Abstract
Oligomannose glycans are of interest as HIV vaccine components but are subject to mannosidase degradation in vivo. Herein, we report the synthesis of oligosaccharides containing a thio linkage at the non-reducing end. A thio-linked dimannose donor participates in highly stereoselective glycosylations to afford tri- and tetramannose fragments. STD NMR studies show that these glycans are recognized by HIV antibody 2G12, and we confirm that the reducing terminal S-linkage confers complete stability against x. manihotis mannosidase.
Carbohydrate or glycoconjugate vaccines1 are in use or development for prevention of bacterial infections,2, 3 cancer4 and HIV.5–7 In HIV vaccine development, there is significant interest in elicitation of antibodies that can bind to the Manα1→2Man moieties of high mannose (Man9GlcNAc2) glycans;8–11 however, we and others have shown that, for glycoconjugate vaccines, mannosidase trimming degrades this motif so that the antibody response is directed against the glycan core or other structures in the glycoconjugate.12, 13 A possible solution to this problem is chemical stabilization of the Manα1→2Man linkage against enzymatic hydrolysis, in particular using sulfur14–20 in the glycosidic linkage. Indeed, antibodies raised against some S-linked glycan analogs exhibit cross-reactivity with the natural oxygen-linked sugars21–25 but such analogs have not been tested in the case of oligomannose vaccines.
Inspired by a report of anomeric alkylation to produce sulfur-linked Manα1→2Man disaccharide,19 we wondered whether a disaccharide donor containing this S linkage (see 1, Scheme 1) would participate in stereospecific glycosylation with anchimeric assistance from the thioether linkage. Glycosyl donors containing simple 2-thio substituents are known,26–33 but only one thio-linked disaccharide donor has been reported, with a gluco- configuration.34 Glycosylation with dimannose derivative 1 would offer an efficient route to serum-stabilized fragments of Man9GlcNAc2, or potentially the whole oligosaccharide.
Scheme 1.
Thioether-linkage-assisted stereospecific glycosylation
To prepare the requisite thio-disaccharide donor, we began from known trityl thioglycoside 4a (Scheme 2).35,18 Exchange to benzyl protecting groups proceeded in 68 % overall yield to afford building block 4b. We wondered whether 5-derived thiolate could displace a 2-triflate derivative with a relatively inert leaving group such as a fluoride already present at C1.
Scheme 2.
Synthesis of S-linked disaccharide donor
Thus, we prepared 1-fluoro glucose derivative 6 by a known protocol including epoxidation of tribenzyl glucal,36 followed by TBAF treatment.37 Following triflation of 6 and triethylsilane/trifluoroacetic acid deprotection of 4b, 5 and 7 were combined and allowed to react in the presence of sodium tert-butoxide to afford the desired disaccharide 8 in 63 % yield.
With dimannose donor 8 in hand, we prepared a suitable monomannose acceptor to produce Man3. Starting from mannose building block 9,19 installation of an azidoethyl linker and deprotection at the 2-position efficiently afforded acceptor 12. Glycosylation of 12 with 1.5 equivalents of 8, in the presence of hafnium trifluoromethanesulfonate38 afforded the desired trisaccharide 13 in 64 % yield as a single stereoisomer. After global deprotection with sodium in liquid ammonia the desired S-Man3 14 was isolated in 62 % yield (Scheme 3). The stability of the thio linkage under dissolving metal conditions has been observed previously,19, 20 but is nevertheless noteworthy. The α configuration of all mannose units was confirmed by carbon-coupled HSQC, which showed all 1JCH to be in the range of 171–178 Hz (see Supporting Information).39, 40
Scheme 3.
Synthesis of S-linked Man3
Similarly, we set about preparation of an S-Man4 containing a reducing-terminal β-mannose analogous to the core mannose in the natural Man9GlcNAc2. We prepared dimannose acceptor 19 by coupling our previously-described β-mannose core 1741 to known building block 16,42 followed by Lev deprotection. 19 coupled smoothly to Man2 fluoride donor 8 (see Scheme 4) in 77 % yield, again as single stereoisomer. This tetrasaccharide was globally deprotected and converted to azide 21 in three steps with an overall yield of 40 %. 21 exhibited three anomeric 1JCH values from 169–174 Hertz for the α linkages, and, as expected, a value of 158 Hz for β linkage (see Supporting Information).
Scheme 4.
Synthesis of S-linked Man4
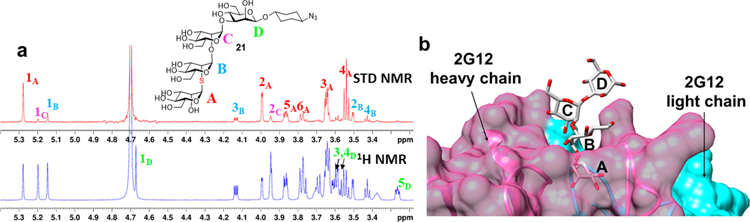
With these S-Man3 and S-Man4 derivatives in hand, we proceeded to study their recognition by HIV broadly neutralizing antibody 2G12, which binds primarily to the linear trimannose (D1) arm of Man9GlcNAc2. STD-NMR (Saturation Transfer Difference NMR) spectroscopy with 25 μM 2G12 IgG and a 200:1 ratio of sugar:antibody showed that, as expected, the greatest saturation transfer is seen for the non-reducing mannose unit in either Man3 or Man4 (SI Figure S1 and Figure 1a). In the case of the Man4 derivative, negligible STD is observed for the reducing-terminal mannose unit. These data are closely analogous to STD NMR data previously acquired for oxygen-linked oligomannose fragments,43, 44 and are consistent with crystal structure data for Man4 bound to 2G12, in which little if any interaction is evident between the antibody and residue D (Figure 1b).
Figure 1.
Binding analysis of S-Man4 to HIV broadly neutralizing antibody 2G12. a) STD-NMR spectrum of S-Man4 (21) with 2G12 IgG. Bottom spectrum (blue) shows the reference 800 MHz 1H NMR whereas the top (red) shows corresponding STD spectrum. See supporting information for details. Numbers indicate selected assignments by carbon number and ring letter. b) Crystal structure for all O-linked Man4 (22) bound to 2G12 (PDB ID 6MSY).
Lastly, we tested natural and sulfur-substituted Man4 derivatives against the action of xanthomonas manihotis mannosidase, which cleaves oligomannose Manα1→2Man and Manα1→3Man linkages. S-Man4 derivative 21 and its oxygen analog 22 were labeled by strain-promoted azide/alkyne cycloaddition with DBCO amine linker 23, in order to facilitate separation and detection of degradation products by LC/MS. After incubation with mannosidase, LC/MS analysis showed no degradation of sulfur-substituted derivative 24 after 48 hours, but nearly complete digestion of natural Man4 derivative 25 to Man1 27 (Scheme 5).
Scheme 5.
Stability of S-Linked Man4 Against Mannosidase Cleavage.
In conclusion, we have demonstrated a facile synthetic route to Man3 and Man4 derivatives with a non-reducing-terminal sulfur linkage that is highly resistant to enzymatic degradation. These derivatives are recognized by an HIV antibody, 2G12, through contacts that are similar to those it makes with the natural oligomannose structure. This synthetic strategy should be readily amenable to preparation of higher branched stabilized oligomannose analogs, suitable for immunogenicity studies in the near future.
Supplementary Material
ACKNOWLEDGMENT
This project was supported by NIH awards AI090475 and AI113737 and Brandeis’ SPROUT program. The 800 MHz NMR in the Landsman Research Facility was purchased with a grant from the NCRR High-End Instrumentation program (S10RR017269) and updated with funds from HHMI and Brandeis University. Dr. Susan Pochapsky of the Brandeis NMR Facility is gratefully acknowledged for assistance with STD NMR.
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c00726.
Procedures, spectra for compounds 4b, 5–8, 10–21, and additional experiments.
Contributor Information
Mahesh Neralkar, Department of Chemistry, Brandeis University, Waltham, Massachusetts, 02454, United States.
Leiming Tian, Department of Chemistry, Brandeis University, Waltham, Massachusetts, 02454, United States.
Richard L. Redman, Department of Chemistry, Brandeis University, Waltham, Massachusetts, 02454, United States
Isaac J. Krauss, Department of Chemistry, Brandeis University, Waltham, Massachusetts, 02454, United States
REFERENCES
- 1.Lang S; Huang X, Carbohydrate Conjugates in Vaccine Developments. Front. Chem 2020, 8, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn A, Bacterial polysaccharide-protein conjugate vaccines. Br. Med. Bull 2004, 70, 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Rappuoli R, Glycoconjugate vaccines: Principles and mechanisms. Sci. Transl. Med 2018, 10. [DOI] [PubMed] [Google Scholar]
- 4.Heimburg-Molinaro J; Lum M; Vijay G; Jain M; Almogren A; Rittenhouse-Olson K, Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horiya S; MacPherson IS; Krauss IJ, Recent strategies targeting HIV glycans in vaccine design. Nat. Chem. Biol 2014, 10, 990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CC; Zheng XJ; Ye XS, Broadly Neutralizing Antibody-Guided Carbohydrate-Based HIV Vaccine Design: Challenges and Opportunities. ChemMedChem 2016, 11, 357–62. [DOI] [PubMed] [Google Scholar]
- 7.Bastida I; Fernández-Tejada A, Synthetic carbohydrate-based HIV-1 vaccines. Drug Discov Today Technol 2020, 35–36, 45–56. [DOI] [PubMed] [Google Scholar]
- 8.Seabright GE; Doores KJ; Burton DR; Crispin M, Protein and Glycan Mimicry in HIV Vaccine Design. J. Mol. Biol 2019, 431, 2223–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calarese DA; Scanlan CN; Zwick MB; Deechongkit S; Mimura Y; Kunert R; Zhu P; Wormald MR; Stanfield RL; Roux KH; Kelly JW; Rudd PM; Dwek RA; Katinger H; Burton DR; Wilson IA, Antibody Domain Exchange Is an Immunological Solution to Carbohydrate Cluster Recognition. Science 2003, 300, 2065. [DOI] [PubMed] [Google Scholar]
- 10.Pejchal R; Doores KJ; Walker LM; Khayat R; Huang P-S; Wang S-K; Stanfield RL; Julien J-P; Ramos A; Crispin M; Depetris R; Katpally U; Marozsan A; Cupo A; Maloveste S; Liu Y; McBride R; Ito Y; Sanders RW; Ogohara C; Paulson JC; Feizi T; Scanlan CN; Wong C-H; Moore JP; Olson WC; Ward AB; Poignard P; Schief WR; Burton DR; Wilson IA, A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 2011, 334, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong L; Lee JH; Doores KJ; Murin CD; Julien JP; McBride R; Liu Y; Marozsan A; Cupo A; Klasse PJ; Hoffenberg S; Caulfield M; King CR; Hua Y; Le KM; Khayat R; Deller MC; Clayton T; Tien H; Feizi T; Sanders RW; Paulson JC; Moore JP; Stanfield RL; Burton DR; Ward AB; Wilson IA, Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol 2013, 20, 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen DN; Xu B; Stanfield RL; Bailey JK; Horiya S; Temme JS; Leon DR; LaBranche CC; Montefiori DC; Costello CE; Wilson IA; Krauss IJ, Oligomannose Glycopeptide Conjugates Elicit Antibodies Targeting the Glycan Core Rather than Its Extremities. ACS Cent Sci 2019, 5, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruxelle JF; Kirilenko T; Qureshi Q; Lu N; Trattnig N; Kosma P; Pantophlet R, Serum alpha-mannosidase as an additional barrier to eliciting oligomannose-specific HIV-1-neutralizing antibodies. Sci. Rep 2020, 10, 7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driguez H, Thiooligosaccharides as tools for structural biology. ChemBioChem 2001, 2, 311–8. [DOI] [PubMed] [Google Scholar]
- 15.Bi J; Zhao C; Cui W; Zhang C; Shan Q; Du Y, Synthesis and affinities of C3-symmetric thioglycoside-containing trimannosides. Carbohydr. Res 2015, 412, 56–65. [DOI] [PubMed] [Google Scholar]
- 16.Kern MK; Pohl NLB, Automated Solution-Phase Synthesis of S-Glycosides for the Production of Oligomannopyranoside Derivatives. Org. Lett 2020, 22, 4156–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belz T; Jin Y; Coines J; Rovira C; Davies GJ; Williams SJ, An atypical interaction explains the high-affinity of a non-hydrolyzable S-linked 1,6-α-mannanase inhibitor. Chem. Commun 2017, 53, 9238–9241. [DOI] [PubMed] [Google Scholar]
- 18.Belz T; Williams SJ, A building block approach to the synthesis of a family of S-linked alpha-1,6-oligomannosides. Carbohydr. Res 2016, 429, 38–47. [DOI] [PubMed] [Google Scholar]
- 19.Norberg O; Wu B; Thota N; Ge JT; Fauquet G; Saur AK; Aastrup T; Dong H; Yan M; Ramstrom O, Synthesis and binding affinity analysis of alpha1–2- and alpha1–6-O/S-linked dimannosides for the elucidation of sulfur in glycosidic bonds using quartz crystal microbalance sensors. Carbohydr. Res 2017, 452, 35–42. [DOI] [PubMed] [Google Scholar]
- 20.Zhong W; Kuntz DA; Ember B; Singh H; Moremen KW; Rose DR; Boons GJ, Probing the substrate specificity of Golgi alpha-mannosidase II by use of synthetic oligosaccharides and a catalytic nucleophile mutant. J. Am. Chem. Soc 2008, 130, 8975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bundle DR; Rich JR; Jacques S; Yu HN; Nitz M; Ling CC, Thiooligosaccharide conjugate vaccines evoke antibodies specific for native antigens. Angew Chem Int Ed Engl 2005, 44, 7725–9. [DOI] [PubMed] [Google Scholar]
- 22.Rich JR; Bundle DR, S-linked ganglioside analogues for use in conjugate vaccines. Org. Lett 2004, 6, 897–900. [DOI] [PubMed] [Google Scholar]
- 23.Wu X; Lipinski T; Paszkiewicz E; Bundle DR, Synthesis and immunochemical characterization of S-linked glycoconjugate vaccines against Candida albicans. Chem. Eur. J 2008, 14, 6474–82. [DOI] [PubMed] [Google Scholar]
- 24.Huo CX; Zheng XJ; Xiao A; Liu CC; Sun S; Lv Z; Ye XS, Synthetic and immunological studies of N-acyl modified S-linked STn derivatives as anticancer vaccine candidates. Org. Biomol. Chem 2015, 13, 3677–90. [DOI] [PubMed] [Google Scholar]
- 25.Kuan TC; Wu HR; Adak AK; Li BY; Liang CF; Hung JT; Chiou SP; Yu AL; Hwu JR; Lin CC, Synthesis of an S-Linked alpha(2-->8) GD3 Antigen and Evaluation of the Immunogenicity of Its Glycoconjugate. Chem. Eur. J 2017, 23, 6876–6887. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto S; Yanagiya Y; Honda T; Ikegami S, A stereocontrolled construction of 2-deoxy-β-glycosidic linkages via 1,2-trans-β-glycosidation of 2-deoxy-2-[(p-methoxyphenyl)thio]glycopyranosyl N,N,N’,N’-tetramethylphosphoroamidates. Chem. Lett 1992, 1511–14. [Google Scholar]
- 27.Toshima K; Nozaki Y; Mukaiyama S; Tatsuta K, Highly β-stereoselective glycosylation by use of 1-O-acetyl-2,6-anhydro-2-thio glycosyl donor for synthesis of 2,6-dideoxy-β-glycosides. Tetrahedron Lett. 1992, 33, 1491–4. [Google Scholar]
- 28.Toshima K; Mukaiyama S; Nozaki Y; Inokuchi H; Nakata M; Tatsuta K, Novel Glycosidation Method Using 2,6-Anhydro-2-thio Sugars for Stereocontrolled Synthesis of 2,6-Dideoxy-α- and -β-glycosides. J. Am. Chem. Soc 1994, 116, 9042–51. [Google Scholar]
- 29.Toshima K; Nozaki Y; Mukaiyama S; Tamai T; Nakata M; Tatsuta K; Kinoshita M, Application of Highly Stereocontrolled Glycosidations Employing 2,6-Anhydro-2-thio Sugars to the Syntheses of Erythromycin A and Olivomycin A Trisaccharide. J. Am. Chem. Soc 1995, 117, 3717–27. [Google Scholar]
- 30.Roush WR; Sebesta DP; James RA, Stereoselective preparation of 2-deoxy-β-glycosides from glycal precursors. 2. Stereochemistry of glycosidation reactions of 2-thiophenyl- and 2-selenophenyl-α-D-glucopyranosyl donors. Tetrahedron 1997, 53, 8837–8852. [Google Scholar]
- 31.Castro-Palomino JC; Simon B; Speer O; Leist M; Schmidt RR, Synthesis of ganglioside GD3 and its comparison with bovine GD3 with regard to oligodendrocyte apoptosis mitochondrial damage. Chem. - Eur. J 2001, 7, 2178–2184. [DOI] [PubMed] [Google Scholar]
- 32.Knapp S; Kirk BA, Glycosylation with 2’-thio-S-acetyl participation. Tetrahedron Lett. 2003, 44, 7601–7605. [Google Scholar]
- 33.Shirahata T; Matsuo J-I; Teruya S; Hirata N; Kurimoto T; Akimoto N; Sunazuka T; Kaji E; Omura S, Improved catalytic and stereoselective glycosylation with glycosyl N-trichloroacetylcarbamate: Application to various 1-hydroxy sugars. Carbohydr. Res 2010, 345, 740–749. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto H; Shimada K; Horito S, Synthesis of α-L-fucopyranosyl disaccharides with thioglycosidic linkages and characterization of α-L-fucosidases from bovine kidney and epididymis by their inhibitory activities. Tetrahedron: Asymmetry 1994, 5, 2351–66. [Google Scholar]
- 35.Matta KL; Girotra RN; Barlow JJ, Synthesis of p-nitrobenzyl and p-nitrophenyl 1-thioglycopyranosides. Carbohydr. Res 1975, 43, 101–9. [DOI] [PubMed] [Google Scholar]
- 36.Cheshev P; Marra A; Dondoni A, Direct epoxidation of D-glucal and D-galactal derivatives with in situ generated DMDO. Carbohydr. Res 2006, 341, 2714–6. [DOI] [PubMed] [Google Scholar]
- 37.Gordon DM; Danishefsky SJ, Displacement reactions of a 1,2-anhydro-alpha-D-hexopyranose: installation of useful functionality at the anomeric carbon. Carbohydr. Res 1990, 206, 361–6. [DOI] [PubMed] [Google Scholar]
- 38.Manabe S; Ito Y, Hafnium(IV) Tetratriflate as a Glycosyl Fluoride Activation Reagent. J. Org. Chem 2013, 78, 4568–4572. [DOI] [PubMed] [Google Scholar]
- 39.Crich D; Li H, Direct Stereoselective Synthesis of β-Thiomannosides. J. Org. Chem 2000, 65, 801–805. [DOI] [PubMed] [Google Scholar]
- 40.Bock K; Pedersen C, A study of 13CH coupling constants in hexopyranoses. J. Chem. Soc,, Perkin Trans 2 1974, 293–297. [Google Scholar]
- 41.MacPherson IS; Temme JS; Habeshian S; Felczak K; Pankiewicz K; Hedstrom L; Krauss IJ, Multivalent Glycocluster Design through Directed Evolution. Angew. Chem. Int. Ed 2011, 50, 11238–11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chayajarus K; Chambers DJ; Chughtai MJ; Fairbanks AJ, Stereospecific synthesis of 1,2-cis glycosides by vinyl-mediated IAD. Org. Lett 2004, 6, 3797–800. [DOI] [PubMed] [Google Scholar]
- 43.Enriquez-Navas PM; Chiodo F; Marradi M; Angulo J; Penades S, STD NMR study of the interactions between antibody 2G12 and synthetic oligomannosides that mimic selected branches of gp120 glycans. ChemBioChem 2012, 13, 1357–65. [DOI] [PubMed] [Google Scholar]
- 44.Enriquez-Navas PM; Marradi M; Padro D; Angulo J; Penades S, A solution NMR study of the interactions of oligomannosides and the anti-HIV-1 2G12 antibody reveals distinct binding modes for branched ligands. Chem. Eur. J 2011, 17, 1547–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.