Abstract
The major clinical problem for patients with cystic fibrosis (CF) is progressive loss of pulmonary function, usually due to chronic bacterial infections. A patient with CF and a lung transplant was severely infected with a previously unidentified gram-negative bacterium. We isolated this organism (strain DS15158) from the patient and characterized it by phylogenetic analysis of the small-subunit rRNA and biochemically by the BIOLOG GN MicroPlate assay, fatty acid analysis, and various standard laboratory tests. No close match to any other organism could be found. Isolate DS15158 represents a new genus-level divergence within the bacterial subdivision α-Proteobacteria on the basis of the 16S rRNA gene analysis.
Cystic fibrosis (CF) is a lethal genetic disease of childhood and the most commonly inherited fatal disease of Caucasians. It can cause disruption of the exocrine function of the pancreas, the intestinal glands, biliary tree, bronchial glands, and sweat glands. Exocrine glands secrete abnormally thick mucus, which obstructs the pancreas and which causes chronic infections of the lungs. CF arises from over 720 different mutations in the gene encoding a plasma membrane protein that is designated the CF transmembrane conductance regulator. Individuals with CF who lack the CF transmembrane conductance regulator are generally subject to chronic microbial inflammations, mostly caused by mucoid Pseudomonas aeruginosa (14). Other microorganisms that are associated with CF are Burkholderia cepacia, Stenotrophomonas maltophilia, Haemophilus influenzae, Staphylococcus aureus, other uncharacterized bacterial species, and atypical mycobacteria (7, 16, 17). Fungi, such as Aspergillus spp. (14) and Candida albicans (9), have also been reported to colonize patients with CF (6). These infections coupled with a misfunctional host immune system contribute to the progressive decline in pulmonary function among CF patients and for most of them can cause death before the age of 30. Treatments vary, but they may include antibiotics, DNase to thin the mucus, aerosols that relieve constriction of the airways, and physical therapy.
We isolated a previously unidentified, very mucoid gram-negative rod (VMGNR) from the respiratory track of a 22-year-old female with CF and end-stage lung disease who had received a bilateral living lobar transplant in February 1998 from two healthy relatives who did not have CF. The perioperative antimicrobial therapy included imipenem, tobramycin, ceftazidime, and aerosolized colistin sulfate. Postoperatively, the imipenem, ceftazidime, and aerosolized colistin sulfate were continued. The immunosuppressive regimen included tacrolimus, mycophenolate mofetil, and methylprednisolone sodium succinate (Solu-Medrol). Cultures of her explants grew P. aeruginosa, Proteus mirabilis, and the VMGNR. Postoperatively, chest X rays showed increasing infiltrates. The patient underwent bronchoscopy with bronchoalveolar lavage (BAL) on the 8th postoperative day. The BAL specimen did not grow any organisms. A BAL specimen obtained on the 16th postoperative day grew Enterococcus, coagulase-negative Staphylococcus sp., and the VMGNR, which was initially identified as “mucoid Pseudomonas sp.” She continued to develop new infiltrates, and on the 28th postoperative day she underwent a bronchoscopy with BAL and transbronchial biopsy. The BAL specimen grew a few colonies of Alcaligenes xylosoxidans that were susceptible to ceftazidime, piperacillin, ciprofloxacin, and imipenem. Her condition slowly improved, and she was discharged from the hospital 6 weeks after the transplant. A follow-up outpatient surveillance bronchoscopy with BAL was performed 2 months posttransplantation, and the BAL specimen did not grow any organisms. The VMGNR has not been recovered from any other specimens during the past year, although during the past 6 months she has developed a slightly elevated leukocyte count with a persistent bandemia of up to 65%.
Conventional biochemical and microbiological tests including the BIOLOG GN MicroPlate assay and whole-cell cellular fatty acid (CFA) analysis showed no match to any species in their extensive databases. Commercially available phylogenetic analysis that was based on only part (500 nucleotides [nt]) of the small-subunit rRNA gene (16S rDNA) sequence derived from strain DS15158 also failed to match this isolate to a known organism. Therefore, to determine the correct relationship of DS15158 to other organisms in the 16S rRNA-based phylogenetic tree, we have used the full-length 16S rRNA for phylogenetic sequence analysis (13, 20). On the basis of this analysis we suggest that isolate DS15158 represents a new genus-level divergence within the bacterial subdivision α-Proteobacteria.
MATERIALS AND METHODS
Laboratory Growth and Susceptibility Tests.
Isolate DS15158 was streaked onto Trypticase soy agar supplemented with 5% sheep blood (BA), MacConkey agar, colistin-nalidixic blood agar, chocolate agar, Mueller-Hinton agar, and colistin-nalidixic acid–phenylethyl alcohol (Rose) blood agar (Hardy Diagnostics, Santa Maria, Calif.). The plates were incubated at 35°C and were examined daily for 5 days. Additional BA plates were incubated at 25 and 42°C. Colonies from 48-h cultures were used to inoculate different identification kits commonly used to identify gram-negative bacteria, according to the directions of the manufacturers: the API 20E system (bioMérieux, St. Louis, Mo.), BBL Crystal Enteric/Nonfermenter ID kit (Becton Dickinson Microbiology Systems, Cockeysville, Md.), and RapID NF Plus system (Remel, Lenexa, Kans.).
The following tests and media were used as described elsewhere (12): tests for oxidase, indole, and catalase with 3% hydrogen peroxide, triple sugar iron agar, and tube tests for citrate, acetamide, methyl red, Voges-Proskauer, indole-nitrate broth, Sims H2S-motility agar, phenylalanine deaminase, Moellers' arginine dihydrolase, and ornithine and lysine decarboxylases.
Initial testing for susceptibility to various antimicrobial agents (see Table 2) was performed by the disk diffusion method according to National Committee for Clinical Laboratory Standards guideline M2-A6 (12a). Subsequent testing was done by the broth microdilution method (National Committee for Clinical Laboratory Standards guideline M7-A4 [12b]) with two different inoculum concentrations corresponding to the no. 0.5 and no. 2 McFarland turbidity standards, respectively. Quantitative cultures were performed to determine the numbers of CFU per milliliter of both concentrations. Incubation was at 35°C for 24 and 40 h for the initial and final interpretations, respectively. The Etest (AB Biodisk, Solna, Sweden) was used to determine or confirm some MIC test results.
TABLE 2.
Susceptibility of bacterial isolate DS15158 to antimicrobial agents
| Antimicrobial agent | MIC (μg/ml)a | Disk diffusion result | Etest MIC (μg/μl) |
|---|---|---|---|
| Levofloxacin | 4 | ||
| Ofloxacin | 8 | ||
| Ciprofloxacin | 4 | ||
| Meropenem | 2 | ||
| Imipenem | 2 | ||
| Ceftriaxone | >32 | ||
| Cefotaxime | >32 | ||
| Cefipime | >32 | ||
| Ceftazidime | >32 | ||
| Ticarcillin-clavulanate | >128/2b | ||
| Piperacillin-tazobactam | >128/4b | ||
| Ampicillin-sulbactam | >32/16b | ||
| Vancomycin | >16 | ||
| Oxacillin | 1 | Rc | >256 |
| Doxycycline | 16 | ||
| Amikacin | 32 | ||
| Tobramycin | R | ||
| Gentamicin | R | ||
| Polymyxin B | R | ||
| Colistin | R |
Broth microdilution method, inoculum of 1.5 × 105 CFU/ml, 24 h of incubation.
Beta-lactam antibiotic–beta-lactamase inhibitor concentration.
R, growth occurred up to the disk.
BIOLOG GN MicroPlate assay.
The BIOLOG GN MicroPlate assay (BIOLOG Inc., Hayward, Calif.) classifies bacterial isolates on the basis of the ability of organisms to oxidize carbon sources (2, 5). The test provides 95 different carbon sources and was performed by the manufacturer with cultures that had been grown at 35°C for 72 h on BA.
CFA analysis.
Whole-cell, long-chain fatty acids were determined with fatty acid methyl esters (FAME) by standard procedures (MIDI Laboratories, Newark, Del.) with cells harvested after incubation for 48 h at 35°C on BA.
Partial 16S rRNA gene analysis.
Partial sequencing of the 16S rRNA gene (500 nts) was performed by MIDI Laboratories by standard procedures, and a phylogenetic analysis based on this partial 16S rDNA fragment was performed. The sequence data were compared to sequences in the MicroSeq database (PE Applied Biosystems), as well as to those in the databases for the Ribosomal Database Project (10) and GenBank (National Center for Biotechnology Information, Bethesda, Md.).
DNA isolation.
DNA from ca. 100 μg of cells was isolated by bead beating as described earlier (8), except that the beads were not pretreated with acid. The lysate was extracted once each with phenol, phenol-chloroform (1:1; vol/vol), and phenol-chloroform-isoamyl alcohol (25:24:1; vol/vol/vol). DNA was precipitated by adding sodium acetate to a final concentration of 0.3 M and 2.5 volumes of ethanol. The pellet was washed with 70% ethanol, dried, resuspended in 10 mM Tris-HCl (pH 8 at 25°C)–1 mM EDTA, and analyzed on a 0.5% agarose gel.
PCR amplification of 16S rDNA.
PCRs were performed with four different primer combinations: 8F (5′-AGAGTTTGATCCTGGCTCAG)–1391R (5′-GACGGGCGGTGWGTRCA; W = A or T, R = A or G), 8F–1492R (5′-GGTTACCTTGTTACGACTT), 515F (5′-GTGCCAGCMGCCGCGGTAA; M = A or C)–1391R, and 515F-1492R, respectively. Each 100-μl PCR mixture contained 8 U of Taq DNA polymerase (Promega), 200 ng of each primer, 300 ng of genomic DNA, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 9 at 25°C), and 0.1% Triton X-100. All PCRs started with an initial denaturation step for 2 min at 94°C, followed by 25 amplification cycles (1 min at 92°C, 1 min at 45°C, and 1 min 30 s at 72°C) and a final extension step for 30 min at 72°C. PCR fragments were purified over Chromabond-100 TE spin columns (Clontech).
Sequencing of PCR products.
Both DNA strands of the different PCR products (50 ng) were sequenced directly by cycle sequencing (24 cycles of 30 s at 96°C, 15 s at 45°C, and 4 min at 60°C) with AmpliTaq FS (PE Applied Biosystems) by using 20 ng of each PCR primer (see above).
Cloning of PCR products and sequencing of rDNA clones.
Additionally, the PCR fragments were cloned into the T-Vector pCPT9 as described elsewhere (15). Transformation of Escherichia coli XL-1 Blue (Stratagene) was performed as described previously (11). Recombinants were selected by the blue or white color of the colonies, and plasmid DNA from several clones for every primer combination was isolated by using the QIAprep plasmid kit (QIAGEN). Both strands of recombinant plasmid DNA were sequenced by cycle sequencing as described for the PCR products (see above), except that 20 ng each of primer T7 (5′-GTAATACGACTCACTATAGGG) and primer SP6 (5′-ATTTAGGTGACACTATAG) and 200 ng of plasmid DNA were used. The sequences were analyzed on an ABI 373A automated DNA sequencer (PE Applied Biosystems) according to the manufacturer's protocol.
Phylogenetic data analysis.
To determine the approximate phylogenetic affiliation of strain DS15158, the sequence data were initially compared to the sequence data available in databases by using BLAST (1). The sequence data were investigated for the presence of chimeric sequences by using CHECK_CHIMERA (10), and the sequences were then aligned with the 16S rRNA sequences in the database of the program package ARB (18) that was manually expanded to 8,472 different 16S rRNA sequences. Data sets that were missing from ARB but that were determined by the BLAST search were retrieved from the GenBank database (5a) and were also aligned. 16S rRNA-based phylogenetic trees were constructed by distance matrix analysis of all available 16S rRNA sequences. A subset of Proteobacteria sequences that were at least 1,350 nts in length was then used to calculate a subtree by the neighbor-joining method of ARB. A subtree was also constructed by using the same data set and the parsimony tool of ARB. We have used a mask that was created manually and that included only regions with unambiguous alignments. The relative confidence of each phylogenetic analysis was estimated by bootstrap analysis, which included 100 replicates (3). Distance matrices were calculated by the neighbor-joining method.
Nucleotide sequence accession number.
The sequence of strain DS15158 was deposited in GenBank (5a) under accession no. AF085496.
RESULTS
Strain DS15158 is a rod-shaped gram-negative bacterium that measures 1.5 to 2 μm in width by 3.5 μm in length. Examination by phase-contrast microscopy revealed a capsule surrounding the cell. Growth occurred at 35 and 42°C but was very slow at 25°C. This organism formed very slimy, nonpigmented colonies on nonselective media, including blood agar, chocolate agar, and Mueller-Hinton agar after 48 h. Growth on MacConkey agar was very slight after 3 days. Growth on Rose blood agar was very slight after 5 days.
Reactions by standard biochemical tests are summarized in Table 1. The API 20E kit system did not produce any positive reactions after 24 and 48 h of incubation. The RapID NF Plus system as well as the BBL Crystal system generated profiles that did not match those of any organism in the corresponding databases. A summary of the individual reactions is included in Table 1.
TABLE 1.
Biochemical characterization of bacterial isolate DS15158
| Characteristic or test | Reactiona |
|---|---|
| Growth on: | |
| BA at: | |
| 37°C | + |
| 42°C | + |
| 25°C | + (slower) |
| MacConkey agar | Poor |
| CNA-PEAb agar | Poor |
| Triple sugar iron agar | Alkaline, no change |
| OF glucose | − |
| Oxidase | + |
| Catalase | + |
| Indole | − |
| Motility | − |
| Esculin hydrolysis | + |
| Gelatin liquefaction | − |
| Urease | + |
| Nitrate reduction | − |
| Nitrite reduction | − |
| H2S production (Sims) | − |
| Methyl red | − |
| Voges-Proskauer | − |
| Utilization of citrate (Simmons) | + |
| MBM acetamide | − |
| Malonate | − |
| Aliphatic thiol | − |
| Growth in 6% NaCl broth | + |
| Arginine dihydrolase (Moellers) | − |
| Lysine decarboxylase (Moellers) | − |
| Ornithine decarboxylase (Moellers) | − |
| Phenylalanine deaminase | − |
| Enzyme production | |
| Phosphatase | + |
| β-Glucosidase | + |
| β-Galactosidase | + |
| N-Acetyl-glucosaminidase | + |
| Phosphorylcholinase | + |
| Proline aminopeptidase | + |
| Pyrollidonyl aminopeptidase | + |
| γ-l-Glutamyl aminopeptidase | + |
| Tryptophan aminopeptidase | + |
| N-Benzyl-arginine aminopeptidase | + |
| Triglyceride hydrolase | + |
| β-Glucuronidase | − |
+, positive; −, negative.
CNA, colistin nalidixic acid blood agar; PEA, phenylethyl alcohol blood agar.
Susceptibilities to various antimicrobial agents are presented in Table 2. The suspension corresponding to the no. 0.5 McFarland standard had 4 × 104 CFU/ml. The suspension corresponding to the no. 2 McFarland standard had 1.5 × 105 CFU/ml. The MICs obtained with the inoculum of 1.5 × 105 CFU/ml are given in Table 2. Incubation beyond 24 h did not significantly change the MICs.
The BIOLOG GN MicroPlate assay showed that the microorganism is capable of using 59 of the 95 carbon sources in the panel (Table 3). The closest match in the BIOLOG, release 3.70, database was to Agrobacterium tumefaciens subgroup A (also known as Agrobacterium radiobacter). The profile values were borderline for acceptability with a similarity value slightly greater than the minimum value of 0.5, and a carbon source distance value of five to seven carbon sources.
TABLE 3.
Carbon sources used by strain DS15158 in the BIOLOG GN MicroPlate assay
| Carbon source | Carbon source | |
|---|---|---|
| 1. Dextrin | 31. Methyl pyruvate | |
| 2. Glycogen | 32. Monomethyl succinate | |
| 3. N-Acetyl-d-galactosamine | 33. Acetic acid | |
| 4. N-Acetyl-d-glucosamine | 34. cis-Aconitic acid | |
| 5. Adonitol | 35. Citric acid | |
| 6. l-Arabinose | 36. Formic acid | |
| 7. d-Arabitol | 37. d-Galactonic acid lactone | |
| 8. Cellobiose | 38. d-Gluconic acid | |
| 9. i-Erythritol | 39. Beta-hydroxybutyric acid | |
| 10. d-Fructose | 40. Alpha-ketoglutaric acid | |
| 11. l-Fucose | 41. d,l-Lactic acid | |
| 12. d-Galactose | 42. Malonic acid | |
| 13. Gentiobiose | 43. Succinic acid | |
| 14. Alpha-d-glucose | 44. Bromosuccinic acid | |
| 15. m-Inositol | 45. Succinamic acid | |
| 16. Alpha-d-lactose | 46. Alaninamide | |
| 17. Lactulose | 47. d-alanine | |
| 18. Maltose | 48. l-Alanine | |
| 19. d-Mannitol | 49. l-alanyl-glycine | |
| 20. d-Mannose | 50. l-asparagine | |
| 21. d-Melibiose | 51. l-aspartic acid | |
| 22. Beta-methyl-d-glucoside | 52. l-glutamic acid | |
| 23. d-Psicose | 53. Glycyl-l-glutamic acid | |
| 24. d-Raffinose | 54. l-Ornithine | |
| 25. l-Rhamnose | 55. l-Proline | |
| 26. d-Sorbitol | 56. l-Serine | |
| 27. Sucrose | 57. d-l-Carnitine | |
| 28. d-Trehalose | 58. Gamma-aminobutyric acid | |
| 29. Turanose | 59. Inosine | |
| 30. Xylitol |
The CFA analysis with FAMEs resulted in a profile that did not match that for any of the organisms in the MIDI Laboratories database. The major fatty acids found in isolate DS15158 are listed in Table 4.
TABLE 4.
FAME constituents of isolate DS15158
| Fatty acid | % Total |
|---|---|
| 13:1 ω 1c | 0.90 |
| Sum in feature 3a | 1.51 |
| 16:0 | 2.19 |
| 17:1 ω 6c | 1.94 |
| 17:0 | 3.29 |
| 16:0 2 OH | 1.86 |
| 16:0 3 OH | 1.52 |
| Sum in feature 6b | 1.91 |
| Sum in feature 7c | 58.84 |
| 18:0 | 2.58 |
| 17:0 2 OH | 2.03 |
| 17:0 3 OH | 1.29 |
| 19:0 cyclo ω 8c | 9.73 |
| 18:1 2 OH | 4.93 |
| 18:0 2 OH | 2.08 |
| 20:3 ω 6,9,12c | 3.40 |
Summed feature 3, 12:0 aldehyde and/or 16:1 iso I and/or 14:0 3 OH.
Summed feature 6, 18:2 ω 6,9,12c and/or 18:0 anteiso.
Summed feature 7, 18:1 ω 7c and/or 18:1 ω 9c.
Analysis of 500 nts of 16S rRNA by MIDI Laboratories resulted in a “no match” interpretation.
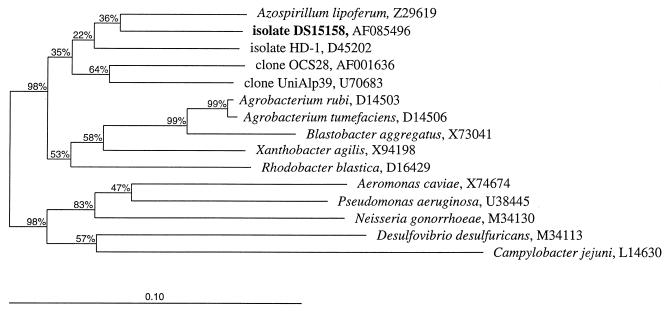
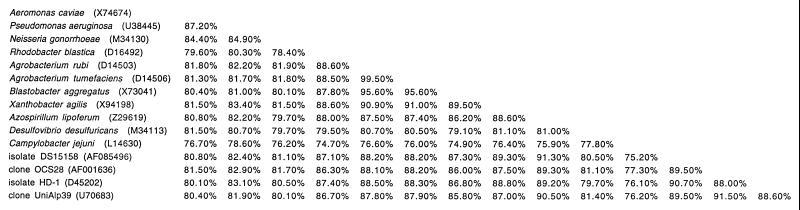
With 1,359 nts, the 16S rDNA sequence data for isolate DS15158 represent almost the full length of the organism's 16S rRNA. All the sequences derived from the PCR products as well as from the recombinant plasmid DNA were identical. No chimeric sequences were detected. On the basis of 16S rRNA phylogenetic analysis, isolate DS15158 belongs to the bacterial subdivision α-Proteobacteria with no specific relative (Fig. 1 and 2). Both the neighbor-joining and the parsimony methods (data not shown) gave similar results with similar bootstrap support. On the basis of the sequence similarities of their 16S rRNAs (91.3%), the closest known relative of isolate DS15158 is Azospirillum lipoferum (Fig. 2).
FIG. 1.
Phylogenetic tree demonstrating the relationship of isolate DS15158 within the bacterial subdivision α-Proteobacteria by comparative analysis of 16S rRNA by using the neighbor-joining method of ARB (18). The bar indicates the number of substitutions per nucleotide position. Bootstrapping was used (100 replicates) to assess support for particular nodes in the tree, and values (in percent) are shown above the nodes. The outgroup is represented by P. aeruginosa and Neisseria gonorrhoeae (bacterial subdivision β-Proteobacteria), Aeromonas caviae (bacterial subdivision γ-Proteobacteria), Desulfovibrio desulfuricans (bacterial subdivision δ-Proteobacteria), and Campylobacter jejuni (bacterial subdivision ɛ-Proteobacteria).
FIG. 2.
Percent similarities of 16S rDNA sequences of 15 Proteobacteria including isolate DS15158. GenBank accession numbers are indicated after each name.
DISCUSSION
Several well-characterized bacteria and fungi are known to cause serious, chronic infections in patients with CF. We describe a bacterium (isolate DS15158) not previously reported to be associated with CF disease and not previously described.
As with other organisms isolated from patients with CF, this strain was resistant to most of the antimicrobial agents that we tested. By the broth microdilution test, this strain was found to be susceptible to oxacillin (MIC, 1 μg/ml). To confirm the unexpected observation, we tested the strain by the oxacillin Etest and by the disk diffusion method. Both of these agar-based systems indicated that the organism was resistant to oxacillin. The reason for the discrepancy between the broth- and the agar-based assays is unclear.
In the clinical laboratory, isolate DS15158 could not be associated with any known genus and species by conventional culture reactions or with identification kits. More sophisticated means of characterizing the isolate were investigated.
We have applied two methods that are commercially available and that are widely used to classify bacterial isolates: the BIOLOG GN MicroPlate assay (2) and CFA analysis by gas chromatography. The combination of both tests is generally sufficient for the identification of a bacterium, assuming that the organism to be studied or at least a close relative thereof is available in the corresponding databases. While CFA analysis gave no matching result, the BIOLOG GN MicroPlate assay suggested a possible relationship of DS15158 to Agrobacterium tumefaciens. Agrobacterium species belong to the bacterial subdivision α-Proteobacteria, and some strains are capable of producing polysaccharide capsules (19). The results of the BIOLOG GN MicroPlate assay show that the bacterium uses virtually every carbohydrate-type carbon source in the panel as well as several carboxylic acids and amino acids. However, for identification purposes the data were borderline. Due to the marginal species match, we doubted whether the strain truly belongs to the genus Agrobacterium.
When phenotypic data are inconclusive, a genotypic approach is necessary. Phylogenetic analysis based on 16S rRNA has been proven to be the most powerful tool for the identification and classification of organisms (13, 20). Initial identification of our isolate based on the analysis of 500 nts of its 16S rDNA also resulted in no match and led to the assumption that DS15158 is a novel and so far undescribed organism. However, a comparative phylogenetic analysis based on ≤500 nts is insufficient for the correct placement of a novel organism in a phylogenetic tree and could even be misleading. We therefore determined the phylogenetic relationship of DS15158 to all other members of the domain Bacteria on the basis of 16S rRNA analysis by using the almost complete (1,359-nt) sequence information for its 16S rDNA. All sequences used for comparison were at least 1,350 nts in length. Isolate DS15158 could not be matched closely with any existing organism in the common databases. However, the high bootstrap support of 98% at the base of each tree derived from the 16S rRNA analysis (Fig. 1) demonstrates that DS15158 clearly belongs to the bacterial subdivision α-Proteobacteria. However, its specific position within the α-Proteobacteria could not be determined due to the lack of a close relative, which is reflected in low bootstrap values (<50%; Fig. 1). On the basis of this phylogenetic approach and as supported by the CFA analysis and the BIOLOG GN MicroPlate assay, we propose that DS15158 represents a new genus-level divergence within the α-Proteobacteria. The carbon source utilization profile of this organism is now contained in an updated BIOLOG database (release 4.0) under the provisional name “Agrobacterium-like—cystic fibrosis.” This will allow laboratories to identify it in the future using a simple, commercially available test.
Although the use of rRNA sequences as a tool to study the natural relatedness of organisms is widely accepted, uncertainties in 16S rDNA sequences can arise because of sequencing errors, amplification errors, and the possibility of microheterogeneity (4). To minimize such errors, both strands of the 16S rDNA represented by PCR products and recombinant plasmid DNAs were sequenced. The fact that all sequences were identical was taken as additional evidence that DS15158 is derived from a pure culture.
DS15158 is a new organism isolated from a patient with CF. The next investigations needed include more detailed characterization and description of the new isolate, determination of its disease-causing potential, and determination of appropriate treatment.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zheng Zhang J Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochner B. Breathprints at the microbial level. ASM News. 1989;55:536–539. [Google Scholar]
- 3.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 4.Fox G E, Wisotzkey J D, Jurtshuk P J., Jr How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 5.Garland J L, Millis A L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol. 1991;57:2351–2359. doi: 10.1128/aem.57.8.2351-2359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.GenBank. [Online.] http://www.ncbi.nlm.nih.gov. [7 July, 1999, last date accessed.]
- 6.Haase G, Skopnik H, Groten T, Kusenbach G, Posselt H G. Long-term fungal culture of sputum from patients with cystic fibrosis. Mycoses. 1991;34:49–52. [PubMed] [Google Scholar]
- 7.Henry D A, Campbell M E, Lipuma J J, Speert D P. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–619. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugenholtz P, Pitulle C, Hershberger K L, Pace N R. Novel division-level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerkmann M-L, Schuppler M, Paul K-D. Red-pigmented Candida albicans in patients with cystic fibrosis. J Clin Microbiol. 1999;37:278. doi: 10.1128/jcm.37.1.278-278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The Ribosomal Database Project. Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniatis T, Fritsch E, Sambrook J. Molecular cloning, a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 1.82–1.84. [Google Scholar]
- 12.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 12a.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility, 6th ed. Approved standard M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12b.National Committee for Clinical Laboratory Standards. Methods for dilution susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 14.Pier G B. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:338–347. [Google Scholar]
- 15.Pitulle C, Pace N R. Novel T-cloning vector for plasmid-based 16S rDNA analysis. BioTechniques. 1999;26:223–224. doi: 10.2144/99262bm08. [DOI] [PubMed] [Google Scholar]
- 16.Segonds C, Heulin T, Marty N, Chabanon G. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J Clin Microbiol. 1999;37:2201–2208. doi: 10.1128/jcm.37.7.2201-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shreve M R, Butler S, Kaplowitz H J, Rabin H R, Stokes D, Light M, Regelmann W E Investigators for the Epidemiologic Study of Cystic Fibrosis; for North American Scientific Advisory Group. Impact of microbiology practice on cumulative prevalence of respiratory tract bacteria in patients with cystic fibrosis. J Clin Microbiol. 1999;37:753–757. doi: 10.1128/jcm.37.3.753-757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Struckman, B. Nonhoff, M. Lenke, A. Ginhart, A. Vilbig, T. Ludwig, A. Bode, K.-H. Schleifer, and W. Ludwig. ARB: a software environment for sequence data. Department of MicrobiologyTechnische Universität MünchenMunich, Germany. [Online.] http://www.mikro.biologie.tu-muenchen.de.
- 19.Sutherland I W. Bacterial surface polysaccharides: structure and function. Int Rev Cytol. 1988;113:187–230. doi: 10.1016/s0074-7696(08)60849-9. [DOI] [PubMed] [Google Scholar]
- 20.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]