Abstract
Purpose
To describe a unique case of Purtscher-like retinopathy after a severe, complicated COVID-19 course which included development of disseminated intravascular coagulation (DIC).
Observations
A 58-year-old male developed blurry vision in the left eye one week after being discharged from the hospital for severe COVID-19 pneumonia and DIC. He had been intubated and ventilated for 5 days. Fundus examination revealed optic nerve hyperemia in the right eye, optic nerve pallor in the left eye, arteriolar attenuation, multiple cotton wool spots and ill-defined areas of retinal whitening in the posterior pole in both eyes. His exam findings were most consistent with Purtscher-like retinopathy in both eyes.
Conclusions and Importance
While several cases of central retinal artery and vein occlusion have been described in COVID-19 patients thus far, there has not been any reported cases of Purtscher-like retinopathy. To the best of our knowledge, this is the first case of Purtscher-like retinopathy in a patient who developed DIC during a severe COVID-19 infection.
Keywords: COVID-19 retinopathy, Purtscher-like retinopathy, Disseminated intravascular coagulation in COVID-19
1. Introduction
The emergence of SARS-CoV-2 has caused a high burden of infection-associated complications in nearly every organ system. Conjunctivitis is the main ocular consequence of COVID-19 reported, with SARS-CoV-2 RNA detected in tear and conjunctival secretions.1,2 Retinal manifestations have also been reported in SARS-CoV-2. A prospective cross-sectional study by Sim et al. of 216 eyes demonstrated retinal microangiopathy in 11% of patients with COVID-19.3 The SERPICO-19 study showed dilation of the retinal vasculature, and a positive correlation between mean vein diameter and disease severity in subjects with COVID-19.4 In a case series of 12 patients with COVID-19, four had subtle cotton wool spots and microhemorrhages along the retinal arcades, but all retained normal visual acuity.5 There have also been several case reports of central retinal artery6, 7, 8, 9 and vein10, 11, 12, 13, 14, 15 occlusion in COVID-19. One study, however, did not find any retinal complications in 46 patients with severe COVID-19 pneumonia.16
To the best of our knowledge, this is the first case of Purtscher-like retinopathy described in a patient with severe COVID-19 pneumonia who also developed disseminated intravascular coagulation (DIC).
2. Case report
A 58-year-old male with a past medical history of gout and chronic thrombocytopenia had seizures, lost consciousness at home and was brought to the Emergency Room via ambulance. In the preceding weeks, his wife and daughter had both tested positive for COVID-19. The patient was febrile (102.3F) and had elevated inflammatory markers including D-dimer of 4770 ng/mL, C-reactive protein of 67.6 mg/L, and leukocytosis of 13.4. A chest X-ray showed multifocal pneumonia with ground-glass opacities. The patient tested positive for COVID-19 using the Roche COBAS SARS-CoV-2 and Influenza A/B PCR test on the LIAT instrument, which is only intended for use under the FDA's Emergency Use Authorization. He was diagnosed with COVID-19 pneumonia and was intubated due to respiratory failure with hypoxia and hypercapnia.
The patient's hospital course was complicated by DIC with bleeding from the nares, oropharynx, and gastrointestinal tract, secondary MRSA bacterial pneumonia, and multifactorial acute metabolic encephalopathy in the setting of respiratory failure, sepsis and decompensated alcoholic cirrhosis. His hemoglobin and hematocrit dropped from 10 g/dL and 31.7% to 6.9 g/dL and 22.1% respectively and his platelet count dropped from 37,000/μL to 32,000/μL. He also had prolonged PT and PTT at 16.6 and 36 seconds respectively. Other laboratory abnormalities included elevated procalcitonin (19.5 ng/mL), lactic acid (16.0 mmol/L), hyperammonemia (130 mcg/dL) and hyperbilirubinemia (2.9 mg/dL). Abdominal ultrasound demonstrated coarsened hepatic echotexture with a nodular capsule and trace ascites, which indicated cirrhosis.
He was placed on life support for 5 days. His treatment regimen included a course of remdesivir and decadron for COVID-19 pneumonia, as well as 6 days of intravenous vancomycin for MRSA pneumonia. He was given fresh frozen plasma, platelets, and packed red blood cells for DIC as well as lactulose and rifaximin for hepatic encephalopathy. He reported an alcohol intake of 12 standard drinks per week and was evaluated by gastroenterology. However, since he had no abdominal pain, nausea or vomiting, serum amylase and lipase were not checked. He was discharged after 15 days of hospitalization.
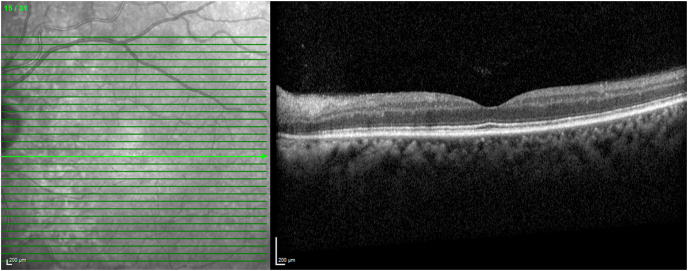
He noted blurry vision in the left eye one week after discharge from the hospital. One month after onset of visual symptoms, he presented to a community-based ophthalmologist with visual acuity of 20/25 in the right eye and 20/400 in the left eye, and a relative afferent pupillary defect in the left eye. Posterior segment exam revealed optic nerve hyperemia in the right eye and pallor in the left eye. There were numerous cotton wool spots with ill-defined areas of retinal whitening in the posterior pole, and arteriolar narrowing bilaterally. He was referred to our practice and on our exam a week later, visual acuity remained 20/25 in the right eye and had improved to 20/80 in the left eye. Cotton wool spots and arteriolar narrowing were again observed (Fig. 1). Areas of retinal whitening had improved and corresponded to inner retinal thinning on optical coherence tomography (OCT) (Fig. 2). These findings were most consistent with Purtscher-like retinopathy.
Fig. 1.
Fundus photographs and fluorescein angiographs of both eyes at presentation. Ultra wide field fundus photograph of the right (A) and left (D) eyes with enlargements of the posterior, poles (B, E) demonstrate presence of optic nerve hyperemia in the right eye and optic nerve pallor in the left eye, cotton wool spots (white arrows) with patches of retinal whitening (blue arrows) in the macula and peripheral white centered hemorrhages (red arrows). Ultra wide field fluorescein angiographs of the right (C) and left (F) eyes show peripheral microaneurysms (red arrows) and peripheral nonperfusion (white stars). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Optical coherence tomograph of the left eye at presentation. Optical coherence tomograph of the left eye demonstrates marked thinning of the inner retinal layers especially affecting the nerve fiber layer and ganglion cell layer.
Peripheral Roth spots, microaneurysms and nonperfusion were also noted bilaterally. Carotid doppler ultrasound demonstrated no significant stenosis bilaterally. The patient denied a history of hypertension, and blood pressure on hospital discharge was 109/57. Laboratory workup with FTA-ABS, quantiferon gold and rheumatoid factor were negative. Serum protein electrophoresis revealed no M spike. Because of a negative review of systems for systemic lupus erythematosus and other autoimmune conditions, a rheumatology consultation was not pursued. MRI brain with and without contrast revealed several nonspecific hyperintense foci on the FLAIR sequences measuring 5mm or less in the periarticular white matter or subcortical white matter of the frontal and parietal lobes bilaterally. The optic nerves were normal and showed no enhancement.
3. Discussion
To the best of our knowledge, this is the first report of Purtscher-like retinopathy in a patient with COVID-19 and DIC. Purtscher retinopathy was originally characterized by multiple areas of retinal whitening in the posterior pole of the eye, known as Purtscher flecken. Other acute fundus abnormalities include cotton wool spots, retinal hemorrhages, and hyperemic optic disc. In addition to these findings, our patient also demonstrated bilateral optic neuropathy, which is consistent with prior reports; in a systematic review of Purtscher and Purtscher-like retinopathy, Miguel et al. reported that 64% of affected patients had optic neuropathy.17 Our patient's OCT findings of diffuse thinning along the inner retinal architecture has been previously reported in Purtscher-like retinopathy as well: Viola et al. demonstrated similar OCT findings in a patient with Purtscher-like retinopathy, septicemic DIC and nephrotic syndrome.18 In addition to the retina, our patient had evidence of hypoxic insult to multiple other organ systems including the lung, liver and brain. He had white matter lesions on brain MRI, which potentially represented leukoencephalopathy and demyelination in the setting of hypoxic insults.19
Purtscher retinopathy is classically associated with trauma, while Purtscher-like retinopathy has been associated with acute pancreatitis, renal failure, surgery, and DIC. In particular, Purtscher-like retinopathy has been reported in patients with DIC and concurrent nephrotic syndrome18 and HELLP syndrome.20 To the best of our knowledge, this is the first case report of Purtscher-like retinopathy documented in a patient with COVID-19 and DIC. A recent meta-analysis demonstrated that DIC occurs in 3% of patients with COVID-19.21 Prognosis is poor for COVID-19 patients who develop DIC: 0.6% of survivors and 71.4% of non-survivors had COVID-19 complicated by DIC.22 Given the enormous global burden of the COVID-19 pandemic, our case illustrates the importance of recognizing the early signs of Purtscher-like retinopathy in patients with COVID-19 as these eye findings may point towards the development of a potentially fatal systemic complication like DIC.
Purtscher-like retinopathy has been related to embolic occlusion of the capillary arterioles from intravascular aggregation of leukocytes, platelets and fibrin in response to abnormal complement activation.23 SARS-CoV-2 is thought to alter complement pathways causing enhanced clot formation, impaired fibrin clearance and increased vascular bed resistance in end organs, thus leading to microthrombosis and a systemic hypercoagulable state.24 Specifically, the virus binds to angiotensin-converting enzyme 2 along endothelial cells and activates two of the three complement pathways, starting with the lectin pathway and then later the classical pathway. This leads to the formation of complement 3b and a consequential increase in both C5a and C5b-9.25 Concentrations of C5a, in particular, has been shown to be abnormally high in patients with COVID-19 26 Derangement of the complement, fibrinolysis and coagulation systems in COVID-19 is thought to cause neutrophils to be unresponsive to anaphylatoxins – thus decreasing their antimicrobial capabilities, macrophages to produce excessive cytokines - hence causing cytokine storms, and endothelial cells to produce tissue factor - which can lead to DIC.27 Whether the patient's Purstcher-like retinopathy is directly caused by DIC or COVID-19 remains indeterminate. However, given the hypothesized pathogenesis of Purtscher-like retinopathy, our patient's eye findings were likely triggered by dysregulation of the complement, fibrinolysis and coagulation pathways in the course of his COVID-19 infection, which included the development of DIC that further exacerbated hypercoagulability and microthrombi formation.
4. Conclusion
This report represents a rare case of Purtscher-like retinopathy in the setting of severe COVID-19 infection and DIC. The described pathogenic pathway should be taken in the context of other events in the natural history of this case.
Patient consent
The patient consented to publication of the case in writing.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
No conflicting relationship exists for any author.
Acknowledgements
The authors gratefully thank R. Tyler Davis, DO for referring and sharing clinical information about the patient with us, and William Graham Jr. for his expertise in image acquisition.
References
- 1.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L., Deng C., Chen X., et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. Dec 2020;98(8):e951–e959. doi: 10.1111/aos.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sim R., Cheung G., Ting D., et al. Retinal microvascular signs in COVID-19. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2020-318236. [DOI] [PubMed] [Google Scholar]
- 4.Invernizzi A., Torre A., Parrulli S., et al. Retinal findings in patients with COVID-19: results from the SERPICO-19 study. EClinicalMedicine. 2020;27:100550. doi: 10.1016/j.eclinm.2020.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinho P.M., Marcos A.A.A., Romano A.C., Nascimento H., Belfort R.J. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya S., Diamond M., Anwar S., Glaser A., Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bapaye M.M., Nair A.G., Bapaye C.M., Bapaye M.M., Shukla J.J. Simultaneous bilateral central retinal artery occlusion following COVID-19 infection. Ocul Immunol Inflamm. Apr 15 2021:1–4. doi: 10.1080/09273948.2021.1891262. [DOI] [PubMed] [Google Scholar]
- 8.Montesel A., Bucolo C., Mouvet V., Moret E., Eandi C.M. Case report: central retinal artery occlusion in a COVID-19 patient. Front Pharmacol. 2020;11:588384. doi: 10.3389/fphar.2020.588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turedi N., Onal Gunay B. Paracentral acute middle maculopathy in the setting of central retinal artery occlusion following COVID-19 diagnosis. Eur J Ophthalmol. 2021;14 doi: 10.1177/1120672121995347. 1120672121995347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahalomi T., Pikkel J., Arnon R., Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient: a case report. Am J Ophthalmol Case Rep. 2020;20:100992. doi: 10.1016/j.ajoc.2020.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walinjkar J.A., Makhija S.C., Sharma H.R., Morekar S.R., Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68(11):2572–2574. doi: 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaba W.H., Ahmed D., Al Nuaimi R.K., Dhanhani A.A., Eatamadi H. Bilateral central retinal vein occlusion in a 40-year-old man with severe coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep. Oct 29 2020;21 doi: 10.12659/AJCR.927691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Invernizzi A., Pellegrini M., Messenio D., et al. Impending central retinal vein occlusion in a patient with coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. Nov 16 2020;28(8):1290–1292. doi: 10.1080/09273948.2020.1807023. [DOI] [PubMed] [Google Scholar]
- 14.Raval N., Djougarian A., Lin J. Central retinal vein occlusion in the setting of COVID-19 infection. J Ophthalmic Inflamm Infect. 2021;11(1):10. doi: 10.1186/s12348-021-00241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatesh R., Reddy N.G., Agrawal S., Pereira A. COVID-19-associated central retinal vein occlusion treated with oral aspirin. BMJ Case Rep. May 19 2021;(5):14. doi: 10.1136/bcr-2021-242987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirraglia M.P., Ceccarelli G., Cerini A., et al. Retinal involvement and ocular findings in COVID-19 pneumonia patients. Sci Rep. Oct 15 2020;10(1):17419. doi: 10.1038/s41598-020-74446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miguel A.I., Henriques F., Azevedo L.F., Loureiro A.J., Maberley D.A. Systematic review of Purtscher's and Purtscher-like retinopathies. Eye (Lond) 2013;27(1):1–13. doi: 10.1038/eye.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viola F., Vezzola D., Villani E., Mapelli C., Barteselli G., Ratiglia R. Purtscher-like retinopathy in septicemic disseminated intravascular coagulation associated with nephrotic syndrome. Eur J Ophthalmol. 2013 Jul-Aug 2013;23(4):601–603. doi: 10.5301/ejo.5000290. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular disease in COVID-19. AJNR Am J Neuroradiol. 2020;41(7):1170–1172. doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart M.W., Brazis P.W., Guier C.P., Thota S.H., Wilson S.D. Purtscher-like retinopathy in a patient with HELLP syndrome. Am J Ophthalmol. 2007;143(5):886–887. doi: 10.1016/j.ajo.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X., Cheng Z., Luo L., et al. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb Res. 2021;201:23–29. doi: 10.1016/j.thromres.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripathy K, Patel BC. Purtscher Retinopathy. Treasure Island (FL): StatPearls Publishing. Accessed June 7, 2021. https://www.ncbi.nlm.nih.gov/books/NBK542167/. [PubMed]
- 24.Java A., Apicelli A.J., Liszewski M.K., et al. The complement system in COVID-19: friend and foe? JCI Insight. 08 06 2020;(15):5. doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo M.W., Kemper C., Woodruff T.M. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020;205(6):1488–1495. doi: 10.4049/jimmunol.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurosawa S., Stearns-Kurosawa D.J. Complement, thrombotic microangiopathy and disseminated intravascular coagulation. J Intens Care. 2014;2(1):65. doi: 10.1186/s40560-014-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]