Abstract
The ideal of experimental methodology in animal research is the reduction or elimination of environmental variables or consistency in their application. In lab animals, diet has been recognized as a very influential response variable. Reproducibility in research using rodents required the development of a unique diet of consistent ingredient and nutrient composition to allow for cross-comparisons of lab results, spatially and temporally. These diets are commonly referred to as standard reference diets (SRDs). The established validity of published nutritional requirements combined with the cooperation of commercial partners led to species-specific reference diets commonly used by the research community. During the last several decades, zebrafish (Danio rerio) have become a widespread alternative animal model, but specific knowledge of their nutrition is lacking. We present a short-term approach for developing an SRD for zebrafish, similar to that eventually attained for rodents over decades. Imminent development of an open-formulation, commercially produced SRD for zebrafish will notably advance translational biomedical science.
Keywords: standard reference diet, zebrafish, process of production, nutrients, ingredients, testing
INTRODUCTION: GOALS
The value of the ethical use of animal models in biomedical research has been demonstrated for decades. A variety of animal models representing multiple phylogenetic classes have been used in these research studies, including invertebrate and vertebrate species. In fact, it has been theorized that for every biological problem there is an appropriate animal of study, termed Krogh’s principle, originally cited by Claude Bernard (3). Despite the large numbers of nonhuman species that have been used in biomedical research, the vast number of recent investigations have focused on relatively few species. These primarily include Bilateria such as rodents, zebrafish, fruit flies, and nematodes. The accumulating wealth of knowledge on their respective genomes in relation to physiological function makes these species attractive for analysis of specific metabolic networks related to human disease onset and progression.
After rodents, zebrafish (Danio rerio) have become one of the most popular tools for biomedical research. Numerous developmental and physiological characteristics make zebrafish highly desirable as a biomedical research animal worldwide. Mass cultures of zebrafish are often provided by care staff to investigators, with the primary goal of culture facilities being to produce high numbers of embryos and larvae for experimentation. Unfortunately, the nutritional requirements of zebrafish, unlike those of rodents, have not been established. Dietary decisions within specific laboratories are often based on hearsay and include the use of live diets or diets formulated for other species, with little or no compositional analysis. Thus, experimental nutritional control is limited. This former approach was understandable due to the lack of nutritional information available. However, with the continued rise in the importance of translational research, and considering that nutrition is often a variable in most studies (whether recognized or not), it is imperative that nutritional guidelines for research animals be established and followed. Without nutritional guidelines, the value of biomedical data becomes more limited, and this omission ultimately limits the use of the model.
The goal of this article is not to review nutritional research on zebrafish, as nutritional data have been recently reviewed (11, 40). Rather, the goals of this review are (a) to provide an understanding of the importance of nutritional standardization in the zebrafish research community and (b) to provide information defining a path to the development of standard reference diets (SRDs) for zebrafish. This information is necessary to maximize the reproducibility and confidence in data sets related to biomedical research. We suggest that this process can be adapted for use in other species to enhance their respective utility in the biomedical sciences.
THE VALUE OF ZEBRAFISH IN BIOMEDICAL RESEARCH
To establish the value of zebrafish in biomedical research would be largely rhetorical. The initial reports describing zebrafish and their potential value in development were published in the 1950s (15); however, George Streisinger was credited with describing the value of zebrafish in genetic analysis, setting the stage for the future development of zebrafish as a biomedical model (reviewed in 7). It is now well established that zebrafish are a valuable research organism in a number of disciplines related to biomedicine, including development (27), neurology and behavior (21), genetic disease (30), animal-microbial interactions (41), gene function and human disease (31), and genetic screening (22). As a homologous animal model in many aspects of human disease, zebrafish continue to grow in value as a research model. Their value in nutritional research, and those morbidities directly related to nutrition, is just beginning to be realized (8, 10, 19, 26, 37, 39, 42–44). Even among nutrition-related morbidities such as obesity and diabetes, most of the published research is still based on studies related to characterizing disease pathologies; however, the underlying role of nutrition in promoting or attenuating these diseases cannot be ascertained due to the lack of dietary control. Lack of nutritional control has been recognized in the zebrafish community, and effective approaches to address these overt shortcomings are now being examined.
DIETS ARE IMPORTANT AS PART OF THE STANDARDS OF CULTURE
Standardization of animal culture conditions and husbandry is paramount to achieving reproducible outcomes in zebrafish. To date, most studies have singularly relied on culture standards related to the physical environment of the fish, including housing, temperature, light, water quality, and enrichment (20, 34, 38). Commercial industries arose to provide controlled standards of housing and water quality in zebrafish culture. Other important areas of standardization include disease monitoring and disease control, as zebrafish can carry an array of pathogens that can disrupt the life cycle. Diet standardization is, however, reduced to best guesses, such as borrowing feeding regimes from other fish including ornamental and production species. Several companies sell diets to the zebrafish research community, and most of these diets are formulated on the basis of general fish nutrition guidelines, perceived or empirical. These diets support growth and reproduction reasonably well. In fact, these diets have been invaluable in moving the research community forward and helping to refine the use of the zebrafish model. Unfortunately, the composition of these diets primarily consists of practical, unrefined ingredients whose identity and quantity are intellectual property. Consequently, the role of specific nutrients in growth, reproductive, and health demographics cannot be accurately evaluated when using these diets. Causative inference leads to the conclusion of the need to use a formulated diet of known ingredients, defined nutrients, and consistent and reproducible quality. These characteristics are the essential hallmarks of an SRD, and the logical development of an SRD is the next step in the evolution of dietary standardization and reproducibility in zebrafish translational research.
THE CONCEPT OF STANDARD REFERENCE DIETS
As we have suggested, the lack of standardized methodology in experimental design, including culture conditions, analytical techniques, and nutritional control, has been recognized as a shortcoming in the ability to confidently compare published research results. Of paramount need for appropriate nutritional control is a diet that can be universally accepted and used as an SRD, allowing for a more direct comparison of outcomes within and among research laboratories. Advocacy of the development and use of SRDs is not novel. Included in a published 1977 report by the American Institute of Nutrition Ad Hoc Committee on Standards of Nutritional Studies was recognition of the need to establish guidelines for nutritional research using rodents as a medical model (1). This report included a recommended SRD (AIN-76). In 1978, the Committee on Laboratory Animal Diets of the National Research Council declared that lack of control of the nutritional variable had not received proper attention in experimental design using live animals. In 1979, the European Inland Fisheries Advisory Council, the International Union of Nutrition Scientists, and the International Commission for Exploration of the Sea Working Group on Standardization of Methodology in Fish Nutrition Research met in Hamburg, Germany, and recommended the use of SRDs for fish nutrition studies (4). The need for SRDs is especially noteworthy for organisms like the zebrafish that are recognized as important biomedical models. In these organisms, SRDs are essential to our understanding of the influence of nutrition on physiological and underlying genomic responses.
The source of nutrition can be responsible for undefined variation in response measured or recorded in research investigations. Such variation can be misleading, whereby similar studies using different diets and feeding management protocols produce completely different results and correspondingly different interpretations. The need for control of diet in research investigations is classically illustrated by the results of a nine-week study that revealed differences in fundamental growth responses corresponding to five different commercially available fish diets and two laboratory-prepared diets (36). Similar results were reported by Fowler et al. (12). This research embodied what is often termed a dietetics study, which simply evaluates diets that have no relationship as part of an experimental design. The diets consist of different mixtures of essential and nonessential macronutrients and micronutrients derived from an array of ingredients and possible micronutrient mixtures. The form (size and shape) and the qualitative and quantitative nutrient and energy contents derived from the ingredients contained in each of these diets collectively influence consumption, digestion, and assimilation of the presumed required nutrients. The results of these studies confirmed the expected wide variation in growth response. The availability and use of multiple commercially available diets, each often characteristically used by a particular research laboratory and across different life stages (juveniles, adults, and brood stock), sometimes spanning many years, underscore the confusion that would inevitably result from the variance of outcomes ascribed in the scientific literature.
Nutrition is truly the overriding issue in the goal of achieving as much control as possible in physiological studies conducted with biomedical models. An SRD based on the application of the best knowledge available would be welcomed on the basis of a level of control and consistency that would be achieved. Once adopted, the SRD should not be viewed as the ideal or most nutritionally efficient diet. Rather, it represents a diet that satisfies nutritional requirements, promotes comparatively high levels of growth, and can serve as the basis for comparisons with altered experimental diets. An SRD ultimately lends confidence to producing healthy zebrafish, a corollary condition that must also be standardized for well-designed biomedical research. Consequently, adhering to the use of an SRD becomes the foundation for comparison of research results and the planning of new experiments and collaborative initiatives. The ultimate end result is an increase in the rate of progress in gaining an understanding of human physiology that can be applied to the practice of medical science.
THE HIERARCHICAL PROCESS OF FORMULATING AN SRD
Provision of a reproducible and readily available diet of known nutrient composition is the foundational, working concept of an SRD. The early experimental diets used to determine nutritional requirements in aquatic organisms, including Chinook salmon (Oncorhynchus tshawytscha) (14), marine shrimp (Penaeus japonicus) (18), and eel (Anguilla japonica) (24), were based on ingredients selected to achieve desired nutrient control that would permit the determinations of certain nutrient requirements. These semipurified diets (defined in Reference 40) were the initial attempts to produce experimental diets that were designed for nutritional control. Essentially, they were the forerunners of SRDs and provided the recognition that diet was a critical part of experimental design. Ideally, an SRD must contain sufficient levels of required nutrients to fulfill the dietary requirements of zebrafish. This objective is guided by the use of existing knowledge that leads to estimates of nutrient requirements for D. rerio based on related species (11). As ongoing research results provide noteworthy advances in the knowledge of nutrient requirements, particularly life-stage differences, changes to nutrient composition can be made.
The recognition of amendments logically requires that an SRD formulation must consist of purified ingredients that are not subject to vagaries in nutrient composition. Examples of purified ingredients include casein, egg white, corn starch, vegetable- and marine-derived oils, soy protein isolate, gelatin, and single cell proteins. Examples of undefined, and therefore undesirable, complex feedstuffs as ingredients would include fish meal, blood meal, soybean meal, ground corn or wheat, and rendered products from terrestrial animal processing for human consumption. The latter group is commonly associated with what are described as practical ingredients (40). These practical ingredients are often included in a chow diet, a term introduced by the Purina Corporation. Practical chow diets are generally formed by using commodity ingredients, which can change with source and time, and are apparently designed to fulfill nutritional requirements that unfortunately cannot be easily defined. Changes in the ingredient composition produced by commercial manufacturers from one batch (lot) to another, and consequently creating a different diet, may occur without the knowledge of investigators. The resulting inconsistency in the nutrient and nonnutrient profiles are subsequently imposed on different studies that are supposedly using the same chow. At times, ingredient contaminants such as phytoestrogens are found in plant-derived ingredients, which can impact a variety of physiological responses that include estrogenic activity, levels of cortisol, and health and growth (28).
In contrast to chow diets, standardization of qualitative and quantitative ingredient composition is essential for any SRD. SRD ingredients must be consistently available in sufficient quantities for commercial production use. Each ingredient must possess a nutrient profile that is characteristically consistent. Nutrients in the SRD must be provided using ingredients that are efficiently digested and assimilated by the model organism. In addition, ingredient selection should ensure that the constituent nutrients remain chemically stable, thereby ensuring a reasonable shelf life, preferably without refrigeration.
NUTRIENT REQUIREMENTS FOR AN SRD
In the process of establishing the nutrient requirements of zebrafish, we are confronted by limited knowledge of their natural diet. Fortunately, this important dietary information is available for other fish species, thereby offering valuable insight into the nutritional requirements of zebrafish. This approach varies from that used by rodent researchers during the last century when empirical determinations of nutrient requirements were published and then summarized by a committee authorized by the American Institute of Nutrition. The convened committee accordingly provided the required information for the development of a diet that promoted growth and health. Unfortunately, that level of empirical information is not available in the zebrafish literature. We therefore rely on the published information that exists for other species of fish that occupy a similar habitat/niche and demonstrate similar feeding habits. Recent research results detailing nutritional physiology and qualitative requirements have been obtained with the use of purified experimental diets that are precursors of SRDs. The amount of information that is available, although lacking specificity for zebrafish, is sufficient for the development of a formulation that can confidently serve as an experimental reference diet that confers acceptable growth and survival.
Historical knowledge about nutrition of terrestrial and aquatic organisms is classified into five nutrient classes: protein, lipids, carbohydrates including fiber, vitamins, and minerals (Figure 1). For the practical development of many formulated diets, nutrients can be divided into two broad classes: (a) macronutrients (usually provided as grams per kilogram of diet), such as protein, lipid, carbohydrate, and fiber, and (b) micronutrients (usually provided as milligrams per kilogram of diet), such as minerals and vitamins (Table 1). Other inclusions, such as bioactive feed components (those components that alter metabolic processes but are not essential nutrients), can be classified as additives. The information provided in this section is thoroughly detailed in two publications (11, 25) and presented to lay the foundation for a choice of nutrients that would compose a proposed SRD.
Figure 1.
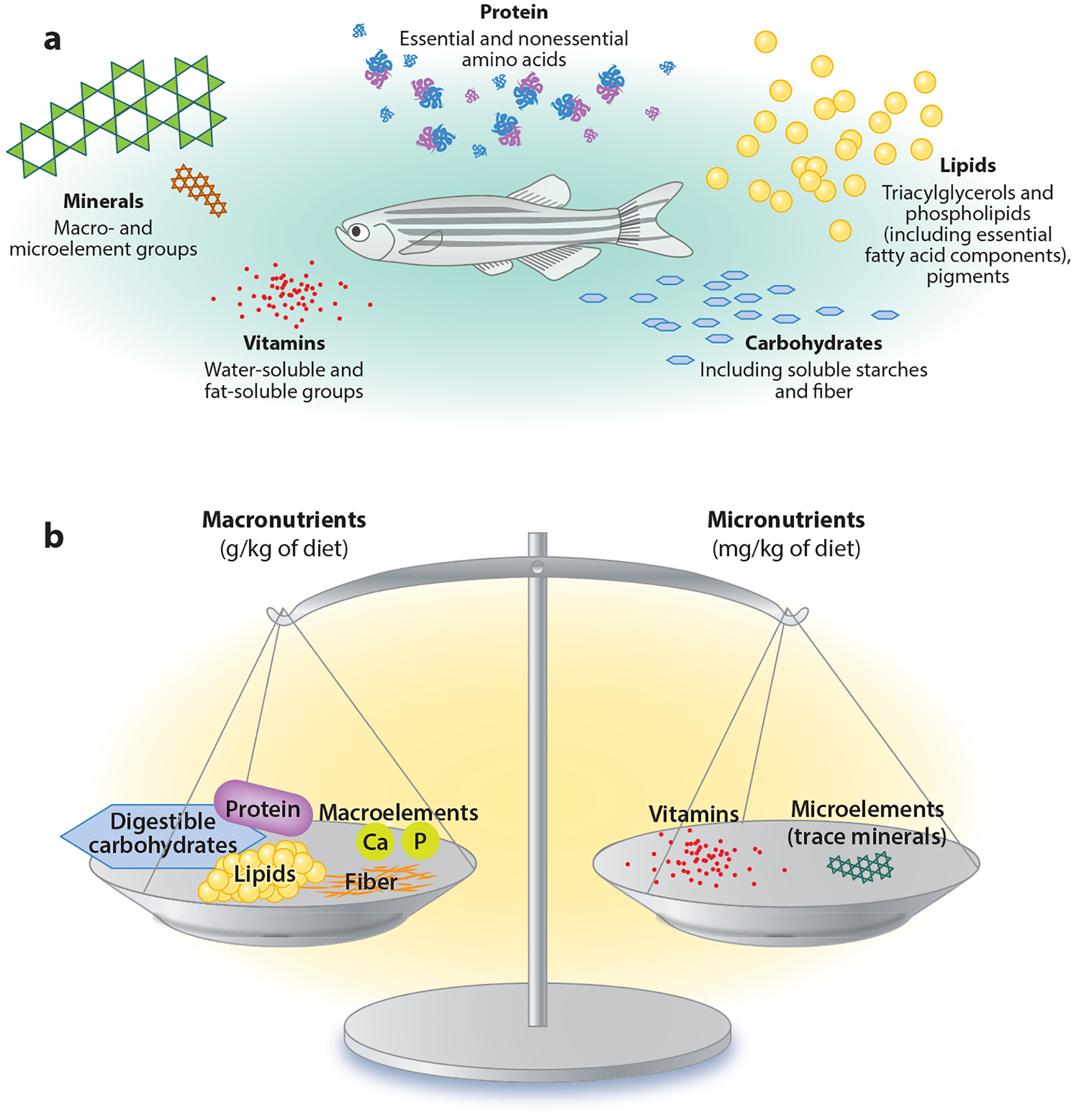
(a) Sources and (b) relative inclusion levels of dietary nutrients in a standard reference diet for zebrafish. Despite the large compositional volume (mass) of macronutrients in a reference diet, micronutrients must be balanced to ensure proper metabolic function and efficiency. Abbreviations: Ca, calcium; P, phosphorus.
Table 1.
Ingredient composition of a hypothetical standard reference diet for zebrafish
| Ingredient | Percent composition | Comment |
|---|---|---|
| Casein (low trace metals) | 24.75 | Protein source |
| Fish protein hydrolysate | 24.75 | Protein source |
| Wheat starch | 8.25 | Carbohydrate source |
| Dextrin (from corn starch) | 5.00 | Carbohydrate source |
| Menhaden fish oil | 2.00 | Lipid source |
| Safflower oil | 4.00 | Lipid source |
| Sunflower lecithin | 2.00 | Lipid source |
| Vitamin mix | 4.00 | NA |
| Mineral mix | 3.00 | NA |
| Canthaxanthin | 2.30 | Pigment source |
| Sodium alginate | 2.00 | Binder/heteropolysaccharide |
| Betaine hydrochloride | 0.15 | Attractant |
| Cholesterol | 0.12 | Sterol source |
| Ascorbyl palmitate | 0.04 | Bonded vitamin C |
| Nucleic acid mixture | 0.2 | Immunity/growth enhancer |
| Diatomaceous earth | 12.44 | Nonnutritive filler |
| Alpha cellulose | 5.00 | Resistant starch fiber |
Abbreviations: NA, not applicable.
Protein
Dietary protein is responsible for most observed growth because it is deposited principally as lean body mass tissue (organs and muscle tissue) and its positive attributes are commonly expressed as weight gain. The requirement for protein is not arbitrary but rather is determined by the cumulative satisfaction of 10 essential amino acids that cannot be endogenously synthesized and therefore require an exogenous dietary source. These 10 essential amino acids are commonly required by all vertebrates. Assuming that all other nutrient requirements are fulfilled, requirements of all essential amino acids must be jointly satisfied to achieve optimum growth. If the level of one essential amino acid is limiting (falls below its dietary requirement), a less than ideal growth rate results. Thus, in the absence of what would be considered an ideal protein source, essential amino acid requirements are usually satisfied by provision of a combination of dietary protein ingredient sources, which themselves are often purified in the form of protein hydrolysates or isolates. In addition to satisfying all essential amino acid requirements, a goal of having them provided in the appropriately proportional amounts can help to enhance growth by promoting their efficient metabolism. For aquatic organisms, crystalline forms of essential amino acids should rarely be included in formulations because these forms are often highly water soluble and leach rapidly in water, thereby creating doubt about the intended full dietary provision. Protein can be metabolically used as a source of energy; however, for diets designed for maximum growth, the ideal is to conserve (spare) the use of protein as an energy source by providing other highly digestible sources of energy, namely lipids and carbohydrates. If possible, no more than two purified protein sources should be used in a reference diet to reduce inherent variability.
Despite being based on the collective satisfaction of essential amino acids, protein requirements are commonly expressed as a percentage of dietary protein (calculated as a percentage of either moisture-free dry weight or ingredients at normal hydration, termed as fed), on the basis of the assumption that all requirements of essential amino acids are met. Most protein levels are assessed by determination of crude protein by Kjeldahl analysis on the basis of the measurement of nitrogen derived from protein (amino acids) and nonprotein sources. Nonprotein sources of nitrogen include nucleic acids and/or other nonamino acid amines and amides. Dietary protein sources derived from ingredients used in the formulation of a reference diet to meet the overall protein requirement must consider the level of purity, qualitative and quantitative amino acid profile, and digestibility. The results of a recent study recommended a level of dietary protein of 37.6% for maximum weight gain and 44.8% for maximum protein retention for D. rerio (9). That recommendation is based on the use of a single protein source, menhaden meal, an ingredient that, with processing, contains a significant amount of highly digestible protein composed of an excellent profile of essential amino acids to meet the corresponding requirements.
Lipids
The second macronutrient is lipid. Fish do not have a specific requirement for lipids, which are classified as either triacylglycerols (triglycerides, the primary sources of energy) or phospholipids (PLs) (polar lipids, components of the lipid layer of cellular membranes). Cholesterol may be an additional lipid of inclusion, depending on dietary requirements. The choice of a lipid source for the preparation of a feed/diet formulation is based on its role as a source of metabolizable energy in conjunction with satisfaction of the requirements of essential fatty acids that are characterized by long carbon chains (≥18) with two to six double (unsaturated) bonds. The level of lipid needed as a source of energy is dependent on whether dietary carbohydrate, another preferred alternative energy source, is efficiently digested and is not associated with unhealthy dietary consequences, such as obesity (12, 33). Thus, the evolutionary feeding habits of species of fish influence the combined amount of lipid and carbohydrate that can be used to address energy requirements. Zebrafish are tropical, omnivorous freshwater fish, and the levels of lipid and essential fatty acid requirements are assumed to be similar to those of species that have similar feeding habits such as tilapia and carp (25). For these species, total dietary lipid levels range from 10% to 18% of dry matter. An adequate dietary level to meet both energy requirements and likewise ensure satisfaction of the requirements of essential fatty acids will commonly range from 12% to 15% of dry matter intake. Fatty acids of both n-6 (plant-derived oils) and n-3 (plant- and animal-derived oils) series are usually required, and n-6 levels are commonly required at a proportionately greater amount by both herbivorous and omnivorous freshwater fish in comparison with carnivorous fish. The essential fatty acid requirements are uniquely reflective of the respective amounts contained in the natural food consumed. There is little information on lipid requirements in D. rerio; however, both n-6:n-3 ratios and total dietary lipid affect adiposity and reproductive health in zebrafish (10). SRDs should include purified sources of several plant or animal oils, as a single source may not provide sufficient levels to satisfy the total essential fatty acid requirements. Due to the energetic and metabolic regulatory value of dietary fatty acids, we believe these nutrients should be a fruitful investigative area of future research.
A fish requirement for exogenous cholesterol has yet to be reported (25), probably because sufficient amounts can most likely be provided as a by-product of the inclusion of a fish oil to meet both the energy requirements and some essential fatty acid requirements. In addition, there is no reported requirement for dietary PL in freshwater fish, and PL is known to be synthesized de novo. However, dietary sources of intact PL have been found to improve survival and/or growth in fish larvae and juveniles (25). Dietary levels of 2% to 4% have most often been determined to be best. To date, the effect of specific PLs has been difficult to determine because most of the studies use a source consisting of a mixture of PLs. Collectively, research results suggest phosphatidylcholine to be the most effective dietary PL (25). Therefore, a separate PL source would appear to be a good choice for inclusion in diets formulated for zebrafish larvae and possibly juveniles. Diogo et al. (6) found that the addition of dietary phosphatidylethanolamine to a purified diet fed to brood stock improved the reproductive performance of zebrafish as measured by sperm quality and egg diameter.
Carbohydrates
Dietary plant-derived carbohydrates in the form of digestible starches are often included in diets for freshwater fish, including zebrafish, as a source of energy. Robison et al. (33) found that at least 5% of the diet had to include carbohydrate and that 5% to 35% of carbohydrate in the diet did not significantly affect total weight gain. The ultimate value of a carbohydrate as a source of energy appears to be limited in most fish species. Efficiency of utilization is based on an array of factors that include quantity and quality and the possibility of adverse interactions among sources. Carbohydrates can effectively reduce the amount of energy derived from dietary protein and lipid sources and thereby support improved feed utilization manifested by protein deposition (growth). In the formulation of a zebrafish diet, provision of a carbohydrate ingredient is advised through the use of highly digestible sources such as purified starch and dextrin (17). A table of ingredients and corresponding dietary levels used in zebrafish dietary formulations can be found in a study by Fowler et al. (11).
Vitamins
Vitamins are organic molecules that are classified as either water or lipid soluble. Biotin, niacin, folic acid, thiamine, riboflavin, pantothenic acid, pyridoxine, cyanocobalamin ascorbic acid, choline, and myoinositol are water-soluble vitamins and are required at dietary levels of either micrograms or milligrams per kilogram for a phylogenetically diverse group of animals. Vitamins E, A, D, and K are lipid soluble. Typically, the vitamin requirements of fish align with those determined for other vertebrates, and this alignment is a reflection of the operation of similar metabolic processes. Vitamin deficiencies result in metabolic or structural aberrations that are sometimes clinically manifested. Lipid-soluble vitamins are commonly stored, even in excess, in tissues. As a result of vitamin storage, their requirements are much more difficult to assess than those of water-soluble vitamins that are readily excreted so that excess tissue levels do not occur.
Minerals
Minerals (elements) are composed of two groups, macro- and microelements that reflect the relative quantities necessary for normal physiological function. Macroelements include calcium, magnesium, potassium, sodium, chlorine, phosphorus, and sulfur and are required at levels of grams per kilogram of diet. Microelements include cobalt, copper, chromium, fluorine, iodine, iron, magnesium, molybdenum, nickel, selenium, silicon, tin, vanadium, and zinc and are required at levels of milligrams per kilogram of diet. These minerals have roles in many physiological processes as cofactors in catalysis of enzymatically controlled chemical reactions and also serve as components of vitamins and hormones. In some cases, the minerals dissolved in the medium in which fish live significantly contribute to the satisfaction of a specific mineral requirement. Consequently, experimental determination of the required dietary level can be difficult. Some minerals may be rendered chemically unavailable by being bound to phytate, a compound characteristically found in meals derived from cereal grains and oil seeds.
INGREDIENT REQUIREMENTS FOR AN SRD
Table 1 presents the ingredient composition of an SRD that has the potential to serve the zebrafish research community. This specific SRD is shown for illustrative purposes and is neither recommended nor promoted. A future diet formulated to desired specifications will most likely be similar to that listed and will potentially serve as an SRD for zebrafish. The inclusion of the proper and adequate levels of nutrients in the SRD must be accompanied by the inclusion of ingredients that will ensure efficient utilization while also contributing to the overall health of zebrafish. As stated previously, the formulation is exclusively composed of chemically defined ingredients that must be essentially void of bioactive food components or antinutritional factors.
A diet similar to a reference diet has been used in recent zebrafish studies (10, 12). The two principal protein sources that compose the formulation, casein and fish protein hydrolysate, are provided to address the need for a variety of protein sources to achieve the desired dietary levels of essential amino acids. All protein sources contain an as-is content of crude protein that exceeds 80% and is highly digestible. The approximate proportion of total dietary protein, calculated as the sum of the percent of protein in each ingredient times the level of ingredient in the formulation, is 40%, a level that should meet the recommended level (25) when protein derived from other ingredients is included in the calculation. Small amounts of crude lipid, fiber, and ash are contained in the predominant protein sources, but their mass provides minimal contribution to the overall dietary content of these macronutrients. In the future, chemically defined single cell protein may prove to be a very satisfactory replacement for one of these protein sources (32). The replacement by a single cell protein ingredient is attractive because these algal-, fungal-, and bacterial-derived protein sources are chemically defined and are characteristically composed of highly digestible protein of consistent chemical composition.
Menhaden oil, safflower oil, and sunflower lecithin are all chemically defined sources of lipid. Menhaden and safflower oils are 100% lipid and sunflower lecithin is approximately 70% lipid, primarily consisting of the PL phosphatidylcholine. The amount of menhaden oil, safflower oil, and sunflower lecithin included in the SRD for zebrafish contributes approximately 7.4% of total dietary lipid and is somewhat lower than levels that have been reported for other species of freshwater fish. The total level of plant-derived sources of lipid exceeds that derived from marine oils and results in a proportionately higher level of dietary n-6 essential fatty acids (from plants) when compared with n-3 essential fatty acids (from marine fish and plants). Marine-sourced oils, such as menhaden oil, contain comparatively high levels of long-carbon-chain fatty acids with multiple double bonds termed long-chain polyunsaturated fatty acids (LC-PUFAs). Although fatty acid requirements of zebrafish are not known, results of a recent investigation suggest that a n-6:n-3 ratio of 4:1 may meet desired growth and reproductive requirements for zebrafish colonies (10).
The two major sources of carbohydrate are wheat starch (~80% carbohydrate) and dextrin, a soluble fiber and polymer of glucose that is 98% carbohydrate. Both sources are highly digestible and serve as an energy source to minimize the use of protein as an energy source. Other starches as possible options for inclusion are rice or corn.
Vitamins and minerals are naturally available in some ingredients of the formulation but are not sufficient to meet dietary requirements. When all supplemental vitamins and minerals are removed from a formulated diet, zebrafish weight gain is reduced (29). Thus, some quantity and quality of vitamins and minerals are needed as part of the formulation to ensure that the full requirements for these micronutrients are satisfied. Sources of minerals include those soluble in freshwater environments that zebrafish inhabit. Vitamin and mineral requirements are known to be shared among all vertebrates, as they are commonly involved in similar metabolic processes. Until specific requirements for individual vitamins and minerals are determined in zebrafish, these nutrients most likely can be estimated on the basis of defined requirements for other vertebrates. At this time, we suggest dietary inclusion of levels in an SRD that are similar to those determined for rodents in SRDs such as AIN-93. Although their inclusion may result in an excess of required levels, it is not uncommon to overformulate a diet with the expectation that some nutrients will be degraded or leached, particularly those that are water soluble. Empirical determinations of these nutrients will eventually be required.
Sodium alginate is a carbohydrate source, more specifically termed a heteropolysaccharide, that also can be used as another source of energy; however, alginate is specifically added to enhance the physical integrity of the diet, serving as a binder to reduce the dissolution of the diet into particles. Use of an effective binder would reduce the rate of leaching of water-soluble nutrients into the aquatic environment. The goal is to minimize dissolution from the time of introduction of the diet into the aquatic medium until eventual consumption. The ingredients of the SRD are thoroughly mixed to create a homogenous distribution of nutrients that are bound together by the alginate.
The proposed SRD also contains a variety of other ingredients that are generally defined as additives. Additives can be nutrients or nonnutrients. Some additive compounds can be classified as bioactive food components. Other nonnutrient compounds are added to enhance the physical integrity of the diet.
Fish are unable to synthesize pigment compounds and therefore depend on exogenous sources that often enter into an array of metabolic pathways to produce other pigment compounds that are then incorporated into the tissue. Similar pigments would be derived from a natural diet in the wild. Canthaxanthin is a carotenoid compound that confers coloration to the zebrafish. It has also been proposed that dietary pigments may also confer some level of immunocompetence to fish (25). Another dietary additive is a mixture of nucleotides. Although no evidence of a lack of ability to synthesize nucleotides exists, the provision of a suitable amount of an exogenous source appears to enhance immune responses and ensure good health and survival (25, 35). Supplemental nucleotides promote growth in a larval zebrafish diet that contains the addition of mixed nucleotides at a level of 0.1% (13). Cholesterol is another added compound that is not based on a demonstrated requirement for zebrafish or other freshwater fish. Ascorbyl palmitate is ascorbic acid (vitamin C) covalently bonded to a saturated fatty acid, palmitic acid. Ascorbyl palmitate is added because it is not soluble in water relative to its highly soluble unbound form (ascorbic acid), thereby assuring that the required level of dietary ascorbic acid can be effectively provided. Betaine hydrochloride is included in the formulation because research has demonstrated that it serves as an effective feed attractant. Effective attraction as a prelude to consumption is essential for the efficient delivery of nutrients, particularly those that are highly water soluble. In addition, betaine is considered an effective osmolyte in protecting animals from stress (5). Furthermore, betaine is involved in the synthesis of methionine, an essential amino acid that is often one of the first to be limiting in a formulated diet. There is no direct evidence for the metabolic value of betaine in a zebrafish diet, but it may confer a health-promoting advantage to the fish.
Alpha cellulose, as a highly resistant starch, is hypothesized to be important in an omnivore diet on the basis of interactions with microbial symbionts as it is in many vertebrate diets. Empirical evidence of its importance has not been determined, and therefore cellulose and acid-washed diatomaceous earth are generally used as fillers on the basis of the assumption that they do not confer direct nutritive value. Their inclusion as ingredients in formulations offers flexibility in amending the ingredient/nutrient composition while still allowing the desired control inherently characteristic of an SRD to be sustained.
LIFE-STAGE CONSIDERATIONS
Life stages of species of fish often exhibit changes in the requirements of particular nutrients that occur during different physiological states. For example, as fish transform from larvae to juveniles, and then to adults and brood stock, growth rates tend to decrease in response to changes in the partitioning of available dietary protein and energy. Results derived from investigations of brood stock of freshwater fish show that the early life stage is uniquely characterized by requirements for higher levels of dietary protein and lipid. Also, characteristically higher dietary levels of n-3 LC-PUFA, ascorbic acid, vitamin E, and pyridoxine (vitamin B6) are required for reproductive health and measured performance (16). The requirements of many of these compounds or elements have not been specifically determined for zebrafish but are estimated on the basis of empirically derived requirements from related species. We hypothesize that at least three different SRDs will be required for zebrafish, including (a) a larval diet influencing positive development and maturation of organ systems, (b) a juvenile diet influencing protein retention and weight gain, and (c) an adult diet that specifically meets somatic and reproductive needs. These life-history diets would probably be characterized by quantitative and/or qualitative modifications of the ingredients of a singular SRD and the corresponding changes in nutrient profiles.
ZEBRAFISH VERSUS RODENT SRDs: A TALE OF TWO ERAS
The existing knowledge for related freshwater species of fish is sufficient for the confident preparation of a formulation to meet or exceed most, if not all, nutritional requirements of D. rerio. As more knowledge of the specific nutritional requirements of D. rerio is revealed, specific changes in the composition of the SRD should be tested over time. Ideally, the use of empirical data sets on zebrafish nutrient requirements would be applied in the formulation of an SRD, but that information is not available. In the last few decades, funded research priorities in the use of molecular technologies have often superseded basic nutritional science. Consequently, studies of nutritional information directly related to animal nutrition are not widely available, nor were they prioritized by the scientific community. Unfortunately, moving forward without dietary standardization might lead to a failure of the use of zebrafish as a model for effective translational research. It is apparent that the approach we have suggested for SRD development in zebrafish is quite different from that used for the development of rodent SRDs. For example, the American Institute of Nutrition established a committee on standards for nutritional studies in rodents that led to a proposed SRD, AIN-76 (1). This SRD was based on years of research and published reports of nutritional studies in rodents and led to a major breakthrough in reproducibility among research labs. Adoption of the use of AIN-76 by the research community ultimately revealed clinical signs of nutritional deficiency that occurred over prolonged use, an observed condition that led to a recommendation of modifications in nutrient content (2). With additional data, a more contemporary diet was formulated and gave rise to AIN-93 (23), which itself was modified to support life stages of growth relative to maintenance. Thus, despite a different approach, a starting point must be launched in zebrafish research, and a similar argument could be made for enhancing the utility of any animal model.
The use of dietary-administered compounds to assess the zebrafish response to exposure to disease, drugs, and presumed toxic compounds has been the subject of numerous investigations using D. rerio. Also, the influence of particular nutrients in the modification of intestinal microbiota and the role of diet in the influence of gene expression (nutrigenomics) (42) and inflammation as well as metabolic diseases such as obesity (8) have also become principal areas of investigation. If use of a designated SRD by a majority of zebrafish research laboratories can become the norm rather than the exception, then this significant paradigm shift will more rapidly advance knowledge that can then be confidently accepted and ultimately applied. With available SRDs, the increase in power of this important animal model cannot be undervalued.
TESTING, AND TESTING AGAIN
Testing a diet and attributing cause and effect to specific ingredients or nutrients is a major challenge facing nutritionists. In early decades, the purity of most ingredients was at commodity levels, with minimal processing (historically termed natural or practical ingredients, as reviewed in 40). Due to increasing demands, many commodity ingredients are now fractioned into component parts for additional commercial value and, for some, an enhanced nutritional value in feed manufacturing. New sources of specific ingredients with improved nutrient profiles are being evaluated almost daily. In the future, the SRDs established today will require periodic review for both ingredient and nutrient profiles. Ongoing testing is necessary not only to guarantee highly reproducible diets but also to test new ingredients and nutrients and to evaluate their principal outcomes in concert with possible effects on animal health. One can debate the responsible entities for ongoing diet development and evaluation, but the scientific community’s pursuit of good science must be the overriding path to change.
In fish, weight gain is often considered the gold standard for the success or failure of an SRD or an experimental diet; however, change in weight is only one of a plethora of outcomes that can be assessed. Other outcomes include survival (which should always be reported); morphology-related phenotypes (length, height, ratios, etc.); various feed, ingredient, and nutrient utilization efficiencies; and response to stress. New omics technologies will broaden and enhance our ability to make critical decisions about the efficacy and importance of ingredients and nutrients. Regardless of the new and improved feedstuffs that become available, ongoing testing is critical to the rigor of science regarding laboratory-held animals, particularly when these animals are used translationally in investigations addressing human health questions that concern and challenge us. A general approach for testing is illustrated in Figure 2.
Figure 2.
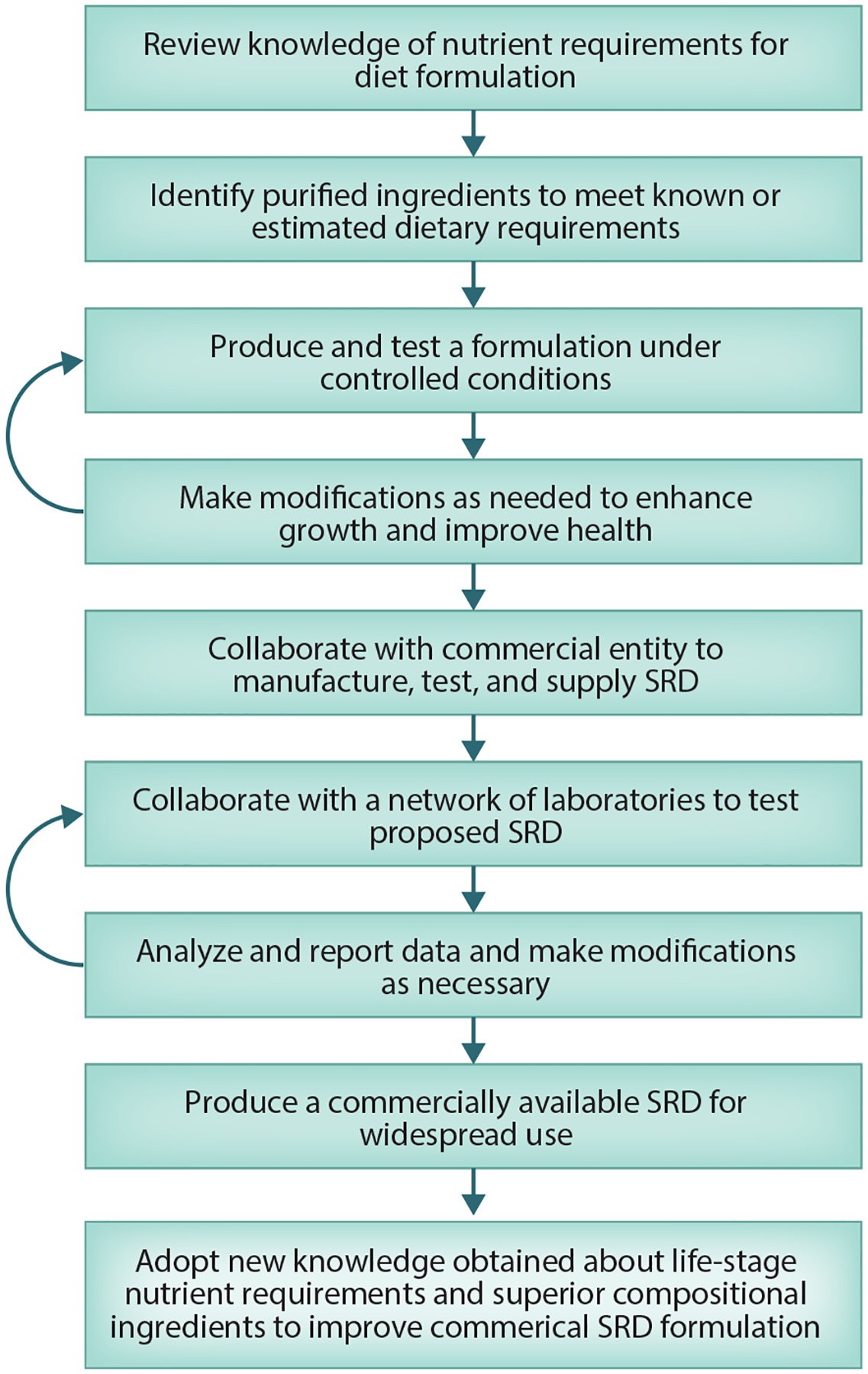
Conceptual flowchart for development and use of a standard reference diet (SRD). Return arrows represent adaptive management paths directed toward achieving an improved formulation.
FUTURE NEEDS: HOW DO WE GET THERE?
Overcoming the bias of perennial use of research diets that have a closed formula and are subject to changes in nutrient composition by a multitude of laboratories devoted to D. rerio research will be difficult. An SRD represents a somewhat radical change in mindset relative to nutritional control of diet as part of experimental designs. Widespread use of a universally accepted SRD will be more difficult to achieve than the actual development of the formulation itself. The change to use of an SRD also may lead to physiological responses that were different from those identified and already reported using undefined research diets. A critical step toward eventual widespread use is adequate testing that includes sufficient supply of a readily available, commercially produced SRD that is temporally consistent in nutritional content with an open (nonproprietary) formula. Participation of commercial suppliers would be based on recognition of the importance of the need for an SRD and its corresponding value relative to progress in the use of zebrafish as a biomedical model. Initial and follow-up testing by an array of designated/interested laboratories that welcome its evaluation would require strict adherence to common testing guidelines founded in the protocol for using the SRD. Once an SRD has been sufficiently tested and accepted, further industry cooperation will be required, specifically through reliable provision of a product in the market-place for purchase. Transition to a diet recognized as the common SRD for use in zebrafish studies is envisioned as occurring through a gradual assimilation by research laboratories. As the number of participating research laboratories increases, the opportunity for comparison and collaboration based on standardized nutritional methodology accordingly increases, leading to a desired reality of producing more meaningful research results, which can be applied confidently.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contributions of many colleagues and friends, past and present, who over several decades contributed in thoughtful and active discussions on the role of standardization in animal diets. We would also like to thank the continued support of the zebrafish research community as we all embrace our collective desire to increase the rigor and reproducibility of zebrafish translational research. This work was supported in part by the National Institutes of Health (NIH) Nutrition and Obesity Research Center (Lab Animal Nutrition Core in the Animal Models Core) at the University of Alabama at Birmingham (P30DK056336) and by the NIH Office of Research Infrastructure Programs.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.AINCSNS (Am. Inst. Nutr. Ad Hoc Comm. Stand. Nutr. Stud.). 1977. Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J. Nutr 107:1340–48 [DOI] [PubMed] [Google Scholar]
- 2.AINCSNS (Am. Inst. Nutr. Ad Hoc Comm. Stand. Nutr. Stud.). 1980. Second report of the ad hoc Committee on Standards for Nutritional Studies. J. Nutr 110:1726 [Google Scholar]
- 3.Bernard C 1865. Introduction à l’étude de la médecine expérimentale Paris: Libr. L’Acad. Imp. Méd. [Google Scholar]
- 4.Castell JD, Kean JC, D’Abramo LR, Conklin DE. 1989. A standard reference diet for crustacean nutrition research. I. Evaluation of two formulations. J. World Aquac. Soc 20:93–99 [Google Scholar]
- 5.Craig SAS. 2004. Betaine in human nutrition. Am. J. Clin. Nutr 80(3):539–49 [DOI] [PubMed] [Google Scholar]
- 6.Diogo P, Martins G, Gavaia P, Pinto W, Dias J, et al. 2015. Assessment of nutritional supplementation in phospholipids on the reproductive performance of zebrafish, Danio rerio (Hamilton, 1822). J. Appl. Ichthyol 31:3–9 [Google Scholar]
- 7.Eisen JS. 2020. History of zebrafish research. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 3–12. London/San Diego: Academic. 1st ed. [Google Scholar]
- 8.Faillaci FF, Milosa F, Critelli RM, Turola E, Schepis F, Villa E. 2018. Obese zebrafish: a small fish for a major human health condition. Anim. Models Exp. Med 1(4):255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes H, Peres H, Carvalho AP. 2016. Dietary protein requirement during juvenile growth of zebrafish (Danio rerio). Zebrafish 13(6):548–55 [DOI] [PubMed] [Google Scholar]
- 10.Fowler LA, Dennis-Cornelius LN, Dawson JA, Barry RJ, Davis JL, et al. 2020. Both dietary ratio of n-6/n-3 fatty acids and total lipid are positively associated with adiposity and reproductive health in zebrafish. Curr. Dev. Nutr 4(4):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler LA, Williams MB, D’Abramo LR, Watts SA. 2020. Zebrafish nutrition—moving forward. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 379–401. London/San Diego: Academic. 1st ed. [Google Scholar]
- 12.Fowler LA, Williams MB, Dennis-Cornelius LN, Farmer S, Barry RJ, et al. 2019. Influence of commercial and laboratory diets on growth, body composition, and reproduction in the zebrafish Danio rerio. Zebrafish 16(6):508–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Ran C, Zhang Z, He S, Min J, Zhou Z. 2017. The growth-promoting effect of dietary nucleotides in fish is associated with an intestinal microbiota-mediated reduction in energy expenditure. J. Nutr 147:781–88 [DOI] [PubMed] [Google Scholar]
- 14.Halver JE, DeLong DC, Mertz ET. 1957. Nutrition of salmonoid fishes: V. Classification of essential amino acids for chinook salmon. J. Nutr 63(1):95–105 [DOI] [PubMed] [Google Scholar]
- 15.Hisaoka KK, Battle HI. 1958. The normal developmental stages of the zebrafish, Brachydanio rerio (Hamilton-Buchanan). J. Morphol 102(2):311–27 [Google Scholar]
- 16.Izquierdo MS, Fernandez-Palacios H, Tacon AGJ. 2001. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197:25–42 [Google Scholar]
- 17.Kamalam BS, Medale F, Panserat S. 2017. Utilization of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27 [Google Scholar]
- 18.Kanazawa A, Shimaya M, Kawasaki M, Kashiwada K. 1970. Nutritional requirements of prawn. I. Feeding on artificial diet. Bull. Jpn. Soc. Sci. Fisheries 36(9):949–54 [Google Scholar]
- 19.Lakstygal AM, de Abreu MS, Lifanov D, Wappler-Guzetta EA, Serikuly N, et al. 2018. Zebrafish models of diabetes-related CNS pathogenesis. Progress Neuro-Psychopharmacol. Biol. Psychiatry 92:48–58 [DOI] [PubMed] [Google Scholar]
- 20.Lawrence C 2016. New frontiers for zebrafish management. In The Zebrafish: Genetics, Genomics, and Transcriptomics, ed. Detrich HW III, Westerfield M, Zon LI, pp. 483–508. Cambridge, MA: Academic. 4th ed. [Google Scholar]
- 21.McArthur KL, Chow DM, Fetcho JR. 2020. Zebrafish as a model for revealing the neuronal basis of behavior. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 593–611. London/San Diego: Academic. 1st ed. [Google Scholar]
- 22.Nichols JT. 2020. Zebrafish as a platform for genetic screening. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 649–56. London/San Diego: Academic. 1st ed. [Google Scholar]
- 23.Nielson FH. 2018. 90th anniversary commentary: the AIN-93 purified diets for laboratory rodents—the development of a landmark article in The Journal of Nutrition and its impact on health and disease research using rodent models. J. Nutr 148(10):1667–70 [DOI] [PubMed] [Google Scholar]
- 24.Nose T, Arai S. 1972. Optimum level of protein in purified diet for eel, Anguilla japonica. Bull. Freshwater Fish. Res. Lab 22:145–55 [Google Scholar]
- 25.NRC (Natl. Res. Counc. Natl. Acad.). 2011. Nutrient Requirements of Fish and Shrimp Washington, DC: Natl. Acad. Press [Google Scholar]
- 26.Oka T, Nishimura Y, Zang L, et al. 2010. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parthak NH, Barresi MJF. 2020. Zebrafish as a model for revealing the neuronal basis of behavior. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 559–82. London/San Diego: Academic. 1st ed. [Google Scholar]
- 28.Pastore MR, Negrato E, Poltronieri C, Barion G, Messina M, et al. 2018. Effects of dietary soy isoflavones on estrogenic, cortisol level, health and growth in rainbow trout, Oncorhynchus mykiss. Aquac. Res 49(4):1469–79 [Google Scholar]
- 29.Paul LT, Fowler LA, Barry RJ, Watts SA. 2013. Evaluation of Moringa oleifera as a dietary supplement on growth and reproductive performance in zebrafish. J. Nutr. Ecol. Food Res 1(4):322–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips JB, Westerfield M. 2020. Zebrafish as a model to understand human genetic diseases. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 619–24. London/San Diego: Academic. 1st ed. [Google Scholar]
- 31.Rissone A, Ledin J, Burgess SM. 2020. Targeted editing of zebrafish genes to understand gene function and human disease pathology. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 637–45. London/San Diego: Academic. 1st ed. [Google Scholar]
- 32.Ritala AS, Häkkinen ST, Toivari M, Wiebe MG. 2017. Single cell protein—state-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol 8:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robison BD, Drew RE, Murdoch GK, Powell M, Rodnick KJ, Settles M. 2008. Sexual dimorphism in hepatic gene expression and the response to dietary carbohydrate manipulation in the zebrafish (Danio rerio). Comp. Biochem. Physiol. Part D Genom. Proteom 3(2):141–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders E, Farmer SC. 2020. Aquatic models: water quality and stability and other environmental factors. ILAR J 60:141–49 [DOI] [PubMed] [Google Scholar]
- 35.Shiau S-S, Gabaudan J, Lin YH. 2015. Dietary nucleotide supplementation enhances immune responses and survival to Streptococcus iniae in hybrid tilapia fed diet containing low fish meal. Aquac. Rep 2:77–81 [Google Scholar]
- 36.Siccardi AJ III, Garris HW, Jones WT, Moseley DB, D’Abramo LR, Watts SA. 2009. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 6(3):275–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teame T, Zhang Z, Ran C, Zhang H, Yang Y, et al. 2019. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front 9(3):68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga ZM, Ekker SC, Lawrence C. 2018. Workshop report: zebrafish and other fish models–description of extrinsic environmental factors for rigorous experiments and reproducible results. Zebrafish 15:533–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts SA, Lawrence C, Powell M, D’Abramo LR. 2016. The vital relationship between nutrition and health in zebrafish. Zebrafish 13(S1):S72–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts SA, Powell M, D’Abramo LR. 2012. Fundamental approaches to the study of zebrafish nutrition. ILAR J 53(2):144–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiles TJ, Guillemin KJ. 2020. Zebrafish as model for investigating animal-microbe interactions. In The Zebrafish in Biomedical Research: Biology, Husbandry, Disease, and Research Applications, ed. Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, pp. 627–32. London/San Diego: Academic. 1st ed. [Google Scholar]
- 42.Williams MB, Watts SA. 2019. Current basis and future directions of zebrafish nutrigenomics. Genes Nutr 14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zang L, Maddison LA, Chen W. 2018. Zebrafish as a model for obesity and diabetes. Front. Cell Dev. Biol 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zang L, Shimada Y, Nishimura N. 2017. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci. Rep 7:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]


