Abstract
This is a review of the development of bumped-kinase inhibitors (BKIs) for the therapy of One Health parasitic apicomplexan diseases. Many apicomplexan infections are shared between humans and livestock, such as cryptosporidiosis and toxoplasmosis, as well as livestock only diseases such as neosporosis. We have demonstrated proof-of-concept for BKI therapy in livestock models of cryptosporidiosis (newborn calves infected with Cryptosporidium parvum), toxoplasmosis (pregnant sheep infected with Toxoplasma gondii), and neosporosis (pregnant sheep infected with Neospora caninum). We discuss the potential uses of BKIs for the treatment of diseases caused by apicomplexan parasites in animals and humans, and the improvements that need to be made to further develop BKIs.
Introduction
Apicomplexa afflict humans and livestock causing morbidity, mortality, and reproductive loss. This review discusses the apicomplexan parasites most likely to respond to a class of compounds called bumped-kinase inhibitors (BKIs), namely Cryptosporidium parvum, C. hominis, Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, Cystoisospora suis, and Besnoitia besnoiti.
Cryptosporidiosis annually afflicts millions of children, less than 2 years-old, with diarrhea in resource limited countries (Khalil et al., 2018). Cryptosporidiosis is responsible for about 25% of moderate-to-severe diarrhea (Kotloff et al., 2013). Cryptosporidiosis is associated with a more than 2-fold increased risk of death compared to children without Cryptosporidium with moderate-to-severe diarrhea; these children with moderate-to-severe diarrhea already have a more than 8-fold increased risk of dying compared to non-ill controls (Kotloff et al., 2013). Furthermore, cryptosporidiosis in children in resource-limited countries is highly associated with stunting, and stunting itself is associated with increased mortality and decreased neurodevelopment (Checkley et al., 1998; Checkley et al., 2015; Delahoy et al., 2018; Khalil et al., 2018; Korpe et al., 2018). Cryptosporidium is also a severe and sometimes fatal infection in immunocompromised patients, such as those with severe HIV or those immunosuppressed for transplantation (Silverlås et al., 2009; Trotz-Williams et al., 2011; White, 2020). For humans, only nitazoxanide is approved for cryptosporidiosis therapy. However, nitazoxanide is only minimally efficacious for malnourished children and immunocompromised individuals, those who need therapy the most (Amadi et al., 2002; Amadi et al., 2009; Checkley et al., 2015). In animal health, cryptosporidiosis also takes a severe toll on newborn calves, and is associated with a 34 kg weight gain failure (Shaw, 2017; Shaw et al., 2020). For treatment of livestock, the only licensed product is halofuginone, which reduces the incidence of cryptosporidiosis about 40% if given metaphylactically. However, halofuginone is not effective as a therapeutic once cryptosporidiosis diarrhea is established (Silverlås et al., 2009; Trotz-Williams et al., 2011).
Toxoplasma gondii infection in pregnancy is associated with fetal loss and fetal and newborn abnormalities in both humans and livestock, such as sheep, goats, and equids (Bigna et al., 2020; Dubey, 2009a, b; Dubey et al., 2020a; Dubey et al., 2020b; Rostami et al., 2019; Stelzer et al., 2019). T. gondii causes infections in the eye (retina) and brain, necessitating drugs that cross the placenta, brain, and eye barriers for penetration. In addition, acute toxoplasmosis can be caused by (partial) immunosuppression and reactivation of parasites from normally dormant tissue cyst stage bradyzoites. Available drugs in humans include pyrimethamine/sulfadiazine combination, clindamycin, spiramycin, and atovaquone. However, none of these drugs are very active and up to 40% of patients with moderate to severe toxoplasmosis have to stop therapy due to drug toxicity (Neville et al., 2015; Sanchez-Sanchez et al., 2018b).
Neospora caninum infection in cattle and sheep leads to repeated abortions and an estimated economic loss of 1.3 billion USD per year (Gonzalez-Warleta et al., 2018; Gonzalez-Warleta et al., 2014; Reichel et al., 2013). Current experimental therapies for neosporosis include ponazuril, toltrazuril, decoquinate, and monensin, but none have been shown to be significantly effective in ruminant pregnancy models (Sanchez-Sanchez et al., 2018b).
Calcium-dependent protein kinase 1 and bumped-kinase inhibitors
Calcium-dependent protein kinase 1 (CDPK1) was first recognized to be important in Toxoplasma gondii gliding motility, exocytosis, and cell entry and exit (Kieschnick et al., 2001; Nagamune and Sibley, 2006). When the crystal structures of T. gondii and Cryptosporidium parvum CDPK1s were solved, it was discovered that the gatekeeper of this protein kinase was glycine (Murphy et al., 2010; Ojo et al., 2010; Wernimont et al., 2010). This allowed selective targeting of these kinases with a class of molecules called “bumped-kinase inhibitors” or BKIs, that take advantage of a hydrophobic pocket that is opened up next to the gatekeeper when small sidechain gatekeepers, such as glycine, are present (Bishop et al., 1998; Bishop and Shokat, 1999) (Figure 1, left). BKIs provide increased potency by inhibiting CDPK1 through the hydrophobic “bump” that fills the pocket next to the glycine gatekeeper. But, more importantly, BKIs are excluded from almost all mammalian protein kinases in that they almost uniformly have gatekeeper side-chain residues larger than the hydrogen of glycine (Figure 1, right). Apicomplexan parasites of human and veterinary public health importance, with a CDPK1 known to have a glycine gatekeeper and thus highly sensitive to inhibition by BKIs, include: C. parvum, C. hominis, T. gondii, Neospora caninum, Sarcocystis neurona, Besnoitia besnoiti, and Cystoisospora suis (Jimenez-Melendez et al., 2017; Murphy et al., 2010; Ojo et al., 2016; Ojo et al., 2010; Ojo et al., 2014; Shrestha et al., 2019).
Figure 1: Bumped Kinase Inhibitors (BKIs) exploit the small gatekeeper structural differences to achieve both potency and specificity.
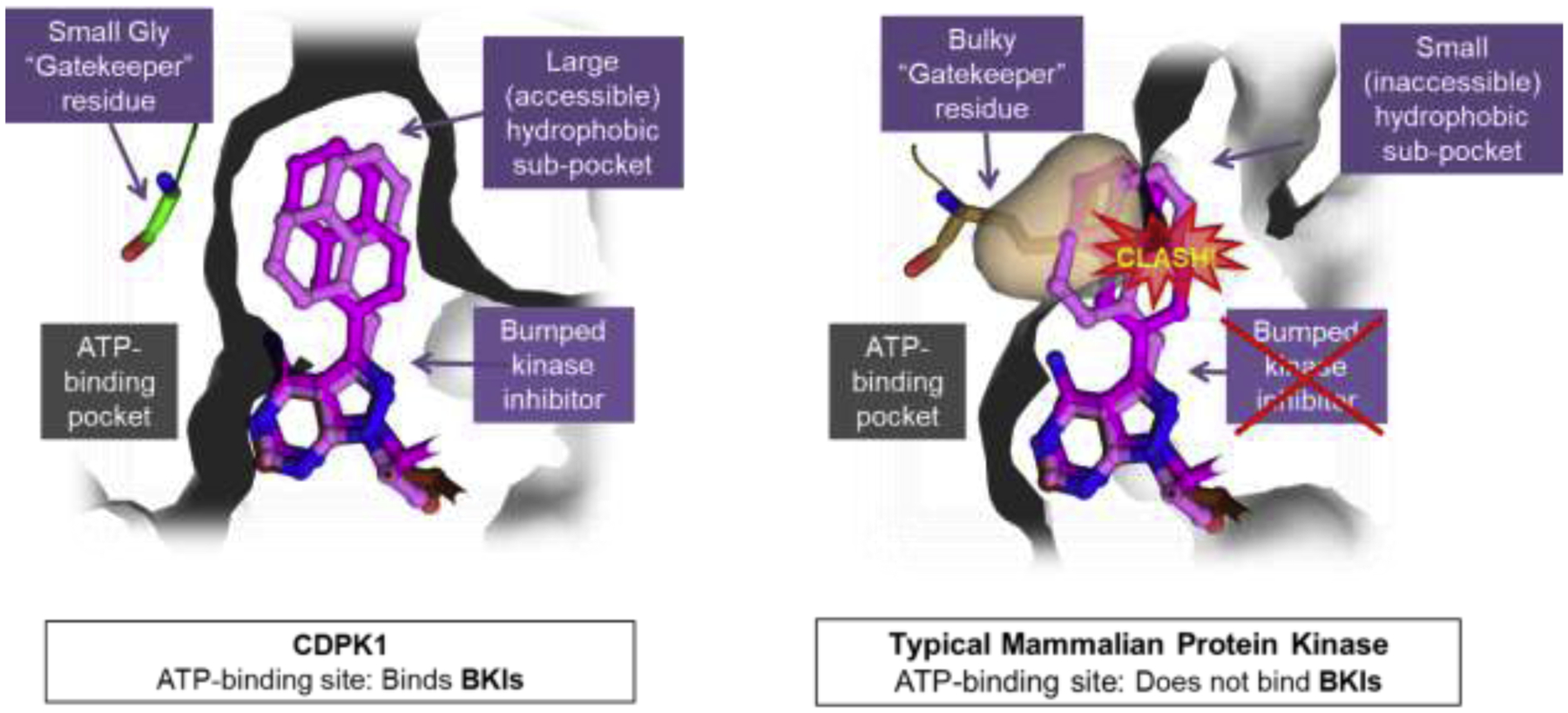
Shown at left is the binding site of T. gondii CDPK1 with a small glycine (Gly) gatekeeper residue, forming a large hydrophobic sub-pocket that allows the BKI to bind at the ATP-binding pocket of CDPK1. On the right is the binding pocket of a typical mammalian kinase with a bulky gatekeeper residue, in this case methionine, demonstrating a clash takes place with the “bump” of BKIs, such that BKIs are excluded from the ATP binding pocket, granting BKIs specificity over almost all mammalian protein kinases.
The most effective BKIs are made on two scaffolds, the pyrazolo[2,3-d]pyrimidine (PP) and the 5-aminopyrazole-4-carboxamide (AC) scaffolds (Figure 2) (Huang et al., 2017; Huang et al., 2015; Johnson et al., 2012; Murphy et al., 2010; Zhang et al., 2014). To date, we have made over 750 BKIs optimized for potency against T. gondii and C. parvum CDPK1 and anti-apicomplexan parasite activity. BKI safety parameters, including limiting mammalian protein kinase activity, pharmacokinetics (PK), including absorption, distribution, metabolism, and excretion properties (ADME), have been investigated.
Figure 2:
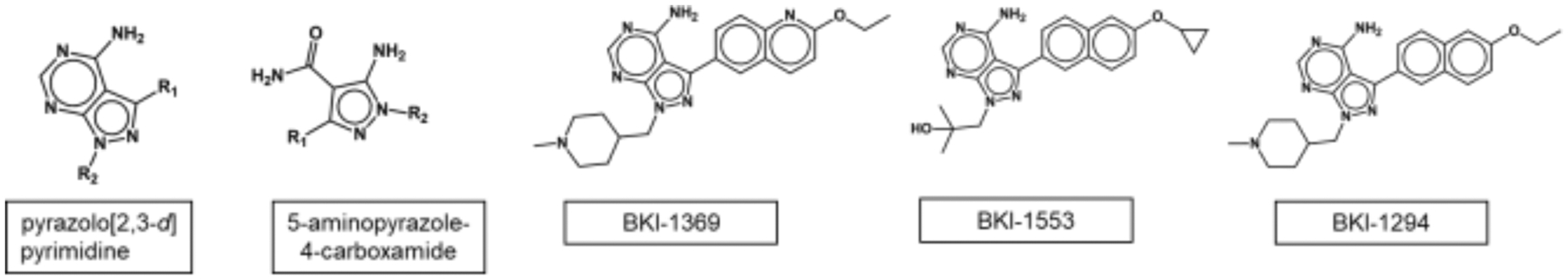
Pyrazolo-pyrimidine and aminopyrazole-carboxamide scaffolds, compounds described in this paper
BKIs for Cryptosporidiosis
Our lead for anti-cryptosporidiosis therapy is a PP compound, BKI-1369 (Figure 2). The characteristics of BKI-1369 are summarized in Table 1. It inhibits 50% of the C. parvum CDPK1 (CpCDPK1) recombinant enzyme phosphorylation activity (IC50) at 0.9 nM, but has no detectable inhibition at 10 μM of mammalian SRC protein kinase activity, which we use as a counterscreen for kinase specificity since SRC has one of the smallest mammalian gatekeepers, threonine (Hulverson et al., 2017b). BKI-1369 inhibits C. parvum nanoluciferase-expressing (Nluc)-UGA strain by 50% (EC50) at 2.4 μM, yet doesn’t inhibit mammalian cell line proliferation up to the limit of solubility, 80 μM (Hulverson et al., 2017a). It does not demonstrate genotoxic properties in the modified AMES assay, has reasonable solubility properties at both pH 2 and pH 6.5, and demonstrates only moderate plasma protein binding in the four species of plasma tested (Arnold et al., 2017). One issue with BKI-1369 is that it blocks the human Ether-à-go-go-Related Gene (hERG) receptor at 2 μM concentration, which is associated with human cardiotoxicity and this may preclude its development for human use. Additionally, BKI-1369 also actively inhibits other strains of C. parvum and C. hominis, the most common species of Cryptosporidium found in humans in the developing world (Table 2) (Hulverson et al., 2017a).
Table 1:
Properties of BKI-1369
| Compound | C. parvum CDPK1 IC50 (μM) | Human SRC IC50 (μM) | NIuc-C. parvum EC50 (μM) | Mammalian cytotox CC50 (μM) | Cardiotox hERG IC50 (μM) | MUTAGEN/GENOTOX | Aqueous SOLUBILITY (μM) | % PLASMA PROTEIN BINDING | |
|---|---|---|---|---|---|---|---|---|---|
| BKI-1369 | 0.0009 | >10 | 2.5 | >80 | 1.5 | (−) | 100 | 77 | 40 |
| Human | Dog | ||||||||
| >80 | 54 | 40 | 76 | ||||||
| Mouse | Rat | ||||||||
IC50: Concentration in micromolar (μM) that gives 50% inhibition of enzyme activity; Human SRC: Proto-oncogene tyrosine-protein kinase SRC; CC50: concentration that yields 50% growth inhibition (cytotoxicity) of mammalian CRL-8155 or HEPG2 cell lines; hERG: human ether-a-go-go related gene, a potassium channel found in heart tissue; Modified AMES test is the AMES test that includes liver microsome metabolized compound; Aqueous endpoint solubility performed at 2 pHs shown; and plasma protein binding shows the bound percentage of compound when incubated with plasma from species shown. Adapted from data in (Hulverson et al., 2017a; Hulverson et al., 2017b)
Table 2:
In vitro potency of BKI-1369 against various Cryptosporidium isolates
| Cryptosporidium Isolate | EC50 (μM) |
|---|---|
| Cryptosporidium Production Lab Iowa C. parvum1 | 0.8 |
| Bunch Grass Farm C. parvum1 | 1.1 |
| C. hominis 1 | 0.3 |
Assayed using high-content imaging for Cryptosporidium proliferation: Melissa Love and Case McNamara, CALIBR/Scripps, La Jolla, CA, adapted from data in (Hulverson et al., 2017a)
Testing of BKI-1369 in animal models of C. parvum and C. hominis demonstrated efficacy leading to profound reductions in parasite shedding, and in clinical models in reduction of diarrhea and better health outcomes. For instance, in the Nluc-C. parvum gamma-interferon knockout mouse model, 60 mg/kg and 30 mg/kg, administered once a day on days 6–10 after infection, led to maximal clearing of infection, but even 5 mg/kg and 15 mg/kg were effective at reducing parasite excretion (Figure 3) (Hulverson et al., 2017a). In the newborn calf model of C. parvum infection, twelve two day-old calves were infected with 5 × 107 C. parvum Iowa strain oocysts (Cryptosporidium Production Lab, University of Arizona), and on day 2 P.I., randomized for treatment with BKI-1369 (5 mg/kg twice a day for 5 days) or vehicle control(Heine et al., 1984; Riggs and Schaefer, 2020). Calves that were treated with BKI-1369 had a 30-fold reduction in total oocyst excretion on days 3–10 P.I., and had almost immediate resolution of diarrhea, with a significant reduction of fecal volume on days 4–8 P.I.. The treated group had a 4.5% weight gain whereas placebo treated animals had a slight weight loss by 10 days P.I. (Hulverson et al., 2017a) (Figure 4). In the gnotobiotic piglet model, eighteen piglets were infected with 106 C. hominis oocysts orally two days after birth, then randomized to treatment or vehicle only, twice a day for five days, and then followed for 10 days after therapy. In this C. hominis model, BKI-1369 administered at 10 mg/kg twice a day was superior to vehicle control in total oocyst excretion (~10-fold reduction of oocysts in feces) (Figure 5). C. hominis DNA excreted in stool, and diarrhea score were also significantly better in BKI-1369 group on days 1–3 post treatment (Lee et al., 2018).
Figure 3: BKI-1369 is effective at reducing nanoluciferase-tagged C. parvum from gamma-interferon knock out mice:
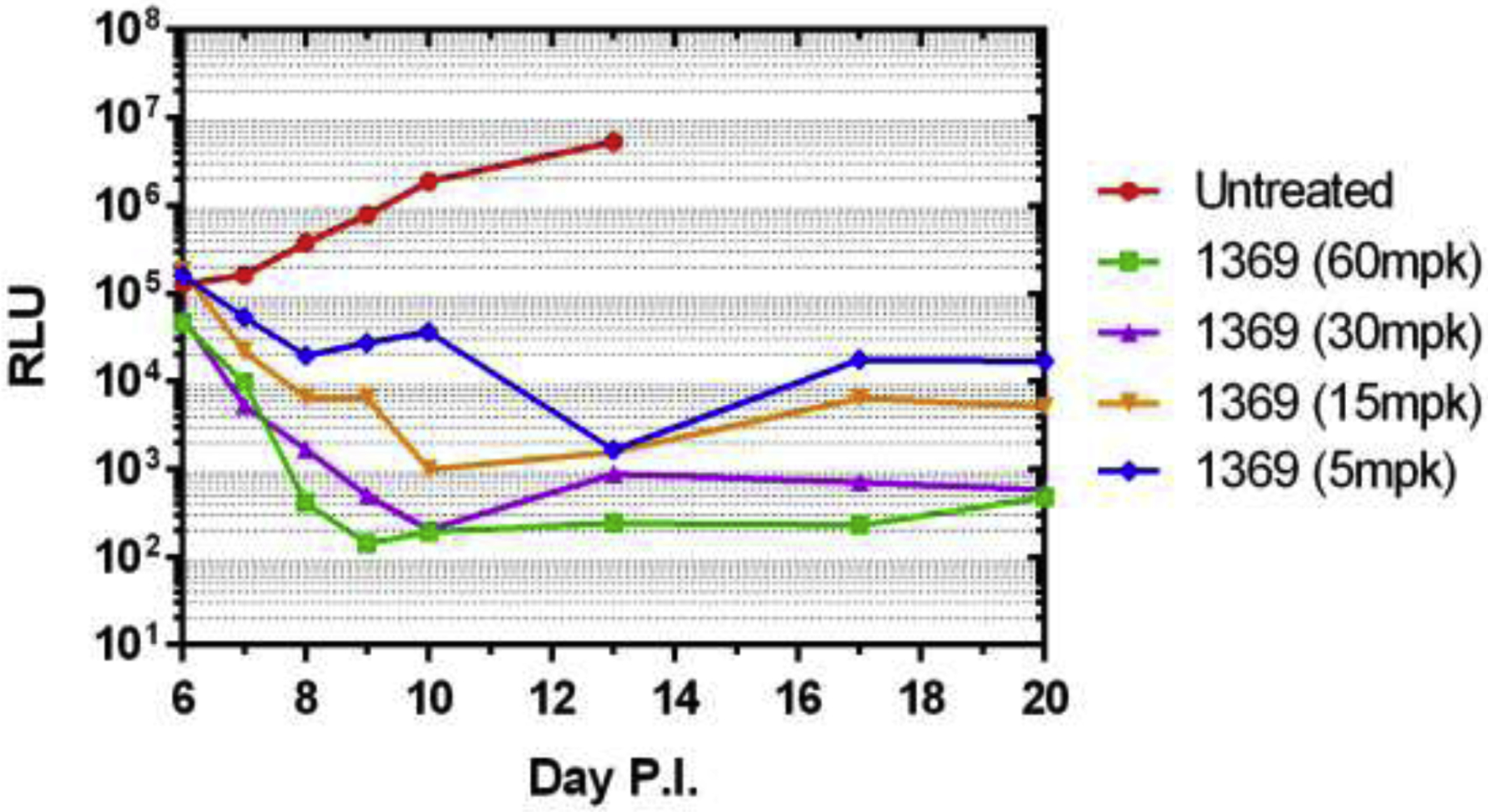
BKI-1369 was administered orally at the doses shown (mpk = milligrams per kg), on day 6–10 post infection (P.I.) with 10,000 oocysts of Nluc-UGA1 C. parvum. The stool was isolated and relative luminescence units (RLU) are shown on the days P.I. Data from that published in (Hulverson et al., 2017a).
Figure 4: C. parvum neonatal calf model demonstrates efficacy of BKI-1369 via reduction in total oocyst excretion, diminished fecal output, and favorable weight gain compared with control infected calves.
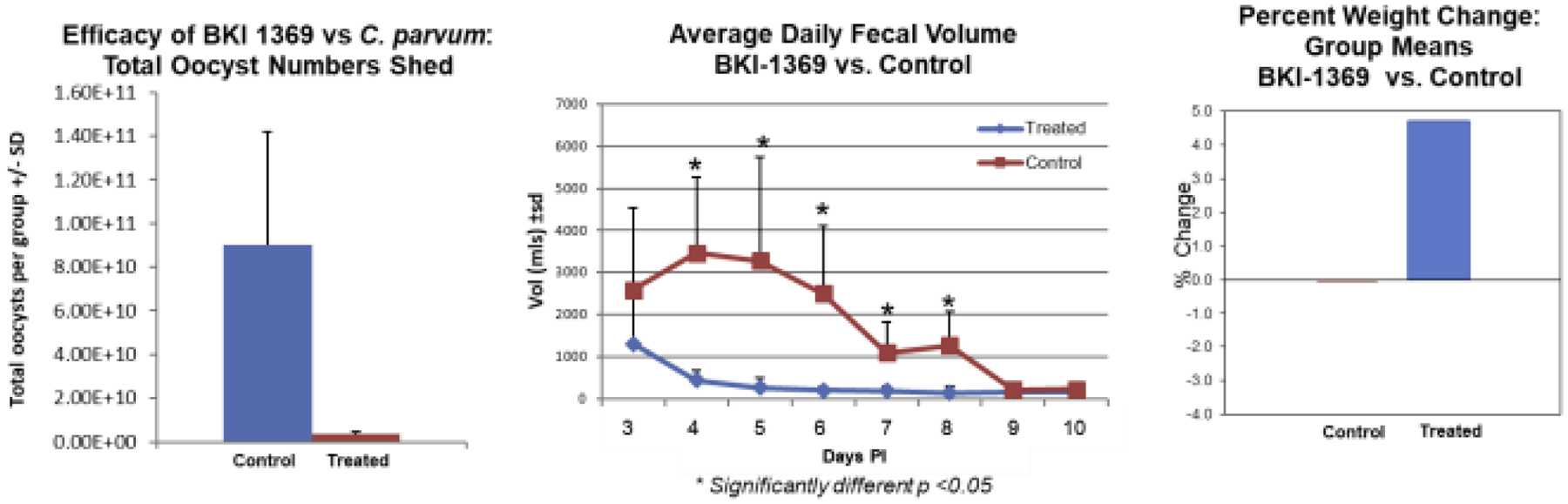
Two-day old calves were infected orally with 5×107 C. parvum (Iowa strain, Cryptosporidium Production Laboratory, Univ. AZ) and two days later begun on BKI-1369 treatment or vehicle alone. Shown, left to right, are (1) the total number of oocysts excreted over the 5 days of treatment and 3 days after treatment in each group of control (vehicle alone treated on days 3–7 post infection (P.I.)) or treated (BKI-1369 5 mg/kg administered twice a day orally); (2) the total daily fecal volume excreted by the calves in each group; and, (3) weight gain or loss in each group over the 10-day observation period. Data replotted from (Hulverson et al., 2017a).
Figure 5: Gnotobiotic piglet C. hominis infection shows a significant reduction in oocysts excreted during and after therapy with BKI-1369.
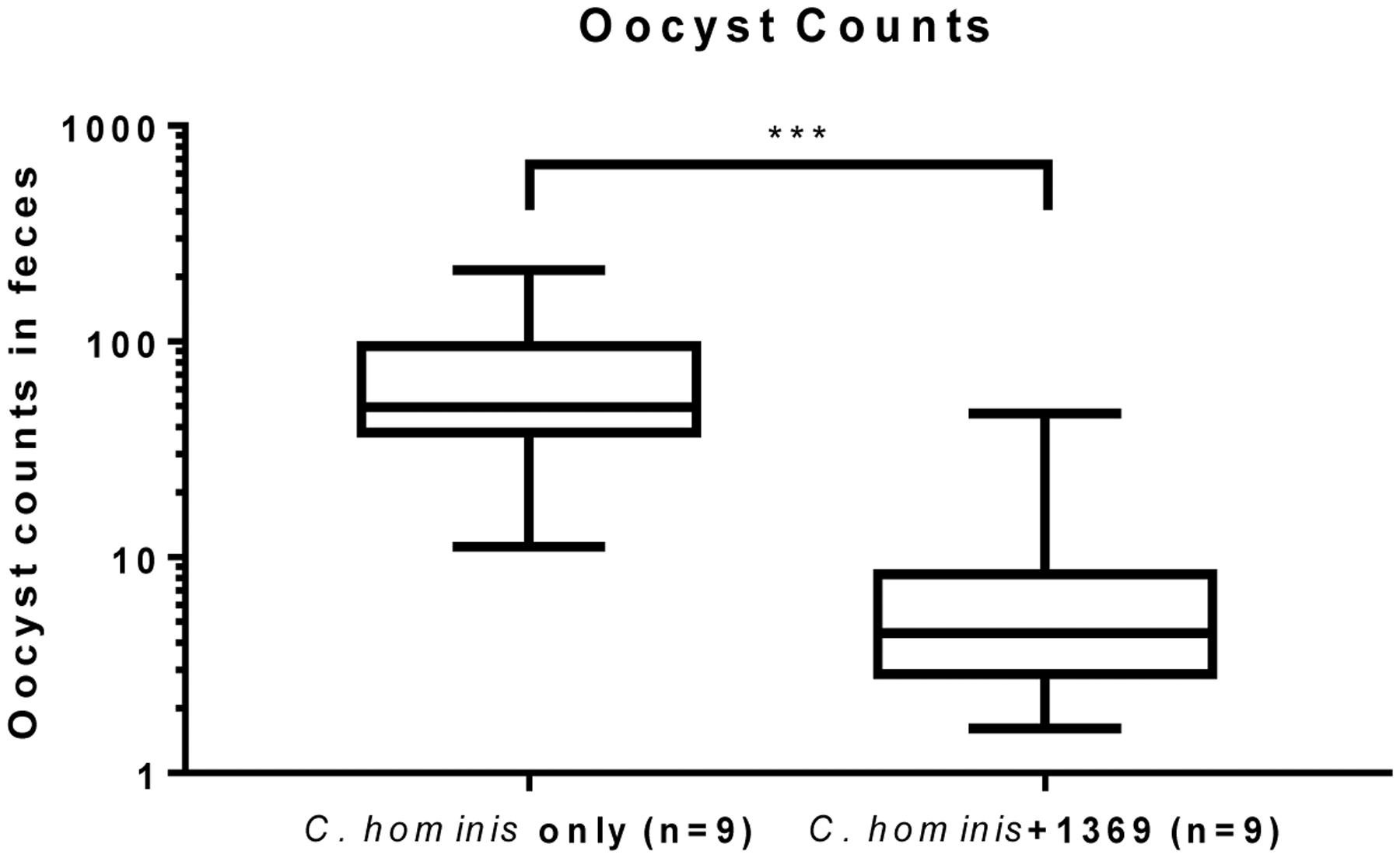
Shown are cumulative oocyst counts from daily rectal swabs taken on days 1–10 post-initiation of BKI-1369 (‘C. hominis +1369’, 10 mg/kg administered orally twice a day for the first 5 days) or vehicle alone (‘C. hominis only’). Data replotted from (Lee et al., 2018).
For human cryptosporidiosis as mentioned above, avoiding hERG inhibition has led us to search for therapeutic alternatives. We have found active compounds on both the PP and AC scaffolds and these are under active investigation for the right balance of safety and efficacy parameters (Huang et al., 2017; Hulverson et al., 2019; Vidadala et al., 2016).
BKIs for Toxoplasmosis
The PK properties for BKIs that drive efficacy for cryptosporidiosis, where drug needs to be delivered to infected gut tissue, are very likely different than the PK properties of BKIs for systemic infections such as toxoplasmosis, where penetration of the blood brain barrier, placenta, and the eye are necessary for optimal therapy (Arnold et al., 2017). BKI-1369 does not reach very high plasma and CNS levels during efficacious therapy for cryptosporidiosis and high exposure in blood or brain is likely to decrease the safety unnecessarily for Cryptosporidium therapy. However, BKIs such as BKI-1294 and BKI-1553 have shown proof-of-concept in small and large animal models of systemic apicomplexan infections, such as toxoplasmosis (Figure 2).
BKI-1294 has a 3 nM IC50 when inhibiting T. gondii CDPK1 (TgCDPK1) and inhibits T. gondii proliferation in vitro at an EC50 of 0.137 μM. BKI-1294 was shown to primarily target TgCDPK1 by demonstration of an 11-fold resistance to BKI-1294 generated by expression of a mutant TgCDPK1 where the gatekeeper changed from glycine to methionine (Johnson et al., 2012). It shares many of the good safety aspects of BKI-1369, such as no measurable mammalian cell cytotoxicity and safety in pregnancy in mice and sheep (Johnson et al., 2012; Sánchez-Sánchez et al., 2019; Winzer et al., 2015). BKI-1294 was shown to be effective in reducing the intraperitoneal T. gondii RH strain by 93% at 30 mg/kg given orally once a day for five days and more than 99% by 100 mg/kg dosed the same way (Doggett et al., 2014). In a mouse model of congenital toxoplasmosis based on oral infection of pregnant mice with 20 oocysts of the T. gondii ME49 strain, only 4 of 55 (7%) of surviving pups treated with BKI-1294 at 50 mg/kg orally once a day for five days had detectable T. gondii in their brains, whereas 67 of 80 (84%) pups treated with placebo were T. gondii infected (Muller et al., 2017b). Thus, in two mouse models, including one involving transplacental and blood brain barrier passage, BKI-1294 appears to be efficacious and safe.
We have gone on to show that BKI-1294 is safe and effective in a sheep pregnancy model (Sánchez-Sánchez et al., 2019). In this model, pregnant sheep (Rasa Aragonesa breed) were infected with 1000 T. gondii sporulated oocysts of the TgShSp1 strain at 90 days gestation. Sheep were treated orally with 100 mg/kg BKI-1294 every other day for 5 treatments or with vehicle alone. An uninfected pregnant control group was treated with BKI-1294 as well. There was 100% fetal loss, in 8 out of 8 infected pregnant ewes by 9 days P.I., in the vehicle treated group. However, in the infected BKI-1294 treated pregnant ewes, fetal mortality was only detected in 2 of 7 ewes (71% reduction in fetal mortality) and upon examination of remaining lambs, there was a 53% reduction of T. gondii vertical transmission (Sánchez-Sánchez et al., 2019).
Unfortunately, BKI-1294 also has an issue with inhibition of hERG at efficacious levels, and thus potential cardiotoxicity. We continue to actively search for a compound without this issue for toxoplasmosis therapy. However, the proof-of-concept experiment with BKI-1294 to block congenital transmission in the sheep model demonstrates unprecedented protection of fetuses during acute toxoplasmosis infection, spurring us to find the ideal BKI for this indication for both human and animal therapy.
BKI-1553 (Figure 2, Compound 32 in the reference) has a 1 nM IC50 when inhibiting T. gondii CDPK1 (TgCDPK1) and inhibits T. gondii proliferation in vitro at an EC50 of 0.060 μM and did not inhibit hERG up to 10 μM (Vidadala et al., 2016). BKI-1553 was very effective at greatly reducing T. gondii in acute mouse infection (Vidadala et al., 2014). In experiments where mice were infected for 5 weeks with ME49 strain T. gondii, to allow establishment of bradyzoites in brain, treatment with BKI-1553 for 2 weeks led to 89% reduction in brain bradyzoites (Vidadala et al., 2016). This demonstrates that BKI therapy can treat the latent form of toxoplasmosis.
BKIs for Neosporosis
BKI-1294 and BKI-1553 (Figure 2) are both active against N. caninum CDPK1 (3 nM and 1 nM IC50s, respectively) and N. caninum growth in vitro (360 nM and 180 nM EC50s, respectively) (Müller et al., 2017; Ojo et al., 2014). In a non-pregnant mouse model of N. caninum infection, treatment with BKI-1294 was found to reduce the brain load of parasites by over 80%, whether 50 mg/kg daily treatment was applied for either 5 or 10 days after infection (Ojo et al., 2014). In a pregnant mouse model using infection with N. caninum Nc-Liv or Nc-Spain7 strains, 50 mg/kg × 5 days demonstrated an 87% reduction in brain infection with both strains; 100% of mice given vehicle alone had brain infection (Winzer et al., 2015). BKI-1553 was also used in a pregnant N. caninum Nc-Spain7 infection mouse model. BKI-1553 led to about 33% increased neonatal mortality at 20 mg/kg daily for 5 days, probably representing an adverse effect of the high levels of BKI-1553 achieved, but surviving BKI-1553 treated fetuses had 44% reduced frequency of N. caninum in the brain compared to 100% infection of the control group (Müller et al., 2017).
Finally, BKI-1553 has demonstrated partial efficacy in a pregnant sheep model of N. caninum infection (Sánchez-Sánchez et al., 2018). In this model, pregnant ewes at day 90 of gestation were infected intravenously with 106 N. caninum tachyzoites of the Nc-Spain7 strain. They were treated at 48 hr. P.I. with either of two dose regimens (#1: 35 mg/kg injected subcutaneously (SQ) at the first dose and 10 mg/kg SQ on a subsequent dose 7 days later; #2: 10 mg/kg administered every other day for 7 total doses). The BKI-1553 dose regimen #1 resulted in a 37% reduction in fetal loss (vehicle control and N. caninum infection led to 100% fetal loss) while the BKI-1553 dose regimen #2 led to a 50% reduction in fetal loss. Parasite detection in fetal brain tissue decreased from 94% in the infected/vehicle treated control group to about 70% in the two treated groups (Sánchez-Sánchez et al., 2018). Though this experiment demonstrated BKI-1553 was not optimal for treating N. caninum in sheep pregnancy, it did demonstrate that partial efficacy could be obtained. Thus, a BKI with better transplacental and brain penetration, and with better pregnancy safety characteristics might be expected to effectively treat N. caninum associated pregnancy loss.
Discussion and Conclusion
BKIs show great promise as a one-health therapeutic for cryptosporidiosis, toxoplasmosis, and neosporosis, as reviewed above (Table 3). In addition to these indications, BKIs show great promise in the therapy of the livestock diseases sarcocystiosis or equine protozoal myeloencephalitis (EPM), cystoisosporosis or epidemic diarrhea in piglets, and besnoitiosis in livestock (Table 3). BKIs have low nanomolar activity against CDPK1 in the parasites that cause these diseases and have nanomolar activity against the parasites in cell culture (Jimenez-Melendez et al., 2017; Ojo et al., 2016; Shrestha et al., 2019). In a mouse model of sarcocystiosis, infection with Sarcocystis neurona was greatly reduced or eliminated with BKI treatment (Ojo et al., 2016). Two studies in the piglet model of cystoisosporosis, where diarrhea was induced with Cystoisospora suis, demonstrated efficacy in abrogating diarrhea and eliminating parasite shedding, and in the most recent study, only two doses of BKI-1369 were required for efficacy (Shrestha et al., 2019; Shrestha et al., 2020). Further optimization of efficacy and safety is likely required for the systemic apicomplexan diseases, especially to provide transplacental and CNS coverage.
Table 3.
BKI potential uses: Animal Health
|
Treatment of sick calves: cryptosporidiosis/scours Moderate/severe crypto → sustained growth detriment, 34 kg average (Hannah Shaw, Moredun Univ) (Shaw, 2017)
|
|
Cryptosporidium metaprophylaxis: prevent disease on contaminated farms Get calves to milk/market earlier & establish immunity
|
|
Therapy or prophylaxis of Neospora caninum: epidemic abortion in cattle Eliminate need for culling
|
Active low nM: Toxoplasma gondii: a frequent cause of sheep/goat abortions
|
| Prophylactic treatment of felines for T. gondii to prevent feline-derived T. gondii infection? |
Active low nM on Sarcocystis neurona: Equine protozoal myeloencephalitis (EPM) in the Americas
|
Active low nM: Cystoisospora suis: epidemic diarrhea in piglets
|
Active low nM on Besnoiti besnoiti: cattle disease in Europe/Central Asia, skin and systemic damage
|
With respect to the human apicomplexan diseases, cryptosporidiosis and toxoplasmosis, further optimization is necessary to remove hERG activity, which is indicative of potential cardiotoxicity in humans. The BKI AC scaffold compounds tend to lack hERG activity and are highly efficacious against C. parvum and T. gondii (Figure 2) (Castellanos-Gonzalez et al., 2016; Huang et al., 2017; Huang et al., 2019; Huang et al., 2015; Hulverson et al., 2019; Schaefer et al., 2016). In addition, transplacental, ocular, and CNS penetration, and balancing efficacy and safety are needed for successful therapy of human toxoplasmosis, and we are conducting new synthesis and testing of BKI AC compounds to find the correct balance of these properties.
Acknowledgments and any additional information concerning research grants, etc.
The authors would like to acknowledge Drs. Wim Hol, Eric T. Larson, and Ethan Merritt for their structural expertise in solving the CDPK1 structure, scientists at AbbVie Inc. who helped with characterizing safety and PK of BKIs including Drs. Dale J. Kempf, Kennan Marsh, James J. Lynch, and Wayne R. Buck, scientists at PATH who have advised us on drug discovery and particularly Dr. Robert Choy, Dr. Anja Joachim and her group at the University of Vienna, Austria, for their work on C. suis, and Dr. Gema Alvarez-Garcia and her group for the work on besnoitiosis. Funding for these studies was provided by National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers R21AI123690, R01AI089441, R01AI111341, R01A1112427, and R01HD080670), the US Department of Agriculture, National Institute of Food and Agriculture (grant numbers 2014-06183 and 2019-07512; Project #ARZT-5704210-A50-133), and the Swiss National Science Foundation (grant number 310030_1846629), the Bill & Melinda Gates Foundation under award number [OPP ID: OPP1160955], and PATH award # [DFI.1850-02-01291794-SUB; and DFI 1850-02-405099]. J. Stone Doggett received funding from VA Merit Review Award BX004522 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Wes Van Voorhis and Tom Kennedy are co-owners of ParaTheraTech Inc., a company that is developing bumped-kinase inhibitors for animal therapy. Otherwise the authors have nothing to report.
Credit Author Statement Vetpar-D-20-14753
Wesley C. Van Voorhis, Conceptualization, Writing - original draft; Writing - review & editing Matthew A. Hulverson, Writing - review & editing
Ryan Choi, Writing - review & editing.
Wenlin Huang, Writing - review & editing
Samuel L. M. Arnold, Writing - review & editing
Deborah A. Schaefer, Writing - review & editing
Dana P. Betzer, Writing - review & editing
Rama S. R. Vidadala, Writing - review & editing
Sangun Lee, Writing - review & editing
Grant R. Whitman, Writing - review & editing
Lynn K. Barrett, Writing - review & editing
Dustin J. Maly, Writing - review & editing
Michael W. Riggs, Writing - review & editing
Erkang Fan, Writing - review & editing
Thomas J. Kennedy, Writing - review & editing
Saul Tzipori, Writing - review & editing
J. Stone Doggett, Writing - review & editing
Pablo Winzer, Writing - review & editing
Nicoleta Anghel, Writing - review & editing
Dennis Imhof, Writing - review & editing
Joachim Müller, Writing - review & editing
Andrew Hemphill, Writing - review & editing
Ignacio Ferre, Writing - review & editing
Roberto Sanchez-Sanchez, Writing - review & editing
Luis Miguel Ortega-Mora, Writing - review & editing
Kayode K. Ojo, Writing - review & editing
References
- Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P, 2002. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360, 1375–1380. [DOI] [PubMed] [Google Scholar]
- Amadi B, Mwiya M, Sianongo S, Payne L, Watuka A, Katubulushi M, Kelly P, 2009. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis 9, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SLM, Choi R, Hulverson MA, Schaefer DA, Vinayak S, Vidadala RSR, McCloskey MC, Whitman GR, Huang W, Barrett LK, Ojo KK, Fan E, Maly DJ, Riggs MW, Striepen B, Van Voorhis WC, 2017. Necessity of Bumped Kinase Inhibitor Gastrointestinal Exposure in Treating Cryptosporidium Infection. J Infect Dis 216, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigna JJ, Tochie JN, Tounouga DN, Bekolo AO, Ymele NS, Youda EL, Sime PS, Nansseu JR, 2020. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: a systematic review, modelling and meta-analysis. Sci Rep 10, 12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Shah K, Liu Y, Witucki L, Kung C, Shokat KM, 1998. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol 8, 257–266. [DOI] [PubMed] [Google Scholar]
- Bishop AC, Shokat KM, 1999. Acquisition of inhibitor-sensitive protein kinases through protein design. Pharmacol Ther 82, 337–346. [DOI] [PubMed] [Google Scholar]
- Castellanos-Gonzalez A, Sparks H, Nava S, Huang W, Zhang Z, Rivas K, Hulverson MA, Barrett LK, Ojo KK, Fan E, Van Voorhis WC, White AC Jr., 2016. A Novel Calcium-Dependent Kinase Inhibitor, Bumped Kinase Inhibitor 1517, Cures Cryptosporidiosis in Immunosuppressed Mice. J Infect Dis 214, 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR, 1998. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. American journal of epidemiology 148, 497–506. [DOI] [PubMed] [Google Scholar]
- Checkley W, White AC Jr., Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr., Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER, 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahoy MJ, Omore R, Ayers TL, Schilling KA, Blackstock AJ, Ochieng JB, Moke F, Jaron P, Awuor A, Okonji C, Juma J, Farag TH, Nasrin D, Panchalingam S, Nataro JP, Kotloff KL, Levine MM, Oundo J, Roellig DM, Xiao L, Parsons MB, Laserson K, Mintz ED, Breiman RF, O’Reilly CE, 2018. Clinical, environmental, and behavioral characteristics associated with Cryptosporidium infection among children with moderate-to-severe diarrhea in rural western Kenya, 2008–2012: The Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 12, e0006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett JS, Ojo KK, Fan E, Maly DJ, Van Voorhis WC, 2014. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob Agents Chemother 58, 3547–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, 2009a. Toxoplasmosis in pigs--the last 20 years. Vet Parasitol 164, 89–103. [DOI] [PubMed] [Google Scholar]
- Dubey JP, 2009b. Toxoplasmosis in sheep--the last 20 years. Vet Parasitol 163, 1–14. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, 2020a. Toxoplasma gondii infections in horses, donkeys, and other equids: The last decade. Research in veterinary science 132, 492–499. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Su C, 2020b. Economic and public health importance of Toxoplasma gondii infections in sheep: 2009–2020. Vet Parasitol 286, 109195. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Warleta M, Castro-Hermida JA, Calvo C, Perez V, Gutierrez-Exposito D, Regidor-Cerrillo J, Ortega-Mora LM, Mezo M, 2018. Endogenous transplacental transmission of Neospora caninum during successive pregnancies across three generations of naturally infected sheep. Vet Res 49, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Warleta M, Castro-Hermida JA, Regidor-Cerrillo J, Benavides J, Alvarez-Garcia G, Fuertes M, Ortega-Mora LM, Mezo M, 2014. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet Res 45, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J, Pohlenz JF, Moon HW, Woode GN, 1984. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J Infect Dis 150, 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Choi R, Hulverson MA, Zhang Z, McCloskey MC, Schaefer DA, Whitman GR, Barrett LK, Vidadala RSR, Riggs MW, Maly DJ, Van Voorhis WC, Ojo KK, Fan E, 2017. 5-Aminopyrazole-4-Carboxamide-Based Compounds Prevent the Growth of Cryptosporidium parvum. Antimicrob Agents Chemother 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Hulverson MA, Choi R, Arnold SLM, Zhang Z, McCloskey MC, Whitman GR, Hackman RC, Rivas KL, Barrett LK, Ojo KK, Van Voorhis WC, Fan E, 2019. Development of 5-Aminopyrazole-4-carboxamide-based Bumped-Kinase Inhibitors for Cryptosporidiosis Therapy. J Med Chem 62, 3135–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ojo KK, Zhang Z, Rivas K, Vidadala RS, Scheele S, DeRocher AE, Choi R, Hulverson MA, Barrett LK, Bruzual I, Siddaramaiah LK, Kerchner KM, Kurnick MD, Freiberg GM, Kempf D, Hol WG, Merritt EA, Neckermann G, de Hostos EL, Isoherranen N, Maly DJ, Parsons M, Doggett JS, Van Voorhis WC, Fan E, 2015. SAR Studies of 5-Aminopyrazole-4-carboxamide Analogues as Potent and Selective Inhibitors of Toxoplasma gondii CDPK1. ACS Med Chem Lett 6, 1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulverson MA, Bruzual I, McConnell EV, Huang W, Vidadala RSR, Choi R, Arnold SLM, Whitman GR, McCloskey MC, Barrett LK, Rivas KL, Scheele S, DeRocher AE, Parsons M, Ojo KK, Maly DJ, Fan E, Van Voorhis WC, Doggett JS, 2019. Pharmacokinetics and In Vivo Efficacy of Pyrazolopyrimidine, Pyrrolopyrimidine, and 5-Aminopyrazole-4-Carboxamide Bumped Kinase Inhibitors against Toxoplasmosis. J Infect Dis 219, 1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulverson MA, Choi R, Arnold SLM, Schaefer DA, Hemphill A, McCloskey MC, Betzer DP, Muller J, Vidadala RSR, Whitman GR, Rivas KL, Barrett LK, Hackman RC, Love MS, McNamara CW, Shaughnessy TK, Kondratiuk A, Kurnick M, Banfor PN, Lynch JJ, Freiberg GM, Kempf DJ, Maly DJ, Riggs MW, Ojo KK, Van Voorhis WC, 2017a. Advances in bumped kinase inhibitors for human and animal therapy for cryptosporidiosis. Int J Parasitol 47, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulverson MA, Vinayak S, Choi R, Schaefer DA, Castellanos-Gonzalez A, Vidadala RSR, Brooks CF, Herbert GT, Betzer DP, Whitman GR, Sparks HN, Arnold SLM, Rivas KL, Barrett LK, White AC Jr., Maly DJ, Riggs MW, Striepen B, Van Voorhis WC, Ojo KK, 2017b. Bumped-Kinase Inhibitors for Cryptosporidiosis Therapy. J Infect Dis 215, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Melendez A, Ojo KK, Wallace AM, Smith TR, Hemphill A, Balmer V, Regidor-Cerrillo J, Ortega-Mora LM, Hehl AB, Fan E, Maly DJ, Van Voorhis WC, Alvarez-Garcia G, 2017. In vitro efficacy of bumped kinase inhibitors against Besnoitia besnoiti tachyzoites. Int J Parasitol 47, 811–821. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Murphy RC, Geiger JA, DeRocher AE, Zhang Z, Ojo KK, Larson ET, Perera BG, Dale EJ, He P, Reid MC, Fox AM, Mueller NR, Merritt EA, Fan E, Parsons M, Van Voorhis WC, Maly DJ, 2012. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem 55, 2416–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, Colombara DV, De Hostos EL, Engmann C, Guerrant RL, Haque R, Houpt ER, Kang G, Korpe PS, Kotloff KL, Lima AAM, Petri WA Jr., Platts-Mills JA, Shoultz DA, Forouzanfar MH, Hay SI, Reiner RC Jr., Mokdad AH, 2018. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health 6, e758–e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieschnick H, Wakefield T, Narducci CA, Beckers C, 2001. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem 276, 12369–12377. [DOI] [PubMed] [Google Scholar]
- Korpe PS, Valencia C, Haque R, Mahfuz M, McGrath M, Houpt E, Kosek M, McCormick BJJ, Penataro Yori P, Babji S, Kang G, Lang D, Gottlieb M, Samie A, Bessong P, Faruque ASG, Mduma E, Nshama R, Havt A, Lima IFN, Lima AAM, Bodhidatta L, Shreshtha A, Petri WA Jr., Ahmed T, Duggal P, 2018. Epidemiology and Risk Factors for Cryptosporidiosis in Children from Eight Low-income Sites: Results from the MAL-ED Study. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM, 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382, 209–222. [DOI] [PubMed] [Google Scholar]
- Lee S, Ginese M, Beamer G, Danz HR, Girouard DJ, Chapman-Bonofiglio SP, Lee M, Hulverson MA, Choi R, Whitman GR, Ojo KK, Arnold SLM, Van Voorhis WC, Tzipori S, 2018. Therapeutic Efficacy of Bumped Kinase Inhibitor 1369 in a Pig Model of Acute Diarrhea Caused by Cryptosporidium hominis. Antimicrob Agents Chemother 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Aguado-Martínez A, Balmer V, Maly DJ, Fan E, Ortega-Mora L-M, Ojo KK, Van Voorhis WC, Hemphill A, 2017. Two Novel Calcium-Dependent Protein Kinase 1 Inhibitors Interfere with Vertical Transmission in Mice Infected with Neospora caninum Tachyzoites. Antimicrobial Agents and Chemotherapy 61, e02324–02316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Aguado-Martinez A, Balmer V, Maly DJ, Fan E, Ortega-Mora LM, Ojo KK, Van Voorhis WC, Hemphill A, 2017a. Two Novel Calcium-Dependent Protein Kinase 1 Inhibitors Interfere with Vertical Transmission in Mice Infected with Neospora caninum Tachyzoites. Antimicrob Agents Chemother 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Aguado-Martinez A, Ortega-Mora LM, Moreno-Gonzalo J, Ferre I, Hulverson MA, Choi R, McCloskey MC, Barrett LK, Maly DJ, Ojo KK, Van Voorhis W, Hemphill A, 2017b. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J Antimicrob Chemother 72, 2334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BGK, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CLMJ, White AC, Merritt EA, Van Voorhis WC, Maly DJ, 2010. Discovery of Potent and Selective Inhibitors of CDPK1 from C. parvum and T. gondii. Acs Medicinal Chemistry Letters 1, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamune K, Sibley LD, 2006. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol Biol Evol 23, 1613–1627. [DOI] [PubMed] [Google Scholar]
- Neville AJ, Zach SJ, Wang X, Larson JJ, Judge AK, Davis LA, Vennerstrom JL, Davis PH, 2015. Clinically Available Medicines Demonstrating Anti-Toxoplasma Activity. Antimicrob Agents Chemother 59, 7161–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo KK, Dangoudoubiyam S, Verma SK, Scheele S, DeRocher AE, Yeargan M, Choi R, Smith TR, Rivas KL, Hulverson MA, Barrett LK, Fan E, Maly DJ, Parsons M, Dubey JP, Howe DK, Van Voorhis WC, 2016. Selective inhibition of Sarcocystis neurona calcium-dependent protein kinase 1 for equine protozoal myeloencephalitis therapy. Int J Parasitol 46, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, Derocher AE, Inampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, Napuli AJ, Maly DJ, Verlinde CL, Buckner FS, Parsons M, Hol WG, Merritt EA, Van Voorhis WC, 2010. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol 17, 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo KK, Reid MC, Kallur Siddaramaiah L, Muller J, Winzer P, Zhang Z, Keyloun KR, Vidadala RS, Merritt EA, Hol WG, Maly DJ, Fan E, Van Voorhis WC, Hemphill A, 2014. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS One 9, e92929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel MP, Alejandra Ayanegui-Alcerreca M, Gondim LF, Ellis JT, 2013. What is the global economic impact of Neospora caninum in cattle - the billion dollar question. Int J Parasitol 43, 133–142. [DOI] [PubMed] [Google Scholar]
- Riggs MW, Schaefer DA, 2020. Calf Clinical Model of Cryptosporidiosis for Efficacy Evaluation of Therapeutics. Methods Mol Biol 2052, 253–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A, Riahi SM, Contopoulos-Ioannidis DG, Gamble HR, Fakhri Y, Shiadeh MN, Foroutan M, Behniafar H, Taghipour A, Maldonado YA, Mokdad AH, Gasser RB, 2019. Acute Toxoplasma infection in pregnant women worldwide: A systematic review and meta-analysis. PLoS Negl Trop Dis 13, e0007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Sánchez R, Ferre I, Re M, Ramos JJ, Regidor-Cerrillo J, Pizarro Díaz M, González-Huecas M, Tabanera E, Benavides J, Hemphill A, Hulverson MA, Barrett LK, Choi R, Whitman GR, Ojo KK, Van Voorhis WC, Ortega-Mora LM, 2019. Treatment with Bumped Kinase Inhibitor 1294 Is Safe and Leads to Significant Protection against Abortion and Vertical Transmission in Sheep Experimentally Infected with Toxoplasma gondii during Pregnancy. Antimicrob Agents Chemother 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Sanchez R, Ferre I, Re M, Regidor-Cerrillo J, Blanco-Murcia J, Ferrer LM, Navarro T, Pizarro Diaz M, Gonzalez-Huecas M, Tabanera E, Benavides J, Ortega-Mora LM, 2018a. Influence of dose and route of administration on the outcome of infection with the virulent Neospora caninum isolate Nc-Spain7 in pregnant sheep at mid-gestation. Vet Res 49, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Sánchez R, Ferre I, Re M, Vázquez P, Ferrer LM, Blanco-Murcia J, Regidor-Cerrillo J, Pizarro Díaz M, González-Huecas M, Tabanera E, García-Lunar P, Benavides J, Castaño P, Hemphill A, Hulverson MA, Whitman GR, Rivas KL, Choi R, Ojo KK, Barrett LK, Van Voorhis WC, Ortega-Mora LM, 2018. Safety and efficacy of the bumped kinase inhibitor BKI-1553 in pregnant sheep experimentally infected with Neospora caninum tachyzoites. Int J Parasitol Drugs Drug Resist 8, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Sanchez R, Vazquez P, Ferre I, Ortega-Mora LM, 2018b. Treatment of Toxoplasmosis and Neosporosis in Farm Ruminants: State of Knowledge and Future Trends. Curr Top Med Chem 18, 1304–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer DA, Betzer DP, Smith KD, Millman ZG, Michalski HC, Menchaca SE, Zambriski JA, Ojo KK, Hulverson MA, Arnold SL, Rivas KL, Vidadala RS, Huang W, Barrett LK, Maly DJ, Fan E, Van Voorhis WC, Riggs MW, 2016. Novel Bumped Kinase Inhibitors Are Safe and Effective Therapeutics in the Calf Clinical Model for Cryptosporidiosis. J Infect Dis 214, 1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw HJ, 2017. A reduction in weight gain in beef calves with clinical cryptosporidiosis. In: Apicowplexa, La Escorial, Spain, October 12, 2017. [Google Scholar]
- Shaw HJ, Innes EA, Morrison LJ, Katzer F, Wells B, 2020. Long-term production effects of clinical cryptosporidiosis in neonatal calves. Int J Parasitol 50, 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha A, Ojo KK, Koston F, Ruttkowski B, Vidadala RSR, Dorr CS, Navaluna ED, Whitman GR, Barrett KF, Barrett LK, Hulverson MA, Choi R, Michaels SA, Maly DJ, Hemphill A, Van Voorhis WC, Joachim A, 2019. Bumped kinase inhibitor 1369 is effective against Cystoisospora suis in vivo and in vitro. Int J Parasitol Drugs Drug Resist 10, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha A, Ruttkowski B, Greber P, Whitman GR, Hulverson MA, Choi R, Michaels SA, Ojo KK, Van Voorhis WC, Joachim A, 2020. Reduced treatment frequencies with bumped kinase inhibitor 1369 are effective against porcine cystoisosporosis. Int J Parasitol Drugs Drug Resist 14, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverlås C, Björkman C, Egenvall A, 2009. Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis. Prev Vet Med 91, 73–84. [DOI] [PubMed] [Google Scholar]
- Stelzer S, Basso W, Benavides Silvan J, Ortega-Mora LM, Maksimov P, Gethmann J, Conraths FJ, Schares G, 2019. Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol 15, e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams LA, Jarvie BD, Peregrine AS, Duffield TF, Leslie KE, 2011. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves. The Veterinary record 168, 509. [DOI] [PubMed] [Google Scholar]
- Vidadala RS, Ojo KK, Johnson SM, Zhang Z, Leonard SE, Mitra A, Choi R, Reid MC, Keyloun KR, Fox AM, Kennedy M, Silver-Brace T, Hume JC, Kappe S, Verlinde CL, Fan E, Merritt EA, Van Voorhis WC, Maly DJ, 2014. Development of potent and selective Plasmodium falciparum calcium-dependent protein kinase 4 (PfCDPK4) inhibitors that block the transmission of malaria to mosquitoes. Eur J Med Chem 74, 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidadala RS, Rivas KL, Ojo KK, Hulverson MA, Zambriski JA, Bruzual I, Schultz TL, Huang W, Zhang Z, Scheele S, DeRocher AE, Choi R, Barrett LK, Siddaramaiah LK, Hol WG, Fan E, Merritt EA, Parsons M, Freiberg G, Marsh K, Kempf DJ, Carruthers VB, Isoherranen N, Doggett JS, Van Voorhis WC, Maly DJ, 2016. Development of an Orally Available and Central Nervous System (CNS) Penetrant Toxoplasma gondii Calcium-Dependent Protein Kinase 1 (TgCDPK1) Inhibitor with Minimal Human Ether-a-go-go-Related Gene (hERG) Activity for the Treatment of Toxoplasmosis. J Med Chem 59, 6531–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernimont AK, Artz JD, Finerty P Jr., Lin YH, Amani M, Allali-Hassani A, Senisterra G, Vedadi M, Tempel W, Mackenzie F, Chau I, Lourido S, Sibley LD, Hui R, 2010. Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium. Nat Struct Mol Biol 17, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC Jr., 2020. Cryptosporidiosis (Cryptosporidium Species), In: Bennett JE, MD; Dolin Raphael, MD; Blaser Martin J., MD (Ed.) Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, Ninth Edition. Elsevier, pp. 3410–3420. [Google Scholar]
- Winzer P, Muller J, Aguado-Martinez A, Rahman M, Balmer V, Manser V, Ortega-Mora LM, Ojo KK, Fan E, Maly DJ, Van Voorhis WC, Hemphill A, 2015. In Vitro and In Vivo Effects of the Bumped Kinase Inhibitor 1294 in the Related Cyst-Forming Apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob Agents Chemother 59, 6361–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ojo KK, Vidadala R, Huang W, Geiger JA, Scheele S, Choi R, Reid MC, Keyloun KR, Rivas K, Siddaramaiah LK, Comess KM, Robinson KP, Merta PJ, Kifle L, Hol WG, Parsons M, Merritt EA, Maly DJ, Verlinde CL, Van Voorhis WC, Fan E, 2014. Potent and selective inhibitors of CDPK1 from T. gondii and C. parvum based on a 5-aminopyrazole-4-carboxamide scaffold. ACS Med Chem Lett 5, 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


