Abstract
Simple Summary
The worldwide COVID-19 emergency has had an important impact on healthcare systems with the need to assist infected patients and also treat non-deferrable oncological conditions. In urology, the main concern has been for patients with bladder cancer, the tenth most common malignancy, where the quality and the alacrity of treatment has a clear well-demonstrated impact on the survivor. The aim of our Italian multi-institutional retrospective study was to assess the impact of the COVID-19 outbreak on diagnosis and treatment of non-muscle invasive bladder cancer. We observed a significant delay between diagnosis and surgical treatment, with a lower adherence to the standard therapeutic scheme such as BCG intravesical instillation and urological guidelines. We also recorded a different attitude in treatment depending on the patients’ location in Italy. Further investigation could show the impact of the pandemic on the survival of these patients.
Abstract
Background: To investigate the impact of COVID-19 outbreak on the diagnosis and treatment of non-muscle invasive bladder cancer (NMIBC). Methods: A retrospective analysis was performed using an Italian multi-institutional database of TURBT patients with high-risk urothelial NMIBC between January 2019 and February 2021, followed by Re-TURBT and/or adjuvant intravesical BCG. Results: A total of 2591 patients from 27 institutions with primary TURBT were included. Of these, 1534 (59.2%) and 1056 (40.8%) underwent TURBT before and during the COVID-19 outbreak, respectively. Time between diagnosis and TURBT was significantly longer during the COVID-19 period (65 vs. 52 days, p = 0.002). One thousand and sixty-six patients (41.1%) received Re-TURBT, 604 (56.7%) during the pre-COVID-19. The median time to secondary resection was significantly longer during the COVID-19 period (55 vs. 48 days, p < 0.0001). A total of 977 patients underwent adjuvant intravesical therapy after primary or secondary resection, with a similar distribution across the two groups (n = 453, 86% vs. n = 388, 86.2%). However, the proportion of the patients who underwent maintenance significantly differed (79.5% vs. 60.4%, p < 0.0001). Conclusions: The COVID-19 pandemic represented an unprecedented challenge to our health system. Our study did not show significant differences in TURBT quality. However, a delay in treatment schedule and disease management was observed. Investigation of the oncological impacts of those differences should be advocated.
Keywords: bladder cancer, SARS-CoV-2, intravesical BCG, trans-urethral resection of bladder tumor, Re-TURBT
1. Introduction
The American Cancer Society estimates about 83,730 new diagnoses of bladder cancer (BC) and 17,200 deaths in 2021 [1]. BC is the fourth most common cancer in men, but it is less common in women [2]. About 75% of newly diagnosed BCs are identified as non-muscle invasive (NMIBC) disease, i.e., limited to the mucosa (Ta and carcinoma in situ (CIS)) or to the lamina propria (T1) [3].
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the related disease, coronavirus disease 2019 (COVID-19), quickly generated a tragic health emergency in Italy due to the concurrent need to provide assistance to infected patients, and at the same time, to treat all the non-deferrable oncological and benign conditions [4].
The associated reallocation of resources needed to properly assist critically ill COVID-19 patients caused a similar redistribution of the activities of several medical disciplines not primarily involved in the care of COVID-19 patients [5]. Furthermore, the suspension of all outpatient and non-urgent activities, added to the restrictions in the scheduling of non-deferrable procedures, determined a major reorganization of urological activities [6,7,8,9,10].
For these reasons, it was challenging to meet the suggested timescales for NMIBC management [11]. In fact, non-muscle invasive bladder cancer (NMIBC) is an extremely time-sensitive disease due to its pathological characteristics, and prompt diagnosis and therapy are required for better clinical outcomes. Any delay in care concerning both time to diagnosis and time to treatment is associated with a higher pathological stage and a poor prognosis, especially for high-grade (HG) NMIBC [12].
This was the reason why, in 2006, a Canadian consortium of experts proposed a recommended maximum wait time of <14 days in cases of high-risk NMIBC and of <42 days in other types of NMIBC from the onset of symptoms and GP referral [13]. Regarding surgery, Rouprêt et al. also suggested that patients with NMIBC should undergo TURBT in < 1 month as prolonged surgical waiting time has an undeniable impact on the clinical outcomes, quality of life and anxiety of patients [3,14]. Moreover, a residual T1 HG/G3 tumor at Re-TURBT confers a worse prognosis in patients with primary T1 HG/G3 treated with maintenance BCG, and patients are very likely to fail BCG therapy alone [15].
Furthermore, in high-grade tumors, a full dose of BCG therapy lasting 3 years is associated with a reduction in recurrence, but not with a lower progression or a better overall survival; this implies that a shorter treatment is associated with worse outcomes [16].
Taking it all together, the aim of this multicenter study was to investigate the impact of the COVID-19 outbreak on the diagnosis and treatment of NMIBC.
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
The study was conducted as retrospective and all participating sites provided institutional data sharing agreements prior to the initiation of the trial. Each participant enrolled in the study signed an informed consent before undergoing intravesical BCG therapy according to the European Association of Urology (EAU) [17], Good Clinical Practice (GCP) guidelines [18], ethical principles of the latest version of the Declaration of Helsinki and General Data Protection Regulation (GDPR).
We performed a retrospective analysis of our Italian multi-institutional database of patients who underwent TURBT ± Re-TURBT followed by adjuvant intravesical BCG or MMC for histologically confirmed urothelial high-risk NMIBC between January 2019 and February 2021. The range of the study time was symmetrically distributed in order to obtain a balanced period of enrollment that would allow stratification of our cohort with regard to the pre-COVID-19 vs. COVID-19 period. We set 9 March 2020, as the reference line to define treatments that occurred within the Italian SARS-CoV-2 outbreak.
All the participants’ institutions were grouped into three different geographical areas, according to the Italian macro-regions (Northern, Central and Southern Italy), and were further stratified according to their case volume contribution, which was presented as quartile variations for each center’s enrollment.
Days from diagnosis to primary TURBT, from TURBT to Re-TURBT and from TURBT/Re-TURBT to adjuvant intravesical treatment initiation were collected for the whole cohort and presented together with demographic, clinic-pathological characteristics and all available covariates that could potentially influence the time to treatment during the pre- COVID-19 vs. COVID-19 period.
Patients with primary muscle-invasive disease (MIBC), non-urothelial carcinoma, with incomplete/missing data, or who received treatment for the specific diagnosis of interest later than 1 year after diagnosis, and 6 months following primary resection or Re-TURBT were excluded together with those patients who were treated with non-curative intervention.
2.2. Statistical Analysis
Statistical analyses as well as reporting and interpretation of the findings were conducted according to established guidelines and consisted of three analytical steps [19]. First, descriptive statistics were used to summarize the pertinent study information. The association between clinical, demographic, and peri-treatment variables reported as percentages (%), and/or median (IQR) during the pre-COVID-19 and COVID-19 period were tested by Student’s t-test or Fisher’s Exact for continuous variables and by the Pearson Chi-squared or Mann–Whitney U test for categorical variables when appropriate.
Second, the univariate effect of the COVID-19 period on time to treatment outcomes was explored by the Kaplan–Meier product-limit method. The log-rank test assessed crude subgroup differences subsequently adjusted for multiple confounders appropriate for the topic of interest.
Third, three separated sets of univariate logistic regression models were developed by testing each potential factor (both dichotomized or continuous variables) influencing the observed median time to TURBT, Re-TURBT and adjuvant intravesical treatment, with significance set at p ≤ 0.05. Subsequent specific multivariable stepwise regression models (forward selection) were further generated by selecting those predictive variables that were significant upon univariate analysis, by entering and removing limits set at p = 0.05 and p = 0.10, respectively. In particular, covariates for each endpoint consisted of center-based, diagnostic-based, tumor-based, and COVID-19 period features as listed below in the respective tables.
Finally, the locally weighted scatter-plot smoother (LOWESS) function was used on the sole sub-group of COVID-19 period patients to graphically depict the predicted probability of a median longer time to intervention according to the three different geographical regions of provenience and according to the single-center volume case quartile distribution.
3. Results
3.1. Study Cohort Characteristics
According to pre-established criteria, the final cohort who received at least the primary TURBT consisted of n = 2591 patients who underwent resection from a total of n = 27 academic or non-academic institutions through the whole of Italy. The majority of the enrolling centers were from Northern Italy with n = 14 institutions followed by n = 5 and n = 8 institutions from Southern and Central Italy, respectively, with similar correspondence relative to the regions’ influence in terms of case recruitment. The whole study cohort baseline and first TURBT peri-operative characteristics were divided according to COVID-19 period and are summarized in Table 1. Of these, n = 1534 (59.2%) patients underwent primary resection before the COVID-19 outbreak and n = 1056 (40.8%) patients during COVID-19. There was only a slight but significant difference between the pre and COVID-19 period in terms of the percentage of recruitment, especially within the Northern institutions (50.7% vs. 45.3%). Out of the whole cohort, the median case volume was 74 (49–109) patients for each center, with a significant difference in terms of patients treated within the pre and COVID-19 period, especially for those among the 4th quartile volume distribution (59.3% vs. 50%).
Table 1.
Descriptive characteristics of the study cohort. In bold, value < 0.05.
| Primary TURBT Demographic and Clinic-Pathological Features | |||||
|---|---|---|---|---|---|
| Variables | Pre-Covid-19 Period | % | Covid-19 Period | % | p-Value |
| Sample size, n (%) | 1535 | 59.2 | 1056 | 40.8 | |
| Regions of provenience, n (%) | <0.0001 | ||||
| Northern Italy | 778 | 50.7 | 478 | 45.3 | |
| Central Italy | 380 | 24.8 | 351 | 33.2 | |
| Southern Italy | 377 | 24.6 | 227 | 21.5 | |
| Center volume case, quartiles | <0.0001 | ||||
| 1st quartile | 134 | 8.7 | 72 | 6.8 | |
| 2nd quartile | 211 | 13.7 | 189 | 17.9 | |
| 3rd quartile | 280 | 18.2 | 267 | 25.3 | |
| 4th quartile | 910 | 59.3 | 528 | 50.0 | |
| Median age, years (IQR) | 74 (68–81) | 74 (66–81) | 0.247 | ||
| Gender, n (%) | 0.429 | ||||
| Male | 1222 | 79.6 | 854 | 80.9 | |
| Female | 313 | 20.4 | 202 | 19.1 | |
| Smoking status, n (%) | 0.001 | ||||
| Never | 629 | 41.0 | 450 | 42.6 | |
| Active | 860 | 56.0 | 599 | 56.7 | |
| Former | 46 | 3.0 | 7 | 0.7 | |
| ACCI score, n (%) | 0.011 | ||||
| 0–2 | 504 | 32.8 | 297 | 28.1 | |
| ≥3 | 1031 | 67.2 | 759 | 71.9 | |
| Hematuria at diagnosis, n (%) | 0.539 | ||||
| No | 509 | 33.2 | 338 | 32.0 | |
| Yes | 1026 | 66.8 | 718 | 68.0 | |
| Dysuria at diagnosis, n (%) | 0.001 | ||||
| No | 1132 | 73.7 | 837 | 79.3 | |
| Yes | 403 | 26.3 | 219 | 20.7 | |
| ER access at diagnosis, n (%) | 0.086 | ||||
| No | 1310 | 85.3 | 874 | 82.8 | |
| Yes | 225 | 14.7 | 182 | 17.2 | |
| Diagnosis modality, n (%) | <0.0001 | ||||
| Ultrasound | 781 | 50.9 | 517 | 49.0 | |
| CT scan | 172 | 11.2 | 143 | 13.5 | |
| Cystoscopy | 446 | 29.1 | 361 | 34.2 | |
| All combined | 136 | 8.9 | 35 | 3.3 | |
| Urinary cytology, n (%) | 0.432 | ||||
| Not performed | 925 | 60.3 | 663 | 62.8 | |
| Negative for TCC | 239 | 15.6 | 154 | 14.6 | |
| Positive for TCC | 371 | 24.2 | 239 | 22.6 | |
| Diagnostic tumor findings | |||||
| Tumor focality, n (%) | 0.478 | ||||
| Unifocal | 885 | 57.7 | 594 | 56.3 | |
| Multifocal | 650 | 42.3 | 462 | 43.8 | |
| Ureteral orifice involvement, n (%) | 0.034 | ||||
| No | 1463 | 95.3 | 1024 | 97.0 | |
| Yes | 72 | 4.7 | 32 | 3.0 | |
| Concomitant Hydronephrosis, n (%) | 0.359 | ||||
| No | 1411 | 91.9 | 981 | 92.9 | |
| Yes | 124 | 8.1 | 75 | 7.1 | |
| Concomitant UTUC, n (%) | 0.003 | ||||
| No | 1463 | 95.3 | 1030 | 97.5 | |
| Yes | 72 | 4.7 | 26 | 2.5 | |
| Perioperative characteristics | |||||
| Median time from diagnosis to TURBT, days (IQR) | 52 (29–75) | 65 (33–84) | 0.002 | ||
| Tumor size, n (%) | 0.469 | ||||
| <3 cm | 1136 | 74.0 | 768 | 72.7 | |
| ≥3 cm | 399 | 26.0 | 288 | 27.3 | |
| Tumor T stage, n (%) | 0.105 | ||||
| T0/Tx | 48 | 3.1 | 40 | 3.8 | |
| Ta | 625 | 40.7 | 434 | 41.1 | |
| T1 | 776 | 50.6 | 516 | 48.9 | |
| ≥T2 | 35 | 2.3 | 40 | 3.8 | |
| Tis | 51 | 3.3 | 26 | 2.5 | |
| Detrusor in the specimen, n (%) | 0.136 | ||||
| Present | 1162 | 75.7 | 826 | 78.2 | |
| Absent | 373 | 24.3 | 230 | 21.8 | |
| Tumor histology, n (%) | 0.563 | ||||
| TCC | 1476 | 96.2 | 1020 | 96.6 | |
| Other | 59 | 3.8 | 36 | 3.4 | |
| CIS, n (%) | 0.376 | ||||
| Absent | 1380 | 89.9 | 964 | 91.3 | |
| Pure CIS | 51 | 3.3 | 26 | 2.5 | |
| Concomitant CIS | 104 | 6.8 | 66 | 6.3 | |
| LVI, n (%) | 0.058 | ||||
| Absent | 1465 | 95.4 | 990 | 93.8 | |
| Present | 70 | 4.6 | 66 | 6.3 | |
| Operator experience, n (%) | 0.347 | ||||
| ≥100 TURBTs | 1251 | 81.5 | 845 | 80.0 | |
| <100 TURBTs | 284 | 18.5 | 211 | 20.0 | |
| Perioperative intravesical CHT, n (%) | 0.007 | ||||
| None | 1485 | 96.7 | 1008 | 95.5 | |
| Mitomycin-C | 26 | 1.7 | 37 | 3.5 | |
| Epirubicin | 24 | 1.6 | 11 | 1.0 | |
The diagnostic modality strategies to detect BC were found to be slightly, but significantly different across the COVID-19 period. In particular, there was a minimal trend toward more direct visual inspection of the suspected lesions with more cystoscopies performed (29.1% vs. 34.2%), while the choice of a combined diagnostic strategy was clearly reduced down to only 3% of the sample.
Ultimately, no further significant or clinically relevant differences were identified among the demographic variables, diagnostic tumor features, perioperative characteristics, and histopathological findings.
3.2. Time from Diagnosis to Primary TURBT
The time from identification of a bladder lesion to primary resection was significantly longer during the COVID-19 period with a median of 65 (33–84) days vs. 52 (29–75) (p = 0.002).
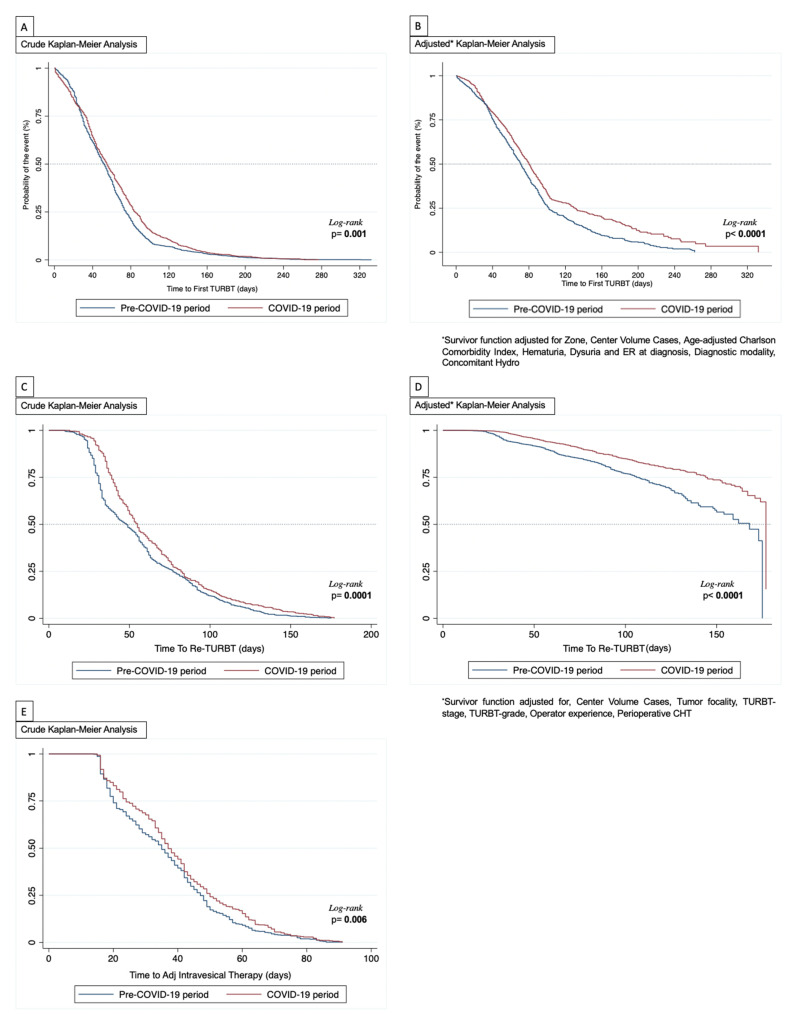
Kaplan–Meier analysis showed that the 30-days to TURBT residual function was 72.6% (95%CI: 69.9–74.4) and 76.7% (95%CI: 74.2–79.3) during the pre vs. COVID-19 period, respectively. Similarly, at 60 and 90-days the residuals for those who had not yet undergone TURBT were 41.1% (95%CI: 38.6–43.6), 45.6% (95%CI: 42.6–48.6) and 14.1% (95%CI: 12.3–15.8), 21.3% (95%CI: 18.8–23.8), respectively (log-rank, p = 0.001; Figure 1A). The same tendency was observed when the residual function was adjusted for the factors independently influencing the median time to TURBT (Figure 1B). Additionally, multivariable logistic regression analysis showed that a primary resection during the COVID-19 period was an independent predictor for delayed median time to TURBT (OR, 1.26, 95%CI: 1.06–1.51; Table 2). Finally, when analyzing only the last sub-group of patients who underwent TURBT during the COVID-19 period, the LOWESS function depicted an increased predicted probability to receive a primary resection with a median time > 65 days in the Northern centers, while this prediction was linearly reduced for Central and Southern centers (Figure 2A). Interestingly, the probability of a longer time to primary resection was almost exponentially increased among those institutions with a baseline higher case volume (Figure 2B).
Figure 1.
Kaplan–Meier analysis. (A) Crude analysis for time to first TURBT; (B) Adjusted analysis for time to first TURBT; (C) Crude analysis for time to Re-TURBT; (D) Adjusted analysis for time to Re-TURBT; (E): Crude analysis for time to adjuvant intravesical therapy.
Table 2.
Descriptive characteristics of Re-TURBT cohort. In bold, value < 0.05.
| Re-TURBT Demographic and Clinic-Pathological Features | |||||
|---|---|---|---|---|---|
| Variables | Pre-COVID-19 Period | % | COVID-19 Period | % | p Value |
| Sample size, n (%) | 604 | 56.7 | 462 | 43.3 | |
| Regions of provenience, n (%) | 0.015 | ||||
| Northern Italy | 283 | 46.9 | 223 | 48.3 | |
| Central Italy | 76 | 12.6 | 83 | 18.0 | |
| Southern Italy | 245 | 40.6 | 156 | 33.8 | |
| Center case volume, quartiles | <0.0001 | ||||
| 1st quartile | 65 | 10.8 | 25 | 5.4 | |
| 2nd quartile | 52 | 8.6 | 40 | 8.7 | |
| 3rd quartile | 85 | 14.1 | 146 | 31.6 | |
| 4th quartile | 402 | 66.6 | 251 | 54.3 | |
| Median age, years (IQR) | 74 (65–80) | 74 (67–80) | 0.332 | ||
| Gender, n (%) | 0.621 | ||||
| Male | 495 | 82.0 | 384 | 83.1 | |
| Female | 109 | 18.0 | 78 | 16.9 | |
| ACCI score, n (%) | 0.567 | ||||
| Perioperative features, n (%) | |||||
|
Median time to Re-TURBT, days (IQR) |
48 (31–77) | 55 (39–82) | <0.0001 | ||
| Re-TURBT T stage, n (%) | 0.714 | ||||
| T0/Tx | 352 | 58.3 | 258 | 55.8 | |
| Ta | 103 | 17.1 | 81 | 17.5 | |
| T1 | 86 | 14.2 | 76 | 16.5 | |
| ≥T2 | 23 | 3.8 | 13 | 2.8 | |
| Tis | 40 | 6.6 | 34 | 7.4 | |
| Tumor Grade (WHO 2004), n (%) | 0.100 | ||||
| Negative | 354 | 58.6 | 258 | 55.8 | |
| LG | 56 | 9.3 | 31 | 6.7 | |
| HG | 194 | 32.1 | 173 | 37.4 | |
| CIS, n (%) | 0.399 | ||||
| Not applicable | 515 | 85.3 | 381 | 82.5 | |
| Pure CIS | 49 | 8.1 | 48 | 10.4 | |
| Concomitant CIS | 40 | 6.6 | 33 | 7.1 | |
| Operator experience, n (%) | 0.264 | ||||
| ≥100 TURBTs | 134 | 22.2 | 116 | 25.1 | |
| <100 TURBTs | 470 | 77.8 | 346 | 74.9 | |
Figure 2.
LOWES functions. (A) Predicted probability time to first TURB > 65 days among institutions; (B) Predicted probability time to first TURB > 65 days among center volume percentiles; (C) Predicted probability time to Re-TURB > 55 days among institutions; (D) Predicted probability time to Re-TURB > 55 days among center volume percentiles.
3.3. Time from TURBT to Secondary Resection (Re-TURBT)
Within the study population, n = 1066 (41.1%) received Re-TURBT with n = 604 (56.7%) during the pre-COVID-19 and n = 462 (43.3%) during COVID-19 period. The median time to secondary resection was significantly longer during the COVID-19 period with a median of 55 (39–82) days vs. 48 (31–77) days, respectively (p < 0.0001) (Table 3).
Table 3.
Descriptive characteristics of adjuvant intravesical therapy cohort. In bold, value < 0.05.
| Adjuvant Intravesical Therapy Demographic and Treatment Schedule | |||||
|---|---|---|---|---|---|
| Variables | Pre-COVID-19 Period | % | COVID-19 Period | % | p Value |
| Sample size, n (%) | 527 | 53.9 | 450 | 46.1 | |
| Regions of provenience, n (%) | <0.0001 | ||||
| Northern Italy | 298 | 56.5 | 220 | 48.9 | |
| Central Italy | 44 | 8.3 | 113 | 25.1 | |
| Southern Italy | 185 | 35.1 | 117 | 26.0 | |
| Center case volume, quartiles | <0.0001 | ||||
| 1st quartile | 34 | 6.5 | 37 | 8.2 | |
| 2nd quartile | 33 | 6.3 | 44 | 9.8 | |
| 3rd quartile | 46 | 8.7 | 124 | 27.6 | |
| 4th quartile | 414 | 78.6 | 245 | 54.4 | |
| Median age, years (IQR) | 74 (68–80) | 73 (65–79) | 0.038 | ||
| Gender, n (%) | 0.209 | ||||
| Male | 429 | 81.4 | 380 | 84.4 | |
| Female | 98 | 18.6 | 70 | 15.6 | |
| ACCI score, n (%) | 0.276 | ||||
| 0–2 | |||||
| ≥3 | 382 | 72.5 | 340 | 75.6 | |
| Median time to Adj Intravesical Therapy, days (IQR) | 35 (20–47) | 37 (24–50) | |||
| Intravesical Drug, n (%) | 0.905 | ||||
| Mitomycin-C | 74 | 14.0 | 62 | 13.8 | |
| BCG | 453 | 86.0 | 388 | 86.2 | |
| Intravesical Adj schedule, n (%) | <0.0001 | ||||
| Only Induction | 94 | 17.8 | 143 | 31.8 | |
| Induction + Maintenance | 419 | 79.5 | 272 | 60.4 | |
| SWOG BCG maintenance, n (%) | <0.0001 | ||||
| 3 months | 27 | 5.1 | 53 | 11.8 | |
| 6 months | 49 | 9.3 | 44 | 9.8 | |
| 12 months | 131 | 24.9 | 19 | 4.2 | |
| >12 months | 139 | 26.4 | 65 | 14.4 | |
The Kaplan Meier analysis showed that the 30, 60 and 90-days to Re-TURBT residual function were 76% (95%CI: 72.6–79.4) vs. 91.8% (95%CI: 89.3–84.3), 37.4% (95%CI: 36.4–46.3) vs. 43.7% (95%CI: 39.2–48.2) and 17.2% (95%CI: 14.2–20.2) vs. 19.5% (95%CI: 15.9–23.1) during the pre and COVID-19 period, respectively (log-rank, p < 0.0001; Figure 1C), even after adjusting for confounders as shown in Figure 1D. Similar to the first TURBT, the multivariable logistic regression analysis showed that the COVID-19 period was an independent predictor for experiencing delayed time to secondary resection (OR: 1.30, 95%CI: 1.05–1.71; Table 4). Of note, as depicted from the LOWESS function only from the COVID-19 months and similarly to what was observed for the primary TURBT analysis, there was a comparable trajectory for the predicted probability of experiencing a median time to Re-TURBT > 55 days among the Northern through to the Southern institutions (Figure 2C). Differently, the probability of having a delayed Re-TURBT was significantly diminished if the surgery was performed in an institution enrolling a high case volume (i.e., 3rd or 4th quartile volume distribution; Figure 2D).
Table 4.
Univariable and multivariable logistic regression analysis for delayed time to secondary resection. In bold, value < 0.05.
| Subgroups and/or Continuous Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | ||
| Region of provenience | Northern Italy | Ref | -- | ||
| Central Italy | 1.42 (0.87–2.37) | 0.63 | |||
| Southern Italy | 0.97 (0.66–1.85) | 0.54 | |||
| Center volume case | 1st quartile | Ref | -- | ||
| 2nd quartile | 0.70 (0.46–1.06) | 0.09 | |||
| 3rd quartile | 1.05 (0.75–1.49) | 0.77 | |||
| 4th quartile | 0.90 (0.65–1.24) | 0.53 | |||
| Age, years | Continuous | 1.01 (0.98–1.02) | 0.06 | ||
| Age, years | <70 | Ref | -- | ||
| ≥70 | 1.04 (0.88–1.23) | 0.66 | |||
| Gender | Male | Ref | -- | ||
| Female | 1.01 (0.83–1.22) | 0.94 | |||
| ACCI, score | 0–2 | Ref | -- | Ref | -- |
| ≥3 | 2.13 (1.72–2.56) | <0.0001 | 1.80 (1.44–2.26) | < 0.0001 | |
| Hematuria at diagnosis | No | Ref | -- | Ref | -- |
| Yes | 0.66 (0.56–0.78) | <0.0001 | 0.80 (0.66–0.97) | 0.023 | |
| Dysuria at diagnosis | No | Ref | -- | Ref | -- |
| Yes | 0.75 (0.63–0.90) | 0.002 | 0.87 (0.71–1.06) | 0.16 | |
| ER access at diagnosis | No | Ref | -- | Ref | -- |
| Yes | 0.68 (0.55–0.84) | 0.001 | 0.76 (0.59–0.97) | 0.029 | |
| Diagnosis modality | Ultrasound | Ref | -- | Ref | -- |
| CT scan | 1.38 (1.08–1.76) | 0.011 | 1.47 (0.78–1.94) | 0.19 | |
| Cystoscopy | 1.27 (1.07–1.52) | 0.008 | 1.33 (0.66–1.62) | 0.26 | |
| All combined | 2.51 (1.79–3.53) | < 0.0001 | 1.42 (0.74–2.73) | 0.292 | |
| Urinary cytology | Not performed | Ref | -- | ||
| Negative for TCC | 0.84 (0.67–1.04) | 0.112 | 1.28 (0.61–1.56) | 0.21 | |
| Positive for TCC | 0.49 (0.41–0.60) | <0.0001 | 0.55 (0.44–0.68) | < 0.0001 | |
| Tumor focality | Unifocal | Ref | -- | ||
| Multifocal | 0.93 (0.80–1.09) | 0.37 | |||
| Ureteral orifice involvement | No | Ref | -- | ||
| Yes | 1.19 (0.80–1.77) | 0.38 | |||
| Concomitant Hydronephrosis | No | Ref | -- | Ref | -- |
| Yes | 0.56 (0.42–0.76) | 0.001 | 0.69 (0.49–1.96) | 0.27 | |
| Concomitant UTUC | No | Ref | -- | ||
| Yes | 0.79 (0.52–1.18) | 0.24 | |||
| TURBT period | Pre-COVID-19 | Ref | -- | Ref | -- |
| COVID-19 | 1.32 (1.11–1.62) | 0.032 | 1.26 (1.06–1.51) | 0.01 | |
3.4. Time from TURBT/Re-TURBT to Adjuvant Intravesical Therapy
The sample who underwent adjuvant intravesical therapy was limited to n = 977 patients, accounting for n = 527 (53.9%) and n = 450 (46.1%) during the pre and COVID-19 period. As expected, the vast majority of the patients who received adjuvant BCG were equally distributed across the non-COVID-19 or COVID-19 period (n = 453, 86% vs. n = 388, 86.2%, respectively; Table 5). In addition, the proportion of patients who underwent induction plus a maintenance course during the COVID-19 period was reduced when compared to the non-COVID-19 period (79.5% vs. 60.4%, p < 0.0001), while among patients in a maintenance course only, the SWOG schedule was longer than 12 months (24.9% vs. 4.2%, p < 0.0001).
Table 5.
Univariable and multivariable logistic regression analysis for delayed time to adjuvant intravesical therapy (induction). In bold, value < 0.05.
| Subgroups and/or Continuous Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | ||
| Region of provenience | Northern Italy | Ref | -- | ||
| Central Italy | 1.25 (0.86–1.83) | 0.25 | |||
| Southern Italy | 0.49 (0.11–2.19) | 0.59 | |||
| Center volume case, quartiles | 1st quartile | Ref | -- | Ref | -- |
| 2nd quartile | 1.43 (0.79–2.61) | 0.24 | 1.19 (0.61–2.11) | 0.34 | |
| 3rd quartile | 0.49 (0.29–0.81) | 0.006 | 0.58 (0.39–1.06) | 0.24 | |
| 4th quartile | 0.47 (0.30–0.74) | 0.001 | 0.64 (0.45–0.89) | 0.0013 | |
| ACCI, score | 0–2 | Ref | -- | ||
| ≥3 | 1.57 (2.23–1.11) | 0.001 | |||
| Tumor focality, n | Unifocal | Ref | -- | Ref | -- |
| Multifocal | 0.73 (0.57–0.93) | 0.01 | 0.75 (0.58–0.99) | 0.039 | |
| Tumor size, cm | <3 cm | Ref | -- | ||
| ≥3 cm | 1.26 (0.96–1.66) | 0.1 | |||
| Tumor stage TNM | Ta | Ref | -- | Ref | -- |
| T1 | 0.55 (0.42–0.72) | <0.0001 | 0.69 (0.51–0.93) | 0.017 | |
| Tis | 1.66 (0.67–4.13) | 0.273 | |||
| Tumor Grade, WHO 2004 | LG | Ref | -- | Ref | -- |
| HG | 0.22 (0.12–0.40) | <0.0001 | 0.25 (0.10–0.62) | <0.0001 | |
| Detrusor in the specimen | No | Ref | -- | ||
| Yes | 0.62 (0.46–0.84) | 0.002 | |||
| Tumor histology | TCC | Ref | -- | ||
| Other | 1.16 (0.59–2.30) | 0.67 | |||
| Concomitant CIS | No | Ref | -- | Ref | -- |
| Yes | 0.55 (0.37–0.83) | 0.005 | 0.71 (0.46–1.09) | 0.12 | |
| LVI | No | Ref | -- | ||
| Yes | 1.63 (0.92–2.90) | 0.1 | |||
| Operator experience | ≥100 TURBTs | Ref | -- | Ref | -- |
| <100 TURBTs | 1.63 (1.23–2.18) | 0.001 | 1.42 (1.04–1.95) | 0.028 | |
| Perioperative CHT | No | Ref | -- | Ref | -- |
| Yes | 3.36 (1.22–9.23) | 0.019 | 4.77 (1.57–14.50) | 0.006 | |
| Concomitant Hydronephrosis | No | Ref | -- | ||
| Yes | 0.98 (0.64–1.52) | 0.94 | |||
| Concomitant UTUC | No | Ref | -- | ||
| Yes | 1.21 (0.70–2.09) | 0.5 | |||
| Re-TURBT period | Pre-COVID-19 | Ref | -- | Ref | -- |
| COVID-19 | 1.32 (1.03–1.68) | 0.026 | 1.30 (1.05–1.71) | 0.036 | |
Kaplan–Meier analysis showed that the 30 and 60-days from last TURBT to adjuvant intravesical therapy were 57.1% (95%CI: 52.9–61.3) vs. 67.6% (95%CI: 63.2–71.9), and 9.1% (95%CI: 6.7–11.6) vs. 15.3% (95%CI: 12–18.7) during the pre and COVID-19 period, respectively (log-rank, p = 0.006; Figure 1E). Although the COVID-19 period was a risk factor upon univariate analysis (OR: 1.25, 95%CI: 0.97–1.61), multivariable logistic regression analysis showed it was not independently associated with delayed time to the beginning of adjuvant intravesical therapy (OR: 1.11, 95%CI: 0.84–1.38; Table 6).
Table 6.
Univariable and multivariable logistic regression analysis for delayed time to adjuvant intravesical therapy (maintenance). In bold, value < 0.05.
| Subgroups and/or Continuous Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | ||
| Region of provenience | Northern Italy | Ref | -- | ||
| Central Italy | 0.96 (0.66–1.41) | 0.85 | |||
| Southern Italy | 1.16 (0.55–1.28) | 0.72 | |||
| Center volume case, quartiles | 1st quartile | Ref | -- | Ref | -- |
| 2nd quartile | 0.71 (0.37–1.36) | 0.31 | 0.92 (0.42–1.99) | 0.83 | |
| 3rd quartile | 0.60 (0.30–0.92) | 0.034 | 1.03 (0.52–2.06) | 0.93 | |
| 4th quartile | 0.30 (0.17–1.54) | <0.0001 | 0.51 (0.28–0.94) | 0.03 | |
| ACCI, score | 0–2 | Ref | -- | ||
| ≥3 | 0.70 (0.49–1.02) | 0.063 | |||
| Adjuvant Intravesical Drug | Mitomycin-C | Ref | -- | Ref | -- |
| BCG | 0.25 (0.16–0.38) | <0.0001 | 0.37 (0.23–0.59) | <0.0001 | |
| Tumor stage at TURBT/Re-TUR | Ta | 0.88 (0.55–1.41) | 0.60 | ||
| T1 | 1.62 (1.00–2.63) | 0.05 | |||
| Tis | 1.60 (0.90–2.84) | 0.11 | |||
| Tumor Grade, WHO 2004 | LG | 0.92 (0.49–1.74) | 0.79 | ||
| HG | 1.43 (1.00–2.04) | 0.05 | |||
| Concomitant CIS at TURBT/Re-TUR | No | Ref | -- | ||
| Yes | 1.23 (0.57–2.62) | 0.60 | |||
| Adjuvant Intravesical period | Pre-COVID-19 | Ref | -- | Ref | -- |
| COVID-19 | 1.25 (0.97–1.61) | 0.008 | 1.11 (0.84–1.38) | 0.35 | |
4. Discussion
As shown in our study, the COVID-19 outbreak led to a delay in surgical therapy (TURBT and Re-TURBT). To mitigate the potential impact of procedure deferral, the EAU proposed additional guidelines to help urologists in their activity [20,21]. The EAU categorized diagnoses of NMIBC into four priority groups according to clinical harm: low priority patients, who should be postponed by 6 months (small papillary recurrences < 1 cm and/or history of Ta/1 low-grade BC); intermediate (BC > 1cm) and high priority patients (high-risk BC or macroscopic hematuria) who should be not postponed beyond 3–4 months and 6 weeks, respectively. In addition, immediate radical cystectomy has been suggested in case of high-risk NMIBC or BCG failure while, reasonably, emergencies should be diagnosed and treated as soon as possible (e.g., macroscopic hematuria with clot retention) [20].
Our study showed that the diagnostic strategies used to detect BCs changed during the COVID-19 period. More specifically, a minimal trend toward more direct visual inspection of the suspected lesions was observed, with more cystoscopies performed (29.1% vs. 34.2%). We can hypothetically explain this trend by the lower outpatient activity (such as the US) determined by resource optimization for the pandemic, and also by with limited use of urinary biomarkers due to their accuracy, availability and high costs. Moreover, we found a reduced use of combined diagnostic strategy, down to only 3% of the sample.
Secondly, the time to treatment during the COVID-19 period was significantly prolonged when compared to times before the pandemic (65 vs. 52 days). In addition, the decreased activity of general practitioners as well as the residents in small or medium-sized cities could have further impacted those delays [22]. The length of the surgical wait time is of crucial importance in BC and patients should undergo a TURBT within 30 days. A delay of over 68 days in this procedure worsens the overall survival at 1, 3 and 5 years as reported by Wallace et al. and therefore, we expect inauspicious outcomes for the sample of patients of the COVID-19 period in the future [23].
Interestingly, our study shows how the probability of a longer time to primary resection was almost exponentially increased among those institutions with a higher case volume baseline, which tends to coincide with the Northern centers. This indicates more difficulties in hospital organization due to the higher number of hospitalized COVID-19 cases. In fact, the reallocation of medical personnel to new COVID-19 wards and the associated reduction in active personnel due to the COVID-19 infection, produced a dramatic change in routine clinical and surgical practice, as already demonstrated by Naspro et al. [24,25].
Similarly, time to Re-TURBT was prolonged during the COVID-19 pandemic (55 vs. 48 days), even though EAU guidelines for NMIBC suggest the second resection 2–6 weeks after the initial TURBT. In contrast with time to first treatment, high volume centers had a shorter time to Re-TURBT when compared to institutions with a smaller volume of cases, which were located in Central and Southern Italy. It is clear that there are many socioeconomic differences across Italy and this results in better organization in the Northern part where oncological hubs were created for better management of patients with BC. Oncologic hub hospitals must fulfill specific requirements, which include: the role as a referral center with high surgical volume and experience; low risk for complications and prolonged hospitalization; the ability to treat oncologic patients in dedicated spaces in order to preserve immunosuppressed subjects from possible COVID-19 infections; the presence of sustainable resources for infrastructural, medical and paramedical necessities aimed to reduce the deferral of cancer patients during the COVID-19 pandemic [26].
Regarding BCG therapy, our results showed that the percentage of patients treated with immunotherapy during the COVID-19 period was comparable to the pre-COVID-19 era (86.2 vs. 86%). We noticed a delay in the 30- and 60- days from last TURBT to adjuvant intravesical BCG administration across the two periods, 57.1% vs. 67.6% and 9.1% vs. 15.3%, respectively.
In addition, our study showed a reduced proportion of patients who underwent BCG therapy (induction + maintenance) after surgery during the COVID-19 period (60.4%) with more difficulty in following the SWOG schedule longer than 12 months. Patients involved in a BCG scheme in the years before the COVID-19 pandemic had more difficulties in maintaining the immunotherapy. As we have learnt from BCG shortages in past, the difference between 3 years maintenance compared to 1 year of maintenance was significant regarding recurrence rate, although no effect on progression or death has been reported [27].
A delay in cancer treatment and disturbances in cancer care during the COVID-19 period was also reported by Schimdt et al., who outlined a significant disruption to cancer care during the pandemic and a decrease in outpatient visits at tertiary institutions in New York and Boston [28]. Similar findings were also reported in case of patients diagnosed with oral squamous cell carcinoma, with a treatment delay in 2020 of 45 days compared to 35 days in the 2010–2019 period (p = 0.004) [29]. A systematic review concluded that patients and caregivers experienced delays in screening, treatment and care of cancer during the COVID-19 pandemic [30].
5. Limitations of the Study
To our knowledge, this is the first study to test the impact of the SARS-CoV-2 pandemic on the management of HG-NMIBC, in particular on time to treatment, time to Re-TURBT and BCG administration. Some limits should be taken into consideration. Firstly, our study is based on a retrospective analysis of data, which implies the impossibility of predicting the impact of the pandemic on the clinical outcomes of our sample of patients. To better understand the role of the lack of NMIBC management, a long-term follow-up of the same patients should be conducted in the next few years. Secondly, the distribution of the sample of patients was not uniform across Italy, because of the difficulties in collecting data from those non-academic institutions overwhelmed by COVID-19 emergencies.
6. Future Perspectives
The COVID-19 pandemic not only determined a redistribution of the activities of several medical disciplines but also had a clear impact on oncological patient therapies, as we demonstrated for HG-NMIBC. The actual impact of SARS-CoV-2 on clinical outcomes is still to be understood. To reduce the delay in BC management several diagnostic strategies can be implemented. Firstly, we recommend better adherence to the guidelines in order to obtain better stratification of patients with HG-NMIBC [31]. Secondly, the Vesical Imaging-Reporting and Data System (VI-RADS) may offer a reliable first-step diagnostic tool in identifying and prioritizing patients who would benefit from immediate intervention [32]. Thirdly, the expansion of the role of urinary biomarkers in diagnostic and surveillance pathways could be a feasible strategy to solve the waiting times for cystoscopies [33,34].
7. Conclusions
The COVID-19 pandemic represented a novel and groundbreaking challenge to our health system, and also heavily influenced the training and education of urology residents. According to our study, although TURBT quality was not significantly affected by the COVID-19 pandemic, a delay in treatment schedules and disease management was observed. Further, the oncological impact should be investigated, in order to assess the whole impact of the COVID-19 pandemic on the outcomes of patients with NMIBC.
Acknowledgments
The authors would like to express their deepest gratitude to Fondazione Muto Onlus in Naples for the support of the publication of this manuscript.
Author Contributions
Conceptualization, M.F. and F.D.G.; methodology, M.F., F.D.G.; formal analysis, F.D.G.; data collection, G.C., G.M.B., L.C. (Luigi Cormio), R.H., R.C., D.A., A.S., M.M. (Martina Maggi)., F.P., M.M. (Matteo Manfredi), C.F., A.A., A.T. (Alessandro Tafuri), P.B., C.T. (Carlo Terrone), M.B., E.C., E.I., E.M., L.B., G.I.R., M.M (Massimo Madonia), A.T. (Alessandro Tedde), A.V., C.S., G.L. (Giovanni Liguori), C.T. (Carlo Trombetta), E.B., R.S., F.D.M., M.R., M.D.V., N.L., L.S. (Lorenzo Spirito), F.C. (Felice Crocetto), F.C. (Francesco Cantiello), R.D., S.M.D.S., M.M. (Michele Marchioni), L.S. (Luigi Schips), P.P., L.C. (Luca Carmignani), A.C., F.S., P.G., B.B., F.D., E.Z., R.P., R.M.S., V.P., G.L. (Giuseppe Lucarelli), P.D., F.M.G.B., G.M., M.C., O.d.C.; writing—original draft preparation, M.F., M.C.; writing—review and editing, M.F., M.C.; visualization and supervision, M.F., O.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Informed consent was obtained from all individual participants included in the study. All study procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The research project was based on retrospective data collection.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Bourgade V., Drouin S.J., Yates D.R., Parra J., Bitker M.O., Cussenot O., Rouprêt M. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J. Urol. 2014;32:475–479. doi: 10.1007/s00345-013-1045-z. [DOI] [PubMed] [Google Scholar]
- 4.Dong M., Zhang X., Yang K., Liu R., Chen P. Forecasting the COVID-19 transmission in Italy based on the minimum spanning tree of dynamic region network. PeerJ. 2021;9:e11603. doi: 10.7717/peerj.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panarello D., Tassinari G. One year of COVID-19 in Italy: Are containment policies enough to shape the pandemic pattern? Socioecon. Plann. Sci. 2021:101120. doi: 10.1016/j.seps.2021.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ficarra V., Novara G., Abrate A., Bartoletti R., Crestani A., De Nunzio C., Giannarini G., Gregori A., Liguori G., Mirone V., et al. Urology practice during the COVID-19 pandemic. Minerva Urol. Nefrol. 2020;72:369–375. doi: 10.23736/S0393-2249.20.03846-1. [DOI] [PubMed] [Google Scholar]
- 7.Busetto G.M., Del Giudice F., Mari A., Sperduti I., Longo N., Antonelli A., Cerruto M.A., Costantini E., Carini M., Minervini A., et al. How Can the COVID-19 Pandemic Lead to Positive Changes in Urology Residency? Front. Surg. 2020;7:563006. doi: 10.3389/fsurg.2020.563006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo G., Buonerba L., Ingenito C., Crocetto F., Buonerba C., Libroia A., Sciarra A., Ragone G., Sanseverino R., Iaccarino S., et al. Clinical Characteristics of Metastatic Prostate Cancer Patients Infected with COVID-19 in South Italy. Oncology. 2020;98:743–747. doi: 10.1159/000509434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito C., Masieri L., Castagnetti M., Crocetto F., Escolino M. Letter to the Editor: Robot-Assisted and Minimally Invasive Pediatric Surgery and Urology During the COVID-19 Pandemic: A Short Literature Review. J. Laparoendosc. Adv. Surg. Tech. A. 2020;30:915–918. doi: 10.1089/lap.2020.0251. [DOI] [PubMed] [Google Scholar]
- 10.Rajwa P., Przydacz M., Krajewski W., Kuffel B., Zapala P., Krzywon A., Cortez A.J., Dybowski B., Stamirowski R., Jarzemski M., et al. Changing patterns of urologic emergency visits and admissions during the COVID-19 pandemic: A retrospective, multicenter, nationwide study. Arch. Med. Sci. 2021;17:1262–1276. doi: 10.5114/aoms.2020.98364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J., Wang Y., Weng H., Wang D., Han F., Huang Q., Deng T., Wang X., Jin Y. Management of non-muscle-invasive bladder cancer: Quality of clinical practice guidelines and variations in recommendations. BMC Cancer. 2019;19:1054. doi: 10.1186/s12885-019-6304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babjuk M., Burger M., Compérat E.M., Gontero P., Mostafid A.H., Palou J., van Rhijn B.W.G., Rouprêt M., Shariat S.F., Sylvester R., et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Surgical Wait Times (SWAT) Initiative Consensus document: Recommendations for optimal surgical wait times for patients with urological malignancies. Can J. Urol. 2006;13((Suppl. 3)):62–64. [PubMed] [Google Scholar]
- 14.Rouprêt M., Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Cowan N.C., Dominguez-Escrig J.L., Gontero P., Mostafid A.H. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur. Urol. 2021;79:62–79. doi: 10.1016/j.eururo.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Ferro M., Vartolomei M.D., Cantiello F., Lucarelli G., Di Stasi S.M., Hurle R., Guazzoni G., Busetto G.M., De Berardinis E., Damiano R., et al. High-Grade T1 on Re-Transurethral Resection after Initial High-Grade T1 Confers Worse Oncological Outcomes: Results of a Multi-Institutional Study. Urol. Int. 2018;101:7–15. doi: 10.1159/000490765. [DOI] [PubMed] [Google Scholar]
- 16.Oddens J., Brausi M., Sylvester R., Bono A., van de Beek C., van Andel G., Gontero P., Hoeltl W., Turkeri L., Marreaud S., et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: One-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 2013;63:462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Babjuk M., Böhle A., Burger M., Capoun O., Cohen D., Compérat E.M., Hernández V., Kaasinen E., Palou J., Rouprêt M., et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 18.Jocham D. Implementation of clinical urological studies and therapy trials using GCP (good clinical practice) guidelines. Urol. A. 1994;33:532–535. [PubMed] [Google Scholar]
- 19.Assel M., Sjoberg D., Elders A., Wang X., Huo D., Botchway A., Delfino K., Fan Y., Zhao Z., Koyama T., et al. Guidelines for reporting of statistics for clinical research in urology. BJU Int. 2019;123:401–410. doi: 10.1111/bju.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribal M.J., Cornford P., Briganti A., Knoll T., Gravas S., Babjuk M., Harding C., Breda A., Bex A., Rassweiler J.J. European Association of Urology Guidelines Office Rapid Reaction Group: An organisation-wide collaborative effort to adapt the European Association of Urology guidelines recommendations to the coronavirus disease 2019 era. Eur. Urol. 2020;78:21–28. doi: 10.1016/j.eururo.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mottrie A. ERUS (EAU Robotic Urology Section) guidelines during COVID-19 emergency. Eur. Urol. 2020;25:1–6. [Google Scholar]
- 22.Mielczarek Ł., Zapała P., Krajewski W., Nowak Ł., Bajkowski M., Szost P., Szabłoński W., Zapała Ł., Poletajew S., Dybowski B., et al. Diagnostic and treatment delays among patients with primary bladder cancer in Poland: A survey study. Cent. Eur. J. Urol. 2020;73:152–159. doi: 10.5173/ceju.2020.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace D., Bryan R., Dunn J., Begum G., Bathers S., Group W.M.U.R. Delay and survival in bladder cancer. BJU Int. 2002;89:868–878. doi: 10.1046/j.1464-410X.2002.02776.x. [DOI] [PubMed] [Google Scholar]
- 24.Roscigno M., Naspro R., Piccichè A., Muttin F., Angiolilli D., Deiana G., Pezzoli F., Da Pozzo L.F. A Snapshot from the Department of Urology in Bergamo Evaluating the Timeline of the SARS-CoV-2 Outbreak: Which Patients Are We Missing? Eur. Urol. Focus. 2020;6:1120–1123. doi: 10.1016/j.euf.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naspro R., Da Pozzo L.F. Urology in the time of corona. Nat. Rev. Urol. 2020;17:251–253. doi: 10.1038/s41585-020-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mistretta F.A., Luzzago S., Molendini L.O., Ferro M., Dossena E., Mastrilli F., Musi G., de Cobelli O. A guide for oncologic patient management during Covid-19 pandemic: The initial experience of an Italian oncologic hub with exemplificative focus on uro-oncologic patients. Cancers. 2020;12:1513. doi: 10.3390/cancers12061513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fankhauser C.D., Teoh J.Y., Mostafid H. Treatment options and results of adjuvant treatment in nonmuscle-invasive bladder cancer (NMIBC) during the Bacillus Calmette-Guérin shortage. Curr. Opin. Urol. 2020;30:365–369. doi: 10.1097/MOU.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A.L., Bakouny Z., Bhalla S., Steinharter J.A., Tremblay D.A., Awad M.M., Kessler A.J., Haddad R.I., Evans M., Busser F., et al. Cancer Care Disparities during the COVID-19 Pandemic: COVID-19 and Cancer Outcomes Study. Cancer Cell. 2020;38:769–770. doi: 10.1016/j.ccell.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metzger K., Mrosek J., Zittel S., Pilz M., Held T., Adeberg S., Ristow O., Hoffmann J., Engel M., Freudlsperger C., et al. Treatment delay and tumor size in patients with oral cancer during the first year of the COVID-19 pandemic. Head Neck. 2021;43:3493–3497. doi: 10.1002/hed.26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhada S., Stewart D., Cheema E., Hadi M.A., Paudyal V. Cancer services during the COVID-19 pandemic: Systematic review of patients’ and caregivers’ experiences. medRxiv. 2021;13:5875–5887. doi: 10.1101/2021.03.19.21253949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torreggiani A., Colombo R., Gontero P., Lapini A., Sanseverino R., Serretta V. Clinical practice and adherence to the diagnosis and treatment of NMIBC guidelines: A report of a recognition based clinical cases study. Urologia. 2015;82:58–70. doi: 10.5301/uro.5000107. [DOI] [PubMed] [Google Scholar]
- 32.Panebianco V., Del Giudice F., Leonardo C., Sciarra A., Catalano C., Catto J.W.F. VI-RADS Scoring Criteria for Alternative Risk-adapted Strategies in the Management of Bladder Cancer During the COVID-19 Pandemic. Eur. Urol. 2020;78:e18–e20. doi: 10.1016/j.eururo.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng K., Vinnakota K., Sharma A., Kelly J., Dasgupta P., Vasdev N. Urinary biomarkers to mitigate diagnostic delay in bladder cancer during the COVID-19 era. Nat. Rev. Urol. 2021;18:185–187. doi: 10.1038/s41585-020-00419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferro M., La Civita E., Liotti A., Cennamo M., Tortora F., Buonerba C., Crocetto F., Lucarelli G., Busetto G.M., Del Giudice F. Liquid Biopsy Biomarkers in Urine: A Route towards Molecular Diagnosis and Personalized Medicine of Bladder Cancer. J. Pers. Med. 2021;11:237. doi: 10.3390/jpm11030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.