Abstract
Vitamin D deficiency is a severe worldwide health issue. Edible mushrooms are an excellent vitamin D2 source and have gained popularity worldwide as a nutritional food. The objective of this study was to investigate the conversion efficiency of ergosterol to vitamin D2 in Agaricus bisporus and Cordyceps militaris mushrooms under ultraviolet (UV) irradiation directly through dry powder or in ethanol suspension (1:20 g/mL, solid to liquid ratio). Several parameters of UV irradiation conditions such as the material form (dry powder or dry powder in ethanol suspension), exposure time (30, 60, or 120 min), wavelength type (UV-C, UV-B, or UV-A), wavelength combination (UV-C plus UV-B, UV-C plus UV-A, UV-B plus UV-A, or UV-C plus UV-B plus UV-A), and wavelength sequence (UV-C → UV-B, UV-C → UV-A, UV-B → UV-A, or UV-C → UV-B → UV-A), were optimized. Under the optimal UV irradiation conditions (dry powder in ethanol suspension irradiated with UV-C at 40 cm for 120 min), vitamin D2 concentrations increased from not detectable to 72 μg/g (dw) in the A. bisporus dry powder and 1104 μg/g (dw) (about 15-fold increase) in the ethanol suspension. After UV irradiation, the vitamin D2 concentration increased from undetectable to 57 μg/g (dw) in the C. militaris dry powder. In contrast, UV irradiation increased the concentration to 877 μg/g (dw) (about 15-fold higher) in the ethanol suspension. Comparison of the effect of various wavelength combinations showed that UV-C irradiation is more effective than UV-A or UV-B. Furthermore, when irradiated by UV-C at a 40 cm irradiation distance in the ethanol suspension, the increase in vitamin D2 in A. bisporus and C. militaris mushrooms was time- or dose-dependent. The conversion rate of vitamin D2 was low to undetectable under dry powder irradiation, but its ergosterol loss rate was higher than in ethanol suspension irradiation. The ergosterol loss rate in dry C. militaris mushrooms was higher than in the dry A. bisporus mushroom powder. Ultraviolet irradiation in ethanol suspension could greatly increase the vitamin D2 concentration than directly on the dry powder and thus make edible mushrooms more practical as a natural vitamin D source for consumers after entirely removing the ethanol.
1. Introduction
Vitamin D plays an essential role in calcium and phosphorus metabolism and skeletal and neuromuscular homeostasis.1 Vitamin D deficiency is a major global health issue, particularly in North America, Europe, and the Middle East, where sunlight exposure is limited, especially during the winter. Rickets is a classic symptom of vitamin D deficiency in children, while long-term vitamin D deficiency in adults can eventually cause osteoporosis.2 In addition, insufficient vitamin D intake often affects the endocrine pancreas and the immune system, leading to various diseases.3 Vitamin D deficiency is known to be associated with various diseases, such as cancer,4 heart disease,5 arterial stiffness,6 neuropsychiatric disorders,7 and diabetes.8 Furthermore, vitamin D has been used as an adjunct to treat COVID-19, which left more than three million people dead as of May 18, 2021.9
Mushrooms are a unique source of compounds such as polyphenols, amino acids (i.e., ergothioneine), polysaccharides (i.e., β-glucans), terpenoids, vitamins (i.e., vitamin D2), and sterols (i.e., ergosterol). These active components have been linked to antioxidant, anticancer, antidiabetic, anti-inflammatory, hepatoprotective, antiallergic, antimicrobial, and antiviral activities.10Cordyceps militaris has been extensively studied for its pharmacological activities such as antitumor, anti-inflammation, and immune regulation.11 Because of their beneficial properties, mushrooms have become attractive as functional foods or a source of ingredients that can be extracted and incorporated into food products.
Mushrooms usually contain very little vitamin D2, as many are grown in the dark. But mushrooms are notable for being rich in ergosterol (a precursor of vitamin D2), which can be transformed to vitamin D2 by applying natural or artificial ultraviolet (UV) irradiation.12 UV light consists of three subregions of wavelengths: UV-C (190–290 nm), UV-B (290–320 nm), and UV-A (320–400 nm).13 The exposure of mushrooms to UV irradiation triggers vitamin D2 formation. However, the transformation efficiency is affected by many factors, including different types of mushrooms,14 UV wavelengths,15 irradiation times,16 irradiation intensities or doses,17 irradiation parts,18 and moisture contents.19 Previous research has focused on the fresh or dried mushrooms directly treated with single-wavelength UV-A, UV-B, or UV-C irradiation.17,20 For example, the concentration of vitamin D2 in shiitake mushrooms was increased after exposure to UV-B at a dose of 25 kJ/m2. As the exposure area increased, the vitamin D2 concentration was increased more effectively by exposure slices than exposure to the gill or pileus of the whole mushrooms.17a UV-C irradiation provides an efficient way to increase the vitamin D2 concentration in mushrooms.20 Improving the nutritional value of mushrooms with UV irradiation, making it a facile available source of vitamin D, deserves further investigation.
Fresh mushrooms deteriorate rapidly after harvest because of the high water content. Therefore, drying is typically used to extend its shelf life. Therefore, exposing dried edible fungi to UV irradiation to increase the vitamin D2 content can further increase the nutritional value of edible fungi and improve the current situation of vitamin D deficiency. However, most existing literature focused on the content changes of the vitamin D2 single compound. However, in the process of ultraviolet irradiation of dried edible fungi, the changes in ergosterol and vitamin D2 content in edible fungi under various irradiation conditions are of great significance for the development and application of foods and drugs to enhance ergosterol and vitamin D2. Still, there are a few studies on ultraviolet irradiation to improve vitamin D2 of C. militaris.
Hence, this study focused on the ergosterol and vitamin D2 content treated with UV irradiation in Agaricus bisporus and C. militaris mushroom powders directly or in ethanol suspension with different irradiation distances and times, different single wavelengths, various combinations of wavelengths, and different sequences of combined wavelengths.
2. Materials and Methods
2.1. Materials
Fresh A. bisporus (Strain W-192) and C. militaris (Strain JN-168) mushrooms were purchased from a local supermarket supplied by Yinong Co. (Mianxian, Shaanxi, China, 33.15° N and 106.67° E) and Meiao Co. (Dongying, Shandong, China, 38.15° N and 118.50° E), respectively. The analytical-grade anhydrous alcohol and the chromatographic-grade formic acid and acetonitrile were purchased from Aladdin Co. (Shanghai, China). The standard vitamin D2 and ergosterol were provided by Aldrich Co. (GR, Shanghai, China).
2.2. Sample Preparation
The preparation of the mushroom powder was carried out by reference methods21.
2.3. UV Irradiation
2.3.1. Exposure with Different Irradiation Material Forms
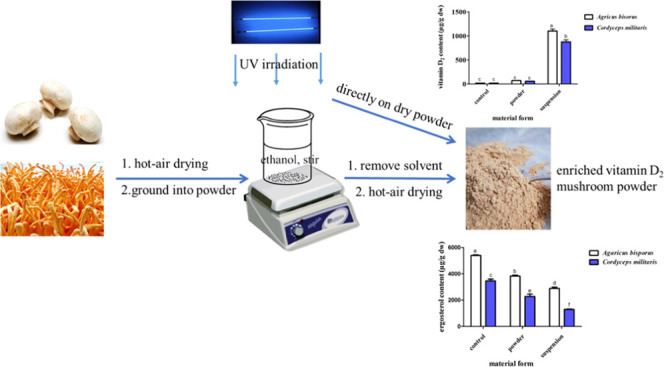
The mushroom dry powder (4.0 g) and anhydrous alcohol (80 mL) were mixed in a 250 mL glass beaker with a material–liquid ratio of 1:20 (g/mL). The mixtures were exposed to UV-C irradiation in a UV chamber (the length, width, and height are 40, 50, and 50 cm, respectively) with nine UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) at a distance of 40 cm from the UV lamps for 2 h with magnetic stirring at 500 rpm/min (C-MAG HS7 digital magnetic stirrer, IKA Co., Staufen, Germany). The irradiation intensity was 2.74 mW/cm2, and the calculated exposure dose was 197.28 kJ/m2. Ethanol was then entirely removed at 40 °C under 0.1 MPa by a rotary evaporator (RV 10, IKA Co., Staufen, Germany). The mushroom powder was further dried21 and named as the suspension sample. Correspondingly, identical quantities of A. bisporus and C. militaris mushroom powders were directly treated with UV-C radiation for 2 h by interval stirring using a stainless steel fork every 10 min in a beaker. The obtained mushroom solid was named as the powder sample. Conversely, the A. bisporus or C. militaris mushroom powder free of any irradiation was used as a control sample.21,22
2.3.2. Exposure to UV-C with Different Irradiation Times and Distances
The mixtures (mushroom dry powder or dry powder in ethanol suspension) were irradiated by UV-C at a distance of 30 or 40 cm from UV lamps in a UV chamber (the length, width, and height are 40, 50, and 50 cm, respectively) with nine UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) for 0, 30, 60, or 120 min with magnetic stirring at 500 rpm/min. The exposure intensities were 3.29 and 2.74 mW/cm2 at distances of 30 and 40 cm, respectively. The sample processing method used was the same as described in Section 2.3.1.21
2.3.3. Irradiation with Different Single Wavelengths
The mixtures (mushroom dry powder or dry powder in ethanol suspension) were irradiated with UV-A, or UV-B, or UV-C at a distance of 10 cm from the UV lamps in a UV chamber (the length, width, and height are 30, 27, and 16 cm, respectively) for 2 h. The exposure intensities were 4.81, 1.67, or 1.11 mW/cm2, respectively. The sample processing method used was the same as described in Section 2.3.1.21
2.3.4. Irradiation with Different Combinations of Wavelengths
The mixtures (mushroom dry powder or dry powder in ethanol suspension) were exposed to UV-C plus UV-B, UV-C plus UV-A, UV-B plus UV-A, or UV-C plus UV-B plus UV-A radiation at a distance of 10 cm from the UV lamps in a UV chamber (the length, width, and height are 30, 27, and 16 cm, respectively) for 2 h. The sample processing method is the same as described in Section 2.3.1.21
2.3.5. Irradiation with Different Sequences of Combined Wavelengths
The mixtures (mushroom dry powder or dry powder in ethanol suspension) were exposed to different sequences of combined wavelengths, i.e., UV-C (2 h) → UV-B (2 h), UV-C (2 h) → UV-A (2 h), UV-B (2 h) → UV-A (2 h), or UV-C (2 h) → UV-B (2 h) → UV-A (2 h) at a distance of 10 cm from the UV lamps in a UV chamber (the length, width, and height are 30, 27, and 16 cm, respectively). The sample processing method is the same as described in Section 2.3.1.21
2.4. Sample Preparation
Ergosterol and vitamin D2 were extracted without separation following our previous method.21
2.5. Qualitative and Quantitative Determination of Ergosterol and Vitamin D2
Qualitative and quantitative determination of ergosterol and vitamin D2 was conducted by HPLC analysis following our reported method.21
2.6. Statistical Analysis
Analysis of variance (ANOVA) was used to analyze the data. Significant differences between treatments were determined using Duncan’s HSD. SPSS 16.0 for Windows was used to conduct all statistical procedures. A significance level of p < 0.05 was selected to separate the mean for all analyses.
3. Results and Discussion
3.1. Influence of Different Material Forms on the Vitamin D2 and Ergosterol Concentrations in Mushrooms A. Bisporus and C. Militaris Exposed to UV-C Irradiation with Different Material Forms
Figure 1 shows the influence on vitamin D2 and ergosterol concentrations in mushrooms A. bisporus and C. militaris exposed to UV-C irradiation with different material forms. Material forms significantly affect the vitamin D2 content. In both mushrooms, A. bisporus and C. militaris, the content of vitamin D2 irradiated upon dry powder was remarkably lower than that irradiated in ethanol suspension.
Figure 1.
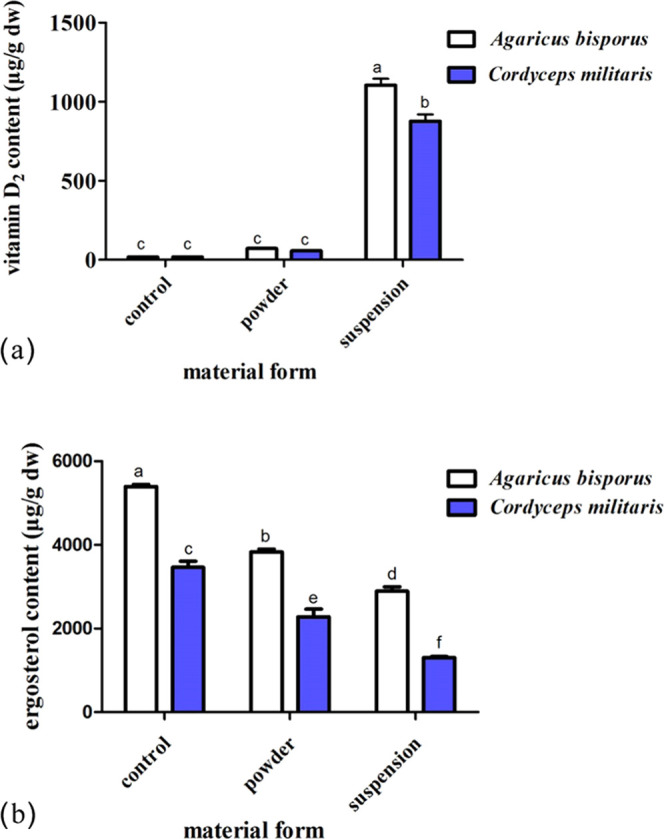
Influence of different material forms on the vitamin D2 (a) and ergosterol (b) concentrations in mushrooms A. bisporus and C. militaris exposed to UV irradiation with different material forms. Different letters indicate significant differences (p < 0.05).
The results indicated that the content of vitamin D2 in mushrooms A. bisporus and C. militaris could significantly increase by exposure to UV-C in an alcohol suspension. The vitamin D2 content was increased by 6152 and 4845% in mushrooms A. bisporus and C. militaris, respectively (p < 0.05) (Figure 1a). Nevertheless, the ergosterol content was significantly lost in A. bisporus and C. militaris after 2 h of treatment, which was about 46 and 62%, respectively (Figure 1b). Therefore, it can be speculated that ergosterol may be transformed into other photoisomerization products such as tachysterol and lumisterol under UV treatment a in dry powder state. However, the structures and concentrations of photoisomerization products need to be further verified in future studies.
For the mushrooms A. bisporus, the vitamin D2 content increased (p < 0.05) with an increased rate of 36 to 72 μg/g for the dry powder form. However, the vitamin D2 content significantly increased (p < 0.05) from not detectable to 1104 μg/g with an increased rate (a value divided by the total duration of the experiment) of 552 μg/h for the ethanol suspension. Therefore, the content of vitamin D2 exposure in ethanol suspension is about 15 times that of dry powder (Figure 1a). In contrast with the control, the content of ergosterol decreased (p < 0.05) to only 29 and 46% for the dry powder and the ethanol suspension, respectively (Figure 1b).
For the mushrooms C. militaris, the vitamin D2 content increased (p > 0.05) with an increased rate of 28–57 μg/g for the dry powder form. However, the vitamin D2 content significantly increased (p < 0.05) from not detectable to 877 μg/g with an increased rate of 438 μg/h for the ethanol suspension (Figure 1a). In contrast with the control, the content of ergosterol decreased (p < 0.05) to only 34 and 62% for the dry powder and the ethanol suspension, respectively (Figure 1b).
It has been reported that 70–80% was the optimum moisture content of mushrooms when exposed to UV irradiation.23 Vitamin D2 could be easily oxidized and photodegraded as the exposure to an oxygen and oxidative atmosphere increased.24 These research studies indicate that mushrooms with UV irradiation in the ethanol suspension were more effective in increasing vitamin D2 concentration compared with direct exposure of the dry powder. This result can be interpreted as follows: when irradiated in ethanol suspension with continuous stirring, the exposure area and UV penetration increased, and the oxidation and photodegradation of vitamin D2 could be avoided.
3.2. Influence of Different Material Forms on the Vitamin D2 and Ergosterol Concentrations in Mushrooms A. Bisporus and C. Militaris Exposed to UV-C Irradiation at Different Times and Distances
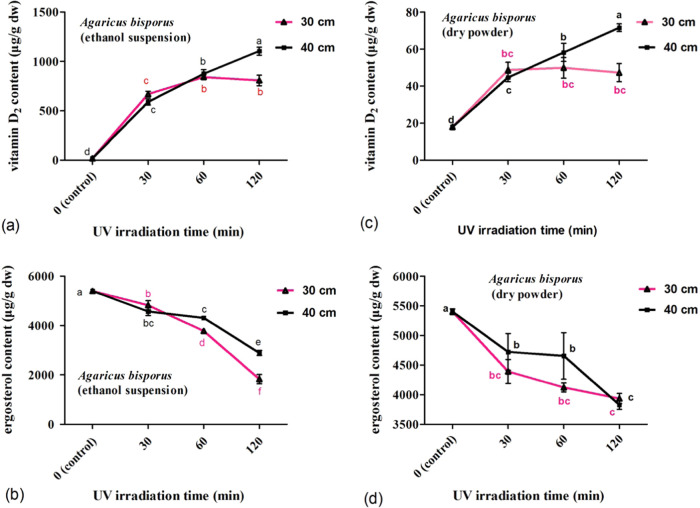
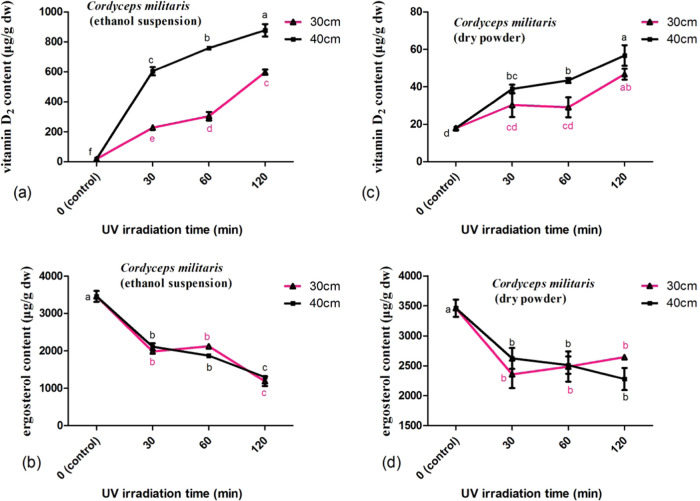
Irradiation with UV-C could increase (p < 0.05) the vitamin D2 content in a time or dose-dependent manner for mushrooms A. bisporus and C. militaris. But the increase rates were decreased with prolonged irradiation time. Correspondingly, the preferable irradiation distance was 40 cm (Figures 2a–d and 3a–d). UV-C irradiation decreased (p < 0.05) the ergosterol content in mushrooms A. bisporus and C. militaris. The content of ergosterol in mushrooms A. bisporus was less impacted than that in mushrooms C. militaris.
Figure 2.
Influence of UV irradiation on the vitamin D2 and ergosterol concentrations in mushrooms A. bisporus exposed to UV irradiation at different times and distances. The concentrations of vitamin D2 (a) and ergosterol (b) in A. bisporus mushroom irradiated in ethanol suspension by various treatments. The concentrations of vitamin D2 (c) and ergosterol (d) in A. bisporus mushroom irradiated upon dry powder by various treatments.
Figure 3.
Influence of UV irradiation at different times and distances on the vitamin D2 and ergosterol concentrations in mushrooms C. militaris. The concentrations of vitamin D2 (a) and ergosterol (b) in the C. militaris mushroom irradiated in ethanol suspension by various treatments. The concentrations of vitamin D2 (c) and ergosterol (d) in the C. militaris mushroom irradiated upon dry powder by various treatments.
For mushroom A. bisporus irradiated in ethanol suspension at 30 cm from the UV lamps, the content of vitamin D2 increased gradually (p < 0.05) from not detectable to 670 and 808 μg/g for 30 and 120 min irradiation, respectively. The increase rates decreased (p < 0.05) steadily from 1340 to 404 μg/h (Figure 2c). The content of ergosterol decreased (p < 0.05) steadily from 89 and 34% for 30 and 120 min irradiation, respectively (Figure 2d). When irradiated in ethanol suspension at a 40 cm distance, the vitamin D2 content in mushrooms A. bisporus increased (p < 0.05) from not detectable to 591 and 1104 μg/g for 30 and 120 min irradiation, respectively. The increase rates decreased (p < 0.05) gradually from 1182 to 552 μg/h (Figure 2c). Correspondingly, the content of ergosterol decreased (p < 0.05) steadily from 85 and 54% for 30 and 120 min irradiation, respectively (Figure 2d).
For mushroom C. militaris irradiated in ethanol suspension 30 cm from the UV lamps, the vitamin D2 content increased (p < 0.05) gradually from not detectable to 304 and 597 μg/g for 30 and 120 min irradiation, respectively. The increase rates decreased (p < 0.05) steadily from 608 to 299 μg/h (Figure 3c).
Correspondingly, the content of ergosterol decreased (p < 0.05) steadily from 57 and 34% for 30 and 120 min irradiation, respectively (Figure 3d). When irradiated in ethanol suspension at a 40 cm distance, the vitamin D2 content in mushrooms C. militaris increased (p < 0.05) from not detectable to 604 and 877 μg/g for 30 and 120 min irradiation, respectively. The increase rates decreased (p < 0.05) gradually from 1208 to 439 μg/h (Figure 3c). Correspondingly, the content of ergosterol decreased (p < 0.05) steadily from 61 and 38% for 30 and 120 min irradiation, respectively (Figure 3d).
Irradiation with UV-C tended to decrease the content of ergosterol for mushrooms A. bisporus and C. militaris. More ergosterol content decreased with the prolonged irradiation time. In addition, this trend in mushrooms C. militaris was more evident than that in mushrooms A. bisporus. With the extension of the irradiation time, the ergosterol content of A. bisporus in ethanol suspension decreased significantly (p < 0.05). The difference was significant among different irradiation distances at the same time. The ergosterol content in C. militaris gradually decreased with an increase in the irradiation time (p > 0.05), and there was no significant difference between different irradiation distances at the same time. For example, with UV-C exposure at 40 cm for 120 min, the content of ergosterol in mushrooms A. bisporus decreased (p < 0.05) to 71 and 54% upon dry powder and in ethanol suspension, respectively. In contrast, the content of ergosterol in mushrooms C. militaris decreased (p < 0.05) to 66 and 38% (Figures 2b,d and 3b,d). Previous studies showed that for fresh white and brown button mushrooms, the content of ergosterol decreased insignificantly for the UV-C treatment of 50, 100, and 200 s.25 UV-C irradiation of fresh white button mushrooms resulted in no significant reduction in the ergosterol content.26
The content of ergosterol in edible mushrooms differed from various strains after UV exposure.13 With the prolonged UV-B or UV-C irradiation time, the content of ergosterol remarkably decreased for mushrooms A. bisporus but inversely increased for mushrooms A. bitorquis and Volvariella volvacea.(13) For example, after UV-B or UV-C irradiation, the content of ergosterol in mushrooms A. bitorquis, V. volvacea, and L. edodes increased to 502% for 2 h, 126% for 1 h, and 125% for 1 h compared with untreatment samples.13 After UV-B irradiation for dry powder, the content of ergosterol increased in button mushrooms but decreased in all other mushroom types.14 This study indicated that the content of ergosterol in mushrooms C. militaris lowered (p < 0.05) from 3461 μg/g of unexposure to 1984 μg/g for 30 min of irradiation in ethanol suspension and then increased (p > 0.05) to 2122 μg/g for 60 min (Figure 3d). Further research is needed to understand the change of ergosterol as a result of UV-C irradiation.
For both mushrooms A. bisporus and C. militaris, UV-C exposure could promote vitamin D2 transformation, but the increased rates in mushrooms C. militaris were lower than in mushrooms A. bisporus. Nevertheless, the increased rates in both A. bisporus and C. militaris mushrooms are far beyond reported by Mau et al.13 This discrepancy most likely is due to the higher irradiation intensity and the suspension of ethanol.
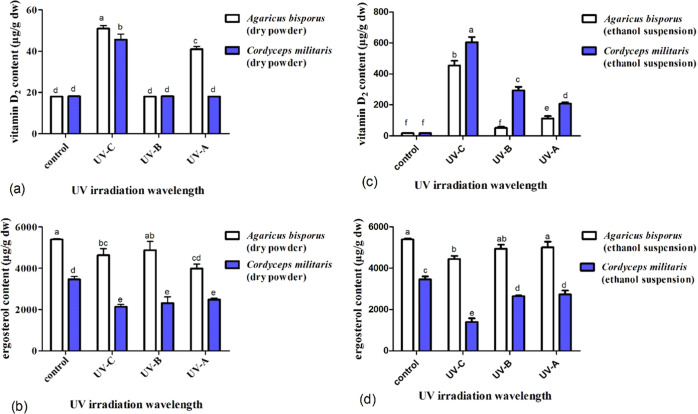
3.3. Effect of UV Irradiation at Different Single Wavelengths on the Vitamin D2 and Ergosterol Concentrations in Mushrooms A. Bisporus and C. Militaris
Various irradiation wavelengths greatly impact the content of vitamin D2, and the influences vary with different mushrooms. For the mushrooms A. bisporus, both UV-C and UV-A exposures are more efficient than UV-B exposure. The vitamin D2 content increased about 2.8 times and 2.3 times upon dry powder and 8.8 times and 2.2 times in ethanol suspension compared to UV-B. There is a significant difference between UV-A and UV-C (Figure 4a,c). When irradiated upon dry powder, the content of vitamin D2 increased from not detectable for the untreated control to 51 μg/g for UV-C (p < 0.05), followed by 41 μg/g for UV-A (p < 0.05) and not detectable for UV-B (p > 0.05), with the increase rates gradually decreased from 26 μg/h for UV-C to 21 μg/h for UV-A (p < 0.05) (Figure 4a). When irradiated in ethanol suspension, the vitamin D2 content increased from not detectable for the untreated control to 454 μg/g for UV-C (p < 0.05), followed by 113 μg/g for UV-A (p < 0.05) and 52 μg/g for UV-B (p > 0.05), with the increase rates gradually decreased from 227 μg/h for UV-C to 57 μg/h for UV-A (p < 0.05) (Figure 4c). Correspondingly, the conversion rate of vitamin D2 was low to undetectable under dry powder irradiation, but its ergosterol loss rate was higher than that in ethanol suspension irradiation. For example, the concentration of ergosterol steadily decreased (p < 0.05) to 90% for UV-B, 74% for UV-A, and 86% for UV-C when irradiated upon dry powder (Figure 4b). When irradiated in ethanol suspension, the concentration of ergosterol steadily decreased (p < 0.05) to 92% for UV-B, 93% for UV-A, and 82% for UV-C (Figure 4d). When irradiated upon dry powder for UV-B, 10% ergosterol was lost even though vitamin D2 was undetectable (Figure 4a,b).
Figure 4.
Influence of UV irradiation at different single wavelengths on the vitamin D2 and ergosterol concentrations in mushrooms A. bisporus and C. militaris. The concentrations of vitamin D2 (a) and ergosterol (b) in A. bisporus and C. militaris mushrooms irradiated upon dry powder by various treatments. The contents of vitamin D2 (c) and ergosterol (d) in A. bisporus and C. militaris mushrooms irradiated in ethanol suspension by various treatments.
For the C. militaris mushrooms, UV-C irradiation is more effective than UV-B and UV-A irradiation. In dry powder, the content of vitamin D2 is 46 μg/g for UV-C compared to undetectable for both UV-B and UV-A. The content of vitamin D2 is approximately 2.1 and 2.9-fold increased in ethanol suspension compared to UV-B and UV-A, and there is a significant difference between UV-B and UV-A (Figure 4a,c). When irradiated in ethanol suspension, the vitamin D2 content increased from not detectable for the untreated control to 605 μg/g for UV-C (p < 0.05), followed by 294 μg/g for UV-B (p < 0.05) and the minimum value of 208 μg/g for UV-A (p > 0.05), with rates gradually decreased (p < 0.05) from 303 μg/h for UV-C, 147 μg/h for UV-B, and 104 μg/h for UV-A (Figure 4c). In contrast, the conversion rate of vitamin D2 was low to undetectable under dry powder irradiation, but its ergosterol loss rate was higher than that in ethanol suspension irradiation. For example, the ergosterol content sharply decreased to 40% for UV-C (p < 0.05), 76% for UV-B (p > 0.05), and 79% for UV-A (p < 0.05) (Figure 4d). When irradiated upon dry powder, the concentration of ergosterol steadily decreased (p < 0.05) to 62% for UV-C, 67% for UV-B, and 72% for UV-A (Figure 4b). When irradiated upon dry powder for UV-B and UV-A, 33 and 29% ergosterol were lost, respectively, even though vitamin D2 was undetectable (Figure 4a,b).
UV irradiation could remarkably increase the content of vitamin D2 in edible mushrooms, and an essential factor is the UV irradiation wavelength. In addition, tachysterol is more likely to be produced by light treatment with a shorter wavelength, while the content of lumisterol is increased with a longer wavelength.14
Exposing the gills of mushrooms to UV-A irradiation for 2 h, one could obtain high levels of vitamin D2. Among the four edible mushrooms tested, the maximum vitamin D2 conversion was obtained for oyster mushrooms. Conversely, the minimum conversion was obtained for button mushrooms under a 2 h exposure in a calculated dosage of 25.2 kJ/m2.23
When exposed to UV-B irradiation, the vitamin D2 content increased for fresh and dried mushrooms.22,27 In addition, dried mushrooms can produce ergocalciferol under UV-B irradiation.27 The content of vitamin D2 in lyophilized mushrooms A. bisporus was 42–119 μg/g (dw), and that in hot-air dried mushrooms was 22–81 μg/g (dw).27 Compared with untreated samples, the vitamin D2 content in the caps and stems increased for both brown and white button mushrooms exposed to UV-C irradiation.25
3.4. Influence of UV Irradiation at Different Combination Wavelengths on the Vitamin D2 and Ergosterol Concentrations in Mushrooms A. Bisporus and C. Militaris
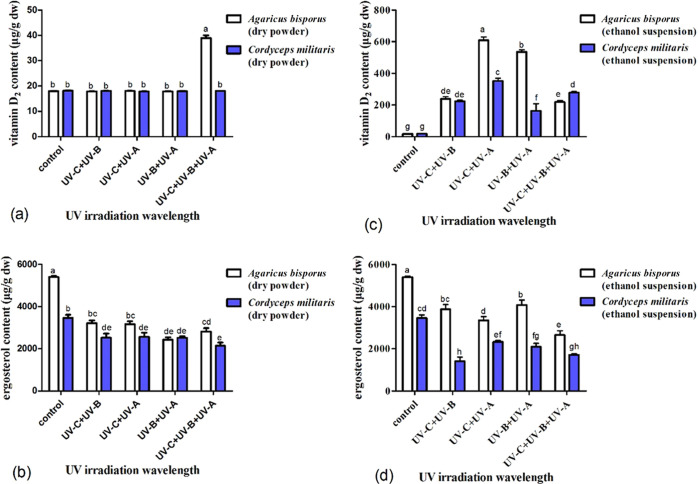
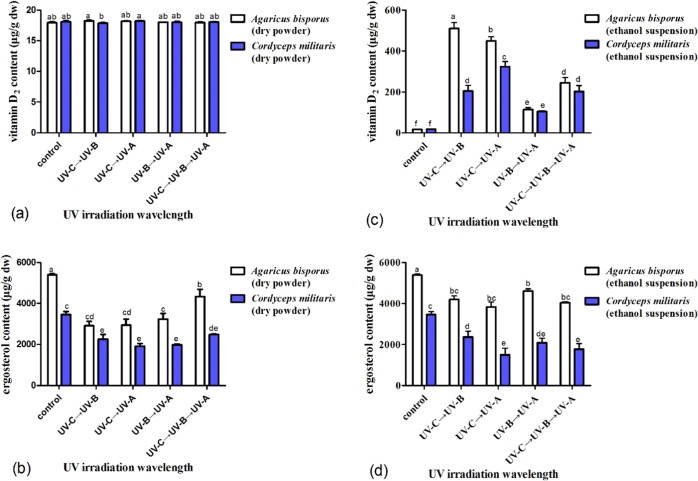
Various combinations of irradiation wavelengths significantly impact the content of vitamin D2, and the influences vary with different mushrooms. For the A. bisporus mushrooms, the content of ergosterol was sharply decreased (p < 0.05) even though vitamin D2 was undetectable when irradiated upon dry powder for UV-C plus UV-B, UV-C plus UV-A, and UV-B plus UV-A. When irradiated upon dry powder, the vitamin D2 concentration only increased (p < 0.05) to 39 μg/g for UV-C plus UV-B plus UV-A, but was still undetectable for UV-C plus UV-B, UV-C plus UV-A, and UV-B plus UV-A (Figure 5a). But the concentration of ergosterol sharply decreased (p < 0.05) to 45% for UV-B plus UV-A, 52% for UV-C plus UV-B plus UV-A, 58% for UV-C plus UV-A, and 60% for UV-C plus UV-B (Figure 5b). When irradiated in ethanol suspension, the vitamin D2 concentration increased (p < 0.05) from not detectable to a maximum value of 609 μg/g for UV-C plus UV-A, followed by 536 μg/g for UV-B plus UV-A, 239 μg/g for UV-C plus UV-B, and a minimum value of 219 μg/g for UV-C plus UV-B plus UV-A, with increase rates steadily decreasing from 305 to 268 (p < 0.05), 120 (p < 0.05), and 110 μg/h (p < 0.05). The difference between UV-C plus UV-A, UV-B plus UV-A, or UV-C plus UV-B was significant, but there was a significant difference between UV-C plus UV-B and UV-C plus UV-B plus UV-A (Figure 5c). Correspondingly, the concentration of ergosterol gradually decreased (p < 0.05) to 76% for UV-B plus UV-A, 72% for UV-C plus UV-B, 62% for UV-C plus UV-A, and 49% for UV-C plus UV-B plus UV-A. The difference in the ergosterol concentration between UV-C plus UV-B plus UV-A and UV-C plus UV-B was insignificant. But there was a significant difference between UV-C plus UV-A, UV-B plus UV-A to UV-C plus UV-B plus UV-A or UV-C plus UV-B (Figure 5d).
Figure 5.
Influence of UV irradiation at different combinations of wavelengths on the vitamin D2 and ergosterol concentrations in mushrooms A. bisporus and C. militaris. The concentrations of vitamin D2 (a) and ergosterol (b) in A. bisporus and C. militaris mushrooms irradiated upon dry powder by various treatments. The contents of vitamin D2 (c) and ergosterol (d) in A. bisporus and C. militaris mushrooms irradiated in ethanol suspension by various treatments.
For the C. militaris mushrooms, when irradiated upon dry powder, the vitamin D2 content is still not detectable for four treatments (Figure 5a), but the content of ergosterol gradually decreased (p < 0.05) to 74% for UV-C plus UV-A, 73% for UV-C plus UV-B and UV-B plus UV-A, and 62% for UV-C plus UV-B plus UV-A (Figure 5b). On the other hand, when irradiated in ethanol suspension, the vitamin D2 content increased (p < 0.05) from not detectable to a maximum value of 352 μg/g for UV-C plus UV-A, followed by 278 μg/g for UV-C plus UV-B plus UV-A, 223 μg/g for UV-C plus UV-B, and a minimum value of 163 μg/g for UV-B plus UV-A, with increase rates steadily decreasing from 176 to 139 (p < 0.05), 112 (p < 0.05), and 82 μg/h (p < 0.05). Therefore, the difference between UV-C plus UV-A, UV-B plus UV-A, UV-C plus UV-B plus UV-A or UV-C plus UV-B was significant. But, there was no significant difference between UV-C plus UV-B and UV-C plus UV-B plus UV-A, similar to the mushrooms A. bisporus (Figure 5c). Correspondingly, the content of ergosterol gradually decreased (p < 0.05) to 67% for UV-C plus UV-A, 61% for UV-B plus UV-A, 50% for UV-C plus UV-B plus UV-A, and 41% for UV-C plus UV-B (Figure 5d).
3.5. Influence of UV Irradiation at Different Sequences of Combined Wavelengths on the Vitamin D2 and Ergosterol Concentrations in Mushrooms A. Bisporus and C. Militaris
Various sequences of combined wavelengths greatly affect the vitamin D2 content, which is different with varied mushrooms. For the A. bisporus mushroom, when irradiated upon dry powder, the vitamin D2 content is still not detectable for four treatments (Figure 6a). But the concentration of ergosterol gradually decreased (p < 0.05) to 80% for UV-C → UV-B → UV-A, 60% for UV-B → UV-A, and 54% for UV-C → UV-B and UV-C → UV-A (Figure 6b). When irradiated in ethanol suspension, the vitamin D2 concentration increased (p < 0.05) from not detectable for the untreated control to a maximum of 510 μg/g for UV-C → UV-B, followed by 449 μg/g for UV-C → UV-A, and 245 μg/g for UV-C → UV-B → UV-A, and a minimum of 114 μg/g for UV-B → UV-A, with the rate of increase decreased gradually from 128 to 112 (p < 0.05), 41 (p < 0.05), and 29 μg/h (p < 0.05). The difference between these four treatments was significant (Figure 6c). Correspondingly, the concentration of ergosterol decreased steadily to 85% for UV-B → UV-A (p < 0.05), 78% for UV-C → UV-B (p < 0.05), 75% for UV-C → UV-B → UV-A (p < 0.05), and 71% for UV-C → UV-A (p < 0.05) (Figure 6d). This result is consistent with the sequential wavelength irradiation of a Lentinus edode ethanol suspension, but the ergosterol loss rate was higher than that of L. edodes treatment.
Figure 6.
Influence of UV irradiation at different sequences of combined wavelengths on the vitamin D2 and ergosterol concentrations in mushrooms A. bisporus and C. militaris. The concentrations of vitamin D2 (a) and ergosterol (b) in A. bisporus and C. militaris mushrooms irradiated upon dry powder by various treatments. The contents of vitamin D2 (c) and ergosterol (d) in A. bisporus and C. militaris mushrooms irradiated in ethanol suspension by various treatments.
For the C. militaris mushrooms, when irradiated upon dry powder, the vitamin D2 concentration is still not detectable for the four treatments (Figure 6a), but the ergosterol concentration gradually decreased (p < 0.05) to 72% for UV-C → UV-B → UV-A, 65% for UV-C → UV-B, 57% for UV-B → UV-A, and 55% for UV-C → UV-A (Figure 6b). When irradiated in ethanol suspension, the vitamin D2 concentration increased (p < 0.05) from not detectable for the untreated control to 322 μg/g for UV-C → UV-A, followed by 205 μg/g for UV-C → UV-B, 203 μg/g for UV-C → UV-B → UV-A, and 105 μg/g for UV-B → UV-A, with the rate of increase gradually decreased (p > 0.05) from 81 to 51, 33, and 26 μg/h. (Figure 6c). This tendency was consistent with the results of sequential wavelength irradiation of Pleurotus ostreatus in ethanol suspension, but the content of vitamin D2 varies with the variety of edible fungi. The concentration of ergosterol steadily decreased to 76% for UV-C → UV-B, 60% for UV-B → UV-A (p < 0.05), 51% for UV-C → UV-B → UV-A (p > 0.05), and 43% for UV-C → UV-A (p > 0.05). But, the difference between UV-C → UV-A, UV-B → UV-A, and UV-C → UV-B → UV-A was insignificant. The ergosterol loss rate of C. militaris irradiated in ethanol suspension was higher than in A. bisporus, shiitake mushrooms, and oyster mushrooms (Figure 6d). For A. bisporus and C. militaris mushrooms, concentrations of ergosterol and vitamin D2 varied with the different sequences of combined wavelength treatments.
After UV irradiation, provitamin D could absorb energy and undergo several reverse photoreactions. The referred isomers mainly include ergosterol, provitamin D2, vitamin D2 tachysterol, and lumisterol. This study focused on the effects of a different single wavelength and combined wavelength treatments on the content of ergosterol and vitamin D2. The transformation mechanism between different isomers remains to be further investigated,
4. Conclusions
The results obtained from the present study demonstrated that the irradiated approach could enormously increase the vitamin D2 content. For both A. bisporus and C. militaris mushrooms, samples exposed to UV irradiation in ethanol suspension could greatly increase the vitamin D2 content than that directly upon dry powder. The vitamin D2 content increased with the increase in the UV irradiation exposure time and dosage. However, the prolonging of exposure time resulted in the reduction of the increase rate. Moreover, the irradiation distance of 40 cm was preferable. UV-A or UV-C treatment or a combination of both were more effective than UV-B irradiation. Under the optimal UV irradiation conditions (dry powder in ethanol suspension with UV-C exposure at 40 cm for 120 min), the concentrations of vitamin D2 increased from not detectable to 1104 μg/g (dw) and 877 μg/g (dw) in A. bisporus and C. militaris mushroom powders in the ethanol suspension, respectively. In addition, the ergosterol content was decreased with the increase in vitamin D2 content, and the ergosterol loss rate in dry C. militaris mushrooms was higher than that in dry A. bisporus mushrooms. Thus, exposure to UV irradiation in ethanol suspension efficiently increases the vitamin D2 content, and consequently, enhances the nutritional value of edible mushrooms, increases natural sources of vitamin D supplementation, and is beneficial to consumer health after thoroughly removing the solvent ethanol.
Acknowledgments
The authors wish to thank Ye Zhang and Yazhou Li for data determination.
This study was funded by the Key Project of Agricultural Science and Technology of Shaanxi Province (2021NY-154), the Postdoctoral Program in Shaanxi University of Technology (SLGBH16–04), and the Research Project of Shaanxi Provincial Education Department (20JS023).
The authors declare no competing financial interest.
References
- Taofiq O.; Fernandes Â.; Barros L.; Barreiro M. F.; Ferreira I. C. F. R. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Tech. 2017, 70, 82–94. 10.1016/j.tifs.2017.10.008. [DOI] [Google Scholar]
- Holick M. F. The vitamin D deficiency pandemic:a forgotten hormone important for health. Public Health Rev. 2010, 32, 267–283. 10.1007/BF03391602. [DOI] [Google Scholar]
- a Arabi S. M.; Bahrami L. S.; Ranjbar G.; Tabesh H.; Norouzy A. The effect of vitamin D supplementation on inflammation in critically ill patients: A systematic review. Pharma Nutrition 2020, 13, 100196 10.1016/j.phanu.2020.100196. [DOI] [Google Scholar]; b Wu Y. F.; Cai Y.; Liu M. Y.; Zhu D. S.; Guan Y. T. The potential immunoregulatory roles of vitamin D in neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2020, 43, 102156 10.1016/j.msard.2020.102156. [DOI] [PubMed] [Google Scholar]
- Messaritakis I.; Koulouridi A.; Sfakianaki M.; Vogiatzoglou K.; Gouvas N.; Athanasakis E.; Tsiaoussis J.; Xynos E.; Mavroudis D.; Tzardi M.; Souglakos J. The role of vitamin D receptor gene polymorphisms in colorectal cancer risk. Cancers 2020, 12, 1379. 10.3390/cancers12061379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar W. A.; Fawzy A.; Allam R. M.; Amer R. M.; Hamed M. S. Maternal vitamin D level and vitamin D receptor gene polymorphism as a risk factor for congenital heart diseases in offspring; An Egyptian case-control study. Genes Dis. 2019, 6, 193–200. 10.1016/j.gendis.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. C.; Hsu C. Y.; Mao P. C. M.; Dreyer G.; Wu F. Z.; Chen C. L. The effects of correction of vitamin D deficiency on arterial stiffness: A systematic review and updated meta-analysis of randomized controlled trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105561 10.1016/j.jsbmb.2019.105561. [DOI] [PubMed] [Google Scholar]
- Kim S. Y.; Jeon S. W.; Lim W. J.; Oh K. S.; Shin D. W.; Cho S. J.; Park J. H.; Kin Y. H.; Shin Y. C. Vitamin D deficiency and suicidal ideation: A cross-sectional study of 157,211 healthy adults. J. Psychosom. Res. 2020, 134, 110125 10.1016/j.jpsychores.2020.110125. [DOI] [PubMed] [Google Scholar]
- a Mirzavandi F.; Babaie S.; Rahimpour S.; Razmpoosh E.; Talenezhad N.; Zarch S. M. A.; Mozaffari-Khosravi H. The effect of high dose of intramuscular vitamin D supplement injections on depression in patients with type 2 diabetes and vitamin D deficiency: A randomized controlled clinical trial. Obesity Medicine 2020, 17, 100192 10.1016/j.obmed.2020.100192. [DOI] [Google Scholar]; b Zhang Q. Q.; Wu Y. C.; Lu Y.; Fei X. Q. Role of vitamin D in risk factors of patients with type 2 diabetes mellitus. Med. Clin. 2020, 154, 151–156. 10.1016/j.medcli.2019.04.019. [DOI] [PubMed] [Google Scholar]
- a Annweiler C.; Cao Z. J.; Sabatier J. M. Point of view: Should COVID-19 patients be supplemented with vitamin D?. Maturitas 2020, 140, 24–26. 10.1016/j.maturitas.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Giménez V. M. M.; Inserra F.; Tajer C. D.; Mariani J.; Ferder L.; Reiter R. J.; Manucha W. Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 2020, 254, 117808 10.1016/j.lfs.2020.117808. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Razdan K.; Singh K.; Singh D. Vitamin D levels and COVID-19 susceptibility: is there any correlation?. Med. Drug Discovery 2020, 7, 100051 10.1016/j.medidd.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]; d WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/.
- a Roncero-Ramos I.; Delgado-Andrade C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. 10.1016/j.cofs.2017.04.002. [DOI] [Google Scholar]; b Kalaras M. D.; Richie J. P.; Calcagnotto A.; Beelman R. B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. 10.1016/j.foodchem.2017.04.109. [DOI] [PubMed] [Google Scholar]; c Ramos M.; Burgos N.; Barnard A.; Evans G.; Preece J.; Graz M.; Ruthes A. C.; Quero A. J.; Martínez-Abad A.; Vilaplana F.; Pham L.; Brouwer A.; Vanderburg B.; Garrigós M. C.; Jiménez A. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. 10.1016/j.foodchem.2019.04.035. [DOI] [PubMed] [Google Scholar]
- Wu T. F.; Shi W. Y.; Chiu Y. C.; Chan Y. Y. Investigation of the molecular mechanism underlying the inhibitory activities of ethanol extract of Bombyx mori pupa-incubated Cordyceps militaris fruiting bodies toward allergic rhinitis. Biomed. Pharmacother. 2021, 135, 111248 10.1016/j.biopha.2021.111248. [DOI] [PubMed] [Google Scholar]
- a Jäpelt R. B.; Jakobsen J. Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013, 4, 136 10.3389/fpls.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Papoutsis K.; Grasso S.; Menon A.; Brunton N. P.; Lyng J. G.; Jacquier J. C.; Bhuyan D. J. Recovery of ergosterol and vitamin D2 from mushroom waste-potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 2020, 99, 351–366. 10.1016/j.tifs.2020.03.005. [DOI] [Google Scholar]; c Jiang Q.; Zhang M.; Mujumdar A. S. UV induced conversion during drying of ergosterol to vitamin D in various mushrooms: Effect of different drying conditions. Trends Food Sci. Technol. 2020, 105, 200–210. 10.1016/j.tifs.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau J. L.; Chen P. R.; Yang J. H. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J. Agric. Food Chem. 1998, 46, 5269–5272. 10.1021/jf980602q. [DOI] [Google Scholar]
- Sapozhnikova Y.; Byrdwell W. C.; Lobato A.; Romig B. Effects of UV-B radiation levels on concentrations of phytosterols, ergothioneine, and polyphenolic compounds in mushroom powders used as dietary supplements. J. Agric. Food Chem. 2014, 62, 3034–3042. 10.1021/jf403852k. [DOI] [PubMed] [Google Scholar]
- Roberts J. S.; Teichert A.; H M. T. Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agric. Food Chem. 2008, 56, 4541–4544. 10.1021/jf0732511. [DOI] [PubMed] [Google Scholar]
- Krings U.; Berger R. G. Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food Chem. 2014, 149, 10–14. 10.1016/j.foodchem.2013.10.064. [DOI] [PubMed] [Google Scholar]
- a Ko J. A.; Lee B. H.; Lee J. S.; Park H. J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. 10.1021/jf073398s. [DOI] [PubMed] [Google Scholar]; b Won D. J.; Kim S. Y.; Jang C. H.; Lee J. S.; Ko J. A.; Park H. J. Optimization of UV irradiation conditions for the vitamin D2-fortified shiitake mushroom (Lentinula edodes) using response surface methodology. Food Sci. Biotechnol. 2018, 27, 417–424. 10.1007/s10068-017-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain P.; Jakobsen J. Dose-response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. J. Agric. Food Chem. 2015, 63, 8156–8161. 10.1021/acs.jafc.5b02945. [DOI] [PubMed] [Google Scholar]
- Calvo M. S.; Babu U. S.; Garthoff L. H.; Woods T. O.; Dreher M.; Hill G.; Nagaraja S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporosis Int. 2013, 24, 197–207. 10.1007/s00198-012-1934-9. [DOI] [PubMed] [Google Scholar]
- Koyyalamudi S. R.; Jeong S. C.; Song C. H.; Cho K. Y.; Pang G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. 10.1021/jf803908q. [DOI] [PubMed] [Google Scholar]
- Hu D. H.; Chen W.; Li X. S.; Yue T. L.; Zhang Z. J.; Feng Z. L.; Li C. L.; Bu X.; Li Q. X.; Hu C. Y.; Li L. D. Ultraviolet irradiation increased the concentration of vitamin D2 and decreased the concentration of ergosterol in shiitake mushroom (Lentinus edodes) and oyster mushroom (Pleurotus ostreatus) powder in ethanol suspension. ACS Omega 2020, 5, 7361–7368. 10.1021/acsomega.9b04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D.-H.; Zhang J.-X. Process optimization of increasing the vitamin D2 content in Flammulina velutipes sporocarp powder by UV irradiation. Chin. Food Sci. Technol. 2018, 43, 89–96. [Google Scholar]
- Jasinghe V. J.; Perera C. O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005, 92, 541–546. 10.1016/j.foodchem.2004.08.022. [DOI] [Google Scholar]
- Vayalil P. K.; Elmets C. A.; Katiyar S. K. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis 2003, 24, 927–936. 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- Guan W.; Zhang J.; Yan R.; Shao S.; Zhou T.; Lei J.; Wang Z. Effects of UV-C treatment and cold storage on ergosterol and vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus). Food Chem. 2016, 210, 129–134. 10.1016/j.foodchem.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Teichmann A.; Dutta P. C.; Staffas A.; Jägerstad M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effects of UV irradiation. LWT - Food Sci. Technol. 2007, 40, 815–822. 10.1016/j.lwt.2006.04.003. [DOI] [Google Scholar]
- Sławińska A.; Fornal E.; Radzki W.; Skrzypczak K.; Zalewska-Korona M.; Michalak-Majewska M.; Parfieniuk E.; Stachniuk A. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. 10.1016/j.foodchem.2015.11.131. [DOI] [PubMed] [Google Scholar]