Abstract
Simple Summary
Even though it is known that (cancer) cells can fuse, it is still less understood how (cancer) cells merge their plasma membranes, thereby giving rise to bi- and multinucleated hybrid cells. Cell-cell fusion is an energy-dependent process and so-called fusogens are a crucial type of membrane-bound proteins, which are mandatory for overcoming plasma membrane hybridization with associated energetic barriers. Viruses and fusogens of human endogenous retroviral elements are a natural reservoir of fusogenic particles and proteins that could cause bi- and multinucleation of cancer cells. Likewise, multinucleated giant cancer cells have been found in several cancers caused by oncogenic viruses suggesting a possible correlation between viruses and fusogens of human endogenous retroviral origin in cancer cell fusion.
Abstract
Cell fusion is a well-known, but still scarcely understood biological phenomenon, which might play a role in cancer initiation, progression and formation of metastases. Although the merging of two (cancer) cells appears simple, the entire process is highly complex, energy-dependent and tightly regulated. Among cell fusion-inducing and -regulating factors, so-called fusogens have been identified as a specific type of proteins that are indispensable for overcoming fusion-associated energetic barriers and final merging of plasma membranes. About 8% of the human genome is of retroviral origin and some well-known fusogens, such as syncytin-1, are expressed by human (cancer) cells. Likewise, enveloped viruses can enable and facilitate cell fusion due to evolutionarily optimized fusogens, and are also capable to induce bi- and multinucleation underlining their fusion capacity. Moreover, multinucleated giant cancer cells have been found in tumors derived from oncogenic viruses. Accordingly, a potential correlation between viruses and fusogens of human endogenous retroviral origin in cancer cell fusion will be summarized in this review.
Keywords: cell-cell fusion, syncytia formation, viruses, cancer
1. Introduction
Cell fusion represents a fundamental biological mechanism, which is mandatory for physiological processes such as fertilization, placentation, myogenesis, osteoclastogenesis and wound healing/tissue repair [1,2,3,4,5,6,7]. Likewise, cell fusion plays an important role during pathophysiological conditions including tumor development. Thus, cancer cell fusion with macrophages [8,9,10,11] or mesenchymal stroma-/stem-like cells [12,13,14] can result in tumor reduction [15], tumor promotion [16], or tumor dormancy [17,18].
Fusion also includes infection of host cells with enveloped viruses [19] and tumor development [20,21]. Even though the process of cell fusion appears phenomenologically simple, like two merging soap bubbles, it is tightly regulated whereby various molecular processes remain to be elucidated [22].
Cell-cell fusion and internalization of enveloped virus content as part of a virus-cell fusion represent multi-step processes that could be subdivided into discrete steps, such as (i) priming the prefusion state, (ii) tight binding to the target membrane, and (iii) additional intermediates between the prefusion and postfusion state [19]. The term “priming the prefusion state” indicates that per se non-fusogenic cells have to be converted first into a fusogenic state before they can hybridize with other cells [3,5,8,23]. Likewise, several pathogenic viruses, such as avian influenza virus, HIV-1, measles virus, respiratory syncytial virus (RSV), Newcastle Disease virus, Ebola Virus and even SARS-CoV-2 must be cleaved by furin or furin-like proteases to become fully activated and to be able to infect cells [24,25]. Another trigger could be a low pH that leads to a conformation change and release of the fusion peptide/loops which then could penetrate into the host cell membrane [4,5,19,26]. The term “tight binding to the target membrane” is self-explanatory since fusion requires a tight cell-cell/virus-cell contact, whereby the two plasma membranes are positioned at a distance not closer than ~10 nm [5]. The actual process of cell fusion by the merging of the plasma membranes is facilitated by so-called fusogens, which are mandatory for overcoming certain energetic barriers and steric formation of distinct (iii) lipid intermediates named “the hallmarks of cell-cell fusion” [5]. These are (a) dehydration of contacting membranes, whereby phospholipid heads are brought to distances of close to 0 nm, (b) hemifusion (merging of the outer monolayers) via a stalk and/or diaphragm intermediates, and (c) opening and expansion of fusion pore(s) from nanometer diameters to multiple microns [5] (Figure 1).
Figure 1.
A hypothetic model of potential virus-mediated stepwise (cancer) cell fusion suggests the formation of diaphragm intermediates by the initial merging of the outer lipid layers of adjacent plasma membranes as hemifusion. Expression of fusogenic factors, e.g., syncytin-1 and the corresponding receptor alanine, serine, and cysteine selective transporter-2 (ASCT-2), together with other viral fusogens such as human endogenous retroviruses (HERV) proteins and further cell fusion inducing factors, such as tumor necrosis factor-α (TNF-α), is required within the cellular fusion partners. Concomitantly, intracellular restructuring of actin cytoskeletal proteins together with an ion gradient and low pH provide a fusion-permissive microenvironment. As a prerequisite, the plasma membranes of the somatic or cancer cells fusion partners in cooperation with the membrane of enveloped viruses have to be localized in close proximity whereby extension of membrane protrusions as lamellipodia can form local fusion pores. Whereas viruses can act as a linker for bridging fusogenic cell membranes the precise molecular role of enveloped viruses to contribute to outer membrane opening, formation of an intermediate hemifusion state, and finally the opening of the inner membrane lipid layers remains enigmatic. Among the various membrane lipids phosphatidylserine (PS) plays an important role in altering the inner lipid membrane structures to enable and finalize the fusion process. Thereby, PS interacts with associated proteins such as annexin V, scramblases, and various cytoskeletal components.
Although it is expected that basic thermodynamic and biophysical requirements encountered during the membrane fusion of enveloped viruses are the same as those occurring during fusion between cells [5,19] the process of cell-cell fusion appears to be more complex. In parallel to the induction of a fusogenic state, hybrid cells must adopt a non-fusogenic state (or post-fusion state) to prevent additional hybridization events, which, i.e., is accompanied by rearrangements of the mixing cytoskeleton and cytoplasms [3,5,19]. Likewise, hybrid cells could either remain in a bi- or multinucleated state, such as osteoclasts and syncytiotrophoblasts [3,8,27], or could undergo heterokaryon-to-synkaryon transition (HST)/ploidy reduction (PR) (HST/PR) [22,28,29,30,31].
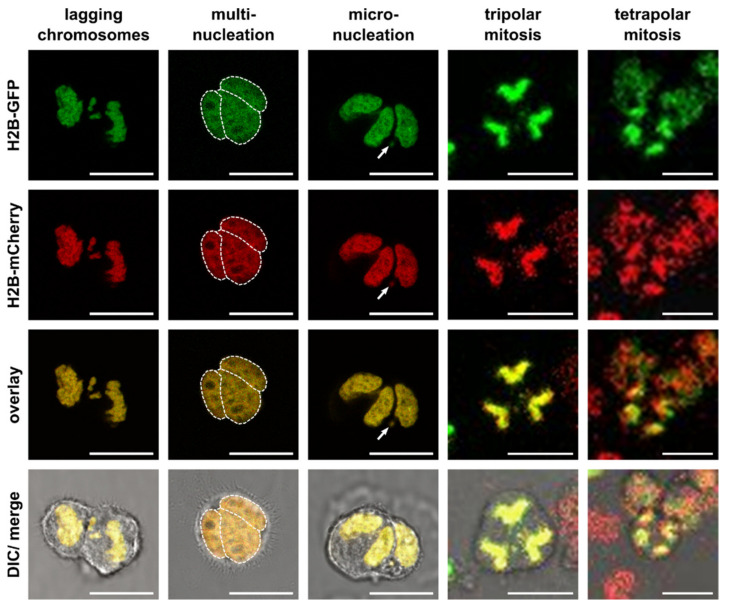
HST/PR is a complex and not yet fully understood mitosis-like process that requires an active cell cycle [30,31,32]. Hybrid cells contain additional copies of centrosomes (one centrosome per parental cell) and both the localization of the centrosomes and attachment of spindle fibers to chromosomes during metaphase and anaphase have an impact whether randomly mixed chromosomes will be equally segregated in a bipolar manner or missegregated due to tri- and multipolar divisions and lagging chromosomes to daughter cells (Figure 2, Videos S1 and S2). In particular, chromosome missegregation has been associated with an overall increased genomic/chromosomal instability (GCIN) due to induction of aneuploidy, multinucleation, micronuclei formation and chromothripsis [22,28,29,30,31,33,34,35,36].
Figure 2.
Changes in the karyotype during HST/PR by lagging chromosomes and multipolar divisions with formation of multinucleation and micronuclei and chromothripsis. Shown are representative images of hybrid cells derived from M13SV1 human breast epithelial cells that were stably transduced with pH2B-GFP (kind gift from Geoff Wahl; Addgene plasmid #11680; http://n2t.net/addgene:11680; RRID: Addgene_11680) or pH2B_mCherry_IRES_puro2 (kind gift from Daniel Gerlich; Addgene plasmid #21045; http://n2t.net/addgene:21045; RRID:Addgene_21045). The plasmids were used to trace green fluorescing GFP-expressing cells and red fluorescing mCherry expressing cells that eventually fuse by forming yellow fluorescing hybrid cells with constitutive expression of both fluorescence genes. Hybrid cells were cultured on chamber slides (ThermoFisher Scientific GmbH, Schwerte, Germany) and images and time-lapse series were recorded using a Leica TCS SP5 confocal laser scanning microscope (Leica, Wetzlar, Germany). Multiple nuclei in multinucleated cells were marked by a dashed line. The arrow points to a micronucleus. Video files of the tri- and tetrapolar cell divisions can be found in the Supplementary Materials Videos S1 and S2, respectively. Bar = 25 µm.
In contrast, virus-infected cells have already reached a post-fusion state directly after virus-host cell membrane fusion that is commonly accompanied by virus replication and cell death. Interestingly, some members of retroviridae (human immunodeficiency virus), paramyxoviridae (Sendai virus), poxviridae (poxvirus), coronaviridae (SARS-CoV and SARS-CoV-2) [37,38,39,40,41,42,43] and reoviridae (Rota virus B) [19,21,26,44,45,46,47] could also lead to syncytium formation, potentially reflecting some kind of immune escape strategy to avoid capturing of free viruses by neutralizing antibodies [37,48,49,50].
The ability of cells to fuse or enveloped viruses to infect host cells is related to fusogens. Certain viral fusogens have already been characterized in detail and grouped into four classes (class I to IV) depending on their structure (see below Section 2) [19,21,26,44,51]. So far, only a small amount of proteins have been identified that facilitate cell fusion. Among them are the well-characterized human fusogens syncytin-1 and syncytin-2. These proteins facilitate the fusion of villous cytotrophoblasts, thereby giving rise to multinucleated syncytiotrophoblasts [52,53,54,55,56]. Syncytin-1 is suggested to be also involved in osteoclastogenesis [57,58] and in cancer cell fusion [59,60,61,62,63,64,65,66,67] (Table 1).
Table 1.
Syncytin-1 expression and mediated fusion in normal human cells and human cancer cells.
| Cell Type | Fusion Partner | Kind Of Fusion | References |
|---|---|---|---|
| villous cytotrophoblasts | villous cytotrophoblasts | homotypic | [52,53,54,55,56] |
| osteoclasts | bi-nucleated osteoclasts | homotypic | [57,58] |
| breast cancer cells | endothelial cells/ mesenchymal stem/stromal cells |
heterotypic | [62,65] [63] |
| colorectal cancer cells | colorectal cancer cells | homotypic | [64] |
| endometrial cancer cells | endometrial cancer cells | homotypic | [61] |
| oral squamous carcinoma cells | HUVEC | heterotypic | [60] |
| prostate cancer cells | prostate cancer cells/ muscle cells |
homotypic/ heterotypic |
[66] |
| seminoma cells | unclear | [67] | |
| urothelial cell carcinoma cells | urothelial cell carcinoma cells | homotypic | [59] |
Interestingly, syncytin-1 and syncytin-2 are of retroviral origin and are related to class I viral fusogens [4,5,7,19,26]. Other known human fusogens include myomaker and myomerger involved in myogenesis [68,69,70,71] as well as Juno (oocyte) and Izumo1 (sperm) that are mandatory for fertilization [72,73,74,75]. In contrast to syncytins, these factors do not share any homologies with viral fusogens suggesting that different classes of fusogens have been developed independently during evolution. Inflammation and inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), have been identified as further triggers/inducers of cell fusion [60,63,76,77,78,79,80,81,82,83] (Figure 1). Other studies revealed that spontaneous cell fusion events can also occur independently of inflammation/inflammatory cytokines [76,77,84] which underscores the heterogeneity and complexity of fusion processes.
Besides the action of fusogens, some physico-chemical conditions and membrane properties necessitate further prerequisites of cell fusion. Among these requirements is the close proximity of plasma membranes between the fusion partners accompanied by the extension of local lamellipodia-containing membrane protrusions [85]. These structures allow crosstalks between plasma membrane phospholipids, in particular phosphatidylserine (PS), PS-binding proteins, reorganization of the actin cytoskeleton and further membrane proteins (for review see: [3,4,5,23,86,87,88,89,90,91,92]). PS exposure in the outer leaflet of the membrane has been observed in virtually all fusogenic cell types, such as myoblasts, macrophages, trophoblasts, sperm and cancer cells (for review see: [4,89]). Moreover, PS exposure and signaling further plays a crucial role during membrane fusion of many enveloped viruses suggesting that PS signaling could represent a uniquely conserved module in cell-cell and viru-cell fusion (for review see: [89]) (Figure 1). Indeed, myogenesis, osteoclastogenesis and syncytization of trophoblasts were markedly impaired by masking PS, removing PS-binding annexins and inhibition/knockdown of professional phospholipid scramblases, such as TMEM16E and TMEM16F that facilitate the translocation of PS from the inner to the outer leaflet of the plasma membrane [91,93,94,95]. Similarly, entry of HIV-1 and alpha herpesvirus into host cells was markedly diminished by inhibition of scramblase activity and blockade of externalized PS [96,97] further substantiating the importance of PS in membrane fusion.
Following the increasing knowledge about cell-cell fusion, the entire process of membrane merging remains complex and requires further elucidation. In this context, virus-host cell and cell-cell fusion demonstrate some similarities that might be helpful for a better understanding of this entire process. However, molecular insights into these interactions might be limited to human fusogens, such as syncytin-1 and -2 that share homologies to class I viral fusogens. For example, myogenesis and osteoclastogenesis are not facilitated by virus-like fusogens but are rather controlled via complex multiprotein fusion machinery [4]. Therefore, common properties so far are represented by PS signaling as a more universal module in cell-cell and virus-cell fusion [89] that might apply to both, physiological and pathophysiological conditions.
Despite these findings, fusion of cancer cells is another not yet fully understood process. The hypothesis that cancer cells could fuse with, e.g., macrophages, was already postulated by the German physician, Otto Aichel, about 110 years ago [98]. Aichel postulated that aneuploidy of cancer cells could be attributed to hybridization with tumor-invading leukocytes and that the combination of extra chromosomes and the qualitative differences in chromosomes from the two cell types could lead to a metastatic phenotype [98,99]. Albeit several studies demonstrated that tumor hybrids could be detected in human cancer patients [100,101,102,103,104,105,106,107,108,109] the understanding of how the fusion of cancer cells is induced and mediated still remains less clear. This also keeps an issue for the hypothesis as to whether the fusion of two normal cells could lead to a malignant transformation of hybrid cells as suggested in several independent studies [110,111,112]. Notably, data of Duelli and colleagues indicated that this process could be attributed to virus-mediated cell-cell fusion [110,111] suggesting that viruses could act as linkers to bridge two cells, thereby causing their hybridization (Figure 1). Indeed, the first hybridoma cells were generated by Sendai virus mediated fusion of plasma cells and myeloma cells [113]. However, whether viruses are common mediators of cell-cell fusion of different cell types including cancer cells remains to be elucidated. Some viruses could lead to syncytia formation, but this has been rather assumed as some kind of immune evasion strategy and not with a malignant conversion of cells [37,48,49,50]. Likewise, a viral infection usually results in the death of the host cells either by the virus itself, induction of apoptosis or by the immune system, which also applies to virus-induced syncytia formation in oncolytic immunotherapy [114,115,116].
2. Viral- and Human Endogenous Retroviral-Derived Fusogens
Fusogens are indispensable for cellular hybridization to overcome fusion-associated energetic barriers [3,4,5,6,7,8,19,20] and thus, have to be expressed by fusion competent cells. Whereas cell fusion could occur in the presence or absence of inflammation [60,63,76,77,78,79,80,81,82,83,84] it can be further concluded that the presence of distinct fusogens and associated relevant factors is either induced or constitutively expressed at basal expression levels.
Besides some extensively characterized human fusogens such as syncytin-1 and -2 [52,53,54,55,56], myomerger, myomaker, actin remodeling proteins [68,70,71,117,118,119,120] as well as Izumo1 and Juno [72,73,74,75], viral fusogens or human endogenous retroviruses (HERVs) also display properties for fusion and synyctia formation capacity associated with a possible role in carcinogenesis and tumor progression.
2.1. Viral Fusogens
Enveloped viruses exhibit predominant capabilities to confer cell fusion due to evolutionary optimized fusogens and cell fusion strategies (for review see: [19,21,26,44,51]) (Figure 1). Depending on their structure, virus types and accompanying viral fusogens are subdivided into four classes (Table 2).
Table 2.
Viral fusogens.
| Class | Virus Family | Virus Strain | Fusogen | Structure | References |
|---|---|---|---|---|---|
| I | Cornaviridae | SARS, MERS, SARS-CoV-2 |
Spike protein (S protein) |
[19,21,26,44,51] | |
| Filoviridae | Ebola | Ebola glycoprotein (GP) | α-helix-rich | [19,21,26,44,51] | |
| Orthomyxoviridae | Influenza virus | Hemagglutinin (HA) | [19,21,26,44,51] | ||
| Paramyxoviridae | Sendai virus | F glycoprotein | [19,21,26,44,51] | ||
| Retroviridae | HIV | glycoprotein 41 (gp41) | [19,21,26,44,51] | ||
| II | Alphaviridae | Semliki Forest virus | E1 glycoprotein | [19,21,26,44,51] | |
| Flaviviridae | Dengue virus, West Nile virus, Zika virus |
E glycoprotein | β-sheet-rich | [19,21,26,44,51] | |
| Matonaviridae | Rubella virus | E1 protein | [19,21,26,44,51] | ||
| III | Baculoviridae | Baculovirus | gp64 glycoprotein | [19,21,26,44,51] | |
| Herpesviridae | Herpes Simplex virus, Varicella Zoster virus |
gB glycoprotein | α-helices and β-sheets | [19,21,26,44,51] | |
| Rhaboviridae | Vesicular Stomatitis virus; Rabies virus |
G glycoprotein | [19,21,26,44,51] | ||
| IV | Reoviridae | Rota virus B | FAST protein | non-structural simplified domains | [19,21,26,44,47,51] |
Class I viral fusogens are α-helix-rich prefusion trimers that arise from coiled-coil structures. Fusion is mediated by the insertion of hydrophobic fusion peptides or loops into membranes concomitant with refolding into postfusion trimers [19,26]. In contrast, class II viral fusogens are β-sheet-rich structures with a transition from dimers to trimers during fusion. These molecules also insert hydrophobic fusion loops into membranes ending in postfusion trimers [19,26]. Class III viral fusogens are trimers composed of both α-helices and β-sheets. In accordance with and in combination with class I and class II properties these viral fusogens insert hydrophobic fusion loops into membranes and form post-fusion trimers [19,26]. Accordingly, a common characteristic of class I to III viral fusogens is represented by facilitated virus-host membrane fusion through conserved mechanisms (for review see: [19,21,26,44,51]). To exhibit their activity, class I to III viral fusogens have to be converted into a fusogenic state and structure first. Mechanisms that contribute to such conformational activation include proteolytic cleavage, receptor binding or a change in pH [4,5,19,24,25,26]. Subsequent release of the fusion peptide/loop, with penetration and incorporation into the host cell membrane promotes fusion of the outer lipid layers by forming a hemifusion state [4,5,19,26]. A following merger of the inner lipid layers opens a fusion pore whereby pore expansion finally concludes the cell-cell merging [4,5,19,26].
Alternatively, class IV viral fusogens that are also termed fusion-associated small transmembrane (FAST) proteins, are much smaller molecules (only 20 to 40 amino acids) and do not directly mediate virus-host membrane fusion [19,26]. Instead, these FAST proteins are expressed after infection and induce syncytium formation (also named multinuclear giant cells (MNGCs)) between infected cells and non-infected adjacent cells that might function as some kind of immune escape strategy to avoid capturing of free viruses by neutralizing antibodies [19,26]. Syncytium/MNGC formation is also characteristic for some members of Retroviridae (human immunodeficiency virus), Paramyxoviridae (Sendai virus), Poxviridae (poxvirus), Herpesviridae (Herpes virus) and even coronaviridae (SARS-CoV and SARS-CoV-2) [37,40,41,48,49,50,51]. Thereby, syncytia/MNGC formation is most likely induced by the expression of viral fusogens in the host cell membrane with subsequent cell-cell fusion [50,51,121,122,123]. This process is similar to the origin of multinuclear syncytiotrophoblasts from syncytin-1 and syncytin-2 with the formation of villous cytotrophoblasts [52,53,54,55,56].
As an example for cell-cell fusion mediated by non-enveloped viruses, Hu and colleagues demonstrated that the human papillomavirus 16 (HPV16) E5 protein is capable to induce bi-nucleated cell formation by cell-cell fusion [124,125]. For this purpose, HPV16 E5 needs to be localized within the plasma membranes to promote cell-cell fusion [125]. However, the mechanism on how this HPV16 protein mediates the merging of plasma membranes remains to be elucidated.
Interestingly, some bacteria are also capable of inducing cell fusion such as the Gram-negative bacteria Burkholderia pseudomallei and the related species Burkholderia thailandensis [126,127,128]. Both strains can relay signals for syncytium formation by cell-cell fusion that might be beneficial for access to nutrients and immune escape. Thereby, cell-cell fusion is mediated by the type VI secretion system 5 (T6SS-5), which is evolutionary, structurally and functionally related to the tail of contractile bacteriophages and the VgrG5 effector [128,129,130,131].
A recently developed novel system for viral protein-mediated delivery and fusion with target cells is represented by the paternally expressed gene 10 (PEG10) [132] This gene product of retroelements as one of the mammalian gag homologs contributes to virus-like capsid formation that may also interact with cellular plasma membranes. Thereby, PEG10 can enable the transport of RNAs such as mRNAs and miRs among others into target cells [132,133]. Accordingly, the PEG10 system can deliver cargo to cells alternative to physiological vehicles like microvesicles or exosomes [134,135]. Moreover, the studies of Segel et al. have demonstrated that the untranslated regions of PEG10 can be reprogrammed by inserting functional mRNAs of certain genes. By developing this system of selective endogenous encapsidation for cellular delivery (SEND), specific RNAs can be addressed, e.g., for therapeutic purposes [132].
2.2. Human Endogenous Retroviruses (HERVs)
About 8% of the human genome is of retroviral origin with more than 400,000 HERV and 240,000 mammalian apparent long terminal repeat (LTR) retrotransposons (MaLRs) copies and related sequences [136,137,138,139]. HERVs are characterized by their 5′LTR-gag-pro-pol-env-3′LTR structure, whereas MaLRs possess a rather simple 5′LTR-ORF-3′LTR structure without a pol gene encoding reverse transcriptase [139]. Most of these ancient remnant elements are non-functional or inactive due to mutations, deletions and/or truncations over time, however, some of them are still active and expressed in certain tissues [136,137,138,139]. Among these prominent HERV-derived proteins are syncytin-1 (HERV-W1) and -2 (HERV-FRD) that mediate the fusion of villous cytotrophoblasts during placentation [52,53,54,55,56]. Syncytins have been identified in a variety of mammals, such as mice [140], rabbits [141], carnivorans [142], ruminants [143], opossum, and kangaroo marsupials [144] indicating an evolutionary benefit of the integration and expression of retroviral elements in placental tissues. In addition to facilitating cell-cell fusion several captured env genes have been proposed to exhibit an immunosuppressive role that is important for preventing maternal rejection of the semi-allogenic fetus during pregnancy [139]. Syncytin-1 and -2 share structural homologies to class I viral fusogens [4,5,7,19,26] and syncytin-1 might also play a role in osteoclastogenesis [57,58] and in cancer cell-cell fusion [59,60,61,62].
In addition to syncytins (HER2V-W1, HERV-FRD) further HERV sequences have been detected in the human genome, such as HERV-H, HERV-K, HERV9, HERV-E, HERV-Fb, HERV-V and HERV3 (HERV-R) (for review see: [138,139]). Interestingly, most HERV-related regulatory elements, such as LTRs, and/or –related proteins are active and expressed in germ cells, pre-implantation embryonic cells and the placenta (for review see: [138,139,145]). For instance, elevated transcription of HERV-H was found in human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (ipSCs). This gene product was suggested to be crucial for maintaining a naïve-like state [146]. HERV-H elements provide functional binding sites for several naïve pluripotency transcription factors, such as LBP9 that drives the expression of pluripotency-associated transcription factors and pluripotency-modulating long noncoding RNA [146]. Indeed, the self-renewal capacity of hESCs and ipSCs was compromised after disruption of LBP9, HERV-H and HERV-H-derived transcripts indicating that HERV-H expression could be a hallmark of naïve-like hESCs and ipSCs [146]. This is substantiated by previous work demonstrating expression of HERV-K RNA and protein in undifferentiated but not in differentiated hESCs and ipSCs [147]. HERV-K expression was regulated by DNA hypomethylation at LTR elements together with transactivation of the stemness marker Oct4 [148]. Other studies revealed that HERV-K and HERV-H transposable elements significantly contributed to chromatin opening during human embryonic genome activation and have been identified as KLF-stimulated enhancers in naïve hESCs [149]. Interestingly, data of Göke et al. revealed a stage-specific expression of HERV elements during early embryogenesis that are related to LTRs of different HERVs, such as LTR3B and LTR14B (oocyte to four-cell; part of HERV-K14), LTR12C (zygote to eight-cell; HERV-9), MLT2A1 (HERV-L) and LTR7B (HERV-H) (both eight-cell), LTR5_Hs (HERV-K) and LTR7B (HERV-H) (both morula) and LTR7Y (HERV-H; blastocyst) [150]. Together, these findings substantiate the important role of HERV elements as regulators of pluripotency during early embryonic development.
Interestingly, nine HERV families and elements, such as HERV-K, HERV-like, HERV-V, HERV-T, HERV-W and HER2V-F, respectively, were significantly up-regulated in both hESCs and human hematopoietic stem cells (hHSCs) [151] suggesting that certain HERV elements might also play a role in the maintenance of a stem cell state in somatic stem cells. Moreover, a differential expression of HERV families and elements was also found in malignant hematopoietic cells, such as transcriptional upregulation of HERV-E family in acute megakaryocytic and erythroid leukemia, upregulation of HERV-Fc in multiple myeloma/plasma cell leukemia, and down-regulation of HERV-K in acute myeloma [151]. Whether differential expression levels of these HERV elements might contribute to the pathogenesis of such hematopoietic disorders by cancer cell fusion is not yet clear. Nonetheless, activation of selective and specific HERV-K elements/transcripts have been identified in various cancers, such as malignant melanoma, breast, ovarian and prostate cancer (for review see: [138,139,145]). Moreover, HERV-K activation was required for expansion and maintenance of a CD133+ melanoma cell subpopulation with stemness features [152]. Likewise, other classes of HERV env mRNAs were expressed in ovarian cancer (ERV3, HERV-E and HERV-K), prostate cancer (HERV-E) [153,154], and kidney cancer [155]. In addition to cancer, HERV elements have been further associated with neurodegenerative and autoimmune diseases, such as amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), rheumatoid arthritis, psoriasis and systemic lupus erythematosus [138,139,145,156,157,158,159]. Whereas these data reflect a correlation between HERV elements and stemness features of (cancer) cells, this also likely indicates an involvement of HERV elements in various diseases including cancer.
As mentioned above, both, syncytin-1 (HERV-W1) and -2 (HERV-FRD) play a pivotal role in placentation by mediating the fusion of villous cytotrophoblasts to multinucleated syncytiotrophoblasts [52,53,54,55,56]. The fusogenic capacity of other HERV env elements has already been investigated, but data indicate that only syncytin-1 and syncytin-2 exhibit a marked fusogenic capacity. Sugimoto and colleagues identified a HERV-Fb1-derived protein in villous cytotrophoblasts and syncytiotrophoblasts, named suppressyn, which inhibits cell-cell fusion through binding to the syncytin-1 receptor ASCT-2 [160]. Expression of ERV3-1 likely promoted fusion of BeWo choriocarcinoma cells [161], but it remains to be elucidated whether ERV3-1 is also capable to mediate the merging of other cell types. Fusogenic properties have also been postulated for the HERV-K env gene, which is expressed in villous and extravillous cytotrophoblast cells of the human placenta [162]. Cells could be infected with a recombinant vesicular stomatitis virus encoding HERV-K env (VSV-HERVK) pseudovirus [163]. Thereby, HERV-K env sequences bound heparin and promoted acidic pH-triggered fusion [163]. However, a distinct role of HERV-K env in cell-cell fusion is less clear. This also applies to HERV-E env proteins expressed in placenta and putatively affecting trophoblast fusion [164].
3. Induction of Cell-Cell Fusion by Virus-Derived Fusogens and Putative Correlation to Tumors
Fusogenic capacities of enveloped viruses and virus-related/derived proteins can stimulate the formation of heterokaryons, cell hybrids, and syncytia. However, these viruses or at least virus-derived fusogens may also be linked to cell-cell fusion and tumor development [21,165] (Figure 1). In addition to its putative role in carcinogenesis cell-cell fusion has been further associated with tumor progression. Indeed, a plethora of studies demonstrated that homotypic (tumor cell × tumor cell) and heterotypic (tumor cell × normal cell) fusion events could give rise to hybrids exhibiting an increased metastatic capacity, an enhanced drug resistance, or even cancer stem/initiating cell properties (for review see: [99,166,167,168,169,170,171,172]). In any case, despite the increasing knowledge about the impact of cell-cell fusion in tumor progression only a few proteins/phospholipids and conditions have been identified so far, which trigger the hybridization of cancer cells.
3.1. Syncytin-1
Several studies have demonstrated that cancer cell fusion is facilitated by syncytin-1 (HERV-W1) [59,60,61,62,63,64,65,66] (Table 1). For instance, the hybridization of human breast cancer cells with endothelial cells was mediated by syncytin-1 [62]. Syncytin-1 expression was found in about 38% of breast tumor specimens [62] that was surprisingly associated with increased recurrence-free survival [65]. In contrast, syncytin-1 expression levels and syncytin-mediated cell-cell fusion were rather correlated to disease progression in urothelial cell carcinoma [59], endometrial carcinoma and pre-stages [61], colorectal cancer [64] and prostate cancer [66]. It remains to be elucidated why cancer cells express high levels of syncytin-1. Yan and colleagues demonstrated that syncytin-1 expression in oral squamous carcinoma cells and ASCT-2 expression in endothelial cells was induced by TNF-α [60], substantiating the well-known correlation of inflammation/inflammatory cytokines as inducers of cell-cell fusion [60,63,76,77,78,79,80,81,82,83]. Constitutive basal expression of syncytin-1 protein was detectable in human MCF-7 and MDA-MB-231 breast cancer cells [62], human SCC-9 squamous carcinoma cells, MG-63 osteocarcinoma cells, Hela cells, and human umbilical vein endothelial cells [60]. Although these proteins are mandatory for overcoming energetic barriers further factors are required for successful cell fusion. As already indicated, PS signaling seems to be a uniquely conserved signaling module in cell fusion [89]. The choriocarcinoma cell line BeWo is commonly used as a model of trophoblast differentiation and cell fusion could be induced by forskolin treatment and up-regulation of syncytin-1 [161,173]. Moreover, Zhang and colleagues showed that the lipid scramblase TMEM16F, which facilitates the translocation of PS from the inner to the outer leaflet of the plasma membrane, is essential for trophoblast fusion [95]. Placentas of TMEM16F knockout mice exhibited deficiency in trophoblast syncytization and aberrant placenta development concomitant with perinatal lethality [95], supporting the relevance of PS signaling in trophoblast fusion. In addition, the placental-specific high temperature requirement factor A 4 (HtrA4) has been identified as another cell fusion associated protein since knockout of this serine protease in BeWo cells failed to undergo forskolin-induced multinucleation [174]. However, the precise role of HtrA4 in cancer cell fusion remains unclear. In any case, PS signaling and syncytin-1 expression can contribute to cancer cell-cell fusion [66,175].
Human prostate cancer cells became fusiogenic after co-cultivation with muscle cells due to an IL-4 and IL-13 induced up-regulation of syncytin-1 and annexin A5 [66]. SiRNA mediated knock-down of annexin A5 expression and likewise blockade of syncytin-1 by a synthetic peptide or shRNA markedly impaired the generation of multinucleated PC3 cells and PC3 × muscle cell heterokaryons [66] supporting the requirement of syncytin-1 and annexin A5 in prostate cancer cell fusion.
Homotypic and heterotypic hybridization of human cancer cells can be facilitated by syncytin-1 together with PS signaling (Figure 1). Albeit syncytin-1 was reported to improve the prognosis of breast cancer patients [65], most data from other carcinoma types rather indicated a relationship between syncyctin-1 and tumor progression [59,61,64,66]. These findings also suggested that fusion of cancer cells in general develops a more malignant phenotype with progression of metastatic lesions [16,20,22,99,167,169,170,172]. Further involvement of syncytin-1 in the fusion of two non-transformed cells undergoing a malignant conversion has not yet been reported and, hence, remains ambiguous.
3.2. Unclear Role of Other HERV Elements in Tumor Development
Various endogenous HERV elements have been associated with tumor progression, whereby HERV-K has been most extensively studied (for review see: [138,139,145,176]). Briefly, mRNA and protein levels of HERV-K elements have been found in various carcinomas, such as melanoma cell lines and tissues [177,178], breast cancer [179,180,181,182], teratocarcinoma [183,184], germ cell tumors [185], prostate cancer cell lines [181,186], ovarian cancer [154], and renal cancer [187]. Moreover, specific antibodies against the HERV-K env protein have been detected in breast cancer and germ cell tumors [182,185] indicating that an adaptive immune response evolved against this viral protein. Additionally, env protein expression of HERV-E and ERV3 was detectable in ovarian cancer [154]. Although fusogenic properties have been discussed for these proteins [163,164], a potential contribution of HERV-K env and HERV-E env to tumor initiation by the merging of (cancer) cells is still unresolved. Nonetheless, several data revealed an implication of HERV elements expression in cancer progression as activators of multiple oncogenic signaling pathways such as Wnt/β-catenin and Ras/ERK signaling and inactivators of tumor suppressor genes (for review see: [176]). For instance, the HERV-K env element is a strong inducer of the Ras/RAF/MAPK/ERK1/2 pathway. This can trigger the induction of several transcription factors, such as ETV4, ETV5 and EGR1, that have been associated with cellular transformation [188]. Moreover, expression of HERV-K env induced epithelial to mesenchymal transition (EMT) in non-tumorigenic MCF10A human breast epithelial cells indicating that this retroviral-derived element might possess oncogenic properties [188]. This was further confirmed by shRNA mediated knockdown of HERV-K env expression, which blocked breast cancer cell proliferation, migration, and invasion due to inhibition of the expression of tumor-associated genes including Ras, p-RSK, and p-ERK [189]. Notably, HERV-K env knockdown also attenuated the ability of breast cancer cells to form tumors and to metastasize [189]. Conversely, overexpression of HERV-K env in shRNAenv knockdown breast cancer cells restored Ras/RAF/MAPK/ERK1/2 signaling concomitant with the reversion of reductions in migration and invasion [189]. Interestingly, HERV-K env overexpression was further correlated with down-regulation of the tumor suppressor p53 [189]. Besides HERV-K env-mediated aberrant signaling in oncogenic signal transduction pathways, the HERV genomes also encodes for long non-coding RNAs, which may also facilitate breast cancer progression. In that regard, high expression levels of the HERV-derived long non-coding RNA TROJAN was found in human triple-negative breast cancer, which was additionally correlated to proliferation and invasion [190]. Of interest, TROJAN increased the degradation of the metastasis-repressing factor ZMYND8 in triple-negative breast cancer cell lines and epigenetically up-regulated metastasis-related genes in multiple cell lines [190]. Furthermore, Zhou and colleagues recently demonstrated that even syncytin-1 (HERV-W1) promoted progression and doxorubicin resistance of hepatocellular carcinoma cells via the inflammation-activated MEK/ERK pathway [191]. In agreement with breast cancer studies shRNA-mediated HERV-K env knockdown significantly reduced in vitro and in vivo growth rates and metastatic spreading of human pancreatic cancer cell lines concomitant with decreased expression of Ras, p-ERK, pRSK, and p-AKT [192]. Silencing of the HERV-K Np9 protein expression inhibited the growth of myeloid and lymphoblastic leukemic cells, whereas the growth of leukemia cells in vitro and in vivo was promoted by Np9 expression suggesting that Np9 might by a potent viral oncogene in human leukemia [193]. Notably, Np9 expression was further correlated to activation of ERK, AKT and Notch1 signaling pathways. Moreover, Np9 promoted up-regulation of β-catenin and increase in the overall number of leukemia stem/progenitor cells indicating that this viral oncogene represents a critical molecular switch of multiple signaling pathways regulating the growth of leukemia stem/progenitor cells [193].
In summary, an increasing body of evidence indicates an active role of HERV elements in tumor initiation and progression due to activation of multiple oncogenic signaling pathways, inhibition of tumor suppressor genes, and expression of HERV-derived long non-coding RNAs (for review see: [176]). Hence, the impact of HERV elements in cancer progression is much more complex than “transduction” of oncogenes and “insertional mutagenesis” by HERV LTRs, which has been previously suggested as the major retroviral tumorigenic mechanisms [138]. While these findings clearly indicate an involvement of HERV elements in cancer initiation and progression, the role of HERV env elements in cancer cell-cell fusion still remains unclear.
3.3. Virus-Mediated Cell-Cell Fusion and Syncytia/PGCC Formation
Multinucleated or so-called polyploid giant cancer cells (PGCCs) have been found in a variety of cancerous tissues and play a prominent role in drug resistance, invasiveness, metastasis, and stemness properties [194,195,196,197,198,199,200,201,202,203,204,205,206]. The predominant mechanisms leading to PGCC formation depend on endoreplication, mitotic slippage, cytokines failure, cell cannibalism, and cell-cell fusion (for review see: [204,207]). Likewise, different triggers of PGCC formation have been identified, such as chemotherapeutics, radiotherapy, hypoxia, oxidative stress, air pollution, UV light, hyperthermia, and oncoviruses (for review see: [204,206,207]).
Oncoviruses as PGCC inducers suggest that multinucleated cells might be derived from virus-facilitated cell merging. This is in agreement with the hypothesis of Duelli and Lazebnik suggesting an impact of virus-facilitated cell-cell fusion in cancer initiation and progression (for review see: [21]). In fact, heterokaryon formation capacity has been demonstrated for the Sendai virus, which facilitates cell merging by acting as a linker to bridge two individual cells [208]. Interestingly, the first monoclonal antibody-producing hybridomas were generated by Sendai virus-mediated fusion of plasma cells and myeloma cells [113]. Bi-nucleation of HPV16 infected cells is facilitated by the HPV16 E5 protein, but the mechanism has not yet been resolved [124,125]. HPV16 is a well-known oncogenic virus strain that could cause head and neck squamous carcinomas and among others cervical, anal, perianal, vulvar, and penile cancers [209,210]. HPV16 E6 and E7 proteins could induce genomic instability due to the generation of mitotic defects by induction of centrosome abnormalities and multipolar divisions, thereby causing aneuploidy/GCIN [211,212]. Hence, E5 mediated cell-cell fusion together with E6/E7-related centrosome abnormalities could play a role in the malignant transformation of HPV16-infected cells. Viable and highly heterogeneous hybrids were derived from Mason-Pfizer monkey virus (MPMV)-facilitated fusion of D551 fibroblasts expressing either HRAS or E1A oncogenes [111]. These findings further support the assumption of virus-facilitated cell-cell fusion as an inducer of a malignant transformation of cells. Interestingly, no MPMV-derived hybrids could be generated from wild-type D551 fibroblasts [111] possibly due to intact tumor suppressor genes/pathways that usually induce senescence or apoptosis in aneuploid (hybrid) cells [33]. Indeed, propagation of chromosome missegregation was inhibited and apoptosis was induced in aneuploid cells with intact tumor suppressors, such as p53 [213,214,215]. Conversely, the lack of tumor suppressors or their mutational inactivation was accompanied by an increased frequency and survival rate of aneuploid cells [216,217,218].
In addition to HPV, further oncogenic viruses could induce bi- and multinucleation, such as Hepatitis B and C virus, Epstein-Barr virus, Kaposi sarcoma virus and Human T-lymphotropic virus 1 (for review see [21,206,219]). However, bi-nucleation and polyploidy (and aneuploidy/GCIN) is rather induced due to a persistent expression of viral oncoproteins that leads to a dysregulation of several important cellular processes and not via cell-cell fusion [206,219]. Thus, viral oncoproteins could activate survival pathways, initiate DNA synthesis and cell cycle progression, activate proto-oncogenes, inactivate tumor suppressors and cause epigenetic modifications [206,219]. Each of these mechanisms alone or in combination could sufficiently cause replication errors, chromosome missegregation and mitotic errors, eventually leading to aneuploid and multinucleated cells including PGCCs. Altogether, cancer initiation and progression by oncogenic viruses appear to be rather attributed to non-cell-cell fusion-dependent mechanisms.
3.4. Oncolytic Virus-Mediated Syncytia Formation in Cancer Therapy
Oncolytic viruses have been suggested as promising candidates for cancer therapy due to their ability to specifically infect and effectively kill cancer cells. These effects are accompanied by an interruption of the immune tolerance and induction of an adaptive immune response against the cancer cells (for review see [115,116]). Limited spreading of oncolytic viruses within the tumor microenvironment remains a key challenge that could be overcome by syncytia formation via virus-mediated and/or viral fusogen-mediated cell-cell fusion (for review see [115,116]). Therefore, natural syncytia viruses such as Newcastle disease virus, Sendai virus, respiratory syncytial virus and measles virus, or so-called engineered syncytia viruses are used for oncolytic virotherapy (for review see [115,116]). Engineered syncytia viruses are generated by insertion of viral fusogens (e.g., FAST proteins, F protein of measles virus) into the backbone of non-fusogenic oncolytic viruses, such as Vesicular stomatitis virus, Herpes simplex virus or Adenovirus (for review see [115,116]). The lytic effect of syncytia formation by oncolytic viruses is multifactorial and related to direct cancer cell killing, due to e.g., induction of apoptosis, necrosis and autophagy, bystander effects of non-infected cells and non-cancer cells, and induction of an adaptive immune response (release of tumor antigens, activation of dendritic cells, cytotoxic CD8 T-cells) (for review see [115,116]). Even though apoptotic cell death and necrosis in virus-derived syncytia has been observed in several studies it became clear that induction of cell death is a highly heterogeneous process [115]. For instance, HIV and measles virus-associated apoptotic cell death is primarily due to amplification of background apoptosis in the wake of cell-to-cell fusion [220], whereas apoptosis in reovirus-induced syncytial cells is initiated due to the FAST protein-induced membrane instability [221]. In contrast, oncolytic virus-induced cell death was not prevented by pan-caspase inhibitors in hepatocellular carcinoma, non-small lung cell cancer cells, and acute myeloma cells [222,223,224] indicating that cell death was likely attributed to necrosis.
The bystander effect has been identified as an important feature in oncolytic virus therapy-induced syncytial cell death. On the one hand, the bystander effect can increase viral spreading throughout cancerous tissues, thereby improving the anti-tumoral potency of oncolytic virus therapy [225]. Likewise, the bystander effect was effective in the induction of apoptosis mediated by the HIV env protein [226]. HIV env expression in infected cells could lead to syncytia formation and activation of multiple pathways that induced mitochondrial apoptosis. Moreover, HIV env-mediated fusion of non-infected cells also resulted in the death of both cells, which was dependent on the mitochondrial pathway of apoptosis, but without the engagement of other multiple pathways [226].
Immunogenic cell death is another important issue in oncolytic virus therapy. In this context, the antitumoral activity of oncolytic viruses is not only related to induction of apoptosis and necrosis, but to a profound induction of a specific immune response against cancer cells. In accordance with multiple processes leading to cell death of infected cancer cells such as induction of apoptosis and necrosis, several mechanisms have been identified how oncolytic virus therapy could activate the innate and adaptive immune system. Pathogen recognition receptors (PRRs) like Toll-like receptors (TLRs) and NOD-like receptors (NLRs) are also expressed by cancer cells [227]. While this has been rather associated with tumor progression due to recognition of damage-associated molecular patterns (DAMPs) and subsequent receptor activation [227], cancer cells expressing PRRs might also recognize oncolytic virus RNA and DNA concomitant with a specific cellular response. Interestingly, virus-cell fusion specifically induced a stimulator of interferon genes (STING) response with subsequent expression of interferon-stimulated genes, in vivo recruitment of leukocytes, and potentiation of signaling via TLR7 and TLR9 [228]. Likewise, the interferon-α/β production was markedly amplified in measles virus-induced syncytial cells [229] suggesting that an antiviral cellular immune response could be fostered by cell-cell fusion. In melanoma, dying syncytia produced more so-called syncytiosomes (syncytia-derived exosomes) than normal cells, which potently loaded dendritic cells and more effectively induced a specific cytotoxic T cell response against melanoma cells expressing the specific tumor antigen gp100 [230]. Likewise, syncytia formation of human Mel888 melanoma cells reversed the suppressive effects of Mel888 on dendritic cells. Moreover, fusing melanoma cells were a more effective source for melanoma gp100 antigen presentation of dendritic cells and induction of a specific cytotoxic T cell response [231].
In summary, these findings underline the fusogenic capacities of viruses in facilitating cell-cell fusion and syncytia formation, which could be beneficial in oncolytic virus cancer therapy.
4. Conclusions and Future Perspectives
Cell-cell fusion has been suggested as a putative driver of tumor initiation and progression [20,99,167,168,172,232,233]. However, despite increasing knowledge the entire mechanism is still scarcely understood. The merging of two plasma membranes is a multi-step process of various proteins and fusogens that have been identified as indispensable mediators of cell-cell fusion to overcome plasma membrane merging-associated energetic barriers [3,4,5,6,7,8,19,20,89]. Enveloped and some non-enveloped viruses could induce syncytia/MNGC formation due to the expression of evolutionarily optimized fusogens [50,51,121,122,123] whereby oncogenic viruses could lead to PGCC formation [21,206,219]. About 8% of the human genome is of retroviral origin [136,137,138,139] and transcripts including env proteins have been identified in normal and tumorigenic tissues with fusogenic properties [138,139,145]). These effects raise the suggestion that viruses and/or fusogens of endogenous retroviral origin may represent natural mediators of cell-cell fusion during tumor initiation and progression. However, only a very few data have been published so far that support this assumption.
MPMV-fusion derived D551 fibroblast hybrids demonstrated tumorigenicity and exhibited a markedly increased GCIN, but only when hybrids were derived from HRAS or E1A expressing fibroblasts [111]. Hybrids derived from wild-type D511 fibroblasts with intact tumor suppressor machinery were not viable [111] which supports the correlation of non-functional tumor suppressors and higher tolerance to aneuploidy/polyploidy [213,214,217,234]. These data likely indicate that viruses could fuse and transform cells with an impaired tumor suppressor machinery. MPMV is a retrovirus with a host range largely restricted to primates that was also detected in human and human cancer cell lines, but displayed no identified pathogenic effect [110]. Likewise, the Sendai virus is a highly transmissible and fusogenic respiratory virus in rodents, but is considered apathogenic in humans and, hence, highly suitable for oncolytic virotherapy [115,116] and vector-based vaccination strategies [235,236]. Although apathogenic and fusogenic viruses might be putative candidates for cell-cell fusion this requires some consecutive prerequisites. These include close contact, infection and uptake of viruses/viral particles from a virus/viral particle producing organisms, successful fusion of cells with a defective tumor suppressor machinery, and survival of resulting hybrids concomitant with malignant transformation. Given that each of these steps is rate-limiting, the likelihood of this sequence of events should be extremely low. Moreover, as stated above, virus-mediated fusion rather leads to the generation of multinucleated and non-viable syncytial cells, which further decreases the overall probability that viruses could cause cellular transformation by cell-cell fusion. Accordingly, the conclusion that virus-mediated hybridization might represent a common event in tumor initiation requires further evidence.
More convincing data are available for HERV elements. Expression of HERV env has been identified in a variety of normal and tumor tissues [138,139,145] and is associated with tumor initiation and progression due to activation of oncogenic signal cascades [188,191,192] and induction of the EMT program [189]. Hence, HERV env elements rather foster tumor progression in a non-cell-cell fusion-dependent manner albeit fusogenic properties have been postulated for HERV-K env and HERV-E env [163,164], but not yet validated. In that regard, it would be interesting to investigate the potential fusogenic capacities of HERV-K env and HERV-E env.
Syncytin-1 (HERV-W1) is a well-characterized fusogen of HERV origin and its involvement in cancer cell fusion has been documented in several studies [59,60,61,62,63,64,65,66] although further molecules and signals are required to conclude this process (Figure 1). Syncytin-1 expression in cancer cells is usually associated with their fusogenic capacity. However, the reason and the benefit of cancer cells to express this fusogen and to fuse with other cells is not clear. Although cell-cell fusion appears as an inefficient process Miroshnychenko et al. suggested that spontaneous somatic cell-cell fusion enables populations of cancer cells to amplify clonal heterogeneity, which may substantially accelerate a tumor’s ability to adapt to new selective pressures [237]. The majority of tumor hybrids will die in a post-hybrid selection process (PHSP) [168] and only a small population, if any, will survive. Likewise, syncytin-1 expression is not sufficient for cell-cell fusion without expression of the ASCT-2 receptor on adjacent cells [62]. Recent findings revealed that even syncytin-1 could induce an oncogenic signaling cascade in hepatocellular carcinoma cells [191], likely indicating another crucial role of this fusogen in tumor progression. In that regard, it would be interesting to investigate whether syncytin-1 induced oncogenic signaling cascades would be also active in other cancer cells and whether these could prevent tumor hybrids from cell death.
Even though viruses and HERV env elements appear to be a natural reservoir of fusogens that could facilitate the merging of two and more cells, their role in cancer cell fusion is still not well understood. So far, syncytin-1 is the only known and to date best characterized fusogen of HERV origin. Its impact on cancer cell fusion and tumor progression has been demonstrated in several studies. Fusogenic properties have been postulated for other HERV env elements, but have not yet been clearly validated. Nonetheless, their impact on tumor progression due to activation of oncogenic signaling pathways is well documented. Oncogenic viruses could cause PGCC formation, but polyploidy is predominantly induced by a persistent expression of viral oncoproteins with dysregulation of several important cellular tumor suppressors and cell cycle regulators rather than by cell-cell fusion.
Hence, despite an inherent fusogenecity, enveloped and some non-enveloped viruses most likely do not foster cancer initiation and progression by facilitating cell-cell fusion. Conversely, the impact of distinct HERV env elements on tumor progression has been demonstrated in several studies including the biological phenomenon of cell-cell fusion. In any case, the mechanism of cell-cell fusion in tumors is still scarcely understood and more research is also necessary to elucidate the impact of virus-derived fusogens in this process.
Abbreviations
| ASCT-2 | alanine, serine, and cysteine selective transporter-2 |
| EMT | epithelial to mesenchymal transition |
| FAST | fusion-associated small transmembrane |
| GCIN | genomic/chromosomal instability |
| HERV | human endogenous retrovirus |
| HST | heterokaryon-to-synkaryon transition |
| HtrA4 | high temperature requirement factor A 4 |
| hESCs | human embryonic stem cells |
| hHSCs | human hematopoietic stem cells |
| HPV | human papillomavirus |
| ipSCs | induced pluripotent stem cells |
| LTR | long terminal repeat |
| MaLR | mammalian apparent LTR retrotransposon |
| MNGCs | multinuclear giant cells |
| MPMV | Mason-Pfizer monkey virus |
| NLR | NOD-like receptor |
| PGCC | polyploid giant cancer cells |
| PR | ploidy reduction |
| PRR | pathogen recognition receptor |
| STING | stimulator of interferon genes |
| TNF-α | tumor necrosis factor-α |
| TLR | Toll-like receptor |
| PEG10 | paternally expressed gene 10 |
| PS | phosphatidylserine |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13215363/s1, Video S1: Video of a tripolar cancer cell division from Figure 2, Video S2: Video of a tetrapolar cancer cell division from Figure 2.
Author Contributions
Conceptualization, T.D. and R.H.; writing—original draft preparation, T.D. and R.H.; documentation, T.L. and J.W. and T.L.; writing—review and editing, T.D. and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dittmar T., Zänker K.S. Cell Fusion in Health and Disease. Volume 1. Springer; Dordrecht, The Netherlands: 2011. [DOI] [PubMed] [Google Scholar]
- 2.Dittmar T., Zanker K.S. Cell Fusion in Health and Disease. Volume 2 Springer; Dordrecht, The Netherlands: 2011. [Google Scholar]
- 3.Aguilar P.S., Baylies M.K., Fleissner A., Helming L., Inoue N., Podbilewicz B., Wang H., Wong M. Genetic basis of cell–cell fusion mechanisms. Trends Genet. 2013;29:427–437. doi: 10.1016/j.tig.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brukman N.G., Uygur B., Podbilewicz B., Chernomordik L.V. How cells fuse. J. Cell Biol. 2019;218:1436–1451. doi: 10.1083/jcb.201901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández J.M., Podbilewicz B. The hallmarks of cell-cell fusion. Development. 2017;144:4481–4495. doi: 10.1242/dev.155523. [DOI] [PubMed] [Google Scholar]
- 6.Iosilevskii Y., Podbilewicz B. Programmed cell fusion in development and homeostasis. Curr. Top. Dev. Biol. 2021;144:215–244. doi: 10.1016/bs.ctdb.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Vargas J., Krey T., Valansi C., Avinoam O., Haouz A., Jamin M., Raveh-Barak H., Podbilewicz B., Rey F.A. Structural basis of eukaryotic cell-cell fusion. Cell. 2014;157:407–419. doi: 10.1016/j.cell.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Helming L., Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Shabo I., Midtbö K., Andersson H., Åkerlund E., Olsson H., Wegman P., Gunnarsson C., Lindström A. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction. BMC Cancer. 2015;15:922. doi: 10.1186/s12885-015-1935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding J., Jin W., Chen C., Shao Z., Wu J. Tumor associated macrophage × cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS ONE. 2012;7:e41942. doi: 10.1371/journal.pone.0041942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clawson G.A., Matters G.L., Xin P., McGovern C., Wafula E., Depamphilis C., Meckley M., Wong J., Stewart L., D’Jamoos C., et al. “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS ONE. 2017;12:e0184451. doi: 10.1371/journal.pone.0184451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitwood C.A., Dietzsch C., Jacobs G., McArdle T., Freeman B.T., Banga A., Noubissi F.K., Ogle B.M. Breast tumor cell hybrids form spontaneously in vivo and contribute to breast tumor metastases. APL Bioeng. 2018;2:031907. doi: 10.1063/1.5024744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melzer C., von der Ohe J., Hass R. In Vivo Cell Fusion between Mesenchymal Stroma/Stem-Like Cells and Breast Cancer Cells. Cancers. 2019;11:185. doi: 10.3390/cancers11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hass R., von der Ohe J., Ungefroren H. Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness. Cancers. 2020;12:3716. doi: 10.3390/cancers12123716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melzer C., von der Ohe J., Hass R. MSC stimulate ovarian tumor growth during intercellular communication but reduce tumorigenicity after fusion with ovarian cancer cells. Cell Commun. Signal. 2018;16:1–9. doi: 10.1186/s12964-018-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melzer C., von der Ohe J., Hass R. Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC) Cell Commun. Signal. 2018;16:2. doi: 10.1186/s12964-018-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartosh T.J., Ullah M., Zeitouni S., Beaver J., Prockop D.J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs) Proc. Natl. Acad. Sci. USA. 2016;113:E6447–E6456. doi: 10.1073/pnas.1612290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melzer C., von der Ohe J., Luo T., Hass R. Spontaneous Fusion of MSC with Breast Cancer Cells Can Generate Tumor Dormancy. Int. J. Mol. Sci. 2021;22:5930. doi: 10.3390/ijms22115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podbilewicz B. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 2014;30:111–139. doi: 10.1146/annurev-cellbio-101512-122422. [DOI] [PubMed] [Google Scholar]
- 20.Duelli D., Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell. 2003;3:445–448. doi: 10.1016/S1535-6108(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 21.Duelli D.M., Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat. Rev. Cancer. 2007;7:968–976. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- 22.Sieler M., Weiler J., Dittmar T. Cell–Cell Fusion and the Roads to Novel Properties of Tumor Hybrid Cells. Cells. 2021;10:1465. doi: 10.3390/cells10061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X., Platt J.L. Molecular and cellular mechanisms of mammalian cell fusion. Adv. Exp. Med. Biol. 2011;713:33–64. doi: 10.1007/978-94-007-0763-4_4. [DOI] [PubMed] [Google Scholar]
- 24.Thomas G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M., Kleine-Weber H., Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell. 2020;78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vance T.D., Lee J.E. Virus and eukaryote fusogen superfamilies. Curr. Biol. 2020;30:R750–R754. doi: 10.1016/j.cub.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huppertz B., Gauster M. Trophoblast Fusion. Adv. Exp. Med. Biol. 2011;713:81–95. doi: 10.1007/978-94-007-0763-4_6. [DOI] [PubMed] [Google Scholar]
- 28.Bjerkvig R., Tysnes B.B., Aboody K.S., Najbauer J., Terzis A.J. Opinion: The origin of the cancer stem cell: Current controversies and new insights. Nat. Rev. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 29.Dörnen J., Sieler M., Weiler J., Keil S., Dittmar T. Cell Fusion-Mediated Tissue Regeneration as an Inducer of Polyploidy and Aneuploidy. Int. J. Mol. Sci. 2020;21:1811. doi: 10.3390/ijms21051811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan A.W., Hickey R.D., Paulk N.K., Culberson A.J., Olson S.B., Finegold M.J., Grompe M. Ploidy Reductions in murine fusion-derived hepatocytes. PLoS Genet. 2009;5:e1000385. doi: 10.1371/journal.pgen.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan A.W., Taylor M.H., Hickey R.D., Hanlon Newell A.E., Lenzi M.L., Olson S.B., Finegold M.J., Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland A.J., Cleveland D.W. Boveri revisited: Chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chunduri N.K., Storchová Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 2019;21:54–62. doi: 10.1038/s41556-018-0243-8. [DOI] [PubMed] [Google Scholar]
- 34.Ganem N.J., Godinho S.A., Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godinho S.A., Kwon M., Pellman D. Centrosomes and cancer: How cancer cells divide with too many centrosomes. Cancer Metastasis Rev. 2009;28:85–98. doi: 10.1007/s10555-008-9163-6. [DOI] [PubMed] [Google Scholar]
- 36.Passerini V., Ozeri-Galai E., de Pagter M.S., Donnelly N., Schmalbrock S., Kloosterman W.P., Kerem B., Storchová Z. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 2016;7:10754. doi: 10.1038/ncomms10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Compton A.A., Schwartz O. They Might Be Giants: Does Syncytium Formation Sink or Spread HIV Infection? PLoS Pathog. 2017;13:e1006099. doi: 10.1371/journal.ppat.1006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss M.A., Zimmer S., Anderson K.W. Role of metastatic potential in the adhesion of human breast cancer cells to endothelial monolayers. Anticancer Res. 2000;20:1425–1433. [PubMed] [Google Scholar]
- 39.Moss M.S., Sisken B., Zimmer S., Anderson K.W. Adhesion of nonmetastatic and highly metastatic breast cancer cells to endothelial cells exposed to shear stress. Biorheology. 1999;36:359–371. [PubMed] [Google Scholar]
- 40.Symeonides M., Murooka T.T., Bellfy L.N., Roy N.H., Mempel T.R., Thali M. HIV-1-Induced Small T Cell Syncytia Can Transfer Virus Particles to Target Cells through Transient Contacts. Viruses. 2015;7:6590–6603. doi: 10.3390/v7122959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornich B.F., Grosskopf A.K., Schlagowski S., Tenbusch M., Kleine-Weber H., Neipel F., Stahl-Hennig C., Hahn A.S. SARS-CoV-2 and SARS-CoV spike-mediated cell-cell fusion differ in the requirements for receptor expression and proteolytic activation. J. Virol. 2021 doi: 10.1128/JVI.00002-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanton C., Nicke B., Schuett M., Eklund A.C., Ng C., Li Q., Hardcastle T., Lee A., Roy R., East P., et al. Chromosomal instability determines taxane response. Proc. Natl. Acad. Sci. USA. 2009;106:8671–8676. doi: 10.1073/pnas.0811835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweeney E.C., Palmer R.A., Pfuller U. Crystallization of the ribosome inactivating protein ML1 from Viscum album (mistletoe) complexed with beta-D-galactose. J. Mol. Biol. 1993;234:1279–1281. doi: 10.1006/jmbi.1993.1682. [DOI] [PubMed] [Google Scholar]
- 44.Más V., Melero J.A. Entry of enveloped viruses into host cells: Membrane fusion. Subcell. Biochem. 2013;68:467–487. doi: 10.1007/978-94-007-6552-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shmulevitz M., Duncan R. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 2000;19:902–912. doi: 10.1093/emboj/19.5.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan K.M.C., Son S., Schmid E.M., Fletcher D.A. A viral fusogen hijacks the actin cytoskeleton to drive cell-cell fusion. eLife. 2020;9 doi: 10.7554/eLife.51358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diller J.R., Parrington H.M., Patton J.T., Ogden K.M. Rotavirus Species B Encodes a Functional Fusion-Associated Small Transmembrane Protein. J. Virol. 2019;93 doi: 10.1128/JVI.00813-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faísca P., Desmecht D. Sendai virus, the mouse parainfluenza type 1: A longstanding pathogen that remains up-to-date. Res. Vet. Sci. 2007;82:115–125. doi: 10.1016/j.rvsc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Okada Y. [3] Sendai virus-induced cell fusion. Methods Enzymol. 1993;221:18–41. doi: 10.1016/0076-6879(93)21005-s. [DOI] [PubMed] [Google Scholar]
- 50.Rawling J., Cano O., Garcin M., Kolakofsky D., Melero J.A. Recombinant sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus. J. Virol. 2011;85:2771–2780. doi: 10.1128/JVI.02065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leroy H., Han M., Woottum M., Bracq L., Bouchet J., Xie M., Benichou S. Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci. 2020;21:9644. doi: 10.3390/ijms21249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huppertz B., Bartz C., Kokozidou M. Trophoblast fusion: Fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron. 2006;37:509–517. doi: 10.1016/j.micron.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Malassiné A., Handschuh K., Tsatsaris V., Gerbaud P., Cheynet V., Oriol G., Mallet F., Evain-Brion D. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta. 2005;26:556–562. doi: 10.1016/j.placenta.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Mi S., Lee X., Li X., Veldman G.M., Finnerty H., Racie L., LaVallie E., Tang X.Y., Edouard P., Howes S., et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 55.Muir A., Lever A.M., Moffett A. Human endogenous retrovirus-W envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J. Gen. Virol. 2006;87:2067–2071. doi: 10.1099/vir.0.81412-0. [DOI] [PubMed] [Google Scholar]
- 56.Vargas A., Moreau J., Landry S., LeBellego F., Toufaily C., Rassart E., Lafond J., Barbeau B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 2009;392:301–318. doi: 10.1016/j.jmb.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 57.Møller A.M.J., Delaisse J.-M., Søe K. Osteoclast Fusion: Time-Lapse Reveals Involvement of CD47 and Syncytin-1 at Different Stages of Nuclearity. J. Cell. Physiol. 2017;232:1396–1403. doi: 10.1002/jcp.25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Søe K., Andersen T.L., Hobolt-Pedersen A.S., Bjerregaard B., Larsson L.I., Delaisse J.M. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone. 2011;48:837–846. doi: 10.1016/j.bone.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Yu H., Liu T., Zhao Z., Chen Y., Zeng J., Liu S., Zhu F. Mutations in 3′-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene. 2014;33:3947–3958. doi: 10.1038/onc.2013.366. [DOI] [PubMed] [Google Scholar]
- 60.Yan T.L., Wang M., Xu Z., Huang C.M., Zhou X.C., Jiang E.H., Zhao X.P., Song Y., Song K., Shao Z., et al. Up-regulation of syncytin-1 contributes to TNF-α-enhanced fusion between OSCC and HUVECs partly via Wnt/β-catenin-dependent pathway. Sci. Rep. 2017;7:40983. doi: 10.1038/srep40983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strick R., Ackermann S., Langbein M., Swiatek J., Schubert S.W., Hashemolhosseini S., Koscheck T., Fasching P.A., Schild R.L., Beckmann M.W., et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-beta. J. Mol. Med. 2007;85:23–38. doi: 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 62.Bjerregaard B., Holck S., Christensen I.J., Larsson L.I. Syncytin is involved in breast cancer-endothelial cell fusions. Cell. Mol. Life Sci. 2006;63:1906–1911. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melzer C., von der Ohe J., Hass R. In vitro fusion of normal and neoplastic breast epithelial cells with human mesenchymal stroma/stem cells (MSC) partially involves TNF receptor signaling. Stem Cells. 2018;36:977–989. doi: 10.1002/stem.2819. [DOI] [PubMed] [Google Scholar]
- 64.Fei F., Li C., Wang X., Du J., Liu K., Li B., Yao P., Li Y., Zhang S. Syncytin 1, CD9, and CD47 regulating cell fusion to form PGCCs associated with cAMP/PKA and JNK signaling pathway. Cancer Med. 2019;8:3047–3058. doi: 10.1002/cam4.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsson L.I., Holck S., Christensen I.J. Prognostic role of syncytin expression in breast cancer. Hum. Pathol. 2007;38:726–731. doi: 10.1016/j.humpath.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Uygur B., Leikina E., Melikov K., Villasmil R., Verma S.K., Vary C.P.H., Chernomordik L.V. Interactions with Muscle Cells Boost Fusion, Stemness, and Drug Resistance of Prostate Cancer Cells. Mol. Cancer Res. 2019;17:806–820. doi: 10.1158/1541-7786.MCR-18-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benešová M., Trejbalová K., Kovářová D., Vernerová Z., Hron T., Kučerová D., Hejnar J. DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas. Retrovirology. 2017;14:20. doi: 10.1186/s12977-017-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millay D.P., O’Rourke J.R., Sutherland L.B., Bezprozvannaya S., Shelton J.M., Bassel-Duby R., Olson E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499:301–305. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leikina E., Gamage D.G., Prasad V., Goykhberg J., Crowe M., Diao J., Kozlov M.M., Chernomordik L.V., Millay D.P. Myomaker and Myomerger Work Independently to Control Distinct Steps of Membrane Remodeling during Myoblast Fusion. Dev. Cell. 2018;46:767–780.e7. doi: 10.1016/j.devcel.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bi P., Ramirez-Martinez A., Li H., Cannavino J., McAnally J.R., Shelton J.M., Sánchez-Ortiz E., Bassel-Duby R., Olson E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science. 2017;356:323–327. doi: 10.1126/science.aam9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinn M.E., Goh Q., Kurosaka M., Gamage D.G., Petrany M.J., Prasad V., Millay D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017;8:15665. doi: 10.1038/ncomms15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue N., Ikawa M., Isotani A., Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 73.Chalbi M., Barraud-Lange V., Ravaux B., Howan K., Rodriguez N., Soule P., Ndzoudi A., Boucheix C., Rubinstein E., Wolf J.P., et al. Binding of sperm protein Izumo1 and its egg receptor Juno drives Cd9 accumulation in the intercellular contact area prior to fusion during mammalian fertilization. Development. 2014;141:3732–3739. doi: 10.1242/dev.111534. [DOI] [PubMed] [Google Scholar]
- 74.Bianchi E., Wright G.J. Cross-species fertilization: The hamster egg receptor, Juno, binds the human sperm ligand, Izumo1. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140101. doi: 10.1098/rstb.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kato K., Satouh Y., Nishimasu H., Kurabayashi A., Morita J., Fujihara Y., Oji A., Ishitani R., Ikawa M., Nureki O. Structural and functional insights into IZUMO1 recognition by JUNO in mammalian fertilization. Nat. Commun. 2016;7:12198. doi: 10.1038/ncomms12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies P.S., Powell A.E., Swain J.R., Wong M.H. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS ONE. 2009;4:e6530. doi: 10.1371/journal.pone.0006530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johansson C.B., Youssef S., Koleckar K., Holbrook C., Doyonnas R., Corbel S.Y., Steinman L., Rossi F.M.V., Blau H.M. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nygren J.M., Liuba K., Breitbach M., Stott S., Thorén L., Roell W., Geisen C., Sasse P., Kirik D., Björklund A., et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat. Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 79.Hotokezaka H., Sakai E., Ohara N., Hotokezaka Y., Gonzales C., Matsuo K., Fujimura Y., Yoshida N., Nakayama K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-α, lipopolysaccharide, or peptidoglycan. J. Cell. Biochem. 2007;101:122–134. doi: 10.1002/jcb.21167. [DOI] [PubMed] [Google Scholar]
- 80.Skokos E.A., Charokopos A., Khan K., Wanjala J., Kyriakides T.R. Lack of tnf-α–induced MMP-9 production and abnormal e-cadherin redistribution associated with compromised fusion in MCP-1–null macrophages. Am. J. Pathol. 2011;178:2311–2321. doi: 10.1016/j.ajpath.2011.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiler J., Dittmar T. Minocycline impairs TNF-α-induced cell fusion of M13SV1-Cre cells with MDA-MB-435-pFDR1 cells by suppressing NF-κB transcriptional activity and its induction of target-gene expression of fusion-relevant factors. Cell Commun. Signal. 2019;17:71. doi: 10.1186/s12964-019-0384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weiler J., Mohr M., Zänker K.S., Dittmar T. Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells. Cell Commun. Signal. 2018;16:14. doi: 10.1186/s12964-018-0226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song K., Zhu F., Zhang H.-Z., Shang Z.-J. Tumor necrosis factor-α enhanced fusions between oral squamous cell carcinoma cells and endothelial cells via VCAM-1/VLA-4 pathway. Exp. Cell Res. 2012;318:1707–1715. doi: 10.1016/j.yexcr.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Skinner A.M., Grompe M., Kurre P. Intra-hematopoietic cell fusion as a source of somatic variation in the hematopoietic system. J. Cell Sci. 2012;125:2837–2843. doi: 10.1242/jcs.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hass R. Role of MSC in the Tumor Microenvironment. Cancers. 2020;12:2107. doi: 10.3390/cancers12082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chernomordik L.V., Zimmerberg J., Kozlov M.M. Membranes of the world unite! J. Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jahn R., Lang T., Südhof T.C. Membrane Fusion. Cell. 2003;112:519–533. doi: 10.1016/S0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 88.Ogle B.M., Cascalho M., Platt J.L. Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 89.Whitlock J.M., Chernomordik L.V. Flagging fusion: Phosphatidylserine signaling in cell–cell fusion. J. Biol. Chem. 2021;296:100411. doi: 10.1016/j.jbc.2021.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melzer C., Von Der Ohe J., Hass R. Involvement of Actin Cytoskeletal Components in Breast Cancer Cell Fusion with Human Mesenchymal Stroma/Stem-Like Cells. Int. J. Mol. Sci. 2019;20:876. doi: 10.3390/ijms20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hochreiter-Hufford A.E., Lee C.S., Kinchen J.M., Sokolowski J.D., Arandjelovic S., Call J.A., Klibanov A.L., Yan Z., Mandell J.W., Ravichandran K.S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park D., Tosello-Trampont A.-C., Elliott M.R., Lu M., Haney L.B., Ma Z., Klibanov A.L., Mandell J.W., Ravichandran K.S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 93.Verma S.K., Leikina E., Melikov K., Gebert C., Kram V., Young M.F., Uygur B., Chernomordik L.V. Cell-surface phosphatidylserine regulates osteoclast precursor fusion. J. Biol. Chem. 2018;293:254–270. doi: 10.1074/jbc.M117.809681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitlock J.M., Yu K., Cui Y.Y., Hartzell H.C. Anoctamin 5/TMEM16E facilitates muscle precursor cell fusion. J. Gen. Physiol. 2018;150:1498–1509. doi: 10.1085/jgp.201812097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y., Le T., Grabau R., Mohseni Z., Kim H., Natale D.R., Feng L., Pan H., Yang H. TMEM16F phospholipid scramblase mediates trophoblast fusion and placental development. Sci. Adv. 2020;6:eaba0310. doi: 10.1126/sciadv.aba0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azab W., Gramatica A., Herrmann A., Osterrieder N. Binding of alphaherpesvirus glycoprotein H to surface α4β1-integrins activates calcium-signaling pathways and induces phosphatidylserine exposure on the plasma membrane. mBio. 2015;6:e01552-15. doi: 10.1128/mBio.01552-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaitseva E., Zaitsev E., Melikov K., Arakelyan A., Marin M., Villasmil R., Margolis L.B., Melikyan G.B., Chernomordik L.V. Fusion Stage of HIV-1 Entry Depends on Virus-Induced Cell Surface Exposure of Phosphatidylserine. Cell Host Microbe. 2017;22:99–110.e7. doi: 10.1016/j.chom.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aichel O. Über Zellverschmelzung mit quantitativ abnormer Chromosomenverteilung als Ursache der Geschwulstbildung. In: Roux W., editor. Vorträge und Aufsätze Über Entwicklungsmechanik der Organismen. Wilhelm Engelmann; Leipzig, Germany: 1911. pp. 1–115. [Google Scholar]
- 99.Pawelek J.M., Chakraborty A.K. Fusion of tumour cells with bone marrow-derived cells: A unifying explanation for metastasis. Nat. Rev. Cancer. 2008;8:377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 100.Chakraborty A., Lazova R., Davies S., Backvall H., Ponten F., Brash D., Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34:183–186. doi: 10.1038/sj.bmt.1704547. [DOI] [PubMed] [Google Scholar]
- 101.LaBerge G., Duvall E., Grasmick Z., Haedicke K., Galan A., Pawelek J. A melanoma patient with macrophage-cancer cell hybrids in the primary tumor, a lymph node metastasis and a brain metastasis. Cancer Genet. 2021;256–257:162–164. doi: 10.1016/j.cancergen.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Laberge G.S., Duvall E., Grasmick Z., Haedicke K., Pawelek J. A Melanoma Lymph Node Metastasis with a Donor-Patient Hybrid Genome following Bone Marrow Transplantation: A Second Case of Leucocyte-Tumor Cell Hybridization in Cancer Metastasis. PLoS ONE. 2017;12:e0168581. doi: 10.1371/journal.pone.0168581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lazova R., Laberge G.S., Duvall E., Spoelstra N., Klump V., Sznol M., Cooper D., Spritz R.A., Chang J.T., Pawelek J.M. A Melanoma Brain Metastasis with a Donor-Patient Hybrid Genome following Bone Marrow Transplantation: First Evidence for Fusion in Human Cancer. PLoS ONE. 2013;8:e66731. doi: 10.1371/journal.pone.0066731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yilmaz Y., Lazova R., Qumsiyeh M., Cooper D., Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: Visualization of putative BMT–tumor hybrids by FISH. Bone Marrow Transplant. 2005;35:1021–1024. doi: 10.1038/sj.bmt.1704939. [DOI] [PubMed] [Google Scholar]
- 105.Gast C.E., Silk A.D., Zarour L., Riegler L., Burkhart J.G., Gustafson K.T., Parappilly M.S., Roh-Johnson M., Goodman J.R., Olson B., et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018;4:eaat7828. doi: 10.1126/sciadv.aat7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manjunath Y., Mitchem J.B., Suvilesh K.N., Avella D.M., Kimchi E.T., Staveley-O’Carroll K.F., Deroche C.B., Pantel K., Li G., Kaifi J.T. Circulating giant tumor-macrophage fusion cells are independent prognosticators in non-small cell lung cancer patients. J. Thorac. Oncol. 2020 doi: 10.1016/j.jtho.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 107.Ramakrishnan M., Mathur S.R., Mukhopadhyay A. Fusion-derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer Res. 2013;73:5360–5370. doi: 10.1158/0008-5472.CAN-13-0896. [DOI] [PubMed] [Google Scholar]
- 108.Clawson G.A., Matters G.L., Xin P., Imamura-Kawasawa Y., Du Z., Thiboutot D.M., Helm K.F., Neves R.I., Abraham T. Macrophage-Tumor Cell Fusions from Peripheral Blood of Melanoma Patients. PLoS ONE. 2015;10:e0134320. doi: 10.1371/journal.pone.0134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andersen T.L., Boissy P., Sondergaard T.E., Kupisiewicz K., Plesner T., Rasmussen T., Haaber J., Kolvraa S., Delaisse J.M. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: A new type of cancer-host partnership? J. Pathol. 2007;211:10–17. doi: 10.1002/path.2078. [DOI] [PubMed] [Google Scholar]
- 110.Duelli D.M., Hearn S., Myers M.P., Lazebnik Y. A primate virus generates transformed human cells by fusion. J. Cell Biol. 2005;171:493–503. doi: 10.1083/jcb.200507069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duelli D.M., Padilla-Nash H.M., Berman D., Murphy K.M., Ried T., Lazebnik Y. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr. Biol. 2007;17:431–437. doi: 10.1016/j.cub.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 112.Zhou X., Merchak K., Lee W., Grande J.P., Cascalho M., Platt J.L. Cell Fusion Connects Oncogenesis with Tumor Evolution. Am. J. Pathol. 2015;185:2049–2060. doi: 10.1016/j.ajpath.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 114.Abdullahi S., Jäkel M., Behrend S.J., Steiger K., Topping G., Krabbe T., Colombo A., Sandig V., Schiergens T.S., Thasler W.E., et al. A Novel Chimeric Oncolytic Virus Vector for Improved Safety and Efficacy as a Platform for the Treatment of Hepatocellular Carcinoma. J. Virol. 2018;92 doi: 10.1128/JVI.01386-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krabbe T., Altomonte J. Fusogenic Viruses in Oncolytic Immunotherapy. Cancers. 2018;10:216. doi: 10.3390/cancers10070216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burton C., Bartee E. Syncytia Formation in Oncolytic Virotherapy. Mol. Ther. Oncolytics. 2019;15:131–139. doi: 10.1016/j.omto.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leikina E., Melikov K., Sanyal S., Verma S.K., Eun B., Gebert C., Pfeifer K., Lizunov V.A., Kozlov M.M., Chernomordik L.V. Extracellular annexins and dynamin are important for sequential steps in myoblast fusion. J. Cell Biol. 2012;200:109–123. doi: 10.1083/jcb.201207012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abmayr S.M., Pavlath G.K. Myoblast fusion: Lessons from flies and mice. Development. 2012;139:641–656. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Horsley V., Pavlath G.K. Forming a multinucleated cell: Molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 120.Simionescu A., Pavlath G.K. Molecular Mechanisms of Myoblast Fusion Across Species. Adv. Exp. Med. Biol. 2011;713:113–135. doi: 10.1007/978-94-007-0763-4_8. [DOI] [PubMed] [Google Scholar]
- 121.Novick S.L., Hoekstra D. Membrane penetration of Sendai virus glycoproteins during the early stages of fusion with liposomes as determined by hydrophobic photoaffinity labeling. Proc. Natl. Acad. Sci. USA. 1988;85:7433–7437. doi: 10.1073/pnas.85.20.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moss B. Membrane fusion during poxvirus entry. Semin. Cell Dev. Biol. 2016;60:89–96. doi: 10.1016/j.semcdb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 124.Hu L., Ceresa B.P. Characterization of the plasma membrane localization and orientation of HPV16 E5 for cell–cell fusion. Virology. 2009;393:135–143. doi: 10.1016/j.virol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu L., Plafker K., Vorozhko V., Zuna R.E., Hanigan M.H., Gorbsky G.J., Plafker S.M., Angeletti P.C., Ceresa B.P. Human papillomavirus 16 E5 induces bi-nucleated cell formation by cell–cell fusion. Virology. 2009;384:125–134. doi: 10.1016/j.virol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.French C.T., Toesca I.J., Wu T.-H., Teslaa T., Beaty S.M., Wong W., Liu M., Schröder I., Chiou P.-Y., Teitell M.A., et al. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc. Natl. Acad. Sci. USA. 2011;108:12095–12100. doi: 10.1073/pnas.1107183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kespichayawattana W., Rattanachetkul S., Wanun T., Utaisincharoen P., Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: A possible mechanism for cell-to-cell spreading. Infect. Immun. 2000;68:5377–5384. doi: 10.1128/IAI.68.9.5377-5384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whiteley L., Meffert T., Haug M., Weidenmaier C., Hopf V., Bitschar K., Schittek B., Kohler C., Steinmetz I., West T.E., et al. Entry, Intracellular Survival, and Multinucleated-Giant-Cell-Forming Activity of Burkholderia pseudomallei in Human Primary Phagocytic and Nonphagocytic Cells. Infect. Immun. 2017;85:e00468-17. doi: 10.1128/IAI.00468-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ku J.W.K., Chen Y., Lim B.J.W., Gasser S., Crasta K.C., Gan Y.-H. Bacterial-induced cell fusion is a danger signal triggering cGAS–STING pathway via micronuclei formation. Proc. Natl. Acad. Sci. USA. 2020;117:15923–15934. doi: 10.1073/pnas.2006908117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwarz S., Singh P., Robertson J.D., Leroux M., Skerrett S.J., Goodlett D.R., West T.E., Mougous J.D. VgrG-5 is a burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect. Immun. 2014;82:1445–1452. doi: 10.1128/IAI.01368-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toesca I.J., French C.T., Miller J.F. The type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect. Immun. 2014;82:1436–1444. doi: 10.1128/IAI.01367-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Segel M., Lash B., Song J., Ladha A., Liu C.C., Jin X., Mekhedov S.L., Macrae R.K., Koonin E.V., Zhang F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373:882–889. doi: 10.1126/science.abg6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kingwell K. Hitting SEND on mRNA delivery. Nat. Rev. Drug Discov. 2021;20:738. doi: 10.1038/d41573-021-00146-z. [DOI] [PubMed] [Google Scholar]
- 134.Melzer C., Rehn V., Yang Y., Bähre H., Von Der Ohe J., Hass R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers. 2019;11:798. doi: 10.3390/cancers11060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luo T., von der Ohe J., Hass R. MSC-Derived Extracellular Vesicles in Tumors and Therapy. Cancers. 2021;13:5212. doi: 10.3390/cancers13205212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Parseval N., Heidmann T. Human endogenous retroviruses: From infectious elements to human genes. Cytogenet. Genome Res. 2005;110:318–332. doi: 10.1159/000084964. [DOI] [PubMed] [Google Scholar]
- 137.Bannert N., Kurth R. Retroelements and the human genome: New perspectives on an old relation. Proc. Natl. Acad. Sci. USA. 2004;101:14572–14579. doi: 10.1073/pnas.0404838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Katoh I., Kurata S. Association of endogenous retroviruses and long terminal repeats with human disorders. Front. Oncol. 2013;3:234. doi: 10.3389/fonc.2013.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meyer T.J., Rosenkrantz J., Carbone L., Chavez S. Endogenous Retroviruses: With Us and against Us. Front. Chem. 2017;5:23. doi: 10.3389/fchem.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dupressoir A., Marceau G., Vernochet C., Benit L., Kanellopoulos C., Sapin V., Heidmann T. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc. Natl. Acad. Sci. USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Heidmann O., Vernochet C., Dupressoir A., Heidmann T. Identification of an endogenous retroviral envelope gene with fusogenic activity and placenta-specific expression in the rabbit: A new “syncytin” in a third order of mammals. Retrovirology. 2009;6:107. doi: 10.1186/1742-4690-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cornelis G., Heidmann O., Bernard-Stoecklin S., Reynaud K., Veron G., Mulot B., Dupressoir A., Heidmann T. Ancestral capture of syncytin-Car1, a fusogenic endogenous retroviral envelope gene involved in placentation and conserved in Carnivora. Proc. Natl. Acad. Sci. USA. 2012;109:E432–E441. doi: 10.1073/pnas.1115346109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cornelis G., Heidmann O., Degrelle S., Vernochet C., Lavialle C., Letzelter C., Bernard-Stoecklin S., Hassanin A., Mulot B., Guillomot M., et al. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc. Natl. Acad. Sci. USA. 2013;110:E828–E837. doi: 10.1073/pnas.1215787110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cornelis G., Vernochet C., Carradec Q., Souquere S., Mulot B., Catzeflis F., Nilsson M.A., Menzies B., Renfree M., Pierron G., et al. Retroviral envelope gene captures andsyncytinexaptation for placentation in marsupials. Proc. Natl. Acad. Sci. USA. 2015;112:E487–E496. doi: 10.1073/pnas.1417000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Friedli M., Trono D. The developmental control of transposable elements and the evolution of higher species. Annu. Rev. Cell Dev. Biol. 2015;31:429–451. doi: 10.1146/annurev-cellbio-100814-125514. [DOI] [PubMed] [Google Scholar]
- 146.Wang J., Xie G., Singh M., Ghanbarian A.T., Raskó T., Szvetnik A., Cai H., Besser D., Prigione A., Fuchs N.V., et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature. 2014;516:405–409. doi: 10.1038/nature13804. [DOI] [PubMed] [Google Scholar]
- 147.Fuchs N.V., Loewer S., Daley G.Q., Izsvák Z., Löwer J., Löwer R. Human endogenous retrovirus K (HML-2) RNA and protein expression is a marker for human embryonic and induced pluripotent stem cells. Retrovirology. 2013;10:115. doi: 10.1186/1742-4690-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grow E.J., Flynn R.A., Chavez S.L., Bayless N.L., Wossidlo M., Wesche D.J., Martin L., Ware C.B., Blish C.A., Chang H.Y., et al. Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells. Nat. Cell Biol. 2015;522:221–225. doi: 10.1038/nature14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pontis J., Planet E., Offner S., Turelli P., Duc J., Coudray A., Theunissen T.W., Jaenisch R., Trono D. Hominoid-Specific Transposable Elements and KZFPs Facilitate Human Embryonic Genome Activation and Control Transcription in Naive Human ESCs. Cell Stem Cell. 2019;24:724–735.e5. doi: 10.1016/j.stem.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Göke J., Lu X., Chan Y.-S., Ng H.-H., Ly L.-H., Sachs F., Szczerbinska I. Dynamic transcription of distinct classes of endogenous retroviral elements marks specific populations of early human embryonic cells. Cell Stem Cell. 2015;16:135–141. doi: 10.1016/j.stem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 151.Engel K., Wieland L., Krüger A., Volkmer I., Cynis H., Emmer A., Staege M.S. Identification of Differentially Expressed Human Endogenous Retrovirus Families in Human Leukemia and Lymphoma Cell Lines and Stem Cells. Front. Oncol. 2021;11:637981. doi: 10.3389/fonc.2021.637981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Matteucci C., Balestrieri E., Argaw-Denboba A., Sinibaldi-Vallebona P. Human endogenous retroviruses role in cancer cell stemness. Semin. Cancer Biol. 2018;53:17–30. doi: 10.1016/j.semcancer.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 153.Wang-Johanning F., Frost A.R., Jian B., Azerou R., Lu D.W., Chen D.-T., Johanning G.L. Detecting the expression of human endogenous retrovirus Eenvelope transcripts in human prostate adenocarcinoma. Cancer. 2003;98:187–197. doi: 10.1002/cncr.11451. [DOI] [PubMed] [Google Scholar]
- 154.Wang-Johanning F., Liu J., Rycaj K., Huang M., Tsai K., Rosen D.G., Chen D.-T., Lu D.W., Barnhart K.F., Johanning G.L. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int. J. Cancer. 2007;120:81–90. doi: 10.1002/ijc.22256. [DOI] [PubMed] [Google Scholar]
- 155.Cherkasova E., Scrivani C., Doh S., Weisman Q., Takahashi Y., Harashima N., Yokoyama H., Srinivasan R., Linehan W.M., Lerman M.I., et al. Detection of an Immunogenic HERV-E Envelope with Selective Expression in Clear Cell Kidney Cancer. Cancer Res. 2016;76:2177–2185. doi: 10.1158/0008-5472.CAN-15-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Talotta R., Atzeni F., Laska M.J. The contribution of HERV-E clone 4-1 and other HERV-E members to the pathogenesis of rheumatic autoimmune diseases. APMIS. 2020;128:367–377. doi: 10.1111/apm.13039. [DOI] [PubMed] [Google Scholar]
- 157.Wang X., Zhao C., Zhang C., Mei X., Song J., Sun Y., Wu Z., Shi W. Increased HERV-E clone 4–1 expression contributes to DNA hypomethylation and IL-17 release from CD4(+) T cells via miR-302d/MBD2 in systemic lupus erythematosus. Cell Commun. Signal. 2019;17:1–11. doi: 10.1186/s12964-019-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bhetariya P.J., Kriesel J.D., Fischer K.F. Analysis of Human Endogenous Retrovirus Expression in Multiple Sclerosis Plaques. J. Emerg. Dis. Virol. 2017;3 doi: 10.16966/2473-1846.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bergallo M., Dapra V., Ponti R., Quaglino P., Novelli M., Fava P., Montanari P., Calvi C., Galliano I., Fierro M. HERV-E expression in peripheral mononuclear cells of patients with psoriasis. Ital. J. Dermatol. Venereol. 2021;156:42–45. doi: 10.23736/S2784-8671.18.06133-3. [DOI] [PubMed] [Google Scholar]
- 160.Sugimoto J., Sugimoto M., Bernstein H., Jinno Y., Schust D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci. Rep. 2013;3:1462. doi: 10.1038/srep01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin L., Xu B., Rote N.S. Expression of endogenous retrovirus ERV-3 induces differentiation in BeWo, a choriocarcinoma model of human placental trophoblast. Placenta. 1999;20:109–118. doi: 10.1053/plac.1998.0337. [DOI] [PubMed] [Google Scholar]
- 162.Kämmerer U., Germeyer A., Stengel S., Kapp M., Denner J. Human endogenous retrovirus K (HERV-K) is expressed in villous and extravillous cytotrophoblast cells of the human placenta. J. Reprod. Immunol. 2011;91:1–8. doi: 10.1016/j.jri.2011.06.102. [DOI] [PubMed] [Google Scholar]
- 163.Robinson-McCarthy L.R., McCarthy K.R., Raaben M., Piccinotti S., Nieuwenhuis J., Stubbs S.H., Bakkers M.J.G., Whelan S.P.J. Reconstruction of the cell entry pathway of an extinct virus. PLOS Pathog. 2018;14:e1007123. doi: 10.1371/journal.ppat.1007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yi J.-M., Kim H.-S. Molecular phylogenetic analysis of the human endogenous retrovirus E (HERV-E) family in human tissues and human cancers. Genes Genet. Syst. 2007;82:89–98. doi: 10.1266/ggs.82.89. [DOI] [PubMed] [Google Scholar]
- 165.Parris G.E. The role of viruses in cell fusion and its importance to evolution, invasion and metastasis of cancer clones. Med. Hypotheses. 2005;64:1011–1014. doi: 10.1016/j.mehy.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 166.Bastida-Ruiz D., Van Hoesen K., Cohen M. The Dark Side of Cell Fusion. Int. J. Mol. Sci. 2016;17:638. doi: 10.3390/ijms17050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hass R., von der Ohe J., Dittmar T. Hybrid Formation and Fusion of Cancer Cells In Vitro and In Vivo. Cancers. 2021;13:4496. doi: 10.3390/cancers13174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hass R., von der Ohe J., Dittmar T. Cancer Cell Fusion and Post-Hybrid Selection Process (PHSP) Cancers. 2021;13:4636. doi: 10.3390/cancers13184636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koulakov A.A., Lazebnik Y. The Problem of Colliding Networks and its Relation to Cell Fusion and Cancer. Biophys. J. 2012;103:2011–2020. doi: 10.1016/j.bpj.2012.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lu X., Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mirzayans R., Murray D. Intratumor Heterogeneity and Therapy Resistance: Contributions of Dormancy, Apoptosis Reversal (Anastasis) and Cell Fusion to Disease Recurrence. Int. J. Mol. Sci. 2020;21:1308. doi: 10.3390/ijms21041308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weiler J., Dittmar T. Cell Fusion in Human Cancer: The Dark Matter Hypothesis. Cells. 2019;8:132. doi: 10.3390/cells8020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Omata W., Iv W.E.A., Vandré D.D., Robinson J.M. Trophoblast cell fusion and differentiation are mediated by both the protein kinase C and a pathways. PLoS ONE. 2013;8:e81003. doi: 10.1371/journal.pone.0081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mansilla M., Wang Y., Lim R., Palmer K., Nie G. HtrA4 is up-regulated during trophoblast syncytialization and BeWo cells fail to syncytialize without HtrA4. Sci. Rep. 2021;11:14363. doi: 10.1038/s41598-021-93520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Noubissi F.K., Harkness T., Alexander C.M., Ogle B.M. Apoptosis-induced cancer cell fusion: A mechanism of breast cancer metastasis. FASEB J. 2015;29:4036–4045. doi: 10.1096/fj.15-271098. [DOI] [PubMed] [Google Scholar]
- 176.Gao Y., Yu X.-F., Chen T. Human endogenous retroviruses in cancer: Expression, regulation and function. Oncol. Lett. 2021;21:121. doi: 10.3892/ol.2020.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Büscher K., Trefzer U., Hofmann M., Sterry W., Kurth R., Denner J. Expression of human endogenous retrovirus K in melanomas and melanoma cell lines. Cancer Res. 2005;65:4172–4180. doi: 10.1158/0008-5472.CAN-04-2983. [DOI] [PubMed] [Google Scholar]
- 178.Schmitt K., Reichrath J., Roesch A., Meese E., Mayer J. Transcriptional profiling of human endogenous retrovirus group HERV-K(HML-2) loci in melanoma. Genome Biol. Evol. 2013;5:307–328. doi: 10.1093/gbe/evt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Golan M., Hizi A., Resau J.H., Yaal-Hahoshen N., Reichman H., Keydar I., Tsarfaty I. Human endogenous retrovirus (HERV-K) reverse transcriptase as a breast cancer prognostic marker. Neoplasia. 2008;10:521-IN2. doi: 10.1593/neo.07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang-Johanning F., Frost A.R., Jian B., Epp L., Lu D.W., Johanning G.L. Quantitation of HERV-K env gene expression and splicing in human breast cancer. Oncogene. 2003;22:1528–1535. doi: 10.1038/sj.onc.1206241. [DOI] [PubMed] [Google Scholar]
- 181.Agoni L., Lenz J., Guha C. Variant splicing and influence of ionizing radiation on human endogenous retrovirus K (HERV-K) transcripts in cancer cell lines. PLoS ONE. 2013;8:e76472. doi: 10.1371/journal.pone.0076472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang-Johanning F., Radvanyi L., Rycaj K., Plummer J.B., Yan P., Sastry K.J., Piyathilake C.J., Hunt K.K., Johanning G.L. Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer Res. 2008;68:5869–5877. doi: 10.1158/0008-5472.CAN-07-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lower R., Boller K., Hasenmaier B., Korbmacher C., Muller-Lantzsch N., Lower J., Kurth R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc. Natl. Acad. Sci. USA. 1993;90:4480–4484. doi: 10.1073/pnas.90.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boller K., König H., Sauter M., Mueller-Lantzsch N., Löwer R., Löwer J., Kurth R. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 1993;196:349–353. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- 185.Sauter M., Roemer K., Best B., Afting M., Schommer S., Seitz G., Hartmann M., Mueller-Lantzsch N. Specificity of antibodies directed against Env protein of human endogenous retroviruses in patients with germ cell tumors. Cancer Res. 1996;56:4362–4365. [PubMed] [Google Scholar]
- 186.Agoni L., Guha C., Lenz J. Detection of Human Endogenous Retrovirus K (HERV-K) Transcripts in Human Prostate Cancer Cell Lines. Front. Oncol. 2013;3:180. doi: 10.3389/fonc.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weyerer V., Strissel P.L., Stöhr C., Eckstein M., Wach S., Taubert H., Brandl L., Geppert C.I., Wullich B., Cynis H., et al. Endogenous Retroviral–K Envelope Is a Novel Tumor Antigen and Prognostic Indicator of Renal Cell Carcinoma. Front. Oncol. 2021;11:657187. doi: 10.3389/fonc.2021.657187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lemaître C., Tsang J., Bireau C., Heidmann T., Dewannieux M. A human endogenous retrovirus-derived gene that can contribute to oncogenesis by activating the ERK pathway and inducing migration and invasion. PLoS Pathog. 2017;13:e1006451. doi: 10.1371/journal.ppat.1006451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhou F., Li M., Wei Y., Lin K., Lu Y., Shen J., Johanning G.L., Wang-Johanning F. Activation of HERV-K Env protein is essential for tumorigenesis and metastasis of breast cancer cells. Oncotarget. 2016;7:84093–84117. doi: 10.18632/oncotarget.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jin X., Xu X.E., Jiang Y.Z., Liu Y.R., Sun W., Guo Y.J., Ren Y.X., Zuo W.J., Hu X., Huang S.L., et al. The endogenous retrovirus-derived long noncoding RNA TROJAN promotes triple-negative breast cancer progression via ZMYND8 degradation. Sci. Adv. 2019;5:eaat9820. doi: 10.1126/sciadv.aat9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou Y., Liu L., Liu Y., Zhou P., Yan Q., Yu H., Chen X., Zhu F. Implication of human endogenous retrovirus W family envelope in hepatocellular carcinoma promotes MEK/ERK-mediated metastatic invasiveness and doxorubicin resistance. Cell Death Discov. 2021;7:177. doi: 10.1038/s41420-021-00562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li M., Radvanyi L., Yin B., Rycaj K., Li J., Chivukula R., Lin K., Lu Y., Shen J., Chang D.Z., et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin. Cancer Res. 2017;23:5892–5911. doi: 10.1158/1078-0432.CCR-17-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen T., Meng Z., Gan Y., Wang X., Xu F., Gu Y., Xu X., Tang J., Zhou H., Zhang X., et al. The viral oncogene Np9 acts as a critical molecular switch for co-activating β-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia. 2013;27:1469–1478. doi: 10.1038/leu.2013.8. [DOI] [PubMed] [Google Scholar]
- 194.Díaz-Carballo D., Saka S., Klein J., Rennkamp T., Acikelli A.H., Malak S., Jastrow H., Wennemuth G., Tempfer C., Schmitz I., et al. A Distinct Oncogenerative Multinucleated Cancer Cell Serves as a Source of Stemness and Tumor Heterogeneity. Cancer Res. 2018;78:2318–2331. doi: 10.1158/0008-5472.CAN-17-1861. [DOI] [PubMed] [Google Scholar]
- 195.Tse G.M., Law B.K., Chan K.F., Mas T.K. Multinucleated stromal giant cells in mammary phyllodes tumours. Pathology. 2001;33:153–156. doi: 10.1080/00313020123549. [DOI] [PubMed] [Google Scholar]
- 196.Zhang Z., Feng X., Deng Z., Cheng J., Wang Y., Zhao M., Zhao Y., He S., Huang Q. Irradiation-induced polyploid giant cancer cells are involved in tumor cell repopulation via neosis. Mol. Oncol. 2021;15:2219–2234. doi: 10.1002/1878-0261.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bojko A., Staniak K., Czarnecka-Herok J., Sunderland P., Dudkowska M., Śliwińska M.A., Salmina K., Sikora E. Improved Autophagic Flux in Escapers from Doxorubicin-Induced Senescence/Polyploidy of Breast Cancer Cells. Int. J. Mol. Sci. 2020;21:6084. doi: 10.3390/ijms21176084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Erenpreisa J., Cragg M.S. Three steps to the immortality of cancer cells: Senescence, polyploidy and self-renewal. Cancer Cell Int. 2013;13:92. doi: 10.1186/1475-2867-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Erenpreisa J., Kalejs M., Ianzini F., Kosmacek E.A., Mackey M.A., Emzinsh D., Cragg M.S., Ivanov A., Illidge T.M. Segregation of genomes in polyploid tumour cells following mitotic catastrophe. Cell Biol. Int. 2005;29:1005–1011. doi: 10.1016/j.cellbi.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 200.Gerstein A.C., Fu M.S., Mukaremera L., Li Z., Ormerod K.L., Fraser J.A., Berman J., Nielsen K. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio. 2015;6:e01340-15. doi: 10.1128/mBio.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mercapide J., Anzanello F., Rappa G., Lorico A. Relationship between tumor cell invasiveness and polyploidization. PLoS ONE. 2012;7:e53364. doi: 10.1371/journal.pone.0053364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pandit S., Westendorp B., de Bruin A. Physiological significance of polyploidization in mammalian cells. Trends Cell Biol. 2013;23:556–566. doi: 10.1016/j.tcb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 203.Puig P.E., Guilly M.N., Bouchot A., Droin N., Cathelin D., Bouyer F., Favier L., Ghiringhelli F., Kroemer G., Solary E. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008;32:1031–1043. doi: 10.1016/j.cellbi.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 204.Was H., Borkowska A., Olszewska A., Klemba A., Marciniak M., Synowiec A., Kieda C. Polyploidy formation in cancer cells: How a Trojan horse is born. Semin. Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 205.Zhang S., Mercado-Uribe I., Xing Z., Sun B., Kuang J., Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2013;33:116–128. doi: 10.1038/onc.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Herbein G., Nehme Z. Polyploid Giant Cancer Cells, a Hallmark of Oncoviruses and a New Therapeutic Challenge. Front. Oncol. 2020;10:567116. doi: 10.3389/fonc.2020.567116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang J., Qiao Q., Xu H., Zhou R., Liu X. Human Cell Polyploidization: The Good and the Evil. Semin. Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 208.Frye L.D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J. Cell Sci. 1970;7:319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- 209.Zur Hausen H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J. Natl. Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 210.Ljubojevic S., Skerlev M. HPV-associated diseases. Clin. Dermatol. 2014;32:227–234. doi: 10.1016/j.clindermatol.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 211.Münger K., Baldwin A., Edwards K.M., Hayakawa H., Nguyen C.L., Owens M., Grace M., Huh K. Mechanisms of Human Papillomavirus-Induced Oncogenesis. J. Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Duensing S., Münger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J. Virol. 2003;77:12331–12335. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Thompson S.L., Compton D.A. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J. Cell Biol. 2010;188:369–381. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Soto M., Raaijmakers J.A., Bakker B., Spierings D.C.J., Lansdorp P.M., Foijer F., Medema R.H. p53 Prohibits Propagation of Chromosome Segregation Errors that Produce Structural Aneuploidies. Cell Rep. 2017;19:2423–2431. doi: 10.1016/j.celrep.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 215.Ohashi A., Ohori M., Iwai K., Nakayama Y., Nambu T., Morishita D., Kawamoto T., Miyamoto M., Hirayama T., Okaniwa M., et al. Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat. Commun. 2015;6:7668. doi: 10.1038/ncomms8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fujiwara T., Bandi M., Nitta M., Ivanova E.V., Bronson R.T., Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 217.Foijer F., Xie S.Z., Simon J.E., Bakker P.L., Conte N., Davis S.H., Kregel E., Jonkers J., Bradley A., Sorger P.K. Chromosome instability induced by Mps1 and p53 mutation generates aggressive lymphomas exhibiting aneuploidy-induced stress. Proc. Natl. Acad. Sci. USA. 2014;111:13427–13432. doi: 10.1073/pnas.1400892111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Michel L., Díaz-Rodriguez E., Narayan G., Hernando E., Murty V.V., Benezra R. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc. Natl. Acad. Sci. USA. 2004;101:4459–4464. doi: 10.1073/pnas.0306069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pietropaolo V., Prezioso C., Moens U. Role of Virus-Induced Host Cell Epigenetic Changes in Cancer. Int. J. Mol. Sci. 2021;22:8346. doi: 10.3390/ijms22158346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Scheller C., Jassoy C. Syncytium formation amplifies apoptotic signals: A new view on apoptosis in HIV infection in vitro. Virology. 2001;282:48–55. doi: 10.1006/viro.2000.0811. [DOI] [PubMed] [Google Scholar]
- 221.Salsman J., Top D., Boutilier J., Duncan R. Extensive syncytium formation mediated by the reovirus FAST proteins triggers apoptosis-induced membrane instability. J. Virol. 2005;79:8090–8100. doi: 10.1128/JVI.79.13.8090-8100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lin E.-H., Salon C., Brambilla E., Lavillette D., Szecsi J., Cosset F.-L., Coll J.-L. Fusogenic membrane glycoproteins induce syncytia formation and death in vitro and in vivo: A potential therapy agent for lung cancer. Cancer Gene Ther. 2010;17:256–265. doi: 10.1038/cgt.2009.74. [DOI] [PubMed] [Google Scholar]
- 223.Higuchi H., Bronk S.F., Bateman A., Harrington K., Vile R.G., Gores G.J. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: Implications for gene therapy. Cancer Res. 2000;60:6396–6402. [PubMed] [Google Scholar]
- 224.Tan L., Jia H., Liu R., Wu J., Han H., Zuo Y., Yang S., Huang W. Inhibition of NF-κB in fusogenic membrane glycoprotein causing HL-60 cell death: Implications for acute myeloid leukemia. Cancer Lett. 2009;273:114–121. doi: 10.1016/j.canlet.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 225.Guedan S., Grases D., Rojas J.J., Gros A., Vilardell F., Vile R., Mercade E., Cascallo M., Alemany R. GALV expression enhances the therapeutic efficacy of an oncolytic adenovirus by inducing cell fusion and enhancing virus distribution. Gene Ther. 2012;19:1048–1057. doi: 10.1038/gt.2011.184. [DOI] [PubMed] [Google Scholar]
- 226.Perfettini J.-L., Castedo M., Roumier T., Andreau K., Nardacci R., Piacentini M., Kroemer G. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12((Suppl. S1)):916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- 227.Pandey S., Singh S., Anang V., Bhatt A.N., Natarajan K., Dwarakanath B.S. Pattern Recognition Receptors in Cancer Progression and Metastasis. Cancer Growth Metastasis. 2015;8:25–34. doi: 10.4137/CGM.S24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Holm C.K., Jensen S.B., Jakobsen M.R., Cheshenko N., Horan K.A., Moeller H.B., Gonzalez-Dosal R., Rasmussen S.B., Christensen M.H., Yarovinsky T.O., et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 2012;13:737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Herschke F., Plumet S., Duhen T., Azocar O., Druelle J., Laine D., Wild T.F., Rabourdin-Combe C., Gerlier D., Valentin H. Cell-cell fusion induced by measles virus amplifies the type i interferon response. J. Virol. 2007;81:12859–12871. doi: 10.1128/JVI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bateman A.R., Harrington K.J., Kottke T., Ahmed A., Melcher A.A., Gough M.J., Linardakis E., Riddle D., Dietz A., Lohse C.M., et al. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 2002;62:6566–6578. [PubMed] [Google Scholar]
- 231.Errington F., Jones J., Merrick A., Bateman A., Harrington K., Gough M., O’Donnell D., Selby P., Vile R., Melcher A. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Ther. 2006;13:138–149. doi: 10.1038/sj.gt.3302609. [DOI] [PubMed] [Google Scholar]
- 232.Clawson G.A. Fusion for moving. Science. 2013;342:699–700. doi: 10.1126/science.1244270. [DOI] [PubMed] [Google Scholar]
- 233.Parris G.E. Historical perspective of cell-cell fusion in cancer initiation and progression. Crit. Rev. Oncog. 2013;18:1–18. doi: 10.1615/CritRevOncog.v18.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 234.Hanks S., Coleman K., Reid S., Plaja A., Firth H., Fitzpatrick D., Kidd A., Méhes K., Nash R., Robin N., et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 2004;36:1159–1161. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- 235.Adderson E., Branum K., Sealy R.E., Jones B.G., Surman S.L., Penkert R., Freiden P., Slobod K.S., Gaur A.H., Hayden R.T., et al. Safety and immunogenicity of an intranasal Sendai virus-based human parainfluenza virus type 1 vaccine in 3- to 6-year-old children. Clin. Vaccine Immunol. 2015;22:298–303. doi: 10.1128/CVI.00618-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Slobod K.S., Shenep J.L., Luján-Zilbermann J., Allison K., Brown B., Scroggs R.A., Portner A., Coleclough C., Hurwitz J.L. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22:3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 237.Miroshnychenko D., Baratchart E., Ferrall-Fairbanks M.C., Velde R.V., Laurie M.A., Bui M.M., Tan A.C., Altrock P.M., Basanta D., Marusyk A. Spontaneous cell fusions as a mechanism of parasexual recombination in tumour cell populations. Nat. Ecol. Evol. 2021;5:379–391. doi: 10.1038/s41559-020-01367-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.