Abstract
Background
Childhood attention deficit-hyperactivity disorder (ADHD) is common in psychotic disorders. However, prevalence estimates vary widely and the impact of ADHD on the severity of psychotic symptoms and associated features is unclear. We used the Philadelphia Neurodevelopmental Cohort (PNC; n= 9498 youth age 8–21), which includes a comprehensive structured interview of clinical symptoms and the Penn Computerized Neurocognitive Battery (CNB), to clarify the prevalence of ADHD in psychosis spectrum (PS) youth and determine if comorbid ADHD is associated with severity of psychotic symptoms and cognitive impairment.
Methods
Prevalence of ADHD among PS youth was established by comparing PS youth to all other youth in the PNC cohort. Cognition was compared between four groups: typically developing (TD), ADHD, PS without ADHD (PS−ADHD), and PS with ADHD (PS+ADHD). To evaluate the impact of ADHD on psychosis symptomatology, severity of positive and negative psychotic symptoms was compared between PS−ADHD and PS+ADHD groups.
Results
ADHD was more prevalent in PS youth compared to non-PS youth (45% vs. 20%). Cognition was significantly impaired in PS youth compared to TD youth, but the presence of ADHD in PS youth was not associated with greater cognitive impairment. Co-morbid ADHD was, however, associated with more severe psychosis symptoms in PS youth.
Conclusion
ADHD is more common among PS youth compared to youth without PS symptoms and is associated with more severe psychotic symptoms, but not severity of cognitive impairment. The association between ADHD and psychotic disorders may be mediated by psychosis symptoms in youth and may manifest a more stable cognitive impairment.
Keywords: Psychosis, psychosis spectrum, cognition, ADHD
1. Introduction
Psychotic disorders are characterized by positive symptoms, such as hallucinations and delusions, and negative symptoms, including amotivation and blunted affect, which for many affected individuals leads to lifelong disability (Andreasen et al., 1995; Bowie et al., 2006). Psychotic symptoms typically emerge during late adolescence and early adulthood and are often accompanied by cognitive impairment, which further contributes to poor functional outcomes (Sheffield et al., 2014; Wingo et al., 2009). In many individuals, frank psychosis is preceded by subtle abnormalities in behavior and cognition during childhood implicating atypical neurodevelopment in the etiology of psychotic disorders.
Neurodevelopmental hypotheses of psychosis are further supported by evidence that childhood cognitive disorders, particularly attention-deficit/hyperactivity disorder (ADHD), are more common in psychotic disorders than in the general population (Nourredine et al., 2021). For example, a longitudinal birth cohort study of 1037 individuals found that 16% of participants diagnosed with a primary psychotic disorder in adulthood were diagnosed with ADHD in childhood (Kim-Cohen et al., 2003). Similarly, a cross-sectional study of 122 first episode psychosis patients reported that 17% met criteria for childhood ADHD (Peralta et al., 2011). While studies consistently find that premorbid ADHD is relatively common in psychotic disorders, prevalence estimates range widely from 15% to 57% (Alaghband-Rad et al., 1995; Dalsgaard et al., 2014; Kim-Cohen et al., 2003; Peralta et al., 2011; Rho et al., 2015; Schaeffer and Ross, 2002; Spencer and Campbell, 1994). Several factors may account for the variable findings. Most studies relied on retrospective self-reporting of childhood ADHD symptoms in adults raising concerns about reliability and validity of ADHD diagnosis (Alaghband-Rad et al., 1995; Dalsgaard et al., 2014; Peralta et al., 2011). Studies with contemporaneous assessment of ADHD, cognitive, and psychotic symptoms are small (Keshavan et al., 2003; Öner and Munir, 2005; Rieder and Nichols, 1979), typically including less than 40 individuals.
In contrast to prevalence studies which, while variable, consistently find elevated rates of ADHD in psychosis, the impact of premorbid ADHD on psychosis phenotypes, including cognitive impairment and psychotic symptoms, is not well understood. Premorbid ADHD in psychotic disorders is associated with poorer academic achievement (Peralta et al., 2011; Rho et al., 2015); however, evidence of greater neuropsychological impairment is mixed. For instance, in 27 schizophrenia participants, those with premorbid ADHD performed worse on measures of set-shifting (Wisconsin Card Sorting Test (WCST) and visual attention (Trails Making Test) but similarly to participants without ADHD on the Continuous Performance Test (CPT) (Donev et al., 2011). Additionally, a study of 24 first-degree relatives of psychotic disorder patients found that relatives with premorbid ADHD demonstrated lower performance IQ compared to relatives without ADHD, but did not significantly differ on the WCST or verbal IQ (Öner and Munir, 2005).
Studies examining the impact of premorbid ADHD on positive and negative symptoms of psychosis have also yielded mixed findings. Rho et al. (2015) found that, in a first episode psychosis population, participants who reported ADHD treatment in childhood had more severe positive and negative symptoms (Rho et al., 2015). In contrast, Peralta et al. (2011) did not find significant differences in symptom severity (reality-distortion, disorganization, and negative symptoms) in first episode patients whose mothers retrospectively reported childhood ADHD symptoms (Peralta et al., 2011). Keshavan et al. (2003) found that first degree relatives with ADHD, as measured through clinical interview, demonstrated more severe psychotic-like experiences and attentional deficits compared to the relatives without ADHD, suggesting psychotic experiences may be exacerbated in high-risk samples with ADHD (Keshavan et al., 2003). Finally, an epidemiological study demonstrated greater ADHD symptoms were associated with paranoia and auditory hallucinations, mediated by dysphoric mood (Marwaha et al., 2015). Similar to studies of neuropsychological functioning, small sample sizes and retrospective reporting of ADHD diagnosis raise concerns about the reliability and generalization of these findings.
To address limitations described above, the current study utilized the Philadelphia Neurodevelopmental Cohort, a large (N=9,498) community-ascertained sample of youths aged 8–21 with concurrent assessment of psychotic symptoms, ADHD symptoms, and cognition. Our investigation had three aims. First, we sought to clarify the prevalence of ADHD in youth with psychosis spectrum (PS) symptoms. Second, we determined the impact of comorbid ADHD on cognitive function in youth with PS symptoms. Finally, we examined the effect of comorbid ADHD on the severity positive and negative psychosis symptoms in PS youth.
2. Materials and methods
2.1. Study participants and clinical characterization
This study used data from the PNC version 3 (Study Accession phs000607.v3.p2) obtained through the Database of Genotypes and Phenotypes (dbGaP) (Calkins et al., 2014). Briefly, the PNC is a population-based sample comprised of 9,498 individuals aged 8–21 selected at random, after stratification by sex, age, and ethnicity, from a larger cohort of individuals that participated in the genetics study at Children’s Hospital of Philadelphia (CHOP) Center for Applied Genomics (Calkins et al., 2015). After removing 2,637 individuals who were missing data, rated as having significant medical co-morbidities (>=3 on the medical rating form), or had a history of a pervasive developmental disorder, 6,861 individuals were included in our sample.
Participant demographics, medical history, psychiatric history, and psychopathology were assessed through a computerized structured interview (GOASSESS) (Calkins et al., 2014). Clinical interviews were conducted with study participants aged 11–21 and informants (e.g. parent) of participants aged 8–17. Embedded within the GOASSESS are the following measures of psychosis symptoms: the PRIME Screen-Revised (PS-R), Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) psychosis screen, and items from the Scale for Prodromal Syndromes (SOPS). The PS-R assesses positive symptoms of psychosis and includes 12 items, which are rated on a scale from 0=Definitely Disagree to 6=Definitely Agree. A total score is calculated by summing up the ratings from each item. The K-SADS psychosis screener is a 7-item measure of positive psychosis that assesses the presence, duration, distress, and impairment of delusions or hallucinations (e.g. “have you ever heard voices when no one is there?”, “have you ever believed in things and later found out they weren’t true, like people being out to get you or talking about you behind your back, or controlling what you do or think?”). The SOPS includes 6 items assessing negative and disorganized symptoms of psychosis (e.g. avolition, expression of emotion, disorganized speech). These items are rated on a scale of 1–6, with 6 being the most severe, and a total score is calculated by summing up the ratings from each item.
For the analyses described below, individuals were classified as PS youth using procedures similar to those described previously (Calkins et al., 2014). Briefly, participants were classified as PS youth if they met one of three conditions: 1) Positive sub-psychosis, which was defined by: an age deviant total PRIME z-score >= 2 standard deviations (SD), or at least 1 item rated 6, or at least 3 items rated 5; 2) Negative or disorganized symptoms defined by an age deviant total SOPS z-score >= 2 SD; or 3) Positive-psychosis as defined by presence of hallucinations or delusions with duration>=1 day resulting in significant impairment or distress (rating >=5) and not occurring in the context of substance use, illness, and medications. The proportions of PS youth that meet each criteria, and the degree of overlap, are reported in Figure S1.
Participants were considered to meet ADHD criteria if they endorsed: 1) >=2 inattentive symptoms or >=2 hyperactive symptoms in two or more contexts for at least 6 months, and 2) had an ADHD severity score >= 5. This definition of ADHD is based on the GOASSESS, which includes six inattention items and three hyperactivity items. Some items combine diagnostic features that are highly related (e.g. “did you often join other people’s conversations OR have trouble waiting your turn”?). In addition, given the equivocal nature of an age cut-off in the DSM-5, as well as examination of symptoms in a developmental sample, we did not require the presence of symptoms before age 12 in our definition; however, in the Supplementary Materials (Figure S2), we report the results of our analyses when an age cut-off of 12 is used in defining the ADHD sample. Finally, typically developing (TD) youth were defined as those that did not meet criteria for psychosis, ADHD, or any other psychopathologies.
2.2. Cognitive assessment
Cognitive abilities were assessed with the Penn CNB (Gur et al., 2012), which includes 14 tests grouped into 5 cognitive domains measuring executive functioning, episodic memory, social cognition, complex cognition, and motor function. The CNB scores were calculated as previously defined (Gur et al., 2012), and a global composite score was calculated by averaging accuracy scores across cognitive domains, excluding the motor domain. Cognitive scores were standardized across the entire sample. The Wide Range Achievement Test (WRAT 4) (Wilkinson and Robertson, 2006) reading subscale was also administered to provide an estimate of general cognitive abilities.
2.3. Clinical symptom severity
Positive symptom severity was calculated by summing the PRIME items, and negative/disorganized symptom severity was calculated by summing the negative/disorganized items from the SOPS. Total PS symptom severity was calculated by summing the PRIME and negative/disorganized symptom severity scores. Item D1 (lack of attention/focus) was removed prior to calculation of symptom scores, due to its clear overlap with symptoms of ADHD.
2.4. Statistical analyses
Chi-square test was used to compare prevalence of ADHD between PS and non-PS youth. To determine the impact of ADHD on cognition in the context of psychosis, the individuals were assigned to one of four groups: typically developing (TD), ADHD, PS without ADHD (PS−ADHD), and PS with ADHD (PS+ADHD). Participants within the ADHD and PS groups (PS+ADHD, PS−ADHD) were included regardless of co-morbid psychopathologies. The Penn CNB composite score was analyzed using one-way ANCOVA analysis with group as a between groups variable and age, race, sex, parental education, and the WRAT4 reading standard score included as covariates. The primary analysis of Penn CNB composite scores was supplemented with additional analyses for each of the five Penn CNB cognitive domains with the critical p-value Bonferroni corrected to account for multiple comparisons (i.e. p=.05 ÷ 5 cognitive domains = .01). Significant ANCOVAs were followed up with pairwise comparisons between the four groups, corrected using the Holm-Bonferroni method, given the large number of sequential comparisons (Holm, 1979). Finally, to determine if comorbid ADHD in PS youth is related to the severity of PS symptoms, the total, positive, and negative/disorganized symptom scores were compared between PS−ADHD and PS+ADHD groups using ANCOVAs controlling for age, race, and sex. Main effects were corrected for multiple comparisons using the Bonferroni method (critical p = .05 ÷ 3 = .017).
To address potential differences in cognitive and symptom patterns based on developmental stage, the analyses described above were run on both the complete cohort aged 8–21 (n=6861) and the sub-sample of adolescent/young adults aged 11–21 (n=4998). Data were analyzed using SPSS (version 26).
3. Results
Demographic data for the total sample and adolescent/young adult subgroup is presented in the Supplemental Materials (Tables S1 and S2). Briefly, within the total and adolescent/young adult samples, there were significant differences between the PS and non-PS groups in race, age, education, parental education, and estimated IQ. PS youth were more likely to be African American, have one year less of parental education on average, and have an estimated premorbid IQ approximately 6 points lower than non-PS youth.
3.1. Prevalence of ADHD in PS youth
As shown in Figures 1A and 1B, the prevalence of ADHD was higher in PS youth compared to non-PS youth in both the total sample (45% vs. 20%; χ2 = 344.51, p < .001) and adolescent/young adult sub-sample (41% vs 17%; χ2 = 302.97, p < .001).
Figure 1:
Prevalence of ADHD in psychosis-specrum (PS) Youth as compared with all other youth in the sample. yo = years old.
Notably, in the total sample, PS youth with ADHD reported more ADHD symptoms than ADHD youth without PS, controlling for age and gender (6.5 vs. 5.7, F(1,1718)=55.22, p<.001). This was true for both inattention and hyperactivity (4.5 vs. 4.1, F(1,1718)=38.87, p<.001; 1.9 vs. 1.6, F(1,1718)=38.37, p<.001). Similar results were observed in the adolescent/young adult sub-sample in regards to total ADHD symptoms (6.2 vs. 5.4, F(1,1109)=44.78, p<.001), inattention (4.4 vs. 4.0, F(1,1109)=33.51, p<.001), and hyperactivity (1.8 vs. 1.4, F(1,1109)=26.39,p<.001).
3.2. Cognition in TD, ADHD, and PS youth with and without ADHD
As shown in Figure 2A, the Penn CNB composite score differed across groups (F(3,4488)=5.39, p = .001). Post-hoc pairwise contrasts indicated that while overall cognitive function was impaired in both PS+ADHD and PS−ADHD (p = .001 and p = .009, respectively), as compared to TD, cognitive ability did not differ between PS+ADHD and PS−ADHD groups (p = .511). Overall cognitive function was also impaired in ADHD youth compared to TD youth (p = .004) and did not differ from the PS groups (p’s > .39). Follow-up analyses of specific Penn CNB cognitive domains revealed main effects of group in executive functioning (F(3,4486)=11.68, p < .001) and complex cognition (F(3,4456)=7.44, p < .001), but not episodic memory (F(3,4467)=0.99, p = .394), social cognition (F(3,4487)=1.65, p = .175), and motor functioning (F(3,4488)=0.28, p = .842). Pairwise contrasts revealed that executive functioning was more impaired in PS+ADHD, PS−ADHD, and ADHD (p’s ≤ .001) compared to TD. This pattern of impairment was also seen in complex cognition (p = .033, p < .001, and p < .001, respectively). Notably, PS−ADHD and PS+ADHD did not differ on any cognitive domain.
Figure 2:
Cognitive performance on the Penn Computerized Neurocognitive Battery (CNB). Marginal means presented for cognitive performance, controlling for age, gender, WRAT4, and parental education. *significant difference between groups after correcting for multiple comparisons.
As shown in Figure 2B, similar results were obtained when the analysis was restricted to the adolescent/young adult sub-sample. A main effect of group was observed for overall cognitive function (F(3,3202)=6.14, p < .001). Post-hoc pairwise contrasts indicated that while overall cognitive function was impaired in both PS+ADHD and PS−ADHD as compared to TD youth (p = .034 and p = .001, respectively), there was no difference between PS+ADHD and PS−ADHD groups (p = .393). Follow-up analyses of specific cognitive domains revealed significant main effects of group in executive functioning (F(3,3201)=10.21, p < .001) and complex cognition (F(3,3195)=7.91, p < .001) but not social cognition (F(3,3202)=2.76, p = .041), episodic memory (F(3,3195)=0.73, p = .532) or motor functioning (F(3,3202)=1.65, p = .176) after multiple-comparison correction. Pairwise contrasts revealed that executive functioning was impaired in PS+ADHD and PS−ADHD compared to TD (both p<.001). Impairment in complex cognition was seen in PS+ADHD and PS−ADHD (p = .036 and p < .001, respectively). Finally, social cognition was slightly better in the ADHD cohort than TD youth (p = .005).
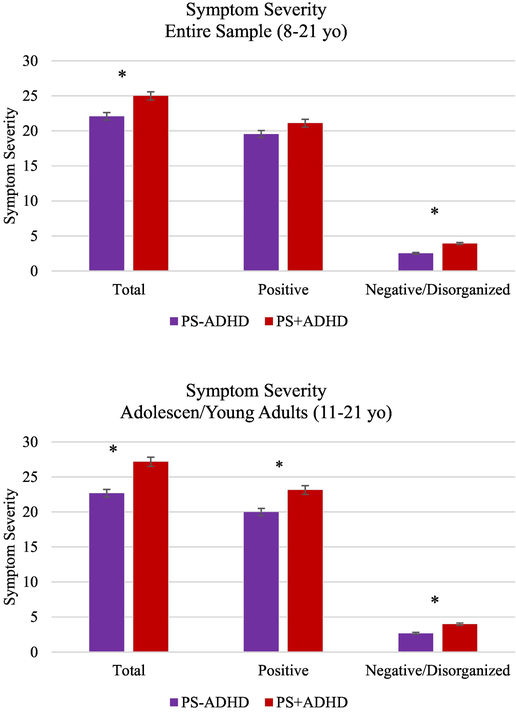
3.3. ADHD and severity of psychotic symptoms in PS Youth
As shown in Figure 3, within the total sample of PS youth, those with co-morbid ADHD had higher total PS symptoms (F(1,1278)=13.44, p < .001), positive symptoms (F(1,1303)=4.23, p =.04), and negative/disorganized symptoms (F(1,1278)=46.88, p<.001), although differences in positive symptoms did not pass multiple comparisons correction. In the adolescent/young adult sub-sample, PS+ADHD showed significantly more severe total (F(1,1118)=28.01, p<.001), positive (F(1, 1139)=15.18, p<.001) and negative/disorganized symptoms (F(1,1118)=33.68, p<.001).
Figure 3:
Group differences in severity of psychosis-spectrum (PS) symptoms as measured by the PRIME Screen. *denotes significant group difference after correcting for multiple comparisons (p<.017)
4. Discussion
In a large, community-ascertained developmental cohort, we found that ADHD is more prevalent in psychosis-spectrum youth than youth without significant psychosis-spectrum symptoms (45% vs. 20%). Cognitive functioning was impaired in all psychosis-spectrum youth to a similar degree, regardless of co-morbid ADHD symptoms. Co-morbid ADHD was, however, associated with worse psychosis-spectrum symptoms. Greater prevalence of ADHD in PS youth suggest that ADHD symptoms may be part of the psychosis prodrome, indicating a potential psychological vulnerability for experiencing ADHD symptoms in PS youth.
Central to the neurodevelopmental model of psychotic disorders is the presence of cognitive and behavioral disruptions during early childhood. Greater prevalence of childhood ADHD in patients with schizophrenia lends support to this model (Dalsgaard et al., 2014). Epidemiological studies have reported 7.2% of youth in the general population have ADHD (Thomas et al., 2015), whereas those who develop schizophrenia have childhood ADHD at rates of over 15%. In the PNC, a developmental cohort of 8–21 year olds reporting current symptoms of psychopathology, we found that youth at risk for psychosis reported significant and distressing ADHD symptoms at a greater rate than all other youth included in the study. In other words, youth with significant positive (e.g. delusional thoughts, hallucinatory experiences), negative (e.g. poor emotional expression) and/or disorganized symptoms also reported difficulties paying attention, problems following instructions, difficulty planning, excessive daydreaming, and hyperactivity in multiple contexts. These ADHD symptoms were reported as distressing and interfered with daily life. Unlike prior reports of schizophrenia-spectrum patients retrospectively reporting diagnosis or symptoms of ADHD in childhood, assessment of ADHD and psychosis symptoms were conducted concurrently during the developmental period, limiting reporting and memory bias. Furthermore, rates of ADHD were compared between PS youth and all other youth in the study, regardless of co-morbidities. Non-PS youth still reported other psychopathologies, including internalizing disorders (e.g. mood and anxiety) and other externalizing disorders (e.g. conduct disorder, oppositional defiant disorder). We were therefore able to capture the unique impact of psychosis-spectrum experiences on rates of ADHD. Our findings strongly suggest that prevalence of ADHD symptoms is significantly increased in the context of psychosis-spectrum symptoms during childhood and adolescence.
Youth at high risk for developing psychotic disorders reliably exhibit cognitive impairment (Byrne et al., 1999), yet it was unknown how co-morbid ADHD symptoms impact cognitive profiles in PS youth. Prior studies, limited by small sample sizes, report mixed findings on the impact of co-morbid ADHD on cognitive impairment in high-risk youth, with some finding worse cognition with co-morbid ADHD (Öner and Munir, 2005) and others finding similar cognitive ability (de la Serna et al., 2010). Here, in samples with >400 individuals per group, we find no differences in cognitive ability between psychosis spectrum youth with and without co-morbid ADHD. Both groups are impaired compared with typically developing youth but, regardless of the cognitive domain, do not differ from one another. These findings are somewhat surprising, particularly in light of reports that high-risk youth with co-morbid ADHD have worse functional outcome (Rho et al., 2015), which is related to cognitive ability in psychotic disorders (Bowie et al., 2006). Yet, given the large sample and concurrent symptom ratings, they are also compelling. They reveal that the presence of ADHD symptoms in PS youth is not related to even greater deficits (e.g. in executive functioning) than are expected for PS youth overall. These findings were observed in the entire cohort (age 8–21) and adolescent/young adults (age 11–21), suggesting consistent impairment in all PS youth, regardless of developmental stage. One interpretation of this finding is that cognitive deficits related to being high-risk for psychosis create a ceiling for cognitive impairment that is not further exacerbated by co-morbid ADHD.
ADHD youth without PS symptoms exhibited cognitive impairment in executive functioning and complex cognition, consistent with prior reports in ADHD (Pennington and Ozonoff, 1996) and consistent with the domains of impairment in PS youth. Unlike PS youth, however, cognitive impairment in ADHD youth was most prominent across the entire cohort and deficits were attenuated in the adolescent/young adult sample. Prior studies have shown that 20–50% of youth with ADHD remit and show improvement in previous behavioral and neuropsychological deficits (Halperin et al., 2008). This possible developmental lag of executive functioning or compensation/recovery of abilities is consistent with reports of delayed prefrontal maturation in ADHD (Shaw et al., 2007). This differential pattern in ADHD and PS youth may serve as an important developmental marker of psychosis-risk. Based on these data, one might expect an individual with ADHD who shows continued cognitive impairment over time, as opposed to stabilization or improvement, would be at greater risk for developing a psychotic disorder. This trajectory that can be monitored by schools and mental health providers in ADHD youth, possibly leading to early intervention.
Similar cognitive profiles between PS+ADHD and PS−ADHD were observed in the context of more severe psychosis spectrum symptoms in the PS+ADHD group. The PS+ADHD group endorsed more severe positive and negative/disorganized symptoms in both investigated age groups. These results are in line with previous studies of high-risk youth, which have shown associations between attentional deficits and positive (Kim et al., 2012) and negative (Hurtig et al., 2011) symptoms associated with psychotic-like experiences. Interestingly, first-episode patients with co-morbid ADHD have been reported to experience onset of psychosis on average two years earlier than first episode patients without ADHD (Rho et al., 2015). It is therefore possible that individuals in the PS+ADHD group are more likely to transition to a psychotic disorder. Co-morbid ADHD has also been related to worse treatment response in first episode patients and more severe positive and negative symptoms at 6-month follow-up, despite similar levels of baseline symptom severity (Elman et al., 1998; Rho et al., 2015). It has therefore been suggested that psychotic disorder patients with co-morbid ADHD are a unique group who are not being adequately served by current treatments (Levy et al., 2015). While our cross-sectional data cannot speak to this hypothesis directly, they lend further support to the notion that presence of ADHD symptoms is related to worse psychosis-spectrum experiences. This was observed in the context of distinct symptom ratings for ADHD and psychosis, meaning symptoms were not “double-counted” across categories. Although speculative, ADHD symptoms (e.g. inattention, impulsivity, distractibility) may reflect prodromal symptoms of psychosis that contribute to functional disturbances and distress in this population.
A strength of this study is the large population-based sample with a high number of PS youth with concurrent clinical and cognitive characterization, based on current (as opposed to retrospective) report. This allowed us to add to the relatively small literature on the link between ADHD and PS symptoms, by characterizing cognitive profiles of PS youth with and without co-morbid ADHD. Despite these strengths, there were several limitations of the current study. These include the use of cross-sectional data, limiting our ability to assess developmental trajectories. For instance, while the data suggests that ADHD is associated with improvements in cognition upon entering adolescence, this cannot be confirmed without a longitudinal cohort. Furthermore, although data was collected through clinical interview, recall of information from memory was still necessary (e.g. how long symptoms lasted for; what contexts they were problematic in), which may have reduced the validity of some of the symptom endorsements. In addition, parental education was used as a proxy for socioeconomic status, however social adversity is associated with both ADHD (Timimi and Taylor, 2004) and psychosis-risk (Heinz et al., 2013) and would be an important third variable to consider in future studies. Finally, the GOASSESS measure did not ask every question on the DSM-5, which may have inflated rates of ADHD across the entire sample.
Overall, the present findings demonstrate higher prevalence of ADHD in PS youth that is associated with more severe psychotic symptoms, but not greater cognitive impairment. These findings may indicate ADHD symptoms as reflecting aspects of the psychosis prodrome. Based on these findings it is recommended that youth with ADHD symptoms – particularly those at other risk for psychosis (e.g. genetically) – are also assessed for PS symptoms and monitored more closely for the emergence of psychosis over time. Trajectory of cognitive deficits (i.e. exacerbation over time) may be an especially useful marker of risk in this population. Such steps may help with early intervention.
Supplementary Material
Acknowledgement
The authors wish to acknowledge the contributions of the Philadelphia Neurodevelopmental Cohort members and those who collected this data for use by the scientific community. We would also like to thank Dr. Meg Benningfield for her comments on our manuscript.
Funding Source
This work was supported by National Institute of Mental Health (NIMH) grant R01 MH115000 (awarded to NDW) and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/National Institute of Health).
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alaghband-Rad J, McKenna K, Gordon CT, Albus KE, Hamburger SD, Rumsey JM, Frazier JA, Lenane MC, Rapoport JL, 1995. Childhood-Onset Schizophrenia: The Severity of Premorbid Course. J. Am. Acad. Child Adolesc. Psychiatry 34, 1273–1283. 10.1097/00004583-199510000-00012 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M, 1995. Symptoms of Schizophrenia: Methods, Meanings, and Mechanisms. Arch. Gen. Psychiatry 52, 341–351. 10.1001/archpsyc.1995.03950170015003 [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD, 2006. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry 163, 418–425. 10.1176/appi.ajp.163.3.418 [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC, 1999. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: Preliminary findings of the Edinburgh High Risk Study (EHRS). Psychol. Med 29, 1161–1173. 10.1017/S0033291799001002 [DOI] [PubMed] [Google Scholar]
- Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, Ruparel K, Wolf DH, Roalf DR, Mentch FD, Qiu H, Chiavacci R, Connolly JJ, Sleiman PMA, Gur RC, Hakonarson H, Gur RE, 2015. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J. Child Psychol. Psychiatry 56, 1356–1369. 10.1111/jcpp.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, Ruparel K, Chiavacci R, Wolf DH, Mentch F, Qiu H, Connolly JJ, Sleiman PA, Hakonarson H, Gur RC, Gur RE, 2014. The psychosis spectrum in a young U.S. community sample: Findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry 13, 296–305. 10.1002/wps.20152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard S, Mortensen PB, Frydenberg M, Maibing CM, Nordentoft M, Thomsen PH, 2014. Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur. Psychiatry 29, 259–263. 10.1016/j.eurpsy.2013.06.004 [DOI] [PubMed] [Google Scholar]
- de la Serna E, Baeza I, Toro J, Andrés S, Puig O, Sánchez-Guistau V, Romero S, Bernardo M, Castro-Fornieles J, 2010. Relationship between clinical and neuropsychological characteristics in child and adolescent first degree relatives of subjects with schizophrenia. Schizophr. Res 116, 159–167. 10.1016/j.schres.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Donev R, Gantert D, Alawam K, Edworthy A, Häβler F, Meyer-Lindenberg A, Dressing H, Thome J, 2011. Comorbidity of schizophrenia and adult attention-deficit hyperactivity disorder. World J. Biol. Psychiatry 12, 52–56. 10.3109/15622975.2011.599212 [DOI] [PubMed] [Google Scholar]
- Elman I, Sigler M, Kronenberg J, Lindenmayer J-P, Doron A, Mendlovic S, Gaoni B, 1998. Characteristics of patients with schizophrenia successive to childhood ADHD.pdf. Isr. J. Psychiatry Relat. Sci 35, 280–286. [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE, 2012. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology 26, 251–265. 10.1037/a0026712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH, 2008. Neuropsychological outcome in adolescents/young adults with childhood ADHD: Profiles of persisters, remitters and controls. J. Child Psychol. Psychiatry Allied Discip 49, 958–966. 10.1111/j.1469-7610.2008.01926.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Deserno L, Reininghaus U, 2013. Urbanicity, social adversity and psychosis. World Psychiatry 12, 187–197. 10.1002/wps.20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, 1979. Board of the Foundation of the Scandinavian Journal of Statistics A Simple Sequentially Rejective Multiple Test Procedure A Simple Sequentially Rejective Multiple Test Procedure. Source Scand. J. Stat. Scand J Stat 6, 65–70. [Google Scholar]
- Hurtig TM, Taanila A, Veijola J, Ebeling H, Mäki P, Miettunen J, Kaakinen M, Joukamaa M, Therman S, Heinimaa M, Järvelin MR, Moilanen I, 2011. Associations between psychotic-like symptoms and inattention/hyperactivity symptoms. Soc. Psychiatry Psychiatr. Epidemiol 46, 17–27. 10.1007/s00127-009-0165-7 [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Sujata M, Mehra A, Montrose DM, Sweeney JA, 2003. Psychosis proneness and ADHD in young relatives of schizophrenia patients. Schizophr. Res. 10.1016/S0920-9964(01)00400-5 [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R, 2003. Prior Juvenile Diagnoses in Adults With Mental Disorder. Arch. Gen. Psychiatry 60, 709. 10.1001/archpsyc.60.7.709 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee YJ, Jang JH, Lim W, Cho IH, Cho SJ, 2012. The relationship between psychotic-like experiences and attention deficits in adolescents. J. Psychiatr. Res 46, 1354–1358. 10.1016/j.jpsychires.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Levy E, Traicu A, Iyer S, Malla A, Joober R, 2015. Psychotic Disorders Comorbid With Attention-Deficit. Can J psychiatry 60, S48–S52. [PMC free article] [PubMed] [Google Scholar]
- Marwaha S, Thompson A, Bebbington P, Singh SP, Freeman D, Winsper C, Broome MR, 2015. Adult attention deficit hyperactivity symptoms and psychosis: Epidemiological evidence from a population survey in England. Psychiatry Res. 229, 49–56. 10.1016/j.psychres.2015.07.075 [DOI] [PubMed] [Google Scholar]
- Nourredine M, Gering A, Fourneret P, Rolland B, Falissard B, Cucherat M, Geoffray MM, Jurek L, 2021. Association of Attention-Deficit/Hyperactivity Disorder in Childhood and Adolescence with the Risk of Subsequent Psychotic Disorder: A Systematic Review and Meta-analysis. JAMA Psychiatry 1–11. 10.1001/jamapsychiatry.2020.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öner Ö, Munir K, 2005. Attentional and neurocognitive characteristics of high-risk offspring of parents with schizophrenia compared with DSM-IV attention deficit hyperactivity disorder children. Schizophr. Res 76, 293–299. 10.1016/j.schres.2005.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S, 1996. Executive functions and developmental psychopathology. J. Child Psychol. Psychiatry Allied Discip 37, 51–87. 10.1111/j.1469-7610.1996.tb01380.x [DOI] [PubMed] [Google Scholar]
- Peralta V, Jalon EGD, Campos MS, Zandio M, Sanchez-Torres A, Cuesta MJ, 2011. The meaning of childhood attention-deficit hyperactivity symptoms in patients with a first-episode of schizophrenia-spectrum psychosis. Schizophr. Res 126, 28–35. 10.1016/j.schres.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Rho A, Traicu A, Lepage M, Iyer SN, Malla A, Joober R, 2015. Clinical and functional implications of a history of childhood ADHD in first-episode psychosis. Schizophr. Res 165, 128–133. 10.1016/j.schres.2015.03.031 [DOI] [PubMed] [Google Scholar]
- Rieder RO, Nichols PL, 1979. Offspring of schizophrenics: III. Hyperactivity and neurological soft signs. Arch. Gen. Psychiatry 10.1001/archpsyc.1979.01780060055006 [DOI] [PubMed] [Google Scholar]
- Schaeffer JL, Ross RG, 2002. Childhood-onset schizophrenia: premorbid and prodromal diagnostic and treatment histories. J. Am. Acad. Child Adolesc. Psychiatry 41, 538–545. 10.1097/00004583-200205000-00011 [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL, 2007. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U. S. A 104, 19649–19654. 10.1073/pnas.0707741104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Gold JM, Strauss ME, Carter CS, MacDonald III AW, Ragland JD, Silverstein SM, Barch DM, 2014. Common and specific cognitive deficits in schizophrenia: Relationships to function. Cogn. Affect. Behav. Neurosci 14. 10.3758/s13415-013-0211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer EK, Campbell M, 1994. Children With Schizophrenia: Diagnosis, Phenomenology, and Pharmacotherapy. Schizophr. Bull 20, 713–725. 10.1093/schbul/20.4.713 [DOI] [PubMed] [Google Scholar]
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P, 2015. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 135, e994–e1001. 10.1542/peds.2014-3482 [DOI] [PubMed] [Google Scholar]
- Timimi S, Taylor E, 2004. ADHD is best understood as a cultural construct. Br. J. Psychiatry 184, 8–9. 10.1192/bjp.184.1.8 [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. WRAT 4: wide range achievement test.
- Wingo AP, Harvey PD, Baldessarini RJ, 2009. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord 11, 113–125. 10.1111/j.1399-5618.2009.00665.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.