Abstract

Various catalysts were used to catalyze the ethoxylation reaction of C12–14 primary alcohols with ethylene oxide. Alcohol ethoxylates with a ratio of ethylene oxide/substrate near 3 were synthesized. The catalysts influenced the reaction rate, molecular weight distribution of adducts, and formation of byproducts. The physicochemical properties of ethoxylates obtained using different catalytic systems were analyzed, and their functional properties, i.e., wetting and permeation, were investigated. The results showed that the products obtained using the catalysts MCT-09 and NAE-03 had a narrower oligomer distribution and excellent wetting properties compared with those obtained using conventional ethoxylation catalysts.
1. Introduction
Alcohol ethoxylates, abbreviated as AEO, are the most important class of nonionic surfactants.1,2 AEO are alcohol–ether mixtures with a certain molecular weight distribution produced by a stepwise additive ethoxylation reaction of fatty alcohols with ethylene oxide in the presence of alkali, acid, or other types of catalysts3 (see Scheme 1).
Scheme 1. Synthesis Route of AEO.
There are three main factors affecting the molecular weight distribution of ethoxylates: first, the catalyst properties; second, the initiator structure; and third, the reactor mass transfer.4 Therefore, for most cases where the reaction device and starting agent type are fixed, the catalyst is the factor affecting the ethoxylation reaction product.5,6 The molecular weight distributions of ethoxylates catalyzed by different kinds of catalysts are very different. Basic catalysts, such as NaOH and KOH, catalyze products with a wider molecular weight distribution and high concentration of unreacted primary alcohols.7,8 Acidic catalysts, such as BF3 and SbCl4 catalyze products with a narrower molecular weight distribution but give rise to more byproducts of the reaction.9,10 In some cases, to obtain a narrow oligomer distribution, heterogeneous catalysts have also been proposed.11 The molecular weight distribution of AEO products directly affects the application of the products.12,13 A narrow distribution is preferable in most cases.14−16 Therefore, the selection of suitable catalysts for the synthesis of narrowly distributed ethoxylated products under suitable reaction conditions has long been the focus of research.
In the current literature, the behavior of the newly proposed catalyst is compared in general only with the classical ones (KOH or NaOH); the aim of this paper is to compare in a homogeneous way the performances of the different catalysts in the ethoxylation reaction to elicidate that the difference in the molecular weight distribution of oligomers can influence their physicochemical properties.
In this paper, products obtained by ethoxylation of a fatty alcohol with classical basic catalysts (NaOH, KOH, NaOCH3, KOCH3) have been compared with different basic (C11H23COONa, MCT-09) and acidic (Mg(ClO4)2, SnCl4 and H3O40PW12·xH2O, NAE-03) catalysts.
The composition and molecular weight distribution of ethoxylates obtained using the various catalytic systems were discussed, and their physical and chemical properties, such as wetting and permeation, were systematically investigated.
2. Results
2.1. Reaction Rates of Fatty Alcohol Ethoxylation with Different Catalytic Systems
In Figure 1, ethylene oxide fed over time has been reported for the different catalytic systems. The basic classical catalysts do not have any induction period and the reaction rate is constant along the time (Figure 1a). In these cases, catalytic species (alcoholate) is formed during the preheating phase in which water or methanol are removed by vacuum
Figure 1.
Ethoxylation reaction rates under different catalytic systems: (a) basic catalytic system and (b) acidic catalytic system.
From the data in Figure 1a, NaOH seems to be more active compared to KOH with an activity of about 66%; however, it has to be considered that the runs were performed with the same weight amount of the catalyst and so with higher molar concentration of NaOH with respect to KOH because of the difference in molar weight. As a matter of fact, the reaction rate is given by the following equation17
And so, it is strictly dependent on the molar concentration of the catalyst. Considering the actual molar concentrations of KOH and NaOH, the difference in activity is around 30%. The lower activity of the corresponding methylate (NaOCH3, KOCH3) is due to the fact that the catalysts are dissolved in methanol and so their final concentration in the reaction mixture is lower than that of the hydroxide. Considering the molar concentration, the activity of methylates is comparable with that of the corresponding hydroxides.
In the case of C11H23COONa, an induction period was observed. This behavior can be justified considering that carboxylates are less nucleophilic (and so less reactive) than alkoxides17 that are formed after the first ethylene oxide (EO) reaction
MCT-09 is a heterogeneous catalyst similar to that used by Hama et al. for the ethoxylation of fatty acid methyl esters18−20 and fatty alcohols.11 At the end of the reaction, the MCT-09 heterogeneous catalyst can be removed by filtration and a transparent product is obtained.
The proposed reaction mechanism for this type of catalyst is based on the bifunctional effect of the base derived from magnesium oxide and the acid derived from the Al ion.11
In the case of acidic catalysts (Lewis type: SnCl4, Mg(ClO4)2; Bronsted type: H3O40PW12·xH2O), no induction time was observed, the initial activity being in the order SnCl44 > Mg(ClO4)2 ≌ H3O40PW12·xH2O. The activity of SnCl4 and H3O40PW12·xH2O was quite constant along the time, while in the case of Mg(ClO4)2, a deactivation was observed.
This behavior can
be explained considering the reaction mechanism.21 In the presence of strong protic acids such
as H3O40PW12·xH2O, the EO ring opening is virtually completed in the
transition state and is followed by the SN1 nucleophilic
attack.
Also, the reaction mechanism in the presence of Lewis
acids (SnCl4, Mg(ClO4)2) is based
on cationic intermediates
and involves the formation of an oxonium ion by the activation of
the EO oxygen atom by the metal. However, in the case of Mg(ClO4)2, we observed a reduction in the reaction rate
probably because Mg2+ interacts preferably with the ether
oxygens of the ethoxylated products, like in the case of crow-ethers.2222
In the case of NAE-03 (see Figure 1b), we notice a completely different behavior with respect to the previously described acidic catalyst. As a matter of fact, NAE-03 showed a long induction period and a subsequent strong increase in the reaction rate. Studies to justify this behavior are ongoing and the results will be published in a future paper. Also, the product obtained with NAE-03 was filtered before physicochemical characterization.
2.2. Selectivity and Oligomer Distribution in Fatty Alcohol Ethoxylation Using Different Catalytic Systems
As can be seen from Table 1, the EO addition number of AEO (EO/S°) determined for the products of the alkaline catalytic system is basically close to the theoretical value (a lower value is obtained with C11H23COONa). For the acidic catalytic system, the addition reaction using magnesium perchlorate and tin tetrachloride catalysts could not achieve high EO addition numbers. Only the actual average EO additions obtained using phosphotungstic acid and NAE-03 catalysts were in agreement with the theoretical value.
Table 1. Average EO Addition Number of AEO (Determined (by Hydroxyl Value; Theoretical Value = 3.00), Poly(ethylene glycol) Concentration (PEG, % W), and Residue Fatty Alcohol C12–C14 Concentration (% W).
| catalyst | hydroxyl value (mg KOH/g) | average EO addition number (EO/S°) | PEG (% W) | fatty alcohol (% W) | |
|---|---|---|---|---|---|
| basic catalyst | KOH | 178 | 2.8 | 3.8 | 13.7 |
| NaOH | 175 | 2.9 | 1.5 | 16.3 | |
| CH3OK | 177 | 2.8 | 3.7 | 10.2 | |
| CH3ONa | 180 | 2.7 | 3.1 | 15.4 | |
| C11H23COONa | 183 | 2.6 | 4.7 | 16.4 | |
| MCT-09 | 180 | 2.7 | 4.4 | 7.1 | |
| acidic catalyst | Mg(ClO4)2 | 198 | 2.1 | 3.3 | 2.4 |
| SnCl4 | 195 | 2.2 | 1.4 | 5.1 | |
| H3O40PW12·xH2O | 180 | 2.7 | 7.7 | 6.5 | |
| NAE-03 | 177 | 2.8 | 6.2 | 10.0 | |
These results can be justified considering the different selectivity of the catalysts. As a matter of fact, during the ethoxylation reaction, different secondary reactions are possible, thus reducing the selectivity.23 The most important one is the formation of poly(ethylene glycol) (PEG). These products can be formed by traces of impurity (like for example water or methanol) in the substrate or by degradation of the ethylene oxide chain of ethoxylated fatty alcohols. From the data reported in Table 1, it can be seen that basic catalysts produce less PEG than acidic catalysts, being, in general, more selective.
From GC data, it is possible to determine the unreacted free alcohol for the different catalytic systems. These data are also reported in Table 1. The results of homogeneous basic catalysts showed that KOH and CH3OK leave less unreacted fatty alcohols with respect to NaOH, CH3ONa, and C11H23COONa. These results are explained by the different equilibrium constant of hydrogen transfer reactions between alcohol and ethoxylate that is strongly dependent on the nature of the cation21,23
The unreacted alcohol concentration using acidic catalysts is, in general, lower than that of basic catalysts, except in the case of the heterogeneous basic catalyst MCT-09 that has a quite similar unreacted alcohol concentration to that obtained with the acidic catalyst H3O40PW12·xH2O. It has to be pointed out that MCT-09 give rise to a lower concentration of byproducts than acidic catalysts and the oligomer distributions are very different. The oligomer distribution obtained with MCT-09 is near that predicted by the Flory distribution,24 while in the case of H3O40PW12·xH2O, a very high concentration of the first adduct is found (see Figure 2). The oligomer distribution in the last case could be described with a Natta–Mantica distribution,25 which is the indication of a complex mechanism with a strong influence of the number of added ethylene oxide molecules on the reaction rate.
Figure 2.
Oligomer distributions obtained with MCT-09 and H3O40PW12·xH2O, compared with the distribution calculated using the Flory model.24
In the case of the NAE-03 catalyst, the obtained oligomer distribution is narrower than that calculated by the Weibull–Nycander Equation,23 which is typical for classical basic catalysts (see Figure 3), and also in this case the distribution could be described by the Natta–Mantica equation.25
Figure 3.
Oligomer Distributions obtained with the NAE-03 catalyst, compared with the distribution calculated using the Weibull–Nycander Equation.23
2.3. Study of the Physicochemical Properties of Primary Alcohol Ethoxylates
The values of the physical properties (viscosity, freezing point, and cloud point) of AEO3 obtained using various catalytic systems are shown in Table 2 (the products with low average EO addition number obtained with Mg(ClO4)2 and SnCl4 were not considered). The data are very close, the more significant difference being the lower viscosity and higher cloud point obtained in the case of MCT-09 and NAE-03 catalysts.
Table 2. Physical Properties of AEO3.
| catalyst | viscosity (MPa·sa) | freezing point (°C) | cloud point (°C) | |
|---|---|---|---|---|
| basic catalyst | KOH | 25 | 2 | 42 |
| NaOH | 20 | 6 | 42 | |
| CH3ONa | 21 | 6 | 44 | |
| CH3OK | 16 | 3 | 41 | |
| C11H23COONa | 18 | 6 | 38 | |
| MCT-09b | 11.7 | 1 | 54 | |
| acidic catalyst | H3O40PW12·xH2O | 16.7 | 0 | 30 |
| NAE-03b | 10.0 | 4 | 54 | |
Test temperature is 25 °C.
Measurements after filtration.
Water penetration into an object with the help of surfactants is called permeability. Excellent penetration properties of surfactants are required in applications such as washing, pesticide spraying, printing, and dyeing. The reason why water can wet and penetrate inside the textile is because the textile fiber is a porous material with huge surface area, which can make the solution flow rapidly along the fiber and enter the fiber void due to capillary action, replacing the air out until the textile is completely wetted. Therefore, the speed of penetration is an important factor when examining the permeability. The most common method to measure surfactant permeability is by measuring the sink time of the canvas piece in the surfactant solution at a specified concentration.
The permeation ability of various AEO3 synthesized using different catalysts is shown in Figure 4, from which it can be seen that the permeation ability of AEO3 is excellent for every case. The permeation ability of these products is in general better than that of C16–C18 ethoxylated alcohols with the same EO/S° value (sink time ∼ 39 s),26 probably because the carbon chain length is shorter and the molecular diffusion rate in the fiber is faster at the same temperature.27
Figure 4.
Water permeability of AEO3 obtained using different catalytic systems.
The study of properties of different AEO3 was continued on one product obtained with a classical catalyst (KOH) and two products obtained with the catalysts that gave a narrow oligomer distribution (MCT-09 and NAE-03). Figure 5 shows the three curves of surface tension vs concentration for KOH, MCT-09, and NAE-03 catalytic systems of AEO3 at 25 °C.
Figure 5.
Variation of surface tension with concentration at 25 °C for AEO3 with different catalytic systems.
From the data reported in Figure 5, it is possible to calculate the critical micelle concentration (CMC), the surface tension at the CMC (γCMC), and other surface parameters. The maximum adsorption amount Γmax and the minimum cross-sectional area Amin of the surfactant at the gas–liquid interface can be calculated according to eqs 1 and 2
| 1 |
| 2 |
where R is the gas constant,
8.314 J/(mol·K); T is the thermodynamic temperature, K; 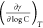 is the rate of change of surface
tension
γ with log of the surfactant concentration at constant temperature
and is the slope of the surface tension curve in a straight line before
the inflection point; n is a constant which, for
nonionic surfactants, is taken as 1; and NA is the Avogadro number, 6.02 × 1023.
is the rate of change of surface
tension
γ with log of the surfactant concentration at constant temperature
and is the slope of the surface tension curve in a straight line before
the inflection point; n is a constant which, for
nonionic surfactants, is taken as 1; and NA is the Avogadro number, 6.02 × 1023.
pC20, the negative logarithmic value of the concentration of surfactant (C20) required to reduce the surface tension of ultrapure water by 20 mN/m was used by Rosen et al.28 to express the efficiency of the surfactant in reducing the surface tension.
The calculated surface parameters are listed in Table 3.
Table 3. Static Surface Tension Parameters.
| catalyst | CMC (mol/L) | γCMC (mN/m) | Γmax (μmol/m2) | Amin (nm2) | pC20 | ΔGmic (kJ/mol) | ΔGads (kJ/mol) |
|---|---|---|---|---|---|---|---|
| KOH | 6.08 × 10–5 | 26.66 | 4.63 | 0.36 | 5.28 | –34.02 | –34.12 |
| MCT-09 | 9.91 × 10–5 | 26.57 | 3.22 | 0.52 | 5.42 | –32.81 | –32.95 |
| NAE-03 | 1.10 × 10–4 | 27.30 | 3.44 | 0.48 | 5.38 | –32.55 | –32.68 |
The three products were relatively close in their ability to reduce surface tension. Among them, the CMC of the narrowly distributed AEO3 obtained using the laboratory-made MCT-09 and NAE-03 catalysts was slightly higher than that of AEO3 obtained by the conventional catalyst KOH. The pC20 value of AEO3 catalyzed by MCT-09 was slightly higher than those of NAE-03 and KOH. This suggests that the surface tension reduction efficiency of narrowly distributed AEO3 would be slightly better than that of conventionally distributed AEO3.
The standard micellar Gibbs energy ΔGmico and the standard adsorption Gibbs energy ΔGads of the surfactant can be calculated by eqs 3 and 4. As can be seen in Table 3, the values of ΔGmico and ΔGads are negative, indicating that adsorption and micellization processes are spontaneous. Moreover, |ΔGmico| > |ΔGads|, indicating that the tendency of surfactant molecules to adsorb at the liquid surface is greater than the tendency to form micelles.
| 3 |
| 4 |
Figure 6 shows the surface tension vs time for AEO3 obtained with KOH, MCT-09, and NAE-03 catalytic systems at constant concentration (0.04 g/L). As can be seen from the figure, the AEO3 curves obtained with the product synthesized using KOH as the catalyst showed a faster decreasing trend than those obtained with the laboratory-made catalysts MCT-09 and NAE-03, indicating that the ethoxylation degree distribution of AEO3 obtained by KOH catalysis was more effective in reducing the surface tension.
Figure 6.
Curves of surface tension with time for AEO3 at 25 °C for different catalytic systems (all solution concentrations are 0.04 g/L).
Wettability can be determined by the contact angle (θ) of the surfactant solution on a hydrophobic film (paraffin film, systems with smaller contact angle have better wettability). It is customary to set θ = 90° as the criterion for wettability. If θ > 90° the system is not wettable, while if θ < 90° it is wettable. Equilibrium contact angle equal to 0 or nondetectable indicates a spreading system. The variation of the contact angle on the paraffin film with time for the aqueous solution of AEO3 obtained with different catalytic systems is shown in Figure 7A. The contact angle of all surfactant solutions on the paraffin film decreased with increasing time. As seen in Figure 7B, upon comparing the aqueous solution of AEO3 obtained with the classical KOH catalyst, the contact angle of the narrowly distributed AEO3 was significantly lower, indicating that the narrowly distributed AEO3 synthesized with MTC-09 and NAE-03 endowed the catalysts with better wetting ability.
Figure 7.
Variation of AEO3 contact angle with time for different catalytic systems: (A) dynamic contact angle of AEO3 at 25 °C and (B) spreading of AEO3 on paraffin film at 25 °C (all solution concentrations are 0.04 g/L).
3. Conclusions
Various catalysts were used to catalyze the ethoxylation reaction of C12–14 primary alcohols with ethylene oxide synthesizing alcohol ethoxylates with EO addition numbers close to 3. The catalysts MCT-09 and NAE-03 give rise to a narrow distribution of the ethoxylation degree with a low concentration of byproducts. The products of all catalytic systems have similar ability to reduce surface tension and have excellent permeability in water. Among them, the narrowly distributed AEO3 synthesized obtained with MCT-09 and NAE-03 catalysts have lower viscosity, higher cloud point, and better wetting properties.
4. Materials and Methods
4.1. Materials
C12–14 primary alcohol was purchased from Sinolight Surfactants Technology Co., Ltd. (Shanghai, China). Ethylene oxide (EO) was purchased from Shanxi Huayan Technology Co., Ltd. (Shanxi, China). Sodium hydroxide (AR) and potassium hydroxide (AR) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China). Sodium methanol (AR), potassium methanol (AR), and sodium laurate (AR) were purchased from Chengdu Aike Reagent Co., Ltd. (Sichuan, China). Magnesium perchlorate (AR), tin chloride (AR), phosphotungstic acid hydrate (AR), and 1-nonanol (GC) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Double-distilled water was used as the solvent in all experiments.
The calcium-based acidic catalyst NAE-03 was used to synthesize fatty alcohol ethoxylates furnished by Sinolight Surfactants Technology Co., Ltd. (Shanghai, China). It contains calcium salts of low molecular weight carboxylic acids and a strong mineral acid. This ethoxylation catalyst is in the form of a homogeneous liquid suspension with pH = 4.
4.2. Preparation of MCT-09
MgO (68.4 g) and 28 g of diatomaceous earth were mixed well and added into 400 mL of aqueous solution containing 23 g of aluminum chloride and stirred for 1 h. Then, the recovered solid was washed, dried, calcined at 500 °C for 1 h, ground, and sieved to obtain the Mg–Al composite catalyst MCT-09. The catalyst powder contains 65% of magnesium oxide, 8.4% of alumina, and 26.6% of diatomaceous earth.
4.3. Ethoxylation of Fatty Alcohols C12–C14
The autoclave is first checked for gas tightness. Afterward, vacuum is drawn and 145 g of fatty alcohol (the mass fractions determined by GC (see below) of C12 and C14 alcohols in the raw primary alcohol are 75 and 25 wt %, respectively) and 0.2 wt % of the target product for the basic catalyst or 1 wt % of the target product for the acidic catalyst are loaded. Then, the system is stirred and heated. When the temperature increases to 105–110 °C, the water and low boiling point substances in the system are removed by vacuum. After replacing the residual air in the autoclave with nitrogen three times, the reaction is induced by introducing ethylene oxide after increasing the temperature to 140–160 °C. The reaction pressure is kept constant at 0.3 ± 0.1 MPa by continuous introduction of ethylene oxide. The reaction temperature is controlled by continuous circulation of thermal fluid in the external jacket of the reactor. The consumption of ethylene oxide is measured by the loss of weight of the reservoir tank and it is stopped when fixed amount is reached (EO/S° = 3). After that, the heating is stopped, allowing the reaction mixture to age until the pressure in the reactor is constant. The reactor is cooled and when the temperature drops below 80 °C, the residual ethylene oxide in the reaction system is removed by vacuum. The material is then discharged to obtain the primary alcohol ethoxylate product.
4.4. Analytical Methods
The theoretical average EO addition number per mole of the initial substrate (EO/S°) can be derived by the consumption of EO during the synthesis process, while the actual average EO/S° can be calculated using the hydroxyl value (I(OH), mg KOH/g) obtained by the acetic anhydride method (GB/T 7384-1996 “Determination of hydroxyl value of nonionic surfactants—Acetic anhydride method”)
where M1 is the average relative molecular weight of AEO
M2 is the average relative molecular mass of primary alcohols (192 g/mol) and M3 is the molecular mass of ethylene oxide (44 g/mol).
Poly(ethylene glycol) (PEG) content was determined according to GB/T 5560-2003 “Determination of nonionic surfactants—polyethylene glycol content and nonionic active substance (adduct) content—Weilbull method”.
The oligomer distribution was obtained by gas chromatographic analysis using a SP-2100 GC-chromatograph with FID as the detector and DB-5 capillary column. The external standard was n-nonanol.
The turbidity point was determined according to the GB 11277-89 standard “Determination of turbidity point index (water number) of surfactants—Nonionic surfactants”
The standard method for wetting power GB/T 11983-2008 “Determination of wetting power of surfactants Submersion method” was used. Every sample was measured seven times to obtain the average value.
The surface tension was measured by a processor tension meter K12 (KRŰSS Company, Germany) using the plate technique at 25.0 ± 0.1 °C.
Different concentrations of surfactant aqueous solution were prepared and the dynamic surface tension was measured using a BP100 bubble pressure tension meter (KRŰSS Company, Germany).
Contact angle values at different concentrations were determined by the KRÜSS DSA255 Drop Shape Analyzer at 25 °C. Paraffin wax film was tightly attached to the slide as a solid substrate.
Acknowledgments
This paper was funded by the Shanxi Province Key R&D Program Project (201903D421034).
The authors declare no competing financial interest.
References
- Bajpai D.; Tyagi V. K. Nonionic Surfactants: An Overview. Tenside, Surfactants, Deterg. 2010, 47, 190–196. 10.3139/113.110062. [DOI] [Google Scholar]
- Tang X.-h.; Wu C.-z.; Li C.-w.; Li B.-j. Characteristics and application of fatty alcohol polyoxyethylene ether. Deterg. Cosmet. 2012, 35, 22–24. [Google Scholar]
- Bialowas E.; Szymanowski J. Catalysts for Oxyethylation of Alcohols and Fatty Acid Methyl Esters. Ind. Eng. Chem. Res. 2004, 43, 6267–6280. 10.1021/ie049898h. [DOI] [Google Scholar]
- Santacesaria E.; Di Serio M.; Garaffa R.; Addino G. Kinetics and Mechanisms of Fatty Alcohol Polyethoxylation. 1. The Reaction Catalyzed by Potassium Hydroxide. Ind. Eng. Chem. Res. 1992, 31, 2413–2418. 10.1021/ie00011a001. [DOI] [Google Scholar]
- Di Serio M.; Russo V.; Santacesaria E.; Tesser R.. The Evolution of the Fed Batch Ethoxylation Reactors to Produce the Non-Ionic Surfactants Front. Chem. Eng. 2021, 3, 10.3389/fceng.2021.644719. [DOI]
- Hreczuch W.; Szymanowski J. Synthesis of surfactants with Narrow-range distribution of the polyoxyethylene chain. J. Am. Oil Chem. Soc. 1996, 73, 73–78. 10.1007/BF02523451. [DOI] [Google Scholar]
- Amaral G. M.; Giudici R. Kinetics and Modeling of Fatty Alcohol Ethoxylation in an Industrial Spray Loop Reactor. Chem. Eng. Technol 2011, 34, 1635–1644. 10.1002/ceat.201100215. [DOI] [Google Scholar]
- Di Serio M.; Tesser R.; Dimiccoli A.; Santacesaria E. Kinetics of ethoxylation and propoxylation of ethylene glycol catalyzed by KOH. Ind. Eng. Chem. Res. 2002, 41, 5196–5206. 10.1021/ie020082v. [DOI] [Google Scholar]
- Olenick A. J.; Parkinson J. K. A comparison of the ethoxylation of a fatty alcohol, fatty acid, and dimethiconol. J. Am. Oil Chem. Soc. 1996, 73, 63–66. 10.1007/BF02523449. [DOI] [Google Scholar]
- Di Serio M.; Iengo P.; Gobetto R.; Bruni S.; Santacesaria E. Ethoxylation of fatty alcohols promoted by an aluminum alkoxide sulphate catalyst. J. Mol. Catal. A: Chem. 1996, 112, 235–251. 10.1016/1381-1169(96)00134-3. [DOI] [Google Scholar]
- Hanna I.Narrow Alcohol Ethoxylates. In Design and Selection of Performance Surfactants; Karsa D. R., Ed.; Sheffield Annual Surfactants Review, CRC Press, 1999; Vol. 2, pp 168–215. [Google Scholar]
- Cox M. F. Effect of alkyl carbon chain length and ethylene oxide content on the performance of linear alcohol ether sulfates. J. Am. Oil Chem. Soc. 1989, 66, 367–374. 10.1007/BF02653293. [DOI] [Google Scholar]
- Dillan K. W. Effects of the ethylene oxide distribution on nonionic surfactant properties. J. Am. Oil Chem. Soc. 1985, 62, 1144–1151. 10.1007/BF02542311. [DOI] [Google Scholar]
- Yang Y.; Wen P.; Guo J.; Sun Y.; Gao Y.; Yang H. Difference of fatty alcohol polyoxyethylene ether derivatives with narrow distribution. Deterg. Cosmet. 2016, 39, 39–42. [Google Scholar]
- Zhang M.; Sun Y.; Liu W.; Guo J.; Liu X. Performance of narrow distribution fatty alcohol polyoxyethylene ether sodium sulfate. China Surfactant Deterg. Cosmet. 2016, 46, 397–399. 10.13218/j.cnki.csdc.2016.07.007. [DOI] [Google Scholar]
- Tian J.; Sun Y.; Guo J. Synthesis of fatty alcohol polyoxyethylene ether with narrow distribution. China Surfactant Deterg. Cosmet. 2019, 49, 174–177. 10.3969/j.issn.1001-1803.2019.03.007. [DOI] [Google Scholar]
- Sun Y.; Luo Y.; Zhang G.; Tian C.. A catalyst for ethoxylation and its application.CN Patent CN1451476A2003.
- Bokhoven J.; Roelofs J.; Jong K.; Koningsberger D. C. Unique structural properties of the Mg-Al hydrotalcite solid base catalyst: an in situ study using Mg and Al K-edge XAFS during calcination and rehydration. Chemistry 2015, 7, 1258–1265. . [DOI] [PubMed] [Google Scholar]
- Kung H. H.; Ko E. I. Preparation of oxide catalysts and catalyst supports - a review of recent advances. Chem. Eng. J. Biochem. Eng. J. 1996, 64, 203–214. 10.1016/S0923-0467(96)03139-9. [DOI] [Google Scholar]
- Hama I.; Okamoto T.; Hidai E.; Yamada K. Direct ethoxylation of fatty methyl ester over Al-Mg composite oxide catalyst. J. Am. Oil Chem. Soc. 1997, 74, 19–24. 10.1007/s11746-997-0113-1. [DOI] [Google Scholar]
- Santacesaria E.; Iengo P.; Di Serio M.. Catalytic and Kinetic Effects in Ethoxylation Processes. In Design and Selection of Performance Surfactants; Karsa D. R., Ed.; Sheffield Annual Surfactants Review, CRC Press, 1999; Vol. 2, pp 146–167. [Google Scholar]
- Smułek W.; łada W. A. Separation of alkali and alkaline earth metals by polyethers using extraction chromatography. Effect of diluent. J. Radioanal. Chem. 1979, 50, 169–178. 10.1007/BF02519954. [DOI] [Google Scholar]
- Schachat N.; Greenwald H. J.. Mechanism of Ethylene Oxide Condensation. Nonionic Surfactants, Surfactant Science Series; Schick M. J., Ed.; Marcel Dekker Inc.: New York, 1967; 1, 8–32. [Google Scholar]
- Flory P. J. Kinetics of the Degradation of Polyesters by Alcohols1. J. Am. Chem. Soc. 1940, 62, 2255–2261. 10.1021/ja01866a001. [DOI] [Google Scholar]
- Natta G.; Mantica E. The Distribution of Products in a Series of Consecutive Competitive Reactions. J. Am. Chem. Soc. 1952, 74, 3152–3156. 10.1021/ja01132a057. [DOI] [Google Scholar]
- Chunli S.Synthesis and Properties of Long Chain Fatty Alcohol Polyoxyethylene Ether Derivatives. M.D. Thesis, Jiangnan University: Wuxi, 2013. [Google Scholar]
- Grundke K.; Zschoche S.; Pöschel K.; Gietzelt T.; Michel S.; Friedel P.; D Jehnichen D.; Neumann A. W. Wettability of Maleimide Copolymer Films: Effect of the Chain Length of n-Alkyl Side Groups on the Solid Surface Tension. Macromolecules 2001, 34, 6768–6775. 10.1021/ma0021592. [DOI] [Google Scholar]
- Rosen M. J.Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, 2004. [Google Scholar]










