Abstract
Altered immune function and inflammation are seen in schizophrenia, however, peripheral inflammatory markers are not consistently elevated in all people, suggesting inflammation may be present only in a subgroup. We measured TNF-α and IL-Iβ in 100 people with schizophrenia or schizoaffective disorder and correlated these with antibodies to gliadin, a protein found in wheat, barley and rye that has been found to be elevated in some people with schizophrenia. We hypothesized that higher peripheral antigliadin antibodies (AGA IgG) would be associated with higher peripheral inflammation as measured by TNF-α and IL-1β. Mean log transformed values of TNF-α, (p = .029) and IL-1β (p = .016) were over twofold higher in people with schizophrenia who had high levels of AGA IgG (≥7 U) compared to those who did not have positivity to AGA IgG. We found a significant positive correlation between AGA IgG and the log transformed TNF-α (r = 0.42, p < .0001) as well as IL-Iβ (r = 0.51, p < .0001). The relationship was independent of cigarette smoking, body mass index and antipsychotic medications. People with schizophrenia having higher levels of AGA IgG show higher levels of peripheral inflammation and may define a subgroup with distinct pathophysiology and potentially novel treatment targets.
Keywords: Schizophrenia, Gliadin, Antibodies, Inflammation, TNFα
1. Introduction
Many lines of evidence suggest that inflammation may play a role in schizophrenia psychopathology (Müller et al., 2015). For example, a pro-inflammatory response with elevated peripheral cytokines is reported in some individuals with schizophrenia relative to controls (Boerrigter et al., 2017). Other evidence that also points to an inflammatory mechanism include genes in the major histocompatibility complex associated with schizophrenia risk, maternal infection as a risk factor for schizophrenia in offspring, and positive effects of some anti-inflammatory agents in clinical trials (Müller et al., 2015). However, it is noteworthy that not all people with schizophrenia have inflammation, suggesting that the contribution of inflammation to schizophrenia may only be in subgroups of individuals (Boerrigter et al., 2017).
Autoimmune and innate immune reactions possibly triggering inflammation have been reported to be higher in schizophrenia compared to healthy controls (Khandaker et al., 2017; Eaton et al., 2006). One such immune response that has been emerging in schizophrenia is the formation of antibodies to gliadin, a component protein of gluten found in wheat, barley and rye (Jackson et al., 2012). Elevated Antigliadin Antibodies (AGA IgG) relative to controls have been reported in numerous recent reports (Sidhom et al., 2012; Dickerson et al., 2016; Okusaga et al., 2016; Cihakova et al., 2017). While inflammation and AGA IgG have not been previously studied or reported in people with schizophrenia, mouse models for gluten sensitivity show that AGA are associated with the production of proinflammatory markers of inflammation (Vijaykrishnaraj et al., 2017).
In a previously published study with 31 schizophrenia patients (distinct from this study) we found that higher AGA IgG was correlated with higher levels of brain neurochemicals such as myoinositol and total choline (Rowland et al., 2017). This is suggestive of inflammation present in the brain (Chang et al., 2013). Severance et al. (2015) recently reported that serum AGA IgG levels measured in the periphery were tightly correlated to AGA IgG levels measured in cerebral spinal fluid in people with schizophrenia, a finding not seen in healthy controls. This suggests that a compromised blood brain barrier in schizophrenia exists which allows the passage of antibodies. This could provoke central inflammation or allow for the passage of cytokines and other immune-related compounds. Since we have seen a correlation of elevated neurochemicals indicative of central inflammation to AGA IgG and a compromised blood brain barrier in schizophrenia may allow for easier passage of antibodies, we would anticipate also seeing inflammatory markers in the periphery of those with high AGA IgG levels.
2. Methods
This study examines the relationship of AGA IgG to two peripheral cytokines reported to be elevated in schizophrenia, TNF-α and IL-1β (Boerrigter et al., 2017). We analyzed these cytokines from frozen serum of 100 participants with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder with previously measured and reported AGA IgG levels (Jackson et al., 2014). The AGA IgG levels were measured previously by an automated enzyme-linked immunosorbent assay (ELISA) method with kits on a single ImmunoCap 100analyzer (Phadia AB, Freiburg, Germany) (Jackson et al., 2014). Positivity was considered ≥7.0 U as provided by the manufacturer. In this follow-up study the stored serum measured had been frozen at −80°F, drawn at the time of the AGA IgG. The serum was measured by Luminex multiplex technology at the Cytokine Core Laboratory at the University of Maryland School of Medicine. The intra- and interclass coefficients of variation for TNF-α were 3.5% and 4.8%, respectively. For IL-1β the values are 5.3% and 4.1% respectively.
Spearman correlations were used to examine the relationship of cytokines to AGA IgG. Additionally we examined differences in cytokines and AGA IgG by variable (antipsychotic medication, Body Mass Index (BMI), smoking status, and diagnosis) using Kruskal-Wallis Chi-Square. We also examined the relationship of peripheral cytokines to psychiatric symptoms as measured by the Brief Psychiatric Rating Scale (BPRS). No participants were included who were taking regularly scheduled anti-inflammatory medications or immune modulating treatments, or maintained on a gluten free diet at the time of the study.
3. Results
3.1. Participants
The demographic and clinical information is listed in Table 1. This population was approximately representative of the schizophrenia population of people seeking treatment in the State of Maryland. Notable, this sample was considered stable and not in acute exacerbation of the illness. This is confirmed by a mean total Brief Psychiatric Rating Scale score of 31.9 ± 6.7.
Table 1.
Demographic and Clinical Information of 100 persons with Diagnosis of Schizophrenia or Schizoaffective Disorder.
| Mean (SD) | |
|---|---|
| Age (years) | 32.5 (9.6) |
| Body Mass Index (kg/m2) (N = 97) | 30.3 (6.8) |
| Total BPRS | 31.9 (6.7) |
| Positive symptoms | 6.2 (3.0) |
| Anxiety/Depression | 5.9 (2.4) |
| Hostility | 4.5 (1.7) |
| Negative symptoms | 6.8 (2.6) |
| Activation | 4.0 (1.4) |
| % (N) | |
| Schizophrenia | 86% (N = 86) |
| Schizoaffective Disorder | 14% (N = 14) |
| Gender (male) | 68% (N = 68) |
| Race (White) | 58% (N = 58) |
| Cigarette Smokers (N = 97) | 76% (N = 74) |
| Antipsychotic medications | |
| Clozapine | 40 (40%) |
| Other Second Generation* | 43 (43%) |
| First Generation | 15 (15%) |
| Unknown | 2 (2%) |
Isncludes aripiprazole, paliperidone, risperidone, quetiapine, ziprasidone, olanzapine, polpharmacy, all groups < 10 per group.
3.2. AGA IgG and inflammatory measures
Of the 100 tested, 9 (9%) were positive for AGA IgG (≥7.0 U). In our previously published paper this was significantly higher than the control samples which were all negative for these antibodies (Jackson et al., 2014). The mean AGA IgG of the schizophrenia group was 2.8 ± 9.4 U. AGA IgG did not differ by age, race, diagnosis, medications, smoking or BMI. Psychiatric symptoms were not correlated to AGA IgG.
We found that mean log transformed values of TNF-α (592.0 ± 619.2 pg/ml vs. 189.5 ± 380.6 pg/ml, t = −2.22, df = 88, p = .029) and IL-1β (947.7 ± 968.0 pg/ml vs. 271.5 ± 572.3 pg/ml; t = −2.47, df = 88, p = .016) were over twofold higher in people with schizophrenia who are positive to AGA IgG compared to schizophrenia participants who did not have positivity to AGA IgG. There was no relationship of the peripheral cytokine measures to antipsychotic medication, BMI, or smoking. Psychiatric symptoms were not correlated to peripheral cytokines with the exception of BPRS activation levels which were higher in those with higher IL-1β (r = 0.75, p = .02), and a trend for those with higher TNF-α (r = 0.61, p = .08). Nonsmokers have higher IL-1β (Chi-Square = 9.8, df = 1, p = .0017) and TNF-α (Chi-Square = 7.4, df = 1, p = .0066).
There was a significant positive correlation between AGA IgG and the log transformed TNF-α (r = 0.42, p < .0001) (Fig. 1) as well as IL-1β (r = 0.51, p < .0001) (Fig. 1), suggesting a robust association of AGA IgG antibodies with peripheral proinflammatory markers. Correlations were not affected by medication, BMI or smoking.
Fig. 1.
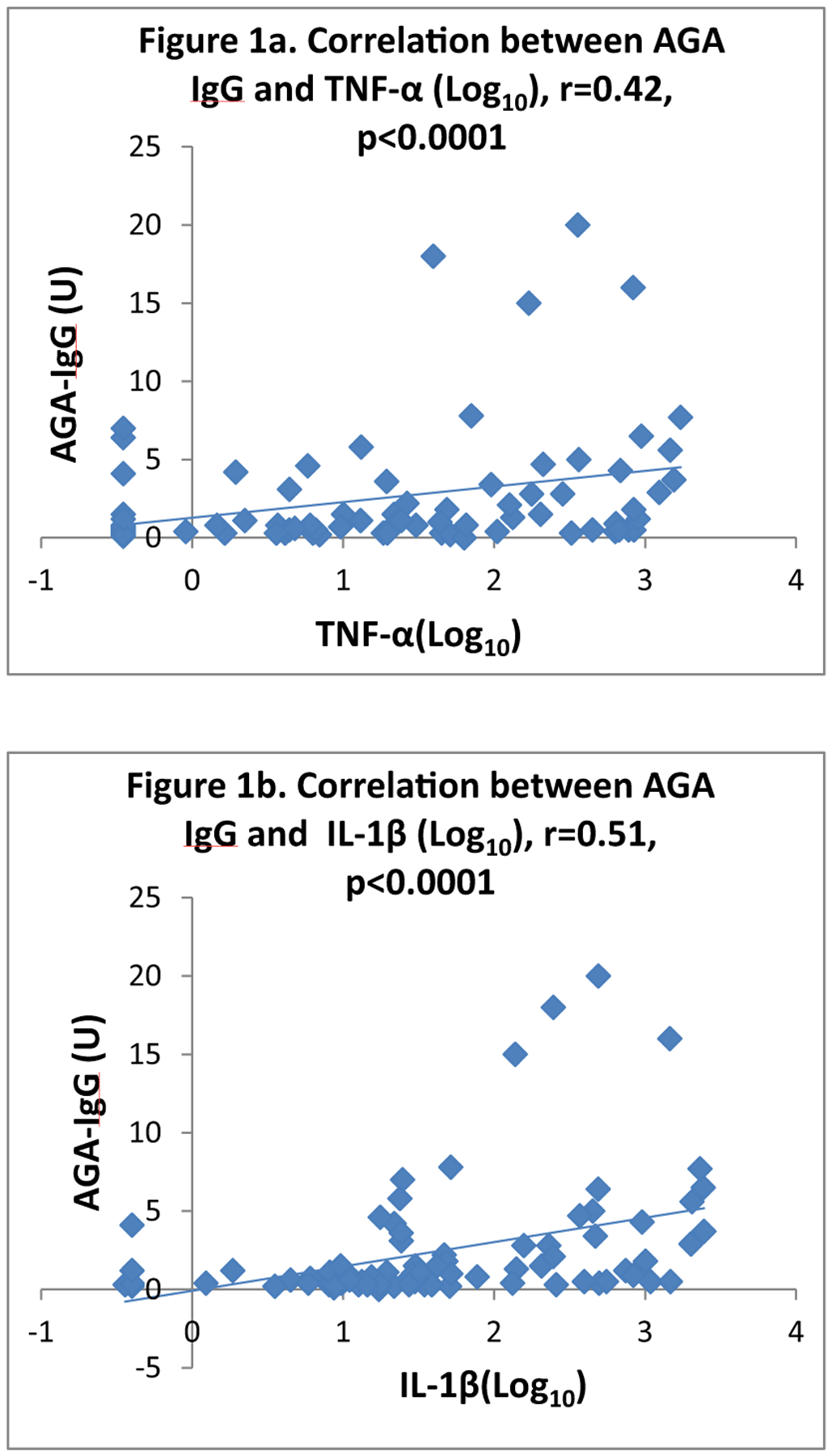
Correlations between AGA IgG and two Cytokines in People with Schizophrenia.
Exploratory analysis examined relationships in the group of 9 who were positive to AGA IgG (≥7.0 U) based on the manufacturer We find that the correlation of AGA IgG and cytokines is not limited to the positive group, but the correlation coefficient values (r) are slightly higher in the N = 9 group suggesting a tight correlation (IL-1β, r = 0.70, p = .036; TNF-α, r = 0.50, p = .175). In the group not considered positive the correlation of IL-1β (r = 0.45, p < .0001) and TNF-α (r = 0.36, p = .0007) with IgG AGA remained significant but was less robust than in the positive group: however, due to the small sample of N = 9, significance is more difficult to achieve. Examining cytokines and their relationship to other variables in the AGA IgG positive group (N = 9) revealed no notable trends or differences.
4. Discussion
These data reflect that higher levels of AGA IgG are correlated with higher levels of two peripheral cytokines, TNF-α and IL-1β. These results suggest that an association exists, but not the causal pathway. Both of these cytokines have been reported to be elevated in previous publications in some people with schizophrenia (Vijaykrishnaraj et al., 2017), however, the linkage of these laboratory results to this specific immune trigger, AGA IgG, have not been previously reported.
The study is limited by a small sample size, lack of specific information on amount of gluten in diets and lack of a healthy control group in which to examine the relationship. We also find lower rates of positivity to AGA IgG using this assay than results published in our more recent work showing about 1/3 of people with positive AGA IgG antibodies (Cihakova et al., 2017). Nonetheless, the correlations suggest that moderate antibody levels below the strong positivity cutoff of this test may also lead to some cytokine response and that the relationship of immune markers and inflammation may exist on a continuum. Future studies should examine these findings in relation to healthy controls, although the study by Severance et al. (2015) suggests that the passage of AGA IgG into the CNS in healthy controls is less prevalent than in schizophrenia.
In conclusion, people with schizophrenia having higher levels of AGA IgG show higher levels of peripheral inflammation and may define a subgroup with distinct pathophysiology and potentially novel treatment targets.
Acknowledgments
This work was funded in part by NIMH R34 MH100776-01 (Eaton and Kelly PI).
Footnotes
Disclosures
Dr. William T. Carpenter, Jr. has served as a consultant for Teva and Allergen. Dr. Alessio Fasano is a consultant for INOVA Diagnostics and Innovate Biopharmaceuticals, Inc. He is a stockholder for Alba Therapeutics and serves as a speaker for Mead Johnson Nutrition. All other authors have no current conflicts to declare. Dr. Kelly has served on an advisory board for Lundbeck.
References
- Müller N, Weidinger E, Leitner B, Schwarz MJ, 2015. The role of inflammation in schizophrenia. Front. Neurosci 9(372). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter D, Weickert TW, Lenroot R, O’Donnell M, Galletly C, Liu D, et al. , 2017. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J. Neuroinflamm 14 (1), 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Dantzer R, Jones PB, 2017. Immunopsychiatry: important facts. Psychol. Med 47 (13), 2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, et al. , 2006. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am. J. Psychiatry 163 (3), 521–528. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL, 2012. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatric Q. 83 (1), 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhom O, Laadhar L, Zitouni M, Ben Alaya N, Rafrafi R, Kallel-Sellami M, et al. , 2012. Spectrum of autoantibodies in Tunisian psychiatric inpatients. Immunol. Invest 41 (5), 538–549. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Schroeder J, Katsafanas E, Schweinfurth L, et al. , 2016. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophrenia Bull. 42 (1), 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaga O, Fuchs D, Reeves G, Giegling I, Hartmann AM, Konte B, et al. , 2016. Kynurenine and tryptophan levels in patients with schizophrenia and elevated antigliadin immunoglobulin G antibodies. Psychosomatic Med. 78 (8), 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihakova D, Eaton WW, Talor MV, Harkus UH, Demyanovich HK, Rodriguez K, et al. , 2017. Gliadin-related antibodies in schizophrenia. Schizophrenia Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishnaraj M, Mohan Kumar BV, Muthukumar SP, Kurrey NK, Prabhasankar P, 2017. Antigen-specific gut inflammation and systemic immune responses induced by prolonging wheat gluten sensitization in BALB/c murine model. J. Proteome Res [DOI] [PubMed] [Google Scholar]
- Rowland LM, Demyanovich HK, Wijtenburg SA, Eaton WW, Rodriguez K, Gaston F, et al. , 2017. Antigliadin antibodies (AGA IgG) are related to neurochemistry in schizophrenia. Front. Psychiatry 8, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T, 2013. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J. Neuroimmune Pharmacol 8 (3), 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Alaedini A, Rohleder C, Enning F, Bumb JM, et al. , 2015. IgG dynamics of dietary antigens point to cerebrospinal fluid barrier or flow dysfunction in first-episode schizophrenia. Brain Behav. Immun 44, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Eaton W, Cascella N, Fasano A, Santora D, Sullivan K, et al. , 2014. Gluten sensitivity and relationship to psychiatric symptoms in people with schizophrenia. Schizophrenia Res. 159 (2–3), 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]


