Abstract
Highly substituted coumarins, privileged and versatile scaffolds for bioactive natural products and fluorescence imaging, are obtained via a Pd(II)-catalyzed direct C–H alkenylation reaction (Fujiwara–Moritani reaction), which has emerged as a powerful tool for the construction and functionalization of heterocyclic compounds because of its chemical versatility and its environmental advantages. Thus, a selective 6-endo cyclization led to 4-substituted coumarins in moderate yields. Selected examples have been further functionalized in C3 through a second intermolecular C–H alkenylation reaction to give coumarin-acrylate hybrids, whose fluorescence spectra have been measured.
Introduction
Coumarins (2H-chromen-2-nes or benzo-α-pyrones) are privileged scaffolds of a large variety of natural products1,2 (e.g., pestalasins A-E3,4 and lamellarins5,6) and/or synthetic molecules, as exemplified by the antioxidant umbelliferone,7 choleretic and antispasmodic hymecromone (4-methylumbelliferone),8 anti-inflammatory toddaculin,9 or anti-HIV agent 4-propylcoumarins10 (Figure 1). Owing to their outstanding optical properties, these coumarin fluorophores have found applications as fluorescent probes and tracers in biology, laser dyes, solar energy collectors, etc.11−13 Additionally, coumarins are widely used as additives in food and cosmetics,14,15 as well as agrochemicals,16 and pharmaceuticals.17 In particular, 4-arylcoumarins, a subgroup of flavonoids, have received great attention because they exhibit important biological activities, such as anti-HIV,18 antibacterial,19 antiprotozoal,20 and cytotoxic properties.21 For example, 5,7,4′-trimethoxy and 5,7-dimethoxy-4-phenylcoumarins have been tested for their anti-inflammatory activity, specifically as COX inhibitors, which opens the door to future developments as these enzymes are also associated with cancer and neuropsychiatric diseases such as schizophrenia.22 The introduction of a fused heterocyclic moiety into the coumarin core usually leads to compounds with promising or even unprecedented properties.23 For example, furo[3,2-g]coumarins (e.g., psoralen, xanthoxin, or bergapten) are used in treatment of several skin disorders (psoriasis and vitiligo).24 Therefore, the development of synthetic methods to construct and functionalize the coumarin core has attracted much attention over the past decades. Classical approaches for the preparation of coumarin derivatives usually involve the acid- or base-catalyzed condensation reactions of phenol derivatives with esters of acrylic acids (the Pechmann reaction) or with carbonyl compounds (Perkin, Wittig reaction, or Knoevenagel-type reactions).25−27 Some milder methods that do not use phenol precursors have also been developed, such as the PIDA/I2-mediated reactions of substituted phenyl acrylic acids28 and visible-light induced synthesis of functionalized coumarins.29
Figure 1.
Selected bioactive compounds with the coumarin core.
Transition-metal-catalyzed, particularly palladium–catalyzed, inter- or intramolecular heteroannulation reactions30,31 represent an excellent alternative for the construction of the coumarin framework.32 Representative examples include the intramolecular Mizoroki–Heck reaction of o-halophenyl 3-alkenoates,33 the tandem intermolecular Heck/cyclization between aryl halides and acrylates,34−36 or the Suzuki cross-coupling/cyclization of 2-(pinacolboronate)phenol and β-brominated dehydroaminoacids.37 In addition, palladium-catalyzed intermolecular carbonylative cyclization of o-halophenols with alkynes38,39 or salicylic aldehydes with benzyl chlorides40 has been reported. More recently, Gilmour et al.41 has reported a Pd(0)-catalyzed cascade annulation of 2-halophenols with β-borylacrylates as ambiphilic C3-synthons to generate C3 and C4-substituted coumarins. However, the transition-metal-catalyzed oxidative coupling reactions42 are even more attractive strategies for the synthesis of heterocyclic scaffolds as coumarins, as they avoid the use of preactivated coupling partners (halides, triflates, boronates, etc.). In this context, the oxidative cyclocarbonylation43 or carboxylation44 of 2-vinylphenols are efficient procedures for the synthesis of 4-substituted coumarins. The inter- or intramolecular hydroarylation of alkynes provides a direct and simple route to these heterocycles from phenols and propiolates or aryl propiolates, respectively.45 The formation of coumarins by addition of phenols and alkynoates via palladium(II)-catalyzed C–H insertion is an excellent example to illustrate the potential of this methodology.46 The palladium(II)-catalyzed intermolecular C–H alkenylation reaction of phenols and acrylates followed by intramolecular C–O formation, first reported by Kitamara et al.47 in 2005 and later developed by Maiti et al.48 and Shi et al,49 has given good results mainly for the synthesis of coumarins with no substituents at the pyrane ring. A related tandem dehydrogenation/oxidative Heck reaction starting from 4-phenylcyclohexanone to generate the intermediate phenol has also been reported.50 Therefore, we decided to investigate the intramolecular C–H alkenylation variant51,52 of aryl alkenoates and cinnamates to prepare 4-alkyl and 4-aryl-substituted coumarins with alkoxy groups at the aromatic ring, a structural feature common to biologically active coumarins (Scheme 1). The strategy involves a 6-endo-trig cyclization, which could be accomplished by controlling the positional selectivity of C–H activation, as we have previously demonstrated in the Pd(II)-catalyzed intramolecular 6-endo C–H alkenylation of N-arylacrylamides53 and N-substituted N-allylanilines54 for the construction of quinolone and dihydroquinoline cores, respectively. The effect of the substitution patterns of the benzene ring and the alkene on the reactivity and regioselectivity would be investigated. In addition, as conjugation of coumarin nucleus with an unsaturated moiety at the C-3 position can play a pivotal role in the anticancer activity of these heterocycles,55 we decided to carry out an intermolecular alkenylation reaction on the coumarin ring with α,β-unsaturated esters to generate new coumarin-acrylate hybrids.
Scheme 1. Synthesis of Coumarins via C–H Alkenylation.
Results and Discussion
We started the study of the reaction conditions (palladium catalyst and oxidant) using cinnamate 1a as the model substrate (Table 1). We selected the reaction conditions that had been previously employed for the 6-endo cyclization of N-aryl acrylamides to quinolones53 as a starting point, carrying out the reactions at room temperature in acetic acid and in the presence of p-TsOH (1 equiv) as an additive. The presence of this acid has been shown to be a determinant to increase the reactivity in this type of reaction.53,54,56,57 However, when palladium acetate was used as the catalyst using copper acetate or p-benzoquinone as oxidants, no conversion at all was observed after 30 h at rt, recovering unreacted 1a (Table 1, entries 1 and 2). The use of t-butyl perbenzoate in combination with copper acetate as an oxidant was required to obtain some reactivity, although low yields of the corresponding coumarin 2a were isolated, observing also the formation of decomposition products (Table 1, entries 3 and 4). A change in the palladium catalyst to bis(acetonitrile)dichloropalladium(II) resulted in a low conversion of the substrate (69% of 1a was recovered) after 8 h, but with a low isolated yield of 2a (Table 1, entry 5). A longer reaction time or higher reaction temperature was detrimental due to decomposition of the substrate (Table 1, entries 6 and 7). The use of a pyridine ligand (L1), a higher catalyst loading (10 mol %), or the use of an aqueous medium for the reaction resulted in increased but still low isolated yields (Table 1, entries 8–10). In no case, open-ring or decarboxylated products could be observed in isolable quantities. It was necessary to use a stronger oxidant such as N-fluoro-2,4,6-trimethylpyridinium triflate (F+) to obtain a complete conversion after 24 h, affording coumarin 2a in good isolated yield (Table 1, entry 11). This oxidant has been successfully employed in related intermolecular C–H alkenylation reactions in which the use of weaker oxidants gave low reactivity.58−60 It is noteworthy that the reaction was completely regioselective toward the formation of the 6-endo cyclization product (coumarin 2a), not observing the formation of the corresponding 5-exo cyclization product, benzofuran-2(3H)-one. Finally, a control experiment was carried out and in the absence of palladium, the reaction did not take place and unreacted 1a was recovered after 24 h at rt.
Table 1. Optimization of 6-Endo Cyclization of 1a.
| entry | [Pd] | [O] | L | t (h) | 2a (%) |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2a | Cu(OAc)2b | - | 30 | -c |
| 2 | Pd(OAc)2a | p-BQb | - | 30 | -c |
| 3 | Pd(OAc)2a | PhCO3tBud | - | 24 | 9 |
| 4 | Pd(dba)2a | PhCO3tBud | - | 24 | 5 |
| 5 | PdCl2(CH3CN)2a | PhCO3tBud | - | 8 | 10e |
| 6 | PdCl2(CH3CN)2a | PhCO3tBud | - | 24 | -f |
| 7 | PdCl2(CH3CN)2a | PhCO3tBud | - | 4g | -f |
| 8 | PdCl2(CH3CN)2a | PhCO3tBud | L1a | 24 | 23 |
| 9 | PdCl2(CH3CN)2h | PhCO3tBud | - | 24 | 25 |
| 10 | PdCl2(CH3CN)2a | PhCO3tBud | - | 24i | 25 |
| 11 | PdCl2(CH3CN)2a | F+d,j | - | 24 | 74 |
5 mol %.
1 equiv.
Starting material was recovered.
Cu(OAc)2 (5 mol %) was used as a co-oxidant.
Conversion: 31%.
Decomposition.
The reaction was carried out under reflux.
10 mol %.
2% wt. PT aqueous solution was used as solvent instead of HOAc.
N-Fluoro-2,4,6-trimethylpyridinium triflate (1.2 equiv) was used as the oxidant
These reaction conditions were applied to a series of cinnamates and enoates 1b–1k. The results are summarized in Table 2.
Table 2. Synthesis of 4-Substituted Coumarins 2a–g.
Yield (%) of the pure isolated compound (rt, 6–24 h).
10 mol % of the catalyst was used; 79% conversion.
75% conversion.
Yield (%) of the pure isolated compound (70 °C, 24 h).
Decomposition.
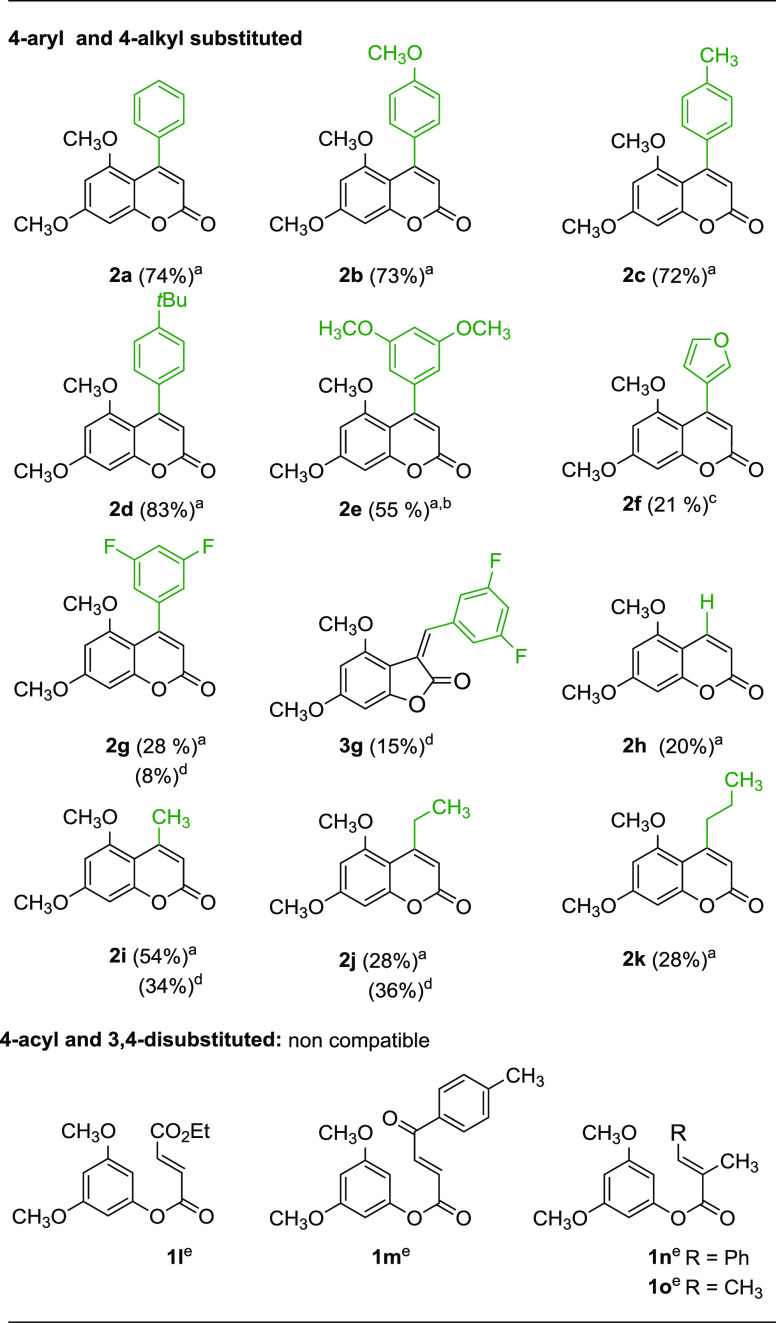
As shown, the results obtained were highly dependent on the substrate employed. Different substitution patterns on the aromatic ring at C4 are tolerated, and coumarins 2b–d were obtained in good isolated yields (Table 2). Nevertheless, the obtention of 2e required the use of a higher catalyst loading (10 mol %). A heteroaromatic ring, such as furan, could also be introduced at C4, although in a lower yield (2f, 21%). When an electron-deficient aryl group was introduced, 2g was obtained in a much lower yield (28%). Interestingly, when the reaction temperature was increased to 70 °C, the regioselectivity of the reaction was lost, isolating both the 6-endo and 5-exo cyclization products (2g and 3g, respectively) in low yields, together with decomposition products. Under these reaction conditions, unsubstituted or 4-alkyl substituted coumarins 2h–k were obtained only in moderate to low yields (Table 2), though no starting material could be recovered. An increase of the reaction temperature to 70 °C did not improve the results. It was also shown that the reaction conditions are not compatible with the presence of acyl groups on the alkene (1l R1 = CO2Et; 1m R1 = COAr) or with the substitution on the α-position of the alkene (1n and 1o R = Ph and CH3, respectively). In these cases, complex mixtures of products were obtained, not isolating coumarins 2l–2o. These results show that it is possible to access the coumarin framework via 6-endo cyclization, but this procedure is not as efficient as it was shown for the synthesis of quinolones from the corresponding amides.53 This difference in reactivity could be attributed to the instability of the esters in acidic media, leading to decomposition when long reaction times are required. Consequently, the reaction conditions were re-evaluated to use an alternative solvent to acetic acid, using in this case pent-5-enoate 1j as the model substrate (Table 3).
Table 3. Cyclization Reaction Conditions for 1j.
| entry | solvent | T (°C) | t (h) | 2j (%) |
|---|---|---|---|---|
| 1a | CH2Cl2 | rt | 24 | 8b |
| 2c | mesitylene/HOAc 4:1 | rt | 96 | 37 |
| 3c | mesitylene/HOAc 4:1 | 40 | 24 | 33 |
| 4c | mesitylene | rt | 96 | -d |
| 5c | mesitylene | 70 | 24 | 49 |
5 mol% of catalyst.
Conversion: 17%.
10 mol% of catalyst.
No p-TsOH was added; no reaction.
The change of acetic acid for a non-protic solvent, such as dichloromethane, led to a low conversion at room temperature (Table 3, entry 1). The use of an apolar solvent, such as mesitylene, in combination with a smaller proportion of acetic acid61 led to an increase of the isolated yield of 2j, although in a longer reaction time (Table 3, entry 2). The reactivity could be recovered at 40 °C, with slight erosion of the yield (Table 3, entry 3). When the reaction was carried out in mesitylene, the presence of p-TsOH was essential, as no reactivity was observed even after 96 h at rt in its absence (Table 3, entry 4). Finally, a moderate yield of 2j could be obtained at 70 °C (Table 3, entry 5). The use of these reaction conditions (mesitylene, 70 °C) resulted in a significant increase in the yield of coumarin 2h (69% vs 20%) and 4-alkyl substituted coumarins 2i–2k (Table 4). The yield of 4-aryl substituted coumarin 2g could not be improved under these conditions, although in this case, the formation of the 5-exo product 3g was not observed by NMR. Finally, the extension to different substitution patterns in the aromatic ring was also studied (Table 4). It was clear that an electron-rich aromatic ring is a determinant to obtain reactivity under these conditions.
Table 4. Synthesis of 4-Substituted Coumarins in Mesitylene.
Yield (%) of the pure isolated compound.
5 mol% of catalyst.
Two mechanistic pathways could be proposed for this type of oxidative alkenylation reaction. On one hand, arene metalation with an electrophilic Pd(II) species would give an aryl-Pd(II) species I, followed by syn-migratory insertion onto the alkene to give II, and syn elimination, after isomerization to III, to afford coumarin 2.53 Alternatively, the alkene could be first activated by the palladium catalyst (IV), followed by anti-addition of the aromatic ring to give also III, and syn elimination would lead to 2 (Scheme 2). In both cases, an electron-rich aromatic ring would be more reactive. As shown on Table 4, 6,7-dialkoxycoumarins 2p, 2q, and 2s could be obtained regioselectively, although in lower yields, as the 5,6,7-trimethoxy-substituted coumarin 2r, but no unreacted substrate was recovered. Although other aromatic substitution patterns were tested, no reactivity was observed with 3,5-dimethylphenyl, 3-methoxyphenyl, and 3-methoxy-5-methyl cinnamates, while complex mixtures of products were formed when using the corresponding (E)-but-2-enoates (see the Supporting Information for the substrates tested, 1t–1y).
Scheme 2. Mechanistic Pproposals for the 6-Endo Cyclization: Arene Metalation vs Alkene Activation.

The selective functionalization of the coumarin skeleton is also an important issue as, in addition to the biological properties, the extension of the conjugation could affect, for instance, their photophysical properties.62,63 Thus, several procedures have been developed for the C3 alkenylation of coumarins. For instance, palladium-catalyzed decarboxylative couplings have been described for the functionalization of coumarins with cinnamic acids64 and for the reaction of coumarin-3-carboxylic acid with acrylates.65 In addition, the selective C3 alkenylation of non-substituted coumarins has also been efficiently accomplished through the oxidative Heck reaction.66 Thus, we decided to study the derivatization of selected 4-substituted coumarins through a second intermolecular C–H alkenylation reaction.
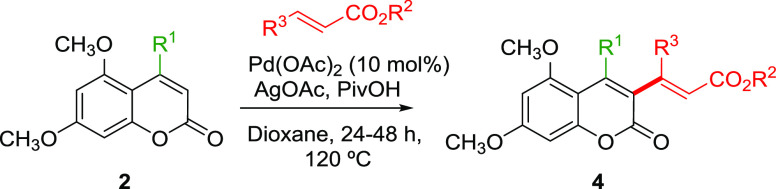
First, we tested the conditions used for the 6-endo-cyclization, but no intermolecular alkenylation reaction with t-butyl acrylate was observed. After some experimentation, we found that the best results were obtained using the reaction conditions previously developed for the alkenylation of quinolones.53 However, in most cases, conversions were low and 30–40% of the starting material was recovered (Table 5). When longer reaction times or higher temperatures were used, complex mixtures of products were isolated. We tried to optimize the reaction conditions by changing the oxidant or the palladium source, but the results could not be improved (see the Supporting Information). As shown in Table 5, C4 aryl- and methyl-substituted coumarins 2a,d,e,i could be alkenylated with methyl or t-butyl acrylate using palladium acetate in the presence of silver acetate and pivalic acid obtaining alkenylated coumarins 4a–f in moderate yields (Table 5, entries 1–6). Substitution on the β-position of the alkene is also possible, obtaining 4g in moderate yield (Table 5, entry 7).
Table 5. Intermolecular C–H Alkenylation of Coumarins 2.
| entry | 2 | R1 | R2 | R3 | 4 | yield (%)a |
|---|---|---|---|---|---|---|
| 1 | 2a | Ph | CH3 | H | 4a | 31 |
| 2 | 2a | Ph | t-Bu | H | 4b | 31 |
| 3 | 2d | 4(tBu)C6H4 | CH3 | H | 4c | 52 |
| 4 | 2e | 3,4(OMe)C6H4 | CH3 | H | 4d | 28 |
| 5 | 2i | CH3 | CH3 | H | 4e | 45 |
| 6 | 2i | CH3 | t-Bu | H | 4f | 53 |
| 7 | 2i | CH3 | CH3 | CH3 | 4 g | 44 |
Yield (%) of the pure isolated compound; in all cases, 30–40% of the unreacted starting material was also recovered.
As 3-vinyl and 3-styryl coumarins62,63 can be interesting fluorophores, the absorption and emission properties of selected coumarins 2a,d,e and 4a–d were also studied (see Table 6, Figure 2, and the Supporting Information). All coumarins showed UV–vis absorption, only one absorption band around λmax. = 375 nm for 4-aryl coumarins 2a,d,e and around λabs. = 300 nm for their C3 alkenylated derivatives 4a–d. This shift to a lower wavelength upon introduction of the acrylate moiety reflects the loss of coplanarity of the aromatic ring and the coumarin nucleus, thus decreasing delocalization. C3 alkenylation in the coumarin core leads to considerable changes in the fluorescence properties of coumarins 4a–d, whose maximum emission were more blue-shifted than those of coumarins 2 (λem. = 452–480 nm vs λem. = 435–446 nm) and presented higher Stoke shifts (up to 180) (Table 6). Fluorescent organic dyes with large Stoke shift are essential for biological applications.67−69
Table 6. Absorption and Emission Maxima of Selected Coumarins 2 and 4.
| entry | compd | λabsa (nm) | λemb (nm) | ΔStokec (nm) |
|---|---|---|---|---|
| 1 | 2a | 375 | 435 | 60 |
| 2 | 2d | 377 | 441 | 64 |
| 3 | 2e | 376 | 446 | 70 |
| 4 | 4a | 301 | 453 | 152 |
| 5 | 4b | 302 | 452 | 150 |
| 6 | 4c | 300 | 480 | 180 |
| 7 | 4d | 300 | 455 | 155 |
The maximum absorption wavelength in acetonitrile (2.3 mM).
Excited at the maximum absorption wavelength in acetonitrile (2.3 mM).
Stoke shift = λem – λabs.
Figure 2.
Excitation and emission spectra of coumarins 4a–d at 2.3 mM in acetonitrile.
In conclusion, it has been shown that 4-aryl-substituted coumarins can be obtained in moderate to good yields via palladium(II)-catalyzed C–H alkenylation reactions. 4-Aryl-substituted derivatives can be obtained in acetic acid at room temperature, while mesitylene at 70 °C is required to obtain moderate yields of 4-alkyl-substituted coumarins, avoiding decomposition in an acidic medium. However, the procedure is limited to an electron-rich aromatic ring for the cyclization to proceed efficiently. The coumarin skeleton can be further functionalized via C3 intermolecular alkenylation. Additionally, the fluorescence spectra of selected coumarins have been measured to provide perspective for potential applications.
Experimental Section
General Experimental Methods
Melting points were measured in unsealed capillary tubes. The NMR spectra were obtained at 300 MHz for 1H and 75.5 MHz for 13C or at 500 MHz for 1H and 125.7 MHz for 13C using CDCl3 as solvent and internal standard at 20–25 °C. Distortionless enhancement by polarization transfer (DEPT) experiments and 2D correlation experiments (COSY, heteronuclear single-quantum coherence (HSQCed), or heteronuclear multiple bond correlation (HMBC)) were used when necessary for the assignments of individual 13C and 1H resonances. Electron impact (EI, 70 eV), chemical ionization (CI, 230 eV), or electrospray ionization (ESI+) sources were used for obtaining the mass spectra. A TOF detector was employed for the obtention of exact mass. The IR spectra were obtained using an ATR instrument. The fluorescence spectra were measured using a Jasco FP-6500 spectrofluorimeter using acetonitrile as solvent at 2.3 mM. The emission spectra were acquired by irradiating the sample at its maximum absorbance. Both excitation and emission spectra were collected with a 3 nm slit bandwidth and were recorded at 25 °C. TLC was carried out with 0.2 mm-thick silica gel plates. Visualization was accomplished by UV light. Flash column chromatography was carried out on silica gel (230–400 mesh). All solvents used in reactions were purified according to standard procedures. Palladium catalysts were purchased from Aldrich and were used without further purification: Pd(OAc)2, 98% purity, and PdCl2(CH3CN)2, 99% purity.
Intramolecular Alkenylation of Enoates 1 and Synthesis of Coumarins 2: General Procedure
1-Fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (1.2 mmol), Cu(OAc)2 (0.05 mmol), p-TsOH (1 mmol), and PdCl2(CH3CN)2 (0.05 or 0.1 mmol) were added sequentially over a solution of the corresponding enoate 1 (1 mmol) in HOAc or mesitylene (4 mL). The mixture was stirred at room temperature or at 70 °C for 4–24 h. The solvent was removed under vacuum and the residue was dissolved in EtOAc (5 mL). The solution was washed with a 2 M aqueous solution of Na2CO3 (2 × 15 mL) and brine (2 × 15 mL). The aqueous phase was re-extracted with EtOAc (10 mL) and the combined organic extracts were dried (Na2SO4) and concentrated in vacuo. Flash column chromatography (silica gel, hexane/EtOAc) afforded coumarins 2.
5,7-Dimethoxy-4-phenyl-2H-chromen-2-one (2a)
Coumarin 2a was prepared from 1a (101.7 mg, 0.36 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (130.7 mg, 0.43 mmol), Cu(OAc)2 (3.3 mg, 0.018 mmol), p-TsOH (69.1 mg, 0.36 mmol), and PdCl2(CH3CN)2 (4.7 mg, 0.018 mmol) in HOAc (1.4 mL). The mixture was stirred at room temperature for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 8/2) afforded 2a (75 mg, 74%) as a solid, whose data are coincidental with those reported:46 mp (CH2Cl2): 164–166 °C [lit.46 mp (CH2Cl2/hexanes) 164–165 °C]; IR (ATR) 1713 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.42 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.01 (s, 1H, H3), 6.23 (d, J = 2.3 Hz, 1H, H6), 6.53 (d, J = 2.3 Hz, 1H, H8), 7.22–7.30 (m, 2H, H3′, H5′), 7.32–7.41 (m, 3H, H2′, H4′, H6′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.4 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.8 (C6), 103.6 (C4a), 112.7 (C3), 127.1 (C2′, C6′), 127.3 (C3′, C5′), 127.9 (C4′), 139.8 (C1′), 155.7 (C4), 157.2 (C8a), 158.2, 163.4 (C5, C7), 160.9 (CO); MS (CI) m/z (rel intensity) 284 (MH+ + 1, 18), 283 (MH+, 100), 282 (34), 254 (7); HRMS (CI) Calcd. for C17H15O4 [MH+]: 283.0965; found, 283.0958.
5,7-Dimethoxy-4-(4-methoxyphenyl)-2H-chromen-2-one (2b)
Coumarin 2b was prepared from 1b (117.6 mg, 0.37 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (131.2 mg, 0.45 mmol), Cu(OAc)2 (3.5 mg, 0.019 mmol), p-TsOH (72.2 mg, 0.37 mmol), and PdCl2(CH3CN)2 (4.9 mg, 0.019 mmol) in HOAc (1.5 mL). The mixture was stirred at room temperature for 6 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2b (85.5 mg, 73%) as a solid, whose data are coincidental with those reported:70 mp (CH2Cl2): 153–155 °C [lit.70 mp (methanol) 151–152 °C]; IR (ATR) 1716 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.46 (s, 3H, OCH3), 3.84 (s, 6H, 2 × OCH3), 5.95 (s, 1H, H3), 6.22 (d, J = 2.4 Hz, 1H, H6), 6.48 (d, J = 2.4 Hz, 1H, H8), 6.88 (d, J = 8.8 Hz, 2H, H3′, H5′), 7.19 (d, J = 8.8 Hz, 2H, H2′, H6′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.3 (OCH3), 55.4 (OCH3), 55.7 (OCH3), 93.6 (C8), 95.7 (C6), 103.6 (C4a), 112.5 (C3), 112.7 (C3′, C5′), 128.7 (C2′, C6′), 132.0 (C1′), 155.5 (C4), 157.2 (C8a), 158.3 (C4′), 159.6, 163.3 (C5, C7), 160.9 (CO); MS (CI) m/z (rel intensity) 314 (MH+ + 1, 20), 313 (MH+, 100), 312 (60), 284 (18); HRMS (CI) Calcd. for C18H17O5 [MH+]: 313.1071; found, 313.1061.
5,7-Dimethoxy-4-(p-tolyl)-2H-chromen-2-one (2c)
Coumarin 2c was prepared from 1c (105.9 mg, 0.36 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (123.2 mg, 0.43 mmol), Cu(OAc)2 (3.2 mg, 0.018 mmol), p-TsOH (67.5 mg, 0.36 mmol), and PdCl2(CH3CN)2 (4.6 mg, 0.018 mmol) in HOAc (1.4 mL). The mixture was stirred at room temperature for 24 h, and after the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc t 8/2) afforded 2c (75.9 mg, 72%) as a solid, whose data are coincidental with those reported:70 mp (CH2Cl2): 131–133 °C [lit.70 mp (ethanol/water) 131–133 °C]; IR (ATR) 1712 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 2.42 (s, 3H, CH3), 3.47 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 6.00 (s, 1H, H3), 6.25 (d, J = 2.4 Hz, 1H, H6), 6.53 (d, J = 2.4 Hz, 1H, H8), 7.15–7.21 (m, 4H, H2′, H3′, H5′, H6′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 21.3 (CH3), 55.4 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.8 (C6), 103.6 (C4a), 112.7 (C3), 127.2 (C2′, C6′), 128.0 (C3′, C5′), 136.9 (C4′), 137.8 (C1′), 155.8 (C4), 157.2 (C8a), 158.3, 161.0 (C5, C7), 163.3 (CO); MS (ESI+) m/z (rel intensity) (MH+ + 1, 15), 297 (MH+, 100); HRMS (ESI+) Calcd. for C18H17O4 [MH+]: 297.1127; found, 297.1132.
4-[4-(tert-Butyl)phenyl]-5,7-dimethoxy-2H-chromen-2-one (2d)
Coumarin 2d was prepared from 1d (122.0 mg, 0.36 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (124.4 mg, 0.43 mmol), Cu(OAc)2 (3.3 mg, 0.018 mmol), p-TsOH (68.2 mg, 0.36 mmol), and PdCl2(CH3CN)2 (4.7 mg, 0.018 mmol) in HOAc (1.4 mL). The mixture was stirred at room temperature for 24 h, and after the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 8/2) afforded 2d (101.0 mg, 83%) as a solid: mp (CH2Cl2): 176–178 °C; IR (ATR) 1718 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 1.38 (s, 9H, C(CH3)3), 3.43 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.02 (s, 1H, H3), 6.25 (d, J = 2.4 Hz, 1H, H6), 6.53 (d, J = 2.4 Hz, 1H, H8), 7.19–7.22 (m, 2H, H3′, H5′), 7.39–7.42 (m, 2H, H2′, H6′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 31.3 (C(CH3)3), 34.7 (C(CH3)3), 55.5 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.8 (C6), 103.7 (C4a), 112.5 (C3), 124.2 (C2′, C6′), 127.0 (C3′, C5′), 136.8 (C4′), 151.1 (C1′), 155.8 (C4), 157.2 (C8a), 158.3, 161.0 (C5, C7), 163.3 (CO); MS (ESI+) m/z (rel intensity) 340 (MH+ + 1, 17), 339 (MH+, 100); HRMS (ESI+) Calcd. for C21H23O4 [MH+]: 339.1596; found, 339.1600.
4-(3,5-Dimethoxyphenyl)-5,7-dimethoxy-2H-chromen-2-one (2e)
Coumarin 2e was prepared from 1e (115.6 mg, 0.34 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (116.5 mg, 0.40 mmol), Cu(OAc)2 (3.0 mg, 0.017 mmol), p-TsOH (63.9 mg, 0.34 mmol), and PdCl2(CH3CN)2 (8.6 mg, 0.034 mmol) in HOAc (1.4 mL). The mixture was stirred at room temperature for 24 h, and after the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 8/2) afforded 2e (63.9 mg, 55%, 79% conversion) as a solid: mp (CH2Cl2): 126–128 °C; IR (ATR) 1718 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.49 (s, 3H, OCH3), 3.81 (s, 6H, 2 × OCH3), 3.87 (s, 3H, OCH3), 6.02 (s, 1H, H3), 6.25 (d, J = 2.4 Hz, 1H, H6), 6.41 (d, J = 2.3 Hz, 2H, H2′, H6′), 6.49 (t, J = 2.3 Hz, 1H, H4′), 6.52 (d, J = 2.4 Hz, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.5 (2 × OCH3), 55.6 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.9 (C6), 100.0 (C4′), 103.5 (C4a), 105.4 (C2′, C6′), 112.4 (C3), 141.7 (C1′), 155.5 (C4), 157.1 (C8a), 158.2 (C7), 160.0 (C3′, C5′), 160.9 (C5), 163.4 (CO); MS (ESI+) m/z (rel intensity) 344 (MH+ + 1, 16), 343 (MH+, 100); HRMS (ESI+) Calcd. for C19H19O6 [MH+]: 343.1182; found, 343.1180.
4-(Furan-3-yl)-5,7-dimethoxy-2H-chromen-2-one (2f)
Coumarin 2f was prepared from 1f (98.1 mg, 0.36 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (124.1 mg, 0.43 mmol), Cu(OAc)2 (3.2 mg, 0.018 mmol), p-TsOH (68.0 mg, 0.36 mmol), and PdCl2(CH3CN)2 (4.6 mg, 0.018 mmol) in HOAc (1.4 mL). The mixture was stirred at room temperature for 24 h, and after the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 8/2) afforded 2f (20.6 mg, 21%, 75% conversion) as a solid: mp (CH2Cl2): 153–155 °C; IR (ATR) 1714 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.67 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.09 (s, 1H, H3), 6.30 (d, J = 2.4 Hz, 1H, H6), 6.49–6.52 (m, 2H, H8, H4′), 7.45 (t, J = 1.8 Hz, 1H, H5′), 7.56–7.57 (m, 1H, H2′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.3 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.7 (C6), 103.4 (C4a), 112.1 (C4′), 112.5 (C3), 125.0 (C3′), 140.3 (C2′), 142.0 (C5′), 146.7 (C4), 157.3, 158.2, 160.8 (C8a, C5, C7), 163.3 (CO); MS (ESI+) m/z (rel intensity) 274 (MH+ + 1, 12), 273 (MH+, 100); HRMS (ESI+) Calcd. for C15H13O5 [MH+]: 273.0763; found, 273.0769.
4-(3,5-Difluorophenyl)-5,7-dimethoxy-2H-chromen-2-one (2g)
Coumarin 2g was prepared from 1g (87.5 mg, 0.27 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (94.9 mg, 0.33 mmol), Cu(OAc)2 (3.0 mg, 0.016 mmol), p-TsOH (79.3 mg, 0.27 mmol), and PdCl2(CH3CN)2 (8.5 mg, 0.032 mmol) in mesitylene (1.1 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 8/2) afforded 2g (25.8 mg, 30%) as a solid: mp (CH2Cl2): 170–172 °C; IR (ATR) 1724 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.48 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 5.97 (s, 1H, H3), 6.23 (d, J = 2.4 Hz, 1H, H6), 6.51 (d, J = 2.4 Hz, 1H, H8), 6.72–6.91 (m, 3H, H2′, H4′, H6′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.6 (OCH3), 55.8 (OCH3), 93.7 (C8), 95.8 (C6), 102.8 (C4a), 103.2 (t, J = 25.2 Hz, C4′), 110.9–112.7 (m, C2′, C6′), 112.7 (C3), 142.8 (t, J = 10.1 Hz, C1′), 153.0 (C4), 157.1 (C8a), 157.9 (C5), 160.3 (C7), 162.2 (dd, J = 248.1, 13.2 Hz, C3′, C5′), 163.8 (CO); MS (EI) m/z (rel intensity) 319.1 (M+ + 1, 19), 318.1 (M+, 100), 291.1 (16), 290.1 (93), 275.1 (39), 247 (12), 188 (14), 175 (15), 69 (10); HRMS (CI) Calcd. for C17H13 F2O4 [MH+]: 319.0776; found, 319.0770.
5,7-Dimethoxy-2H-chromen-2-one (2h)
Coumarin 2h was prepared from 1h (94.4 mg, 0.45 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (157.4 mg, 0.54 mmol), Cu(OAc)2 (4.1 mg, 0.023 mmol), p-TsOH (131.6 mg, 0.45 mmol), and PdCl2(CH3CN)2 (5.9 mg, 0.023 mmol) in mesitylene (1.8 mL). The mixture was stirred at 70 °C for 4 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2h (64.9 mg, 69%) as a white solid, whose data are coincidental with those reported:46 mp (CH2Cl2): 144–146 °C [lit.46 mp (CH2Cl2/pentanes) 143–145 °C]; IR (ATR) 1724 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.82 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.15 (d, J = 9.7 Hz, 1H, H3), 6.28 (d, J = 2.1 Hz, 1H, H6), 6.41 (d, J = 2.1 Hz, 1H, H8), 7.96 (d, J = 9.7 Hz, 1H, H4); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.8 (OCH3), 55.9 (OCH3), 92.8 (C8), 94.8 (C6), 104.0 (C4a), 110.9 (C3), 138.7 (C4), 156.8 (C8a), 157.0, 163.7 (C5, C7), 161.5 (CO); MS (EI) m/z (rel intensity) 207 (M+ + 1, 12), 206 (M+, 100), 178 (79), 163 (48), 135 (22); HRMS (CI) Calcd. for C11H11O4 [MH+]: 207.0652; found, 207.0658.
5,7-Dimethoxy-4-methyl-2H-chromen-2-one (2i)
Coumarin 2i was prepared from 1i (84.2 mg, 0.38 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (131.5 mg, 0.45 mmol), Cu(OAc)2 (3.4 mg, 0.018 mmol), p-TsOH (72.1 mg, 0.38 mmol), and PdCl2(CH3CN)2 (9.8 mg, 0.036 mmol) in mesitylene (1.5 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2i (50.3 mg, 60%) as a white solid, whose data are coincidental with those reported:46 mp (CH2Cl2): 166–168 °C [lit.46 mp (methanol): 168–170 °C; IR (ATR) 1740 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 2.50 (d, J = 1.2 Hz, 3H, CH3), 3.82 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 5.91 (br s, 1H, H3), 6.26 (d, J = 2.4 Hz, 1H, H6), 6.39 (d, J = 2.4 Hz, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 24.2 (CH3), 55.6 (OCH3), 55.7 (OCH3), 93.4 (C8), 95.4 (C6), 104.8 (C4a), 111.3 (C3), 154.5 (C8a), 156.9 (C4), 159.1, 162.8 (C5, C7), 161.0 (CO); MS (EI) m/z (rel intensity) 221 (M+ + 1, 13), 220 (M+, 100), 193 (11), 192 (91), 178 (10), 177 (73), 149 (13); HRMS (CI) Calcd. for C12H13O4 [MH+]: 221.0808; found, 221.0811.
4-Ethyl-5,7-dimethoxy-2H-chromen-2-one (2j)
Coumarin 2j was prepared from 1j (61.0 mg, 0.26 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (94.3 mg, 0.31 mmol), Cu(OAc)2 (2.4 mg, 0.013 mmol), p-TsOH (49.9 mg, 0.26 mmol), and PdCl2(CH3CN)2 (6.8 mg, 0.026 mmol) in mesitylene (1 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2j (29.8 mg, 49%) as a solid, whose data are coincidental with those reported:71 mp (CH2Cl2): 141–143 °C [lit.71 mp (EtOH:H2O) 146–148 °C]; IR (ATR) 1710 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 1.21 (t, J = 7.3 Hz, 3H, CH3), 2.94 (q, J = 7.3 Hz, 2H, CH2), 3.84 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.00 (s, 1H, H3), 6.30 (d, J = 2.4 Hz, 1H, H6), 6.45 (d, J = 2.4 Hz, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 13.5 (CH3), 29.4 (CH2), 55.7 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.6 (C6), 104.3 (C4a), 109.7 (C3), 157.2 (C8a), 158.8 (C4), 159.9, 162.6 (C5, C7), 161.4 (CO); MS (EI) m/z (rel intensity) 235 (M+ + 1, 15), 234 (M+, 100), 207 (12), 206 (86), 205 (16), 191 (52), 161 (12), 103 (11); HRMS (CI) Calcd. for C13H15O4 [MH+]: 235.0965; found, 235.0966.
5,7-Dimethoxy-4-propyl-2H-chromen-2-one (2k)
Coumarin 2k was prepared from 1k (77.0 mg, 0.31 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (112.4 mg, 0.37 mmol), Cu(OAc)2 (2.9 mg, 0.015 mmol), p-TsOH (59.4 mg, 0.31 mmol), and PdCl2(CH3CN)2 (8.0 mg, 0.030 mmol) in mesitylene (1.2 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2k (37.9 mg, 50%) as a solid, whose data are coincidental with those reported:72 mp (CH2Cl2): 109–111 °C [lit.72 mp (CHCl3) 113.5–115 °C]; IR (ATR) 1713 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 1.00 (t, J = 7.3 Hz, 3H, CH2–CH2–CH3), 1.52–1.69 (m, 2H, CH2–CH2–CH3), 2.82–2.88 (m, 2H, CH2–CH2–CH3), 3.84 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 5.96 (s, 1H, H3), 6.30 (d, J = 2.4 Hz, 1H, H6), 6.45 (d, J = 2.4 Hz, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 14.0 (CH2–CH2–CH3), 22.8 (CH2–CH2–CH3), 38.5 (CH2–CH2–CH3), 55.7 (OCH3), 55.8 (OCH3), 93.6 (C8), 95.6 (C6), 104.3 (C4a), 110.7 (C3), 157.3 (C8a), 158.2 (C4), 158.7, 162.6 (C5, C7), 161.3 (CO); MS (EI) m/z (rel intensity) 249.1 (M+ + 1, 15), 248.1 (M+, 100), 233.1 (36), 220.1 (63), 205.1 (41), 192.1 (56), 191.1 (28), 178 (15), 177.1 (25), 161.1 (22), 146 (15), 91 (10), 77.1 (12), 69 (14); HRMS (CI) Calcd. for C14H17O4 [MH+]: 249.1121; found, 249.1123.
6,7-Dimethoxy-4-phenyl-2H-chromen-2-one (2p)
Coumarin 2p was prepared from 1p (60 mg, 0.21 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (75.9 mg, 0.25 mmol), Cu(OAc)2 (2.2 mg, 0.010 mmol), p-TsOH (40.2 mg, 0.21 mmol), and PdCl2(CH3CN)2 (5.6 mg, 0.021 mmol) in mesitylene (1.0 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2p (17.8 mg, 30%) as an oil, whose data are coincidental with those reported:73 IR (ATR): 1715 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.77 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 6.26 (s, 1H, H3), 6.88 (s, 1H, H8), 6.94 (s, 1H, H5), 7.46–7.56 (m, 5H, Ph); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 56.3 (OCH3), 56.4 (OCH3), 100.3 (C8), 107.5 (C5), 111.4 (C4a), 112.4 (C3), 128.3, 128.9, 129.7 (C2′, C3′, C4′, C5′, C6′), 135.8 (C1′), 146.1 (C4), 150.2 (C8a), 152.9, 155.6 (C6, C7), 161.4 (CO); MS (EI) m/z (rel intensity): 282 (M+, 100), 254 (23), 239 (16), 207 (27), 168 (10), 155 (12), 139 (15), 127 (17); HRMS (ESI+) Calcd. for C17H15O4 [MH+]: 283.0965; found, 283.0957.
6,7-Dimethoxy-4-methyl-2H-chromen-2-one (2q)
Coumarin 2q was prepared from 1q (60.1 mg, 0.27 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (98.9 mg, 0.33 mmol), Cu(OAc)2 (5.1 mg, 0.027 mmol), p-TsOH (52.2 mg, 0.27 mmol), and PdCl2(CH3CN)2 (7.1 mg, 0.027 mmol) in mesitylene (1.3 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 2q (20.3 mg, 34%) as a solid, whose data are coincidental with those reported:71 mp (CH2Cl2): 128–129 °C [lit.71 mp (EtOH:H2O) 128–129 °C]; IR (ATR): 1715 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 2.41 (d, J = 1.0 Hz, 3H, CH3), 3.94 (s, 6H, 2 × OCH3), 6.17 (d, J = 1.0 Hz, 1H, H3), 6.85 (s, 1H, H5), 6.94 (s, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 18.9 (CH3), 56.3 (OCH3), 56.4 (OCH3), 100.1 (C8), 105.2 (C5), 112.4 (C4a), 112.5 (C3), 146.2 (C4), 149.2 (C8a), 152.3, 159.1 (C6, C7), 161.5 (CO); MS (EI) m/z (rel intensity): 220 (M+, 100), 192 (10), 149 (14), 1201 (10); HRMS (ESI+) Calcd. for C12H13O4 [MH+]: 221.0808; found, 221.0820.
5,6,7-Trimethoxy-4-methyl-2H-chromen-2-one (2r)
Coumarin 2r was prepared from 1r (674 mg, 0.24 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (88.3 mg, 0.31 mmol), Cu(OAc)2 (4.4 mg, 0.024 mmol), p-TsOH (48.8 mg, 0.24 mmol), and PdCl2(CH3CN)2 (6.2 mg, 0.024 mmol) in mesitylene (1.0 mL). The mixture was stirred at 70 °C for 72 h, and after the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 8/2) afforded 2r (13.0 mg, 18%) as an oil, whose data are coincidental with those reported:74 IR (ATR): 1735 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 2.56 (d, J = 1.1 Hz, 3H, CH3), 3.85 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 6.04 (d, J = 1.1 Hz, 1H, H3), 6.64 (s, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 23.0 (CH3), 56.2 (OCH3), 61.0 (OCH3), 61.3 (OCH3), 96.2 (C8), 108.1 (C4a), 113.0 (C3), 139.2 (C6), 151.3 (C8a), 151.6 (C4), 153.6, 156.3 (C5, C7), 160.1 (CO); MS (EI) m/z (rel intensity): 250 (M+, 90), 235 (100), 207 (75), 163 (39), 149 (14), 69 (17), 53 (14), 51 (13); HRMS (ESI+) Calcd. for C13H15O5 [MH+]: 251.0914; found, 251.0939.
4-Methyl-6,7-methylenedioxy-2H-chromen-6-one (2s)
Coumarin 2s was prepared from 1s (61.5 mg, 0.30 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (101.1 mg, 0.35 mmol), Cu(OAc)2 (5.1 mg, 0.015 mmol), p-TsOH (57.7 mg, 0.30 mmol), and PdCl2(CH3CN)2 (8.0 mg, 0.027 mmol) in mesitylene (1.5 mL). The mixture was stirred at 70 °C for 96 h, and after the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 7/3) afforded 2s (13.0 mg, 20%) as a solid, whose data are coincidental with those reported:75 mp (CH2Cl2): 120–122 °C; IR (ATR): 1735 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 2.37 (s, 3H, CH3), 6.07 (s, 2H, OCH2O), 6.17 (s, 1H, H3), 6.83 (s, 1H, H5), 6.96 (s, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 19.1 (CH3), 98.4 (OCH2O), 102.1 (C8), 102.3 (C5), 112.2 (C4a), 113.8 (C3), 144.9 (C4), 150.6 (C8a), 150.9, 152.4 (C6, C7), 161.2 (CO); MS (EI) m/z (rel intensity): 204 (M+, 100), 176 (72), 96 (15); HRMS (ESI+) Calcd. for C11H9O4 [MH+]: 205.0495; found, 205.0523.
(Z)-3-(3,5-Difluorobenzylidene)-4,6-dimethoxybenzofuran-2(3H)-ne (3g)
Compound 3g was obtained from 1g (123.8 mg, 0.39 mmol), 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate (135.5 mg, 0.46 mmol), Cu(OAc)2 (3.6 mg, 0.019 mmol), p-TsOH (74.6 mg, 0.39 mmol), and PdCl2(CH3CN)2 (5.1 mg, 0.019 mmol) in HOAc (1.5 mL). The mixture was stirred at 70 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 8/2) afforded 2g (9.8 mg, 8%) and 3g (18.3 mg, 15%) as a solid: mp (CH2Cl2): 202–204 °C; IR (ATR): 1774 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.85 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 6.26 (d, J = 2.0 Hz, 1H, H5), 6.32 (d, J = 2.0 Hz, 1H, H7), 6.79–6.88 (m, 1H, H4′), 7.49–7.63 (m, 1H, H2′, H6′), 7.80 (s, 1H, C=CH–Ar); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 55.8 (OCH3), 55.9 (OCH3), 89.3 (C7), 94.3 (C5), 104.7–105.8 (m, C3a, C4′), 113.5–113.9 (m, C2′, C6′), 122.6 (C3), 136.4 (C=CH–Ar), 136.9 (t, J = 10.0 Hz, C1′), 155.1 (C7a), 157.0 (C4), 162.6 (dd, J = 247.5, 13.0 Hz, C3′, C5′), 163.1 (C6), 165.8 (CO); MS (EI) m/z (rel intensity) 319 (M+ + 1, 19), 318 (M+, 100), 275 (28), 188 (12), 175 (11), 69 (10); HRMS (ESI+) Calcd. for C17H13F2O4 [MH+]: 319.0776; found, 319.0783.
C3 Intermolecular C–H Alkenylation of Coumarins 2: General Procedure
Over a solution of coumarins 2 (1 mmol) in 1,4-dioxane (5 mL), AgOAc (3 mmol), PivOH (10 mmol), and Pd(OAc)2 (0.1 or 0.2 mmol) were added. The mixture was stirred vigorously for 5 min, and then the corresponding acrylate (2 or 4 mmol) was added. The reaction was heated at 120 °C for 24–48 h, and then the reaction was allowed to cool down to room temperature. The mixture was filtered through diatomaceus earth and the filtrate was concentrated in vacuo. Flash column chromatography (silica gel, hexane/EtOAc) afforded coumarins 4.
Methyl (E)-3-(5,7-Dimethoxy-2-oxo-4-phenyl-2H-chromen-3-yl)acrylate (4a)
Coumarin 4a was prepared from 2a (83.2 mg, 0.29 mmol), AgOAc (0.15 g, 0.88 mmol), PivOH (0.30 g, 2.95 mmol), methylacrylate (0.06 mL, 0.59 mmol), and Pd(OAc)2 (6.6 mg, 0.029 mmol). The reaction was heated at 120 °C for 24 h, and after this, additional amounts of methyl acrylate (0.06 mL, 0.59 mmol) and Pd(OAc)2 (6.6 mg, 0.029 mmol) were added, and it was heated again at 120 °C for 24 h. After the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 4a (33.4 mg, 31%) as a solid: mp (CH2Cl2) 194–196 °C; IR (ATR): 1710 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.29 (s, 3H, CH=CH–COOCH3), 3.65 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.15 (d, J = 2,4 Hz, 1H, H6), 6.49 (d, J = 2,4 Hz, 1H, H8), 7.06–7.15 (m, 4H, H2′, H4′, H6′, CH=CH–COOCH3), 7.34–7.47 (m, 3H, H3′, H5′, CH=CH–COOCH3); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 51.4 (CH=CH–COOCH3), 55.7 (OCH3), 55.9 (OCH3), 93.1 (C8), 96.0 (C6), 104.7 (C4a), 115.7 (C3), 121.9 (CH=CH–COOCH3), 127.1 (C2′, C6′), 127.8 (C3′, C5′), 127.9 (C4′), 137.2 (CH=CH–COOCH3), 137.7 (C1′), 155.8 (C4), 156.0 (C8a), 159.1, 164.4 (C5, C7) 159.2 (CO), 168.4 (COOCH3); MS (CI) m/z (rel intensity) 368 (MH+ + 1, 19), 367 (MH+, 100), 336 (7), 335 (41); HRMS (ESI+) Calcd. for C21H19O6 [MH+]: 367.1176; found, 367.1180.
tert-Butyl (E)-3-(5,7-Dimethoxy-2-oxo-4-phenyl-2H-chromen-3-yl)acrylate (4b)
Coumarin 4b was prepared from 2a (82.4 mg, 0.29 mmol), AgOAc (0.15 g, 0.88 mmol), PivOH (0.30 g, 2.92 mmol), tert-butyl acrylate (85 μL, 0.58 mmol), and Pd(OAc)2 (6.6 mg, 0.029 mmol). The reaction was heated at 120 °C for 24 h, and after the work-up, flash column chromatography (silica gel, CH2Cl2) afforded 4b (37.3 mg, 31%, 44% conversion) as a solid: mp (CH2Cl2) 148–150 °C; IR (ATR): 1732 (COO), 1706 (COOtBu) cm–1; 1H NMR (CDCl3, 300 MHz): δ 1.41 (s, 9H, C(CH3)3), 3.31 (s, 3H, OCH3), 3.89 (OCH3), 6.17 (d, J = 2.4 Hz, 1H, H6), 6.52 (d, J = 2.4 Hz, 1H, H8), 7.02–7.15 (m, 4H, H2′, H4′, H6′, CH=CH–COOtBu), 7.35–7.47 (m, 3H, H3′, H5′, CH=CH–COOtBu); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 28.1 (C(CH3)3), 55.7 (OCH3), 55.9 (OCH3), 79.9 (C(CH3)3), 93.1 (C8), 96.0 (C6), 104.8 (C4a), 115.9 (C3), 124.4 (CH=CH–COOtBu), 127.1 (C3′, C5′), 127.7 (C4′), 127.9 (C2′, C6′), 135.9 (CH=CH–COOtBu), 137.8 (C1′), 155.4, 155.9 (C4, C8a), 159.1, 159.2 (C5, C7), 164.2 (CO), 166.9 (COOtBu); MS (ESI+) m/z (rel intensity) 410 (MH+ + 1, 9), 409 (MH+, 45), 354 (16), 353 (100); HRMS (ESI+) Calcd. for C25H25O6 [MH+]: 409.1651; found, 409.1653.
Methyl (E)-3-{4-[4-(tert-Butyl)phenyl]-5,7-dimethoxy-2-oxo-2H-chromen-3-ylacrylate (4c)
Coumarin 4c was prepared from 2d (85.0 mg, 0.25 mmol), AgOAc (0.13 g, 0.75 mmol), PivOH (0.26 g, 2.51 mmol), methyl acrylate (45 μL, 0.50 mmol), and Pd(OAc)2 (5.6 mg, 0.025 mmol). The reaction was heated at 120 °C for 24 h, and after this, additional amounts of methyl acrylate (45 μL, 0.50 mmol) and Pd(OAc)2 (5.6 mg, 0.025 mmol) were added, and it was heated again at 120 °C for 24 h. After the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 8/2) afforded 4c (55.4 mg, 52%, 63% conversion) as a solid: mp (CH2Cl2): 159–162 °C; IR (ATR): 1726 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 1.40 (s, 9H, C(CH3)3), 3.28 (s, 3H, COOCH3), 3.67 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 6.16 (d, J = 2.4 Hz, 1H, H6), 6.50 (d, J = 2.4 Hz, 1H, H8), 7.04–7.06 (m, 2H, H3′, H5′), 7.16 (d, J = 15.8 Hz, 1H, CH=CH–COOCH3), 7.24 (d, J = 15.8 Hz, 1H, CH=CH–COOCH3), 7.44–7.47 (m, 2H, H2′, H6′); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 31.3 (C(CH3)3), 34.7 (C(CH3)3), 51.4 (COOCH3), 55.8 (OCH3), 55.8 (OCH3), 93.1 (C8), 96.1 (C6), 105.0 (C4a), 115.7 (C3), 121.8 (CH=CH–COOCH3), 124.7 (C3′, C5′), 126.9 (C2′, C6′), 134.6 (C1′), 137.4 (CH=CH–COOCH3), 150.8 (C4′), 156.0 (C4, C8a), 156.3, 159.2 (C5, C7), 164.3 (CO), 168.3 (COOCH3); MS (ESI+) m/z (rel intensity) 424 (MH+ + 1, 24), 423 (MH+, 100), 391 (20), 318 (11); HRMS (ESI+) Calcd. for C25H27O6 [MH+]: 423.1808; found, 423.1801.
Methyl (E)-3-[4-(3,5-Dimethoxyphenyl)-5,7-dimethoxy-2-oxo-2H-chromen-3-yl]acrylate (4d)
Coumarin 4d was prepared from 2e (69.2 mg, 0.20 mmol), AgOAc (0.10 g, 0.61 mmol), PivOH (0.21 g, 2.02 mmol), methyl acrylate (36 μL, 0.40 mmol), and Pd(OAc)2 (4.5 mg, 0.020 mmol). The reaction was heated at 120 °C for 24 h, and after this, additional amounts of methyl acrylate (36 μL, 0.40 mmol) and Pd(OAc)2 (4.5 mg, 0.020 mmol) were added, and it was heated again at 120 °C for 24 h. After the work-up, flash column chromatography (silica gel, petroleum ether/EtOAc 6/4) afforded 4d (24.2 mg, 28%, 57% conversion) as a solid: mp (CH2Cl2) 207–209 °C; IR (ATR): 1722 cm–1 (C=O); 1H NMR (CDCl3, 300 MHz): δ 3.40 (s, 3H, COOCH3), 3.69 (s, 3H, OCH3), 3.81 (s, 6H, 2 × OCH3), 3.88 (s, 3H, OCH3), 6.19 (d, J = 2.4 Hz, 1H, H6), 6.28–6.29 (m, 2H, H2′, H6′), 6.49–6.51 (m, 2H, H8, H4′), 7.15–7.27 (m, 2H, CH=CH–COOCH3); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 51.5 (COOCH3), 55.5 (2 × OCH3), 55.9 (OCH3), 56.0 (OCH3), 93.1 (C8), 96.1 (C6), 99.8 (C4′), 104.6 (C4a), 105.5 (C2′, C6′), 115.5 (C3), 122.0 (CH=CH–COOCH3), 137.2 (CH=CH–COOCH3), 139.5 (C1′), 155.5 (C4), 156.0 (C8a), 159.2, 160.6 (C5, C7), 164.4 (CO), 168.2 (COOCH3); MS (ESI+) m/z (rel intensity) 428 (MH+ + 1, 19), 427 (MH+, 100), 396 (10), 395 (56), 353 (24); HRMS (ESI+) Calcd. for C23H23O8 [MH+]: 427.1393; found, 427.1393.
Methyl (E)-3-(5,7-Dimethoxy-4-methyl-2-oxo-2H-chromen-3-yl)acrylate (4e)
Coumarin 4e was prepared from 2i (81.3 mg, 0.37 mmol), AgOAc (0.19 g, 1.11 mmol), PivOH (0.38 g, 3.69 mmol), methyl acrylate (0.07 mL, 0.74 mmol), and Pd(OAc)2 (8.5 mg, 0.04 mmol). The reaction was heated at 120 °C for 48 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 6/4) afforded 4e (50.7 mg, 45%) as a solid: mp (CH2Cl2) 199–201 °C; IR (ATR): 1713 cm–1 (COO, COOCH3); 1H NMR (CDCl3, 300 MHz): δ 2.72 (s, 3H, CH3), 3.79 (s, 3H, COOCH3), 3.85 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 6.30 (d, J = 2,4 Hz, 1H, H6), 6.40 (d, J = 2,4 Hz, 1H, H8), 7.07 (d, J = 15,7 Hz, 1H, CH=CH–COOCH3), 7.82 (d, J = 15,7 Hz, 1H, CH=CH–COOCH3); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 19.7 (CH3), 51.7 (CH=CH–COOCH3), 55.8 (OCH3), 55.9 (OCH3), 93.1 (C8), 96.0 (C6), 106.5 (C4a), 115.5 (C3), 123.1 (CH=CH–COOCH3), 136.3 (CH=CH–COOCH3), 154.5 (C4), 155.8 (C8a), 159.0, 163.5 (C5, C7), 159.8 (CO), 168.4 (COOCH3); MS (CI) m/z (rel intensity) 306 (MH+ + 1, 14), 305 (MH+, 100), 274 (10), 273 (85); HRMS (CI) Calcd. for C16H17O6 [MH+]: 305.1020; found, 305.1021.
tert-Butyl (E)-3-(5,7-Dimethoxy-4-methyl-2-oxo-2H-chromen-3-yl)acrylate (4f)
Coumarin 4f was prepared from 2i (60 mg, 0.27 mmol), AgOAc (0.14 g, 0.82 mmol), PivOH (0.28 g, 2.72 mmol), tert-butyl acrylate (0.08 mL, 0.54 mmol), and Pd(OAc)2 (6.2 mg, 0.03 mmol). The reaction was heated at 120 °C for 24 h, and after the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 4f (49.8 mg, 53%) as a solid: mp (CH2Cl2): 159–161 °C; IR (ATR): 1716 (COO), 1695 (COOtBu) cm–1; 1H NMR (CDCl3, 300 MHz): δ 1.51 (s, 9H, C(CH3)3), 2.69 (s, 3H, CH3), 3.83 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.28 (d, J = 2.4 Hz, 1H, H6), 6.38 (d, J = 2.4 Hz, 1H, H8), 6.97 (d, J = 15.7 Hz, 1H, CH=CH–COOtBu), 7.71 (d, J = 15.7 Hz, 1H, CH=CH–COOtBu); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 19.6 (CH3), 28.2 (C(CH3)3), 55.7 (OCH3), 55.9 (OCH3), 80.3 (C(CH3)3), 93.1 (C8), 95.9 (C6), 105.5 (C4a), 115.6 (C3), 125.4 (CH=CH–COOtBu), 135.0 (CH=CH–COOtBu), 154.0 (C8a), 155.7 (C4), 159.1, 159.7 (C5, C7), 163.4 (COO), 167.3 (COOtBu); MS (CI) m/z (rel intensity) 347.1 (MH+, 6), 346.1 (20), 292.1 (10), 291 (52), 290.1 (100), 274.1 (10), 273.1 (63), 262.1 (12), 245.1 (72); HRMS (CI) Calcd. for C19H23O6 [MH+]: 347.1489; found, 347.1507.
Methyl (E)-3-(5,7-Dimethoxy-4-methyl-2-oxo-2H-chromen-3-yl)but-2-enoate (4g)
Coumarin 4g was prepared from 2i (52.1 mg, 0.24 mmol), AgOAc (0.12 g, 0.71 mmol), PivOH (0.24 g, 2.37 mmol), methyl crotonate (0.05 mL, 0.47 mmol), and Pd(OAc)2 (5.4 mg, 0.024 mmol). The reaction was heated at 120 °C for 48 h. After the work-up, flash column chromatography (silica gel, hexane/EtOAc 7/3) afforded 4g (33.5 mg, 44%) as a solid: mp (CH2Cl2): 153–155 °C; IR (ATR): 1706 cm–1 (COO, COOCH3); 1H NMR (CDCl3, 300 MHz): δ 2.39 (d, J = 1.3 Hz, 3H, C(CH3)=CH(COOCH3)), 2.48 (s, 3H, C–CH3), 3.73 (s, 3H, C(CH3)=CH(COOCH3)), 3.85 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 5.76–5.79 (m, 1H, C(CH3)=CH(COOCH3)), 6.31 (d, J = 2,4 Hz, 1H, H6), 6.43 (d, J = 2,4 Hz, 1H, H8); 13C{1H} NMR (CDCl3, 75.5 MHz): δ 19.4 (C(CH3)=CH(COOCH3)), 20.7 (C–CH3), 51.1 (C(CH3)=CH(COOCH3)), 55.7 (OCH3), 55.8 (OCH3), 93.2 (C8), 95.8 (C6), 104.8 (C4a), 111.4 (C3), 121.6 (C(CH3)=CH(COOCH3)), 148.8 (C(CH3)=CH(COOCH3)), 152.6 (C4), 155.8 (C8a), 159.4, 159.5 (C5, C7), 162.6 (CO), 166.5 (C(CH3)=CH(COOCH3)); MS (ESI+) m/z (rel intensity) 319 (MH+, 100), 288 (17), 287 (71); HRMS (ESI+) Calcd. for C17H19O6 [MH+]: 319.1176, found, 319.1171.
Acknowledgments
Ministerio de Economía y Competitividad (FEDER CTQ2016-74881-P), Ministerio de Ciencia e Innovación (PID2019-104148GB-I00), and Gobierno Vasco (IT1045-16) are gratefully acknowledged for their financial support. A.C.-M. wishes to thank Gobierno Vasco for a grant. Technical and human support provided by Servicios Generales de Investigación SGIker (UPV/EHU, MINECO, GV/EJ, ERDF and ESF) is also acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03469.
Preparation procedures for the substrates 1, additional substrates tested in the cyclization, additional essays for the intermolecular alkenylation reaction of 2i, copies of the 1H and 13C NMR spectra of compounds 2–4, and the fluorescence spectra for selected compounds 2 and 4 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Estévez-Braun A.; González A. G. Coumarins. Nat. Prod. Rep. 1997, 14, 465–475. 10.1039/np9971400465. [DOI] [PubMed] [Google Scholar]
- Vazquez-Rodriguez S.; Matos M.; Borges F.; Uriarte E.; Santana L. Bioactive Coumarins from Marine Sources: Origin, Structural Features and Pharmacological Properties. Curr. Top. Med. Chem. 2015, 15, 1755–1766. 10.2174/1568026615666150427125916. [DOI] [PubMed] [Google Scholar]
- Xu J.; Kjer J.; Sendker J.; Wray V.; Guan H.; Edrada R.; Müller W. E. G.; Bayer M.; Lin W.; Wu J.; Proksch P. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 2009, 17, 7362–7367. 10.1016/j.bmc.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Rateb M. E.; Ebel R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- Fan H.; Peng J.; Hamann M. T.; Hu J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287. 10.1021/cr078199m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T.; Ishibashi F.; Iwao M.. Lamellarin alkaloids: Isolation, synthesis, and biological activity. In The Alkaloids: Chemistry and Biology; Vol. 83, Knolker H. J. Ed.; Elsevier: London, 2020; pp. 1–112. [DOI] [PubMed] [Google Scholar]
- Garg S. S.; Gupta J.; Sharma S.; Sahu D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 2020, 152, 105424. 10.1016/j.ejps.2020.105424. [DOI] [PubMed] [Google Scholar]
- Nagy N.; Kuipers H. F.; Frymoyer A. R.; Ishak H. D.; Bollyky J. B.; Wight T. N.; Bollyky P. L. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front. Immunol. 2015, 6, 123. 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai M.; Watanabe A.; Yoshida I.; Mishima T.; Nakamura M.; Nishikawa K.; Morimoto Y. Evaluation of Aculeatin and Toddaculin Isolated from Toddalia asiatica as Anti-inflammatory Agents in LPS-Stimulated RAW264 Macrophages. Biol. Pharm. Bull. 2018, 41, 132–137. 10.1248/bpb.b17-00607. [DOI] [PubMed] [Google Scholar]
- McKee T. C.; Fuller R. W.; Covington C. D.; Cardellina J. H.; Gulakowski R. J.; Krepps B. L.; McMahon J. B.; Boyd M. R. New Pyranocoumarins Isolated from Calophyllum lanigerum and Calophyllum teysmannii. J. Nat. Prod. 1996, 59, 754–758. 10.1021/np9603784. [DOI] [PubMed] [Google Scholar]
- Duval R.; Duplais C. Fluorescent natural products as probes and tracers in biology. Nat. Prod. Rep. 2017, 34, 161–193. 10.1039/C6NP00111D. [DOI] [PubMed] [Google Scholar]
- Tasior M.; Kim D.; Singha S.; Krzeszewski M.; Ahn K. H.; Gryko D. T. π-Expanded coumarins: synthesis, optical properties and applications. J. Mater. Chem. C 2015, 3, 1421–1446. 10.1039/C4TC02665A. [DOI] [Google Scholar]
- Cao D.; Liu Z.; Verwilst P.; Koo S.; Jangjili P.; Kim J. S.; Lin W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. 10.1021/acs.chemrev.9b00145. [DOI] [PubMed] [Google Scholar]
- Ungureanu I. M.; Van Ommeren E.; Givaudan S. A.. Switzerland Off-taste masking sweetener. PCT Int. Appl. WO 2010100158 A1 20100910, Sep. 10, 2010; Chem. Abstr. 2010, 153, 381750.
- Orlow S. J.; Komatsu L. N.. (New York University, USA) Coumarins as melanogenesis modifiers for cosmetics and pharmaceuticals. PCT Int. Appl. WO 2012103487 A1 20120802, Jan. 27, 2017; Chem. Abstr. 2012, 157, 305798.
- Guan A.-Y.; Liu C.-L.; Li M.; Zhang H.; Li Z.-N.; Li Z.-M. Design, synthesis and structure-activity relationship of novel coumarin derivatives. Pest Manage. Sci. 2011, 67, 647–655. 10.1002/ps.2103. [DOI] [PubMed] [Google Scholar]
- Stefanachi A.; Leonetti F.; Pisani L.; Catto M.; Carotti A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250–284. 10.3390/molecules23020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Liu X.; De Clercq E. NF-κB: the inducible factors of HIV-1 transcription and their inhibitors. Mini-Rev. Med. Chem. 2009, 9, 60–69. [DOI] [PubMed] [Google Scholar]
- Verotta L.; Lovaglio E.; Vidari G.; Finzi P. V.; Neri M. G.; Raimondi A.; Parapini S.; Taramelli D.; Riva A.; Bombardelli E. 4-Alkyl- and 4-phenylcoumarins from Mesua ferrea as promising multidrug resistant antibacterials. Phytochemistry 2004, 65, 2867–2879. 10.1016/j.phytochem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Pierson J.-T.; Dumètre A.; Hutter S.; Delmas F.; Laget M.; Finet J.-P.; Azas N.; Combes S. Synthesis and antiprotozoal activity of 4-arylcoumarins. Eur. J. Med. Chem. 2010, 45, 864–869. 10.1016/j.ejmech.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Combes S.; Barbier P.; Douillard S.; McLeer-Florin A.; Bourgarel-Rey V.; Pierson J.-T.; Fedorov A. Y.; Finet J. P.; Boutonnat J.; Peyrot V. Synthesis and Biological Evaluation of 4-Arylcoumarin Analogues of Combretastatins. Part 2. J. Med. Chem. 2011, 54, 3153–3162. 10.1021/jm901826e. [DOI] [PubMed] [Google Scholar]
- Revankar H. M.; Bukhari S. N. A.; Kumar G. B.; Qin H.-L. Coumarins scaffolds as COX inhibitors. Bioorg. Chem. 2017, 71, 146–159. 10.1016/j.bioorg.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Medina F. G.; Marrero J. G.; Macías-Alonso M.; González M. C.; Córdova-Guerrero I.; Teissier García A. G.; Osegueda-Robles S. Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. 10.1039/C4NP00162A. [DOI] [PubMed] [Google Scholar]
- Rodrigues J. L.; Rodrigues L. R. Biosynthesis and heterologous production of furanocoumarins: perspectives and current challenges. Nat. Prod. Rep. 2021, 38, 869–879. 10.1039/D0NP00074D. [DOI] [PubMed] [Google Scholar]
- Garazd M. M.; Garazd Y. L.; Khilya V. P. Neoflavones. 2. Methods for synthesizing and modifying 4-arylcoumarins. Chem. Nat. Compd. 2005, 41, 245–271. 10.1007/s10600-005-0126-7. [DOI] [Google Scholar]
- Fedorov A. Y.; Nyuchev A. V.; Beletskaya I. P. Catalytic methods of creation and functionalization of the coumarin skeleton. Chem. Heterocycl. Comp. 2012, 48, 166–178. 10.1007/s10593-012-0980-8. [DOI] [Google Scholar]
- Vekariya R. H.; Patel H. D. Recent Advances in the Synthesis of Coumarin Derivatives via Knoevenagel Condensation: A Review. Synth. Commun. 2014, 44, 2756–2788. 10.1080/00397911.2014.926374. [DOI] [Google Scholar]
- Li J.; Chen H.; Zhang-Negrerie D.; Du Y.; Zhao K. Synthesis of coumarins via PIDA/I2-mediated oxidative cyclization of substituted phenylacrylic acids. RSC Adv. 2013, 3, 4311–4320. 10.1039/c3ra23188g. [DOI] [Google Scholar]
- Singh J.; Sharma A. Visible Light-Induced Synthesis of Functionalized Coumarins. Adv. Synth. Catal. 2021, 363, 3411–3438. 10.1002/adsc.202100306. [DOI] [Google Scholar]
- de Meijere A.; Bräse S.; Oestreich M. Eds. Metal-Catalyzed Cross-Coupling Reactions and More 3 Volume Set; Wiley-VCH: Wenheim, Germany, 2014. [Google Scholar]
- Larhed M. Ed. Science of Synthesis: Cross Coupling and Heck-Type Reactions 3, Metal-Catalyzed Heck-Type Reactions and C–C Cross-Coupling via C–H Activation; Thieme: Stuttgart, Germany, 2013. [Google Scholar]
- Priyanka S. R. K.; Katiyar D. Recent Advances in Transition-Metal-Catalyzed Synthesis of Coumarins. Synthesis 2016, 48, 2303–2322. [Google Scholar]
- Catellani M.; Chiusoli G. P.; Marzolini G.; Rossi E. Designing a catalytic synthesis of 4-methylcoumarin from ortho-iodophenyl 3-butenoate: ring closure and isomerization control. J. Organomet. Chem. 1996, 525, 65–69. 10.1016/S0022-328X(96)06489-3. [DOI] [Google Scholar]
- Battistuzzi G.; Cacchi S.; De Salve I.; Fabrizi G.; Parisi L. M. Synthesis of Coumarins in a Molten n-Bu4NOAc/n-Bu4NBr Mixture through a Domino Heck Reaction/Cyclization Process. Adv. Synth. Catal. 2005, 347, 308–312. 10.1002/adsc.200404297. [DOI] [Google Scholar]
- Ulgheri F.; Marchetti M.; Piccolo O. Enantioselective Synthesis of (S)- and (R)-Tolterodine by Asymmetric Hydrogenation of a Coumarin Derivative Obtained by a Heck Reaction. J. Org. Chem. 2007, 72, 6056–6059. 10.1021/jo0705667. [DOI] [PubMed] [Google Scholar]
- Chen J.; Liu W.; Zhou L.; Zhao Y. Palladium catalyzed Heck-arylation/cyclization cascade: An environmentally benign and efficient synthesis of 4-arylcoumarins in water. Tetrahedron Lett. 2018, 59, 2526–2531. 10.1016/j.tetlet.2018.05.032. [DOI] [Google Scholar]
- Queiroz M.-J. R. P.; Abreu A. S.; Calhelha R. C.; Carvalho M. S. D.; Ferreira P. M. T. New strategies for the synthesis of heteroannulated 2-pyridinones, substituted 2-quinolinones and coumarins from dehydroamino acid derivatives. Tetrahedron 2008, 64, 5139–5146. 10.1016/j.tet.2008.03.057. [DOI] [Google Scholar]
- Kadnikov D. V.; Larock R. C. Palladium-Catalyzed Carbonylative Annulation of Internal Alkynes: Synthesis of 3,4-Disubstituted Coumarins. J. Org. Chem. 2003, 68, 9423–9432. 10.1021/jo0350763. [DOI] [PubMed] [Google Scholar]
- Kadnikov D. V.; Larock R. C. Palladium-catalyzed carbonylative annulation of terminal alkynes: synthesis of coumarins and 2-quinolones. J. Organomet. Chem. 2003, 687, 425–435. 10.1016/S0022-328X(03)00786-1. [DOI] [Google Scholar]
- Wu X.-F.; Wu L.; Jackstell R.; Neumann H.; Beller M. A general palladium-catalyzed carbonylative synthesis of chromenones from salicylic aldehydes and benzyl chlorides. Chem. – Eur. J. 2013, 19, 12245–12248. 10.1002/chem.201301774. [DOI] [PubMed] [Google Scholar]
- Wienhold M.; Molloy J. J.; Daniliuc C. G.; Gilmour R. Coumarins by Direct Annulation: β-Borylacrylates as Ambiphilic C3- Synthons. Angew. Chem., Int. Ed. 2021, 60, 685–689. 10.1002/anie.202012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei A.; Shi W.; Liu W.; Zhang H.; He C.. Oxidative Cross-Coupling Reactions; Wiley-VCH: Weinheim, 2016, 10.1002/9783527680986. [DOI] [Google Scholar]
- Ferguson J.; Zeng F.; Alper H. Synthesis of Coumarins via Pd-Catalyzed Oxidative Cyclocarbonylation of 2-Vinylphenols. Org. Lett. 2012, 14, 5602–5605. 10.1021/ol302725x. [DOI] [PubMed] [Google Scholar]
- Sasano K.; Takaya J.; Iwasawa N. Palladium(II)-Catalyzed Direct Carboxylation of Alkenyl C–H Bonds with CO2. J. Am. Chem. Soc. 2013, 135, 10954–10957. 10.1021/ja405503y. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. Synthesis of Heterocycles via Transition-Metal-Catalyzed Hydroarylation of Alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. 10.1039/C3CS60369E. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Toste F. D.; Greenman K. Atom Economy Palladium-Catalyzed Formation of Coumarins by Addition of Phenols and Alkynoates via a Net C-H Insertion. J. Am. Chem. Soc. 2003, 125, 4518–4526. 10.1021/ja0286573. [DOI] [PubMed] [Google Scholar]
- Aoki S.; Oyamada J.; Kitamura T. Formation of Coumarins by Palladium(II)-Catalyzed Reaction of Phenols with Ethyl Acrylates. Bull. Chem. Soc. Jpn. 2005, 78, 468–472. 10.1246/bcsj.78.468. [DOI] [Google Scholar]
- Sharma U.; Naveen T.; Maji A.; Manna S.; Maiti D. Palladium-Catalyzed Synthesis of Benzofurans and Coumarins from Phenols and Olefins. Angew. Chem., Int. Ed. 2013, 52, 12669–12673. 10.1002/anie.201305326. [DOI] [PubMed] [Google Scholar]
- Zhang X.-S.; Li Z.-W.; Shi Z.-J. Palladium-Catalyzed Base-Accelerated Direct C–H Bond Alkenylation of Phenols to Synthesize Coumarin Derivatives. Org. Chem. Front. 2014, 1, 44–49. 10.1039/C3QO00010A. [DOI] [Google Scholar]
- Kim D.; Min M.; Hong S. One-pot catalysis of dehydrogenation of cyclohexanones to phenols and oxidative Heck coupling: expedient synthesis of coumarins. Chem. Commun. 2013, 49, 4021–4023. 10.1039/c3cc41296b. [DOI] [PubMed] [Google Scholar]
- Suna E.; Shubin K.. Intramolecular Coupling via C(sp2)-H Activation. In Science of Synthesis. Cross Coupling and Heck-Type Reactions 3. Metal-Catalyzed Heck-Type Reactions and C-H Couplings via C-H Activation; Larhed M., Ed.; Georg Thieme Verlag: Stuttgart, 2013; pp. 643–653. [Google Scholar]
- Carral-Menoyo A.; Sotomayor N.; Lete E. Palladium-catalysed Heck-type alkenylation reactions in the synthesis of quinolines. Mechanistic insights and recent applications. Catal. Sci. Technol. 2020, 10, 5345–5361. 10.1039/D0CY00789G. [DOI] [Google Scholar]
- Ortiz-de-Elguea V.; Sotomayor N.; Lete E. Two consecutive Palladium(II)-promoted C-H alkenylation reactions for the synthesis of 3-alkenylquinolones. Adv. Synth. Catal. 2015, 357, 463–473. 10.1002/adsc.201400931. [DOI] [Google Scholar]
- Carral-Menoyo A.; Sotorríos L.; Ortiz-de-Elguea V.; Diaz-Andrés A.; Sotomayor N.; Gómez-Bengoa E.; Lete E. Intramolecular Palladium(II)-Catalyzed 6-endo C–H Alkenylation Directed by the Remote N-Protecting Group: Mechanistic Insight and Application to the Synthesis of Dihydroquinolines. J. Org. Chem. 2020, 85, 2486–2503. 10.1021/acs.joc.9b03174. [DOI] [PubMed] [Google Scholar]
- Sandhu S.; Bansal Y.; Silakari O.; Bansal G. Coumarin hybrids as novel therapeutic agents. Bioorg. Med. Chem. 2014, 22, 3806–3814. 10.1016/j.bmc.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Carral-Menoyo A.; Misol A.; Gómez-Redondo M.; Sotomayor N.; Lete E. Palladium(II)-Catalyzed Intramolecular C–H Alkenylation for the Synthesis of Chromanes. J. Org. Chem. 2019, 84, 2048–2060. 10.1021/acs.joc.8b03051. [DOI] [PubMed] [Google Scholar]
- Carral-Menoyo A.; Ortiz-de-Elguea V.; Martinez-Nunes M.; Sotomayor N.; Lete E. Palladium-Catalyzed Dehydrogenative Coupling: An Efficient Synthetic Strategy for the Construction of the Quinoline Core. Mar. Drugs 2017, 15, 276. 10.3390/md15090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rubia A.; Urones B.; Gómez-Arrayás R.; Carretero J. C. PdII-Catalyzed C-H Olefination of N-(2-Pyridyl)sulfonyl Anilines and Arylalkylamines. Angew. Chem., Int. Ed. 2011, 50, 10927–10931. 10.1002/anie.201105611. [DOI] [PubMed] [Google Scholar]
- Urones B.; Gomez-Arrayas R.; Carretero J. C. PdII-Catalyzed Di-o-olefination of Carbazoles Directed by the Protecting N-(2-Pyridyl)sulfonyl Group. Org. Lett. 2013, 15, 1120–1123. 10.1021/ol400206k. [DOI] [PubMed] [Google Scholar]
- García-Rubia A.; Laga E.; Cativiela C.; Urriolabeitia E. P.; Gómez-Arrayás R.; Carretero J. C. Pd-Catalyzed Directed ortho-C-H Alkenylation of Phenylalanine Derivatives. J. Org. Chem. 2015, 80, 3321–3331. 10.1021/jo502912m. [DOI] [PubMed] [Google Scholar]
- Schiffner J. A.; Oestreich M. All-Carbon-Substituted Quaternary Carbon Atoms in Oxindoles by an Aerobic Palladium(II)-Catalyzed Ring Closure onto Tri- and Tetrasubstituted Double Bonds. Eur. J. Org. Chem. 2011, 1148–1154. 10.1002/ejoc.201001526. [DOI] [Google Scholar]
- Gordo J.; Avó J.; Parola A. J.; Lima J. C.; Pereira A.; Branco P. S. Convenient Synthesis of 3-Vinyl and 3-Styryl Coumarins. Org. Lett. 2011, 13, 5112–5115. 10.1021/ol201983u. [DOI] [PubMed] [Google Scholar]
- Avó J.; Martins S.; Parola A. J.; Lima J.; Ramalho J.; Branco P.; Pereira A. A Family of Styryl Coumarins: Synthesis, Spectroscopic, Photophysical and Photechemical Properties. ChemPlusChem 2013, 78, 789–792. 10.1002/cplu.201300118. [DOI] [PubMed] [Google Scholar]
- Wang X.; Li S.; Pan Y.; Wang H.; Chen Z.; Huang K. Regioselective Palladium-Catalyzed Decarboxylative Cross-Coupling Reaction of Alkenyl Acids with Coumarins: Synthesis of 3-Styrylcoumarin Compounds. J. Org. Chem. 2015, 80, 2407–2412. 10.1021/jo502572j. [DOI] [PubMed] [Google Scholar]
- Jafarpour F.; Zarei S.; Barzegar Amiri Olia M.; Jalalimanesh N.; Rahiminejadan S. Palladium-Catalyzed Decarboxylative Cross-Coupling Reactions: A Route for Regioselective Functionalization of Coumarins. J. Org. Chem. 2013, 78, 2957–2964. 10.1021/jo302778d. [DOI] [PubMed] [Google Scholar]
- Min M.; Kima Y.; Hong S. Regioselective palladium-catalyzed olefination of coumarins via aerobic oxidative Heck reactions. Chem. Commun. 2013, 49, 196–198. 10.1039/C2CC37676H. [DOI] [PubMed] [Google Scholar]
- Lavis L. D.; Raines R. T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142–155. 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavis L. D.; Raines R. T. Bright Building Blocks for Chemical Biology. ACS Chem. Biol. 2014, 9, 855–866. 10.1021/cb500078u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.; Hao Y.; Zheng M.; Chen Y. A fluorescent dye with large Stokes shift and high stability: synthesis and application to live cell imaging. RSC Adv. 2017, 7, 7604–7609. 10.1039/C6RA27547H. [DOI] [Google Scholar]
- Boland G. M.; Donnelly D. M. X.; Finet J.-P.; Rea M. D. Synthesis of neoflavones by Suzuki arylation of 4-substituted coumarins. J. Chem. Soc. Perkin. Trans. 1 1996, 21, 2591–2597. [Google Scholar]
- Prateeptongkum S.; Duangdee N.; Thongyoo P. Facile Iron(III) Chloride Hexahydrate Catalyzed Synthesis of Coumarins. ARKIVOC 2015, 2015, 248–258. 10.3998/ark.5550190.p008.947. [DOI] [Google Scholar]
- Sekino E.; Kumamoto T.; Tanaka T.; Ikeda T.; Ishikawa T. Concise Synthesis of Anti-HIV-1 Active (+)-Inophyllum B and (+)-Calanolide A by Application of (−)-Quinine-Catalyzed Intramolecular Oxo-Michael Addition. J. Org. Chem. 2004, 69, 2760–2767. 10.1021/jo035753t. [DOI] [PubMed] [Google Scholar]
- Crecente-Campo J.; Pilar Vázquez-Tato M.; Seijas J. A. Microwave-Promoted, One-Pot, Solvent-Free Synthesis of 4-Arylcoumarins from 2-Hydroxybenzophenones. Eur. J. Org. Chem. 2010, 4130–4135. 10.1002/ejoc.201000051. [DOI] [Google Scholar]
- Tang B.-C.; Wang M.; Ma J.-T.; Wang Z.-X.; Wu Y.-D.; Wu A.-X. Palladium-Acid Co-Catalyzed Cleavage of Alkynoates to Construct Dibenzo[c,h]xanthene Derivatives. Adv. Synth. Catal. 2018, 360, 4023–4028. 10.1002/adsc.201800770. [DOI] [Google Scholar]
- Zhao B.; Xu B. Visible-Light Promoted Oxidative Cyclization of Cinnamic Acid Derivatives using Xanthone as the Photocatalyst. Org. Biomol. Chem. 2021, 19, 568–573. 10.1039/D0OB02417A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.