Abstract
Background:
Esophagectomy is associated with postoperative delirium but its pathophysiology is not well defined. We conducted this study to measure the relationship among serum biomarkers of inflammation and neuronal injury and delirium incidence and severity in a cohort of esophagectomy patients.
Methods:
Blood samples were obtained from patients at preoperative and postoperative days 1 and 3, and analyzed for S100 calcium-binding protein B, C-Reactive Protein, interleukins 8, 10, tumor necrosis factor-alpha, insulin like growth factor 1. Delirium was assessed using Richmond Agitation Sedation Scale and Confusion Assessment Method for Intensive Care Unit twice daily. Delirium severity was assessed using Delirium Rating Scale Revised-98 once daily.
Results:
Samples from 71 patients were included. Preoperative biomarker concentrations were not associated with postoperative delirium. Significant differences in change in concentrations from preoperative to postoperative day 1 were seen in interleukin-8 (delirium: 38.6 interquartile range (IQR): 29.3, 69.8; no delirium: 24.8 IQR: 16.0, 41.7, p=0.022), and interleukin-10 (delirium: 26.1 IQR: 13.9, 36.7; no delirium: 12.4 IQR: 7.7, 25.7, p=0.025). Greater postoperative increase in S100 calcium-binding protein B (Spearman r=0.289, p=0.020) and lower levels of Insulin-like Growth Factor 1 were correlated with greater delirium severity (Spearman r=−0.27, p=0.040). Greater CRP change quartiles were associated with higher delirium incidence adjusting for severity of illness (OR=1.68, 95% CI: 1.03,2.75, p=0.037) or comorbidities (OR=1.70, 95% CI: 1.05,2.76, p=0.030).
Conclusions:
Differences in change in serum CRP, IL-8 and IL-10 concentrations were associated with postoperative delirium suggesting biomarker measurement early in the postoperative course is associated with delirium.
Keywords: Delirium, Inflammation, systemic, Outcomes, delirium, esophagectomy, biomarkers, c-reactive protein, postoperative delirium
Delirium occurs in up to 64% of patients following major non-cardiac thoracic surgery and is independently associated with increased risk of mortality, cognitive and functional decline, and institutionalization.1–9 Despite its prevalence and significant impact on health outcomes, there are no effective pharmacological treatments for delirium, making it a significant public health threat.10–13 Hence, efforts to characterize biomarkers that could identify patients at risk of developing delirium and potentially advance scientific understanding of delirium pathophysiology are of great importance.14 Current pathophysiologic models of delirium borrow from the neuroinflammatory hypothesis which posits that delirium results from a cytokine-mediated inflammatory syndrome.15,16 After an acute stressor (e.g., surgery), an overabundance of peripheral cytokines such as interleukin (IL)-2, 6, 8, and tumor necrosis factor (TNF)-alpha cross a disrupted blood-brain barrier resulting in microglial and astrocyte activation. Activation of these cells is also associated with release of S-100 Calcium Binding Protein B (S-100β), a marker of neuronal injury. Neuronal damage leads to cholinergic failure and brain network disruption culminating in clinical manifestation of delirium.17–19 The pathophysiologic model also takes into account the loss of neurotrophic factors such as IGF-1 (insulin-like growth factor-1), further impairing the ability for neurons to repair and survive.
Recent studies evaluating the neuro-inflammatory hypothesis in the perioperative population have not comprehensively examined the cytokine-mediated inflammatory syndrome.20–25 In a large cohort of older patients undergoing major non-cardiac thoracic surgery, pre-operative C-Reactive Protein (CRP), an acute phase reactant, was associated with an increased risk for postoperative delirium.21 The study did not, however, include measures of astrocyte and glial activation, or neurotrophic factors, which may be of importance in delirium development. Previous studies in emergent surgery and critical care settings have demonstrated an association between S-100β and postoperative delirium incidence and severity.26–33 Nonetheless, this relationship, and the timing of biomarker measurement has not been examined in an elective esophagectomy population to our knowledge. The identification of perioperative inflammatory and neuronal injury blood-based biomarkers may aid in identification of at-risk individuals, facilitate administration of perioperative prevention strategies, and lead to personalized treatments to reduce the risk of postoperative complications. Therefore, the purpose of our analysis was to identify peripheral biomarkers associated with postoperative delirium incidence and severity in patients undergoing esophagectomy. We hypothesized that increased markers of neuronal injury (S-100β), systemic inflammation (CRP), pro-inflammatory cytokines (IL-6, IL-8, and IL-10), and lower levels of neuroprotective factors (IGF-1) will be associated with increased postoperative delirium.
Material and Methods
This is a secondary analysis of blood samples collected from patients enrolled in a randomized double-blind placebo controlled single center trial testing the feasibility and acceptability of haloperidol prophylaxis vs. placebo to prevent postoperative delirium. All patients in this analysis underwent esophagectomy. The study was performed at Indiana University Simon Cancer Center, and received ethical approval from the Indiana University Institutional Review Board. Full details of the study were previously published.1 In brief, English-speaking patients, 18 years of age or older, and undergoing esophagectomy were included. Exclusion criteria included a history of schizophrenia, Parkinson’s Disease, severe dementia, neuroleptic malignant syndrome, pregnancy, alcohol abuse, breastfeeding, a haloperidol allergy, ≥500 millisecond QT prolongation, and current use of levodopa or a cholinesterase inhibitors. In order to analyze change in biomarker values and their relationship with delirium, we excluded patients without blood samples from both preoperative visit and postoperative day one. Hence, each patient included in the study had blood samples available from the 2 different time points for analysis.
Outcomes
Delirium and Coma
Level of consciousness and delirium were assessed twice daily by trained research personnel (once in the morning between 9:00 am-11:00 am, and once in the afternoon between 3:00pm-5:00pm) beginning on postoperative day 1 using the validated scales of the Richmond Agitation Sedation Scale (RASS), and Confusion Assessment Method for the Intensive Care Unit (CAM-ICU), respectively, and continuing until hospital discharge.34–35 Participants were considered eligible for CAM-ICU assessment if their RASS score was between −3 and +4. Coma was defined as a RASS score of −4 or −5. Patients were considered as having delirium if they had any positive CAM-ICU on either the morning or afternoon assessment during the hospitalization. The CAM-ICU score was determined by examining the patient for (a) acute or fluctuating changes in mental status, (b) inattention, (c) altered level of consciousness, and (d) disorganized thinking. Patients were considered delirious if they displayed (a) and (b), plus (c) and/or (d).
Delirium Severity
Delirium severity was assessed once daily using the Delirium Rating Scale Revised-98 (DRS-R-98) administered by trained research staff.36 The DRS-R-98 captures impairments in attention; short- and long-term memory; visuospatial ability and orientation; perceptual and sleep–wake cycle disturbances; abnormalities of language, thought process, and content; motor agitation or retardation; and mood lability. It is a 16-item scale, with the severity scale having 13 items (rated 0–3 each, maximum 39 points) and higher scores indicating greater delirium severity.
Biomarkers
Based on review of the scientific literature related to delirium pathophysiology and the neuroinflammatory hypothesis, the following biomarkers of inflammation were selected a priori for analysis: IL-6, 8, 10; TNF-A; and CRP.21–26,28–33 IGF-1 was selected as biomarker for neuroprotection, and S-100β as a marker of astrocyte and glial activation. Details of blood sample collection and biomarker sampling techniques are provided in the supplementary materials.
Other Data Collection
Study records and electronic medical records were reviewed to determine length of hospital and intensive care unit stay, participant demographics (age, sex, and race), comorbidities (to compute Charlson Comorbidity Index), and severity of illness (computed using Acute Physiology and Chronic Health Evaluation (APACHE II) score). Activities of daily living (assessed by Katz scale) and instrumental activities of daily living (assessed by Lawton scale) were collected by patient self-report. Clinical data including mechanical ventilation, and American Society of Anesthesiologists physical status (ASA) were obtained from the medical record.
Statistical Analysis
Demographic and clinical characteristics between patients with and without delirium were examined using the Fischer’s exact test (categorical) and the non-parametric Wilcoxon rank sum test (continuous) due to data being non-normally distributed. Correlations were examined using Spearman correlation coefficients. To explore potential non-linear relationship between biomarkers and postoperative delirium and to minimize influence of potential outliers, we categorized each biomarker into quartile groups. We used logistic regression models to test the association of biomarker change quartiles and postoperative delirium incidence adjusting for APACHE II scores and randomization. Biomarker change quartiles were used as categorical and as linear trend variables in separate logistic models. All analyses were conducted using SAS v9.4. Significance was noted at p<0.05.
Results
After applying eligibility criteria, 71 patients (out of 84 in the parent trial who were in the esophagectomy group) were included in the final analysis. Reasons for exclusion were: n=5 had esophagectomy procedure cancelled due to intraoperative findings; n=5 did not have blood samples from preoperative time point; n=2 did not have blood samples on postoperative day one; and, n=1 did not have blood samples from both preoperative and postoperative day one time points. Demographic and clinical characteristics are shown in Table 1. Median age of the subjects was 62.6 years (interquartile range (IQR): 52.9,69.3), 19.7% were female, and 1.4% were African American. Approximately 95.8% of the patients in our sample required mechanical ventilation after surgery. Twenty-six (26/71, 36.7%) patients developed postoperative delirium during the follow up period, with 15/26 (57.7%), 5/26 (19.2%), and 1/26 (3.8%) screening positive for delirium on POD 1, 2, and 3, respectively. Patients with delirium had higher median APACHE II scores (delirium: 24.0 IQR: 18.0,27.0; no delirium: 17.0 IQR: 13.0,24.0, p=0.012).
Table 1.
Baseline demographics, clinical characteristics, and randomization status of the study sample
| Variables | All (n=71) |
No Delirium (n=45) |
Delirium (n=26) |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) | 62.6 (52.9, 69.3) | 61.7 (52.1, 67.9) | 65.9 (57.8, 70.6) | 0.147 |
| Female, n (%) | 14 (19.7%) | 7 (15.6%) | 7 (27.9%) | 0.354 |
| African-American, n (%) | 1 (1.4%) | 0 (0.0%) | 1 (3.8%) | 0.366 |
| Education | 0.738 | |||
| High School Graduate, n (%) | 40 (58.0%) | 27 (61.4%) | 13 (52.0%) | |
| Some College, n (%) | 17 (24.6%) | 10 (22.7%) | 7 (28.0%) | |
| Bachelor’s Degree or more, n (%) | 12 (17.4%) | 7 (15.9%) | 5 (20.0%) | |
| Clinical and Functional Characteristics | ||||
| Mechanically Ventilated, n (%) | 68 (95.8%) | 43 (95.6%) | 25 (96.2%) | 1.000 |
| APACHE II, median (IQR) | 18.0 (15.0, 25.0) | 17.0 (13.0, 24.0) | 24.0 (18.0, 27.0) | 0.012 |
| Charlson Comorbidity Index, median (IQR) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 3.0 (2.0, 5.0) | 0.119 |
| Activities of Daily Living (ADL), median (IQR) | 6.0 (6.0, 6.0) | 6.0 (6.0, 6.0) | 6.0 (6.0, 6.0) | 0.469 |
| Instrumental Activities of Daily Living (IADL), median (IQR) | 8.0 (7.0, 8.0) | 8.0 (7.0, 8.0) | 8.0 (7.0, 8.0) | 0.871 |
| ASA Class | 1.000 | |||
| ASA I, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| ASA II, n (%) | 1 (1.4%) | 1 (2.2%) | 0 (0.0%) | |
| ASA III, n (%) | 70 (98.6%) | 44 (97.8%) | 26 (100.0%) | |
| Esophagectomy Technique | 1.000 | |||
| Ivor Lewis, n (%) | 63 (88.7%) | 40 (88.9%) | 23 (88.5%) | |
| Other, n (%) | 8 (11.3%) | 5 (11.1%) | 3 (11.5%) | |
| Randomization | ||||
| Intervention, n (%) | 34 (47.9%) | 25 (55.6%) | 9 (34.6%) | 0.139 |
ASA: American Society of Anesthesiologists; APACHE II: Acute Physiology and Chronic Health Evaluation
Biomarkers at Preoperative Baseline
There were no significant differences in median levels of S100, CRP, IGF, IL-8, IL-10 or TNF-A at preoperative baseline between patients with and without postoperative delirium (see Table 2).
Table 2.
Median biomarker concentration values at preoperative, and change from preoperative baseline to postoperative day 1 and 3 by delirium status
| Biomarker | No Delirium | Delirium | P-valuea | ||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | ||
| Preoperative | |||||
| S-100β | 42 | 0.2 (0.2, 0.4) | 23 | 0.2 (0.1, 0.3) | 0.560 |
| CRP | 45 | 0.9 (0.6, 2.7) | 26 | 0.8 (0.7, 5.4) | 0.862 |
| IGF1 | 45 | 97.9 (64.2, 133.4) | 26 | 95.0 (69.8, 128.5) | 0.863 |
| IL-8 | 44 | 15.5 (9.4, 30.9) | 26 | 13.3 (8.9, 21.6) | 0.372 |
| IL-10 | 45 | 2.3 (1.5, 10.8) | 26 | 1.5 (1.5, 7.8) | 0.653 |
| TNFA | 44 | 12.3 (8.2, 19.9) | 26 | 12.3 (8.2, 16.8) | 0.908 |
| Change from preoperative to POD1 b | |||||
| S-100β | 42 | 1.0 (0.7, 1.5) | 23 | 1.3 (0.8, 1.6) | 0.414 |
| CRP | 45 | 16.8 (0.2, 35.4) | 26 | 30.2 (21.3, 43.9) | 0.050 |
| IGF1 | 44 | −5.1 (−29.2, 32.1) | 26 | −15.9 (−43.8, 0.0) | 0.113 |
| IL-8 | 44 | 24.8 (16.0, 41.7) | 26 | 38.6 (29.3, 69.8) | 0.022 |
| IL-10 | 45 | 12.4 (7.7, 25.7) | 26 | 26.1 (13.9, 36.7) | 0.025 |
| TNFA | 44 | 0.3 (−1.4, 4.1) | 26 | 3.0 (0.0, 10.0) | 0.066 |
| Change from preoperative to POD3 c | |||||
| S-100β | 36 | 0.5 (0.3, 0.8) | 22 | 0.5 (0.3, 0.9) | 0.695 |
| CRP | 39 | 40.4 (11.8, 49.2) | 25 | 32.9 (1.2, 44.6) | 0.193 |
| IGF1 | 38 | −33.0 (−61.3, −0.7) | 25 | −40.3 (−75.4, −26.5) | 0.199 |
| IL-8 | 39 | 11.7 (6.7, 22.8) | 25 | 17.2 (9.2, 23.0) | 0.160 |
| IL-10 | 39 | 0.4 (0.0, 6.4) | 25 | 2.9 (0.0, 8.2) | 0.231 |
| TNFA | 39 | 1.6 (−0.3, 6.7) | 25 | 1.6 (−0.5, 6.3) | 0.847 |
p values are from Wilcoxon rank sum test and unadjusted for other covariates. CRP: C-Reactive Protein; IGF: Insulin-like growth factor; IL: Interleukin; POD: Postoperative day; S100B: S100 Calcium Binding Protein B. TNFA: Tumor necrosis factor alpha.
Calculated as postoperative day 1 minus preoperative baseline;
Calculated as postoperative day 3 minus preoperative baseline.
Change in Biomarkers from Preoperative to Postoperative Day 1 (POD1)
As shown in Table 2 and Figure 1, there were significant differences in change in preoperative to postoperative day 1 biomarker concentrations between patients with and without delirium in the following: IL-8 (delirium: 38.6 IQR: 29.3,69.8; no delirium: 24.8 IQR: 16.0,41.7, p=0.022), and IL-10 (delirium: 26.1 IQR: 13.9,36.7; no delirium: 12.4 IQR: 7.7,25.7, p=0.025). When the analysis was limited to patients who developed delirium on postoperative day 1, there were no significant differences in change in biomarker concentrations (see Supplementary Table 1). In patients who developed delirium at any point in the postoperative period, median values of CRP on postoperative day 1 were significantly higher in patients with delirium compared to those without delirium (delirium: 35.4 IQR: 25.0,50.0; no delirium: 22.7 IQR: 2.2,42.7, p=0.031) as shown in Supplementary Table 2.
Figure 1. Change in median biomarker concentrations from preoperative to postoperative day 1 by delirium status.
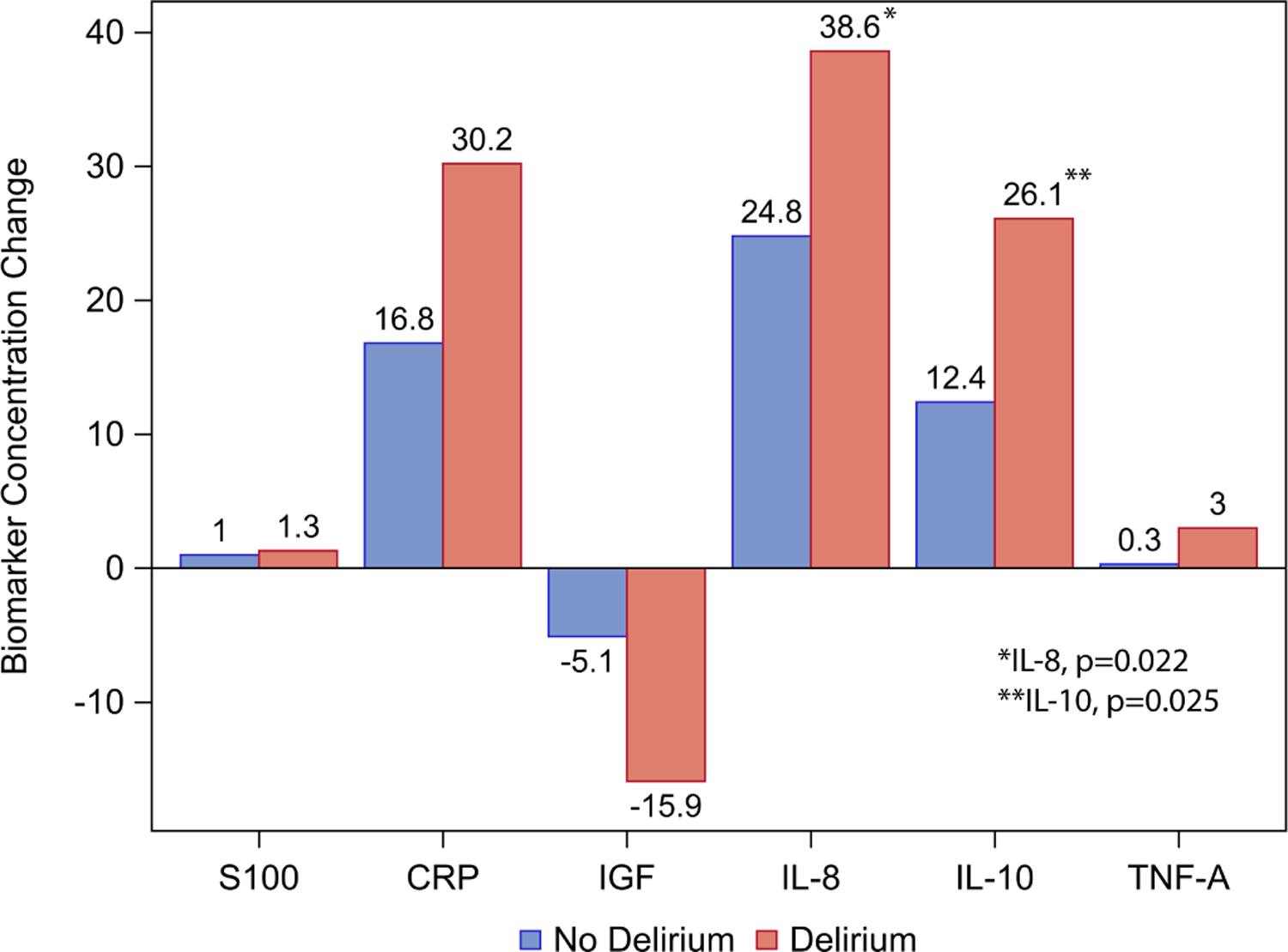
IL-8 p=0.022, IL-10 p=0.025, p>0.05 for all other biomarkers. CRP: C-Reactive Protein; IGF: Insulin-like growth factor; IL: Interleukin; POD: Postoperative day; S100B: S100 Calcium Binding Protein B. TNFA: Tumor necrosis factor alpha.
Change in Biomarkers from Preoperative to Postoperative Day 3 (POD3)
There were no significant differences in change in biomarker concentrations from preoperative to postoperative day 3 (Table 2). There were also no differences in median biomarker concentrations in the patients with and without delirium on postoperative day 3 (Supplementary Table 1).
Association Between Biomarker Change and Delirium Incidence
Biomarker change (from preoperative to POD 1) quartiles as categorical variables were not significantly different in delirium incidence adjusting for severity of illness score (APACHE II) (Supplementary Table 3) or Charlson Comorbidity Index (Supplementary Table 4) and randomization group. Figure 2 shows the percentage of patients with delirium by quartiles of biomarker change (from preoperative to POD 1) and suggests linear trends for the quartiles. Logistic models using linear trends for biomarker change quartiles showed that greater CRP change quartiles are associated with higher delirium incidence adjusting for severity of illness (OR=1.68, 95% CI: 1.03,2.75, p=0.037, Supplementary Table 3) or adjusting for Charlson Comorbidity Index (OR=1.70, 95% CI: 1.05,2.76, p=0.030, Supplementary Table 4).
Figure 2. Percentage of patients with delirum by quartiles of change in biomarker values from preoperative to postoperative day 1.
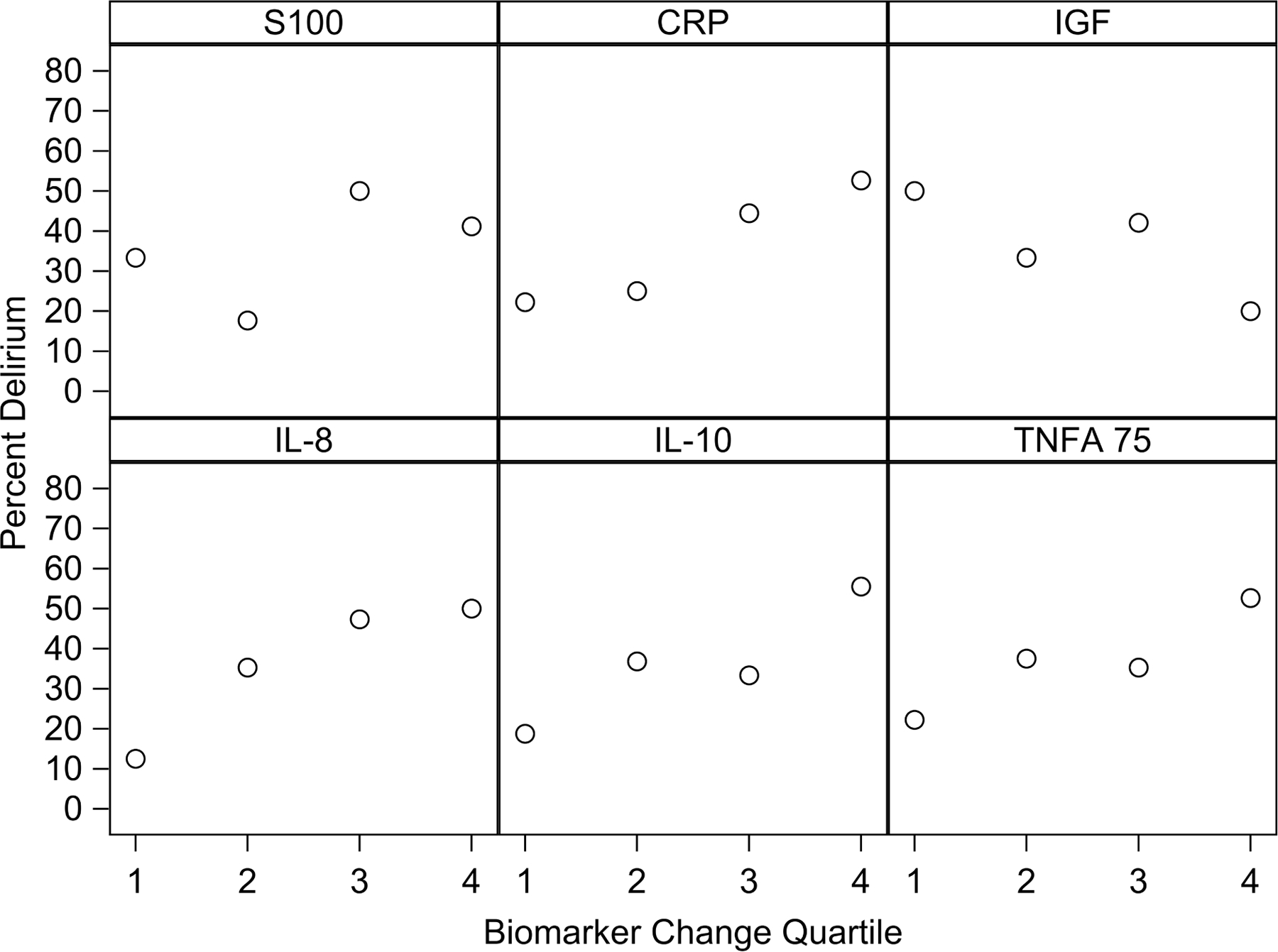
CRP: C-Reactive Protein; IGF: Insulin-like growth factor; IL: Interleukin; POD: Postoperative day; S100B: S100 Calcium Binding Protein B. TNFA: Tumor necrosis factor alpha.
Correlation Between Biomarker Change (preoperative to postoperative data 1) and Delirium Severity
Greater postoperative increase in levels of S100B, a biomarker of glial cell activation and neuronal injury, were significantly correlated with increased delirium severity as assessed by mean DRS-R-98 (Spearman r=0.289, p=0.020). Smaller changes in levels of IGF1, a biomarker of neuronal proliferation, were also correlated with higher delirium severity scores (Spearman r=−0.27, p=0.040), as shown in Supplementary Table 5. Other biomarker changes did not show significant correlation with delirium severity (see Supplementary Table 5). As shown in Supplementary Table 6, IL-10 was significantly correlated with IL-8 (Spearman r=0.528, p<0.001) and TNF-A (Spearman r=0.475, p<0.001), IL-8 was significantly correlated with CRP (Spearman r=0.330, p=0.005) and TNF-A (Spearman r=0.777, p<0.001), and CRP was correlated with TNF-A (Spearman r=0.298, p=0.012). Correlations of other biomarkers with each other at postoperative day one, and correlations of biomarkers with APACHE-II are shown in Supplementary Table 6.
Effect of Infectious Complications on Results
Only five out of 71 patients experienced sepsis, pneumonia, respiratory failure or pulmonary embolism within the blood sample collection window (i.e., on or prior to postoperative day 3). When the analysis was repeated after removing the five patients with complications, results were similar to those obtained in all 71 patients with the only exception that median concentrations of CRP measured on postoperative day one by delirium status were no longer significant (delirium: 32.8 IQR: 23.8,49.3; no delirium: 22.7 IQR: 1.4,42.9, p=0.060, Supplementary Table 2).
Comment
Our study has four significant and clinically relevant findings. First, concentrations of the selected biomarkers measured at the preoperative visit were not associated with development of postoperative delirium. Second, higher quartiles of changes in CRP from preoperative to postoperative day one were associated with postoperative delirium after adjusting for severity of illness or comorbidities. Third, greater change in postoperative day one levels of S-100β and smaller changes in levels of IGF-1, markers of neuronal injury and neuroproliferation, respectively, were significantly correlated with greater delirium severity. Fourth, changes in IL-8 and IL-10 biomarker concentrations from preoperative to postoperative day one were significantly associated with the development of delirium during the hospital stay, emphasizing the importance of collection timing of these candidate biomarkers, even when delirium occurs later in the hospital course.
Our findings differ from other large, well-conducted biomarker studies in the non-cardiac surgery population.21,27,32,37–38 In those studies, the biomarker signature of delirium was characterized by increased preoperative levels of CRP, and increased levels of IL-2 and IL-6 at preoperative, postoperative recovery, and postoperative day two (IL-8 and IL-10 were not significantly associated with delirium).21, 37–40 Our study extends this work by adding evaluation of biomarkers of neuronal injury and neuronal proliferation in a specialized thoracic surgery population at high-risk for delirium. Our study’s contrasting findings may be due to 3 major reasons: 1) the higher percentage of patients in our study had delirium on postoperative day one; 2) use of different blood sample collection timepoints (postoperative days one and three) likely identified changes in biomarker concentrations in the early postoperative period representing a stress response to the surgery; and, 3) the smaller sample size of our study.
This study has notable strengths and limitations. Strengths of this study include the chronological analysis of variation in inflammatory marker levels as blood samples, a priori selection of inflammatory biomarkers, and assessment of delirium incidence and severity using validated assessment tools. These results expand our understanding of neuronal injury biomarkers associated with postoperative delirium in patients undergoing elective esophagectomy.
There are also several important limitations. First, due to the small number of blood samples, results were not adjusted for multiple comparisons or certain potential confounders such as hypoxia or pain. Second, given that only 26 patients in this study developed delirium (which had a median duration of 1 day), we were not able to evaluate the collective utilities of the candidate biomarkers as a panel. Ongoing biomarker research using larger cohort studies are planned for confirmation and validation of this study’s findings. Third, while differences in randomization to haloperidol between the two groups did not reach statistical significance, we acknowledge that 59% of delirium-negative patients in our analysis were allocated to haloperidol intervention in the parent trial. In the parent study, differences in delirium incidence between the two arms of the trial did not achieve statistical significance (delirium incidence in haloperidol intervention: 23.8% vs. delirium incidence in placebo arm: 40.5%, p=0.16). Given the trend towards lower delirium incidence in the haloperidol esophagectomy group, we cannot entirely exclude some biological effect of haloperidol on our findings. We adjusted for randomization assignment in our current analysis to address this limitation. Finally, our analysis was performed on serum rather than plasma, did not include cerebrospinal fluid biomarker concentrations, or measure serum anticholinergic activity. Future studies with these biological measures may refine our understanding of delirium pathophysiology.
In conclusion, we found differences in change in serum CRP, IL-8 and IL-10 concentrations from preoperative to postoperative day one were associated with postoperative delirium in patients undergoing esophagectomy, consistent with biomarker studies in other surgical populations. Future studies of biological vulnerability and response to surgical stress are needed in order to translate these findings to bedside care.
Supplementary Material
Abbreviations
- ASA
American Society of Anesthesiologists
- APACHE-II
Acute Physiology and Chronic Health Evaluation-II Score
- CAM-ICU
Confusion Assessment Method for the Intensive Care Unit
- CRP
C-Reactive Protein
- DRS-R-98
Delirium Rating Scale Revised 98
- IGF1
Insulin-like Growth Factor 1
- IL
Interleukin
- IQR
Interquartile Range
- OR
Odds Ratio
- POD
Postoperative Day
- RASS
Richmond Agitation Sedation Scale
- S-100β
S-100 Calcium Binding Protein B
- TNF-A
Tumor Necrosis Factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan BA, Perkins AJ, Campbell NL, et al. Preventing Postoperative Delirium After Major Noncardiac Thoracic Surgery-A Randomized Clinical Trial. J Am Geriatr Soc 2018;66(12):2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Sigua NL, Manchanda S, et al. Preoperative STOP-BANG Scores and Postoperative Delirium and Coma in Thoracic Surgery Patients. Ann Thorac Surg 2018;106(4):966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong DM, Kim JA, Ahn HJ, Yang M, Heo BY, Lee SH. Decreased Incidence of Postoperative Delirium in Robot-assisted Thoracoscopic Esophagectomy Compared With Open Transthoracic Esophagectomy. Surg Laparosc Endosc Percutan Tech 2016;26(6):516–522. [DOI] [PubMed] [Google Scholar]
- 4.Markar SR, Smith IA, Karthikesalingam A, Low DE. The clinical and economic costs of delirium after surgical resection for esophageal malignancy. Ann Surg 2013;258(1):77–81. [DOI] [PubMed] [Google Scholar]
- 5.Khan BA, Perkins AJ, Gao S, et al. The Confusion Assessment Method for the ICU-7 Delirium Severity Scale: A Novel Delirium Severity Instrument for Use in the ICU. Crit Care Med 2017;45(5):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha A, Krasnow RE, Mossanen M, et al. A contemporary population-based analysis of the incidence, cost, and outcomes of postoperative delirium following major urologic cancer surgeries. Urol Oncol 2018;36(7):341.e315–341.e322. [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 2016;12(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balas MC, Happ MB, Yang W, Chelluri L, Richmond T. Outcomes Associated With Delirium in Older Patients in Surgical ICUs. Chest 2009;135(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan BA, Perkins AJ, Campbell NL, et al. Pharmacological Management of Delirium in the Intensive Care Unit: A Randomized Pragmatic Clinical Trial. J Am Geriatr Soc 2019;67(5):1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med 2018;379(26):2506–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van den Boogaard M, Slooter AJC, Bruggemann RJM, et al. Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium: The REDUCE Randomized Clinical Trial. Jama 2018;319(7):680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013;1(7):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018;33(11):1428–1457. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry 2009;65(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol 2010;119(6):737–754. [DOI] [PubMed] [Google Scholar]
- 17.Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res 2011;14(6):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses 2011;77(1):140–143. [DOI] [PubMed] [Google Scholar]
- 19.Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018;37:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen H, Shao Y, Chen J, Guo J. Insulin-Like Growth Factor-1, a Potential Predicative Biomarker for Postoperative Delirium Among Elderly Patients with Open Abdominal Surgery. Curr Pharm Des 2016;22(38):5879–5883. [DOI] [PubMed] [Google Scholar]
- 21.Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher C-Reactive Protein Levels Predict Postoperative Delirium in Older Patients Undergoing Major Elective Surgery: A Longitudinal Nested Case-Control Study. Biol Psychiatry 2017;81(2):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL-2 and TNF-alpha concentrations are associated with postoperative delirium in patients undergoing coronary-artery bypass graft surgery. Int Psychogeriatr 2014;26(5):845–855. [DOI] [PubMed] [Google Scholar]
- 23.Wilson K, Broadhurst C, Diver M, Jackson M, Mottram P. Plasma insulin growth factor-1 and incident delirium in older people. Int J Geriatr Psychiatry 2005;20(2):154–159. [DOI] [PubMed] [Google Scholar]
- 24.Capri M, Yani SL, Chattat R, et al. Pre-Operative, High-IL-6 Blood Level is a Risk Factor of Post-Operative Delirium Onset in Old Patients. Front Endocrinol (Lausanne) 2014;5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan BA, Zawahiri M, Campbell NL, Boustani MA. Biomarkers for delirium--a review. J Am Geriatr Soc 2011;59 Suppl 2:S256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beishuizen SJ, Scholtens RM, Vellekoop AE, et al. Timing Is Critical in Determining the Association Between Delirium and S100 Calcium-Binding Protein B. J Am Geriatr Soc 2015;63(10):2212–2214. [DOI] [PubMed] [Google Scholar]
- 27.Beishuizen SJ, Scholtens RM, van Munster BC, de Rooij SE. Unraveling the Relationship Between Delirium, Brain Damage, and Subsequent Cognitive Decline in a Cohort of Individuals Undergoing Surgery for Hip Fracture. J Am Geriatr Soc 2017;65(1):130–136. [DOI] [PubMed] [Google Scholar]
- 28.Van Munster BC, Korevaar JC, Korse CM, Bonfrer JM, Zwinderman AH, de Rooij SE. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry 2010;25(3):234–239. [DOI] [PubMed] [Google Scholar]
- 29.Van Munster BC, Bisschop PH, Zwinderman AH, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn 2010;74(1):18–23. [DOI] [PubMed] [Google Scholar]
- 30.Van Munster BC, Korse CM, de Rooij SE, Bonfrer JM, Zwinderman AH, Korevaar JC. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol 2009;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes CG, Pandharipande PP, Thompson JL, et al. Endothelial Activation and Blood-Brain Barrier Injury as Risk Factors for Delirium in Critically Ill Patients. Crit Care Med 2016;44(9):e809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hov KR, Bolstad N, Idland AV, et al. Cerebrospinal Fluid S100B and Alzheimer’s Disease Biomarkers in Hip Fracture Patients with Delirium. Dement Geriatr Cogn Dis Extra 2017;7(3):374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan BA, Farber MO, Campbell N, et al. S100 calcium binding protein B as a biomarker of delirium duration in the intensive care unit - an exploratory analysis. Int J Gen Med 2013;6:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166(10):1338–1344. [DOI] [PubMed] [Google Scholar]
- 35.Ely EW, Inouye SK, Bernard GR, et al. Delirium in Mechanically Ventilated Patients: Validity and Reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 36.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001;13(2):229–242. [DOI] [PubMed] [Google Scholar]
- 37.Vasunilashorn SM, Ngo L, Inouye SK, et al. Cytokines and Postoperative Delirium in Older Patients Undergoing Major Elective Surgery. J Gerontol A Biol Sci Med Sci 2015;70(10):1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knaak C, Vorderwulbecke G, Spies C, et al. C-reactive protein for risk prediction of post-operative delirium and post-operative neurocognitive disorder. Acta Anaesthesiol Scand 2019;63(10):1282–1289. [DOI] [PubMed] [Google Scholar]
- 39.Pol RA, van Leeuwen BL, Izaks GJ, et al. C-reactive protein predicts postoperative delirium following vascular surgery. Ann Vasc Surg 2014;28(8):1923–1930. [DOI] [PubMed] [Google Scholar]
- 40.Khan BA, Perkins AJ, Prasad NK, et al. Biomarkers of Delirium Duration and Delirium Severity in the ICU. Crit Care Med 2020;48(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


