Abstract
The pathway by which atypical protein kinase C (aPKC) contributes to nerve growth factor (NGF) signaling is poorly understood. We previously reported that in PC12 cells NGF-induced activation of mitogen-activated protein kinase (MAPK) occurs independently of classical and nonclassical PKC isoforms, whereas aPKC isoforms were shown to be required for NGF-induced differentiation. NGF-induced activation of PKC-ι was observed to be dependent on phosphatidylinositol 3-kinase (PI3K) and led to coassociation of PKC-ι with Ras and Src. Expression of dominant negative mutants of either Src (DN2) or Ras (Asn-17) impaired activation of PKC-ι by NGF. At the level of Raf-1, neither PKC-ι nor PI3 kinase was required for activation; however, PKC-ι could weakly activate MEK. Inhibitors of PKC-ι activity and PI3K had no effect on NGF-induced MAPK or p38 activation but reduced NGF-stimulated c-Jun N-terminal kinase activity. Src, PI3K, and PKC-ι were likewise required for NGF-induced NF-κB activation and cell survival, whereas Ras was not required for either survival or NF-κB activation but was required for differentiation. IKK existed as a complex with PKC-ι, Src and IκB. Consistent with a role for Src in regulating NF-κB activation, an absence of Src activity impaired recruitment of PKC-ι into an IKK complex and markedly impaired NGF-induced translocation of p65/NF-κB to the nucleus. These findings reveal that in PC12 cells, aPKCs comprise a molecular switch to regulate differentiation and survival responses coupled downstream to NF-κB. On the basis of these findings, Src emerges as a critical upstream regulator of both PKC-ι and the NF-κB pathway.
The pheochromocytoma cell line PC12 is a well-utilized model for study of neurotrophic factors such as nerve growth factor (NGF). Treatment of these cells with NGF induces differentiation and survival. The NGF signaling cascade begins with the sequential action of a Src-Ras cassette (26), leading to the activation of mitogen-activated protein kinase (MAPK). Inhibition of MEK, the upstream MAPK kinase, blocks NGF-induced differentiation (43), thus suggesting that MAPK plays a critical role in cell differentiation. However, MAPK activation is not absolutely required for differentiation of PC12 cells, since bone morphogenic protein 2 can induce differentiation in the absence of MAPK activation (18). NGF also leads to the activation of both the p38 kinase (39) and c-Jun N-terminal kinase (JNK) (17, 38). In addition, phosphatidylinositol 3-kinase (PI3K) is activated and required for NGF-mediated differentiation (19, 22, 25). PI3K is also required for NGF survival signaling (59).
The protein kinase C (PKC) superfamily, composed of 11 isoforms (51), has been implicated in mediating NGF responses as well. PC12 cells express all 11 isoforms of PKC, and each is activated in response to NGF (56, 57), implying that each plays a role in mediating NGF responses. Inhibition of PKC by sphingosine blocks NGF-induced neurite outgrowth (16), and microinjection of PKC antibodies inhibits NGF-induced neurite outgrowth and c-Fos expression (3). However, downregulation of PKC with chronic phorbol ester treatment, resulting in removal of classical and nonclassical PKC (cPKC and nPKC) pools, has no effect on NGF-induced neurite outgrowth (46) or NGF-induced MAPK activation (33). We demonstrated that the phorbol ester-sensitive PKC isoforms (α, β, γ, δ, and ɛ) were not required for NGF differentiation and further demonstrated that NGF activated the phorbol ester-insensitive atypical PKC (aPKC) isoforms, ι/λ and ζ (9, 56). Moreover, removal of aPKCs was observed to block NGF-induced differentiation of PC12 cells only in the absence of other PKCs, demonstrating a hierarchal relationship between aPKCs and other PKC isoforms activated by NGF (9). Recently, FEZ1 (fasciculation and elongation protein zeta 1), a brain-specific transcript which is the mammalian homologue of UNC-76, a protein involved in axonal outgrowth and fasciculation in Caenorhabditis elegans, has been identified as a PKC-ζ binding protein whose expression in PC12 cells stimulates differentiation (28). These findings further underscore the importance of aPKC in control of neuronal responses.
Overexpression of aPKCs enhances NGF responsiveness as well as survival of differentiated cells through an NF-κB pathway (58). These findings are in keeping with the ability of aPKCs to bind IKKβ and directly regulate the κB pathway (29). Members of the aPKC subfamily comprised of iota/lambda and zeta isoforms are highly homologous. Furthermore, aPKCs are activated by a wortmannin-sensitive, PI3K-dependent pathway (2) involving phosphorylation of aPKC by PDK1 (31). Additionally, we have recently shown that aPKCs can bind Src, resulting in tyrosine phosphorylation of aPKC (49). Little is understood regarding the precise placement of the aPKCs in relation to known components of the NGF survival/differentiation signaling pathway(s). To broaden our understanding of aPKC's position in these pathways, we undertook a detailed study to map the relationship of PKC-ι to components of the Src-Ras/Raf-1/MAPK, p38, and JNK pathways utilizing NF-κB as a downstream target. In addition, the relationship of PI3K to this signal network, as well as its role in activation of aPKC, Ras-MAPKs, and NF-κB, was studied. We find that both Ras and Src lie upstream of aPKC, regulated by PI3K. In PC12 cells, neither aPKC nor PI3K is directly required for activation of MAPK. Furthermore, NGF-induced activation of NF-κB required Src. Our findings reveal that two discrete NGF signaling pathways are operative; differentiation employs the Ras-Src/aPKC cassette, whereas survival is restricted to the Src-PI3K/aPKC pathway. Atypical PKC-ι emerges as a component of both differentiation and survival signaling pathways regulated by a novel Src kinase pathway.
MATERIALS AND METHODS
Materials.
NGF was purchased from Harlan BioProducts for Science (Indianapolis, Ind.). The p38 inhibitor SB203580, the MEK inhibitor PD98059, Ras antibody, pseudosubstrate peptide to cPKC, and chelerythrine chloride were from Calbiochem (La Jolla, Calif.). The phosphospecific antibodies to MAPK, JNK, and p38 were obtained from New England Biolabs, Beverly, Mass. Raf-1, MEK, PKC-ι, IKK, and c-Jun (S-63, KM-1) antibodies and recombinant MEK were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif. Antibody to c-Src and p34 Src peptide substrate was obtained from Upstate Biotechnology, Lake Placid, N.Y. Both myristoylated and nonmyristoylated forms of PKC-ι pseudosubstrate peptide were synthesized by the Center for Macromolecular Structure, University of Kentucky, Lexington. LY294002 and wortmannin were purchased from BioMol, Indianapolis, Inc. NF-κB oligonucleotide was from Promega (Madison, Wis.). PC12 cells overexpressing c-Src under the control of the cytomegalovirus promoter (Src cells) and Src− cells (Src DN2 expressing the K295R mutant [kinase dead] form of chicken Src) were kindly provided by Simon Halegoua (Stony Brook, N.Y.). PC12 cells (M-M17-26) expressing the dominant inhibitory mutant RasN17, which interferes with normal Ras function, were provided by Geoffery Cooper (Harvard Medical School, Boston, Mass.). PC12 cells expressing a temperature-sensitive v-Src were provided by Gordon Guroff (National Institutes of Health). Jorge Moscat (Centro de Biología Molecular, Universidad Autonoma, Madrid, Spain) kindly provided aPKC constructs. Polyclonal antibody to JNK and purified glutathione S-transferase fused to amino acids 1 to 79 of c-Jun [GST-cJun(1-79)] was a gift from Guisheng Zhou, Baylor College of Medicine, Houston, Tex.
Cell culture.
PC12 cells were grown on collagen-coated plastic culture dishes in Dulbecco's modified Eagle's medium with 10% horse serum and 5% fetal calf serum, as described elsewhere (56–58). Prior to treatment with NGF, the cells were starved overnight by incubation in a ratio of 1 ml of conditioned medium to 5 ml of serum-free medium.
aPKC activity.
PC12 cells were incubated for different times with NGF, extracted in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM EGTA, protease inhibitors), and immunoprecipitated with affinity-purified rabbit polyclonal antibody (20). Immunoprecipitates were washed five times with lysis buffer containing 0.5 M NaCl. For immune complex kinase assay, the samples were resuspended in kinase buffer (35 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 0.5 mM EGTA, 0.1 mM CaCl2, 1 mM p-nitrophenyl phosphate) containing 1 μg of myelin basic protein (MBP) and 5 μCi (100 μM) of [γ-32P]ATP for 30 min at 30°C in a final volume of 40 μl. To assess the specificity for PKC-ι, assays were done in the presence or absence of 40 μM pseudosubstrate peptide (SIYRRGARRWRKL) or irrelevant control peptide (IETVDNKASTRAY), both preincubated for 5 min. Reactions were terminated by addition of concentrated sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by exposure to X-ray film and quantitation with a computer-interfaced densitometer. MBP phosphorylation was normalized to the amount of PKC-ι in each sample and thus expressed as specific activity in arbitrary units.
Coimmunoprecipitation of PKC-ι with Ras or Src.
The procedure used was essentially as previously described by Diaz-Meco et al. (11). PC12 cells were lysed with a mixture of 30 mM HEPES (pH 7.5), 1% Triton X-100, 10% glycerol, 10 mM NaCl, 5 mM MgCl2, 1 mM Na3VO4, 2 mM phenylmethylsulfonyl fluoride (PMSF), 25 mM NaF, 1 mM EGTA, and protease inhibitors. The lysates were immunoprecipitated by addition of pan-Ras antibody. Immune complexes were recovered by addition of protein A-agarose followed by incubation overnight at 4°C. The beads were washed twice with 20 mM Tris-HCl (pH 7.5)–500 mM NaCl–1% Triton X-100–0.1% β-mercaptoethanol, followed by a final wash in buffer containing 10 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 25 mM NaF, 100 μM Na3VO4, leupeptin (20 μg/ml), pepstatin A (1 μg/ml), and aprotinin (4 μg/ml). The immune complexes were separated by SDS-PAGE on a 12% gel, transferred to nitrocellulose, and Western blotted with PKC-ι, Ras, or Src antibody; proteins were identified by enhanced chemiluminescence.
Purification of aPKCs.
In brief, Sf9 cells were infected with recombinant virus. The cells were lysed in a mixture of 20 mM Tris-HCl (pH 7.5), 5 mM EDTA, 43 mM β-mercaptoethanol, 1% Triton X-100, PMSF, and protease inhibitors. The lysate was clarified by centrifugation at 10,000 × g for 30 min, and aPKC was purified as previously described (60).
In vitro phosphorylation of MEK-1.
Phosphorylation of MEK was conducted using immunoprecipitated Raf-1 to which purified PKC-ι was added at various concentrations. The reactions were conducted in the presence or absence of PKC-ι inhibitor pseudosubstrate peptide (SIYRRGARRWRKL). Kinase reactions were performed for 30 min at 30°C in 50 μl of this buffer with 10 μCi of [γ-32P]ATP, with or without 1 μg of the natural substrate MEK1. Reactions were terminated by the addition of SDS sample buffer and analyzed on SDS–10% polyacrylamide gels.
Raf-1 protein kinase activity.
PC12 cells were stimulated with NGF and lysed in buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.2 mM Na3VO4, 0.2 mM PMSF, 1% Triton X-100, 0.5% NP-40, and protease inhibitors. Lysates were clarified by centrifugation and precleared twice for 1 h with 25 μl of protein A-Sepharose. Raf-1 was immunoprecipitated by addition of Raf-1 antibody and inverted end-over-end for 1 h at 4°C, followed by addition of 30 μl of anti-goat antibody for an additional 1.5 h. The immune complex was washed three times in lysis buffer and twice in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 7)–10 mM MnCl2–1 μg of aprotinin/ml. Kinase reactions were performed for 30 min at 30°C in 50 μl of this buffer with 10 μCi of [γ-32P]ATP, with or without 1 μg of the natural substrate MEK1 (48, 53). Reactions were terminated by the addition of SDS sample buffer and analyzed on SDS–10% polyacrylamide gels.
Activation of Raf-1, p38, MAP, and JNK.
Measurement of Raf-1 activation was also determined by electrophoretic mobility shift assay (EMSA) and visualized by Western blotting, indicative of Raf-1 phosphorylation and activation (53). p38, MAP, and JNK were determined by immunoblotting with phosphospecific antibodies. The blots were stripped by addition of 0.1 M glycine (pH 2) followed by neutralizing and reprobed with antibody to p38, MAP, and JNK. Alternatively, the activity of JNK was also measured by immune complex kinase assay as previously described with GST-cJun(1-79) as the substrate (17, 37). The radioactive protein bands were scanned with a computer-interfaced densitometer to determine the relative changes in substrate phosphorylation.
NF-κB EMSA.
Cell extracts were prepared in high-salt detergent buffer (Totex; 20 mM HEPES [pH 7.9], 350 mM NaCl, 20% [vol/vol] glycerol, 1% [wt/vol] NP-40, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 0.5 mM dithiothreitol [DTT], 0.1% PMSF, 1% aprotinin) (58). The cells were harvested by centrifugation, washed in ice-cold phosphate-buffered saline, resuspended in 4 volumes of Totex buffer, incubated on ice for 30 min, and then centrifuged for 5 min at 13,000 × g at 4°C. The protein content of the supernatant was determined, and equal amounts of protein (20 μg) were added to a reaction mixture containing 20 μg of bovine serum albumin, 2 μg of poly(dI-dC), 2 μl of buffer D+ (20 mM HEPES [pH 7.9], 20% glycerol, 100 mM KCl, 0.5 mM EDTA, 0.25% NP-40, 2 mM DTT, 0.1% PMSF), 4 μl of buffer F (20% Ficoll 400, 100 mM HEPES, 300 mM KCl, 10 mM DTT, 0.1% PMSF), and 100,000 cpm of a 32P-labeled oligonucleotide (5′ AGTTGAGGGGACTTTCCCAGGC 3′) in a final volume of 20 μl. Samples were incubated at room temperature for 25 min. For supershift assays, 2 to 5 μg of antibody was added to the protein and allowed to incubate overnight, followed by inclusion in an assay. Excess AP-1 (5′ CGCTTGATGAGTCAGCCGGAA 3′) or NF-κB oligonucleotide was included as a negative control. The samples were resolved on a 6% Tris-glycine polyacrylamide gel. The gel was dried and exposed to X-ray film for 24 to 72 h.
Coprecipitation of IκB, Src, and PKC-ι.
A previously defined (1) coprecipitation protocol was used. In brief, PC12 cells were washed in phosphate-buffered saline and lysed in ice-cold lysis buffer (20 mM Tris [pH 7.8], 150 mM NaCl, 1 mM CaCl2, 0.2% deoxycholic acid, 0.2% NP-40, 1 mM Na3VO4, protease inhibitors). The lysates were incubated with anti-IKK followed by protein A, and the beads containing the complexes were washed extensively with lysis buffer.
Measure of Src activity.
As previously described (1), Src was immunoprecipitated and washed in kinase lysis buffer (1.5% NP-40, 150 mM NaCl, 25 mM Tris [pH 8], 25 mM NaF, 100 mM NaVO3) and then preincubated with 15 ml of kinase reaction buffer (25 mM HEPES [pH 7.4], 150 mM NaCl, 5 mM MnCl2, 100 μM NaVO3), for 10 min at 22°C. The kinase reaction was initiated by addition of 5 μCi of [γ-32P]ATP (6,000 Ci/mmol), 5 μM ATP, and 1 μg of p34 c-Src and was terminated after 2 min by addition of an equal volume of 2× SDS sample buffer; the mixture was boiled, dried, and exposed to X-ray film.
Cell survival and differentiation.
To assess cell death or differentiation, PC12 cells were plated and after 3 days washed five times with serum-free medium (59). Cultures were treated in the presence or absence of 100 ng of NGF per ml. After 48 h, the cells and their medium were collected. Trypan blue was added to examine cell viability. An aliquot of the cells was counted, and the percentage cells that stained trypan blue positive was determined. Alternatively, PC12 differentiation was determined by scoring for neurite outgrowth 6 days after addition of NGF. The percentage cells that possessed neurites was determined by counting treatments in triplicate. A minimum of 500 cells were counted within a treatment group. The percentage of cells with neurites was calculated based on an individual cell bearing one process with an extension which was greater in length than two cell diameters. Clumped cells were not included in the scoring process.
RESULTS
Activation of aPKC requires Ras, Src and PI3K.
PKC-ι antibody obtained from Santa Cruz has been previously shown not to cross-react with other PKC isoforms or with the highly homologous PKC-ζ (40). To validate that the immune complex kinase assay accurately measures PKC-ι activity, several approaches were undertaken. Previously, we have shown that NGF-induced PKC-ι activation results in an approximately twofold increase in aPKC activity using an in situ assay (56). Similarly, upon stimulation of PC12 cells with NGF, the activity of PKC-ι, measured in the immune complex kinase assay, was increased approximately twofold (Table 1). Thus, measure of NGF-activated PKC-ι using this assay was similar to previous findings employing a separate method. The aPKCs lack the C2 domain and consequently do not bind, nor are they downregulated by phorbol esters (51). Prolonged treatment with phorbol myristate acetate (PMA) results in PC12 cells that are devoid of cPKC and nPKC isoforms (56). Treatment of PC12 cells with 1 μM PMA for 48 h prior to NGF stimulation failed to alter the level of PKC-ι activity. Peptides complementary to the pseudosubstrate region have previously been shown to serve as highly competitive selective inhibitors of specific PKC isoforms (30). To further validate the assay of PKC-ι, the cells were treated with pseudosubstrate peptides to which an N-terminal myristic acid had been added to facilitate their diffusion through the plasma membrane. Treatment of the cells with pseudosubstrate peptide to the cPKC isoforms failed to inhibit recovery of NGF-stimulated PKC-ι activity, whereas treatment with pseudosubstrate peptide to atypical PKC-ι diminished the activity of NGF-stimulated immune-complexed kinase enzyme. Last, PKC-ι antibody was preincubated with peptide antigen prior to immunoprecipitation. In this case, recovery of NGF-stimulated PKC-ι was blocked by inclusion of the peptide. Collectively, these findings validate the measure of PKC-ι activity by the immune complex kinase assay.
TABLE 1.
Validation of immune complex kinase assay for measure of PKC-ι activity
| Treatmenta | Mean relative activity of PKC-ι (%) ± SD |
|---|---|
| NGF | 100 |
| PMA + NGFb | 100 |
| cPKC PP + NGFc | 98 ± 3 |
| aPKC PP + NGFc | 50 ± 2 |
| Peptide + NGFd | |
| 1:1 | 10 ± 3 |
| 1:2 | 0 |
| cPKC PPe | |
| 25 μM + NGF | 98 ± 5 |
| 50 μM + NGF | 88 ± 6 |
| 100 μM + NGF | 100 |
| aPKC PPe | |
| 25 μM + NGF | 42 ± 4 |
| 50 μM + NGF | 22 ± 5 |
| 100 μM + NGF | 8 ± 2 |
PC12 cells were treated with NGF (100 ng/ml) for 15 min, followed by immune complex kinase assay as described in Materials and Methods. Alternatively, cells were treated with various agents as indicated.
Cells were treated with 1 μM PMA for 48 h to downregulate cPKC and nPKC prior to stimulation with NGF.
Lysates obtained from NGF-treated cells were incubated with 15 μM pseudosubstrate peptide (PP) specific for either cPKC (SIYRRGARRWRKLYRANG) or aPKC (MPARKGALRQ) for 1 h prior assay of PKC-ι activity.
PKC-ι antibody was incubated 1:1 with peptide antigen or 1:2, as indicated, prior to addition of NGF-stimulated lysates and measurement of PKC-ι activity.
PC12 cells were incubated with myristoylated, cell-permeable, pseudosubstrate peptide specific for either cPKC or aPKC for 1 h prior to stimulation with NGF.
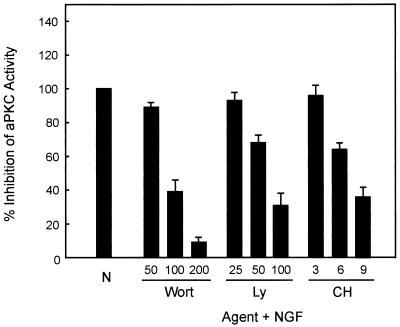
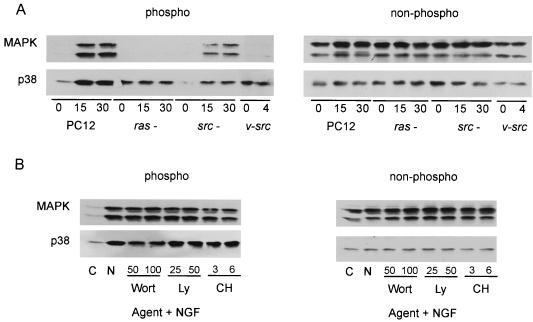
Previous studies have demonstrated that phosphatidylinositol-3,4,5-triphosphate (PIP3) is required for aPKC activation (41). In PC12 cells, NGF rapidly activates PI3K, resulting in production of PIP3 (45). Thus, we hypothesized that elimination of PI3K activity may abrogate NGF-induced activation of PKC-ι. To further determine the role that PI3K plays in activation of PKC-ι by NGF, PC12 cells were pretreated with either wortmannin or LY294002, potent and specific inhibitors of PI3K (22). On the other hand, chelerythrine chloride has been shown to be a potent and specific inhibitor of aPKC (30) and thus was used as a means to inhibit PKC-ι activity and to serve as a control in these experiments. NGF-induced activation of PKC-ι was sensitive to PI3K (Fig. 1), and chelerythrine chloride pretreatment of PC12 cells likewise inhibited NGF-induced aPKC activity. At higher doses of all three inhibitors, aPKC activity was significantly reduced. Thus, NGF-induced activation of aPKC is sensitive to both chelerythrine chloride and PI3K.
FIG. 1.
Effects of pharmacological agents on aPKC activity. PC12 cells were pretreated with either wortmannin (Wort; 50, 100, or 200 nM), LY294002 (Ly; 25, 50, or 100 μM), or chelerythrine chloride (CH; 3, 6, or 9 μM) for 1 h prior to addition of NGF (100 ng/ml) for 15 min. PC12 cell lysates (400 μg) were analyzed for aPKC activity in triplicate by immune complex kinase assay using MBP as substrate. Data shown are the means ± standard error of the means of three independent experiments. N, NGF.
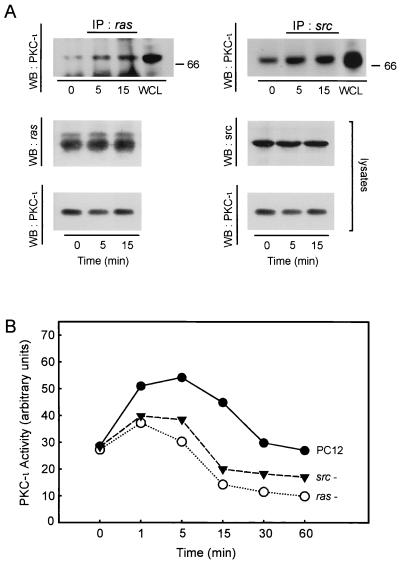
Since NGF responses consist of a Ras-Src cassette (26) and aPKCs have been reported to bind Ras (11) and interact with Src (49), we initially determined whether NGF would stimulate coassociation of Ras and Src with PKC-ι. Coimmunoprecipitation studies reveal that both Ras and Src bind to PKC-ι (Fig. 2A). A high level of basal interaction between PKC-ι and Src was observed; however, NGF stimulated increases in PKC-ι that was complexed to both Ras and Src, although the degree of stimulation was far more dramatic for Ras. Since PKC-ι bound both proteins, the requirement of either Ras or Src in NGF-induced activation of PKC-ι was likewise examined. As shown in Fig. 2B, the absence of either Ras or Src activity had a profound effect on NGF-stimulated PKC-ι, each decreasing the kinetics of PKC-ι activation in response to NGF. Measure of PKC-ι activity was normalized to the amount of PKC-ι in the assay; moreover, all three cell lines expressed equivalent amounts of PKC-ι. Thus, the amount of PKC-ι did not contribute to differences in the magnitude of activation. Interestingly, only partial inhibition of PKC-ι activity in the absence of either Src or Ras was observed, indicating that perhaps other factors likely contribute to regulation of PKC-ι.
FIG. 2.
PKC-ι coassociates with Ras and Src. (A) PC12 cells were stimulated with NGF (100 ng/ml) as indicated for 0 to 15 min. Cell lysates (500 μg) were prepared and immunoprecipitated (IP) with antibody to either Ras or Src followed by Western blotting (WB) with PKC-ι antibody. Included in the analysis as a standard was a PC12 cell whole cell lysate (WCL; 70 μg). As positive control, the cell lysates (50 μg) were analyze by immunoblotting with antibody to Ras, Src, or PKC-ι. (B) Control, Ras−, or Src− PC12 cells were stimulated with NGF (100 ng/ml) for 0 to 60 min followed by analysis of aPKC activity in triplicate by immune complex kinase assay using MBP as substrate. This experiment was repeated two other times with similar results.
Raf-1 is regulated by Ras independently of Src and PKC-ι.
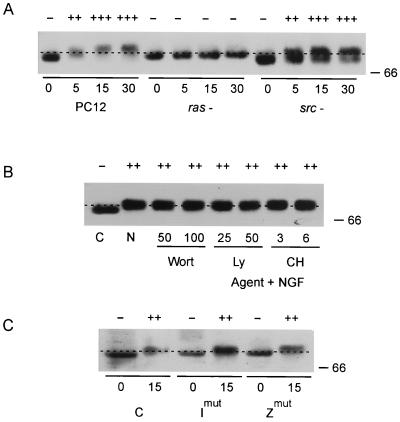
To further dissect the aPKC signaling pathway, the relationship of Src/Ras and PKC-ι to NGF-induced activation of Raf-1 was studied. Overexpression of Raf-1 has been shown to stimulate neurite outgrowth of PC12 cells (54), demonstrating a sufficiency of overexpression requirement of Raf-1 for the differentiation response. In addition, NGF activates the Raf-MAPK cascade (55). Using a cotransfection approach, aPKC has also been reported to activate MEK independently of Raf-1 (48), suggesting that a Raf-1-independent pathway also exists. Therefore, we used several approaches to explore the regulation of NGF-induced activation of Raf-1 by PKC-ι. As a control, Raf-1 activation by NGF was validated in cells that were lacking Ras or Src activity (Fig. 3A). Raf activation occurs via phosphorylation; therefore, a hypershift of p74 Raf-1 mobility on SDS-PAGE can be used as an indicator of Raf-1 activation. Cell lysates prepared from PC12, Src−, or Ras− cells stimulated with NGF were analyzed for Raf activation by EMSA (Fig. 3A). NGF-induced Raf-1 activity was dependent on Ras and independent of Src, thus validating assay of Raf-1 activity by gel shift and accounting for any possible differences in clonal variants of these PC12 cells. Agents that were shown to inhibit activation of aPKC (Fig. 1) had little effect on NGF-induced activation of Raf-1 (Fig. 3B). The inability of aPKC to modulate Raf-1 was further confirmed by transfecting mutants for either PKC-ι or PKC-ζ that confer a dominant negative phenotype (5, 6). Cell lysates obtained from transfected cells stimulated with NGF were confirmed to possess diminished levels of aPKC activity (data not shown). An absence of aPKC activity had no effect on NGF-induced Raf-1 activation (Fig. 3C). Moreover, overexpression of aPKCs failed to enhance NGF-induced activation of Raf-1 (58). These findings (Fig. 3B and C) were likewise validated by measure of Raf-1 activity in an immune complex kinase assay using MEK as the substrate: inhibitors of aPKC or PI3 kinase had little effect on Raf-1 kinase (data not shown). Collectively, these findings demonstrate that aPKC does not directly influence NGF-induced activation of the Raf-1 node of the MAPK signaling cascade in PC12 cells and are in line with previous work defining MEK as the critical point of regulation (48).
FIG. 3.
Positioning of Ras, Src, and aPKC relative to Raf-1. (A) Control, Ras−, or Src− PC12 cells were stimulated with NGF (100 ng/ml) for 0 to 30 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (7.5% polyacrylamide) and then immunoblotted with Raf-1 antibody. (B) PC12 cells were pretreated with either wortmannin (Wort; 50 or 100 nM), LY294002 (Ly; 25 or 50 μM), or chelerythrine chloride (CH; 3 or 6 μM) for 1 h prior to addition of NGF (100 ng/ml) for 15 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (7.5% polyacrylamide) and then immunoblotted with Raf-1 antibody. C, control; N, NGF. (C) PC12 cells were transfected with control construct, mutant PKC-ι (Imut), or mutant PKC-ζ (Zmut). Thereafter, the cells were stimulated with NGF (100 ng/ml) for 0 or 15 min, as indicated. After lysis, equal protein aliquots were resolved by SDS-PAGE (7.5% polyacrylamide) and then immunoblotted with Raf-1 antibody. Samples were scored + based on the ability of NGF to stimulate Raf-1 hyperphosphorylation/activation as indicated by an increase in relative molecular weight or gel shift (–––). These experiments were repeated two other times with similar results. Bands at 66 kDa are indicated on the right.
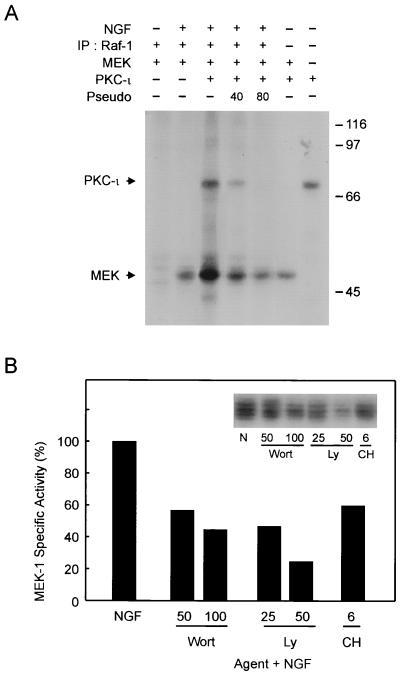
Since previous studies have suggested that aPKC may be capable of activating MEK directly (6, 48), we undertook an analysis to explore the regulation of MEK itself by aPKC in PC12 cells. In an in vivo-in vitro kinase assay, aPKC was observed to directly phosphorylate MEK (Fig. 4A). The inclusion of aPKC pseudosubstrate peptide blocked aPKC-induced phosphorylation of MEK in a dose-dependent fashion. Moreover, aPKC synergized with Raf-1 to stimulate phosphorylation of MEK. To explore the position of aPKC and PI3 kinase in relation to MEK activation, NGF-stimulated MEK activity was assessed (Fig. 4B). We observed that NGF-stimulated MEK activity was dependent on PI3K, aPKC, and Src. Collectively, these findings support regulation of MEK by aPKC independently of Raf-1 (48).
FIG. 4.
PKC-ι activates MEK. (A) PC12 cells were stimulated with NGF (100 ng/ml) for 15 min followed by immunoprecipitation (IP) of Raf-1 and subjected to an in vitro kinase reaction as indicated with [γ-32P]ATP in the presence of 1 μg of recombinant MEK1. The samples were separated by SDS-PAGE (10% polyacrylamide) followed by autoradiography. Autophosphorylated PKC-ι and phosphorylated MEK1 are shown. Sizes are indicated in kilodaltons. (B) MEK1 activity was measured using immune complex kinase assay as previously described (48). The relative changes in activity was normalized to that obtained with NGF treatment. Similar results were obtained in two other experiments. N, NGF.
aPKCs and PI3K fail to regulate p38, MAPK, or JNK.
In other systems, aPKCs have been reported to lie upstream of MAPK (5, 6) as well as JNK (58). Overexpression of aPKCs in PC12 cells was observed to slightly enhance MAPK and markedly enhanced NGF-activated JNK (58). In addition, aPKC was directly capable of activating JNK, thus positioning aPKCs upstream of JNK. Using a complementary pharmacological approach, we mapped the relationship of Ras, Src, and aPKC to downstream activation of p38, MAPK, and JNK. NGF has been shown to stimulate the activation of all three kinases, JNK, MAPK, and p38 (19, 22, 39). Activation of MAPK by NGF was Ras dependent and slightly independent of Src, whereas activation of p38 was independent of Ras and dependent on Src (Fig. 5A). Since NGF-induced aPKC activity was shown to be chelerythrine chloride and PI3K sensitive (Fig. 1), these inhibitors were used to explore the relative position of aPKC to p38, MAPK, and JNK. Pretreatment of PC12 cells with these inhibitors failed to diminish NGF-stimulated MAPK or p38 activities (Fig. 5B), consistent with a previous study reporting a lack of effect of PI3K on NGF-stimulated MAPK activity (25). Due to the poor quality of the blots obtained with the phospho-JNK antibodies, the effects of aPKC inhibitors on NGF-stimulated JNK kinase activity were examined using an immune complex kinase assay with GST-cJun(1-79) as the substrate. NGF stimulated JNK activation ∼2-fold, similar to what has been previously reported (17). Activation of JNK by NGF was dependent on Ras, Src, aPKC, and PI3K. In addition, constitutive activation of v-Src had a profound effect on the activity of JNK (Table 2). These observations were confirmed by measure of activated c-Jun phosphorylated at S63 (data not shown). Placement of aPKC upstream of JNK parallels our previous findings using a genetic approach (58).
FIG. 5.
Positioning of Ras, Src, and aPKC relative to MAPK and p38. (A) Control, Ras−, or Src− PC12 cells were stimulated with NGF (100 ng/ml) for 0 to 30 min. After lysis, equal protein aliquots (70 μg) were resolved by SDS-PAGE (12% polyacrylamide) and then immunoblotted with either anti-phospho-MAPK or -p38 antibody. The blots were stripped and reprobed with a nonphospho-specific antibody to MAPK or p38. (B) PC12 cells were pretreated with either wortmannin (Wort; 50 or 100 nM), LY294002 (Ly; 25 or 50 μM), or chelerythrine chloride (CH; 3 or 6 μM) for 1 h prior to addition of NGF (100 ng/ml) for 15 min. After lysis, equal protein aliquots (70 μg) were resolved by SDS-PAGE (12% polyacrylamide) and then immunoblotted with anti-phospho-MAPK or -p38 antibody. This experiment was repeated three times with similar results. C, control; N, NGF.
TABLE 2.
Regulation of JNK activity by Src, Ras, aPKC, and PI3Ka
| Treatment | JNK activity (fold stimulation) |
|---|---|
| PC12 control | 1 |
| PC12 + NGF | 2.8 ± 0.4 |
| Ras− + NGF | 1.5 ± 0.3 |
| Src− + NGF | 1.2 ± 0.4 |
| v-Src | 4.2 ± 0.6 |
| PC12 + Wort + NGF | 1.1 ± 0.3 |
| PC12 + LY + NGF | 1.3 ± 0.4 |
| PC12 + CH + NGF | 1.1 ± 0.3 |
Cell lysates were prepared from the indicated PC12 cell cultures that had been treated with NGF (100 ng/ml) for 15 min. Alternatively, cells were treated with either wortmannin (Wort; 100 nM), LY294002 (LY; 50 μM), or chelerythrine chloride (CH; 6 μM) for 1 h prior to addition of NGF. PC12 cells expressing a temperature-sensitive v-Src were shifted to 37°C for 4 days to activate Src. JNK activity was measured using 400 μg of total protein in an immune complex kinase assay (17) with GST-cJun(1-79) as substrate. Data are means ± standard error of the means of three independent experiments.
Since aPKC has been positioned upstream of MAPK in other systems, while overexpression in PC12 cells failed to drastically enhance NGF-induced MAPK activation (58), several alternative approaches were used to further explore the relationship of aPKCs to MAPK. First, transient transfection of a kinase-dead PKC-ζ or PKC-ι failed to inhibit NGF-stimulated MAPK. Second, treatment of the cells with myristoylated pseudosubstrate to aPKCs failed to inhibit NGF-stimulated MAPK. Last, delivery of either antisense or sense oligonucleotides to PC12 cells also failed to inhibit NGF-stimulated MAPK (data not shown). Collectively, these findings further document direct regulation on the MAPK pathway in PC12 cells by the aPKCs. These findings are consistent with regulation of aPKC by the alternative JNK kinase pathway (58) (Table 2). Taken together, these results demonstrate that aPKC, Src, and PI3K are components of a newly defined pathway which is distinct from the classical Ras-MAPK signal cascade.
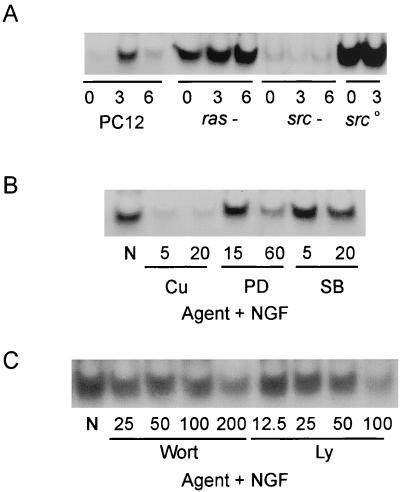
NGF-induced activation of NF-κB is dependent on PKC-ι, Src, JNK, and PI3K and independent of Ras.
aPKC lies upstream of NF-κB in the NGF signaling pathway (58), consistent with its placement in other signaling paradigms (5, 10, 12). One essential step in the activation of NF-κB is the phosphorylation of IκB by either IKKα or IKKβ. Moreover, aPKC has been shown to lie upstream of IKKβ, regulating the kinase via phosphorylation (29), thus documenting direct interaction between aPKC and the NF-κB pathway. To further dissect the relationship of aPKC to this downstream transcription target, the requirement of Ras or Src for NGF-induced activation of NF-κB was examined. NGF-induced activation of NF-κB has previously been characterized as prolonged (29). Activation of NF-κB by NGF was observed to require Src but not Ras (Fig. 6A). Consistent with a Src requirement, overexpression of Src enhanced basal NF-κB levels. To determine the requirement of either JNK, MAPK, or p38 kinase, PC12 cells were pretreated with specific pharmacological agents that target these signal transduction pathways. JNK is specifically inhibited by curcumin (8), MEK and MAPK are inhibited by PD98059 (43), and p38 is inhibited by SB202190 (39). The ability of these agents to impair NGF-induced activation of NF-κB was evaluated. Inhibition of JNK markedly inhibited NGF-induced NF-κB (Fig. 6B). Activation of NF-κB was sensitive to MEK and relatively insensitive to p38. The role of PI3K in mediating NGF-induced NF-κB was likewise examined (Fig. 6C). Pretreatment with LY294002 or wortmannin, potent inhibitors of PI3K, resulted in a dose-dependent inhibition of NGF-induced NF-κB activity.
FIG. 6.
Positioning of Ras, Src, and aPKC relative to NF-κB. (A) PC12, Ras−, Src−, or cells overexpressing c-Src were stimulated with NGF (100 ng/ml) for 0 to 6 h. After lysis, equal protein aliquots were subjected to EMSA analysis. (B) PC12 cells were pretreated with curcumin (Cu; 5 or 20 μM), PD98059 (PD; 15 or 60 μM), or SB202190 (SB; 5 or 20 μM) followed by addition of NGF (100 ng/ml) for 3 h. After lysis, equal protein aliquots were subjected to EMSA analysis. (C) PC12 cells were pretreated with either wortmannin (Wort; 25 to 200 nM) or LY294002 (Ly; 12.5 to 100 μM) for 1 h prior to addition of NGF (100 ng/ml) for 3 h. After lysis, equal protein aliquots were subjected to EMSA analysis. This experiment was repeated twice with similar results.
To address the position of PI3K relative to Src, we also examined the effect of wortmannin or LY294002 on NGF-induced Src kinase activity. Neither inhibitor of PI3K blocked Src activity (data not shown). Thus, Src regulates aPKC independently of PI3K. This finding is in keeping with a lack of influence of PI3K on Src-mediated tyrosine phosphorylation of PKC-ι (M. L. Vandenplas, M. L. Seibenhener, and M. W. Wooten, submitted for publication). Therefore, aPKC is modulated independently by both Src and PI3K.
Previous studies have shown that NGF promotes survival of PC12 cells in a serum-free environment independent of Ras (59) but dependent on PI3K. It has also been suggested that two distinct nonoverlapping pathways are required for mediation of NGF function (24): the Ras pathway is the primary regulator of differentiation, whereas PI3K regulates a separate survival signaling pathway in PC12 cells. aPKC, on the other hand, has been shown to block differentiation of PC12 cells (9), whereas overexpression of aPKC markedly enhanced both survival and differentiation (58). These findings support the existence of two pathways, one which overlaps with components of the other. Therefore, studies were undertaken to map the positions of Ras-Src, PKC-ι, PI3K, p38, MAPK, and JNK to determine which elements of the differentiation pathway overlapped with components of the survival pathway in PC12 cells (Table 3). As previously reported (59), Ras was required for differentiation but not survival. Src, on the other hand, was required for both survival and differentiation. Moreover, overexpression of Src enhanced survival of PC12 cells in a serum-free environment. Both survival and differentiation were dependent on PI3K. Removal of cPKC or nPKC by PMA downregulation had little effect on survival or differentiation. By comparison, removal of aPKC by treatment with myristoylated pseudosubstrate peptide or chelerythrine chloride blocked both survival and differentiation responses. Inhibition of either MAPK or p38 had no effect on survival but was required for differentiation. On the other hand, inhibition of JNK blocked survival, similar to removal of aPKC. Collectively, these findings reveal that the Ras-MAPK pathway plus the Src-aPKC-PI3K pathway is required for differentiation, whereas survival is exclusive to components of the Src pathway, independently of Ras-MAPK cassette.
TABLE 3.
Effects of Src, Ras, PKC, and PI3K on survival and differentiation responses of PC12 cellsa
| Treatment | % Survival | % Differentiation |
|---|---|---|
| PC12 − NGF | 48 ± 4 | 0 |
| PC12 + NGF | 74 ± 6 | 60 ± 6 |
| Ras− − NGF | 49 ± 5 | 0 |
| Ras− + NGF | 48 ± 7 | 0 |
| Src− − NGF | 8 ± 2 | 0 |
| Src− + NGF | 29 ± 6 | 38 ± 6 |
| SrcO − NGF | 66 ± 4 | 0 |
| SrcO + NGF | 84 ± 8 | 75 ± 6 |
| LY, 50 μM | 12 ± 4 | 0 |
| LY + NGF | 48 ± 3 | 0 |
| W, 100 nM | 22 ± 7 | 0 |
| W + NGF | 52 ± 6 | 0 |
| PMA, 1 μM | 47 ± 5 | 0 |
| PMA + NGF | 85 ± 6 | 79 ± 8 |
| aPKC, 50 μM | 5 ± 1 | 0 |
| aPKC + NGF | 24 ± 3 | 30 ± 4 |
| cPKC, 50 μM | 23 ± 3 | 0 |
| cPKC + NGF | 59 ± 6 | 54 ± 5 |
| CH, 6 μM | 2 ± 1 | 0 |
| CH + NGF | 42 ± 6 | 32 ± 8 |
| PD, 60 μM | 60 ± 4 | 0 |
| PD + NGF | 79 ± 6 | 0 |
| SB, 20 μM | 69 ± 6 | 0 |
| SB + NGF | 73 ± 6 | 0 |
| Cu, 20 μM | 0 | 0 |
| Cu + NGF | 56 ± 6 | 37 ± 4 |
PC12 cells were plated and after 3 days washed five times with serum-free medium and cultured with or without NGF in a serum-free environment for 48 h; then all cells in the well were removed, stained with trypan blue, and counted. To score cells for differentiation, the cells were cultured with NGF for a total of 6 days and scored as described in Materials and Methods. The experiment was conducted using triplicate treatments and repeated twice. Cells were pretreated with LY294002 (LY), wortmannin (W), aPKC (myristoylated pseudosubstrate peptide), cPKC (myristoylated pseudosubstrate peptide), chelerythrine chloride (CH), PD98059 (PD), SB202190 (SB), or curcumin (Cu), for 1 h prior to addition of NGF. PMA was added for 48 h to remove cPKC and nPKC isoforms (56).
Src regulates formation of an IKK–PKC-ι complex and mediates the upstream portion of an NF-κB survival signaling pathway.
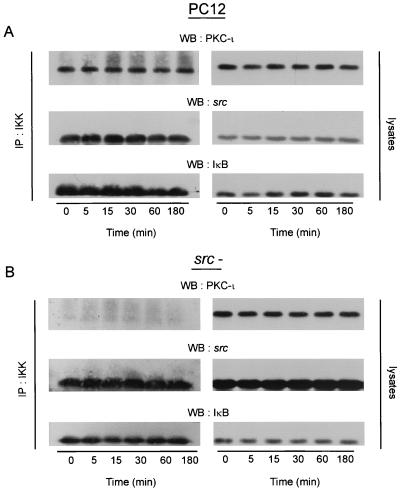
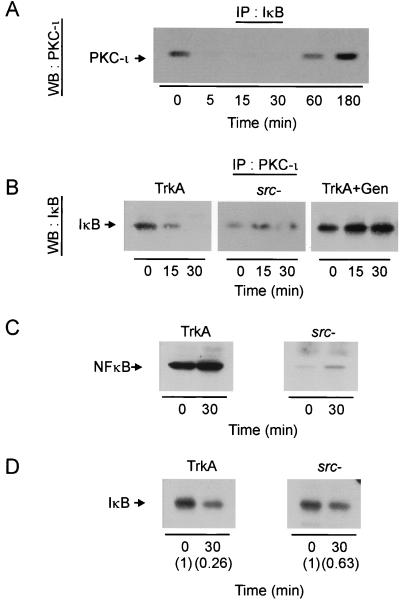
aPKC can form a complex with IKK, phosphorylating the kinase to lead to activation of the NF-κB pathway (29). Moreover, Src regulates aPKC by increasing its tyrosine phosphorylation state and modulating enzyme activity (49; Vandenplas et al., submitted). Since Src− cells fail to activate NF-κB, we hypothesized that Src may play a role in the coupling of aPKC with the NF-κB pathway via modulating the interaction of tyrosine-phosphorylated PKC-ι with IKK. To test this idea, IKK was immunoprecipitated after NGF stimulation from equivalent protein of control and Src− PC12 cells (Fig. 7). IKK was constitutively associated with PKC-ι, Src, and IκB, whereas in Src− cells a drastic reduction in the recovery of PKC-ι complexed with IKK was observed, even though Src/IκB levels were similar between both sets of immunoprecipitates (compare Fig. 7). Pretreatment of PC12 cells with genistein, a potent and selective tyrosine kinase inhibitor, likewise reduced the amount of PKC-ι complexed to IKK (data not shown). Thus, interaction of IKK with PKC-ι was dependent on Src tyrosine kinase activity as determined by two independent means. Activation of NF-κB is preceded by signal-induced IκB degradation. After NGF treatment, PKC-ι dissociates from IκB (Fig. 8A) in a time frame coincident with activation of PKC-ι and Src-induced tyrosine phosphorylation of PKC-ι, along with parallel activation of NF-κB (58; Vandenplas, submitted). To test the requirement for Src activity in this process, the association of PKC-ι/IκB was examined in Src− cells and TrkA cells (Fig. 8B). Upon treatment of the cells with NGF, PKC-ι dissociates from the IκB complex (Fig. 8A and B). However, in cells that were deficient in Src, no such dissociation was observed; rather, a low level of basal coassociation between PKC-ι and IκB was noted, consistent with the amount of PKC-ι/IκB observed in the IKK complex in the absence of Src activity (Fig. 7). Treatment with genistein likewise blocked dissociation of PKC-ι/IκB. These results demonstrate that Src tyrosine directly participates in the coupling of PKC-ι to the NF-κB pathway. Moreover, Src activity was required to stimulate translocation of p65/NF-κB to the nucleus (Fig. 8C). Last, the degradation of IκB was examined in Src− cells. NGF failed to stimulate IκB degradation in Src− (37%) compared to TrkA (74%) cells (Fig. 8D). Taken together, the uncoupling of IKK/PKC-ι (Fig. 7) parallels a lack of NGF-stimulated NF-κB activation (Fig. 6 and 8C, and 8D) and diminished PKC-ι activity observed in Src− cells (Fig. 1). These data demonstrate that Src tyrosine kinase plays a role in modulating the interaction between IKK and PKC-ι, whereas an absence of Src activity abrogates the signal-induced dissociation of PKC-ι and IκB. Thus, regulation of PKC-ι/IκB interaction would thus account for the absence of NF-κB DNA binding previously observed in PC12 cells deficient in Src activity (Fig. 6A).
FIG. 7.
Src regulates the association of IKK with aPKC. PC12 cells, parental or Src−, were treated with NGF (100 ng/ml) for the times indicated, and lysates (500 μg) were immunoprecipitated with anti-IKK. The immunoprecipitates (IP:IKK) or lysates (50 μg), as a positive control, were Western blotted (WB) with PKC-ι, Src, and IκB antibodies. This experiment was repeated twice with similar results.
FIG. 8.
Src is required for PKC-ι/IκB coassociation. (A) PC12 cells were treated with NGF (100 ng/ml) for the times indicated, and lysates were immunoprecipitated (IP) with anti-IκB. The precipitates were Western blotted (WB) with PKC-ι antibody. (B) PC12 cells, cells pretreated with genistein (Gen; 20 μM), or Src− cells were treated with NGF (100 ng/ml) for the times indicated, and lysates were immunoprecipitated with anti-PKC-ι. The precipitates were blotted with IκBα. (C) PC12 or Src− cells were exposed to NGF (100 ng/ml) for 30 min. Nuclei were isolated and immunoblotted with anti-p65/NF-κB antibody. (D) PC12 or Src− cells were exposed to NGF (100 ng/ml) for 30 min. Whole cell lysates were prepared (50 μg) and immunoblotted with antibody to IκB. The blots were scanned, and the relative change in intensity of IκB is shown in parentheses.
Collectively, these findings demonstrate that aPKC, PI3K, and Src are principal components of a survival signaling pathway, coupled to regulation of NF-κB, which cross talk with elements of the differentiation pathway through JNK (Fig. 9). Our findings support the existence of one pathway for differentiation which encompasses elements of a distinct survival signaling cascade.
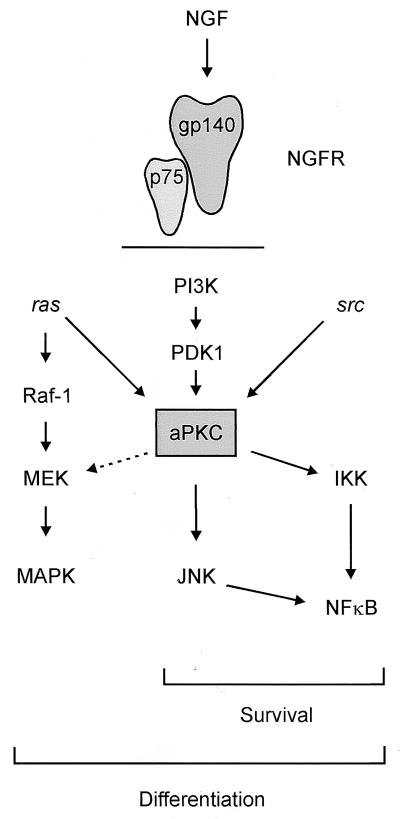
FIG. 9.
Model illustrating the relative positioning of aPKC within the NGF signaling cascades for survival and differentiation. aPKC can activate MEK and lies upstream of JNK. Both Ras and Src lie upstream of aPKC, as does PI3K, whereas aPKC lies upstream of NF-κB, directly interacting with IKK. Differentiation requires components of both the Src and Ras pathways. The differentiation pathway overlaps with components of the survival pathway through Src, aPKC, and PI3K. Survival signaling is Ras independent but dependent on PI3K, Src, aPKC, and NF-κB. Our findings support a model whereby aPKC occupies a critical node, capable of interacting with Ras-MAPK through modulation of MEK and JNK and upstream of NF-κB. NGFR, NGF receptor.
DISCUSSION
In this study, we show that a PKC does not reside as a component of the Ras-MAPK pathway but instead is a component of a parallel, distinct signaling pathway. Our data demonstrate that this pathway is regulated in a novel fashion by c-Src. Both Src and Ras have been shown to induce neurite extension similar to NGF (4, 42, 50). A Src-Ras signaling cassette for NGF signaling was initially proposed by microinjection studies, with Src positioned upstream of Ras (26). Persistent activation of the Ras-Raf-MAPK pathway was observed to be insufficient to sustain PC12 differentiation (52). In addition, activation of pp60 Src was also required for PC12 differentiation (26). In lieu of a linear Src-Ras cascade, these data support existence of a parallel Src cascade as being important for PC12 differentiation. Support for such a parallel cascade has likewise been obtained in H19-7 cells, which are rat E17 hippocampal cells that have been conditionally immortalized with a retroviral vector expressing a temperature-sensitive simian virus (27). At the nonpermissive temperature, when T antigen is inactivated, the cells differentiate. Under these conditions, no activation of Ras-Raf-MAPK by v-Src was detected. Similar observations were likewise made in this study, whereby v-Src failed to induce the MAPK pathway but significantly enhanced JNK activity. Thus, Src and Ras appear to activate two discrete pathways, JNK and MAPK. Since both Ras and Src pathways are required for differentiation to ensue, the observation of inhibition of MEK and abrogation of neurite outgrowth (43) implies that MEK is a likely convergent point at which the two pathways overlap. Synergistic induction of neurite outgrowth by v-Src and activated MEK would support the existence of a separate pathway that can cross talk at the level of MEK to support differentiation of PC12 cells. Our findings are consistent with previous work in other systems (6, 48, 53), demonstrating that aPKC can modulate MEK. Thus, aPKC acting as a MEK kinase may directly serve as a convergence point for the Ras/MAPK and Src/JNK pathways.
Overexpression of aPKC has previously been shown to modulate the JNK pathway, whereas removal of aPKC using a full-length antisense construct blocked NGF-induced JNK activity (58). Consistent with these findings, inhibition of aPKC by pharmacological manipulation likewise blocked NGF-induced activation of JNK. Thus, in PC12 cells, aPKC positions itself upstream of JNK. In addition, JNK activation by NGF is influenced by PI3K, as both wortmannin and LY294002 impaired NGF effects, which is in keeping with the ability of Src but not Ras to regulate NGF-induced JNK activity. This finding is consistent with the ability of v-Src expression to enhance activation of JNK, concomitant with formation of neurites. Although JNK has been more commonly studied in stress signaling, it is also activated by epidermal growth factor (34). Previous studies (25) revealed that overexpression of PI3K resulted in activation of JNK but not MAPK. Our findings position PI3K upstream of JNK. We conclude that Src, PI3K, aPKC, and JNK are components of a newly defined signaling cascade, consistent with the demonstrated ability of JNK to modulate neurite outgrowth (23) as well as survival and activation of NF-κB (32). The adapter protein Crk is also involved in TrkA signaling (36). In keeping with the ability of v-Src to modulate JNK, JNK activation by v-Src is blocked by dominant negative mutants of Crk (13). On the basis of these findings, we propose that a Src–PI3K–aPKC–JNK–NF-κB pathway exists, comprising the survival signaling cascade in PC12 cells.
While survival is Ras independent, confirming observations made by others (59), Src is required for survival responses. In keeping with Src's role in cell survival, overexpression of Src was observed to enhance cell survival in a serum-free environment to the extent that overexpression of PKC-ι did (58). In addition, Src overexpression resulted in constitutive activation of NF-κB. Moreover, only removal of Src or aPKCs drastically promoted cell death. These results demonstrate that neuroprotection can be mimicked by NF-κB activation and that cell viability is reduced by NF-κB inhibition. In this regard, inhibition of PI3K blocked cell survival and NGF-induced NF-κB activation. These results strongly support a pathway for NF-κB activation exerted by NGF that involves PI3K, Src, and aPKC, which collaborate to provide a neuroprotective function. This finding is in keeping with constitutive activation of NF-κB being capable of promoting resistance to apoptosis (15), and also the ability of NF-κB activation to promote survival in other systems (21). NGF induction of the NF-κB pathway is profoundly influenced by JNK compared to MAPK or p38, thus suggesting that modulation of JNK in PC12 cells may have a more direct influence on survival than would alterations in MAPK or p38. This is in fact the case, as inhibition of JNK abrogated NGF-induced κB activity and likewise blocked cell survival as well as differentiation. Therefore, we hypothesize that overexpression of any element of the Src-PI3K-aPKC-JNK pathway would promote increased NF-κB activity and cell survival.
Previous studies have shown that aPKC plays a critical role in κB-dependent transcription. Recent findings document that aPKCs directly regulate IKK in vitro and in vivo (29). Moreover, removal of aPKC was likewise observed to block NGF-induced NF-κB activation, whereas overexpression of aPKC enhanced NF-κB activity and survival of PC12 cells in a serum-free environment (58). The results presented herein further define a critical role of Src in activation of NF-κB and place Src, as well as PKC-ι, within a common signaling cascade regulating NF-κB. This is in keeping with recent observations made by this lab, demonstrating that Src binds aPKC and is likewise tyrosine phosphorylated (49; Vandenplas et al., submitted). Moreover, tyrosine phosphorylation of aPKC is mediated by Src independently of PI3K (Vandenplas, submitted for publication); likewise, PI3K modulates aPKC activity independently of an effect on Src. Thus, PI3K lies in a pathway distinct from Src, although tied to regulation of NF-κB through aPKC. In addition, evidence presented herein supports Src-induced modulation of aPKC as contributing to regulation of coupling between IKK/aPKC, NF-κB, and cell survival. In the absence of Src activity, aPKC fails to couple with IKK and likewise blocks NF-κB activation via inhibiting dissociation from IκB the resting complex, resulting in a direct effect on both survival and differentiation. Thus, Src emerges as a critical regulator necessary for coupling of PKC-ι to the κB pathway. In this regard, overexpression of Src, analogous to PKC-ι overexpression (58), enhances NF-κB activity as well as survival responsiveness.
Association of aPKC with the κB pathway in other cells occurs through p62, the aPKC binding protein (44), which localizes the PKC-ι to the receptor complex (47). Since Src modulates aPKC interaction with IKK, it stands to reason that Src may also play a role in directing the association of p62 with the receptor complex. Studies are under way to address this possibility. FEZ1, another recently identified aPKC binding protein (28), may serve to scaffold critical elements of the NGF receptor with the differentiation pathway, since this protein is homologous to UNC-76, a protein involved in axonal outgrowth and fasciculation in C. elegans. The differences between the two binding proteins, p62 and FEZ1, and their relationship to NGF responses warrant consideration since the scaffolding of particular signal cascades and the factors that regulate these interactions are likely to be critical to directing signal specificity. It is possible that the role of each binding protein is to scaffold aPKC to a particular signal pathway.
Src family kinases have previously been implicated in regulation of ostoeclastogenesis (1), T-cell function (14), and memory impairment (35). Similar to observations made herein, tumor necrosis factor induction of NF-κB in murine bone marrow macrophages is likewise mediated by Src, through formation of a long-lived complex between IκB and Src (1). In T cells, v-Src is capable of activating NF-κB (14). Also, an absence of Src family member Fyn has been associated with abnormal hippocampal development, defective long-term potentiation, and impaired memory (35), processes which have been coupled to the activation of aPKCs. Thus, regulation of aPKCs by Src-mediated tyrosine phosphorylation may be common to a number of other systems.
In summary, our study reveals that aPKC, regulated by a novel Src-kinase pathway, plays a critical role in activation of NF-κB. Due to the position that aPKC occupies, it plays a role in modulating both differentiation as well as survival signaling pathways. Thus, our observations are consistent with aPKC playing a critical role in regulating NGF responses in PC12 cells.
ACKNOWLEDGMENTS
We thank numerous investigators who provided cDNAs, reagents, and cell lines for this study. We thank members of our laboratory for fruitful discussion.
This research was funded by NINDS grant RO2-NS33661 and the Auburn University Biogrants Program.
REFERENCES
- 1.Abu-Amer Y, Ross F P, McHugh K P, Livolsi A, Peyron J, Teitelbaum S L. Tumor necrosis factor-α activation of nuclear transcription factor-κB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of IκBα. J Biol Chem. 1998;273:29417–29423. doi: 10.1074/jbc.273.45.29417. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S, Mizuno K, Hirai S, Kazlauskas A, Ohno S. EGF and PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 3.Altin J G, Wetts R, Riabowol K T, Bradshaw R A. Testing the in vivo role of protein kinase C and c-fos in neurite outgrowth by microinjection of antibodies into PC12 cells. Mol Biol Cell. 1992;3:323–333. doi: 10.1091/mbc.3.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induced morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- 5.Berra E, Diaz-Meco M T, Dominguez I, Municio M M, Sanz L, Lozano J, Chapkin R S, Moscat J. Protein kinase C ζ isoform is critical for mitogenic signal transduction. Cell. 1993;74:555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- 6.Berra E, Diaz-Meco M T, Lozano J, Frutos S, Municio M M, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C ζ. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berra E, Municio M M, Sanz L, Frutos S, Diaz-Meco M T, Moscat J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol Cell Biol. 1997;17:4346–4354. doi: 10.1128/mcb.17.8.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y-R, Tan T-H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17:173–178. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- 9.Coleman E S, Wooten M W. Nerve growth factor-induced differentiation of PC12 cells employs the PMA-insensitive protein kinase C-ζ isoform. J Mol Neurosci. 1994;5:39–57. doi: 10.1007/BF02736693. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Meco M T, Berra E, Municio M M, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera M T, Alcami J, Paya C V, Arenzana-Seisdedos F, Virelizier J L, Moscat J. A dominant negative protein kinase C ζ subspecies blocks NF-κB activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Meco M T, Lozano J, Municio M M, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C ζ. J Biol Chem. 1994;269:31706–31710. [PubMed] [Google Scholar]
- 12.Diaz-Meco M T, Municio M M, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ζ and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–111. doi: 10.1128/mcb.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolfi F, Garcia-Guzman M, Ojaniemi M, Nakamura H, Matsuda M, Vuori K. The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc Natl Acad Sci USA. 1998;95:15394–15399. doi: 10.1073/pnas.95.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eicher D M, Tan T-H, Rice N R, O'Shea J J, Kennedy I C S. Expression of v-src in T cells correlates with nuclear expression of NF-κB. J Immunol. 1994;152:2710–2719. [PubMed] [Google Scholar]
- 15.Giri D K, Aggarwal B B. Constitutive activation of NF-κB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 16.Hall F L, Fernyhough P, Ishii D N, Vuillet P R. Suppression of nerve growth factor-directed neurite outgrowth in PC12 cells by sphingosine an inhibitor of protein kinase C. J Biol Chem. 1988;263:4460–4466. [PubMed] [Google Scholar]
- 17.Heasley L E, Storey B, Fanger G R, Butterfield L, Zamarripa J, Blumberg D, Maue R A. GTPase-deficient Gα16 and Gαq induce PC12 cell differentiation and persistent activation of c-Jun NH2-terminal kinases. Mol Cell Biol. 1996;16:648–656. doi: 10.1128/mcb.16.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki S, Hattori A, Sato M, Tsujimoto M, Kohno M. Characterization of the bone morphogenetic protein-2 as a neurotrophic factor. J Biol Chem. 1996;271:17360–17365. doi: 10.1074/jbc.271.29.17360. [DOI] [PubMed] [Google Scholar]
- 19.Jackson T R, Blader I J, Hammonds-Odie L P, Burga C R, Cooke F, Hawkins P T, Wolf A G, Heldman K A, Theibert A B. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamieson L, Carpenter L, Biden T J, Fields A P. Protein kinase C ι activity is necessary for Bcr-Abl-mediated resistance to drug-induced apoptosis. J Biol Chem. 1999;274:3927–3930. doi: 10.1074/jbc.274.7.3927. [DOI] [PubMed] [Google Scholar]
- 21.Jimi E, Nakmura I, Ikebe T, Akiyama S, Takahashi N, Suda T. Activation of NF-κB is involved in the survival of osteoclasts promoted by interleukin-1. J Biol Chem. 1998;273:8799–8805. doi: 10.1074/jbc.273.15.8799. [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onoderam K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 23.Kita Y, Kimura K D, Kobayashi M, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Nagata S, Fukui Y. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the Rac-JNK signal transduction pathway. J Cell Sci. 1998;111:907–915. doi: 10.1242/jcs.111.7.907. [DOI] [PubMed] [Google Scholar]
- 24.Klesse L J, Meyers K A, Marshall C J, Parada L F. Nerve growth factor induces survival and differentiation through two distinct signaling cascades in PC12 cells. Oncogene. 1999;18:2055–2068. doi: 10.1038/sj.onc.1202524. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Nagata S, Kita Y, Nakatsu N, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Saitoh I, Fukui Y. Expression of a constitutively active phosphatidylinositol 3-kinase induces process formation in rat PC12 cells. J Biol Chem. 1997;272:16089–16092. doi: 10.1074/jbc.272.26.16089. [DOI] [PubMed] [Google Scholar]
- 26.Kremer N E, D'Arcangelo G, Thomas S M, DeMarco M, Brugge J S, Halegoua S. Signal transduction by nerve growth factor and fibroblast growth factor in PC12 cells requires a sequence of Src and Ras actions. J Cell Biol. 1991;115:809–819. doi: 10.1083/jcb.115.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo W-L, Chung K-C, Rosner M R. Differentiation of central nervous system neuronal cells by fibroblast-derived growth factor requires at least two signaling pathways: roles for Ras and Src. Mol Cell Biol. 1997;17:4633–4643. doi: 10.1128/mcb.17.8.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K. Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C ζ-interacting protein. J Cell Biol. 1999;144:403–411. doi: 10.1083/jcb.144.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lallena M J, Diaz-Meco M T, Bren G, Paya C V, Moscat J. Activation of IκB kinase B by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher E C. Evidence of ζ protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- 31.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Hsu H, Goedde D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd E D, Wooten M W. pp42/44 MAP kinase is a component of the neurogenic pathway utilized by nerve growth factor in PC12 cells. J Neurochem. 1992;59:1099–1109. doi: 10.1111/j.1471-4159.1992.tb08352.x. [DOI] [PubMed] [Google Scholar]
- 34.Logan S K, Falasca M, Hu P, Schlessinger J. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowell C A, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda M, Hashimoto Y, Muroya K, Hasegawa H, Kurata T. Crk proteins bind to two guanine nucleotide-releasing proteins for the Ras family and modulates nerve growth factor-induced activation of Ras in PC12 cells. Mol Cell Biol. 1994;14:5495–5500. doi: 10.1128/mcb.14.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller B S, Shankavaram U T, Horney M J, Gore A C S, Kurtzm D T, Rosenweig S A. Activation of cJun NH2-terminal kinase/stress-activated protein kinase by insulin. Biochemistry. 1996;35:8769–8775. doi: 10.1021/bi952651r. [DOI] [PubMed] [Google Scholar]
- 38.Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 39.Morooka T, Nishida E. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem. 1998;273:24285–24288. doi: 10.1074/jbc.273.38.24285. [DOI] [PubMed] [Google Scholar]
- 40.Murray N R, Fields A P. Atypical protein kinase C iota protects human leukemia cells against drug-induced apoptosis. J Biol Chem. 1997;272:27521–27524. doi: 10.1074/jbc.272.44.27521. [DOI] [PubMed] [Google Scholar]
- 41.Nakanishi H, Brewer K A, Exton J H. Activation of the ζ isozyme of protein kinase C by phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 42.Noda M, Ko M, Ogura A, Liu D G, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- 43.Pang L, Sawada T, Decker S J, Saltiel A R. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 44.Puls A, Schmidt S, Grawe F, Stabel S. Interaction of protein kinase C ζ with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA. 1997;94:6191–6196. doi: 10.1073/pnas.94.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raffioni S, Bradshaw R A. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor and nerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci USA. 1992;89:9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinhold D S, Neet K E. The lack of a role for protein kinase C in neurite extension and in the induction of ornithine decarboxylase by nerve growth factor in PC12 cells. J Biol Chem. 1989;264:3538–3544. [PubMed] [Google Scholar]
- 47.Sanz L, Sanchez P, Lallena M J, Diaz-Meco M T, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seibenhener M L, Rohem J, White W O, Neidigh K B W, Vandenplas M L, Wooten M W. Identification of Src as a novel atypical protein kinase C interacting protein. Mol Cell Biol Res Commun. 1999;2:28–31. doi: 10.1006/mcbr.1999.0140. [DOI] [PubMed] [Google Scholar]
- 50.Thomas S M, Hayes M, D'Arcangelo G, Armstrong R D, Meyer B E, Ziberstein A, Brugge J S, Halegoua S. Induction of neurite outgrowth by v-src mimics critical aspects of nerve growth factor-induced differentiation. Mol Cell Biol. 1991;11:4739–4750. doi: 10.1128/mcb.11.9.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:1134–1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- 52.Vaillancourt R R, Heasley L E, Zamarripa J, Storey B, Valius M, Kazlauskas A, Johnson G L. Mitogen-activated protein kinase activation is insufficient for growth factor receptor-mediated PC12 cell differentiation. Mol Cell Biol. 1995;15:3644–3653. doi: 10.1128/mcb.15.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Dijk M C M, Hilkmann H, van Blitterswijk W J. Platelet-derived growth factor activation of mitogen-activated protein kinase depends on the sequential activation of phosphatidylcholine-specific phospholipase C, protein kinase C-ζ and Raf-1. Biochem J. 1997;325:303–307. doi: 10.1042/bj3250303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood K W, Qi H, D'Arcangelo G, Armstrong R C, Roberts T M, Halegoua S. The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: a potential role for cellular raf kinases in neuronal growth factor signal transduction. Proc Natl Acad Sci USA. 1993;90:5016–5020. doi: 10.1073/pnas.90.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood K W, Sarnecki C, Roberts T M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, raf-1, and rsk. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 56.Wooten M W, Zhou G, Seibenhener M L, Coleman E S. A role for ζ-protein kinase C in nerve growth factor-induced differentiation of PC12 cells. Cell Growth Differ. 1994;5:395–403. [PubMed] [Google Scholar]
- 57.Wooten M W, Zhou G, Wooten M C, Seibenhener M L. Transport of protein kinase C isoforms to the nucleus of PC12 cells by nerve growth factor: association of atypical zeta PKC with the nuclear matrix. J Neurosci Res. 1997;49:393–403. doi: 10.1002/(sici)1097-4547(19970815)49:4<393::aid-jnr1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 58.Wooten M W, Seibenhener M L, Zhou G, Vandenplas M L, Tan T H. Overexpression of atypical PKC in PC12 cells enhances NGF responsiveness and survival through an NF-kappaB dependent pathway. Cell Death Differ. 1999;6:753–764. doi: 10.1038/sj.cdd.4400548. [DOI] [PubMed] [Google Scholar]
- 59.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 60.Zhou G, Seibenhener M L, Wooten M W. Nucleolin is a substrate of ζ-protein kinase C this is phosphorylated in response to nerve growth factor. J Biol Chem. 1997;272:31130–31137. doi: 10.1074/jbc.272.49.31130. [DOI] [PubMed] [Google Scholar]