Abstract

The design, synthesis, and physicochemical characterization of conjugates are arduous and tedious processes. Several synthetic pathways for polymeric conjugation have been reported; however, conjugation through monomers with suitable reaction conditions can be a simple and robust approach. In the present study, three different conjugates of hydrophilic N-2-hydroxypropyl methacrylamide (HPMA) and hydrophobic polycaprolactone (PCL) were synthesized. The followed synthetic pathway not only was simple and robust but also reduced the overall synthetic steps as well as harsh reaction conditions significantly. In a nutshell, three conjugates, i.e., N-2-hydroxypropyl methacrylamide and polycaprolactone (HP-PCL), n-butanol-polycaprolactone-N-2-hydroxypropyl methacrylamide (nBu-PCL-HP), and isoamyl alcohol-polycaprolactone-N-2-hydroxypropyl methacrylamide (ISAL-PCL-HP), were synthesized through this simple synthetic strategy following the monomer conjugation approach along with exhaustive spectroscopic and rheological characterization. The conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were characterized by Fourier transform infrared (FT-IR) and NMR (13C and 1H) spectroscopies. The size and ζ potential of conjugates were determined through the dynamic light scattering (DLS) method. The nBu-PCL-HP conjugate displayed a hexagonal-like shape, as evidenced by scanning electron microscopy (SEM) with an obtained size of 237.9 ± 0.21 nm. X-ray diffraction (XRD) analysis proved the crystalline nature of nBu-PCL-HP conjugates. The results of smartly synthesized conjugates intrigued us to study their flow properties in detail. Rheological evaluation resulted in their non-Newtonian type of flow with the best-fit behavior for all of the conjugates followed as per the Herschel–Bulkley and power-law models applied herein. Conclusively, the synthesized HPMA and PCL conjugates may have applications in the preparation of blends, fibers, etc. in the future. The study portrayed that the explored synthetic scheme using monomers and initiators could be a suitable approach for the synthesis of HPMA and PCL conjugates.
1. Introduction
Polymers have revolutionized the world with their state-of-art properties and applicability in biomedical sciences as well as other industries. Polymers are booming ubiquitously in every branch of science, ranging from automobiles to health care. Ironically, polymers are continuously giving progressive results in medical and industrial fields.1,2 Polymer therapeutics involve the use of conjugates and polymeric nanocarriers for drug delivery and other biomedical applications. This approach is one of the most fruitful approaches over conventional drug delivery. The two top-selling marketed products—white blood cell booster, Neulasta (a modified PEG), and Copaxone (glatiramer)—and many more are corroborating the success story of polymer therapeutics.3−5 In fact, the properties of two different polymers are usually combined by converting them into copolymers of A–B type so that the applicability of both sections of the developed copolymer can be exploited for intended therapeutic applications.6
Designing conjugates and their synthesis is a complex and arduous process, and one has to carefully fine-tune the entire steps. The right choice of solvents, moisture sensitivity, presence of impurities in the product, and interference of some acidic or basic reagents are the major drawbacks of polymerization reactions. This is mostly observed in the case of popular polymerization reactions such as reversible addition fragmentation chain transfer (RAFT), atom transfer radical polymerization (ATRP), free radical polymerization (FRP), etc.7,8 Sometimes, tuning synthetic pathways with minimum use of organic solvents may lead to the development of some newer environmental-friendly and industrially applicable materials or conjugates.
N-2-Hydroxypropyl methacrylamide (HPMA)-based conjugates have been explored extensively as carriers for the delivery of various anticancer and other drugs in the recent past.3−5 The functional groups of HPMA offer fruitful conjugation possibilities. HPMA-conjugate-based formulations have entered clinical trials due to their unique properties.9−13 PCL is a biodegradable polymer that has immense potential in therapeutic delivery and tissue engineering. The amicable, tailored, and tunable (very low glass-transition temperature and melting point) properties of PCL have provided outstanding results. The sustainable and versatile nature of PCL is the main reason for its applicability in polymer therapeutics. It is used for manufacturing scaffolds, nanofilms, nanofibers, nanoparticles, and microspheres for various applications.14,15
The different industrial applications of polymers are dependent on their economic viability plus simple and safe synthesis strategy. Merely the simpler synthetic pathway may not be the only requirement for its various applications, but other properties such as flow properties can be crucial in deciding the appropriate applicability of the synthesized polymers or their conjugates. Therefore, physicochemical properties including rheological behaviors of polymeric materials and conjugates must be studied before their intended or expected use. Rheological properties can play a crucial role in defining the physical properties of polymers and their conjugates. Flow properties are important (i) to access the viscosity of prepared conjugated systems, (ii) to examine their quality, and (iii) to identify the mechanism of drug delivery16 (if at all of these are to be used for biomedical applications). Surprisingly, there is scarcity of published literature available on the physicochemical behavior of HPMA and its conjugates. Nevertheless, many fundamental questions remained unanswered as to how the synthesized conjugates behave rheologically. What parameters will affect the viscosity or flow properties of the prepared conjugates? Considering these questions, this study was planned, which is a step ahead of our previous conjugation work related to HPMA in drug delivery.17−20
In the present study, a simple and robust conjugation strategy based on monomer conjugation17,18,21−24 was explored for the synthesis of HPMA-PCL conjugates. Three different conjugates, i.e., N-2-hydroxypropyl methacrylamide and polycaprolactone (HP-PCL), n-butanol-polycaprolactone-N-2-hydroxypropyl methacrylamide (nBu-PCL-HP), and isoamyl alcohol-polycaprolactone-N-2-hydroxypropyl methacrylamide (ISAL-PCL-HP), were synthesized. The present study also reported the use of two initiators—n-butanol (nBu) and isoamyl alcohol (ISAL)—along with ring-opening polymerization of PCL for the synthesis of HPMA-PCL conjugates. Both HPMA and PCL are potential polymers in the biomedical field; however, only limited work has been focused on synthesizing the conjugates of N-2-hydroxypropyl methacrylamide (HPMA) and polycaprolactone (PCL). Earlier evidence from benchwork studies indicated that the HPMA-PCL conjugates were prepared mostly by RAFT.25−28 The majority of these reports studied the synthesis and characterization and focused on drug delivery and biological assays in exploration of their potential applications. However, in the present study, attempts were made to report not only a robust and simple conjugation strategy but also rheological characterization of the developed conjugates (HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP) so as to explore their possible biomedical and other applications.
2. Results and Discussion
Three conjugates based on HPMA and PCL were synthesized following different synthetic pathways (Figure 1). However, N-2-hydroxypropyl methacrylamide-polycaprolactone (HP-PCL) resulted in a low percent yield of 12.26%. The reason for this may be the low reactivity of the secondary alcohol functionality present in HPMA, which fails to open the ring of ε–CL fully.18 Additionally, two initiators nBu and ISAL were explored, which resulted in high percent yields of HPMA- and PCL-based conjugates n-butanol-polycaprolactone-N-2-hydroxypropyl methacrylamide (nBu-PCL-HP) and isoamyl alcohol-polycaprolactone-N-2-hydroxypropyl methacrylamide (ISAL-PCL-HP).
Figure 1.
Synthetic schemes for (A) HP-PCL (HP-PCL), (B) n-butanol-PCL-HPMA (nBu-PCL-HP), and (C) isoamyl alcohol-PCL-HPMA (ISAL-PCL-HP) conjugates.
2.1. Characterization of HPMA, HP-PCL (N-2-Hydroxypropyl methacrylamide-Polycaprolactone) (HP-PCL), nBu-PCL-HP (n-Butanol-PCL-HPMA), and ISAL-PCL-HP (Isoamyl alcohol-PCL-HPMA) Conjugates
2.1.1. FT-IR Spectroscopy
FT-IR spectra of prepared conjugates are shown in Figure 2. In Figure 2a, absorption peaks at 2946.79 and 2866.69 cm–1 as well as at 1467.03, 1370.66, and 1467.03 cm–1 show CH stretching and bending, respectively, of the terminal methyl group present in HP-PCL. Absorption peaks at 1629.44 cm–1 and 707. 58, 731.77, 963.21, and 1106.57 cm–1 were observed for CH stretching and bending of the methylene group present at the terminal position of HPMA. In the FT-IR spectrum of HP-PCL, a peak was noticed at 3439.91 cm–1 for H-bonded alcohol, confirming that the hydroxyl group of HPMA reacted with PCL and formed an ester bond between HPMA and PCL. The absorption peak noticed at 1727.69 cm–1 suggested the presence of an amide group in the structure of HPMA. No extra peak in the absorption wavelength was observed, which could be ascribed to the lack of impurities in the synthesized HP-PCL conjugate. Hence, all of the intended absorption peaks for the HP-PCL conjugate were present.
Figure 2.
FT-IR spectra of prepared conjugates: (a) HP-PCL, (b) nBu-PCL-HP, and (c) ISAL-PCL-HP.
In the FT-IR spectrum (Figure 2b), the absorption peak was observed at 3362.41 cm–1 (H-bonded), suggesting that the hydroxyl group present in HPMA was involved in the formation of the ester bond with ε-CL. Further, absorption peaks at 1382.93, 1278.79, 1215.37, and 1164.66 cm–1 confirmed the presence of CO stretching of the ester bond. Peaks at 2973.77, 2713.25, 1382.93, and 1447.41 cm–1 were noticed for CH stretching and bending of the terminal methyl group of n-butanol. Peaks at 1608.16 cm–1 for (CH2=CH2) stretching of methylene groups and peaks at 510.92, 572.80, 599.06, and 709.23 cm–1 for methylene groups were present in the nBu-PCL chain. Absorption peaks at 1648.89 and 1692.83 cm–1 for (C=O) stretching and 1565.48 and 1608.18 cm–1 for (N–H) bending of the amide group were present in HPMA.
In Figure 2c, the FT-IR spectrum of ISAL-PCL-HP and the absorption peak at 3424.69 cm–1 (H-bonded) clearly indicated the formation of an ester bond between ISAL and PCL. The presence of an absorption peak at 1730.72 cm–1 (C=O) stretching for the ester bond indicated the successful synthesis of ISAL-PCL-HP. Further, the absorption peaks at 1262.94, 1215.50, 1193.26, and 1163.82 cm–1 showed the presence of CO stretching of the ester bond. Absorption peaks at 2935.06 and 1446.30, 1400.36 cm–1 for the CH stretching vibration band and bending of the methyl group were present in the chemical structure of ISAL-PCL. Peaks at 809.75, 942.77, 999.09, and 1063.65 cm–1 for C–H bending of methylene groups present in the ISAL-PCL were also observed. Absorption peaks at 1648.23 and 1565.88 cm–1 for (N–H) bending of the amide group were present in the HPMA spectrum, which confirmed the presence of HPMA along with the ISAL-PCL chain in ISAL-PCL-HP conjugates. The absence of newer peaks or a shift in the ISAL-PCL-HP FT-IR spectrum indicated the lack of impurities in the synthesized conjugate.
2.1.2. UV–Vis Spectroscopy
UV–Vis spectroscopy was performed for pure monomers and all of the synthesized conjugates (Figure 3). Results inferred sharp absorption peaks for HPMA and ε-CL and showed maximum absorption at wavelengths <300 and >300 nm, respectively. HP-PCL showed a sharp peak that is overlapped by HPMA, suggesting the presence of a copolymer HPMA in the conjugate HP-PCL. The presence of a carbonyl group in nBu-PCL-HP may be clearly seen at wavelength 280 nm. Conjugate ISAL-PCL-HP showed a longer absorption peak due to the presence of a carbonyl group at wavelength 320 nm. All of the characteristic peaks were intensified and clearly visible in the UV–vis spectra (Figure 3). However, NMR spectroscopy is a more reliable technique with less chances of error in characterization of conjugates.
Figure 3.
UV–visible spectra of monomers ε-CL and HPMA and synthesized conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP.
2.1.3. NMR Spectroscopy (1H and 13C)
2.1.3.1. HPMA
The HPMA copolymer was synthesized and characterized by FT-IR and NMR spectroscopies at each step (Figure S1).
2.1.3.2. HP-PCL
The 1H NMR spectrum for the HP-PCL conjugate showed characteristic chemical shifts (in ppm) at 0.83 (a, multiplet, CH2, PCL), 1.22 (b, CH2, PCL), 2.31 (c, CH2, PCL), 4.10 (d, OH, PCL), 1.60 (e, CH3, HPMA), 3.39 (h, CH2, HPMA), 7.12 (L, CH), 5.28 (f, =CH2, HPMA), and 7.66 (g, NH, HPMA), as shown in Figure 4A.
Figure 4.
1H NMR spectra of (A) HP-PCL, (B) nBu-PCL-HP, and (C) ISAL-PCL-HP conjugates.
2.1.3.3. nBu-PCL-OH, nBu-PCL-COOH, and nBu-PCL-HP
nBu-PCL-OH conjugates showed characteristic shifts in the 1H NMR spectrum. Chemical shifts values (in ppm) of 0.83 (a, CH3, nBu), 1.49 (b, multiplet, nBu), 4.17 (c, CH2 of nBu attached to the ester group of the PCL chain), 2.27 (d, CH2, PCL), 1.51 (e, L, multiplet, CH2, PCL), 1.26 (f, CH2, PCL), 1.28 (k, multiplet, CH3, nBu), 3.61 (g, CH2 attached to the alcohol end group of PCL), and 4.35 (h, the OH group of PCL) confirmed the synthesis of nBu-PCL-OH (Figure S2A). In the 13C NMR spectrum for nBu-PCL-OH, chemical shift values (in ppm) of 13.27 (a, alkyl carbon, nBu), 19.56 (b, nBu), 31.19 (c, methylene carbon, nBu), 63.35 (d, methylene carbon attached to the ester group), 176.86 (e, carbon of the ester group present in between nBu and PCL), 32.25 (f, nBu), 24.42 (g, h, CH2 of the caprolactone polymer chain), 27.55 (k, methylene group carbon, PCL polymer chain), and 60.56 (j, methylene carbon attached to the alcohol group of PCL) also confirmed the successful synthesis of nBu-PCL-OH (Figure S2B). The characteristic peaks of nBu-PCL-COOH were clearly observed in the 1H NMR spectrum (data not included), and after the confirmation of synthesis, the final step for synthesis of nBu-PCL-HP was continued. In the 1H NMR spectrum of nBu-PCL-HP, the chemical shift values (in ppm) at 0.83 (a, CH3, nBu), 1.50 and 1.53 (b, c, CH2, multiplet, nBu), 4.03 (e, CH2 attached to the ester group), 2.32 (d, k CH2, nBu), 1.13 (g, CH2, multiplet), 1.74 (f, CH2, multiplet), 1.61 (h, CH2, multiplet), 1.25 (p, CH3), 3.60 (q, CH2 attached to the amine group of HPMA), 8.22 (R, NH of HPMA), 5.63 (S, terminal CH2 of HPMA), 5.77 (y, double-bonded CH2 of HPMA), and 1.86 (t, terminal CH3 of HPMA) confirmed the successful synthesis (Figure 4B). Further confirmation was also done by 13C NMR spectroscopy. The chemical shift values observed (in ppm) at 11.41 (a, nBu) and the confirmation peaks of ester formation among nBu, PCL, and HPMA were seen at 173.85 ppm (b, c) and 168. 36 ppm (d, amide present in HPMA) (Figure S3).
2.1.3.4. ISAL-PCL-OH, ISAL-PCL-COOH, and ISAL-PCL-HP
The synthesized isoamyl-based conjugates of HP and PCL were characterized by 1H and 13C NMR spectroscopies. In the 1H NMR spectrum, ISAL-PCL-OH showed the characteristic shift for a proton of alcohol at 0.84 ppm (1, CH3), 1.66 ppm (2, CH3, multiplet, ISAL), 3.46 ppm (8, terminal OH), 4.04 ppm (3, CH2 attached to the ester group formed between ISAL and PC), 2.27 ppm (4, CH2), 1.45 ppm (5, CH2), 1.32 ppm (6, CH2), 1.38 ppm (7, CH2), and 1.52 ppm (9, multiplet, CH2) (Figure S4). The obtained peaks in 1H NMR confirmed the synthesis of ISAL-PCL-OH. For the second step, the 1H NMR spectrum of ISAL-PCL-COOH with peaks at 1.02 ppm (a, CH3), 1.86 ppm (b, multiplet, CH2), 3.05 ppm (c, CH2), 2.58 ppm (d, CH2 attached to the ester group), and 3.71 ppm (e, CH2 attached to the ester group) (Figure S5a) confirmed the synthesis. Further confirmation of the COOH group formed in the ISAL-PCL-COOH intermediate was provided by the 13C NMR spectrum (Figure S5b). The chemical shift values (in ppm) were seen at 19.15 (b) and 43.26 (e) (methylene carbon of the long polymer chain), and the confirmatory peak of carboxylic acid was observed at 168.10 ppm for ISAL-PCL-COOH.
In the 1H NMR spectrum of ISAL-PCL-HP, peaks were observed at 0.90 ppm [a] (CH3), 1.85 ppm [b] (CH), 4.06 ppm [c, h] (CH2), 2.29 ppm [e], methylene, 1.31 ppm [f] (CH2), 1.46 ppm [g] (CH2), 1.54 ppm [s] (CH2), 1.85 ppm [P] (terminal, CH3), 1.12 [L] (CH3), 5.33 and 5.63 ppm [M] (double-bonded CH2 of HPMA), which confirmed the synthesis of ISAL-PCL-HP (Figure 4C). In the 13C NMR spectrum, peaks at 21 ppm [e] (alkyl carbon), 140 ppm [J], and 24 ppm [d] (methylene carbon) were seen. Further confirmation of the prepared ester was provided by peaks at 173.21 ppm [a, b, c] (ester group), 17.8 ppm [O] (C attached to terminal alkyl), and 120 ppm [f] (CH2) for the synthesized ISAL-PCL-HP (Figure S6). The percent yield of all of the conjugates is listed in Table 1.
Table 1. Particle size, ζ Potential, Polydispersity Index (PDI), and Percent Yield (%Y) of the Synthesized Conjugates: HP-PCL, nBu-PCL-HP, ISAL-PCL-HP, ISAL-PCL, and nBu-PCL.
| sample code | conjugate | size (nm) | ζ potential | PDI | percent yield (% Y) |
|---|---|---|---|---|---|
| HP-PCL | N-2-hydroxypropyl methacrylamide-polycaprolactone | 212.12 ± 0.11 | –20.7 ± 0.01 | 0.11 ± 0.01 | 12.26 |
| nBu-PCL-HP | n-butanol-polycaprolactone- N-2-hydroxypropyl methacrylamide | 237.9 ± 0.21 | –11.60 ± 0.11 | 0.56 ± 0.31 | 96.16 |
| ISAL-PCL-HP | Isoamyl alcohol-polycaprolactone- N-2-hydroxypropyl methacrylamide | 198.6 ± 0.24 | –14.3 ± 0.13 | 0.46 ± 0.11 | 95.68 |
| ISAL-PCL | Isoamyl alcohol-polycaprolactone | 233.5 ± 0.14 | –17.40 ± 0.05 | 1.00 ± 0.01 | |
| nBu-PCL | n-butanol-polycaprolactone | 163.3 ± 0.01 | –8.26 ± 0.04 | 0.212 ± 0.23 |
The direct conjugation of HPMA and PCL resulted in a conjugate (HP-PCL) with a very low percent yield (12.26%); this may be due to the presence of the less reactive secondary alcohol group of HPMA. Therefore, an alternative method was followed for the synthesis of conjugates of HPMA and PCL through two different initiators: n-butanol (nBu) and isoamyl alcohol (ISAL). This approach resulted in a higher yield of conjugates (nBu-PCL-HP and ISAL-PCL-HP). The three conjugates were further evaluated for their rheological behavior.
2.2. Average Molecular Weight (Mn)
The structural compositions of HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were confirmed by FT-IR, 1H NMR, and 13C NMR spectroscopies. The number average molecular weight (Mn) of these synthesized conjugates was calculated by substituting the appropriate values obtained in eqs 1 and 2. Using salient features of 1H NMR spectra (Figure 4A–C), the monomer units obtained in HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were 3.03, 5.49, and 6.39, respectively (Table 2). Similarly, the Mn values of the synthesized conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were calculated to be 782.16, 2374.58, and 2857.41 g/mol, respectively. The Mn value calculated for HP-PCL was less than the Mn value for nBu-PCL-HP and ISAL-PCL; thus, the synthesized HP-PCL can be considered as an oligomer. Mn values of HP-PCL, nBu-PCL, nBu-PCL-HP, ISAL-PCL, and ISAL-PCL-HP are listed in Tables S1–S5. Although SEC and GPC can be the best methods to determine the molecular weights of the synthesized conjugates, the NMR-signal-based methods also give an idea about the number average molecular weight (Mn) of the conjugates. The degree of polymerization (DP) values calculated for HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were found to be 5.431, 32.03, and 32.41, respectively.
Table 2. Number Average Molecular Weight (Mn) of the Synthesized Conjugates Calculated Using Integral Values of Protons from 1H NMR Spectra.
| conjugate | monomer units | Mn by NMR (g/mol) | repeating units | end groups |
|---|---|---|---|---|
| HP-PCL | 3.03 | 782.16 | e, f, g, h, L, K | a, b, c |
| nBu-PCL-OH | 8.35 | 1571.97 | d, l, e, f, g, k | a, b, c, k |
| nBu-PCL-HP | 5.49 | 2374.58 | e, f, g, k | a, b, c, d, p, q, s, t |
| ISAL-PCL-OH | 4.33 | 875.91 | 4, 5, 6, 7, 8 | 1, 2, 3, 9 |
| ISAL-PCL-HP | 6.39 | 2857.41 | e, f, g, h | a, b, s, L, M, P |
2.3. Solubility Study
A qualitative solubility analysis of the synthesized conjugates was performed. HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were dissolved in water and different organic–inorganic solvents. HP-PCL and nBu-PCL-HP were soluble in water, while ISAL-PCL-HP was partially soluble in water; however, using a cosolvent, the solubility can be improved. In organic solvents, such as 1,2-dioxane, dry DCM, hexane, etc., the conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were freely soluble. A detailed solubility analysis of conjugates is reported in Table 3. Phase solubility analysis studies gave an insight into the aqueous/nonaqueous solubility of synthesized conjugates. This data may be helpful for future applicability of conjugates.
Table 3. Solubility of Prepared Conjugates: HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP in Different Solventsa.
| conjugate |
|||
|---|---|---|---|
| solvents | HP-PCL | nBu-PCL-HP | ISAL-PCL-HP |
| dichloromethane (DCM) | + | + | + |
| methanol | + | + | ± |
| acetone | ± | – | – |
| ethanol | + | + | + |
| 1,4 dioxane | + | + | + |
| hexane | + | + | + |
| water | + | + | ± |
+, soluble; −, insoluble; ±, partially soluble.
2.4. Physical Characterization
2.4.1. Size, ζ Potential, and PDI (Polydispersity Index)
The sizes of the prepared conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were found to be 212.12 ± 0.11, 237.9 ± 0.21, and 198.6 ± 0.24 nm, respectively (Table 1). The negative ζ potential observed might be due to the presence of ester groups in the synthesized conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP. PDI values for HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP were 0.11 ± 0.01, 0.56 ± 0.31, and 0.46 ± 0.11, respectively (Table 1). The low PDI indicates a better homogeneity and a uniform distribution. Conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP showed narrow polymer dispersity values of 0.11 ± 0.01, 0.56 ± 0.31, and 0.46 ± 0.11, respectively. Conjugates prepared by the monomer approach exhibited a very low polymer dispersity and suggested that the prepared copolymer system may be beneficial in therapeutic applications. However, the conjugates prepared by RAFT and FRP polymerization methods show a high dispersity index.29 High polymer dispersity causes hindrance in the clinical applicability of polymeric therapeutics. Size and ζ potential measurements were interpreted by the DLS method.
2.5. Electron Microscopy (EM)
The surface morphology of synthesized conjugates (HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP) was analyzed through SEM (Figure 5). SEM images of HP-PCL indicated the agglomerated particles of conjugates in the form of sheets (Figure 5A). In Figure 5B, SEM images of nBu-PCL-HP indicated the hexagonal-shaped particles’ presence, which were uniformly distributed on the surface with no cluster formation. The size of these particles was in the range of 400–500 nm. SEM results indicated that the particle size was approximately equal for nBu-PCL-HP and ISAL-PCL-HP. However, a nonuniform particle size is depicted in Figure 5C for ISAL-PCL-HP but less agglomerated than HP-PCL conjugates.
Figure 5.
Scanning electron microscopy (SEM) images of conjugates (A) HP-PCL (scale bar of (I) 50 μm and (II) 10 μm), (B) nBu-PCL-HP (scale bar of (I) 400 nm and (II) 500 nm), and (C) ISAL-PCL-HP (scale bar of (I) 5 μm and (II) 4 μm).
2.6. XRD (X-Ray Diffraction)
The powdered XRD patterns for nBu-PCL-HP and HPMA are shown in Figure 6A,B, respectively. HPMA is a crystalline copolymer, which showed sharp peaks at 2θ of 16.2, 19.60, 21.10, 22.86, 26.32, 30.1, 31.8, 36.01, 44.9, and 48.2°. However, the XRD pattern of powdered nBu-PCL-HP showed some sharp peaks at 14.6, 22.1, 24.0, 33.3, and 46.8° and revealed a crystalline nature for the nBu-PCL-HP conjugate. The observed sharp peaks may be an indication of the crystalline state of HPMA that remained unchanged during the synthesis of the nBu-PCL-HP conjugate. Also, the peaks observed were a clear indication of the crystalline nature of HPMA, which did not change much in the synthesized nBu-PCL-HP conjugate. Sharp peaks were observed at 33.3 and 46.8°, suggesting that the crystalline nature of nBu-PCL-HP might be due to the presence of HPMA, which is crystalline in nature.
Figure 6.
XRD pattern of the synthesized hexagonal-shaped conjugate (A) nBu-PCL-HP and the pure copolymer (B) HPMA.
2.7. Rheological Evaluations
Rheology of polymers defines important characteristics, which helps in elucidating polymer production in industries. The purpose of rheological evaluation was to determine the effect of applied forces on the flow properties of the three synthesized conjugates. The difference in the structures of the prepared conjugates can be determined by a viscosity measurement as a function of the shear rate. Viscosity determination is the prime requisite step for designing new polymeric materials. The rheological analysis of conjugates was performed for monomers HPMA and ε-CL and conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP. Samples were prepared as an aqueous solution with a concentration of 1 mg/mL. Two rheological models were applied—the Herschel–Bulkley model and the power-law model—for the determination of flow characteristics of conjugates. The prepared conjugates showed a shear-thinning behavior (Figure 7) in the diluted form.30,31
Figure 7.
Viscosity vs shear rate as a function of a 1 mg/mL solution for HPMA, PCL, and prepared conjugates (HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP) analyzed at (A) 25 and (B) 40 °C.
2.7.1. Viscosity vs Shear Rate
First, the effect of shear rate on the viscosity of the prepared conjugates was investigated. In the flow curves (Figure 7), a decrease in the viscosity of conjugates with increasing applied shear rate was noticed. The decrease in the viscosity of HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP conjugates was more than in the monomers HPMA and PCL. This may be ascribed to the fact that the rheology of the prepared conjugates was dependent on the molecular weight, molecular weight distribution, and the branching in the structure. The linear shape of conjugates may be responsible for the reduced viscosity, which also helps in their mobility. At 25 °C, the HP-PCL conjugate showed a viscosity of 6.67 Pa at a low shear rate, and as the shear rate increased, the viscosity started decreasing. In the case of nBu-PCL-HP, a viscosity of 9.32 Pa was observed (Figure 7A) at a shear rate of 1 s–1. Similarly, ISAL-PCL-HP showed a viscosity of 14.33 Pa at a shear rate of 1 s–1. For all three conjugates, a fall in viscosity was noticed with an increasing shear rate. At an elevated temperature of 40 °C, a decrease in viscosity could be seen for the prepared conjugates (Figure 7A). A very low viscosity of 1.04 Pa was found in the case of nBu-PCL-HP, while ISAL-PCL-HP and HP-PCL showed viscosities of 8.70 Pa and 5.41 Pa at a shear rate of 1 s–1. These observations revealed that the nBu-PCL-HP and ISAL-PCL-HP conjugates offered a very low resistance to the flow. This may be more advantageous for film coating or manufacturing of other polymeric materials.
The Herschel–Bulkley and power-law model results with correlation coefficient values (R2) at 25 and 40 °C are given in Table 4.
Table 4. Correlation Coefficient (R2) Values in Different Rheological Models Obtained for HPMA and Conjugates at 25 and 40 °C.
| HP-PCL |
nBu-PCL-HP |
ISAL-PCL-HP |
HPMA |
|||||
|---|---|---|---|---|---|---|---|---|
| models | 25 °C | 40 °C | 25 °C | 40 °C | 25 °C | 40 °C | 25 °C | 40 °C |
| Herschel–Bulkley | 0.9745 | 0.9809 | 0.9599 | 0.9992 | 0.9235 | 0.9646 | 0.9921 | 0.9975 |
| power law | 0.4709 | 0.8977 | 0.7183 | 0.008 | 0.6821 | 0.8762 | 0.7423 | 0.4151 |
In the Herschel–Bulkley model, nBu-PCL-HP (R2 = 0.9599) and HP-PCL (R2= 0.9745) were better fitted than ISAL-PCL-HP (R2= 0.9235) at 25 °C. At an elevated temperature of 40 °C, the conjugates nBu-PCL-HP (R2 = 0.9992), HP-PCL (R2 = 0.9809), and ISAL-PCL-HP (R2 = 0.9646) were also best fitted. The Herschel–Bulkley and power-law model results with correlation coefficient values (R2) for copolymers HPMA and PCL were also calculated (Table 4) at 25 and 40 °C. The significant property of the synthesized conjugates is their shear-thinning behavior, which can be noticed in (Figure 7). It was observed that as the shear rate increased, the viscosity of the prepared conjugates decreased. Results suggested that the conjugates showed non-Newtonian behavior at 25 and 40 °C. The nonlinear graph plotted between the log shear stress and the log shear rate for the Herschel–Bulkley model suggested that nBu-PCL-HP follows a non-Newtonian flow. The flow index values of conjugates were calculated from both of these models as mentioned in Table S6. Similarly, in the power-law model, the nonlinear graph plotted between the log viscosity and the log shear rate described the non-Newtonian flow of nBu-PCL-HP and ISAL-PCL-HP conjugates. Results indicated a low resistance in the flow properties of HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP, which may be due to the lack of chain entanglement of HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP conjugates as they are linear in structure. The significant decrease in viscosity suggested that the nBu-PCL-HP and ISAL-PCL-HP conjugates possessed a high molecular mobility in their structural arrangements.32 It can also be concluded that the decrease in viscosity is an indication of the shear-thinning behavior of the synthesized conjugates.
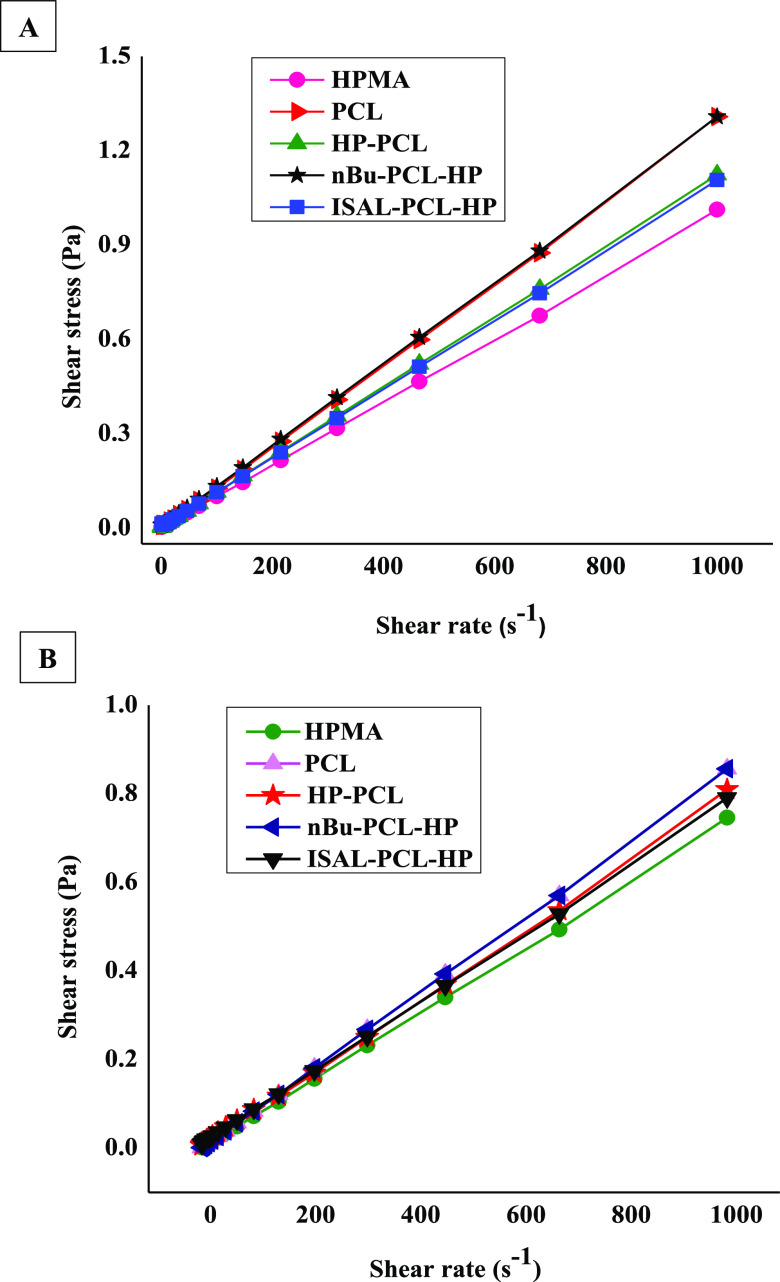
2.7.2. Shear Stress vs Shear Rate
A rheogram plotted between the shear stress and the shear rate depicts (in (Figure 8)) the analysis of conjugates at temperatures of 25 and 40 °C. It was noticed that with the increase in shear rate, the shear stress also increased for all of the prepared conjugates at both temperatures. The appearance of a rheogram was also in a similar fashion. The graph obtained between the shear rate and shear stress seems to be directly proportional, and this indicates that the flow behavior followed by the polymers is of a Newtonian nature. However, when other parameters such as viscosity and shear rate were evaluated and compared, as shown in (Figure 7), the flow behavior of all three conjugates was observed to be non-Newtonian in nature. It can also be observed that the shear-thinning or shear-thickening behavior of polymers initially seems to be like a Newtonian flow; however, later on, the curve turns into such a curvature that decides whether it is a pseudoplastic or dilatant flow behavior of a non-Newtonian nature. Initially, it seems to be Newtonian, and this may be the case with the curve observed in Figure 8A,B for the synthesized conjugates in the present study.
Figure 8.
Shear stress vs shear rate as a function of concentration for 1 mg/mL solutions for HPMA, PCL, and prepared conjugates (HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP) analyzed at (A) 25 and (B) 40 °C.
2.7.3. Torque vs Shear Rate
The rheograms between the shear rate and torque were also plotted at 25 and 40 °C for the synthesized conjugates HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP, as shown in (Figure 9A,B). A similar pattern was observed in both of the rheograms by the synthesized conjugates, which suggested that with the increase of shear rate, the torque also increased.32
Figure 9.
Torque vs shear rate as a function of solutions (1 mg/mL) for HPMA, PCL, and prepared conjugates (HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP) analyzed at (A) 25 and (B) 40 °C.
To further investigate the effect of temperature on the viscosity of the synthesized conjugates, a comparison of rheograms for all of the conjugates was also performed, as shown in (Figure 10). The result of this comparison for HP-PCL at 25 °C showed a sharp decrease in viscosity from 6.67 to 1.87 Pa with an increase in the shear rate (Figure 10A), while at an elevated temperature of 40 °C, the decrease in viscosity was not sharp but gradual. The rheogram plotted for nBu-PCL-HP (Figure 10B) at 25 °C showed a decrease in viscosity from 9.32 to 6.56 Pa with increasing shear rate. However, at an elevated temperature of 40 °C, a sharp decrease in viscosity from 1.03 to 0.77 Pa was observed with increasing shear rate. However, at 40 °C, the viscosity of nBu-PCL-HP was only 1.03 Pa at a shear rate of 1 s–1, but at 25 °C, the viscosity was 9.32 Pa, which was more than the viscosity noticed at 25 °C for the same conjugate. This observation indicated that at a physiological temperature of 37 °C (close to 40 °C), the resistance in flow of nBu-PCL-HP is very low, and this can be beneficial for its use in the preparation of polymer blends and polymer biofilms in the future. Further, the viscosity of ISAL-PCL-HP at 25 °C also decreased from 14.33 to 9.54 Pa at shear rate 1 s–1(Figure 10C). However, at 40 °C, ISAL-PCL-HP showed a viscosity of 8.70 Pa, which was 1.64 times less than the viscosity noticed at 25 °C (Figure 10C). The results concluded that ISAL-PCL-HP and nBu-PCL-HP could provide a low resistance in the flow of the polymeric material prepared from these conjugates and can act as a better candidate for the preparation of polymeric materials based on ISAL-PCL-HP and nBu-PCL-HP in the future.
Figure 10.
Comparative rheogram of viscosity vs shear rate for the prepared conjugates: (A) HP-PCL, (B) nBu-PCL-HP, and (C) ISAL-PCL-HP at 25 and 40 °C.
In the present study, the rheological behavior of the prepared conjugates was exhaustively investigated and the Herschel–Bulkley model and the power-law model were applied to determine the flow characteristics. The significant outcomes of this research work encouraged us to use these conjugates for the preparation of polymeric films, blends, etc. in the future for biomedical purposes.
3. Conclusions
In this study, the HPMA-PCL conjugates were synthesized, exhaustively characterized, and evaluated for rheological properties. Our study explored and analyzed the simple synthetic pathways of HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP conjugates using two monomers and initiators, i.e., nBu and ISAL. Selection of the monomers was a crucial step for the present synthesis. The selected two monomers (HPMA and ε-CL) and two initiators (nBu and ISAL) are being reported in this study for the first time. The direct conjugation approach of synthesizing HP-PCL resulted in the low percent yield. Therefore, we planned to explore an alternative synthetic route that can result in better percent yields. UV–visible, FT-IR, and NMR spectroscopies were used for the characterization and confirmation of synthesis at each step. The Mn value for the synthesized conjugates was calculated using the 1H NMR measurements. Size, ζ potential, and surface morphology of the prepared conjugates were also studied and reported. The DLS and SEM results showed that the prepared nBu-PCL-HP conjugate was of size 237.9 ± 0.21 nm (with ζ potential −11.60 ± 0.11) and was hexagonal in shape, while HP-PCL and ISAL-PCL-HP showed the presence of some agglomerates.
The added rheological studies contribute toward the knowledge on how the conjugates and their flow properties vary at different temperature conditions. To the best of our knowledge, no study has been reported related to the exhaustive spectroscopic, surface morphology, and rheological characterization of HPMA and PCL conjugates in terms of viscosity, shear stress, shear strain, size, and ζ potential. The influence of temperature on the sensitivity of conjugates (from the flow analysis point of view) suggested a decrease in viscosity with increasing shear rate at 25 and 40 °C. Rheology results provided detailed information on the deformation in conjugates. From the rheology results, it can be contemplated that the flow behavior is directly connected to the chemical structure, molecular weight, molecular weight distribution, and branching of the synthesized conjugates. We can conclude that the synthesized nBu-PCL-HP and ISAL-PCL-HP conjugates may be suitable materials for the preparation of polymer blends, polymer melts, polymer films, etc. A comprehensive support for this conclusion was obtained from all sets of experiments. In further protocols, the in vitro anticancer studies conducted in different cancerous cell lines have also given promising and positive results (unpublished data). The results (unpublished data) of these studies are encouraging and significant for possible biomedical applications of these conjugates in future applications. This also indicated the potential use of these conjugates in drug delivery and in other biological applications. Conclusively, the synthesized conjugates of biodegradable materials can be modulated into polymeric nanofibers in the future for various applications.
4. Experimental Section
4.1. Materials
N-2-Hydroxypropyl methacrylamide (HPMA), ε-caprolactone (ε-CL), 4-dimethyl aminopyridine (DMAP), stannous octoate (SnOct)2, acetonitrile (ACN), and diethyl ether were purchased from Sigma-Aldrich. Isoamyl alcohol (ISAL), n-butanol (nBu), 1,2-dioxane, dichloromethane (DCM), acetone, methanol, N, N-dicyclohexyl carbodiimide (DCC), 1-ethyl-3-(dimethylaminopropyl) carbodiimide (EDC), and succinic anhydride (SA) were supplied by CDH Chemicals Pvt. Ltd. India. Dialysis membranes of 50 and 150 KDa were purchased from Hi-Media, India. DCM and 1,2-dioxane were dried before use by a conventional method. All of the other chemicals were used as such as those were received without further purification.
4.1.1. Instruments
All 1H and 13C NMR spectra were recorded using a Bruker Ascend 500 MHz NMR spectrometer (Switzerland). FT-IR analysis was performed using the ATR mode (Perk, M/s Perkin Elmer Co., Waltham, Massachusetts). A UV–visible spectrophotometer (Agilent, Cary UV-Visible India) was used to analyze the prepared samples. The flow behavior of the synthesized conjugates was determined through a rotational modular compact rheometer (air-bearing MCR300, Anton Paar).
4.2. Syntheses of HPMA, HP-PCL (N-2-Hydroxypropyl methacrylamide-polycaprolactone) (HP-PCL), nBu-PCL-HP (n-Butanol-PCL-HPMA), and ISAL-PCL-HP (Isoamyl alcohol-PCL-HPMA) Conjugates
4.2.1. HPMA
The HPMA copolymer was synthesized as per a previously reported protocol33 and characterized by FT-IR17,19 and NMR spectroscopies at each step (Figure S1).
4.2.2. HP-PCL Conjugate
HP-PCL was synthesized (Figure 1A) by conjugating HPMA (N-2-hydroxypropyl methacrylamide) with the ε-CL monomer through the ring-opening polymerization reaction (ROP). Briefly, a mixture of HPMA (250 mg, 1 equiv, 1.76 mmol) and ε-CL (288.34 μL, 1.5 equiv, 1.44 mmol) in toluene was added in a round-bottom flask (RBF) and purged to provide inert conditions to the reaction. Then, Sn(Oct)2 was added and the reaction was allowed to proceed for 24 h at 65 °C. At the end of the reaction, TLC (thin-layer chromatography) was performed in 10% methanol/DCM to monitor the reaction. Finally, a white-colored powdered product was obtained with a low percent yield. The synthesis of HP-PCL was confirmed at each step by FT-IR and NMR spectroscopies. UV–visible spectroscopy of the prepared conjugate was also performed.
4.2.3. nBu-PCL-HP Conjugate
The synthesis of nBu-PCL-HP was performed in three steps. In the first step, nBu-PCL-OH (n-butanol-PCL-OH) was synthesized by ROP and thereafter an intermediate (nBu-PCL-COOH) was prepared and then the final conjugate (Figure 1B), i.e., nBu-PCL-HP (n-butanol-polycaprolactone-HPMA), was synthesized.
4.2.3.1. nBu-PCL-OH
In the first step of the synthesis, nBu (initiator) (194.16 μL, 1 equiv, 2.61 mmol), ε-CL (158.44 μL, 1 equiv, 1.38 mmol), and Sn(Oct)2 (57 μL, 0.1 equiv, 0.14 mmol as the catalyst) were reacted by ROP under an inert atmosphere.34 The reaction mixture was refluxed at 115 °C for 24 h. TLC was carried out in 10% methanol/DCM to monitor the reaction. Finally, an off-white-colored waxy product was obtained. It was purified by adding chilled diethyl ether thrice, and then, a white-colored semisolid product was obtained. The synthesis of the nBu-PCL-OH conjugate was confirmed by FT-IR and NMR spectroscopies.
4.2.3.2. nBu-PCL-COOH
In the next step, nBu-PCL containing an end-functionalized carboxylic acid group was synthesized utilizing SA. Briefly, an SA solution and nBu-PCL-OH (352 mg, 1 equiv, 1.86 mmol) were dissolved in anhydrous DCM and dry dioxane was mixed to maintain the inert conditions. Then, DMAP was added dropwise to the reaction mixture under continuous stirring over a period of 1 h. After DMAP addition, the reaction assembly was kept in an inert atmosphere for 24 h. After completion of the reaction, an off-white solid product was obtained. It was purified by washing with chilled diethyl ether thrice, and DMAP impurities were removed by the dialysis method with continuous replenishing of water from a beaker every 12 h for two days. The structure of the synthesized nBu-PCL-COOH, an intermediate, was confirmed by FT-IR, 1H NMR, and 13C NMR spectroscopies at each synthetic step.
4.2.3.3. nBu-PCL-HP
The nBu-PCL-HP conjugate was synthesized (Figure 1B) with some modifications to the previously reported protocol.35 Briefly, nBu-PCL-COOH (242 mg, 1 equiv, 0.84 mmol) and HPMA (278.78 mg, 1 equiv, 1.93 mmol) were dissolved in 2.5 mL of anhydrous DCM. Then, a solution of DCC and DMAP in dry DCM was added to the reaction mixture over a period of 1 h at room temperature. After 24 h, the reaction was assessed by TLC in a 10% methanol/DCM solution. At the end of the reaction, a creamy waxy solid was obtained, which was washed thrice with cold diethyl ether to remove organic impurities like DMAP by dialysis. The obtained percent yield of nBu-PCL-HP was 96.16%. The synthesized conjugate was characterized by FT-IR and NMR spectroscopies at every step.
4.2.4. ISAL-PCL-HP Conjugate
The synthesis of ISAL-PCL-HP was accomplished following a three-step process. In the first step, ISAL-PCL-OH (isoamyl alcohol-PCL-OH) was synthesized following the ROP method. In the second step, ISAL-PCL-COOH was synthesized followed by the synthesis of the final step where ISAL-PCL-HP was prepared and characterized.
4.2.4.1. ISAL-PCL-OH
In this reaction, ISAL-PCL having an alcohol group was first synthesized using ring-opening polymerization (ROP) of ε-CL and isoamyl alcohol (ISAL) as an initiator (FDA-approved).36 ISAL (100 mg, 1 equiv, 1.13 mmol) was added into ε-CL (377.13 μL, 3 equiv, 3.3 mmol) in the presence of Sn(Oct)2 (54.28 μL, 0.1 equiv, 0.13 mmol), and all of the reactants were degassed before the reaction. Then, the reaction mixture was refluxed in an oil bath at 115 °C for 24 h. TLC was performed in 10% methanol/DCM to check the reaction status. The obtained off-white solid product was dialyzed in a dialysis membrane (5 KDa) with a continuous change of distilled water every 12 h to remove impurities. The purified compound was characterized by NMR spectroscopy.
4.2.4.2. ISAL-PCL-COOH
The synthesized ISAL-PCL conjugate was further conjugated to SA to produce an intermediate. In this reaction, degassing was done to provide an inert atmosphere to the chemical reaction. ISAL-PCL was dissolved in dry DCM, and SA (108 mg, 1 equiv, 1.07 mmol) was added in dry dioxane. Then, DMAP (28.2 mg, 1.5 equiv, 0.23 mmol) dissolved in DCM was added dropwise. The whole assembly was kept under a nitrogen atmosphere for 24 h at 40 °C. The obtained product was white in color and dialyzed in a dialysis membrane of 5 KDa to remove DMAP impurities.37 SA conjugation was performed to provide a carboxylic group that will facilitate the conjugation of ISAL-PCL with HPMA.
4.2.4.3. ISAL-PCL-HP
Finally, the ISAL-PCL-HPMA conjugate was synthesized by reacting an intermediate ISAL-PCL-SA with HPMA. Degassing was done to provide an inert atmosphere. Briefly, ISAL-PCL-SA (56.5 mg, 1 equiv, 1.13 mmol) was dissolved in DCM, and HPMA was added. DCC (19.52 mg, 1 equiv, 0.09 mmol) dissolved in DCM was added dropwise to initiate the reaction.33 Finally, DMAP (13.23 mg, 1 equiv, 0.1 mmol) was added to the reaction assembly. Reaction progress was analyzed by TLC (10% methanol/DCM solution). The obtained creamy waxy product with a percent yield of 95.68% was washed with cold diethyl ether thrice (Figure 1C) and purified by the dialysis membrane method simultaneously and finally stored for future use in a deep freezer (−80 °C).
4.3. Characterization
The synthesized conjugates (HP-PCL, nBu-PCL-HP, ISAL-PCL-HP) were characterized by FT-IR and NMR spectroscopies to confirm the synthesis. UV–vis spectrophotometry was also performed for the prepared conjugates.
4.3.1. FT-IR (Fourier Transform Infrared) Spectroscopy
FT-IR spectra were recorded in the attenuated total reflectance (ATR) mode by the Perkin Elmer instrument. The scan range of 500–4000 cm–1 was set with a resolution of 4 cm–1, and 64 scans were averaged. The detector and sample chamber were purged with dry, CO2-free air to avoid interference from CO2 and moisture.
4.3.2. UV–Visible Spectroscopy
The prepared conjugates were characterized by a UV–visible spectrophotometer (UV–vis Perkin Elmer). The samples were dissolved in DMSO at room temperature and sonicated for 2–4 min each for proper mixing.11 Samples were scanned in a UV–visible spectrophotometer.
4.3.3. NMR Spectroscopy (1H and 13C) and Molecular Weight Determination
1H and 13C NMR spectra were recorded in a Bruker 500 MHz spectrometer (Switzerland). Samples were dissolved in CDCl3, DMSO-d6, or D2O at room temperature depending on the solubility. All of the chemical shifts were recorded in ppm relative to TMS in 1H and 13C NMR spectra as the internal standard. The number average molecular weights (Mn) of the synthesized HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP conjugates were calculated using 1H NMR spectral data following a previously reported method.38,39 The average chain length of the conjugates was calculated following the below-mentioned formula (eq 1) as reported in the literature.38,39
| 1 |
The integration for end groups was calculated for synthesized conjugates by simplifying the equation (eq 2)
| 2 |
where Ii and pi are the integration and the number of protons associated with the ith conjugate signal, respectively, m is the number of conjugate signals used, IE and pE are the integration and number of protons associated with the end group, respectively, and nP indicates the number of monomer units present in the synthesized conjugates. The degree of polymerization (DP) for the synthesized conjugates was also calculated by dividing the number average molecular weight with the molecular weight of the monomer unit.40
4.4. Solubility Analysis
Solubility is the property of a material to get dissolved in organic and inorganic solvents. The hydrophobic and hydrophilic parts of copolymers affect the solubility of prepared HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP conjugates. To investigate the solubility of these conjugates, different solvents were selected.27 The phase solubility analysis method was followed to elucidate the solubility.
4.5. Particle Size, ζ Potential, and Polydispersity Index (PDI)
Synthesized conjugates were characterized by a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Zetasizer is based on the principle of photon correlation spectroscopy (PCS), where the average particle size was calculated by measuring the width of particle size distribution. The samples were suspended in 1 mL of deionized water, and the solutions were filtered by a syringe filter (0.22 μm) before measurement.
4.6. Surface Morphology
The surface morphology of the prepared amphiphilic conjugates was analyzed by a scanning electron microscope (EVO 18, ZEISS) at a magnification of 10 000×. Vacuum-dried samples were placed on copper grid using double-edged carbon tape, and gold coating of 10 nm was applied prior to analysis with a spinner system for 5–10 min.41 After that, samples were placed in a scanning electron microscope, and a working distance of 5 mm was used with a 5 kV accelerating voltage and a 3 nm spot size. Images were captured using a Nova nanoSEM field emission scanning electron microscope.
4.7. XRD (X-ray Diffraction)
XRD was used to investigate the crystallographic structures of HPMA and the prepared conjugates (nBu-PCL-HP) to examine the purity of the synthesized conjugate.42 HP-PCL and ISAL-PCL-HP were not tested for XRD studies due to their waxy nature, leading to a sticky end product, which was not suitable for XRD analysis. Wide-range X-ray diffraction measurements were carried out using a PANalytical Empyrean, equipped with a Cu Kα radiation source. The X-rays were generated at 40 mA and 45 kV. Data were obtained from 5 to 60° (diffraction angle 2θ) at a step size of 0.01 °C and a scanning speed of 5° per min radiation. For optimum results, a minimum of two analyses were performed for each sample. All of the measurements were taken at room temperature.
4.8. Rheological Studies
Rheology was performed to study the flow behavior of synthesized conjugates; HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP for various rheological parameters like viscosity, shear stress, etc. as a function of concentration and temperature. The samples were prepared with a concentration of 1% w/v in distilled water using a bath sonicator and then analyzed through a rotational modular compact rheometer (air-bearing MCR300, Anton Paar). Samples were equilibrated at 25 ± 0.1 and 40 ± 0.1 °C for 5 min before measurements. The viscosity was measured with varying shear rates from 1 to 1000 (s–1) in a logarithmic fashion with the data acquisition time varying in a logarithmic scale from 10 to 5 s at 25 and 40 °C. The measurements were carried out on MCR 702 with a P-PTD 200 base plate and CP50-1 geometry. The gap was set to 0.11 mm. The viscosities of the conjugates (HP-PCL, nBu-PCL-HP, and ISAL-PCL-HP) were measured at two temperatures (25 and 40 °C) as a function of the shear rate in an upward sweep from 1 to 1000 s–1 by measuring at 20 points with a gradual decrease in the temperature. Observations were collected, recorded, and analyzed using Rheoplus V.3.61 software (Anton Paar). All of the measurements were done in triplicate. The Herschel–Bulkley model and the power-law model were used to investigate the rheological behavior of the synthesized conjugates in an aqueous solution with a concentration of 1 mg/mL. The data of rheological measurements were fitted to the Herschel–Bulkley model (eq 3) and the power-law model (eq 4), which were used to determine the flow behavior of samples.26,29,39 The models used for non-Newtonian fluid identification can be given by the following equations
| 3 |
| 4 |
where “a” is the shear stress, “b” is the strain rate, “y°” is the yield stress of the material, “K” is the consistency factor, and “n” is the flow index. Indeed, the behavior of a fluid is dependent on these three Herschel–Bulkley parameters.
Acknowledgments
This work was supported by the University Grants Commission (UGC) New Delhi, India, and the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), New Delhi, India, through funding to Dr. U.G. The authors would like to acknowledge Mayank Varshney for rheological studies (Anton Paar). The authors are thankful to Mr. Sukruth for measurements of rheological parameters. The authors acknowledge Dr. Satish Shilpi, Ravishankar College of Pharmacy, Bhopal, India, for lyophilization-related studies and Komal Kanwar from the Department of Physics, Central University of Rajasthan, for XRD analysis. The authors acknowledge the facility and support provided by the Malaviya National Institute of Technology (MNIT), Jaipur, India, for the particle characterization (such as size and morphology analyses).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04243.
FT-IR, 1H NMR, and 13C NMR spectroscopies of monomers and intermediates prepared during synthesis; calculated average molecular weight (Mn) values of these intermediates or conjugates; and flow index of the synthesized conjugates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Peters W.; Brandon H.; Jerina K. L.; Wolf C.; Young V. L.. The History of Biomaterials Used for Breast Augmentation. In Biomaterials in Plastic Surgery; Breast Implants, Woodhead Publishing: Cambridge, UK, 2012; pp 1–39. [Google Scholar]
- Curtis J.; Colas A.. Medical Application of Silicones. In Biomaterials Science, 3rd ed.; Ratner B. D.; Hoffman A. S.; Schoen F. J.; Lemons J. E., Eds.; Academic Press: Oxford, UK, 2013; pp 1106–1116. [Google Scholar]
- Duncan R. The Dawning Era of Polymer Therapeutics. Nat. Rev. Drug Discovery 2003, 2, 347–360. 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Liu X. M.; Miller S. C.; Wang D. Beyond Oncology Application of HPMA Copolymers in Noncancerous Disease. Adv. Drug Delivery Rev. 2010, 62, 258–271. 10.1016/j.addr.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. Polymer Therapeutics: Top 10 Selling Pharmaceuticals-What next?. J. Controlled Release 2014, 190, 371–380. 10.1016/j.jconrel.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Petrova S.; Klepac D.; Konefał R.; Kereiche S.; Kovaacik L.; Filippov S. K. Synthesis and Solution Properties of PCL-b-PHPMA Di-copolymers Containing Stable Nitroxyl Radicals. Macromolecules 2016, 49, 5407–5416. 10.1021/acs.macromol.6b01187. [DOI] [Google Scholar]
- Stenzel M. H. RAFT Polymerization: An Avenue to Functional Polymeric Micelles for Drug Delivery. Chem. Commun. 2008, 2008, 3486–3503. 10.1039/b805464a. [DOI] [PubMed] [Google Scholar]
- Tsarevsky N. V.; Matyjaszewski k. “Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev. 2007, 107, 2270–2299. 10.1021/cr050947p. [DOI] [PubMed] [Google Scholar]
- Vasey P. A.; Kaye S. B.; Morrison R.; Twelves C.; Wilson P.; Duncan R.; Thomson A. H.; Murray L. S.; Hilditch T. E.; Murray T.; Burtles S.; Fraier D.; Frigerio E.; Cassidy J. Phase I Clinical and Pharmacokinetic Study of PK1 [N-(2- Hydroxypropyl) methacrylamide Copolymer Doxorubicin]: First Member of a New Class of Chemotherapeutic Agents Drug-Polymer Conjugates. Clin. Cancer Res. 1999, 5, 83–94. [PubMed] [Google Scholar]
- Rademaker-Lakhai J. M.; Terret C.; Howell S. B.; Baud C. M.; Boer R. F. D.; Pluim D.; Beijnen J. H.; Schellens J. H. M.; Droz J. P. A. Phase I and Pharmacological Study of the Platinum Polymer AP5280 Given as An Intravenous Infusion Once Every 3 Weeks in Patients with Solid Tumors. Clin. Cancer Res. 2004, 10, 3386–3395. 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]
- Seymour L. W.; Ferry D. R.; Anderson; Hesslewood S.; Julyan P. J.; Poyner R.; Doran J.; Young A. M.; Burtles S.; Kerr D. J. Hepatic Drug Targeting: Phase I Evaluation of Polymer Bound Doxorubicin. J. Clin. Oncol. 2002, 20, 1668–1676. 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- Seymour L. W.; Ferry D. R.; Kerr D. J.; Rea D.; Whitlock M.; Poyner R.; Boivin C.; Hesslewood S.; Twelves C.; Blackie R.; Schatzlein A.; Jodrell D.; Bissett D.; Calvert H.; Lind M.; Robbins A.; Burtles S.; Duncan R.; Cassidy J. Phase II Studies of Polymer-Doxorubicin (PK1, FCE28068) in the Treatment of Breast, Lung and Colorectal Cancer. Int. J. Oncol. 2009, 34, 1629–1636. 10.3892/ijo_00000293. [DOI] [PubMed] [Google Scholar]
- Nowotnik D. P.; Cvitkovic E. A Review of the Development of an HPMA DACH Platinum Polymer Therapeutic. Adv. Drug Delivery Rev. 2009, 61, 1214–1219. 10.1016/j.addr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Dash T. K.; Konkimalla V. B. Polymeric Modification and Its Implication in Drug Delivery: Poly-ϵ-Caprolactone (PCL) as a Model Polymer. Mol. Pharmceutics 2012, 9, 2365–2379. 10.1021/mp3001952. [DOI] [PubMed] [Google Scholar]
- Dash T. K.; Konkimalla V. B. Poly-ϵ-caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Controlled Release 2012, 158, 15–33. 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Ghica M. V.; Hirjau M.; Lupuleasa D.; Dinu-Pirvu C. E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. 10.3390/molecules21060786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S.; Khan I.; Gothwal A.; Pachouri P. K.; Bhaskar N.; Gupta U. D.; et al. Conjugated and Entrapped HPMA-PLA Nano-Polymeric Micelles Based Dual Delivery of First Line Anti TB Drugs: Improved and Safe Drug Delivery against Sensitive and Resistant Mycobacterium Tuberculosis. Pharm. Res. 2017, 34, 1944–1955. 10.1007/s11095-017-2206-3. [DOI] [PubMed] [Google Scholar]
- Rani S.; Sahoo R. K.; Nakhate K. T.; Ajazuddin; Gupta U. Biotinylated HPMA Centered Polymeric Nanoparticles for Bortezomib Delivery. Int. J. Pharm. 2020, 579, 119173 10.1016/j.ijpharm.2020.119173. [DOI] [PubMed] [Google Scholar]
- Rani S.; Gothwal A.; Pandey P. K.; Chuahan D. S.; Pachouri P. K.; Gupta U. D.; Gupta U. HPMA-PLGA Based Nanoparticles for Effective In Vitro Delivery of Rifampicin. Pharm. Res. 2019, 36, 19. 10.1007/s11095-018-2543-x. [DOI] [PubMed] [Google Scholar]
- Rani S.; Gupta U. HPMA-based Conjugates in Anticancer Therapeutics. Drug Discovery Today 2020, 25, 997–1012. 10.1016/j.drudis.2020.04.007. [DOI] [PubMed] [Google Scholar]
- Filippov S. K.; Chytil P.; Konarev P. V.; Dyakonova M.; Papadakis C. M.; Zhigunov A.; Plestil J.; Stepanek P.; Etrych T.; Ulbrich K.; Svergun D. I. Macromolecular HPMA-Based Nanoparticles with Cholesterol for Solid-Tumor Targeting: Detailed Study of the Inner Structure of a Highly Efficient Drug Delivery System. Biomacromolecules 2012, 13, 2594–2604. 10.1021/bm3008555. [DOI] [PubMed] [Google Scholar]
- Cui Y.; Shan W.; Liu M.; Wu L.; Huang Y. A Strategy for Developing Effective Orally Delivered Nanoparticles Through Modulation of the Surface “Hydrophilicity/Hydrophobicity Balance. J. Mater. Chem. B 2017, 5, 1302. 10.1039/C6TB02475K. [DOI] [PubMed] [Google Scholar]
- Najafi M.; Kordalivand N.; Moradi M. A.; Dikkenberg J. V. D.; Fokkink R.; Friedrich H.; Sommerdijk N. A. J. M.; Hembury M.; Vermonden T. Native Chemical Ligation for Cross-Linking of Flower-Like Micelles. Biomacromolecules 2018, 19, 3766–3775. 10.1021/acs.biomac.8b00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere K. W. M.; Soliman B. G.; Rijkers D. T. S.; Hennink W. E.; Vermonden T. Thermoresponsive Injectable Hydrogels Cross-Linked by Native Chemical Ligation. Macromolecules 2014, 47, 2430–2438. 10.1021/ma5000927. [DOI] [Google Scholar]
- Lele B. S.; Lereux J. C. Synthesis and Micellar Characterization of Novel Amphiphilic A-B-A Tricopolymers of N-(2-Hydroxypropyl) Methacrylamide or N-Vinyl-2-pyrrolidone with Poly (caprolactone). Macromolecules 2002, 35, 6714–6723. 10.1021/ma020433h. [DOI] [Google Scholar]
- Krimmer S. G.; Pan H.; Liu J.; Yang J.; et al. Synthesis and Characterization of Poly(ε-caprolactone)-block-poly[N-(2-hydroxypropyl) methacrylamide] Micelles for Drug Delivery. Macromol. Biosci. 2011, 11, 1041–1051. 10.1002/mabi.201100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac D.; Kostkova H.; Petrova S.; et al. Interaction of Spin-Labeled HPMA-Based Nanoparticles with Human Blood Plasma Proteins–Introduction of Protein-Corona Free Polymer Nanomedicines. Nanoscale 2018, 10, 6194–6204. 10.1039/C7NR09355A. [DOI] [PubMed] [Google Scholar]
- Sponchioni M.; Morosi L.; Lupi M.; Palmiero U. C. Poly (HPMA)-based Copolymers with Biodegradable Side Chains Able to Self-Assemble into Nanoparticles. RSC Adv. 2017, 7, 50981–50992. 10.1039/C7RA11179G. [DOI] [Google Scholar]
- Chytil P.; Sirovaa M.; Koziolova E.; Ulbrich K.; Rihovaa B.; Etrych T. The Comparison of In Vivo Properties of Water-Soluble HPMA-Based Polymer Conjugates with Doxorubicin Prepared by Controlled Raft or Free Radical Polymerization. Phys. Res. 2015, 64, 41–49. 10.33549/physiolres.933137. [DOI] [PubMed] [Google Scholar]
- Rueda M. M.; Auschera M. C.; Fulchirona R.; Periec T.; Martinb G.; Sonntag P.; Cassagnau P. Rheology and Applications of Highly Filled Polymers: A Review of Current Understanding. Prog. Polym. Sci., Jpn. 2017, 66, 22–53. 10.1016/j.progpolymsci.2016.12.007. [DOI] [Google Scholar]
- Kennedy J. R. M.; Kent K. E.; Brown J. R. Rheology of Dispersions of Xanthan Gum, Locust Bean Gum and Mixed Biopolymer Gel with Silicon Dioxide Nanoparticles. Materials Sci. Eng., C 2015, 48, 347–353. 10.1016/j.msec.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Abdelhalim M. A. K. The Rheological Properties of Different GNPs. Lipids Health Dis. 2012, 11, 14 10.1186/1476-511X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytil P.; Etrych T.; Kriz J.; Subr V.; Ulbrich K. N-(2-Hydroxypropyl)methacrylamide-based Polymer Conjugates with pH-Controlled Activation of Doxorubicin for Cell-Specific or Passive Tumour Targeting Synthesis by RAFT Polymerisation and Physicochemical Characterisation. Eur. J. Pharm. Sci. 2010, 41, 473–482. 10.1016/j.ejps.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Qiu L. Y.; Bae Y. H. Self-assembled Polyethylenimine-Graft-poly(e-caprolactone) Micelles as Potential Dual Carriers of Genes and Anticancer Drugs. Biomaterials 2007, 28, 4132–4142. 10.1016/j.biomaterials.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponchioni M.; Morosi L.; Lupi M.; Palmiero U. C. Poly (HPMA)-Based Copolymers with Biodegradable Side Chains Able to Self-Assemble into Nanoparticles. RSC Adv. 2017, 7, 50981–50992. 10.1039/C7RA11179G. [DOI] [Google Scholar]
- Dragone G.; Mussatto S. I.; Oliveira J. M.; Teixeira J. A. Characterisation of Volatile Compounds in an Alcoholic Beverage Produced by Whey Fermentation. Food Chem. 2009, 112, 929–935. 10.1016/j.foodchem.2008.07.005. [DOI] [Google Scholar]
- Wang C. H.; Hsuie G. H. Polymer-DNA Hybrid Nanoparticles Based on Folate-polyethylenimine-block-poly(L-lactide). Bioconjugate Chem. 2005, 16, 391–396. 10.1021/bc049754j. [DOI] [PubMed] [Google Scholar]
- Wackerly J. W.; Dunne J. F. Synthesis of Polystyrene and Molecular Weight Determination by 1H NMR End-Group Analysis. J. Chem. Educ. 2017, 94, 1790–1793. 10.1021/acs.jchemed.6b00814. [DOI] [Google Scholar]
- Izunobi J. U.; Higginbotham C. L. Polymer Molecular Weight Analysis by 1H NMR Spectroscopy. J. Chem. Educ. 2011, 88, 1098–1104. 10.1021/ed100461v. [DOI] [Google Scholar]
- Czajka A.; Armes S. P. In Situ SAXS Studies of a Prototypical RAFT Aqueous Dispersion Polymerization Formulation: Monitoring the Evolution in Copolymer Morphology During Polymerization-induced Self-Assembly. Chem. Sci. 2020, 11, 11443–11454. 10.1039/D0SC03411H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanraj S.; Murugan D.; Rengarajan A.; Rajiv S. Anticancer Activity of Starch/Poly[N-(2-Hydroxypropyl)Methacrylamide]: Biomaterial Film to Treat Skin Cancer. Int. J. Biol. Macromol. 2014, 70, 116–123. 10.1016/j.ijbiomac.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Mohanraj S.; Rajiv S. Preparation and Characterization of Camptothecin-Loaded Alginate/Poly[N-(2-Hydroxypropyl) Methacrylamide] Hydrogel Beads for Anticancer Treatment. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 781–790. 10.1080/00914037.2016.1269104. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.