Abstract
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide due to its high degree of malignancy, high incidence, and low survival rate. However, the underlying mechanisms of hepatocarcinogenesis remain unclear. Long non coding RNA (lncRNA) has been shown as a novel type of RNA. lncRNA by acting as ceRNA can participate in various biological processes of HCC cells, such as tumor cell proliferation, migration, invasion, apoptosis and drug resistance by regulating downstream target gene expression and cancer-related signaling pathways. Meanwhile, lncRNA can predict the efficacy of treatment strategies for HCC and serve as a potential target for the diagnosis and treatment of HCC. Therefore, lncRNA serving as ceRNA may become a vital candidate biomarker for clinical diagnosis and treatment. In this review, the epidemiology of HCC, including morbidity, mortality, regional distribution, risk factors, and current treatment advances, was briefly discussed, and some biological functions of lncRNA in HCC were summarized with emphasis on the molecular mechanism and clinical application of lncRNA-mediated ceRNA regulatory network in HCC. This paper can contribute to the better understanding of the mechanism of the influence of lncRNA-mediated ceRNA networks (ceRNETs) on HCC and provide directions and strategies for future studies.
Keywords: Epidemiology, HCC, ceRNA, ceRNET, Mechanism, Function
Current epidemiology of HCC
Liver cancer, primarily hepatocellular carcinoma (HCC) [1], is the sixth most common cancer type and the fourth leading cause of cancer deaths worldwide. It was estimated in 2018 that there were 782, 000 deaths and 841, 000 new cases of liver cancer throughout the world, with a higher incidence in men than in women in many parts of the world [2, 3] (Fig. 1A). Moreover, the incidence and mortality rates of HCC vary widely across the globe (Fig. 1B), with the highest incidence rates in Eastern Asia and sub-Saharan Africa [4], followed by Southern Europe and lower rates in Europe and some regions of America [5].
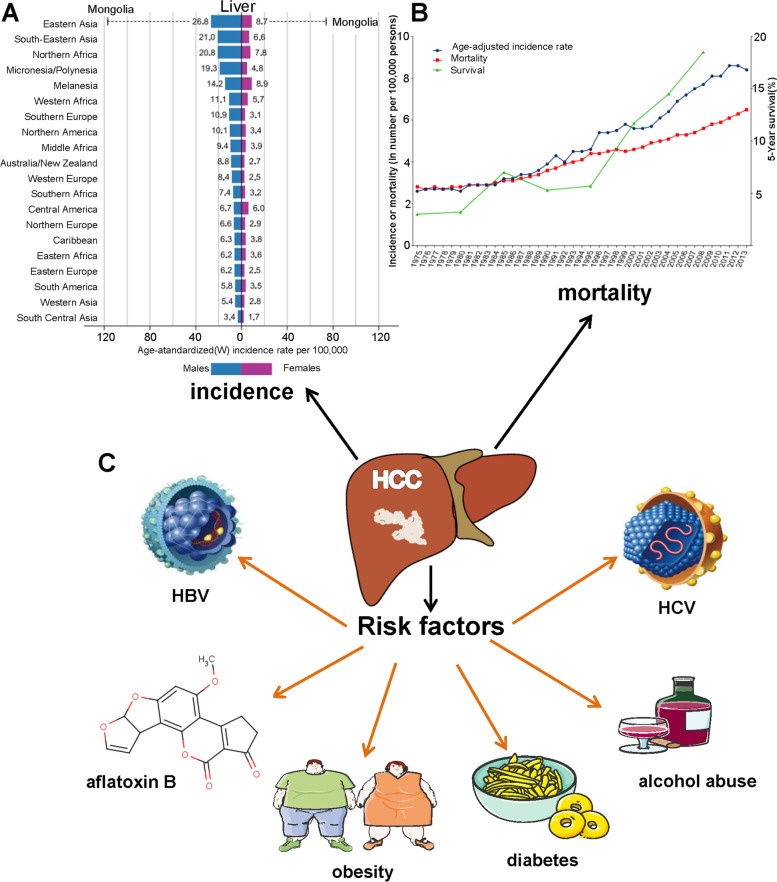
Fig. 1.
Incidence and risk factors of HCC. A Bar graph of Region-adjusted incidence of HCC (per 100, 000) worldwide in 2018, stratified by Sex. B Age - specific incidence and mortality rates (per 100, 000), and 5-year relative survival for HCC in the US from 1975 to 2013. C Risk factors: HBV, HCV obesity, aflatoxin B, diabetes, and alcohol abuse
The etiologic factors of liver cancer are very complicated. The leading causes of the HCC with high incidence include chronic hepatitis B virus infections (HBV), hepatitis C virus (HCV) infections, and food intake contaminated with aflatoxin B (Fig. 1C) [6–9]. Other risk factors include chemical carcinogens exposure, obesity, diabetes, and alcohol abuse (Fig. 1C) [10–13], and the HCC incidence has been on the rise in recent years. Meanwhile, there are significant geographical differences in the risk factors for HCC, mirroring the variation in global distribution patterns. In Asia (notably China) and Africa, the chronic HBV infection with high prevalence has caused an increase in the incidence rates of HCC [14]. In addition, HCV infection with high incidence has become the leading virus-related cause of HCC in some countries, such as Singapore, Japan, Australia, Europe, and the United States of America [15].
Statistics indicate that 5-years survival for HCC is 18%, suggesting that only 30–40% of patients are diagnosed in the early stage [16]. Curative-intent treatments for early-stage HCC include surgical resection, ablation, liver transplantation (LT) and transarterial chemoembolization (TACE) [17]. However, HCC is characterized by a high recurrence and metastasis rate, low detection rate for the curable stages, and ineffective therapeutic options [18]. The incidence and mortality of HCC that is accepted as cancer with poor prognosis and reduced survival are still on the rise. Hence, effective treatment strategies to improve the diagnosis, prevention, and treatment ability of HCC are of urgency.
In recent years, a new and promising molecular-targeted therapy based on the carcinogenic mechanism of HCC has been applied in clinical treatment. Proper understanding of the molecular mechanism of hepatocarcinogenesis and identification of target molecules and signal pathways related to tumor phenotype are essential for designing better treatments and preventing the disease [19]. Noncoding RNA (ncRNA), an RNA molecule that is widely expressed in organisms and cannot encode proteins, has been found to mediate normal cell processes and to be involved in various human diseases, including cancer [20–23].
The emerging roles of competing endogenous RNA (ceRNA) in HCC
Noncoding RNA (ncRNA) can be divided into two major categories: long ncRNA and short ncRNA according to whether the length is greater than 200 nucleotides. It can also be subdivided into long no coding RNA (lncRNA), circular RNA (circRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), microRNA (miRNA), small interfering RNA (siRNA), and small nucleolar RNA (snoRNA) [24–27], etc. Among them, lncRNA and miRNA mainly mediate post-transcriptional regulation, the dysregulation of which was found associated with many malignant tumors [28].
Long noncoding RNA (lncRNA) is a subtype of ncRNA with a transcriptional length of more than 200 nucleotides and was once considered to be unable to encode proteins [29]. However, with the development of research, it was discovered that a small amount of lncRNA could encode polypeptides. Numerous identified lncRNAs are transcribed by RNA polymerase II. They may be 5′-capped and polyadenylated, usually located in the nuclear and cytoplasmic [30–32]. The lncRNA-related data have been reported and annotated in LncBase and other databases. Compared with protein-coding genes, lncRNAs can be transcribed from almost every site in multiple genomes in transcription directions.
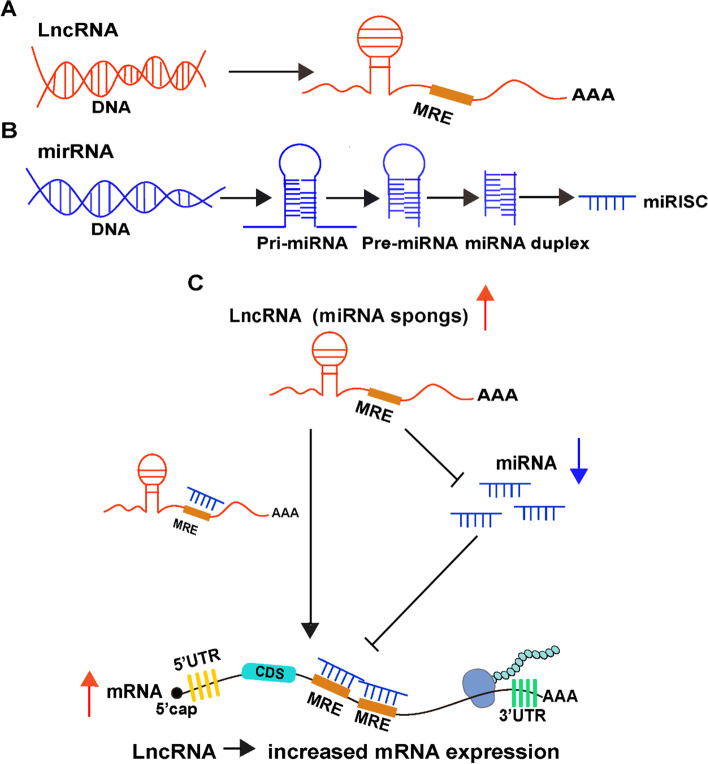
The ceRNA functions via a novel interaction mechanism between RNAs instead of deoxyribonucleic acid. In addition to directly regulating target genes, lncRNA can act as a miRNA sponge to bind miRNAs competitively [33], as suggested by the ceRNA hypothesis established in 2011, thereby weakening the inhibition of miRNA on target gene mRNA, indirectly improving the expression level of target genes, and regulating biological functions without being affected by protein translation (Fig. 2). Hence, these lncRNAs are known as competitive endogenous RNA (ceRNA).
Fig. 2.
The mechanisms of lncRNA related ceRNA regulatory networks. A-B Biogenesis of long noncoding RNA (lncRNA) and microRNA (miRNA). C lncRNAs as competing endogenous RNAs and mRNAs share a pool of miRNAs, and the competition for miRNAs leads to dynamic regulation of the expression level of mRNAs
Studies have shown that ceRNA, especially lncRNA are involved in all aspects of cancer. However, these long noncoding RNAs (lncRNA) have been considered as “junk genes” [34], while the functions of lncRNAs are found complex and diverse, which are characterized by biological regulation and explicit evolutionary conservatism [35].
Additionally, lncRNAs show more cell type-specific expression patterns than protein-coding genes, which supports the hypothesis that lncRNA is of functional importance. LncRNAs can be used as the organizational framework of subcellular structure to regulate protein localization or activity, and some lncRNAs can regulate global or local gene expression in trans or cis by influencing RNA polymerase II recruitment or inducing chromatin remodeling [36].
LncRNA can regulate gene expression through multiple mechanisms, such as directing chromatin remodeling complexes to specific sites, mediating mammalian X chromosome inactivation, and regulating DNA methylation and histone modification to induce epigenetic modification of DNA [37]. At the biological level, Different lncRNAs can mediate gene expression at the transcriptional, post-transcriptional and epigenetic levels.
Moreover, lncRNA is also involved in tumor biological processes, such as tumor proliferation, invasion, and metastasis. It has been reported that the dysregulated expression of lncRNA that can act as an oncogene or tumor suppressor gene may cause various cancers [38–40].
The lncRNA-mediated ceRNA networks (ceRNETs) functions by modulating signaling pathways in HCC
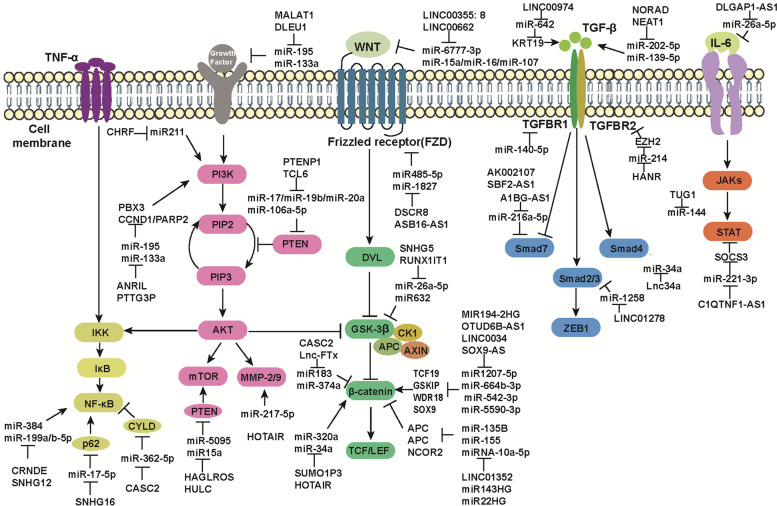
More attention has been paid to molecular mechanisms that play a key role in the development of HCC. It has been observed that many cell signaling pathways are essential in regulating critical cell processes, such as cell proliferation, cell cycle progression, and apoptosis [41–43]. As a ceRNA, LncRNA also participates in HCC cell proliferation, epithelial to mesenchymal transition (EMT), invasion and metastasis by regulating signaling pathways in HCC, such as nuclear factor κB (NF-kB) pathway, phosphatidylinositol 3′-kinase(PI3K)-Akt signaling pathway, Wnt/β-catenin pathway, TGF-β pathway, and Janus kinase signal transducer and activator of transcription (JAK/STAT) pathway, which are very important in the process of carcinogenesis (Fig. 3).
Fig. 3.
Schematic representation of five major signaling pathways. Schematic representation of five major signaling pathways involved in lncRNAs related ceRNETs regulation in HCC
NF-κB pathway
NF-kB is a critical eukaryotic transcription regulator of inflammation [44]. Abnormal activation of NF-kB pathway often occurs in the early stage of HCC and is associated with the growth and invasion of HCC and EMT in HCC [45]. It was found that the lncRNA-mediated ceRNA networks can play biological functions by regulating NF-kB pathway. Oncogenic lncRNAs can exert the part of ceRNAs by activating NF-κB signaling in HCC. For instance, lncRNA SNHG16 can regulate p62 expression by sponging miR-17-5p, thereby mediating the NF-κB signaling pathway to promote proliferation, migration, and invasion of HCC cells and inhibit apoptosis [46]. LncRNA CRNDE can accelerate the progression of HCC by acting as the ceRNA of miR-384 to activate the NF-κB pathway [47]. LncRNA SNHG12 can promote the expression of MLK3 by competitively binding miR-199a/b-5p, thus activating the NF-κB pathway to promote the initiation and metastasis of HCC [48].
On the contrary, tumor suppressor lncRNAs acting as ceRNA may perform their biological function by inactivating the NF-κB pathway in HCC. For example, lncRNA CASC2, an important inhibitor of HCC, can up-regulate the expression of CYLD by competitively binding miR-362-5p, thereby inhibiting the NF-κB pathway to promote the growth and metastasis of HCC [49]. In conclusion, lncRNA-miRNA-NF-κB regulation network is expected to be a potential therapeutic target for HCC treatment.
PI3K/AKT pathway
In nearly half of cases of HCC, the PI3K/AKT pathway was highly activated and was involved in critical cellular processes such as cell growth, survival regulation, and metabolism [50]. It has been reported that lncRNAs can play their biological functions by acting as miRNA ceRNAs to mediate the PI3K/Akt pathway. Among them, oncogenic lncRNAs by acting as ceRNAs can perform their biological effects by activating the PI3K/AKT pathway in HCC.
For example, lncRNA ANRIL can up-regulate the expression of PBX3 by acting as the ceRNA of miR-144, thereby activating the PI3K/AKT signaling pathway to promote the HCC growth, migration, and invasion, or inhibit apoptosis of HCC cells [51]. LncRNA CHRF and HOTAIR can activate the PI3K/AKT pathway and PI3K/AKT/MMP-2/9 pathway by sponging miR-211 and miR-217-5p, respectively, thereby promoting the viability, proliferation, and EMT process of HCC cells [52]. LncRNA PTTG3P can promote the expression of CCND1 and PARP2 by competitively binding miR-383, thus affecting the PI3K/AKT signaling pathway to promote the growth and metastasis of HCC cells [53]. LncRNA MALAT1 and DLEU1 can promote the expression of EGFR and IGF-1R by acting as the ceRNA of miR-195 and miR-133a, respectively, thereby activating the downstream PI3K/Akt signaling pathway to accelerate the HCC progression [54]. The expression of PTEN was reported to be decreased in nearly half of all HCC tumors, leading to the activation of the PI3K/Akt/mTOR pathway to promote HCC tumorigenesis. LncRNA HAGLROS and HULC can up-regulate the expression of ATG12 and P62 by sponging miR-5095 and miR15a, respectively, thereby affecting the PTEN/PI3K/AKT/mTOR signaling pathway to promote HCC cell survival and inhibit apoptosis [55].
However, tumor suppressor lncRNAs can exert the function of ceRNAs by inactivating PI3K/AKT or PI3K/AKT/mTOR in HCC. For example, lncRNA PTENP1 can up-regulate the expression of PTEN and other target genes by acting as ceRNAs of miR-17, miR-19b, and miR-20a, thereby inactivating PI3K/Akt/mTOR pathway to inhibit the HCC growth, proliferation, and migration and induce apoptosis of HCC cells [56]. LncRNA TCL6, a tumor suppressor lncRNA, can promote PTEN protein level by sponging miR-106a-5p, thus negatively regulating the PI3K/Akt signaling pathway in HCC to inhibit the HCC proliferation, migration, and invasion [57]. Therefore, lncRNA-mediated ceRNA networks can play their biological functions in HCC by mediating PI3K/AKT or PI3K/AKT/mTOR pathways and serve as promising targets for HCC treatment.
Wnt/β-catenin pathway
Abnormal activation of Wnt/β-catenin signaling pathway is critical in the initiation and development of cancer. The elucidation on the regulatory mechanism of Wnt/β-Catenin pathway can provide new insights into anti-cancer therapy. lncRNAs can act as miRNA sponges by mediating the Wnt/β-catenin pathway in HCC to regulate the HCC progression, as supported by more studies.
Oncogenic lncRNAs can function as ceRNAs by activating Wnt/β-Catenin signaling in HCC. LncRNA SUMO1P3 can activate the Wnt/β-catenin pathway by inhibiting miR-320a, thereby accelerating the aggressive progression of HCC [58]. LncRNA LINC00355: 8 and SNHG5 can promote the expression of Wnt10b and GSK3β by sponging miR-6777-3p and miR-26a-5p, respectively, thereby activating the Wnt/β-catenin signaling pathway to promote HCC growth and EMT [59]. LncRNA LINC00662 can promote the expression and secretion of WNT3a by sponging miR-15a, miR-16, and miR-107, thus activating the Wnt/β-catenin signal transduction pathway to accelerate the HCC progression [60]. LncRNA HOTAIR can enhance the Wnt/β-catenin signaling pathway by down-regulating miR-34a, thereby promoting the drug resistance of HCC to paclitaxel [61]. LncRNA MIR194-2HG and OTUD6B-AS1 can up-regulate the expression of Transcription Factor 19 (TCF19) and GSK3B Interacting Protein (GSKIP) by competitively binding miR1207-5p and miR-664b-3p, respectively, thereby activating the Wnt/β-catenin signaling pathway and further promoting the proliferation and invasion of HCC cells [62]. LncRNA LINC00346 and SOX9-AS can promote the expression of WDR18 and SOX9 by acting as the ceRNAs of miR-542-3p and miR-5590-3p, respectively, thereby both regulating the Wnt/β-catenin pathway to promote the proliferation and metastasis of hepatoma cells [63]. LncRNA ASB16-AS1 and DSCR8 can up-regulate the expression of Frizzled Class Receptor 4 (FZD4) and FZD7 by sponging miR-1827 and miR485-5p, respectively, thus activating Wnt/β-catenin pathway and promoting the development of HCC [64].
However, tumor-suppressor lncRNAs that can act as ceRNAs, can perform their biological functions by inactivating the Wnt/β-catenin pathway in HCC. LncRNA LINC01352 directly binds to miR-135B and acts as a competitive endogenous RNA to promote the expression of miR-135B target gene APC Regulator of WNT Signaling Pathway (APC), thereby negatively regulating Wnt/β-catenin signaling to inhibit liver tumor development [65]. LncRNA RUNX1-IT1 can promote the expression of Glycogen Synthase Kinase 3 Beta (GSK3B) by competitively binding miR632, thereby inactivating the Wnt/β-catenin pathway of hepatoma cells to inhibit the proliferation, migration, and differentiation of cancer stem cells [66]. LncRNA CASC2 and Lnc-FTx can inactivate the Wnt/β-catenin pathway by down-regulating miR183 and miR-374a, respectively, thereby inhibiting EMT and invasion of HCC cells [67]. LncRNA MIR143HG and MIR22HG can act as ceRNAs of miR-155 and miRNA-10a-5p to up-regulate the expression of APC and Nuclear Receptor Corepressor 2 (NCOR2), thereby negatively regulating the Wnt/β-catenin pathway to inhibit the proliferation and metastasis of HCC [68]. In conclusion, the lncRNA-miRNA-Wnt/β-catenin regulatory network can provide rationales and insights for novel strategies of HCC treatment.
TGF-β pathway
Studies have found the critical role of TGF-β signaling pathway in regulating cell growth, apoptosis, and differentiation, which also participates in the tumorigenesis and metastasis of HCC [69–72]. More importantly, lncRNA can regulate the progress of HCC by mediating the TGF-β signal pathway in HCC to play the role of miRNA sponge. Among them, oncogenic lncRNAs as ceRNAs can exert their biological function by activating the TGF-β signal pathway in HCC [73]. LncRNA NORAD can activate the TGF-β pathway by acting as the ceRNA of miR-202-5p and removing inhibitory effect of miR-202-5p on TGFBR1 and TGFBR2, thereby promoting the migration and invasion of HCC cells [74]. LncRNA LINC01278 and Lnc34a can up-regulate the expression of SMAD Family Member 4 (SMAD4) and SMAD2/3 by competitively binding miR-1258 and miR-34a, respectively, thereby activating TGF-β signaling to promote bone metastasis of HCC [75]. LncRNA AK002107 and SBF2-AS1 both target Transforming Growth Factor Beta Receptor 1 (TGFBR1) by sponging miR-140-5p, thereby promoting migration and invasion of hepatoma cells [76]. LncRNA HANR can up-regulate the expression of Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit (EZH2) by competitively binding miR-214, thereby affecting the level of TGFBR2 to promote the HCC progression [77]. LncRNA NEAT1 can up-regulate the expression of TGF-β1 by competitively binding hsa-miR-139-5p, thus inducing the progression of HCC [78]. LncRNA HULC can inhibit Zinc Finger E-Box Binding Homeobox 1 (ZEB1) by competitively binding miR-200a, thereby promoting HCC progression and EMT [79]. LncRNA LINC00974 can promote the expression of Keratin 19 (KRT19) by sponging miR-642, thereby activating the TGF-β signaling pathway to expedite the proliferation and invasion of HCC [80].
On the contrary, tumor suppressor lncRNAs, by acting as ceRNAs, can exert its biological function by inactivating the TGF-β1 pathway in HCC [81–83]. For example, lncRNA A1BG-AS1 can promote the expression of Smad7 by acting as the ceRNA of miR-216a-5p, thereby inhibiting the proliferation and invasion of HCC [84]. Therefore, the lncRNA-mediated ceRNA network by mediating the TGF-β pathway can provide novel ideas for HCC treatment.
JAKs/STAT pathway
The JAK/STAT pathway can be activated by multiple cytokines that is involved in many important cellular processes, such as cell differentiation, proliferation, and apoptosis [85–88]. The abnormal activation of the JAK/STAT signal can cause tumor cell migration and invasion in HCC [89]. Moreover, the lncRNA-mediated ceRNA network can exert its biological functions by regulating the JAK/STAT pathway, in which lncRNA, acting as ceRNA, can perform its biological function by regulating the JAK/STAT pathway in HCC. For example, lncRNA DLGAP1-AS1 can increase the level of oncogenic cytokine IL-6 by competitively binding miR-26a-5p, thereby promoting the occurrence and EMT of HCC through the JAK2/STAT3 signaling pathway [90]. LncRNA TUG1 can activate the JAK2/STAT3 pathway by inhibiting miR-144, thereby promoting the proliferation and migration of HCC cells [91]. On the contrary, the tumor suppressor lncRNA C1QTNF1-AS1 can up-regulate the Suppressor of Cytokine Signaling 3 (SOCS3) by sponging miR-221-3p, thereby inhibiting the proliferation, migration, and invasion of HCC cells through the JAK/STAT signaling pathway [92]. Therefore, lncRNA-miRNA-JAK/STAT regulatory network is a promising therapeutic target for HCC, which is expected to improve clinical efficacy as a personalized therapy.
Clinical application potential of lncRNA-mediated ceRNET in HCC
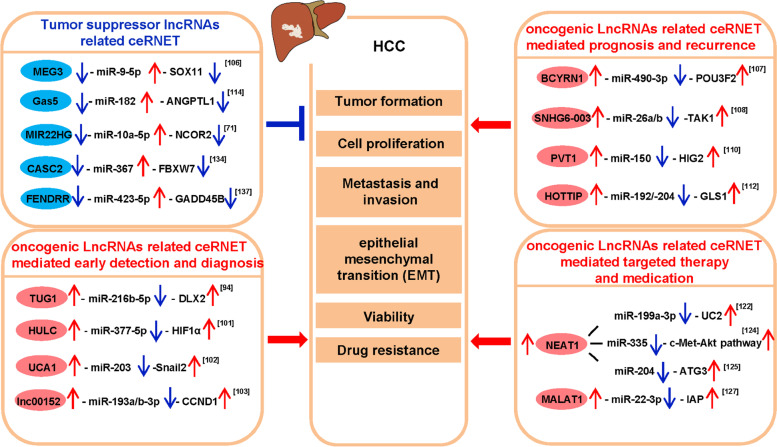
Currently, the most common detection methods for HCC are alpha-fetoprotein (AFP) detection and imaging examination. AFP is a conventional tumor biomarker for the clinical diagnosis of HCC. However, AFP has low sensitivity and insufficient specificity in the diagnosis of HCC, failing to detect about 30% of patients with early-stage HCC [93]. Liver ultrasound is effective in early tumor diagnosis and treatment, but its effectiveness in clinical practice needs to be improved [94]. Meanwhile, among the anti-cancer drug regimens for HCC, the first-line treatments such as sorafenib and lenvatinib can only prolong a short survival time for patients with advanced HCC [95]. Therefore, the search for new biomarkers to improve the diagnosis rate of HCC at an early stage is of urgency. A substantial body of evidence has demonstrated that lncRNA can act as ceRNA and can be used as biomarkers or even treatment targets for HCC (Fig. 4). Therefore, further studies on lncRNA may provide novel insights into the detection and diagnosis, prognosis and recurrence, and targeted medication and treatment of HCC.
Fig. 4.
Summary of lncRNAs related ceRNETs mediated functions in HCC. LncRNAs can regulate HCC progressions, such as tumor formation, cell proliferation, metastasis and migration, epithelial–mesenchymal transition (EMT), and drug resistance
LncRNA related ceRNET mediated early detection and diagnosis potential in HCC
Although AFP is regarded as a gold standard biomarker in the detection and diagnosis of HCC, its sensitivity and specificity are insufficient, thus giving urgency of new biomarkers with better clinical significance. Several lncRNAs have been identified as ceRNAs in many cancers, including HCC. A growing body of evidence suggests that lncRNAs can act as ceRNA and related ceRNETs may play a significant role in clinical applications as diagnostic biomarkers for HCC.
Taurine Up-Regulated 1 (TUG1) has been proved to be a proto-oncogene, and the up-regulated TUG1 expression in the HCC group is correlated with the Barcelona Clinic Liver Cancer (BCLC) staging and tumor size compared with the control group [96]. Notably, the combined detection of TUG1 and AFP can improve the accuracy of HCC diagnosis [97]. Besides, TUG1 can enhance the expression of Distal-Less Homeobox 2 (DLX2) by acting as a ceRNA for miR-216b-5p, thereby promoting the growth and metastasis of HCC cells [98].
LINC00152 plays an oncogenic role in human malignancies, including HCC [99]. The expression levels of HULC and LINC00152 in HCC patients were marked higher than those in the control group, and the combined detection of HULC, LINC00152 and AFP enjoyed a high diagnostic sensitivity and specificity, with an Area under Curve (AUC) value of 0.89 [100]. Similarly, the expression levels of UCA1 and LINC00152 in HCC patients were significantly higher than those in patients with benign hepatopathy and healthy controls, and the combined detection of HULC, LINC00152 and AFP had even higher sensitivity and specificity of diagnosis, with AUC value of 0.912 and sensitivity and specificity of 82.9 and 88.2%, respectively [101]. HULC can promote the expression of Hypoxia Inducible Factor 1 Subunit Alpha (HIF1A) by sponging miR-377-5p, thus enhancing the proliferation and invasion of HepG2 cells [102]. Urothelial Cancer Associated 1 (UCA1) can activate the expression of transcription factor Snail2 by acting as the ceRNA of miR-203, thus promoting the proliferation, invasion, and EMT of hepatoma cells [103]. LINC00152 can promote the progression of the HCC cell cycle by sponging miR-193a/b-3p to regulate its target gene CCND1 [104]. In short, the three ceRNETs of HULC/miR-377-5p/HIF1α, UCA1-miR-203-Snail2 and lNC00152-miR-193a/b-3p-CCND1 are all promising targets for HCC detection and diagnosis.
However, some tumor suppressor genes have an active function in the HCC diagnosis. Maternally Expressed 3 (MEG3) is a tumor suppressor that is involved in the occurrence and development of various malignancies, including HCC. Both in vivo and in vitro experiments have confirmed that MEG3 can inhibit the proliferation and induce apoptosis of HCC cells, and Cox regression analysis found that MEG3 expression is an independent prognostic factor for HCC patients [105]. Meanwhile, MEG3 is a risk factor for elevated human AFP [106]. Therefore, MEG3 is a potential biomarker for predicting the prognosis of HCC. In addition, MEG3 can enhance SRY-Box Transcription Factor 11 (SOX11) expression by acting as a ceRNA for miR-9-5p, thereby inhibiting growth but promoting apoptosis of HCC cells [107]. Therefore, the ceRNET may provide new insights into the pathogenesis of HCC and provide a potential diagnostic marker for HCC.
On the other hand, technologies for assaying circRNAs have been greatly improved, but error and bias still affect detection accuracy. Theoretically, RNA-seq can identify circRNAs accurately within populations of different RNAs that constitute complete transcriptomes [108–111]. However, algorithms for analyzing the next-generation sequencing data often apply different problem-solving rules to minimize false positives and false negatives, leading to incorrect discoveries and misinterpretation in data.
Other variables that may confound interpretation of data include the introduction of sequence artifacts during library preparation, sequence-specific influences on splicing, biases resulting from rarity of circRNAs and difficulties with classifying splice junctions as true positives. Enriching circRNA populations by treating extracts with RNase R to eliminate linear RNAs is now widely used in studies employing microarray assays [112]. Direct assay of the fractions of circularized transcripts that are derived from parental genes will assist with interpretations about regulatory functions.
Because of the implication with the etiology of HCC, circRNAs can be used as potential markers of the malignancy and targets of therapeutic intervention and therapeutic agents. The field is at a critical development stage, thus giving importance of the clarification of relevant studies reconciling disparate observations, standardization of methodology, and devising methods to exclude possible artifacts.
LncRNA related ceRNET mediated prognosis and recurrence potential in HCC
Although liver transplantation (LT) and partial liver resection (LR) are effective treatments for HCC, the prognosis is not ideal due to metastasis and recurrence. Recent studies have shown that lncRNA as a ceRNA can predict postoperative recurrence and survival time of HCC patients.
Brain Cytoplasmic RNA 1 (BCYRN1) is found of prognostic value besides its diagnostic function in HCC. Overexpression of BCYRN1 can promote the growth and migration of HCC cells in vitro with being overexpressed in HCC samples. Therefore, BCYRN1 can serve as a prognostic marker for HCC [113]. Meanwhile, BCYRN1 can promote the expression of POU3F2 by sponging miR-490-3p, and the ceRNET mechanism is more significant in regulating the prognosis of HCC [114].
LncRNA SNHG6–003 can promote cell proliferation and induce drug resistance in HCC cells, and its high expression is correlated with the poor prognosis of HCC patients. SNHG6–003 can regulate the expression of transforming growth factor-β activated kinase 1 (TAK1) by acting as the ceRNA of miR-26a/b [115]. Therefore, targeted study on this ceRNET is expected to find a therapeutic strategy for HCC.
Human lncRNA-PVT1 expression can be up-regulated in HCC tissues, and patients with high expression of lncRNA-PVT1 had poor clinical prognosis. Human lncRNA-PVT1 can promote the proliferation, cell cycle and stem cell-like properties of HCC cells [116], as demonstrated by in vitro and in vivo experiments. Meanwhile, the up-regulation of miR-150 expression by knockout of PVT1 gene can reduce the expression of Hypoxia Inducible Gene 2 (HIG2) and inhibit the proliferation, invasion, and iron metabolism balance of HCC [117]. Therefore, this ceRNET will contribute to the study of the pathogenesis of HCC.
Multivariate analysis showed that the high expression of HOXA Distal Transcript Antisense RNA (HOTTIP) was an independent risk factor for tumor recurrence and shorter overall survival after LT. In vitro studies showed that down-regulation of HOTTIP expression could reduce the aggressiveness of tumor cells and increase their chemo-sensitivity. Therefore, the expression level of HOTTIP can be a predictor of tumor recurrence after LT in HCC patients [118]. In addition, the miR-192/− 204-HOTTIP axis may inhibit the viability of HCC cells by down-regulating GLS1, and the combined indicators of miR-192/− 204 and HOTTIP are more significantly correlated with the prognosis of HCC.
Nevertheless, some tumor suppressor genes are risk factors for poor prognosis of HCC. Multivariate analysis revealed that the up-regulation of lncRNA Gas5 expression was an independent predictor of poor recurrence free survival (RFS) (HR: 1.287, 95% CI: 1.027 ~ 1.612, P = 0.028). Methylation data showed that the methylation status of 5 CpG sites (cg07177756, cg17025683, cg16290996, cg03044573 and cg06644515) was moderately negatively correlated with the expression of Growth Arrest Specific 5 (Gas5) (P = − 0.54, P < 0.001). Therefore, Gas5 plays a vital role in HCC prognosis [119]. Moreover, Gas5 spongs miR-182 can up-regulate anti-metastatic protein Angiopoietin Like 1 (ANGPTL1) expression and then inhibit tumor metastasis, which can play a crucial role in the therapeutic intervention against the progression of HCC [120].
Another tumor suppressor gene is miR22HG. The silico and experimental analyses showed that overexpression of miR22HG could inhibit the proliferation, invasion, and metastasis of HCC cells, while low expression of miR22HG is related to tumor progression and poor prognosis of HCC patients. Therefore, miR22HG is a potential prognostic biomarker [121]. Moreover, miR22HG can suppress the activation of the Wnt/β-catenin pathway by sponging miR-10a-5p to up-regulate the expression of the target gene NCOR2, thereby inhibiting the progression of HCC [68]. In conclusion, ceRNET can provide new clues for the development of novel prognostic biomarkers for HCC patients.
The potential of LncRNA related ceRNET as therapy target in HCC
Conventional cancer treatments are effective only in some patients with the major issue of drug resistance. Therefore, exploration of the potential mechanism of HCC resistance and effective drug-specific targets that target tumor cells without adversely affecting normal cells will help to improve the cure rate of HCC. LncRNA has been considered a potential biomarker candidate for targeted therapy and medication in cancer due to its higher specific expression in tumor cells, less side effects, and critical role in mediating resistance [122].
LncRNA NEAT1 plays a carcinogenic role in various tumors, including gastric cancer, lung cancer, colorectal cancer and liver cancer [123]. Recent studies have shown that NEAT1, as a ceRNA, can be a new target for HCC therapy. NEAT1 expression can be up-regulated in HCC tissues, and NEAT1 can promote the expression of Uridine-Cytidine Kinase 2 (UCK2) by competitively binding to the tumor suppressor miR-199a-3p, so as to maintain the growth of HCC cells [124]. More importantly, NEAT1-mediated ceRNET can affect the drug resistance of HCC. Studies showed that sorafenib resistance could be induced by inhibiting sorafenib induced apoptosis by activating the c-Met-Akt pathway in vivo and in vitro [125]. However, NEAT1 can enhance the drug resistance of HCC cells to sorafenib by sponging miR-335 to relieve the inhibition of the c-Met-Akt pathway [126]. Meanwhile, NEAT1 can also act as the ceRNA of miR-204 to up-regulate the expression of Autophagy Related 3 (ATG3), thus promoting HCC autophagy and enhancing the resistance of HCC cells to sorafenib [127].
Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) is one of the most widely studied lncRNAs in cancer therapy. lncRNA MALAT1 was found to play an oncogenic role in the occurrence and development of HCC and act as a proto-oncogene to regulate the expression of apoptosis inhibitor IAPS [128]. MALAT1 can act as the ceRNA of miR-22-3p to cause the abnormal expression of the target gene inhibitor of apoptosis proteins (IAPs) [129]. Meanwhile, MALAT1 is involved in BA-induced apoptosis in HCC, and betulinic acid (BA) can inhibit the expression of IAPs and MALAT1 in HCC. BA is a natural drug extracted from various Chinese herbal medicines [130] with satisfactory anti-cancer activity, selectively cytotoxic to cancer cells but not to normal cells, which can induce autophagy and apoptosis of cancer cells [131]. BA can affect IAP in HCC by activating miR-22-3p to target MALAT1, thus inducing the death of HCC cells [132]. Therefore, MALAT1 is a potential biomarker involved in the drug treatment mechanism of HCC.
Similarly, some lncRNAs also play an essential role in the treatment of HCC as tumor suppressor factors. Cancer Susceptibility 2 (CASC2) is a useful liver cancer cell regulator whose expression can be down-regulated in multiple cancers, including HCC [133]. Overexpression of CASC2 can promote the apoptosis of HCC cells and inhibit cell proliferation and metastasis [134]. In addition, CASC2 can promote F-Box and WD Repeat Domain Containing 7 (FBXW7) by sponging miR-367, thereby inhibiting EMT, migration, and invasion of HCC cells [135]. Meanwhile, CASC2 plays a key role in the drug resistance of HCC. CASC2 can improve the sensitivity of HCC cells to cisplatin (DDP) by downregulating the expression of miR-222, which provides a promising therapeutic strategy for overcoming DDP resistance in HCC [136].
FOXF1 Adjacent Non-Coding Developmental Regulatory RNA (FENDRR) is related to the progression of various tumors. In HCC, FENDRR can inhibit the growth and metastasis of HCC cells by down-regulating Glypican 3 (GPC3) expression [137]. FENDRR can also act as the ceRNA of miR-423-5p to up-regulate Growth Arrest and DNA Damage Inducible Beta (GADD45B), inhibit HCC growth, promote the apoptosis of HCC cells, and regulate the immune escape of HCC mediated by Tregs. Therefore, ceRNET associated with FENDRR is a new therapeutic target worth consideration.
Outlook
HCC is one of the most complex cancers with increasing incidence and mortality rate and various potential pathogenic mechanisms. Many risk factors contribute to the initiation of HCC, and the rates of metastasis and recurrence of HCC remain high, giving huge challenges to the current treatment strategies. So far, an increasing number of preclinical studies showed that lncRNA is a good diagnostic and prognostic biomarker for HCC treatment. Many lncRNAs can influence the cellular biological processes of HCC, such as cell proliferation, invasion, migration, apoptosis, and EMT, by competitively binding miRNAs of ceRNA and mRNA, which lays a foundation for promising clinical indicators for early detection, diagnosis, prognosis, recurrence, therapy and targeted medication of HCC.
However, studies on the detailed mechanism of the ceRNA network and its relationship with HCC are still in the initial stage. Although increasing attention has been paid to lncRNA as ceRNA in HCC, less was to the interaction between lncRNA-mediated ceRNA regulatory networks in HCC. Therefore, it is necessary to study the identity, role, and mechanism of ceRNAs in different malignant stages of HCC. To date, studies on ncRNAs that act as ceRNAs in HCC have primarily involved overexpression and knockout assays in cells and animals.
Moreover, other factors can affect ceRNA activity, such as subcellular location and ceRNA component abundance, interactions with RNA binding proteins, RNA editing, and ceRNA affinity in the endogenous cellular context. However, whether the results of overexpression assays can truly reflect the spontaneous ceRNA crosstalk during carcinogenesis in patients with HCC remains unknown. Therefore, more animal experiments and clinical trials should be conducted for verification.
Additionally, the majority of identified ceRNA interactions show single binding partners, although ceRNA crosstalk in large interconnected networks. Apart from direct interactions via shared miRNAs, secondary and indirect interactions might also significantly affect ceRNA modulation.
Therefore, further investigations on ceRNAs should focus not only on identifying binary ceRNA interactions but on the network analysis of potential complex miRNA and ceRNA networks. Moreover, the scale-free network property of ceRNA regulation also poses a challenge when selecting HCC-related molecular therapeutic targets. Targeting nonessential nodes within regulatory networks could cause ineffective therapeutic responses, as cancer cells may overcome the resulting damage through alternative signaling pathways. Therefore, therapeutic targets situated in a hub position of a ceRNET should be considered in future screening studies for HCC therapeutic targets.
In conclusion, the recently developed research techniques and computational methods will support a more comprehensive study on lncRNA that acts as ceRNA and mediated ceRNET in HCC. These findings can not only contribute to a better understanding of the pathogenesis and progress of HCC but also help to create new directions and strategies for the prevention, diagnosis, and treatment of HCC.
Acknowledgements
We would like to thank Dr. Wei Zhang for data analysis and critical discussion of the manuscript.
Authors’ contributions
W.L., D.F. and Y.S. designed the study; All authors collected data, performed the statistical analyses and interpreted the data; W.L., Y.S., J.Y. and D.F. wrote the manuscript; Y.S, J.-B.L., and J.D. contributed equally to this work. All authors contributed to the final version of the manuscript and approved the final manuscript.
Funding
This study was supported partly by grants from National Key R&D Program of China (2018YFE0110200), National Natural Science Foundation of China (81972214), Key Program of Hunan Provincial Department of Science and Technology (2020WK2020 and 2019NK2111), Natural Science Foundation of Hunan Province of China (2020JJ4278), High Technology Industry S & T Innovation Leading Project of Hunan Province (2020NK2001), the Scientific Research Project of Hunan Provincial Education Department (20C0622), Nantong Science and Technology Bureau Project (jc2019132 and xg02002–4).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
All of the named authors have agreed to submit the paper in its present form.
Competing interests
No potential conflicts of interest were disclosed.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi Shi, Ji-Bin Liu and Jing Deng contributed equally to this work.
Contributor Information
Yu-Shui Ma, Email: mayushui@tongji.edu.cn.
Fu Da, Email: fu800da900@126.com.
Wen Li, Email: liwendream@163.com.
References
- 1.Huang J, Zheng Y, Xiao X, Liu C, Lin J, Zheng S, et al. A circulating long noncoding RNA panel serves as a diagnostic marker for hepatocellular carcinoma. Dis Markers. 2020;2020:5417598. doi: 10.1155/2020/5417598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 1873;2020(1):188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang Q, Chen J, Luo H, Tan N, Gao H, Zhang X, et al. Decrease in Chitinase 3-like protein 1 levels reflects improvement in liver fibrosis after HCV eradication. Dis Markers. 2020;2020:8539804. doi: 10.1155/2020/8539804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han R, Nusbaum O, Chen X, Zhu Y. Valeric acid suppresses liver Cancer development by acting as a novel HDAC inhibitor. Mol Ther Oncolytics. 2020;19:8–18. doi: 10.1016/j.omto.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42:S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan JB, Lai CC, Jhu JW, Gongol B, Marin TL, Lin SC, et al. Insulin and metformin control cell proliferation by regulating TDG- mediated DNA demethylation in liver and breast Cancer cells. Mol Ther Oncolytics. 2020;18:282–294. doi: 10.1016/j.omto.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma YS, Wu TM, Qian B, Liu YS, Ding H, Fan MM, et al. KDM5A silencing transcriptionally suppresses the FXYD3-PI3K/AKT axis to inhibit angiogenesis in hepatocellular cancer via miR-433 up-regulation. J Cell Mol Med. 2021;25(8):4040–4052. doi: 10.1111/jcmm.16371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang DG, Gong CC, Wu XJ, Ren X, Sedaka RS, Chen WC, et al. P21-activated kinase 5 potentiates the chemoresistant phenotype of liver cancer. Signal Transduct Target Ther. 2021;6(1):47. doi: 10.1038/s41392-020-00409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao S, Hossain T, Mahmoudi T. 3D human liver organoids: An in vitro platform to investigate HBV infection, replication and liver tumorigenesis. Cancer Lett. 2021;506:35–44. doi: 10.1016/j.canlet.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Zhang C. The role of liver sinusoidal endothelial cells in cancer liver metastasis. Am J Cancer Res. 2021;11(5):1845–1860. [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda H, Takai A, Iguchi E, Mishima M, Arasawa S, Kumagai K, et al. Oncogenic transcriptomic profile is sustained in the liver after the eradication of the hepatitis C virus. Carcinogenesis. 2021;42(5):672–684. doi: 10.1093/carcin/bgab014. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Wen H, Peng B, Weng J, Zeng F. HFD-induced TRAF6 upregulation promotes liver cholesterol accumulation and fatty liver development via EZH2-mediated miR-429/PPARα axis. Mol Ther Nucleic Acids. 2021;24:711–727. doi: 10.1016/j.omtn.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin X, Lu Y, Xie S, Chen Y, Jiang X, Song S, et al. miR-155 accelerates the growth of human liver Cancer cells by activating CDK2 via targeting H3F3A. Mol Ther Oncolytics. 2020;17:471–483. doi: 10.1016/j.omto.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau KC, Joshi SS, Gao S, Giles E, Swidinsky K, van Marle G, Bathe OF. Urbanski SJ, Terrault NA, Burak KW, Osiowy C, Coffin CS. Oncogenic HBV variants and integration are present in hepatic and lymphoid cells derived from chronic HBV patients. Cancer Lett. 2020;480:39–47. doi: 10.1016/j.canlet.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Z, Wei-Qi J, Jin D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim Biophys Acta Rev Cancer. 1874;2020(1):188382. doi: 10.1016/j.bbcan.2020.188382. [DOI] [PubMed] [Google Scholar]
- 16.Lee IC, Lei HJ, Chau GY, Yeh YC, Wu CJ, Su CW, et al. Predictors of long-term recurrence and survival after resection of HBV-related hepatocellular carcinoma: the role of HBsAg. Am J Cancer Res. 2021;11(7):3711–3725. [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Li H, Liu J, Du C, He T, Luo X, et al. The short-term efficacy of DEB-TACE loaded with epirubicin and raltitrexed in the treatment of intermediate and advanced primary hepatocellular carcinoma. Am J Transl Res. 2021;13(8):9562–9569. [PMC free article] [PubMed] [Google Scholar]
- 18.Meng F, Liu J, Lu T, Zang L, Wang J, He Q, et al. SNHG1 knockdown upregulates miR-376a and downregulates FOXK1/snail axis to prevent tumor growth and metastasis in HCC. Mol Ther Oncolytics. 2021;21:264–277. doi: 10.1016/j.omto.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma YS, Chu KJ, Ling CC, Wu TM, Zhu XC, Liu JB, et al. Long noncoding RNA OIP5-AS1 promotes the progression of liver hepatocellular carcinoma via regulating hsa-miR-26a -3p/EPHA2 axis. Mol Ther - Nucl Acids. 2020;21:229–241. doi: 10.1016/j.omtn.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Ma J, Kong FF, Yang D, Yang H, Wang C, Cong R, et al. lncRNA MIR210HG promotes the progression of endometrial cancer by sponging miR-337-3p/137 via the HMGA2-TGF-β/Wnt pathway. Mol Ther Nucleic Acids. 2021;24:905–922. doi: 10.1016/j.omtn.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SM, Pang J, Zhang KJ, Zhou ZY, Chen FY. lncRNA MIR503HG inhibits cell proliferation and promotes apoptosis in TNBC cells via the miR-224-5p/HOXA9 axis. Mol Ther Oncolytics. 2021;21:62–73. doi: 10.1016/j.omto.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang L, Guo L, Wang J, Huang J, Lou X, Zhao M. Oncolytic virotherapy armed with an engineered interfering lncRNA exhibits antitumor activity by blocking the epithelial mesenchymal transition in triple-negative breast cancer. Cancer Lett. 2020;479:42–53. doi: 10.1016/j.canlet.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Yang W, Li N, Chen X, Ma F, Yang J, et al. LncRNA MCF2L-AS1 aggravates proliferation, invasion and glycolysis of colorectal cancer cells via the crosstalk with miR-874-3p/FOXM1 signaling axis. Carcinogenesis. 2021;42(2):263–271. doi: 10.1093/carcin/bgaa093. [DOI] [PubMed] [Google Scholar]
- 24.Ma YS, Liu JB, Lin L, Zhang H, Wu JJ, Shi Y, et al. Exosomal microRNA-15a from mesenchymal stem cells impedes hepatocellular carcinoma progression via downregulation of SALL4. Cell Death Discov. 2021;7(1):224. doi: 10.1038/s41420-021-00611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohosova J, Kubickova A, Slaby O. lncRNA PVT1 in the Pathogenesis and Clinical Management of Renal Cell Carcinoma. Biomolecules. 2021;11(5):664. doi: 10.3390/biom11050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Qian Y, Gao K, Zheng W, Wu G, He Q, et al. LncRNA BRCAT54 inhibits the tumorigenesis of non-small cell lung cancer by binding to RPS9 to transcriptionally regulate JAK-STAT and calcium pathway genes. Carcinogenesis. 2021;42(1):80–92. doi: 10.1093/carcin/bgaa051. [DOI] [PubMed] [Google Scholar]
- 27.Miao C, Zhou W, Wang X, Fang J. The research Progress of exosomes in osteoarthritis, With Particular Emphasis on the Mediating Roles of miRNAs and lncRNAs. Front Pharmacol. 2021;12:685623. doi: 10.3389/fphar.2021.685623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Liu X, Cui X, Tan Y, Wang Q, Wang Y, et al. LncRNA PRADX-mediated recruitment of PRC2/DDX5 complex suppresses UBXN1 expression and activates NF-κB activity, promoting tumorigenesis. Theranostics. 2021;11(9):4516–4530. doi: 10.7150/thno.54549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You B, Sun Y, Luo J, Wang K, Liu Q, Fang R, et al. Androgen receptor promotes renal cell carcinoma (RCC) vasculogenic mimicry (VM) via altering TWIST1 nonsense-mediated decay through lncRNA-TANAR. Oncogene. 2021;40(9):1674–1689. doi: 10.1038/s41388-020-01616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napoli M, Li X, Ackerman HD, Deshpande AA, Barannikov I, Pisegna MA, et al. Pan-cancer analysis reveals TAp63-regulated oncogenic lncRNAs that promote cancer progression through AKT activation. Nat Commun. 2020;11(1):5156. doi: 10.1038/s41467-020-18973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Yang T, Tang H, Sha Z, Chen R, Chen L, et al. Inhibition of lncRNA MAAT controls multiple types of muscle atrophy by cis- and trans-regulatory actions. Mol Ther. 2021;29(3):1102–1119. doi: 10.1016/j.ymthe.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shigeyasu K, Toden S, Ozawa T, Matsuyama T, Nagasaka T, Ishikawa T, et al. The PVT1 lncRNA is a novel epigenetic enhancer of MYC, and a promising risk-stratification biomarker in colorectal cancer. Mol Cancer. 2020;19(1):155. doi: 10.1186/s12943-020-01277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, He D, Fu Y, Zhang R, Guo H, Wang Z, et al. A novel lncRNA ARST represses glioma progression by inhibiting ALDOA- mediated actin cytoskeleton integrity. J Exp Clin Cancer Res. 2021;40(1):187. doi: 10.1186/s13046-021-01977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C, Zheng H, Luo Y, Kong Y, An M, Li Y, et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Invest. 2021;131(8):e146431. doi: 10.1172/JCI146431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey M, Mukhopadhyay A, Sharawat SK, Kumar S. Role of microRNAs in regulating cell proliferation, metastasis and chemoresistance and their applications as cancer biomarkers in small cell lung cancer. Biochim Biophys Acta Rev Cancer. 1876;2021(1):188552. doi: 10.1016/j.bbcan.2021.188552. [DOI] [PubMed] [Google Scholar]
- 36.Yang CP, Yang WS, Wong YH, Wang KH, Teng YC, Chang MH, et al. Muscle atrophy-related myotube-derived exosomal microRNA in neuronal dysfunction: targeting both coding and long noncoding RNAs. Aging Cell. 2020;19(5):e13107. doi: 10.1111/acel.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kottorou A, Dimitrakopoulos FI, Tsezou A. Non-coding RNAs in cancer- associated cachexia: clinical implications and future perspectives. Transl Oncol. 2021;14(7):101101. doi: 10.1016/j.tranon.2021.101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J, Li X, Zhang F, Zhu L, Chen Y. Transcriptome wide analysis of long non-coding RNA-associated ceRNA regulatory circuits in psoriasis. J Cell Mol Med. 2021;25(14):6925–6935. doi: 10.1111/jcmm.16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao C, Bai L, Yang Y, Huang J. Dysregulation of lncRNAs in rheumatoid arthritis: biomarkers, Pathogenesis and Potential Therapeutic Targets. Front Pharmacol. 2021;12:652751. doi: 10.3389/fphar.2021.652751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B, Liang RS, Wu XY, Lin YJ. LncRNA TUG1 inhibits neuronal apoptosis in status epilepticus rats via targeting the miR-421/mTOR axis. Cell Signal. 2020;76:109787. doi: 10.1016/j.cellsig.2020.109787. [DOI] [PubMed] [Google Scholar]
- 41.Bao Z, Zheng Q, Li L. Oncogenic roles and mechanisms of lncRNA AGAP2-AS1 in human solid tumors. Am J Transl Res. 2021;13(2):757–769. [PMC free article] [PubMed] [Google Scholar]
- 42.Hull R, Mbita Z, Dlamini Z. Long non-coding RNAs (LncRNAs), viral oncogenomics, and aberrant splicing events: therapeutics implications. Am J Cancer Res. 2021;11(3):866–883. [PMC free article] [PubMed] [Google Scholar]
- 43.Flores-Huerta N, Silva-Cázares MB, Arriaga-Pizano LA, Prieto-Chávez JL, López-Camarillo C. LncRNAs and microRNAs as essential regulators of Stemness in breast Cancer stem cells. Biomolecules. 2021;11(3):380. doi: 10.3390/biom11030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu B, Xu X, Yi P, Hao Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J Cell Mol Med. 2020;24(18):10855–10865. doi: 10.1111/jcmm.15714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo F, Wang H, Jiang M, Yang Q, Xiang Q, Zhou H, et al. TDP-43 induces EMT and promotes hepatocellular carcinoma metastasis via activating Wnt/β-catenin signaling pathway. Am J Cancer Res. 2020;10(10):3285–3301. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong JH, Xiang X, Wang YY, Liu X, Qi LN, Luo CP, et al. The lncRNA SNHG16 affects prognosis in hepatocellular carcinoma by regulating p62 expression. J Cell Physiol. 2020;235:1090–1102. doi: 10.1002/jcp.29023. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, Yu C, Zhan L, Pan Y, Chen L, Sun C. LncRNA CRNDE promotes hepatic carcinoma cell proliferation, migration and invasion by suppressing miR-384. Am J Cancer Res. 2016;6(10):2299–2309. [PMC free article] [PubMed] [Google Scholar]
- 48.Lan T, Ma W, Hong Z, Wu L, Chen X, Yuan Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:11. doi: 10.1186/s13046-016-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni F, Zhao H, Cui H, Wu Z, Chen L, Hu Z, et al. MicroRNA-362-5p promotes tumor growth and metastasis by targeting CYLD in hepatocellular carcinoma. Cancer Lett. 2015;356:809–818. doi: 10.1016/j.canlet.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Zhu D, Zhang X, Liu Y, Wang J, Yan L. Tanshinone IIA regulates fibroblast proliferation and migration and post-surgery arthrofibrosis through the autophagy-mediated PI3K and AMPK-mTOR signaling pathway. Am J Transl Res. 2021;13(2):565–584. [PMC free article] [PubMed] [Google Scholar]
- 51.Ma Y, Zhang H, Li G, Hu J, Liu X, Lin L. LncRNA ANRIL promotes cell growth, migration and invasion of hepatocellular carcinoma cells via sponging miR-144. Anti-Cancer Drugs. 2019;30:1013–1021. doi: 10.1097/CAD.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 52.Li YC, Li YN, Xu XS. The long noncoding RNA cardiac hypertrophy-related factor plays oncogenic roles in hepatocellular carcinoma by downregulating microRNA-211. J Cell Biochem. 2019;120:13361–13371. doi: 10.1002/jcb.28611. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Q, Zhang W, Wang Z, Liu S. Long non-coding RNA PTTG3P functions as an oncogene by sponging miR-383 and up-regulating CCND1 and PARP2 in hepatocellular carcinoma. BMC Cancer. 2019;19(1):731. doi: 10.1186/s12885-019-5936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J, Ye C, Huang Y, Huang D, Tang L, Hou W, et al. Corilagin suppresses RANKL-induced osteoclastogenesis and inhibits oestrogen deficiency-induced bone loss via the NF-κB and PI3K/AKT signalling pathways. J Cell Mol Med. 2020;24(18):10444–10457. doi: 10.1111/jcmm.15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei H, Hu J, Pu J, Tang Q, Li W, Ma R, et al. Long noncoding RNA HAGLROS promotes cell proliferation, inhibits apoptosis and enhances autophagy via regulating miR-5095/ATG12 axis in hepatocellular carcinoma cells. Int Immunopharmacol. 2019;73:72–80. doi: 10.1016/j.intimp.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 56.Qian YY, Li K, Liu QY, Liu ZS. Long non-coding RNA PTENP1 interacts with miR-193a-3p to suppress cell migration and invasion through the PTEN pathway in hepatocellular carcinoma. Oncotarget. 2017;8(64):107859–107869. doi: 10.18632/oncotarget.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo LH, Jin M, Wang LQ, Xu GJ, Lin ZY, Yu DD, et al. Long noncoding RNA TCL6 binds to miR-106a-5p to regulate hepatocellular carcinoma cells through PI3K/AKT signaling pathway. J Cell Physiol. 2020;235:6154–6166. doi: 10.1002/jcp.29544. [DOI] [PubMed] [Google Scholar]
- 58.Wu S, Chen S, Lin N, Yang J. Long non-coding RNA SUMO1P3 promotes hepatocellular carcinoma progression through activating Wnt/β-catenin signalling pathway by targeting miR-320a. J Cell Mol Med. 2020;24:3108–3116. doi: 10.1111/jcmm.14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou F, Lei Y, Xu X, Zhou H, Liu H, Jiang J, et al. LINC00355:8 promotes cell proliferation and migration with invasion via the MiR-6777-3p/Wnt10b axis in hepatocellular carcinoma. J Cancer. 2020;11(19):5641–5655. doi: 10.7150/jca.43831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian X, Wu Y, Yang Y, Wang J, Niu M, Gao S, et al. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling. Mol Oncol. 2020;14:462–483. doi: 10.1002/1878-0261.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duan Y, Chen J, Yang Y, Qu Z, Lu Y, Sun D. LncRNA HOTAIR contributes Taxol-resistance of hepatocellular carcinoma cells via activating AKT phosphorylation by down-regulating miR-34a. Biosci Rep. 2020;40(7):BSR20201627. doi: 10.1042/BSR20201627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong S, Xue H, Li Y, Li P, Ma F, Liu M, et al. The long noncoding RNA OTUD6B-AS1 enhances cell proliferation and the invasion of hepatocellular carcinoma cells through modulating GSKIP/Wnt/beta-catenin signalling via the sequestration of miR-664b-3p. Exp Cell Res. 2020;395(1):112180. doi: 10.1016/j.yexcr.2020.112180. [DOI] [PubMed] [Google Scholar]
- 63.Zhang N, Chen X. A positive feedback loop involving the LINC00346/beta-catenin/MYC axis promotes hepatocellular carcinoma development. Cell Oncol. 2020;43(1):137–153. doi: 10.1007/s13402-019-00478-4. [DOI] [PubMed] [Google Scholar]
- 64.Yao X, You G, Zhou C, Zhang D. LncRNA ASB16-AS1 promotes growth and invasion of hepatocellular carcinoma through regulating miR-1827/FZD4 Axis and activating Wnt/beta-catenin pathway. Cancer Manag Res. 2019;11:9371–9378. doi: 10.2147/CMAR.S220434. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Huang P, Xu Q, Yan Y, Lu Y, Hu Z, Ou B, et al. HBx/ERalpha complex-mediated LINC01352 downregulation promotes HBV-related hepatocellular carcinoma via the miR-135b-APC axis. Oncogene. 2020;39(18):3774–3789. doi: 10.1038/s41388-020-1254-z. [DOI] [PubMed] [Google Scholar]
- 66.Sun L, Wang L, Chen T, Shi Y, Yao B, Liu Z, et al. LncRNA RUNX1-IT1 which is downregulated by hypoxia-driven histone deacetylase 3 represses proliferation and cancer stem-like properties in hepatocellular carcinoma cells. Cell Death Dis. 2020;11:95. doi: 10.1038/s41419-020-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu F, Yuan JH, Huang JF, Yang F, Wang TT, Ma JZ, et al. Long noncoding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR-374a. Oncogene. 2016;35:5422–5434. doi: 10.1038/onc.2016.80. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y, Zhou Y, Huan L, Xu L, Shen M, Huang S, et al. LncRNA MIR22HG inhibits growth, migration and invasion through regulating the miR-10a-5p/NCOR2 axis in hepatocellular carcinoma cells. Cancer Sci. 2019;110:973–984. doi: 10.1111/cas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melchionna R, Trono P, Tocci A, Nisticò P. Actin cytoskeleton and regulation of TGFβ signaling: exploring their links. Biomolecules. 2021;11(2):336. doi: 10.3390/biom11020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corona A, Blobe GC. The role of the extracellular matrix protein TGFBI in cancer. Cell Signal. 2021;84:110028. doi: 10.1016/j.cellsig.2021.110028. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Yang S, Li S, Qu Y, Wang HY, Liu J, et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-β trap enhances antitumor efficacy of CAR-T cell therapy. Mol Ther Oncolytics. 2021;21:144–157. doi: 10.1016/j.omto.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu P, Li Z, Wang Y, Yu X, Shao X, Li YX, et al. miR-18a contributes to preeclampsia by downregulating Smad2 (full length) and reducing TGF-β signaling. Mol Ther Nucleic Acids. 2020;22:542–556. doi: 10.1016/j.omtn.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landry NM, Dixon IMC. Fibroblast mechanosensing, SKI and Hippo signaling and the cardiac fibroblast phenotype: Looking beyond TGF-β. Cell Signal. 2020;76:109802. doi: 10.1016/j.cellsig.2020.109802. [DOI] [PubMed] [Google Scholar]
- 74.Yang X, Cai JB, Peng R, Wei CY, Lu JC, Gao C, et al. The long noncoding RNA NORAD enhances the TGF-beta pathway to promote hepatocellular carcinoma progression by targeting miR-202-5p. J Cell Physiol. 2019;234(7):12051–12060. doi: 10.1002/jcp.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang WJ, Tian XP, Bi SX, Zhang SR, He TS, Song LY, et al. The beta-catenin/TCF-4-LINC01278-miR-1258-Smad2/3 axis promotes hepatocellular carcinoma metastasis. Oncogene. 2020;39(23):4538–4550. doi: 10.1038/s41388-020-1307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang YH, He GL, Huang SZ, Zhong KB, Liao H, Cai L, et al. The long noncoding RNA AK002107 negatively modulates miR-140-5p and targets TGFBR1 to induce epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Oncol. 2019;13(5):1296–1310. doi: 10.1002/1878-0261.12487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Jin Q, Jin X, Liu T, Lu X, Wang G, He N. A disintegrin and metalloproteinase 8 induced epithelial-mesenchymal transition to promote the invasion of colon cancer cells via TGF-β/Smad2/3 signalling pathway. J Cell Mol Med. 2020;24(22):13058–13069. doi: 10.1111/jcmm.15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Q, Wei S, Li L, Bu Q, Zhou H, Su W, et al. miR-139-5p sponged by LncRNA NEAT1 regulates liver fibrosis via targeting beta-catenin/SOX9/TGF-beta1 pathway. Cell Death Discov. 2021;7(1):243. doi: 10.1038/s41420-021-00632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ, et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7(27):42431–42446. doi: 10.18632/oncotarget.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang J, Zhuo H, Zhang X, Jiang R, Ji J, Deng L, et al. A novel biomarker Linc00974 interacting with KRT19 promotes proliferation and metastasis in hepatocellular carcinoma. Cell Death Dis. 2014;5(12):e1549. doi: 10.1038/cddis.2014.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang D, He Y, Mo Q, Liu E, Li X, Huang L, Zhang Q, Chen F, Li Y, Shao H. PRICKLE1, a Wnt/PCP signaling component, is overexpressed and associated with inferior prognosis in acute myeloid leukemia. J Transl Med. 2021;19(1):211. doi: 10.1186/s12967-021-02873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou MY, Cai L, Feng XW, Mu YR, Meng B, Liu FY, et al. Lentivirus-mediated overexpression or silencing of aquaporin 1 affects the proliferation, migration and invasion of TNF-α-stimulated rheumatoid arthritis fibroblast-like Synoviocytes by Wnt/β-catenin signaling pathway. J Inflamm Res. 2021;14:1945–1957. doi: 10.2147/JIR.S312783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng S, Liu J, Hailiang L, Wen J, Zhao Y, Li X, et al. Amplification of RAD54B promotes progression of hepatocellular carcinoma via activating the Wnt/β-catenin signaling. Transl Oncol. 2021;14(8):101124. doi: 10.1016/j.tranon.2021.101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bai J, Yao B, Wang L, Sun L, Chen T, Liu R, et al. lncRNA A1BG-AS1 suppresses proliferation and invasion of hepatocellular carcinoma cells by targeting miR-216a-5p. J Cell Biochem. 2019;120(6):10310–10322. doi: 10.1002/jcb.28315. [DOI] [PubMed] [Google Scholar]
- 85.Mohan CD, Rangappa S, Nayak SC, Sethi G, Rangappa KS. Paradoxical functions of long noncoding RNAs in modulating STAT3 signaling pathway in hepatocellular carcinoma. Biochim Biophys Acta Rev Cancer. 1876;2021(1):188574. doi: 10.1016/j.bbcan.2021.188574. [DOI] [PubMed] [Google Scholar]
- 86.Li M, Xu Y, Liang J, Lin H, Qi X, Li F, et al. USP22 deficiency in melanoma mediates resistance to T cells through IFNγ-JAK1-STAT1 signal axis. Mol Ther. 2021;29(6):2108–2120. doi: 10.1016/j.ymthe.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Y, Nie AQ, Chen S, Wang MM, Yin R, Tang BH, et al. Downregulation of renal MRPs transporters in acute lymphoblastic leukemia mediated by the IL-6/STAT3/PXR signaling pathway. J Inflamm Res. 2021;14:2239–2252. doi: 10.2147/JIR.S310687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ni H, Sun H, Zheng M, Bian T, Liu J, Li X, et al. Mining database for the expression and clinical significance of STAT family in head and neck squamous cell carcinomas. Transl Oncol. 2021;14(1):100976. doi: 10.1016/j.tranon.2020.100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang Y, Chen P, Hu K, Dai G, Li J, Zheng D, et al. Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation. J Cell Mol Med. 2021;25(3):1568–1582. doi: 10.1111/jcmm.16256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin Y, Jian Z, Jin H, Wei X, Zou X, Guan R, et al. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/β-catenin pathway. Cell Death Dis. 2020;11:34. doi: 10.1038/s41419-019-2188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lv J, Kong Y, Gao Z, Liu Y, Zhu P, Yu Z. LncRNA TUG1 interacting with miR-144 contributes to proliferation, migration and tumorigenesis through activating the JAK2/STAT3 pathway in hepatocellular carcinoma. Int J Biochem Cell Biol. 2018;101:19–28. doi: 10.1016/j.biocel.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Zhang B, Ding M, Lu S, Zhou H, Sun D, et al. C1QTNF1-AS1 regulates the occurrence and development of hepatocellular carcinoma by regulating miR-221-3p/SOCS3. Hepatol Int. 2019;13:277–292. doi: 10.1007/s12072-019-09944-5. [DOI] [PubMed] [Google Scholar]
- 93.Xue J, Cao Z, Cheng Y, Wang J, Liu Y, Yang R, et al. Acetylation of alpha-fetoprotein promotes hepatocellular carcinoma progression. Cancer Lett. 2020;471:12–26. doi: 10.1016/j.canlet.2019.11.043. [DOI] [PubMed] [Google Scholar]
- 94.Liu Q, Li B. The diagnostic value of ultrasound detection of the fetal middle cerebral artery, umbilical artery blood flow and fetal movement reduction in fetal distress. Am J Transl Res. 2021;13(4):3529–3535. [PMC free article] [PubMed] [Google Scholar]
- 95.Guo Y, Xu J, Du Q, Yan Y, Geller DA. IRF2 regulates cellular survival and Lenvatinib-sensitivity of hepatocellular carcinoma (HCC) through regulating β-catenin. Transl Oncol. 2021;14(6):101059. doi: 10.1016/j.tranon.2021.101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mohyeldeen M, Ibrahim S, Shaker O, Helmy H. Serum expression and diagnostic potential of long non-coding RNAs NEAT1 and TUG1 in viral hepatitis C and viral hepatitis C-associated hepatocellular carcinoma. Clin Biochem. 2020;84:38–44. doi: 10.1016/j.clinbiochem.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 97.Dai Q, Deng J, Zhou J, Wang Z, Yuan XF, Pan S, et al. Long non-coding RNA TUG1 promotes cell progression in hepatocellular carcinoma via regulating miR-216b-5p/DLX2 axis. Cancer Cell Int. 2020;20:8. doi: 10.1186/s12935-019-1093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z, Shen J, Chan MT, Wu WK. TUG1: a pivotal oncogenic long non-coding RNA of human cancers. Cell Prolif. 2016;49:471–475. doi: 10.1111/cpr.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deng X, Zhao XF, Liang XQ, Chen R, Pan YF, Liang J. Linc00152 promotes cancer progression in hepatitis B virus-associated hepatocellular carcinoma. Biomed Pharmacother. 2017;90:100–108. doi: 10.1016/j.biopha.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 100.Cai Q, Wang Z, Wang S, Weng M, Zhou D, Li C, et al. Long non-coding RNA LINC00152 promotes gallbladder cancer metastasis and epithelial-mesenchymal transition by regulating HIF-1α via miR-138. Open Biol. 2017;7(1):160247. doi: 10.1098/rsob.160247. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Li J, Wang X, Tang J, Jiang R, Zhang W, Ji J, et al. HULC and Linc00152 act as novel biomarkers in predicting diagnosis of hepatocellular carcinoma. Cell Physiol Biochem. 2015;37:687–696. doi: 10.1159/000430387. [DOI] [PubMed] [Google Scholar]
- 102.Zhao J, Liu Y, Zhang W, Zhou Z, Wu J, Cui P, et al. Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle. 2015;14:3112–3123. doi: 10.1080/15384101.2015.1078034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao Y, Xiong JB, Zhang GY, Liu Y, Jie ZG, Li ZR. Long noncoding RNA UCA1 regulates PRL-3 expression by sponging MicroRNA-495 to promote the progression of gastric Cancer. Mol Ther Nucleic Acids. 2020;19:853–864. doi: 10.1016/j.omtn.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Ma P, Wang H, Sun J, Liu H, Zheng C, Zhou X, et al. LINC00152 promotes cell cycle progression in hepatocellular carcinoma via miR-193a/b-3p/CCND1 axis. Cell Cycle. 2018;17(8):974–984. doi: 10.1080/15384101.2018.1464834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, Wu N, Wang J, Li Z. LncRNA MEG3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. J Cell Mol Med. 2019;23(10):6708–6719. doi: 10.1111/jcmm.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuo H, Tang J, Lin Z, Jiang R, Zhang X, Ji J, et al. The aberrant expression of MEG3 regulated by UHRF1 predicts the prognosis of hepatocellular carcinoma. Mol Carcinog. 2016;55:209–219. doi: 10.1002/mc.22270. [DOI] [PubMed] [Google Scholar]
- 107.Liu Z, Chen JY, Zhong Y, Xie L, Li JS. lncRNA MEG3 inhibits the growth of hepatocellular carcinoma cells by sponging miR-9-5p to upregulate SOX11. Braz J Med Biol Res. 2019;52:e8631. doi: 10.1590/1414-431X20198631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo SS, Li BX, Zou DB, Yang SJ, Sheng LX, Ouyang GF, et al. Tip of the iceberg: roles of circRNAs in hematological malignancies. Am J Cancer Res. 2020;10(2):367–382. [PMC free article] [PubMed] [Google Scholar]
- 109.Vasantharajan SS, Eccles MR, Rodger EJ, Pattison S, McCall JL, Gray ES, et al. The epigenetic landscape of circulating tumour cells. Biochim Biophys Acta Rev Cancer. 1875;2021(2):188514. doi: 10.1016/j.bbcan.2021.188514. [DOI] [PubMed] [Google Scholar]
- 110.Lin X, Chen W, Wei F, Xie X. TV-circRGPD6 nanoparticle suppresses breast Cancer stem cell-mediated metastasis via the miR-26b/YAF2 Axis. Mol Ther. 2021;29(1):244–262. doi: 10.1016/j.ymthe.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang T, Chen Y, Goodale D, Allan AL, Ronald JA. A survivin-driven, tumor- activatable minicircle system for prostate cancer theranostics. Mol Ther Oncolytics. 2021;20:209–219. doi: 10.1016/j.omto.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang M, Xu Y, Zhang Y, Li B, Lou G. Circular RNA circE2F2 promotes malignant progression of ovarian cancer cells by upregulating the expression of E2F2 protein via binding to HuR protein. Cell Signal. 2021;84:110014. doi: 10.1016/j.cellsig.2021.110014. [DOI] [PubMed] [Google Scholar]
- 113.Ming XL, Feng YL, He DD, Luo CL, Rong JL, Zhang WW, et al. Role of BCYRN1 in hepatocellular carcinoma pathogenesis by lncRNA-miRNA-mRNA network analysis and its diagnostic and prognostic value. Epigenomics. 2019;11(10):1209–1231. doi: 10.2217/epi-2018-0218. [DOI] [PubMed] [Google Scholar]
- 114.Ding S, Jin Y, Hao Q, Kang Y, Ma R. LncRNA BCYRN1/miR-490-3p/POU3F2, served as a ceRNA network, is connected with worse survival rate of hepatocellular carcinoma patients and promotes tumor cell growth and metastasis. Cancer Cell Int. 2020;20:6. doi: 10.1186/s12935-019-1081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao C, Zhang T, Zhang D, Xie L, Zou X, Lei L, et al. The long non-coding RNA, SNHG6-003, functions as a competing endogenous RNA to promote the progression of hepatocellular carcinoma. Oncogene. 2017;36:1112–1122. doi: 10.1038/onc.2016.278. [DOI] [PubMed] [Google Scholar]
- 116.Xu Y, Luo X, He W, Chen G, Li Y, Li W, et al. Long non-coding RNA PVT1/miR-150/HIG2 Axis regulates the proliferation, invasion and the balance of Iron metabolism of hepatocellular carcinoma. Cell Physiol Biochem. 2018;49:1403–1419. doi: 10.1159/000493445. [DOI] [PubMed] [Google Scholar]
- 117.Wang F, Yuan JH, Wang SB, Yang F, Yuan SX, Ye C, et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60:1278–1290. doi: 10.1002/hep.27239. [DOI] [PubMed] [Google Scholar]
- 118.Ge Y, Yan X, Jin Y, Yang X, Yu X, Zhou L, Han S, Yuan Q, Yang M. MiRNA-192 and miRNA-204 directly suppress lncRNA HOTTIP and interrupt GLS1-mediated Glutaminolysis in hepatocellular carcinoma. PLoS Genet. 2015;11(12):e1005726. doi: 10.1371/journal.pgen.1005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Q, Yang H, Cheng P, Han Q. Bioinformatic analysis of the prognostic value of the lncRNAs encoding snoRNAs in hepatocellular carcinoma. Biofactors. 2019;45(2):244–252. doi: 10.1002/biof.1478. [DOI] [PubMed] [Google Scholar]
- 120.Liu W, Zhan J, Zhong R, Li R, Sheng X, Xu M, Lu Z, Zhang S. Upregulation of long noncoding RNA_GAS5 suppresses cell proliferation and metastasis in laryngeal Cancer via regulating PI3K/AKT/mTOR signaling pathway. Technol Cancer Res Treat. 2021;20:1533033821990074. doi: 10.1177/1533033821990074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang DY, Zou XJ, Cao CH, Zhang T, Lei L, Qi XL, et al. Identification and functional characterization of long non-coding RNA as a tumor suppressor for hepatocellular carcinoma. Theranostics. 2018;8:3751–3765. doi: 10.7150/thno.22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Venit T, Dowaidar M, Gestin M, Mahmood SR, Langel Ü, Percipalle P. Transcriptional profiling reveals ribosome biogenesis, microtubule dynamics and expression of specific lncRNAs to be part of a common response to cell- penetrating peptides. Biomolecules. 2020;10(11):1567. doi: 10.3390/biom10111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J, Li K, Wang R, Chen S, Wu J, Li X, et al. The interplay between ATF2 and NEAT1 contributes to lung adenocarcinoma progression. Cancer Cell Int. 2020;20:594. doi: 10.1186/s12935-020-01697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q, Cheng Q, Xia M, Huang X, He X, Liao J. Hypoxia-induced lncRNA-NEAT1 sustains the growth of hepatocellular carcinoma via regulation of miR-199a-3p/UCK2. Front Oncol. 2020;10:998. doi: 10.3389/fonc.2020.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, Gu Y, Cheng X, Jiang H, Huang Y, Zhang Y, et al. Upregulation lnc-NEAT1 contributes to colorectal cancer progression through sponging miR-486-5p and activating NR4A1/Wnt/β-catenin pathway. Cancer Biomark. 2020;30(3):309–319. doi: 10.3233/CBM-201733. [DOI] [PubMed] [Google Scholar]
- 126.Mu L, Zhao H, Yang Y, Song R. Long noncoding RNA NEAT1 aggravates sorafenib-resistance in non-small cell lung cancer via regulating miRNA-335/c-met. J BUON. 2021;26(2):345–352. [PubMed] [Google Scholar]
- 127.Li X, Zhou Y, Yang L, Ma Y, Peng X, Yang S, et al. LncRNA NEAT1 promotes autophagy via regulating miR-204/ATG3 and enhanced cell resistance to sorafenib in hepatocellular carcinoma. J Cell Physiol. 2020;235:3402–3413. doi: 10.1002/jcp.29230. [DOI] [PubMed] [Google Scholar]
- 128.Chen F, Zhong Z, Tan HY, Guo W, Zhang C, Cheng CS, et al. Suppression of lncRNA MALAT1 by betulinic acid inhibits hepatocellular carcinoma progression by targeting IAPs via miR-22-3p. Clin Transl Med. 2020;10:e190. doi: 10.1002/ctm2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng J, Pang CH, Du W, Wang L, Sun LG, Xing ZY. An allele of rs619586 polymorphism in MALAT1 alters the invasiveness of meningioma via modulating the expression of collagen type V alpha. J Cell Mol Med. 2020;24(17):10223–10232. doi: 10.1111/jcmm.15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang H, Li W, Gu W, Yan Y, Yao X, Zheng J. MALAT1 accelerates the development and progression of renal cell carcinoma by decreasing the expression of miR-203 and promoting the expression of BIRC5. Cell Prolif. 2019;52:e12640. doi: 10.1111/cpr.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wüpper S, Lüersen K, Rimbach G. Cyclodextrins, natural compounds, and plant bioactives-a nutritional perspective. Biomolecules. 2021;11(3):401. doi: 10.3390/biom11030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Z, Dang C, Xing E, Zhao M, Shi L, Sun J. Overexpression of CASC2 improves cisplatin sensitivity in hepatocellular carcinoma through sponging miR-222. DNA Cell Biol. 2019;38:1366–1373. doi: 10.1089/dna.2019.4882. [DOI] [PubMed] [Google Scholar]
- 133.Ju B, Liu Z, Nai C, Zhu X. Long non-coding RNA CASC2 induces apoptosis and autophagy in human colon cancer cells via modulation of TRIM16 expression. Am J Transl Res. 2020;12(6):2695–2702. [PMC free article] [PubMed] [Google Scholar]
- 134.Li QY, Yang K, Liu FG, Sun XG, Chen L, Xiu H, et al. Long noncoding RNA CASC2c inhibited cell proliferation in hepatocellular carcinoma by inactivated ERK1/2 and Wnt/β-catenin signaling pathway. Clin Transl Oncol. 2020;22:302–310. doi: 10.1007/s12094-019-02223-7. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang C, et al. Long non-coding RNA CASC2 suppresses epithelial-mesenchymal transition of hepatocellular carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer. 2017;16:123. doi: 10.1186/s12943-017-0702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao L, Zhang Y, Zhang Y. Long noncoding RNA CASC2 regulates hepatocellular carcinoma cell oncogenesis through miR-362-5p/Nf-κB axis. J Cell Physiol. 2018;233:6661–6670. doi: 10.1002/jcp.26446. [DOI] [PubMed] [Google Scholar]
- 137.Wang B, Xian J, Zang J, Xiao L, Li Y, Sha M, et al. Long non-coding RNA FENDRR inhibits proliferation and invasion of hepatocellular carcinoma by down-regulating glypican-3 expression. Biochem Biophys Res Commun. 2019;509:143–147. doi: 10.1016/j.bbrc.2018.12.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.