Abstract

In this paper, cellulose chitosan composite aerogels were prepared through sol–gel and freeze-drying processes. The porous morphology of the aerogels was controlled by adjusting the cellulose concentration. Within a certain range, as the concentration of cellulose increases, the pore diameter of the composite aerogel becomes smaller and the pore structure becomes denser. The cellulose–chitosan composite aerogel can successfully separate the oil–water mixture without asphalt and showed stable filtration performance. The filtration speed is basically unchanged after a slight decrease and can be maintained at about 90% of the initial filtration speed within 30 min. The filtration speed can reach up to 9315 kg·h–1·m–2. When filtering bituminous oil–water mixtures, the filtration rate decreased significantly, with a 50% drop in 30 min. After adding the asphalt stabilizer poly(styrene-alt-octadecyl maleimide) (SNODMI), which is made in our laboratory, the effect of aerogel filtering the asphalt-containing oil–water mixture is obviously improved, and the downward trend of filtration speed is obviously improved. The combination of SNODMI and cellulose–chitosan has great application potential in the field of asphalt-containing oil–water separation.
1. Introduction
Oil–water separation is an important process in crude oil production and environment protection for the following reasons:1−3 (1) the repeated occurrence of offshore crude oil spills.4−6 (2) The production of heavy crude oil through secondary and tertiary oil extraction, which may contain water as high as 90%. (3) The salty crude oil to be eluted with water to avoid the risk of corrosion to subsequent process equipment.7,8 Therefore, it is of great significance to develop efficient technologies for oil–water separation. The traditional methods include physical methods such as centrifugal separation, flotation, and gravity separation; chemical approaches such as salting out, flocculation, and acidification; electrochemical methods including electrolytic separation and electromagnetic separation; and biological treatment including oxidation pond method and activated sludge method.2,3,9−18 Among these methods, adsorption and filtration are the most widely used methods.19−25
Cellulose porous materials have the advantages of abundant raw materials, simple preparation, excellent wettability, and excellent mechanical properties.26−30 They have been widely used for the adsorption and separation processes. Yue31 et al. reported a composite aerogel with banana peel and waste paper as the raw materials. Through a combination of freeze casting, freeze drying, and thermal cracking processes, an aerogel with a layered porous structure was prepared and employed for oil adsorption and separation of water-in-oil emulsions. This aerogel shows high hydrophobicity (149.3°), super lipophilicity (0°), and high porosity in the air. Its oil absorption capacity can reach 35–115 g/g. The aerogel can also separate water-in-oil emulsions containing emulsifiers with an efficiency over 99.6%. Zhang32 et al. reported a nanocellulose crystal aerogel with antibacterial activity and capability for oil–water separation. Cyanuryl chloride (CYCH) was attached to nanocellulose crystals (NCCs), and chloropropyltriethoxysilane was used for cross-linking to prepare a hydrogel based on modified nanocellulose crystals. This aerogel has a three-dimensional porous structure and hydrophobicity and can adsorb organic solvents of up to 16 g/g. It exhibits stable adsorption capacity in 10 adsorption and desorption cycles. In addition, this chlorinated aerogel has been shown to have an effective bactericidal effect against Staphylococcus aureus and Escherichia coli O157:H7. Georgouvelas et al. presented a one-step method for the manufacturing cellulose-based functional membranes, which exhibited high water permeability and efficient adsorption of metal ions.33−35
Cellulose-based porous materials are also often used as filtering and separating materials.15,30,36−38 He37 et al. prepared an aerogel filtration membrane made of cellulose nanofibers for the oil–water separation process. This aerogel has superior hydrophilicity and underwater oleophobicity, excellent wettability, and pothole-like surface structure. Driven by gravity, the filtration tests on the free oil/water mixture all showed a 100% separation effect. The efficiency of separating the oil–water emulsion also reached 98.6%. In order to enhance the hydrophilicity of the cellulose aerogel, other ingredients can be added during the preparation process. Meng38 et al. produced a stable super-hydrophilic (contact angle near 0°) and underwater superoleophobic (contact angle over 150°) cellulose–chitosan (CECS) aerogel. In the preparation process, chitosan self-assembled into micrometer size particles on the aerogel surface. In addition, the network structure of cellulose was destroyed, and more hydrophilic −OH groups were exposed. The hydrophilicity and surface roughness gave the CECS aerogel the characteristics of ultra-oleophobic underwater. The CECS aerogel effectively separated the free oil–water mixture and oil–water emulsion even after many cycles.
When separating more complex oil–water mixtures, more complicated problems were encountered.29,32,39−44 In the oil–water separation experiment of Cao45 et al., super-hydrophobic polyurethane sponge embedded with perfluorinated graphene oxide effectively separated the oil–water mixture. However, the separation rate was considerably reduced when filtering the mixture of crude oil and water. Bochner de Araujo46 et al. reported that asphalt, one of the main components of crude oil, spontaneously emulsifies the oil–water mixture when there is no external force, forming a large quantity of emulsion droplets, which also reduces the efficiency of oil–water separation. Therefore, it is necessary to study the separation of oil–water mixtures containing asphalt.
In our previous work,47 the polydicyclopentadiene (PDCPD)-based aerogel membrane with super-lipophilic and super-hydrophobic properties was prepared to filter the asphalt-containing oil–water mixture. By using an asphalt stabilizer, poly(styrene-alt-octadecyl maleimide) (SNODMI), the asphalt–toluene mixture can pass through the aerogel membrane and be separated from water. In this paper, we successfully prepared composite aerogels of cellulose and chitosan, which possess superhydrophilicity and underwater oleophobic properties. When this membrane was employed to separate the asphalt-containing oil–water mixture, water can permeate the membrane and be separated from the asphalt/toluene mixture, which was stabilized by an asphalt stabilizer. The filtration efficiency and the influence of the asphalt stabilizer have been investigated.
2. Results and Discussion
2.1. Synthesis of Aerogels
CECS aerogels were prepared by the sol–gel process, and the concentrations of cellulose in the wet gel were controlled to be 1, 1.5, and 2.0 wt % and the corresponding aerogels were marked as A1.0, A1.5, and A2.0, respectively.
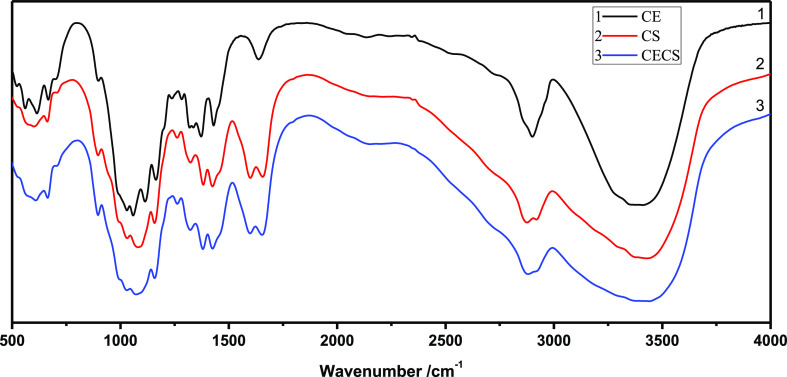
Figure 1 shows the FTIR spectra of CS, CE, and CE–CS aerogel. The characteristic peaks of CE were located at 1050, 1430, 2890, and 3348 cm–1 corresponding to C–O–C, −CH2, C–H, −OH stretching bands, respectively. The peaks of chitosan at 3300–3450 cm–1 correspond to the stretching of −OH and −NH; two peaks at 1650 and 1550 cm–1 correspond to amides I and amides II.48 For CECS aerogel, the characteristic bands of both cellulose and chitosan can be observed, which proves the composition of the CECS aerogel.
Figure 1.
FTIR spectra of CE, CS, and CECS aerogels.
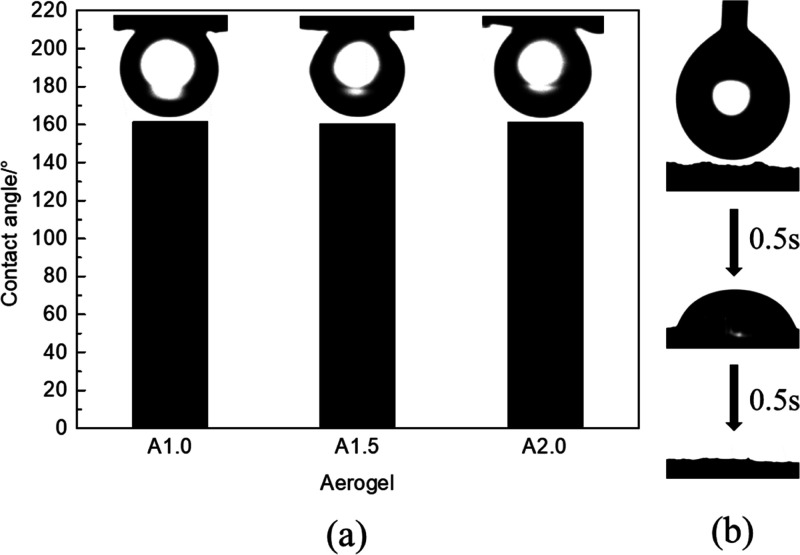
Figure 2 shows the specific surface wettability of CECS aerogels. Figure 2b presents the measuring process of the water contact angle on the surface of aerogel in the air. When the water droplets contacted the aerogel, they spread to the aerogel surface and were absorbed by the aerogel within 1 s, showing the superhydrophilicity of the aerogel. Figure 2a shows the contact angles of toluene droplet on the surface of three different aerogel membranes submerged under water. The measured contact angles were 161.4, 160.0, and 161° for A1.0, A1.5, and A2.0, respectively. These results indicate the aerogel’s underwater oleophobic properties, which is not affected by the aerogel cellulose concentration.
Figure 2.
Wetting behavior of toluene and water. (a) Contact angle of toluene under water and (b) water wettability of the CECS aerogel in air.
Figure 3 shows the SEM photographs of three CECS aerogels made from different cellulose concentrations. It can be seen that the cellulose aerogel is composed of a cellulose network cross-linked and intertwined. As the cellulose concentration in the aerogel increases, the cellulose network becomes denser, and the pore size becomes smaller.
Figure 3.
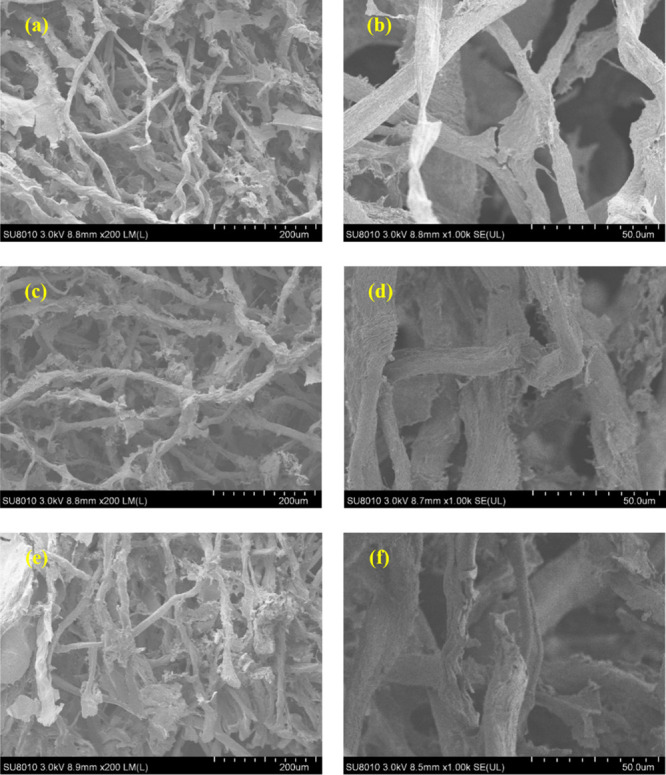
SEM photographs of CECS aerogels: A1.0: (a), (b); A1.5: (c,d); and A2.0: (e,f).
2.2. Separation of Simple Oil–Water Mixtures
The CECS aerogel can be used to separate simple oil–water mixtures as a result of its hydrophilicity, underwater oleophobicity, and porous structure. As shown in Figure 4, deionized water can pass through the aerogel membrane easily and the toluene, dyed by Sudan III, cannot pass through. The obtained filtrate is a toluene-free aqueous phase. After stopping adding water during filtration, the toluene and water mixture in the filtering device will eventually be completely separated, so that the liquid phase that has not passed through the aerogel membrane is a non-aqueous toluene phase.
Figure 4.
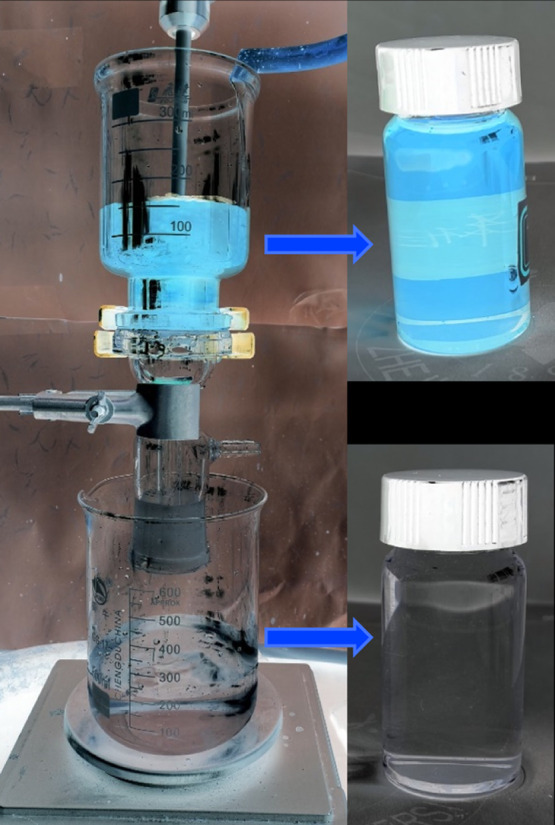
Separation of simple oil–water mixtures with the CECS aerogel.
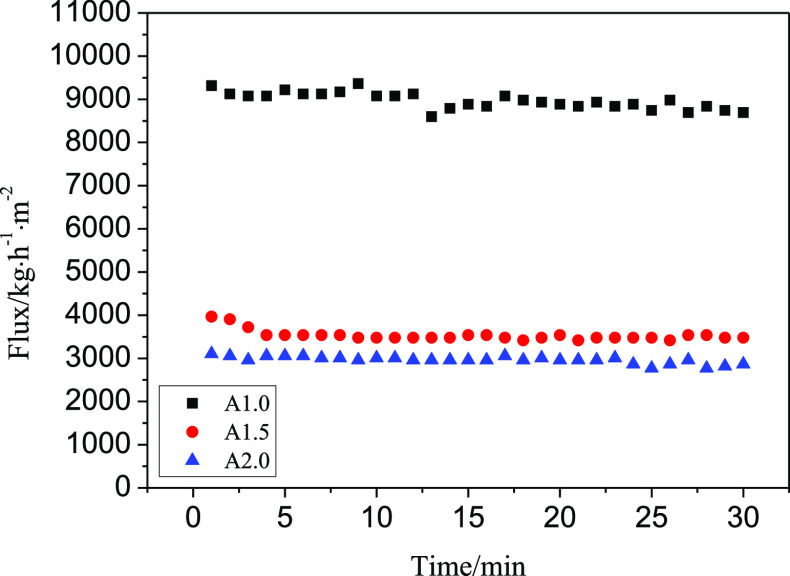
Figure 5 presents the change in filtration speed during the continuous separation process of simple oil–water mixtures. CECS aerogel membranes with different concentrations can all successfully separate simple oil–water mixtures. The filtration speed gradually decreases with the increased cellulose concentration. This is because the lower the concentration of the aerogel, the larger the aerogel pores, and the higher the aerogel porosity, which makes it easier for water to permeate the aerogel membrane and the filtration speed increased. It can be seen that the CECS aerogel membrane exhibits good stability when filtering simple oil–water mixtures. The filtration rate changed insignificantly after a slight decrease, which proves that the aerogel membrane can be used for long-term simple oil–water separation.
Figure 5.
Filtration speed for simple oil–water separation.
2.3. Separation of Oil–Water Mixture with Asphalt
In order to investigate the effect of asphalt on oil–water separation, an asphalt–toluene solution (3 wt %) was prepared. The oil–water mixture was prepared with a volume ratio of toluene: water at 3:7. The oil–water mixture with asphalt was filtrated using A1.0, A1.5, and A2.0. An asphalt stabilizer, SNODMI, was added to the asphalt–toluene solution to investigate the effect of asphalt stabilizers on the separation of asphalt-containing oil–water mixtures.
After adding asphalt, the oil–water mixture becomes more viscous, and the mixture exhibits a certain degree of emulsification after stirring. When filtering with the CECS aerogel, deionized water can pass through the hydrophilic aerogel membrane, while toluene-containing asphalt cannot due to the aerogel membrane’s underwater oleophobic properties. As shown in Figure 6, the toluene-containing oil–water mixture can also be finally separated into a toluene-free aqueous phase and a non-aqueous toluene phase.
Figure 6.

Separation of asphalt-containing oil–water mixtures with CECS aerogels.
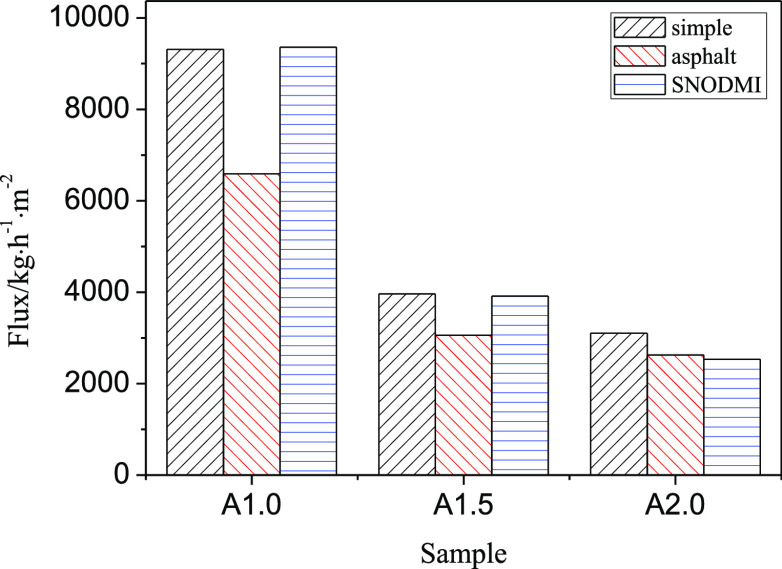
Figure 7 shows the initial filtration rate when different oil–water mixtures were separated with the aerogel membrane. After adding asphalt, the filtration speed of aerogel membranes with different concentrations decreases to a certain extent. When the asphalt stabilizer was used, the filtration rate of A1.0 and A1.5 with larger pore diameters were greatly improved, which were close to the filtration rate of the simple oil–water mixtures. On the other hand, the results of the A2.0 aerogel with the smallest pore diameters did not change much.
Figure 7.
Effect of asphalt and asphalt stabilizer on an initial filtration speed.
In order to further investigate the effects of asphalt and asphalt stabilizer on the filtration rate, the asphalt-containing oil–water mixture with and without a stabilizer were subjected to continuous filtration experiments. In order to better compare the change of the filtering rate, the rate at the first minute of each filtering experiment is taken as the initial speed, and the subsequent filtering rate is expressed as a percentage of the initial speed.
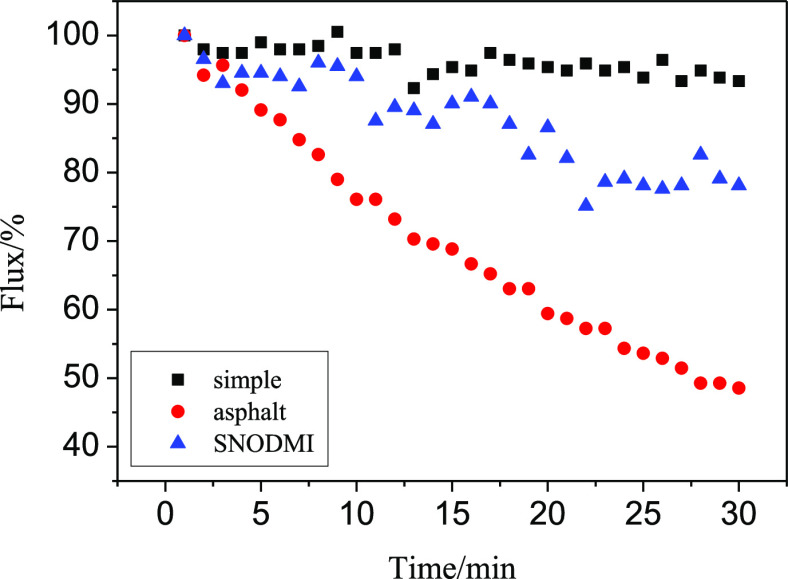
As can be seen from Figure 8, the filtration rate of A1.0 greatly decreased with time after adding asphalt. In 30 min, it was decreased to 48.6% of the initial filtration rate. When using the asphalt stabilizer, the filtration speed rate was only reduced to 78.1% of the initial filtration speed after 30 min. For comparison, the filtration rate of the simple oil–water system was only reduced to 93.3% of its initial value after 30 min. This is because asphalt as a natural emulsifier can emulsify the oil–water mixture,47 so that a greater driving force is required for oil–water separation during filtration, thereby reducing the filtration speed. In addition, when toluene-containing asphalt came in contact with the aerogel membrane, asphalt adhered to the aerogel surface and formed large particles to block the aerogel pores. Asphalt stabilizers can help asphalt better stabilized in toluene without precipitation, thereby reducing the number of asphalt particles attached to the aerogel film and reducing the size of the asphalt particles. Therefore, the filtration efficiency was improved when asphalt stabilizers were used.
Figure 8.
Effect of asphalt and asphalt stabilizer on a filtration speed of A1.0.
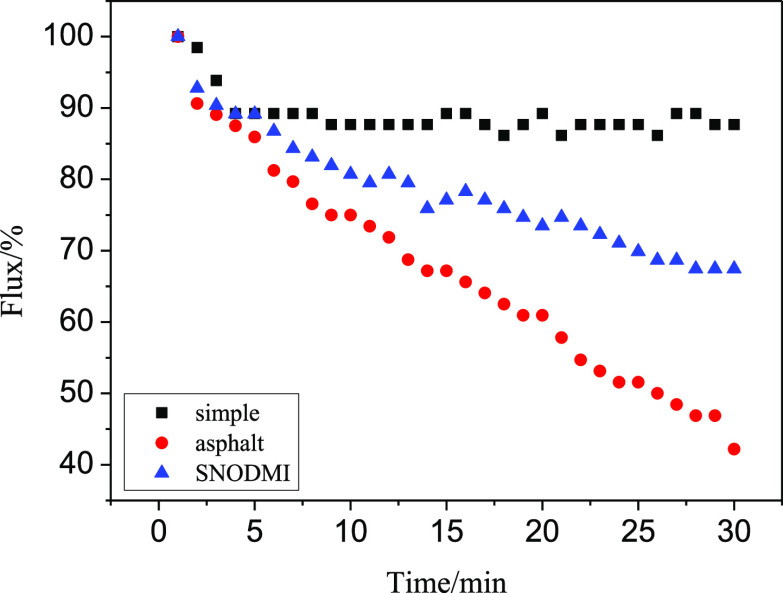
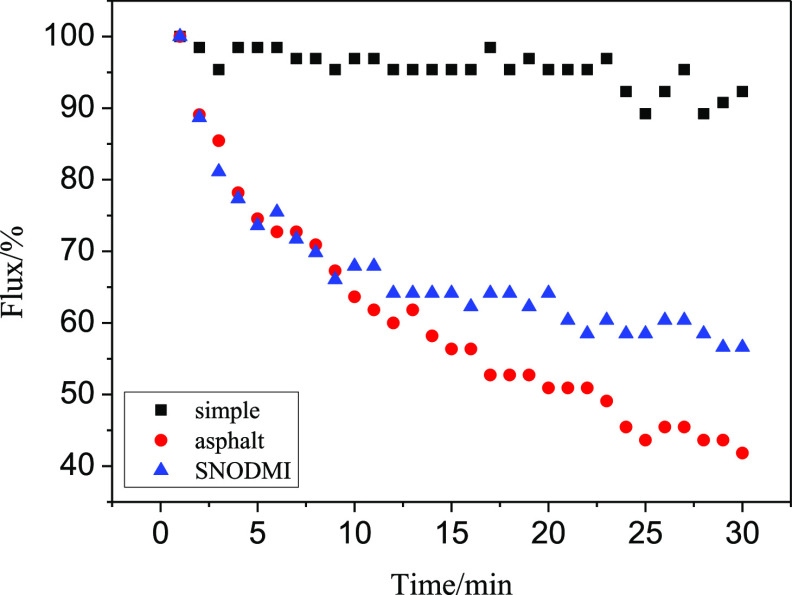
As can be seen from Figures 9 and 10, the aerogel membranes A1.5 and A2.0 showed the same characteristics as A1.0 when filtering asphalt-containing oil–water mixtures: the filtration rate of A1.5 and A2. 0 decreased to 42.2 and 41.8% of their initial value after 30 min, respectively. After adding asphalt stabilizers, the filtration speeds of A1.5 and A2.0 at 30 min were increased to 67.5 and 56.6% of their initial value, respectively.
Figure 9.
Effect of asphalt and asphalt stabilizer on a filtration speed of A1.5.
Figure 10.
Effect of asphalt and asphalt stabilizer on a filtration speed of A2.0.
In order to further investigate the effect of asphalt on the aerogel membrane when filtering the oil–water mixture containing asphalt, the filtered aerogel membrane was observed with SEM. Figure 11a,c,e is the SEM images of the A1.0, A1.5, and A2.0 aerogel membranes after filtering the asphalt-containing oil–water mixture without using the asphalt stabilizer. A large number of asphalt particles were attached to the aerogel fiber skeleton, and the channels inside the aerogel were severely blocked. As the diameter of the cellulose pores decreases, the blockage becomes more serious. Figure 11b,d,e is the SEM images of the A1.0, A1.5, and A2.0 aerogel membranes after filtering the asphalt-containing oil–water mixture with the asphalt stabilizer. The number of asphalt particles on the fiber skeleton is significantly reduced, and the size of the asphalt particles is also significantly decreased. As the aerogel pore diameter increases, the positive effects of the asphalt stabilizer become more pronounced. These changes are in line with the above trends in filtration rate and validate the speculated mechanism on the influence of asphalt and asphalt stabilizers.
Figure 11.
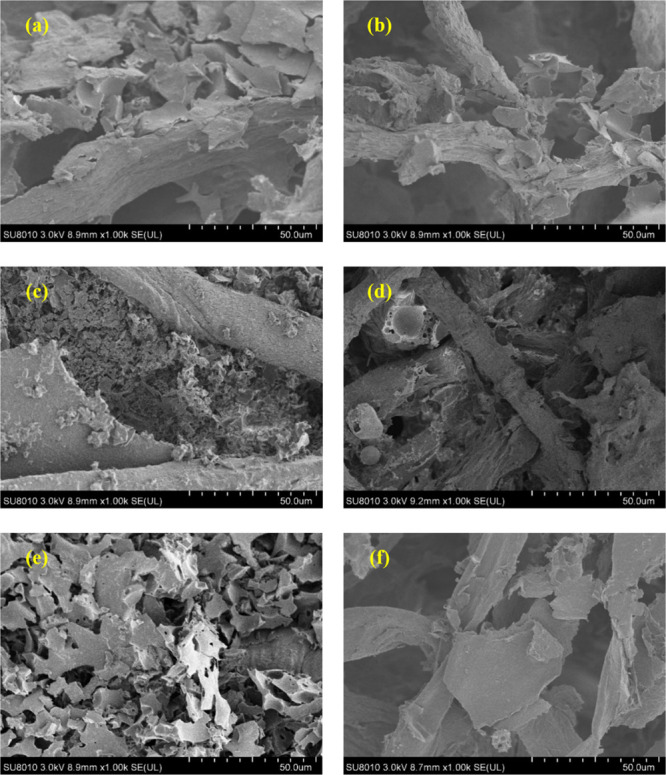
SEM photographs of CECS aerogels after filtering: asphalt-containing oil–water mixture without SNODMI: A1.0: (a), A1.5: (c), and A2.0: (e); asphalt-containing oil–water mixture with SNODMI: A1.0: (b), A1.5: (d), and A2.0: (f).
3. Conclusions
In this paper, a composite aerogel of cellulose and chitosan was successfully prepared by sol–gel method and freeze-drying method. The pore size of the aerogel was adjusted by controlling the concentration of cellulose. The filtering performance of the aerogels with different pore sizes was tested in subsequent oil–water separation experiments. The effect of asphalt on aerogel filtration oil–water mixture was studied. The results show that asphalt can decrease the filtration speed. After 30 min of continuous filtration, the filtration speeds were reduced to less than 50% of the initial speed. This is because asphalt can adhere to the surface of the aerogel during filtration, blocking the filtration channel and reducing filtration speed. After using the asphalt stabilizer, the negative effect of asphalt on the filtration speed was greatly lessened. The asphalt stabilizer reduces the tendency of asphalt to adhere to the aerogel membrane by better stabilizing asphalt in toluene, thereby improving the filtration speed. Therefore, the combination of CECS aerogel and asphalt stabilizer SNODMI has promising application potential in the separation of asphalt-containing oil–water mixture.
4. Materials and Methods
4.1. Materials
Medical cotton was purchased from the HeNan Piaoan Group. Chitosan (90% deacetylation) was supplied by J&K Scientific Ltd. Urea, sodium hydroxide, and ethanol of analytical grade were provided by Aladdin. Toluene was obtained from Jiangsu Yonghua Fine Chemicals Co., Ltd. SNODMI, the asphalt stabilizer, was synthesized in our laboratory.47
4.2. Preparation of CECS Aerogels
CECS aerogels were prepared using sol–gel and freeze-drying processes. The cellulose solution was prepared according to the adapted method.48 A certain amount of medical cotton was dissolved in a 100 g solution, which consisted of 7 wt % NaOH, 12 wt % urea, and 81 wt % H2O to obtain a homogeneous cellulose solution at −20 °C. The chitosan solution was produced by dissolving 1.5 g of chitosan with 100 g of solution that consisted of 5 wt % NaOH, 6 wt % urea, and 89 wt % H2O. The cellulose solution and the chitosan solution were mixed at 5 °C for 20 min. The homogeneous solution was poured into a container to form a film with a thickness of 0.25 cm. The container was placed in the refrigerator for 48 h. The obtained hydrogel was immersed into deionized water to coagulate and regenerate and washed to neutrality with deionized water. After washing, the sample was freeze-dried at −50 °C for 24 h to obtain a CECS aerogel.
4.3. Preparation of Different Oil–Water Mixtures
Oil–water mixture: 70 mL of deionized water, 30 mL of toluene, and a small amount of coloring agent (Sudan III) were mixed for 1 h.
Oil–water mixture containing asphalt: the asphalt/toluene solution was prepared by dissolving 0.783 g of asphalt in 30 mL of toluene. This solution was added to 70 mL of deionized water to prepare an oil–water mixture containing asphalt.
The oil–water mixture containing asphalt and SNODMI: 0.054 g of SNODMI and 0.783 g of asphalt were dissolved in 30 mL of toluene sequentially, followed by the addition of 70 mL of deionized water.
4.4. Oil–Water Separation Experiment
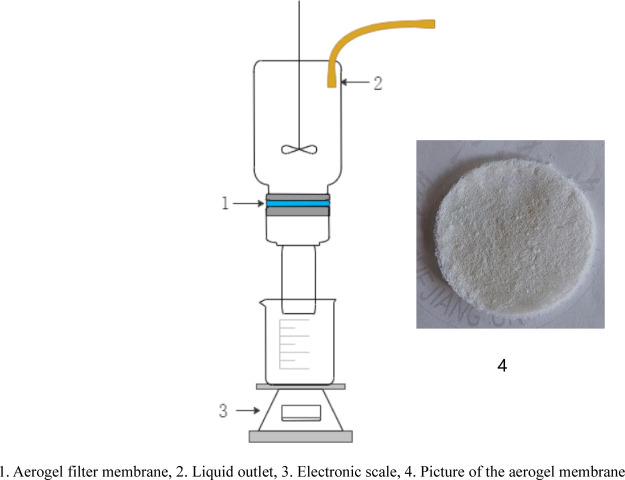
As shown in Figure 12, a continuous filtering device was designed. Water was continuously fed to the filtering device at a speed equal to the rate of water passing through the membrane. Therefore, the total amount and composition of the oil–water mixture to be filtered were kept constant. A stirrer was employed to obtain the uniform oil–water mixture and keep the liquid in the mixture in contact with the membrane. The filtrate was weighted by the electronic balance scale to measure the filtering rate.
Figure 12.
Device for filtering.
4.5. Characterization Method
FTIR spectra were recorded using a Thermo Fisher’s Nicolet 5700 infrared spectrometer. The DTGS KBr detector was used with a wavenumber scanning range of 400–4000 cm–1 and a resolution of 0.1 cm–1. The water contact angle on the aerogel surface was measured using the OCA 20 video optical contact angle measuring instrument from Data physics of Germany. Hitachi SU 8010, a field emission scanning electron microscope, was employed to observe the morphologies.
Author Contributions
ZY. and J.H. involved in conceptualization, methodology, investigation, and writing—review and editing. Z.Y. involved in investigation, data curation, and writing—original draft. B.L. involved in the investigation.
The authors declare no competing financial interest.
References
- Gossen L. P.; Velichkina L. M. Environmental problems of the oil-and-gas industry (Review). Pet. Chem. 2006, 46, 67–72. 10.1134/s0965544106020010. [DOI] [Google Scholar]
- Mysore D.; Viraraghavan T.; Jin Y. C. Oil/water separation technology - A review. J. Residuals Sci. Technol. 2006, 3, 5–14. [Google Scholar]
- Padaki M.; Surya Murali R.; Abdullah M. S.; Misdan N.; Moslehyani A.; Kassim M. A.; Hilal N.; Ismail A. F. Membrane technology enhancement in oil-water separation. A review. Desalination 2015, 357, 197–207. 10.1016/j.desal.2014.11.023. [DOI] [Google Scholar]
- Nordvik A. B.; Simmons J. L.; Bitting K. R.; Lewis A.; Strøm-Kristiansen T. Oil and water separation in marine oil spill clean-up operations. Spill Sci. Technol. Bull. 1996, 3, 107–122. 10.1016/s1353-2561(96)00021-7. [DOI] [Google Scholar]
- Adebajo M. O.; Frost R. L.; Kloprogge J. T.; Carmody O.; Kokot S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. 10.1023/a:1027484117065. [DOI] [Google Scholar]
- Kammerer M.; Mastain O.; Le Dréan-Quenech’du S.; Pouliquen H.; Larhantec M. Liver and kidney concentrations of vanadium in oiled seabirds after the Erika wreck. Sci. Total Environ. 2004, 333, 295–301. 10.1016/j.scitotenv.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Rana M. S.; Sámano V.; Ancheyta J.; Diaz J. A. I. A review of recent advances on process technologies for upgrading of heavy oils and residua. Fuel 2007, 86, 1216–1231. 10.1016/j.fuel.2006.08.004. [DOI] [Google Scholar]
- Chopra S.; Lines L. Introduction to this Special Section : Heavy Oil. Leading Edge 2008, 27, 1104–1106. 10.1190/1.2978971. [DOI] [Google Scholar]
- Chen G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. 10.1016/j.seppur.2003.10.006. [DOI] [Google Scholar]
- Hanafy M.; Nabih H. I. Treatment of oily wastewater using dissolved air flotation technique. Energy Sources, Part A 2007, 29, 143–159. 10.1080/009083190948711. [DOI] [Google Scholar]
- Suzuki Y.; Maruyama T. Removal of emulsified oil from water by coagulation and foam separation. Sep. Sci. Technol. 2005, 40, 3407–3418. 10.1080/01496390500423755. [DOI] [Google Scholar]
- Chakrabarty B.; Ghoshal A. K.; Purkait M. K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. 10.1016/j.memsci.2008.08.007. [DOI] [Google Scholar]
- Ge J.; Zhao H.-Y.; Zhu H.-W.; Huang J.; Shi L.-A.; Yu S.-H. Advanced Sorbents for Oil-Spill Cleanup: Recent Advances and Future Perspectives. Adv. Mater. 2016, 28, 10459–10490. 10.1002/adma.201601812. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Carrott P. J. M.; Ribeiro Carrott M. M. L.; Suhas Low-Cost Adsorbents: Growing Approach to Wastewater Treatmenta Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. 10.1080/10643380801977610. [DOI] [Google Scholar]
- He X.; Zhang L.; Meng D.; Wu J. From hydrogel to aerogel: A green fabrication of multifunctional polyimide absorbents. Eur. Polym. J. 2017, 89, 461–467. 10.1016/j.eurpolymj.2017.02.039. [DOI] [Google Scholar]
- Li A.; Lin R.; Lin C.; He B.; Zheng T.; Lu L.; Cao Y. An environment-friendly and multi-functional absorbent from chitosan for organic pollutants and heavy metal ion. Carbohydr. Polym. 2016, 148, 272–280. 10.1016/j.carbpol.2016.04.070. [DOI] [PubMed] [Google Scholar]
- Zhang W. F.; Liu N.; Cao Y. Z.; Lin X.; Liu Y. N.; Feng L. Superwetting Porous Materials for Wastewater Treatment: from Immiscible Oil/Water Mixture to Emulsion Separation. Adv. Mater. Interfaces 2017, 4, 1600029. 10.1002/admi.201700029. [DOI] [Google Scholar]
- Zheng T.; Wang J.; Wang Q.; Meng H.; Wang L. Research trends in electrochemical technology for water and wastewater treatment. Appl. Water Sci. 2017, 7, 13–30. 10.1007/s13201-015-0280-4. [DOI] [Google Scholar]
- Van der Bruggen B.; Vandecasteele C.; Van Gestel T.; Doyen W.; Leysen R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. 10.1002/ep.670220116. [DOI] [Google Scholar]
- Geise G. M.; Lee H.-S.; Miller D. J.; Freeman B. D.; Mcgrath J. E.; Paul D. R. Water Purification by Membranes: The Role of Polymer Science. J. Polym. Sci., Part B: Polym. Phys. 2010, 48, 1685–1718. 10.1002/polb.22037. [DOI] [Google Scholar]
- Wang K.; Liu X.; Tan Y.; Zhang W.; Zhang S.; Li J. Two-dimensional membrane and three-dimensional bulk aerogel materials via top-down wood nanotechnology for multibehavioral and reusable oil/water separation. Chem. Eng. J. 2019, 371, 769–780. 10.1016/j.cej.2019.04.108. [DOI] [Google Scholar]
- Mazrouei-Sebdani Z.; Salimian S.; Khoddami A.; Shams-Ghahfarokhi F. Sodium silicate based aerogel for absorbing oil from water: the impact of surface energy on the oil/water separation. Mater. Res. Express 2019, 6, 085059. 10.1088/2053-1591/ab1eed. [DOI] [Google Scholar]
- Zhang S.; Zhang N.; Qian L.; Liu Y.; Ren H.; Li Y. Facile preparation of high strength aerogel based on polysulfonamide. Mater. Lett. 2019, 250, 159–162. 10.1016/j.matlet.2019.05.024. [DOI] [Google Scholar]
- Yuan J.; Zhang M.; Xia M.; Cao W.; Du M.; Dou J.; Zhao D. Novel high-capacity and reusable carbonaceous sponges for efficient absorption and recovery of oil from water. Appl. Surf. Sci. 2019, 487, 398–408. 10.1016/j.apsusc.2019.05.036. [DOI] [Google Scholar]
- Alghunaimi F. I.; Alsaeed D. J.; Harith A. M.; Saleh T. A. Synthesis of 9-octadecenoic acid grafted graphene modified with polystyrene for efficient light oil removal from water. J. Cleaner Prod. 2019, 233, 946–953. 10.1016/j.jclepro.2019.05.239. [DOI] [Google Scholar]
- Liu H.; Geng B.; Chen Y.; Wang H. Review on the Aerogel-Type Oil Sorbents Derived from Nanocellulose. ACS Sustainable Chem. Eng. 2017, 5, 49–66. 10.1021/acssuschemeng.6b02301. [DOI] [Google Scholar]
- Cervin N. T.; Aulin C.; Larsson P. T.; Wågberg L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. 10.1007/s10570-011-9629-5. [DOI] [Google Scholar]
- Korhonen J. T.; Kettunen M.; Ras R. H. A.; Ikkala O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. 10.1021/am200475b. [DOI] [PubMed] [Google Scholar]
- Shi J.; Lu L.; Guo W.; Sun Y.; Cao Y. An environment-friendly thermal insulation material from cellulose and plasma modification. J. Appl. Polym. Sci. 2013, 130, 3652–3658. 10.1002/app.39615. [DOI] [Google Scholar]
- Granström M.; née Pääkkö M. K.; Jin H.; Kolehmainen E.; Kilpeläinen I.; Ikkala O. Highly water repellent aerogels based on cellulose stearoyl esters. Polym. Chem. 2011, 2, 1789–1796. 10.1039/c0py00309c. [DOI] [Google Scholar]
- Yue X.; Zhang T.; Yang D.; Qiu F.; Li Z. Hybrid aerogels derived from banana peel and waste paper for efficient oil absorption and emulsion separation. J. Cleaner Prod. 2018, 199, 411–419. 10.1016/j.jclepro.2018.07.181. [DOI] [Google Scholar]
- Zhang Y.; Yin M.; Lin X.; Ren X.; Huang T.-S.; Kim I. S. Functional nanocomposite aerogels based on nanocrystalline cellulose for selective oil/water separation and antibacterial applications. Chem. Eng. J. 2019, 371, 306–313. 10.1016/j.cej.2019.04.075. [DOI] [Google Scholar]
- Georgouvelas D.; Abdelhamid H. N.; Li J.; Edlund U.; Mathew A. P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. 10.1016/j.carbpol.2021.118044. [DOI] [PubMed] [Google Scholar]
- Abdelhamid H. N.; El-Bery H. M.; Metwally A. A.; Elshazly M.; Hathout R. M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. 10.1016/j.carbpol.2019.03.024. [DOI] [PubMed] [Google Scholar]
- Soliman M.; Sadek A. A.; Abdelhamid H. N.; Hussein K. Graphene oxide-cellulose nanocomposite accelerates skin wound healing. Res. Vet. Sci. 2021, 137, 262–273. 10.1016/j.rvsc.2021.05.013. [DOI] [PubMed] [Google Scholar]
- Du Y.; Li Y.; Wu T. A superhydrophilic and underwater superoleophobic chitosan-TiO2 composite membrane for fast oil-in-water emulsion separation. RSC Adv. 2017, 7, 41838–41846. 10.1039/c7ra08266e. [DOI] [Google Scholar]
- He Z.; Zhang X.; Batchelor W. Cellulose nanofibre aerogel filter with tuneable pore structure for oil/water separation and recovery. RSC Adv. 2016, 6, 21435–21438. 10.1039/c5ra27413c. [DOI] [Google Scholar]
- Meng G.; Peng H.; Wu J.; Wang Y.; Wang H.; Liu Z.; Guo X. Fabrication of Superhydrophobic Cellulose/Chitosan Composite Aerogel for Oil/Water Separation. Fibers Polym. 2017, 18, 706–712. 10.1007/s12221-017-1099-4. [DOI] [Google Scholar]
- Baig N.; Alghunaimi F. I.; Saleh T. A. Hydrophobic and oleophilic carbon nanofiber impregnated styrofoam for oil and water separation: A green technology. Chem. Eng. J. 2019, 360, 1613–1622. 10.1016/j.cej.2018.10.042. [DOI] [Google Scholar]
- Li Y.; Zou G.; Zhang X.; Yang S. Y.; Wang Z. H.; Chen T.; Zhang L.; Lei J.; Zhu W. K.; Duan T. Bio-inspired and assembled fungal hyphae/carbon nanotubes aerogel for water-oil separation. Nanotechnology 2019, 30, 275601. 10.1088/1361-6528/ab0be3. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhu L.; Grishkewich N.; Tam K. C.; Yuan J.; Mao Z.; Sui X. CO2 -Responsive Cellulose Nanofibers Aerogels for Switchable Oil- Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 9367–9373. 10.1021/acsami.8b22159. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wang H. Ultra-hydrophobic and mesoporous silica aerogel membranes for efficient separation of surfactant-stabilized water-in-oil emulsion separation. Sep. Purif. Technol. 2019, 212, 597–604. 10.1016/j.seppur.2018.11.078. [DOI] [Google Scholar]
- Xie A.; Chen Y.; Cui J.; Lang J.; Li C.; Yan Y.; Dai J. Facile and green fabrication of superhydrophobic sponge for continuous oil/water separation from harsh environments. Colloids Surf., A 2019, 563, 120–129. 10.1016/j.colsurfa.2018.12.009. [DOI] [Google Scholar]
- Zhou L.; Zhai S.; Chen Y.; Xu Z. Anisotropic Cellulose Nanofibers/Polyvinyl Alcohol/Graphene Aerogels Fabricated by Directional Freeze-drying as Effective Oil Adsorbents. Polymers 2019, 11, 712. 10.3390/polym11040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N.; Guo J.; Boukherroub R.; Shao Q.; Zang X.; Li J.; Lin X.; Ju H.; Liu E.; Zhou C.; Li H. Robust superhydrophobic polyurethane sponge functionalized with perfluorinated graphene oxide for efficient immiscible oil/water mixture, stable emulsion separation and crude oil dehydration. Sci. China: Technol. Sci. 2019, 62, 1585–1595. 10.1007/s11431-019-9533-y. [DOI] [Google Scholar]
- Bochner de Araujo S.; Merola M.; Vlassopoulos D.; Fuller G. G. Droplet Coalescence and Spontaneous Emulsification in the Presence of Asphaltene Adsorption. Langmuir 2017, 33, 10501–10510. 10.1021/acs.langmuir.7b02638. [DOI] [PubMed] [Google Scholar]
- Lin B.; Wang Z.; Zhu Q.-j.; Binti Hamzah W. N.; Yao Z.; Cao K. Aerogels for the separation of asphalt-containing oil-water mixtures and the effect of asphalt stabilizer. RSC Adv. 2020, 10, 24840–24846. 10.1039/d0ra00544d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. L.; Wu J. N.; Wang Y. X.; Wang H.; Liu Z. Y.; Shi Y. L.; Guo X. H. A facile approach for preparation of underwater superoleophobicity cellulose/chitosan composite aerogel for oil/water separation. Appl. Phys. A: Mater. Sci. Process. 2016, 122, 516. 10.1007/s00339-016-0049-0. [DOI] [Google Scholar]