the 73 malignant lymph nodes, there were 53 of type III, 11 of type IV, and 9 of types I and II (Table 1). Among the <1 cm lymph nodes, there were 20 of type I, 4 of type II, 8 of type III, and 5 of type IV. Among the 1-2 cm lymph nodes, there were 15 of type I, 10 of type II, 26 of type III, and 7 of type IV. Among the >2 cm lymph nodes, there were 6 of type I, 10 of type II, 27 of type III, and 2 of type IV.
Table 1.
CEUS typing of the 140 superficial lymph node lesions (nos.)
| Subgroup | Type I | Type II | Type III | Type IV |
|---|---|---|---|---|
| Benign (n=67) | 38 (56.72) | 17 (25.37) | 10 (14.93) | 2 (2.99) |
| Malignant (n=73) | 3 (4.11) | 6 (8.22) | 53 (72.60) | 11 (15.07) |
| X2 | 44.181 | 7.488 | 46.958 | 4.706 |
| P | <0.001 | 0.006 | <0.001 | 0.030 |
CEUS showed that the benign lymph nodes were mostly <2 mm, and the malignant lymph nodes were mostly the >2 mm type. The dermal-medullary boundaries of both the benign and malignant lymph nodes were mostly clear. The malignant lymph nodes had more missing hila than the benign lymph nodes. The internal echoes of the benign lymph nodes were mainly uniform. The larger the diameter, the more uneven the internal echo of the malignant lymph nodes. The blood flow patterns of the benign lymph nodes was predominantly hilar, but the malignant lymph nodes had predominantly peripheral or mixed patterns.
Diagnostic efficacy of CEUS, SWE
Referring to the pathological results, the accuracy, sensitivity, and specificity of CEUS in the diagnosis of malignant lymph nodes were 85.00% (119/140), 87.67% (64/73) and 82.09% (55/67), respectively (Table 2). According to the pathological findings, the accuracy, sensitivity and specificity of SWE in the diagnosis of malignant lymph nodes were 89.29% (125/140), 80.82% (59/73), and 98.51% (66/67), respectively (Table 3). There were no significant differences in the accuracy or sensitivity between CEUS and SWE in the diagnosis of malignant lymph nodes (P>0.05), but the specificity of SWE was higher than that of CEUS (P<0.05).
Table 2.
The efficacy of CEUS in diagnosing benign and malignant superficial lymph nodes (En)
| CEUS | Pathology | Total | |
|---|---|---|---|
|
| |||
| Malignant | Benign | ||
| Malignant | 64 | 12 | 76 |
| Benign | 9 | 55 | 64 |
| Total | 73 | 67 | 140 |
Table 3.
The efficacy of shear wave elastography in diagnosing benign and malignant lymph nodes (En)
| Shear wave elastography | Pathology | Total | |
|---|---|---|---|
|
| |||
| Malignant | Benign | ||
| Malignant | 59 | 1 | 60 |
| Benign | 14 | 66 | 80 |
| Total | 73 | 67 | 140 |
SWV values of SWE
The SWE revealed that the SWV values in the benign lymph nodes ranged from 1.51 m/s to 2.55 m/s, with a mean value of (2.11±0.41) m/s. The SWV values in the malignant lymph nodes ranged from 2.48 m/s to 3.99 m/s, with a mean value of (3.22±0.79) m/s. The SWV values in the malignant lymph nodes were significantly higher than those in the benign lymph nodes (P<0.05) (Figure 2). The SWV values for differentiating the benign and malignant lymph nodes were plotted as the ROC curves (AUC=0.9948), suggesting that the SWV values obtained using the SWE had a high value in differentiating the benign and malignant lymph nodes (Figure 3).
Figure 2.
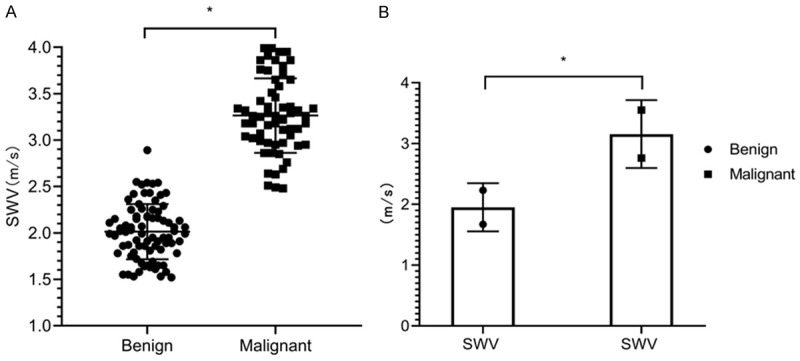
SWV values. A: A scatter plot of the SWV values of the benign and malignant lymph nodes; B: A comparison of the mean SWV values of the benign and malignant lymph nodes, *P<0.05.
Figure 3.
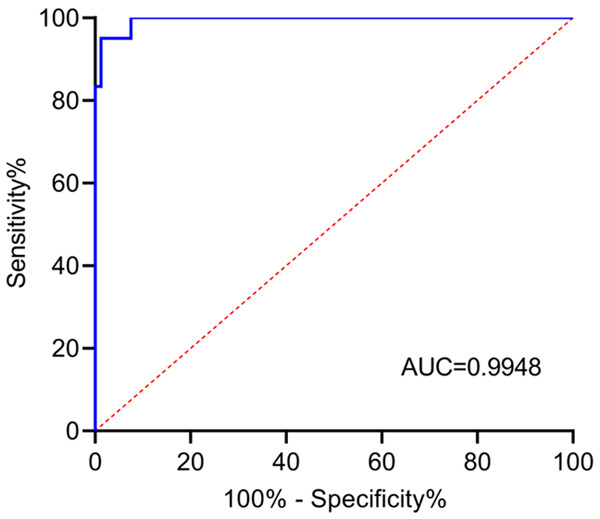
ROC curves of the SWV values used for differentiating the benign and malignant lymph nodes. AUC=0.9948, 95% CI of 0.9882-1.0000, SE=0.003379, P<0.0001.
Discussion
Ultrasound is most commonly used to detect superficial lymph node lesions. However, conventional two-dimensional ultrasound cannot clearly show the tiny blood vessels and fine structures inside the lymph nodes, and some benign and malignant lymph nodes exhibit similar presentations in ultrasound features, so it is insufficient to select conventional two-dimensional ultrasound for the identification of lymph node lesions [13,14].
The appearance and application of CEUS provide a new method for the qualitative diagnosis of lymph node lesions. CEUS enables real-time, dynamic monitoring of the perfusion characteristics of the lymph nodes, so that the necrotic areas can be detected more accurately and the tumor metastatic areas can be determined [15]. Matoba et al. [16] reported that benign lymph nodes were the mostly uniformly perfused. In this study, about half of the benign lymph nodes showed homogeneous perfusion enhancement, but the other benign lymph nodes showed a heterogeneous perfusion enhancement, and a small number showed regular, perfusion-free areas among the benign lymph nodes to which they belonged, and the tubercular lymph nodes were predominant, suggesting that tubercular lymph nodes are dominated by perfusion-free areas. If lymph nodes with inhomogeneous perfusion are all judged to be malignant, the false positive results will be high, results will affect the accuracy of the diagnosis. With regard to the perfusion patterns in the malignant lymph nodes, Barrow-McGee et al. believe that it is mainly inhomogeneous perfusion [17], which is similar to the results in this study. This may be because tumor cells destroy the normal lymph node structures and form tumor trophoblastic vessels, leading to uneven lymph node imaging [18]. In addition, Ling et al. [19] and Chen et al. [20] found that the non-perfused areas in malignant lymph nodes often have no clear boundaries or regular morphology, and they are distributed in sheets, which may be due to the fact that the invasion of tumor tissue is not as restricted as the necrosis caused by benign lesions, and the tumor tissues proliferate at different rates, so the morphology of the perfusion defects is also irregular.
There are many types of elastography. This study examined acoustic radiation force impulse (ARFI) imaging, which is a new sonographic technology that non-invasively assesses qualitative and quantitative tissue elasticity by measuring the shear wave velocities (SWVs) of a selected region of interest (ROI), and SWV is a reflection of the hardness of the tissue [21,22]. ARFI is feasible because the infiltration of malignant tumor tissue alters the hardness of lymph nodes, [23]. The results of this study showed that the SWV values of the benign lymph nodes were significantly lower than that of the malignant lymph nodes. Li et al. [24] also confirmed that there are differences in the SWV values between the benign and the malignant lymph nodes. This may be because the enlarged tissue is also lymphoid tissue with no significant change in hardness, although the volume of the benign lymph nodes increases significantly. As a result, SWE shows a higher gray-scale brightness of the VTI images and also a lower SWV. In contrast, metastatic lymph nodes are affected by the invasion, deposition and diffusion of tumor tissue; and the hardness of the tissue in the lymph nodes can differ significantly. Under the pressure of the acoustic beam, the tissues in the sampling frame area will not be significantly displaced, so the VTI image is darker, and the SWV spreads more quickly [25]. Our comparison of the diagnostic results of CEUS and SWE showed that both methods had a high sensitivity, specificity, and accuracy in the diagnosis of malignant lymph nodes, but the latter had a higher specificity. The SWV values of the benign and malignant lymph nodes were plotted as the ROC curves (AUC=0.9948), indicating that the SWV values obtained by SWE had a high value in differentiating benign and malignant lymph nodes. Similar results were also found in the study of Chen et al. [26], suggesting that both CEUS and SWE can be used in the qualitative diagnosis of superficial lymph node lesions, and the combination of the two methods can be used to ensure a higher diagnostic accuracy.
In conclusion, both CEUS and SWE are valuable in the differentiation of benign and malignant lymph node lesions, and the specificity of SWE is higher compared to SWE, and the SWV value of SWE is effective in the differentiation of benign and malignant lymph nodes. However, the value of the two methods was not further compared in this study, and the diagnostic mechanisms of the two methods were not fully elaborated, so this needs to be discussed in future studies.
Disclosure of conflict of interest
None.
References
- 1.Null M, Agarwal M. Treasure Island (FL): StatPearls Publishing; 2021. Anatomy, lymphatic system. [PubMed] [Google Scholar]
- 2.Thomas SN, Rohner NA, Edwards EE. Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annu Rev Biomed Eng. 2016;18:207–233. doi: 10.1146/annurev-bioeng-101515-014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogoslowski A, Kubes P. Lymph nodes: the unrecognized barrier against pathogens. ACS Infect Dis. 2018;4:1158–1161. doi: 10.1021/acsinfecdis.8b00111. [DOI] [PubMed] [Google Scholar]
- 4.Białek EJ, Jakubowski W. Mistakes in ultrasound diagnosis of superficial lymph nodes. J Ultrason. 2017;17:59–65. doi: 10.15557/JoU.2017.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin SS, Cui QL, Fan ZH, Yang W, Yan K. Diagnostic value of arrival time parametric imaging using contrast-enhanced ultrasonography in superficial enlarged lymph nodes. J Ultrasound Med. 2019;38:1287–1298. doi: 10.1002/jum.14809. [DOI] [PubMed] [Google Scholar]
- 6.Belotta AF, Gomes MC, Rocha NS, Melchert A, Giuffrida R, Silva JP, Mamprim MJ. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J Vet Intern Med. 2019;33:1403–1413. doi: 10.1111/jvim.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Z, Gao S, Wu W, Chen S, Fu S, Zhang J, Chen Y. Clinical value of the VTIQ technology in the differential diagnosis of superficially enlarged lymph nodes. Acta Radiol. 2018;59:836–844. doi: 10.1177/0284185117732601. [DOI] [PubMed] [Google Scholar]
- 8.Piccolo CL, Trinci M, Pinto A, Brunese L, Miele V. Role of contrast-enhanced ultrasound (CEUS) in the diagnosis and management of traumatic splenic injuries. J Ultrasound. 2018;21:315–327. doi: 10.1007/s40477-018-0327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J, Tong W, Luo J, Liang J, Pan F, Zheng Y, Xie X. Does contrast-enhanced ultrasound (CEUS) play a better role in diagnosis of breast lesions with calcification? A comparison with MRI. Br J Radiol. 2020;93:20200195. doi: 10.1259/bjr.20200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, Gao L, Witte RS. Shear-Wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37:855–870. doi: 10.1148/rg.2017160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prativadi R, Dahiya N, Kamaya A, Bhatt S. Chapter 5 ultrasound characteristics of benign vs malignant cervical lymph nodes. Semin Ultrasound CT MR. 2017;38:506–515. doi: 10.1053/j.sult.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Weskott HP. Contrast-enhanced ultrasound in the diagnostic workup of lymph nodes. Radiologe. 2018;58:563–571. doi: 10.1007/s00117-018-0389-1. [DOI] [PubMed] [Google Scholar]
- 13.Białek EJ, Jakubowski W. Mistakes in ultrasound diagnosis of superficial lymph nodes. J Ultrason. 2017;17:59–65. doi: 10.15557/JoU.2017.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei M, Ye L, Quan J, Huang P. Contrast-enhanced ultrasound for the differential diagnosis between benign and metastatic superficial lymph nodes: a meta-analysis. Cancer Manag Res. 2018;10:4987–4997. doi: 10.2147/CMAR.S174751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen Moody A, Cox K, Haigh I, Chen Y, Sharma N. Does contrast enhanced ultrasound (CEUS) of normal/benign axillary lymph nodes in patients with breast cancer identify significant axillary nodal burden? Eur J Radiol. 2020;132:109311. doi: 10.1016/j.ejrad.2020.109311. [DOI] [PubMed] [Google Scholar]
- 16.Matoba M, Tsuji H, Shimode Y, Nagata H, Tonami H. Diagnostic performance of adaptive 4D Volume Perfusion CT for detecting metastatic cervical lymph nodes in head and neck squamous cell carcinoma. AJR Am J Roentgenol. 2018;211:1106–1111. doi: 10.2214/AJR.17.19241. [DOI] [PubMed] [Google Scholar]
- 17.Barrow-McGee R, Procter J, Owen J, Woodman N, Lombardelli C, Kothari A, Kovacs T, Douek M, George S, Barry PA, Ramsey K, Gibson A, Buus R, Holgersen E, Natrajan R, Haider S, Shattock MJ, Gillett C, Tutt AN, Pinder SE, Naidoo K. Real-time ex vivo perfusion of human lymph nodes invaded by cancer (REPLICANT): a feasibility study. J Pathol. 2020;250:262–274. doi: 10.1002/path.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Tan HN, Liang P, Hou P, Cui L, Gao JB. Relationship between one-stop CT spectral perfusion imaging parameters and expression of lymphatic microvessel density and vascular endothelial growth factor-C in axillary lymph nodes of rabbit VX2 breast cancer. Zhonghua Yi Xue Za Zhi. 2019;99:1024–1027. doi: 10.3760/cma.j.issn.0376-2491.2019.13.013. [DOI] [PubMed] [Google Scholar]
- 19.Ling W, Nie J, Zhang D, Yang Q, Jin H, Ou X, Ma X, Luo Y. Role of contrast-enhanced ultrasound (CEUS) in the diagnosis of cervical lymph node metastasis in nasopharyngeal carcinoma (NPC) patients. Front Oncol. 2020;10:972. doi: 10.3389/fonc.2020.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Chen L, Liu J, Wang B, Zhang H. Value of qualitative and quantitative contrast-enhanced ultrasound analysis in preoperative diagnosis of cervical lymph node metastasis from papillary thyroid carcinoma. J Ultrasound Med. 2020;39:73–81. doi: 10.1002/jum.15074. [DOI] [PubMed] [Google Scholar]
- 21.Odéen H, de Bever J, Hofstetter LW, Parker DL. Multiple-point magnetic resonance acoustic radiation force imaging. Magn Reson Med. 2019;81:1104–1117. doi: 10.1002/mrm.27477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst EB, Unnikrishnan S, Wang S, Klibanov AL, Hossack JA, Mauldin FW Jr. The use of acoustic radiation force decorrelation-weighted pulse inversion for enhanced ultrasound contrast imaging. Invest Radiol. 2017;52:95–102. doi: 10.1097/RLI.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan S, Miao LY, Cui LG, Sun PF, Qian LX. Value of shear wave elastography versus contrast-enhanced sonography for differentiating benign and malignant superficial lymphadenopathy unexplained by conventional sonography. J Ultrasound Med. 2017;36:189–199. doi: 10.7863/ultra.16.01014. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Chen M, Cao CL, Zhou LQ, Li SG, Ge ZK, Zhang WH, Xu JW, Cui XW, Dietrich CF. Diagnostic performance of acoustic radiation force impulse elastography for the differentiation of benign and malignant superficial lymph nodes: a meta-analysis. J Ultrasound Med. 2020;39:213–222. doi: 10.1002/jum.15096. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Zhao X, Ji X, Han R, Li P, Du M. Diagnostic value of acoustic radiation force impulse imaging for assessing superficial lymph nodes: a diagnostic accuracy study. Medicine (Baltimore) 2017;96:e8125. doi: 10.1097/MD.0000000000008125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Lin X, Chen X, Zheng B. Noninvasive evaluation of benign and malignant superficial lymph nodes by virtual touch tissue quantification: a pilot study. J Ultrasound Med. 2016;35:571–575. doi: 10.7863/ultra.15.05053. [DOI] [PubMed] [Google Scholar]


