Abstract
High glucose concentrations acutely provoke endothelial cell oxidative stress and are suggested to trigger diabetes-related macro- and microvascular injury in humans. Multiple clinical studies report that acute hyperglycaemia (induced by mixed meal or oral glucose) decreases arterial vascular function in healthy humans. Feeding, however, impacts autonomic output, blood pressure, and insulin and incretin secretion, which may each independently alter vascular function and obscure the effect of acute hyperglycaemia per se. Surprisingly, no studies have examined the acute effects of intravenous glucose-induced hyperglycaemia on both macro- and microvascular function while controlling plasma insulin concentrations. In this randomized study of healthy young adults, we compared macrovascular (i.e. brachial artery flow-mediated dilatation, carotid-femoral pulse wave velocity and post-ischaemic brachial artery flow velocity) and microvascular (heart and skeletal muscle perfusion by contrast-enhanced ultrasound) functional responses to euglycaemia and hyperglycaemia. Octreotide was infused throughout both protocols to prevent endogenous insulin release. Acute intravenous glucose-induced hyperglycaemia enhanced brachial artery flow-mediated dilatation (P = 0.004), increased skeletal muscle microvascular blood volume and flow (P = 0.001), and expanded cardiac muscle microvascular blood volume (P = 0.014). No measure of vascular function changed during octreotide-maintained euglycaemia. Our findings suggest that unlike meal-provoked acute hyperglycaemia, 4 h of intravenous glucose-induced hyperglycaemia enhances brachial artery flow-mediated dilatation, provokes cardiac and skeletal muscle microvascular function, and does not impair aortic stiffness. Previous findings of acute large artery vascular dysfunction during oral glucose or mixed meal ingestion may be due to differences in study populations and meal-induced humoral or neural factors beyond hyperglycaemia per se. (ClinicalTrials.gov number NCT03520569.)
Keywords: cardiac muscle, hyperglycaemia, microvascular perfusion, muscle metabolism, octreotide, skeletal muscle
Introduction
Chronic hyperglycaemia is a major driver of diabetes mellitus (DM) microvascular complications through non-enzymatic glycation and formation of advanced glycation end-products, enhanced reactive oxygen species production, endoplasmic reticulum stress, polyol pathway activation, and other derangements (Shah & Brownlee, 2016; Barrett et al. 2017).
Acute (hours-to-days) exposure to hyperglycaemia provokes oxidative stress in cultured human endothelial cells (Brownlee, 2001; Shah & Brownlee, 2016), which may be an initial trigger for DM-related micro- and macrovascular injury. Moreover, absent DM, acute hyperglycaemia (AH) associates with worsened vascular function in a number of clinical settings (DECODE study group, 1999; Ishihara et al. 2003; Deedwania et al. 2008; Van den Berghe et al. 2009). However, it is uncertain whether AH per se or the underlying conditions that provoked AH are primarily responsible for this vascular dysfunction.
Loader et al. performed a systematic review and meta-analysis of >30 clinical observational studies and controlled trials that examined AH’s effect on macrovascular endothelial function (Loader et al. 2015). In healthy young adults, AH (either from a mixed meal or oral glucose) decreased flow-mediated dilatation (FMD) in 9 of 11 studies while two reported no significant change. The use of oral glucose or high-carbohydrate meals to induce AH in these studies is complicated by their effects on autonomic nervous system output (Lipsitz et al. 1993), blood pressure (Sauder et al. 2012), gut hormone release (Holst, 2007) and insulin secretion, which can each alter vascular function and which were not controlled in the 11 studies cited. Surprisingly, the effect of intravenous (i.v.) glucose-induced AH on arterial FMD has been little studied. One study in healthy young adults reported no effect (Bagg et al. 2000), while FMD declined in response to i.v. glucose-induced AH in older (Ceriello et al. 2008a,b) and overweight or obese subjects (Ceriello et al. 2008a,b; Perkins et al. 2015; Joy et al. 2016).
Many fewer studies have examined the microvascular responses to AH in healthy humans, and the majority of these studies used resistance arteriolar flow (e.g. responses to acetylcholine, post-ischaemic hyperaemia or cutaneous warming) to quantify oral glucose’s effects. Results were near-evenly split between improved (Grasser et al. 2014), unchanged (Charkoudian et al. 2002) or diminished (Akbari et al. 1998; Russell et al. 2018) responses. Once again, contributions by insulin, incretins and autonomic output were neither controlled nor examined in these studies. Two careful clinical studies of healthy young adults used brachial artery glucose infusion to produce local forearm hyperglycaemia without insulin or incretin stimulation and measured forearm blood flow basally and in response to cholinergic stimulation. One reported a flow decrease after 6 h of AH (Williams et al. 1998), and the second reported a slight increase at 6, 12 and 24 h (Houben et al. 1996). Plasma insulin was maintained at basal levels in each forearm study. Another study of healthy young adults utilized i.v. glucose-induced hyperglycaemia plus somatostatin and basal insulin infusion for 6 h and saw a significant increase in forearm blood flow (van Gurp et al. 2005). These studies did not examine macrovascular endothelial function.
Given the multiple, complex physiological responses to meal ingestion, we rationalized that specifically testing AH’s direct macro- and microvascular effects would be best approached using i.v. glucose and controlling endogenous insulin release. To our knowledge, such studies have not been previously performed. We studied healthy young adults in an attempt to identify ‘normal’ vascular responses. To accomplish this, we infused glucose intravenously for 4 h to generate steady-state AH (during co-administration of octreotide (OCT)) and assessed vascular function at three distinct levels of the arterial tree. We measured microvascular perfusion using contrast-enhanced ultrasound in both cardiac and skeletal muscle, resistance arteriolar function using brachial artery post-ischaemic flow velocity (PIFV), and macrovascular function with carotid-femoral pulse wave velocity (cfPWV) and brachial artery FMD. As a control, we tested the effect of euglycaemic OCT infusion on these same vascular endpoints.
Methods
Ethical approval
Each study participant gave written informed consent at their initial visit prior to being carefully screened to verify inclusion/exclusion criteria. All study protocols were approved by the University of Virginia (UVA) Institutional Review Board (Study No. 19948) and met all conditions outlined in the seventh revision of the Declaration of Helsinki for use of human volunteers. This trial was registered with ClinicalTrials.gov on 9 May 2018 (ClinicalTrials.gov number NCT03520569).
Recruitment and study population
We recruited study participants by community flyers and digital advertisements. Healthy young adults met inclusion criteria if they were ≥18 and ≤35 years old, had a body mass index of 18–25 kg m−2, and had fasting plasma glucose <100 mg dl−1 and blood pressure <140/90 mmHg at time of screening. Subjects were excluded if they were current smokers or quit smoking <5 years ago, had a first-degree relative with type 2 DM, were taking vasoactive medications (e.g. anti-hypertensives, diuretics, statins, etc.), were pregnant (i.e. positive pregnancy test) or nursing, had a history of allergy or prior adverse reaction to OCT or Definity®, or had history of significant premorbid disease that could, in the investigator’s opinion, affect outcome measures or subject safety.
Clinical assessment and initial screening
All screening visits and infusion studies were conducted at the UVA Clinical Research Unit (CRU). Screening included a detailed medical history and physical examination along with fasting measures of complete blood count, comprehensive metabolic panel, lipid panel, fasting plasma glucose and serum pregnancy test.
Experimental protocols
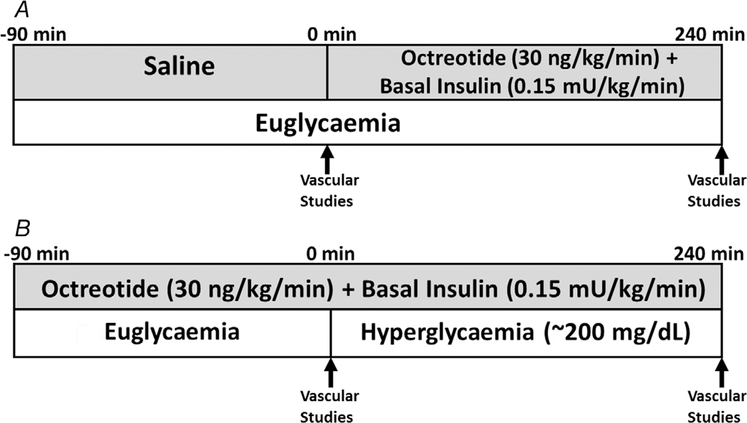
Subjects meeting inclusion criteria were randomized to one of two study protocols (Fig. 1) using a 1:1 allocation with a computer-generated sequence program (Urbaniak & Plous, 2013). After randomization, study personnel were blinded to subject and protocol when evaluating outcome measures. In this randomized crossover trial, the infusion protocols were designed to test the effects of OCT alone (protocol A) or OCT with AH (protocol B) on systemic macrovascular and cardiac and skeletal muscle microvascular function. Protocols A and B were performed ≥2 but ≤4 weeks apart for individual subjects. For each protocol, we measured cfPWV, brachial artery FMD and PIFV, and cardiac and skeletal muscle microvascular perfusion immediately before and at the end of the infusion period (Fig. 1). Study participants were instructed to avoid alcohol, exercise and caffeine for 24 h and fast overnight prior to admission to the CRU. Prior to study initiation, we placed i.v. catheters in the right wrist for blood sampling and in the right antecubital fossa for administration of insulin, glucose, OCT and saline. Studies began with a saline (protocol A) or an OCT infusion (protocol B), with simultaneously infused regular insulin (to maintain plasma insulin near basal levels) and variable-rate glucose (to maintain euglycaemia in protocol A and hyperglycaemia in protocol B). We did not replace glucagon or growth hormone, as there is currently no evidence that acutely suppressing basal levels of either hormone affects vascular function.
Figure 1.
Experimental protocols
Protocol A (euglycaemia).
A 90-min saline infusion was initiated, with baseline vascular function measurements obtained during the final 30 min (Fig. 1A). Then, OCT (30 ng kg−1 min−1) with basal insulin replacement (0.15 mU min−1 kg−1) was infused for 240 min. Plasma glucose (PG) was sampled every 10 min and euglycaemia (EU) was maintained by a variable-rate glucose infusion using the negative feedback principle (DeFronzo et al. 1979). We then repeated vascular measurements over the final 30 min of study. Plasma insulin was sampled every 30 min throughout the protocol, and intercellular adhesion molecule-1 (ICAM-1), interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and protein carbonyls were sampled at baseline and end-of-study.
Protocol B (acute hyperglycaemia).
OCT with basal insulin replacement was continuously infused for 90 min, with baseline vascular function measurements obtained over the final 30 min (Fig. 1B). Then, a primed, continuous variable-rate 20% dextrose infusion was begun to acutely raise and maintain PG at ~200 mg dl−1 using the hyperglycaemic clamp method (DeFronzo et al. 1979). PG was sampled every 5 min and plasma insulin every 30 min, with repeat vascular measurements obtained over the final 30 min of AH. ICAM-1, IL-6, TNF-α and protein carbonyls were again sampled at baseline and end-of-study.
Carotid-femoral pulse wave velocity.
To assess central aortic stiffness, cfPWV was measured non-invasively using a Sphygmacor tonometer (ATCOR USA; Napierville, IL, USA). To minimize the effects of sympathetic activity on cfPWV measurements, participants lay in the supine position for at least 15 min prior to measurement. We measured the distance from the suprasternal notch to the carotid pulse and from the suprasternal notch to the femoral pulse on the same side. For each cfPWV measurement, 10 s of carotid and 10 s of femoral arterial waveforms were recorded. cfPWV measurements were made in duplicate and the mean value was reported. We followed expert consensus (Thijssen et al. 2019; Townsend et al. 2015) and had the same trained observer obtain all vascular measurements in this study. We assessed cfPWV intraobserver reliability by having the observer record three serial cfPWV measurements on the same subject over a 4-h period. The coefficient of variation was 3.63%, indicating good intraobserver reliability (Shechtman, 2013; Thijssen et al. 2019).
Flow-mediated dilatation and post-ischaemic flow velocity.
We measured left brachial artery FMD and left forearm PIVF with the Epiq 7 cardiovascular ultrasound (Philips Medical Systems; Andover, MA, USA) instrument with a linear array probe (L12–3) steadied by a probe-holder as described previously (Jahn et al. 2016). FMD images were analysed using Brachial Analyzer (Medical Imaging Applications, LLC; Coralville, IA, USA) edge detection software by study personnel blinded to subject and protocol. We assessed FMD intraobserver reliability by having the same trained observer record eight serial FMD measurements on the same subject over a 4-h period. Coefficient of variation was 7.41%, indicating good intraobserver reliability (Shechtman, 2013; Thijssen et al. 2019).
Microvascular perfusion by contrast-enhanced ultrasound.
Cardiac (interventricular septum) and forearm skeletal muscle microvascular perfusion were assessed with an Epiq 7 ultrasound system during steady-state infusion of Definity® microbubbles (Lantheus Medical Imaging; North Billerica, MA, USA) using low mechanical index (MI; 0.10) continuous imaging for 20 s (myocardium) or 30 s (forearm) at a framerate of 15 s−1 with a flash at 0.88 MI to initiate a replenishment curve. Four 30-s replenishment curves for forearm and four 20-s replenishment curves for myocardium were acquired at baseline and at the end of the infusion study. Replenishment curves were analysed using Q-Lab (Philips Research; Cambridge, MA, USA) by study personnel blinded to subject and protocol.
Biochemical analyses
Complete blood count, comprehensive metabolic panel, lipid panel, fasting PG, and serum pregnancy tests were assayed at the UVA Clinical Chemistry Laboratory. PG was measured with a YSI 2700 Biochemistry Analyzer (Yellow Springs Instrument Company; Yellow Springs, OH, USA), plasma insulin with an ALPCO insulin ELISA assay (ALPCO; Salem, NH, USA), and ICAM-1, IL-6, TNF-α and protein carbonyls by ELISA assay (R&D Systems; Minneapolis, MN, USA). All assays were read on a Synergy 2 microplate reader (BioTek; Winooski, VT, USA).
Data storage
Study data were stored in a Research Electronic Data Capture (REDCap) (Harris et al. 2019) project file repository hosted at UVA. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical analyses
Sample size.
We used the Hedges g effect size from a prior study that examined the effects of acute hyperglycaemia on brachial artery flow-mediated dilatation in persons without diabetes (Joy et al. 2016) to estimate sample size for the current study. This calculation indicated that a total sample size of ≥10 subjects would have >90% power to detect meaningful differences in vascular function.
Outcomes.
The primary macrovascular and microvascular outcomes for each protocol were change in FMD and microvascular blood volume (MBV), respectively. Main secondary outcomes for each protocol included changes in cfPWV, PIFV, microvascular flow velocity (MFV), microvascular blood flow (MBF), plasma insulin and ICAM-1.
Descriptive summarization.
Patient demographics were summarized using common descriptive statistics. The arithmetic mean and standard deviation, median and interquartile range were used to summarize continuous scaled outcome measures.
Data transformation and summarization.
The pre- and post-intervention outcome measurements for each protocol were rescaled to the natural logarithmic scale (i.e. loge). The analytical outcome data were then derived by subtracting the loge-transformed pre-intervention outcome measurements from the loge-transformed post-intervention outcome variable measurements. For all outcome measurements, the point estimate for the mean pre-intervention outcome measurements, the point estimate for the mean pre- to post-intervention outcome measurement change, and the point estimate for the difference between the mean of the pre-intervention outcome measurements and the mean pre- to post-intervention outcome measurement change for each protocol were converted via natural logarithmic antilog transformation (i.e. ex) to a geometric mean ratio scale.
Outcome measure analyses.
The pre-intervention EU and AH outcome measurements were compared by linear mixed model (LMM) analysis of variance, testing whether pre-intervention measurements were equal. To meet assumptions of the LMM, all outcome measurements were rescaled to the natural logarithmic scale (i.e. loge). The pre- to post-intervention outcome variable change for each protocol was compared by LMM analysis of covariance. Significance was set at α = 0.05 (two-tailed test). Bonferroni adjustment was used to correct for multiple comparisons. All statistical analyses were performed with SAS Studio 3.8 (SAS; Cary, NC).
LMM specification.
The analytical outcome data of each protocol served as the dependent variable measurements of the LMM. An indicator variable to identify the protocol (i.e. A or B) served as one of the LMM independent variables, and the loge-transformed pre-intervention outcome measurements of protocols A and B served as a second LMM independent variable. Note that the loge-transformed pre-intervention measurements were included as part of the LMM so that the between-admission comparison of the mean pre- to post-intervention change in the outcome measure could be standardized to a common reference pre-intervention measurement value.
Hypothesis testing.
Primary hypotheses under the null tested whether the mean within-protocol change in each outcome measure was equal to zero. Secondary hypotheses examined whether the mean pre- to post-intervention outcome measurement change was equivalent between protocols after standardizing the comparison to a common reference pre-intervention measurement value. Specific hypotheses were that AH negatively alters FMD, cfPWV, PIFV, MBV, MFV and MBF.
Results
Baseline subject characteristics and demographics
Table 1 details baseline demographics of the 13 study participants. All had normal BMI, blood pressure and fasting PG. Notably, 10 subjects completed both protocols while three subjects completed only the EU protocol. Reasons for withdrawal were that one subject moved out of the geographic area, one experienced abdominal cramping secondary to OCT and one withdrew due to scheduling difficulties.
Table 1.
Baseline subject characteristics and demographics
| Variable | Value |
|---|---|
| Sex | 7 female; 6 male |
| Age (years) | 25.15 (4.39) |
| Body mass index (kg m−2) | 22.00 (2.08) |
| Systolic blood pressure (mmHg) | 112.15 (10.29) |
| Diastolic blood pressure (mmHg) | 64.85 (6.39) |
| Fasting plasma glucose (mg dl−1) | 87.69 (6.59) |
| Total cholesterol (mg dl−1) | 167.92 (25.18) |
| LDL cholesterol (mg dl−1) | 97.00 (21.68) |
| HDL cholesterol (mg dl−1) | 60.77 (13.02) |
| Triglycerides (mg dl−1) | 62.00 (19.38) |
Data are presented as mean (SD). HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Plasma insulin, glucose, ICAM-1 and inflammatory biomarker concentrations
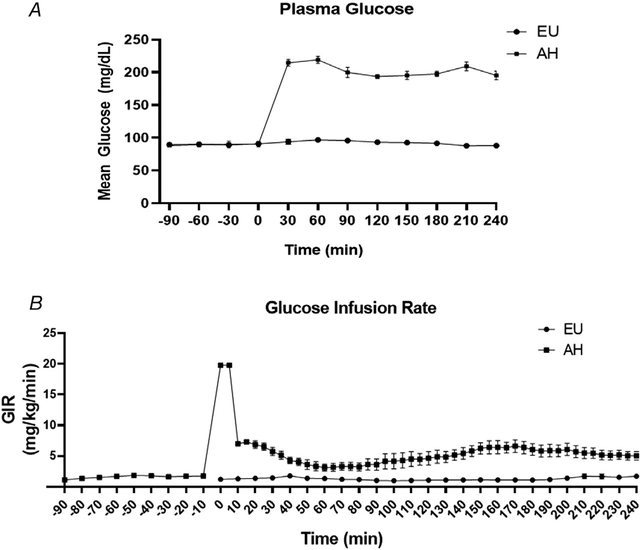
Figure 2 shows the time course for mean PG (Fig. 2A) and mean glucose infusion rate (Fig. 2B) throughout each infusion study. Plasma insulin concentrations during EU and AH did not change from baseline within either protocol (Table 2), indicating that the selected OCT and basal insulin doses maintained the basal insulin and glucose milieu during EU and prevented an endogenous insulin secretory response during AH. Furthermore, there were no between-protocol differences in insulin concentrations during either the pre- or post-intervention periods (Table 2). There were also no significant within- or between-protocol changes in ICAM-1, indicating that neither protocol induced endothelial inflammation/dysfunction (Table 2). Plasma IL-6, TNF-α and protein carbonyl levels in these healthy subjects were below the lower limits of detection for the assays used at both the baseline and end-of-study measurements.
Figure 2. Time course for mean plasma glucose.
(A) and mean glucose infusion rate (B) throughout each infusion protocol
AH, acute hyperglycaemia; EU, euglycaemia; GIR, glucose infusion rate.
Table 2.
Summary statistics for pre- and post-intervention plasma insulin (μIU ml−1) and ICAM-1 (ng ml−1) levels
| Variable | Protocol | Assessment | n a | Mean | SD | CV | GM | Within-protocol P-valueb | Between-protocol P-valuec |
|---|---|---|---|---|---|---|---|---|---|
| Insulin | EU | Pre | 7 | 6.06 | 4.37 | 0.72 | 4.70 | 0.439 | 0.316 |
| Post | 7 | 8.55 | 5.91 | 0.69 | 6.79 | ||||
| AH | Pre | 6 | 14.24 | 14.04 | 0.98 | 7.16 | 0.510 | ||
| Post | 6 | 11.48 | 7.29 | 0.64 | 10.04 | ||||
| ICAM-1 | EU | Pre | 13 | 234.88 | 59.53 | 0.25 | 228.44 | 0.766 | 0.173 |
| Post | 13 | 232.06 | 49.59 | 0.21 | 227.32 | ||||
| AH | Pre | 10 | 222.36 | 43.50 | 0.20 | 218.48 | 0.192 | ||
| Post | 10 | 217.04 | 42.98 | 0.20 | 213.08 |
Six subjects in the euglycaemia protocol and4 subjects in the acute hyperglycaemia protocol had plasma insulin levels below the limits of detection.
Within-protocol P-values reflect the point estimate for the mean pre- to post-intervention change in plasma insulin after conversion to the geometric mean ratio scale.
Between-protocol P-values represent the baseline-corrected ratio of geometric means (RGM) between protocols (i.e. RGM acute hyperglycaemia: RGM euglycaemia). AH, acute hyperglycaemia; CV, coefficient of variation; EU, euglycaemia; GM, geometric mean; ICAM-1, intercellular adhesion molecule-1.
Macrovascular function
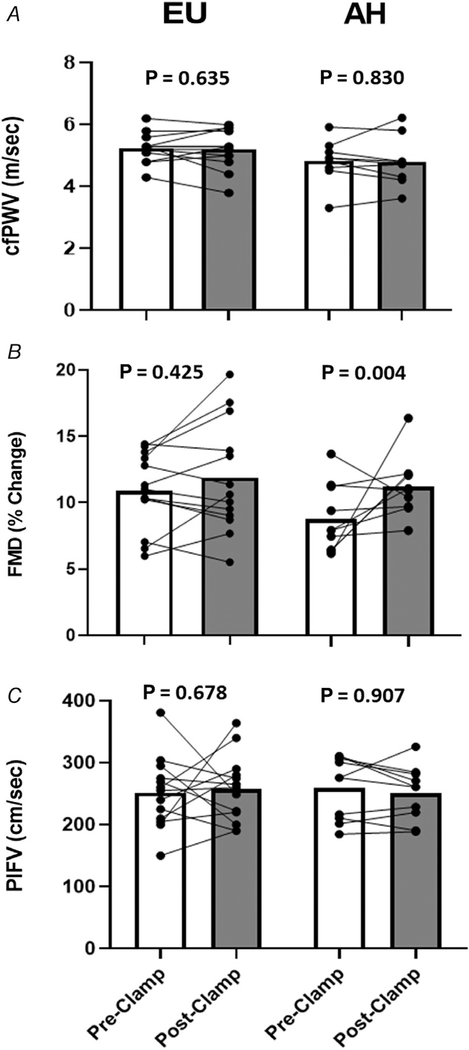
Figure 3 shows the pre- and post-intervention measures of cfPWV, FMD and PIFV in each protocol. FMD did not change with EU but significantly increased with AH (ratio of geometric mean (RGM): 1.34; 95% CI: 1.11, 1.62; P = 0.004) (Table 3). There were no significant pre- to post-intervention changes in cfPWV or PIFV with either protocol.
Figure 3. Paired individual trend lines and group mean bar graphs (pre- and post-insulin clamp) detailing cfPWV (A), FMD (B), and PIFV (C) responses to both the EU and AH protocols.
Data are presented as raw (i.e. arithmetic) values. AH, acute hyperglycaemia; cfPWV, carotid-femoral pulse wave velocity; EU, euglylcaemia; FMD, flow-mediated dilatation; PIFV, post-ischaemic flow velocity.
Table 3.
Within admission and baseline-adjusted between admission post- to pre-intervention geometric mean outcome measure ratios
| Outcome measure | Protocol | Estimate ratio of GMs (RGMs) | 95% CI | P-value |
|---|---|---|---|---|
| FMD | EU | 1.07 | 0.90, 1.26 | 0.425 (0.850) |
| AH | 1.34 | 1.11, 1.62 | 0.004 (0.008) | |
| RGM AH: RGM EUa | 1.15 | 0.93, 1.42 | 0.178 (0.357) | |
| PIFV | EU | 1.03 | 0.90, 1.18 | 0.678 (1.000) |
| AH | 1.01 | 0.87, 1.18 | 0.907 (1.000) | |
| RGM AH: RGM EUa | 1.03 | 0.93, 1.15 | 0.470 (0.939) | |
| cfPWV | EU | 0.99 | 0.94, 1.04 | 0.635 (1.000) |
| AH | 0.99 | 0.94, 1.05 | 0.830 (1.000) | |
| RGM AH: RGM EUa | 1.00 | 0.92, 1.09 | 0.971 (1.000) | |
| Skeletal MBV | EU | 1.10 | 0.92, 1.32 | 0.273 (0.545) |
| AH | 1.68 | 1.37, 2.06 | <0.001 (<0.001) | |
| RGM AH: RGM EUa | 1.50 | 1.15, 1.95 | 0.008 (0.016) | |
| Skeletal MFV | EU | 1.19 | 0.95, 1.48 | 0.124 (0.247) |
| AH | 1.39 | 1.08, 1.79 | 0.012 (0.024) | |
| RGM AH: RGM EUa | 1.13 | 0.89, 1.44 | 0.274 (0.549) | |
| Skeletal MBF | EU | 1.31 | 0.89, 1.93 | 0.162 (0.324) |
| AH | 2.34 | 1.51, 3.63 | 0.001 (0.001) | |
| RGM AH: RGM EUa | 1.68 | 1.06, 2.64 | 0.031 (0.062) | |
| Cardiac MBV | EU | 1.08 | 0.91, 1.29 | 0.356 (0.712) |
| AH | 1.34 | 1.07, 1.67 | 0.014(0.027) | |
| RGM AH: RGM EUa | 1.14 | 0.91, 1.41 | 0.211 (0.423) | |
| Cardiac MFV | EU | 1.16 | 0.93, 1.45 | 0.181 (0.362) |
| AH | 0.89 | 0.67, 1.18 | 0.415 (0.830) | |
| RGM AH: RGM EUa | 0.70 | 0.55, 0.88 | 0.007 (0.014) | |
| Cardiac MBF | EU | 1.25 | 0.94, 1.67 | 0.113 (0.226) |
| AH | 1.24 | 0.86, 1.78 | 0.235 (0.469) | |
| RGM AH: RGM EUa | 0.79 | 0.57, 1.10 | 0.137 (0.274) |
Baseline corrected comparison (Bonferroni-adjusted P-value). AH, acute hyperglycaemia; cfPWV, carotid femoral pulse wave velocity; CI, confidence interval; EU, euglycaemia; FMD, flow-mediated dilatation; GM, geometric mean; MBF, microvascular blood flow; MBV, microvascular blood volume; MFV, microvascular flow velocity; RGM, ratio of geometric mean; PIFV, post-ischaemic flow velocity.
Microvascular function
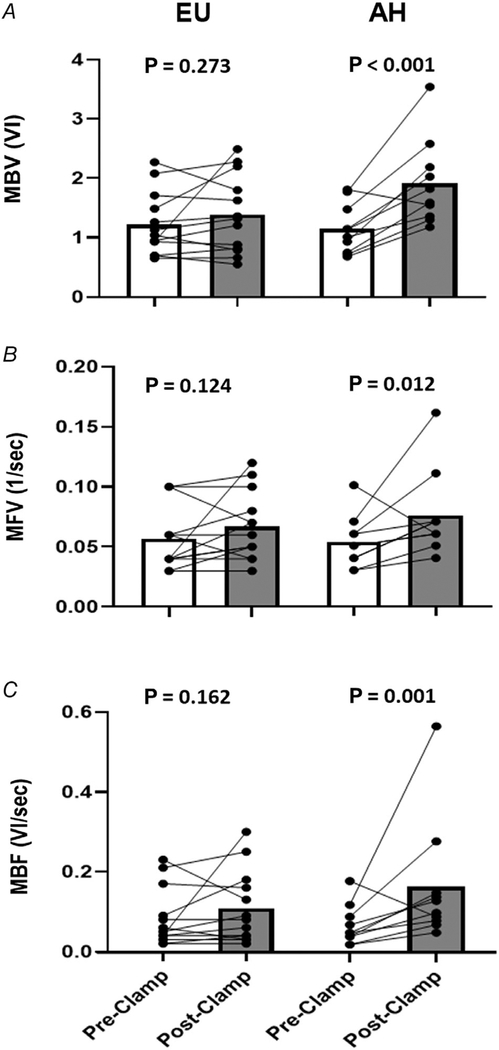
Figure 4 shows the pre- and post-intervention measures for skeletal muscle MBV (upper panel), MFV (middle panel) and MBF (lower panel). MBV is given in arbitrary units of video intensity, MFV in 1/s, and MBF is the product of MBV and MFV (video intensity/s). There were no significant changes from baseline in MBV (RGM: 1.10; 95% CI: 0.92, 1.32; P = 0.273), MFV (RGM: 1.19; 95% CI: 0.95, 1.48; P = 0.124), or MBF (RGM: 1.31; 95% CI: 0.89, 1.93; P = 0.162) during 4 h of EU. By contrast, AH increased skeletal muscle MBV (RGM: 1.68; 95% CI: 1.37, 2.06; P < 0.001), MFV (RGM: 1.39; 95% CI: 1.08, 1.79; P = 0.012) and MBF (RGM: 2.34; 95% CI: 1.51, 3.63; P = 0.001) above baseline (Table 3). MBV, MFV and MBF declined with AH in only the one subject who had the highest baseline values for each. Moreover, the change above baseline was significantly greater for AH compared to EU with both MBV (RGM: 1.50; 95% CI: 1.15, 1.95; P = 0.008) and MBF (RGM: 1.68; 95% CI: 1.06, 2.64; P = 0.031; Bonferroni-adjusted P = 0.062).
Figure 4. Paired individual trend lines and group mean bar graphs (pre- and post-insulin clamp) detailing skeletal muscle MBV (A), MFV (B), and MBF (C) responses to both the EU and AH protocols.
Data are presented as raw (i.e. arithmetic) values. AH, acute hyperglycaemia; EU, euglycaemia; MBF, microvascular blood flow; MBV, microvascular blood volume; MFV, microvascular flow velocity; VI, video intensity.
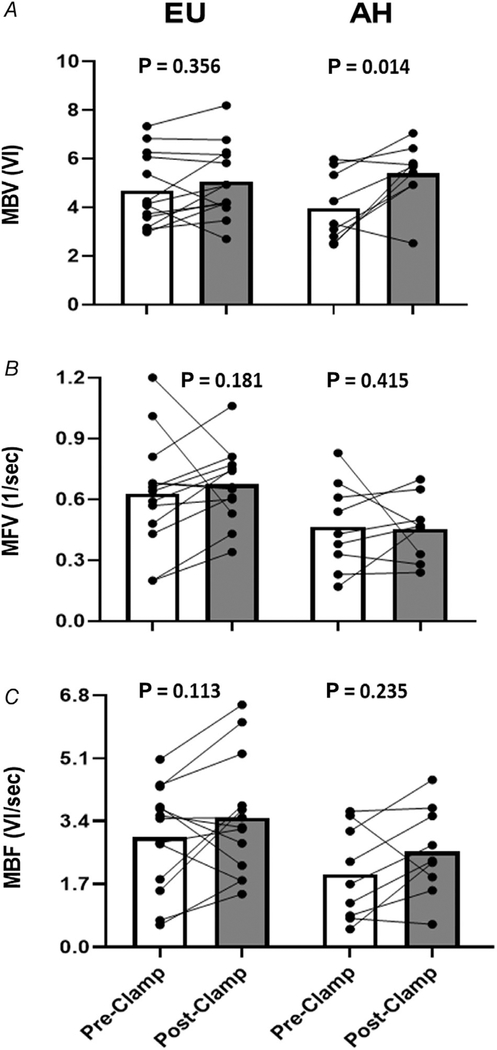
The response of cardiac muscle to 4 h of EU or AH is shown in Fig. 5. As seen in skeletal muscle, EU during OCT infusion did not alter cardiac MBV (RGM: 1.08; 95% CI: 0.91, 1.29; P = 0.356), MFV (RGM: 1.16; 95% CI: 0.93, 1.45; P = 0.181), or MBF (RGM: 1.25; 95% CI: 0.94, 1.67; P = 0.113). By contrast, AH significantly increased cardiac MBV (RGM: 1.34; 95% CI: 1.07, 1.67; P = 0.014) (Table 3). MFV did not change significantly (RGM: 0.89; 95% CI: 0.67, 1.18; P = 0.415) and MBF increased in 7 of 9 subjects, but this trend was not significant (RGM: 1.24; 95% CI: 0.86, 1.78; P = 0.235).
Figure 5. Paired individual trend lines and group mean bar graphs (pre- and post-insulin clamp) detailing cardiac muscle MBV (A), MFV (B), and MBF (C) responses to both the EU and AH protocols.
Data are presented as raw (i.e. arithmetic) values. AH, acute hyperglycaemia; EU, euglycaemia; MBV, microvascular blood volume; MBF, microvascular blood flow; MFV, microvascular flow velocity; VI, video intensity.
Vascular responses by sex
Our study was neither designed nor powered to determine vascular effects by sex, but we did conduct a purely exploratory analysis to determine if any sex-by-protocol interactions existed. There were no such interactions identified for any vascular measure assessed. Sex-by-protocol interaction testing (adjusted for individual baseline responses) identified P-values of 0.760 for cfPWV; 0.804 for FMD; 0.147 for PIFV; 0.605, 0.952 and 0.596 for skeletal muscle MBV, MFV and MBF, respectively; and 0.718, 0.448 and 0.380 for cardiac muscle MBV, MFV and MBF, respectively.
Discussion
The current study provides several novel findings regarding the acute effects of AH on vascular function. First, compared to EU, AH significantly enhanced brachial artery flow-mediated dilatation. This has not been previously reported and contrasts with the consistently reported vascular dysfunction provoked by oral glucose or high carbohydrate meals in healthy young adults (Loader et al. 2015). Almost certainly our use of i.v. glucose delivery and of OCT to block insulin and incretin responses during AH each contributed to the observed result. As OCT infusion during euglycaemia did not affect FMD, we cannot attribute the enhanced FMD in protocol B to a vascular action of OCT. We also note that van Gurp et al. (2005) previously demonstrated that i.v.-induced hyperglycaemia during somatostatin infusion did not alter sympathetic nerve output. Therefore, altered sympathetic tone is unlikely to account for the enhanced FMD seen here. The unaltered ICAM-1 levels, coupled with enhanced FMD, suggests that 4 h of AH did not interfere with the ability of shear stress to stimulate nitric oxide production by brachial artery endothelium. Two prior studies utilized similar pancreatic clamp methodology to assess the effects of i.v. glucose-induced AH on vascular inflammatory biomarkers and endothelial function in non-diabetic (Joy et al. 2016) and overweight and obese (Perkins et al. 2015) humans. Both studies found that 4 h of i.v. glucose-induced AH increased ICAM-1 (and several other inflammatory biomarkers) and decreased brachial artery FMD, neither of which were seen in the current study. However, the recruited populations for these studies had mean BMI of 29 kg m−2 (with range of 23–36 kg m−2) (Joy et al. 2016) and 30.1 kg m−2 (Perkins et al. 2015). Ceriello et al. also used pancreatic clamp methodology to assess the effects of i.v. glucose-induced AH on nitrotyrosine (a biomarker of oxidative stress) and brachial artery FMD in healthy subjects (Ceriello et al. 2008a,b). Their results showed that AH increased nitrotyrosine and reduced FMD in healthy subjects, and that these negative effects were completely prevented with vitamin C administration. These studies, however, also had healthy cohorts with mean age and BMI of 50.3 years and 27.5 kg m−2 (Ceriello et al. 2008a) and 50.5 years and 28.5 kg m−2 (Ceriello et al. 2008b). Obesity has been clearly linked to reduced brachial artery FMD (Ne et al. 2017), and weight loss itself coincides with increased brachial artery FMD (Joris et al. 2015). Brachial artery FMD is also impaired in older adults compared to young, healthy adults (Celermajer et al. 1994; Donato et al. 2007; Gates et al. 2007), and a steady age-related decline begins at age 30 years in men and age 45 years in women (Skaug et al. 2013). These findings underscore the contrast between our testing the effect of isolated AH from i.v. glucose in young, healthy adults versus the effect of oral glucose (with accompanying hormonal and autonomic changes) or the effect of i.v. glucose in overweight and obese or older populations.
We also note that OCT infusion during euglycaemia affected neither cardiac nor skeletal muscle microvascular perfusion. To our knowledge, these results are the first to demonstrate that OCT does not alter cardiac or skeletal muscle microvascular perfusion in humans. This finding is consistent with a prior canine microsphere study that reported no change in total blood flow to either tissue after a high-dose somatostatin infusion (Becker et al. 1982). Likewise, human studies using a similar OCT dose reported no change in large vessel endothelial function (Moller et al. 1995; Beckman et al. 2001; Beckman et al. 2002; Joy et al. 2016), but did not examine the effect on cardiac or skeletal muscle microvascular perfusion. Recent reports have employed similar hyperglycaemic clamp methods with OCT infusion to assess vascular function and inflammatory biomarker levels in overweight and obese humans without DM (Perkins et al. 2015; Joy et al. 2016). Thus, use of OCT provided a useful control for the current study. Inasmuch as OCT did not alter any vascular parameter evaluated in our study, its use to test the specific vascular effect of AH independent of changes in insulin, incretins and autonomic activity that accompany oral glucose or mixed meal ingestion was justified.
The present study also provides the first report of acute cardiac and skeletal muscle microvascular responses to isolated AH. Our use of contrast-enhanced ultrasound allowed specific focus on the muscle compartment at both sites while excluding contributions from skin, subcutaneous adipose and bone that contribute particularly to measures of forearm flow. Our study design unexpectedly revealed that 4 h of isolated AH from i.v. glucose significantly increased MBV in both skeletal and cardiac muscle. The increased MBV indicates an increased endothelial surface area available for nutrient and gas exchange even in the absence of increased total flow. Overall flow (MBV × MFV) likewise increased in skeletal muscle and trended towards increase in cardiac muscle. Thus, AH per se enhanced rather than inhibited microvascular perfusion. Insulin secretion was effectively blocked by OCT infusion, discounting any role for insulin-induced vasodilatation. Additionally, our use of i.v. glucose loading (together with OCT infusion) removed possible contributions of incretin hormones on the vasculature. We note that AH has been reported to increase both retinal (Burgansky-Eliash et al. 2012; Klefter et al. 2015) and renal resting blood flow (Woods et al. 1987; Marre et al. 1999); however, insulin secretion was not controlled in those studies. Accumulating data have also demonstrated increased resting coronary blood flow in DM subjects compared to healthy controls (Meyer & Schwaiger, 1997; Picchi et al. 2011; Sorensen et al. 2020), possibly due to increased resting cardiac metabolic demand (Haas et al. 2019). This flow increase in the resting heart does not appear benign, as studies have confirmed it as an independent predictor of cardiovascular mortality (Gupta et al. 2017) and shown an association with diastolic dysfunction (Haas et al. 2019). Future studies could examine pathophysiological mechanisms linking hyperglycaemia, microvascular function and resting MBF in both health and DM.
A fourth finding is that AH did not adversely affect cfPWV or PIFV. Our cfPWV result confirms findings from other studies demonstrating no cfPWV response to AH in healthy humans (Kobayashi et al. 2015; Williams et al. 2020). PIFV, which principally reflects resistance arteriolar tone, was also not impaired by AH. However, PIFV (like FMD, MBV and MFV) is sensitive to local nitric oxide production and might have been expected to change in concert with these other variables. We cannot at present explain this difference, but considered that PIFV (which like forearm blood flow sums contributions from multiple tissues) might be less sensitive to AH stimulation.
Taken together, neither micro- nor macrovascular function appeared adversely affected by 4 h of isolated hyperglycaemia in the current study. While we are not suggesting that hyperglycaemia has no adverse vascular effects over time, our results do suggest that it is incorrect to conclude that during brief periods of AH, glucose per se inhibits macro- and microvascular function. Our findings also suggest that the widely reported adverse vascular responses to oral glucose may reflect contributions of endocrine and/or neural factors beyond AH itself. i.v. glucose delivery bypasses the gut and subsequent release of many gut-derived vasoactive hormones (Holst, 2019). In contrast, mixed meal ingestion (Alsalim et al. 2015) and oral glucose (Faerch et al. 2015) stimulate incretin and islet hormone release in healthy humans. Recent studies have shown that oral glucose (Russell et al. 2018) and high-glucose mixed meals (Parker et al. 2020) impair, while low-glucose mixed meal ingestion increases (Russell et al. 2018), skeletal muscle microvascular perfusion. As such, our findings support the hypothesis that vascular functional and microvascular perfusion responses may differ by glucose delivery method (Loader et al. 2015) and/or nutritional composition of a meal (Roberts-Thomson et al. 2020; Alvares, 2020). In many of the studies reviewed by Loader et al. (2015), the oral glucose ingestion may confound results by increasing concentrations of both glucose and insulin. For example, insulin is a potent vasodilator that acts through endothelial stimulation (Steinberg et al. 1994; Baron et al. 1995; Barrett et al. 2011; Hoffman, 2015). Beyond this, hyperglycaemia without accompanying changes in plasma insulin has been shown to markedly increase baseline forearm blood flow in both healthy adults (Hoffman et al. 1999) and adolescents with type 1 DM (Dye et al. 2012). Further study is needed to determine specific mechanisms by which AH induces different vascular responses by glucose delivery method and/or meal composition.
There are both strengths and limitations of this study that should be noted. Strengths include: (1) the use of OCT to block endogenous insulin (D’Alessio et al. 1989) and incretin(s) (Plockinger et al. 1999) secretion during AH, which is important given that these hormones have vasodilatory effects (Steinberg et al. 1994; Beckman et al. 2002; Ban et al. 2008; Wang et al. 2020); (2) induction of hyperglycaemia by i.v. glucose, which avoided the sympathetic haemodynamic changes that accompany oral glucose or meal ingestion (Young & Landsberg, 1977; Welle et al. 1981); (3) a study design that allowed us to characterize the microvascular effects of OCT and AH in cardiac and skeletal muscle, which had heretofore been unreported; and (4) enhancement of scientific rigor by blinding study personnel to subject and protocol during analysis of key vascular parameters.
There are several limitations to our study that also warrant consideration. By design, all study participants were healthy and lean with intact vascular function. Those who are older and/or less healthy might respond differently, and we suggest that this be a focus of future investigation. Second, it is possible that the use of OCT has in some unknown manner skewed the vascular response to AH. We recognize that this possibility should not be discounted, though we do note that previous studies using a similar dose of OCT reported no changes in endothelium-dependent vasodilatation (Beckman et al. 2001, 2002; Joy et al. 2016) or forearm perfusion (Moller et al. 1995). Prior work has also reported that OCT infusion does not alter the haemodynamic effects of AH (Marfella et al. 2000). Third, we chose to study the vascular effects of AH over 4 h instead of shorter time periods. Two key considerations led to using a 4-h duration for glucose infusion: (1) increasing plasma free fatty acid concentrations for 4 h impairs vascular function (Steinberg et al. 2000) and vascular insulin sensitivity (Liu et al. 2011), yet we recently observed that 4 h of AH did not inhibit insulin’s microvascular vasodilatory action, suggesting that the acute vascular responses to glucose versus fat nutrient excess diverge (Horton et al. 2020); and (2) the United States Food and Drug Administration limits the allowable daily dose of Definity® microbubble infusion to one vial per day (allowing collection of only a before-and-after treatment image set), and a 4-h duration ensured adequate time for any AH effect(s) to develop. We cannot exclude that a shorter AH duration may yield different results.
Conclusions
We provide the first evidence that AH per se enhanced skeletal muscle microvascular perfusion, cardiac muscle MBV and brachial artery vascular function in the same subjects. We also found that OCT altered neither micro- nor macrovascular function during euglycaemia. Compared to other published findings, our results suggest that vascular responses to AH differ based on the study population (i.e. normal weight vs. overweight/obese) and/or glucose delivery method (i.e. i.v. vs. oral glucose).
Supplementary Material
Key points.
Multiple clinical studies report that acute hyperglycaemia (induced by mixed meal or oral glucose) decreases arterial vascular function in healthy humans. Feeding, however, impacts autonomic output, blood pressure, and insulin and incretin secretion, which may themselves alter vascular function.
No prior studies have examined the effect of acute hyperglycaemia on both macro- and microvascular function while controlling plasma insulin concentrations.
Macrovascular and microvascular functional responses to euglycaemia and hyperglycaemia were compared. Octreotide was infused throughout both protocols to prevent endogenous insulin release.
Acute hyperglycaemia (induced by intravenous glucose) enhanced brachial artery flow-mediated dilatation, increased skeletal muscle microvascular blood volume and flow, and expanded cardiac muscle microvascular blood volume.
Compared to other published findings, the results suggest that vascular responses to acute hyperglycaemia differ based on the study population (i.e. normal weight vs. overweight/obese) and/or glucose delivery method (i.e. intravenous vs. oral glucose).
Funding
This work was supported in part by NIH research grants F32HL14230401, KL2TR003016, and UL1TR003015 (to W.B.H.) and RO1DK073059 and RO1HL142250 (to E.J.B.).
Biography

William Horton is an Assistant Professor of Medicine in the University of Virginia Division of Endocrinology and Metabolism. He completed postdoctoral fellowship training under Eugene Barrett, MD, PhD, where he studied the acute effects of insulin and glucose on micro- and macrovascular function. He lives with type 1 diabetes and seeks to extend this training into new areas of investigation, including the effects of glycaemic variability on cardiovascular disease in persons with type 1 diabetes.
Footnotes
Additional information
Competing interests
All authors have no competing interests to declare or disclose.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW & Veves A (1998). Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J Vasc Surg 28, 687–694. [DOI] [PubMed] [Google Scholar]
- Alsalim W, Omar B, Pacini G, Bizzotto R, Mari A & Ahren B (2015). Incretin and islet hormone responses to meals of increasing size in healthy subjects. J Clin Endocrinol Metab 100, 561–568. [DOI] [PubMed] [Google Scholar]
- Alvares TS (2020). High-glucose mixed meals impair microvascular function: the attenuating effect of exercise. J Physiol 599, 11–12. [DOI] [PubMed] [Google Scholar]
- Bagg W, Whalley GA, Sathu A, Gamble G, Sharpe N & Braatvedt GD (2000). The effect of acute hyperglycaemia on brachial artery flow mediated dilatation in normal volunteers. Aust N Z J Med 30, 344–350. [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ & Husain M (2008). Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117, 2340–2350. [DOI] [PubMed] [Google Scholar]
- Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A & Brechtel G (1995). Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW, Vinik AI & Casellini CM (2017). Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab 102, 4343–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EJ, Wang H, Upchurch CT & Liu Z (2011). Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301, E252–E263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RH, Scholtholt J, Scholkens BA, Jung W & Speth O (1982). A microsphere study on the effects of somatostatin and secretin on regional blood flow in anesthetized dogs. Regul Pept 4, 341–351. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB & Creager MA (2001). Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation 103, 1618–1623. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Garrett LA & Creager MA (2002). Inhibition of protein kinase Cβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res 90, 107–111. [DOI] [PubMed] [Google Scholar]
- Brownlee M (2001). Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820. [DOI] [PubMed] [Google Scholar]
- Burgansky-Eliash Z, Barak A, Barash H, Nelson DA, Pupko O, Lowenstein A, Grinvald A & Rubinstein A (2012). Increased retinal blood flow velocity in patients with early diabetes mellitus. Retina 32, 112–119. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J & Deanfield JE (1994). Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24, 471–476. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Esposito K, Piconi L, Ihnat M, Thorpe J, Testa R, Bonfigli AR & Giugliano D (2008a). Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res Clin Pract 82, 262–267. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M & Giugliano D (2008b). Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57, 1349–1354. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Vella A, Reed AS, Minson CT, Shah P, Rizza RA & Joyner MJ (2002). Cutaneous vascular function during acute hyperglycemia in healthy young adults. J Appl Physiol 93, 1243–1250. [DOI] [PubMed] [Google Scholar]
- D’Alessio DA, Sieber C, Beglinger C & Ensinck JW (1989). A physiologic role for somatostatin 28 as a regulator of insulin secretion. J Clin Invest 84, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECODE study group; European Diabetes Epidemiology Group (1999). Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 354, 617–621. [PubMed] [Google Scholar]
- Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T & Raskin P (2008). Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 117, 1610–1619. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD & Andres R (1979). Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 237, E214–E223. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE & Seals DR (2007). Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res 100, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Dye AS, Huang H, Bauer JA & Hoffman RP (2012). Hyperglycemia increases muscle blood flow and alters endothelial function in adolescents with type 1 diabetes. Exp Diabetes Res 2012, 170380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Sandbaek A, Holst JJ & Jorgensen ME (2015). GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO study. Diabetes 64, 2513–2525. [DOI] [PubMed] [Google Scholar]
- Gates PE, Boucher ML, Silver AE, Monahan KD & Seals DR (2007). Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102, 63–71. [DOI] [PubMed] [Google Scholar]
- Grasser EK, Yepuri G, Dulloo AG & Montani JP (2014). Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults: A randomized cross-over study. Eur J Nutr 53, 1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, Harrington M, Hainer J, Rimoldi O, Dorbala S, Bhatt DL, Blankstein R, Camici PG & Di Carli MF (2017). Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 136, 2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AV, Rosner BA, Kwong RY, Rao AD, Garg R, Di Carli MF & Adler GK (2019). Sex differences in coronary microvascular function in individuals with type 2 diabetes. Diabetes 68, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN & Consortium RE (2019). The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95, 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RP (2015). Hyperglycemic endothelial dysfunction: does it happen and does it matter? J Thorac Dis 7, 1693–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RP, Hausberg M, Sinkey CA & Anderson EA (1999). Hyperglycemia without hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Diabetes Complications 13, 17–22. [DOI] [PubMed] [Google Scholar]
- Holst JJ (2007). The physiology of glucagon-like peptide 1. Physiol Rev 87, 1409–1439. [DOI] [PubMed] [Google Scholar]
- Holst JJ (2019). The incretin system in healthy humans: the role of GIP and GLP-1. Metabolism 96, 46–55. [DOI] [PubMed] [Google Scholar]
- Horton WB, Jahn LA, Hartline LM, Aylor KW, Patrie JT & Barrett EJ (2020). Hyperglycemia does not inhibit insulin’s effects on microvascular perfusion in healthy humans: A randomized crossover study. Am J Physiol Endocrinol Metab 319, E753–E762, [DOI] [PubMed] [Google Scholar]
- Houben AJ, Schaper NC, de Haan CH, Huvers FC, Slaaf DW, de Leeuw PW & Nieuwenhuijzen Kruseman C (1996). Local 24-h hyperglycemia does not affect endothelium-dependent or -independent vasoreactivity in humans. Am J Physiol 270, H2014–H2020. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Umemura T, Nakamura S & Yoshida M (2003). Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am Heart J 146, 674–678. [DOI] [PubMed] [Google Scholar]
- Jahn LA, Hartline L, Rao N, Logan B, Kim JJ, Aylor K, Gan LM, Westergren HU & Barrett EJ (2016). Insulin enhances endothelial function throughout the arterial tree in healthy but not metabolic syndrome subjects. J Clin Endocrinol Metab 101, 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PJ, Zeegers MP & Mensink RP (2015). Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis 239, 21–30. [DOI] [PubMed] [Google Scholar]
- Joy NG, Perkins JM, Mikeladze M, Younk L, Tate DB & Davis SN (2016). Comparative effects of acute hypoglycemia and hyperglycemia on pro-atherothrombotic biomarkers and endothelial function in non-diabetic humans. J Diabetes Complications 30, 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klefter ON, Vilsboll T, Knop FK & Larsen M (2015). Retinal vascular and structural dynamics during acute hyperglycaemia. Acta Ophthalmol 93, 697–705. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Yoshida S & Okamoto T (2015). Arterial stiffness after glucose ingestion in exercise-trained versus untrained men. Appl Physiol Nutr Metab 40, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Ryan SM, Parker JA, Freeman R, Wei JY & Goldberger AL (1993). Hemodynamic and autonomic nervous system responses to mixed meal ingestion in healthy young and old subjects and dysautonomic patients with postprandial hypotension. Circulation 87, 391–400. [DOI] [PubMed] [Google Scholar]
- Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W & Liu Z (2011). Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 96, 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loader J, Montero D, Lorenzen C, Watts R, Meziat C, Reboul C, Stewart S & Walther G (2015). Acute hyperglycemia impairs vascular function in healthy and cardiometabolic diseased subjects: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 35, 2060–2072. [DOI] [PubMed] [Google Scholar]
- Marfella R, Nappo F, De Angelis L, Paolisso G, Tagliamonte MR & Giugliano D (2000). Hemodynamic effects of acute hyperglycemia in type 2 diabetic patients. Diabetes Care 23, 658–663. [DOI] [PubMed] [Google Scholar]
- Marre M, Bouhanick B, Berrut G, Gallois Y, Le Jeune JJ, Chatellier G, Menard J & Alhenc-Gelas F (1999). Renal changes on hyperglycemia and angiotensin-converting enzyme in type 1 diabetes. Hypertension 33, 775–780. [DOI] [PubMed] [Google Scholar]
- Meyer C & Schwaiger M (1997). Myocardial blood flow and glucose metabolism in diabetes mellitus. Am J Cardiol 80, 94A–101A. [DOI] [PubMed] [Google Scholar]
- Moller N, Bagger JP, Schmitz O, Jorgensen JO, Ovesen P, Moller J, Alberti KG & Orskov H (1995). Somatostatin enhances insulin-stimulated glucose uptake in the perfused human forearm. J Clin Endocrinol Metab 80, 1789–1793. [DOI] [PubMed] [Google Scholar]
- Ne JYA, Cai TY, Celermajer DS, Caterson ID, Gill T, Lee CMY & Skilton MR (2017). Obesity, arterial function and arterial structure – a systematic review and meta-analysis. Obes Sci Pract 3, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Morrison DJ, Betik AC, Roberts-Thomson K, Kaur G, Wadley GD, Shaw CS & Keske MA (2020). High-glucose mixed nutrient meal ingestion impairs skeletal muscle microvascular blood flow in healthy young males. Am J Physiol Endocrinol Metab 318, E1014–E1021, [DOI] [PubMed] [Google Scholar]
- Perkins JM, Joy NG, Tate DB & Davis SN (2015). Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab 309, E168–E176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchi A, Limbruno U, Focardi M, Cortese B, Micheli A, Boschi L, Severi S & De Caterina R (2011). Increased basal coronary blood flow as a cause of reduced coronary flow reserve in diabetic patients. Am J Physiol Heart Circ Physiol 301, H2279–H2284. [DOI] [PubMed] [Google Scholar]
- Plockinger U, Holst JJ, Messerschmidt D, Hopfenmuller W & Quabbe HJ (1999). Octreotide suppresses the incretin glucagon-like peptide (7–36) amide in patients with acromegaly or clinically nonfunctioning pituitary tumors and in healthy subjects. Eur J Endocrinol 140, 538–544. [DOI] [PubMed] [Google Scholar]
- Roberts-Thomson KM, Betik AC, Premilovac D, Rattigan S, Richards SM, Ross RM, Russell RD, Kaur G, Parker L & Keske MA (2020). Postprandial microvascular blood flow in skeletal muscle: similarities and disparities to the hyperinsulinaemic euglycaemic clamp. Clin Exp Pharmacol Physiol 47, 725–737. [DOI] [PubMed] [Google Scholar]
- Russell RD, Hu D, Greenaway T, Sharman JE, Rattigan S, Richards SM & Keske MA (2018). Oral glucose challenge impairs skeletal muscle microvascular blood flow in healthy people. Am J Physiol Endocrinol Metab 315, E307–E315. [DOI] [PubMed] [Google Scholar]
- Sauder KA, Johnston ER, Skulas-Ray AC, Campbell TS & West SG (2012). Effect of meal content on heart rate variability and cardiovascular reactivity to mental stress. Psychophysiology 49, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MS & Brownlee M (2016). Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 118, 1808–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechtman O (2013). The coefficient of variation as an index of measurement reliability. In Methods of Clinical Epidemiology, ed. Doi SAR & Williams GM, pp. 39–49.Springer, Berlin, Heidelberg. [Google Scholar]
- Skaug EA, Aspenes ST, Oldervoll L, Morkedal B, Vatten L, Wisloff U & Ellingsen O (2013). Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prev Cardiol 20, 531–540. [DOI] [PubMed] [Google Scholar]
- Sorensen MH, Bojer AS, Pontoppidan JRN, Broadbent DA, Plein S, Madsen PL & Gaede P (2020). Reduced myocardial perfusion reserve in type 2 diabetes is caused by increased perfusion at rest and decreased maximal perfusion during stress. Diabetes Care 43, 1285–1292. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N & Baron AD (1994). Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J & Baron AD (2000). Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49, 1231–1238. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ & Ghiadoni L (2019). Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40, 2534–2547. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM & Weber T (2015). Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension, 66, 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak GC & Plous S (2013). Research Randomizer (Version 4.0) [Computer software]. Retrieved on August 22, 2020, from http://www.randomizer.org/.
- Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R & Mesotten D (2009). Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab 94, 3163–3170. [DOI] [PubMed] [Google Scholar]
- van Gurp PJ, Rongen GA, Lenders JW, Al Nabawy AK, Timmers HJ & Tack CJ (2005). Sustained hyperglycaemia increases muscle blood flow but does not affect sympathetic activity in resting humans. Eur J Appl Physiol 93, 648–654. [DOI] [PubMed] [Google Scholar]
- Wang N, Tan AWK, Jahn LA, Hartline L, Patrie JT, Lin S, Barrett EJ, Aylor KW & Liu Z (2020). Vasodilatory actions of glucagon-like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care 43, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Lilavivat U & Campbell RG (1981). Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism 30, 953–958. [DOI] [PubMed] [Google Scholar]
- Williams JS, Stimpson TV, Tremblay JC, Fenuta AM & Pyke KE (2020). No impact of acute hyperglycaemia on arterial stiffness in the early and late follicular phases of the menstrual cycle in young females. Exp Physiol 105, 174–183. [DOI] [PubMed] [Google Scholar]
- Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC & Creager MA (1998). Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 97, 1695–1701. [DOI] [PubMed] [Google Scholar]
- Woods LL, Mizelle HL & Hall JE (1987). Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am J Physiol 252, F65–F73. [DOI] [PubMed] [Google Scholar]
- Young JB & Landsberg L (1977). Stimulation of the sympathetic nervous system during sucrose feeding. Nature 269, 615–617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.