Abstract
Simple Summary
This review focuses on the effects that a class of drugs, PI3Kδ inhibitors, used for the treatment of patients with lymphoma can have not on the neoplastic cells but on the normal cells and how this effect can modulate the immune response and potentially contribute to the anti-tumor response.
Abstract
The development of small molecules able to block specific or multiple isoforms of phosphoinositide 3-kinases (PI3K) has already been an active field of research for many years in the cancer field. PI3Kδ inhibitors are among the targeted agents most extensively studied for the treatment of lymphoma patients and PI3Kδ inhibitors are already approved by regulatory agencies. More recently, it became clear that the anti-tumor activity of PI3K inhibitors might not be due only to a direct effect on the cancer cells but it can also be mediated via inhibition of the kinases in non-neoplastic cells present in the tumor microenvironment. T-cells represent an important component of the tumor microenvironment and they comprise different subpopulations that can have both anti- and pro-tumor effects. In this review article, we discuss the effects that PI3Kδ inhibitors exert on the immune system with a particular focus on the T-cell compartment.
Keywords: lymphoma, PI3K inhibitors, T-cells, B-cells, macrophages, chemokine, cancer, tumor, immune checkpoint inhibitors
1. Introduction
Phosphoinositide 3-kinases (PI3Ks) are a class of enzymes fundamental in the regulation of cell metabolism, proliferation and survival [1,2,3,4,5,6]. PI3Ks are active in most human cancers, often representing oncogenic drivers due to genetic events directly targeting their coding genes or determining the constitutive activation or upstream components of the signaling cascade [1,4,5,6].
PI3Ks comprise four isoforms p110α (PI3Kα, coded by the PIK3CA gene), p110β (PI3Kβ, coded by PIK3CB), p110δ (PI3Kδ, coded by PIK3CD), and p110γ (PI3Kγ, coded by PIK3CG). These are the catalytic subunits that form heterodimers with regulatory isoforms. The p85α and its splicing variants p55α and p50α (PIK3R1), p85β (PIK3R2), and p55γ (PIK3R3) can bind PI3Kα, PI3Kβ, or PI3Kδ (class IA PI3Ks), while p101 (PIK3R5) or p87 (PIK3R6) bind PI3Kγ (Class IB PI3Ks). PI3Kδ and PI3Kγ are largely restricted to leukocytes, while PI3Kα and PI3Kβ are ubiquitously expressed [1,4,5,6].
The development of small molecules able to block specific or multiple PI3K isoforms is a heavily pursued effort in oncology: Table 1 shows the PI3Kδ inhibitors that have entered clinical development. Multiple preclinical and clinical studies showed the anti-tumor activity of PI3Kδ inhibitors in patients affected by chronic lymphocytic leukemia (CLL), B- and T-cell lymphomas and these data have been extensively summarized elsewhere [3,4,5,6,7,8]. Importantly, PI3Ks are expressed not only in the cancer cells, but also in the non-neoplastic cells, in which PI3K inhibitors contribute to their pro- or anti-tumor effects, and they can be used to improve the response to immunomodulatory and immunotherapeutic agents [9,10,11,12,13,14,15,16,17]. In this review article, we will discuss the effects of inhibiting PI3Kδ isoform on the immune system with a particular focus on the T-cell compartment.
Table 1.
List of P3Kdelta inhibitors sorted by their target, their official name, if assigned, or by their common/alternative name.
| Target | Official Name | Common/Alternative Name | PI3Kδ (IC50, nM) |
PI3Kα (IC50, nM) |
PI3Kβ (IC50, nM) |
PI3Kγ (IC50, nM) |
Adm. Route | Phase # | FDA Approval | On-Going Trials ## |
|---|---|---|---|---|---|---|---|---|---|---|
| PI3Kδ | Acalisib | GS-9820, CAL-120 [18] | 12.7 | 5441 | 3377 | 1389 | p.o | 1 | - | - |
| PI3Kδ | Dezapelisib | INCB040093 [19] | 31 | 28,912 | 3751 | 2297 | p.o | 2 | - | - |
| PI3Kδ | Idelalisib | CAL-101, GS-1101 [20] | 2.5 | 820 | 565 | 89 | p.o | 3 | CLL, FL, SLL ** | Lymphoid tumors |
| PI3Kδ | Leniolisib | CDZ173 [21] | 1.1 | 244 | 424 | 2230 | p.o | 3 ^ | - | APDS/PASLI ^ |
| PI3Kδ | Linperlisib | YY-20394, PI3K(delta)-IN-2 [22] | n.a. | n.a. | n.a. | n.a. | p.o | 2 | - | Lymphoid and solid tumors |
| PI3Kδ | Nemiralisib | GSK2269557 [23] | 9.9 | n.a. | n.a. | n.a. | inh. | 2^ | - | - |
| PI3Kδ | Parsaclisib | INCB050465, IBI-376 [19] | 1.1 | >20,000 | >20,000 | >10,000 | p.o | 3 | - | Lymphoid tumors, myeloid neoplasms |
| PI3Kδ | Puquitinib | XC-302 [24] | 3.3 | 992.8 | 959.2 | 89.8 | p.o | no | - | - |
| PI3Kδ | Seletalisib | UCB-5857 [25] | 12 | 3638 | 2129 | 282 | p.o | 2 | - | No |
| PI3Kδ | Zandelisib | ME-401, PWT143 [26] | 5 | 5022 | 208 | 2137 | p.o | 2 | - | Lymphoid tumors |
| PI3Kδ | - | ACP-319, AMG 319 [27] | 18 | 33,000 | 270 | 85 | p.o | 2 | - | Lymphoid tumors |
| PI3Kδ | - | BGB-10188 [28] | n.a. | n.a. | n.a. | n.a. | p.o | 2 | - | Lymphoid and solid tumors |
| PI3Kδ | - | GS-9901 [29] | 1 | 750 | 100 | 190 | p.o | 1 | - | - |
| PI3Kδ | - | GSK2292767 [23] | n.a. | n.a. | n.a. | n.a. | inh. | 1 ^ | - | - |
| PI3Kδ | - | HMPL-689 [30] | 0.8 | >1000 | 87 | 114 | p.o | 1 | - | Lymphoid tumors |
| PI3Kδ | - | IOA-244, MSC2360844 [31] | 145 | 18,500 | 2850 | >20,000 | p.o | 1 | - | Lymphoid and solid tumors |
| PI3Kδ | - | RV1729 [32] | 12 | 193 | n.a. | 25 | inh. | 1 ^ | - | - |
| PI3Kδ | - | SHC014748M [33] | n.a. | n.a. | n.a. | n.a. | p.o | 2 | - | Lymphoid tumors |
| PI3Kα/PI3Kδ | Copanlisib | BAY 80-6946 [34] | 0.7 | 0.5 | 3.7 | 6.4 | i.v. | 3 | FL *** | Lymphoid and solid tumors |
| PI3Kα/PI3Kδ | Pictrelisib | Pictilisib GDC-0941, RG-7321 [35] | 3 | 3 | 33 | 75 | p.o | 2 | - | - |
| PI3Kα/PI3Kδ | - | TQ-B3525 [36] | n.a. | n.a. | n.a. | n.a. | p.o | 2 | - | Lymphoid and solid tumors |
| PI3Kβ/PI3Kδ | - | AZD8186 [37] | 12 | 35 | 4 | 675 | p.o | 2 | - | Solid tumors |
| PI3Kβ/PI3Kδ | - | KA2237 [38] | 8 | >500 | 19 | >500 | p.o | 1 | - | - |
| PI3Kα/PI3Kδ/PI3Kγ | Taselisib | GDC-0032 [39] | 0.12 | 0.29 | 9.1 | 0.97 | p.o | 3 | - | Lymphoid and solid tumors |
| PI3Kα/PI3Kβ/PI3Kδ | Sonolisib | PX-866 [40] | 2.7 | 5.5 | >300 | 9 | p.o | 2 | - | - |
| PI3Kδ/PI3Kγ | Duvelisib | IPI-145, INK1197 [41] | 2.5 | 1602 | 85 | 27 | p.o | 3 | CLL, FL, SLL **** | Lymphoid tumors |
| PI3Kδ/PI3Kγ | Tenalisib | RP6530 [42] | 24 | >7000 | >3000 | 33 | p.o | 2 | - | Solid tumors |
| PI3Kα/PI3Kδ/BRAF | - | ASN003 [43] | 6 | 16 | 690 | 97 | p.o | 1 | - | - |
| PI3Kδ/CK1ε | Umbralisib | TGR-1202, RP5264 [44] | 22.23 | >9000 | >1000 | >1000 | p.o | 3 | FL, MZL ***** | Lymphoid tumors |
| PI3Kα/PI3Kβ/PI3Kδ/HDAC | Fimepinostat | CUDC-907 [45] | 39 | 19 | 54 | 311 | p.o | 2 | - | Lymphoid and solid tumors |
FDA, U.S. Food and Drug Administration; target IC50 inhibition based on reported kinase inhibition profiles; #, based on http://adisinsight.springer.com/ and on https://clinicaltrials.gov accessed in 15 September 2021; ##, defined as “recruiting” or “not yet recruiting” in https://clinicaltrials.gov accessed in 15 September 2021; APDS/PASLI, Activated phosphoinositide 3-kinase delta syndrome/p110δ-activating mutation causing senescent T-cells, lymphadenopathy and immunodeficiency; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; ^, non in oncology; **, for the treatment of patients with (a) relapsed CLL in combination with rituximab, in patients for whom rituximab alone would be considered appropriate therapy due to other co-morbidities, (b) relapsed FL after at least two prior systemic therapies, (c) relapsed SLL after at least two prior systemic therapies) [46]; ***, for the treatment of adult patients with relapsed FL after at least two prior systemic therapies [47]; **** for the treatment of adult patients with (a) relapsed or refractory CLL/SLL after at least two prior therapies, (b) relapsed or refractory FL after at least two prior systemic therapies [48]; ***** for the treatment of adult patients with (a) relapsed or refractory MZL who have received at least one prior anti-CD20-based regimen and (b) for relapsed or refractory FL who have received at least three prior lines of systemic therapy [49]; inh., inhalation.; p.o., per os; i.v., intravenous.
2. Immune System and Anti-Cancer Immunotherapy
The TME is a complex system comprising the cancer cells, plus proteins and other chemical components of the extracellular matrix (ECM), and “accessory” non-neoplastic cells, such as resident mesenchymal support cells, infiltrating inflammatory immune cells, and endothelial cells. Altogether, the tumor microenvironment (TME) plays a fundamental role in regulating tumor development, both leading to an immune inflammatory response and fueling innate and adaptive immune activity against cancer cells, but also supporting the growth of the latter [50].
Cells and tissues are continuously surveilled by immune cells, which recognize and eliminate emerging cancer cells. Genetically engineered mice deficient for CD8+ cytotoxic T-lymphocyte (CTLs), CD4+ Th1 helper T-cells, or natural killer (NK) cells components of the immune system, show an increased tumor incidence [51,52].
During tumor initiation, naïve T-cells recognize antigens expressed by malignant cells are primed in the draining lymph nodes, are activated, and migrate in the TME. In this niche, immune response eliminates immunogenic cancer cells [53]. NK and cytotoxic CD8+ T-cells eliminate immunogenic proliferating cancer cells [54]. Later on, inflammation is persistent and inflammatory cells are recruited and activated. In many cancers, high presence of tumor-infiltrated T-cells has a good prognostic value [55,56]; instead, high presence of macrophage infiltration often correlates with poor prognosis [57] and tumor-associated inflammatory response has a paradoxical effect of enhancing tumor progression [58,59]. Tumor-promoting effects of immune cells is becoming more and more evident and inflammation provides bioactive factors that helps proliferative growth, angiogenesis, invasion, and metastasis. In the setting of T-cell lymphomas, expression and secretion of immunoinhibitory molecules can be shared by both tumor and non-neoplastic cells [15,17].
CD8+ T-cells are the main players among anti-tumor T-cells. They are primed and activated by antigen presenting cells (APC) to differentiate into CTL, which can directly kill cancer cells [60].
CD4+ T helper 1 (Th-1) cells act through a variety of mechanisms. They massively secrete proinflammatory cytokines, as IL-2, TNF-α and IFN-γ, co-adjuvate CTL cytotoxicity and T-cell priming and activation, help macrophages and NK cells to destroy tumoral cells and facilitate tumor antigens presentation [61,62,63]. Immune infiltrate components of tumors include CD8+ T-cells and Th-1 cytokines, correlating with favorable prognosis in many cancer types [64].
Effector T-cells killing-activity relies on the balance between the capability of tumor antigens to induce an immune response (immunogenic feature) and the existence of signals impairing T-cell functions [53]. This process by which the immune system controls tumoral growth and balances tumor immunogenicity is called immune editing. Tumoral cells with the most immunogenic antigens are recognized and killed in the early stages of tumorigenesis, while the less immunogenic cancer cells escape T-cell control [54,65]. Neoplastic cells are also able to decrease the response of the others innate immunity involved cells as tumor-associated macrophages (TAM) and NK cells [66].
At present, different mechanisms of cancer immune tolerance have been identified. Immune checkpoints signals are negative regulators of effector T-cells, and the two mainly studied molecules in cancers are Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) and Programmed Cell Death 1 (PD-1) [63]. Known ligands of CTLA-4 are CD80 and CD86, while PD-1 binds to its coreceptors PDL-1/2, expressed also by cancer cells, to impair anti-tumor T-cell responses [67]. Immune checkpoint inhibitors (pembrolizumab and nivolumab as anti-PD1; atezolizumab as anti-PD-L1; ipilimumab as anti-CTLA4) became a successful strategy to enhance anti-tumor response in many malignancies [68].
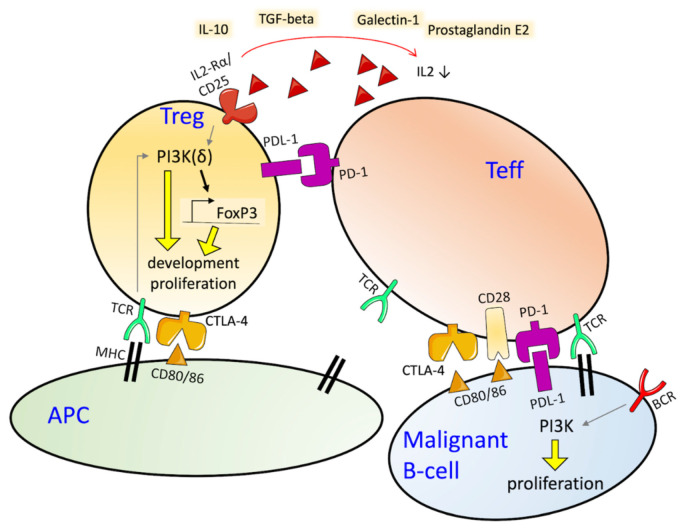
As the tumor grows, cancer cells and signal molecules in the TME recruit regulatory CD4+ T-cells (Tregs), responsible to inhibit T-cell responses, specifically priming, activation and cytotoxicity of effector immune cells (Figure 1) [69]. Tregs contribute to the suppression of uncontrolled clonal expansion and negatively regulate the insurgence of hyper-inflammatory state. In tumors, they are recruited to hamper the immune system and escape immune surveillance [70,71]. Tregs exploit their function through contact-dependent mechanisms—PDL-1, LAG-3 C39/73, CTLA-4 or PD1 are expressed on their cell surface, and lead to effector cell death or to enhance this event—and contact-independent mechanisms—by secreting immune-suppressive cytokines, as IL-10, TGF-β, prostaglandin E2, adenosine, and galectin-1 [72,73], and also recruiting myeloid-derived suppressor cells which contribute to build an immunosuppressive environment [74]. Tumor-infiltrating Treg cells are under pressure of a challenging environment with low oxygen availability, high glucose demand, and a multitude of cytokines and chemokines [75,76]. Tan et al. demonstrated that PI3K-AKT pathway regulates the immunosuppressive capacity of PD-1 deficient Tregs [77]. Tregs are characterized by the expression of the IL-2 receptor alpha chain (CD25), CD4, FOXP3 and CTLA-4. Enrichment of Tregs in tumors can be due to an augmented recruitment, expansion in the TME as a consequence of antigenic exposure, response to cytokine signals or metabolic changes (Figure 1) [53]. Higher numbers of Tregs have been detected in the blood of lymphoma patients than of healthy or cured patients [78,79], and in lymphoma tissues than in reactive lymph nodes [78]. In cutaneous diffuse large B-cell lymphoma, Hodgkin’s lymphoma and Epstein-Barr virus-associated lymphoma, Tregs are recruited by CCR4 ligands or evolve from conventional cells (Tconvs) to Tregs in the TME [80]. High presence of circulating Tregs represents a poor prognostic factor in diffuse large B-cell lymphoma (DLBCL), correlates with high lactate dehydrogenase, advanced stage of the disease [78], and poor survival [72,81].
Figure 1.
Immuno-regulation in the tumor microenvironment. Tregs deprive the surrounding of co-stimulatory signals for effector T-cells (Teff) affinity and activity. They exert their suppressive mechanism by different modalities: depriving IL-2 from the surrounding, therefore reducing it for effector T-cells, by IL-2 binding with CD25; constitutively expressing CTLA-4, which down-regulates CD80/86 expression by antigen-presenting cells (APC) and limits co-stimulatory signals for Teff, together with CD28; immune-suppressive cytokines produced by Tregs decrease APC and Teff signals; PD-1/PD-L1 axis activation inhibits Teff function. In this environment, responders T-cells die by apoptosis or stay dormant and tumor cells are prone to proliferation. Targeting PI3K specifically in Tregs could provide advantage for anti-cancer immunotherapy. TCR: T-cell receptor; MHC: Major Histocompatibility Complex, BCR: B-cell receptor.
3. Targeting PI3Kδ and Treg in Lymphomas
The ideal immune regulatory approach to fight cancer should be able to selectively deprive Tregs in TME, while maintaining a potent immune effector system. Understanding the signaling pathways regulating Tconvs and Tregs mechanisms could help to develop specific Tregs and Tconvs modulation strategies. In vitro and in vivo studies demonstrated that PI3K signaling is fundamental for Treg differentiation and immunosuppressive function, although the precise mechanism is still unclear [82,83,84].
Tregs are dependent on the activity of the PI3Kδ isoform [83,85,86] and studies suggest that loss of PI3K signaling in Tregs leads to increased activity of the BACH2 and FOXO1 transcription factors, which in normal conditions, regulate the expression of key genes in Treg differentiation and function (FOXP3, L-selectin, CCR7 and IFNγ) [87,88,89] (Figure 2). In line with this mechanism, PTEN inhibition impairs Treg function and reduces their immunosuppression ability [90]. PTEN-deficient Tregs could reduce FOXO1 transcription, followed by decreased expression of FOXP3, essential for Treg development [91].
Figure 2.
PI3K pathway in Tregs regulating anti-cancer immunity. Activated AKT pathway through PI3Kδ signaling, the dominant isoform in Tregs, leads to the phosphorylation of the BACH2 and FOXO1 transcription factors and in this form they are sequestrated in the cytoplasm. BACH2 and FOXO1 are regulators of genes involved in Treg differentiation and function, such as FOXP3, L-selectin (SELL), CCR7 and IFNγ. PI3Kδ inhibition suppress Tregs functionality; they are not able to suppress any more anti-tumor responses. Proteins belonging to downstream TCR signaling are also regulated by PI3Kδ, as pS6 phosphorylation and GSK-3β activation, controlling proliferation, survival pathway and downstream degradation of the antiapoptotic protein Mcl-1.
Interestingly, genetic and pharmacological inhibition of PI3Kδ in mice exerts anti-tumor activity via inhibition of Tregs and, possibly, of myeloid-derived suppressor cells [82,85]. In this context, CD8+ CTL can still mediate anti-tumor activity, although an altered balance between regulatory and effector CD4+ T-cells, with effector cells that prevail. Pharmacological targeting of PI3Kδ lead to similar changes compared to genetical inhibition, such as suppression of tumor growth and reduction of immunosuppression, in many cancer models [22,31,82,86,92,93,94,95,96]. The PI3Kδ inhibitor parsaclisib has in vivo antitumor activity against the A20 mouse lymphoma cell lines despite no in vitro anti-tumor activity [96]. Similar data are available for the PI3Kδ inhibitor linperlisib against models of breast carcinoma and colorectal cancer [22]. Hanna et al. demonstrated that PI3Kδ inhibition decreases Tregs numbers, proliferation, and activity in the Eμ-TCL1 model, but also CD8+ effector T-cells numbers and cytotoxicity T-cell ability [94]. In vitro experiments on T-cells from CLL patients, revealed that idelalisib down regulates the expression of crucial genes for T-cell mediated immunity, impairs IFNγ production by CD4 and CD8 T-cells, and decreases the proliferative capacity of T-cells without affecting their survival [93]. Similar results have been reported by Maharaj et al., also using the Eμ-TCL1 model [95]. The PI3Kδ inhibitor idelalisib and the PI3Kδ/γ inhibitor duvelisib, but not the dual PI3Kδ/CK1ε inhibitor umbralisib, determined a reduction of Tregs, which was associated with increased immune-mediated toxicities, in the absence of changes in the CD4/CD8 ratio or in the absolute number of T-cells [95]. In a syngeneic colorectal cancer model, treatment with the PI3Kδ inhibitor IOA-244 increases NK cells, and the ratio of cytotoxic CD8+ T-cells/Tregs [31]. The last observation is supported by data indicating a selective and concentration-dependent suppression of Treg cells but not of the proliferation of CD8+ T-cells [31]. Suppression of Tregs in syngeneic tumors is also reported with the PI3Kα/δ inhibitor copanlisib [92], and with KA2237 [38]. In an in vivo mammary tumor model, PI3Kδ blockade leads tumors to be divided in “non-regressors”, in which tumor growth rate is reduced but tumors continue to grow, and “regressors” where tumors shrink. Tumor infiltrating T-cells in “regressors” are enriched of elements indicating a CD8-specific T-cell response. In both groups of mice Tregs where reduced, although in Tregs from “non-regressor” tumors the expression of the coinhibitory receptor LAG3 is enriched compared to “regressor” and untreated tumors [97].
Exposure of follicular lymphoma (FL) cells, cocultured with follicular dendritic cells derived from normal tonsils, to idelalisib down-regulates the expression of integrins and their ligands, of proangiogenic factors and it determines a disruption of the CD40/CD40L-mediated crosstalk between FL cells and T-cells [98]. The PI3Kδ inhibitor down-regulates CCL22 expression, and this would reduce the recruitment of Tregs and of T follicular helper cells (TFH), both expressing the chemokine receptor CCR4 and supportive for the growth and survival of FL cells [98]. A similar effect is also observed during the generation of high-affinity antibodies in the GC, where PI3Kδ regulates TFH formation and function, activating ICOS, leading to intracellular signaling activation, production of TFH-related cytokines and effector molecules [99]. Moreover, idelalisib appears to increase the sensitivity of FL cells to the BCL2 inhibitor venetoclax, via a reduced PI3Kδ-mediated BAD phosphorylation, and/or via up-regulating the levels of proapoptotic factor HRK, and/or down-regulation of the anti-apoptotic factor BFL-1 [98].
Targeting PI3Kδ isoform with idelalisib stimulates CD8+ T-cells proliferation, maintaining survival, cytokines and granzyme B production. Idelalisib also inhibits Akt phosphorylation (both S473 and T308) in Tregs but not in Tconvs, and abrogates Tregs proliferation without affecting Tconv cells [83,86].
Finally, data collected in syngeneic mouse models mostly suggest that PI3Kδ inhibitors show synergism with immune checkpoint modulators [22,31,92]. However, there are also data demonstrating an important suppression of CD8+ T-cells maturation and killing capacity, antagonizing the effect due to immune checkpoint blockade [100].
4. Potential Toxicities Linked with PI3Kδ Inhibition in T-Cells
Side effects of PI3Kδ inhibitors encompass infections, hepatotoxicity, diarrhea and/or colitis, and pneumonitis [4,5,6,101,102,103,104,105,106] (Table 2). In clinical trials with idelalisib, serious adverse events have also included deaths related to cytomegalovirus infections, pneumonias caused by Pneumocystis jirovencii, in addition to respiratory events possibly caused by infections [107]. These toxicities have been linked with a T-cell immune response impairment induced by PI3Kδ inhibition that could favor such infections or viral reactivations, both by an increase in Treg-mediated immune tolerance mechanisms, and by impairment of the later stages of CD8 differentiation involved in the most potent antiviral activity [93,103]. Interestingly, these toxicities seem more frequent in treatment-naïve than in pre-treated patients and in younger than older individuals, further suggesting that the presence of a still partially preserved immune system is implied [101,103,104], and they might be associated with higher clinical activity [105].
Table 2.
Effect of the PI3Kδ inhibitors on serum levels of secreted factors in the context of clinical trials enrolling patients with lymphoma.
| PI3Kδ Inhibitor | Phase | Lymphoma Subtypes | Decreased Factors | Increased Factors |
|---|---|---|---|---|
| Copanlisib | 1 [108] | FL, WM, DLBCL, BL, MCL, PTCL | CCL2, CCL3, CCL5, CCL15, CCL16, IL-10, IL2RA, CD27, CD5L (cycle 1, day 15) | - |
| Duvelisib | 1 [109] | FL, WM, SLL, MZL | CCL1, CCL4, CCL17, CCL22, CXCL10, CXCL13, IL-10, IL-16, MMP-9, TNFα (cycle 1, day 8) | - |
| Duvelisib | 1 [110] | CLL | CCL1, CCL3, CCL4, CCL17, CCL22, CXCL10, CXCL13, IL-6, IL-10, IL-12p40, MMP-9, MMP-12, TNFα (cycle 1, day 8) | - |
| Duvelisib | 1 [111] | PTCL | IL10, IL-12p40, CXCL13, (cycle 1, day 8) | CCL1, IL6, IL8, IL9, IL15 IL17A, IL-12p70, CD40L, TNFβ |
| Duvelisib | 1 [112] | CLL, FL, WM, SLL, MZL | CCL1, CCL4, CCL17, CCL22, CXCL10, CXCL13, MMP-9, TNFα (cycle 1, day 8) | - |
| Duvelisib | 3 [113] | CLL/SLL | CCL3, CCL4, CCL17, CCL19, CCL22, CXCL13, IL2RA, IL-12p40, IL-10, TNFα (cycle 2, day1); | - |
| Idelalisib | 1 [114] | CLL/SLL | CCL3, CCL4, CCL17, CCL22, CD40L, CCL2, CXCL13, TNFα (within 1 month) | - |
| Tenalisib | 1 [115] | HL | CCL17 | - |
FL, follicular lymphoma; WM, Waldenström’s macroglobulinemia; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; MCL, mantle cell lymphoma; PTCL, peripheral T-cell lymphoma; SLL, small lymphocytic lymphoma; MZL, marginal zone lymphoma; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma.
Although the reduction of Tregs in the TME is an important and attractive therapeutic target, the caveat is that a reduction of Tregs activity, can activate autoimmune reactions [116,117]. For example, the effect on T-cells is believed to cause the severe diarrhea or colitis, which are some of the major side effects in patients receiving PI3Kδ inhibitors [7,102]. A picture similar to graft versus host disease has been described in these patients, with increase infiltration of CD8+ cytotoxic T-cells [118,119], perhaps due to the already mentioned effect of the PI3Kδ inhibitor on the mesenteric B-cells leading to an unleashed activity of Tregs [116].
We have also to consider that the pattern of selectivity for the PI3Kδ isoform versus other class IA or IB members largely varies across the small molecules that have entered the clinical evaluation (Table 1). Their ability to bind isoforms can affect the toxicity profile. An example is given by observed acute insulin resistance, also causing severe hyperglycemia and hyperinsulinemia, seen with compounds that also target PI3Kα, physiologically involved in the glucose homeostasis in muscle, liver, and fat tissues [6,102,120].
5. Effects on T-Cells in the Context of Clinical Trials
While the effect of the PI3Kδ inhibitors on the secretion of chemokines has been studied in many clinical trials enrolling patients with lymphoma (Table 3), only a few studies have explored whether the drugs affect T-cell populations in the peripheral blood (Pb) [114,117,121,122,123] or in the TME [108,124].
Table 3.
Potential toxicities of the PI3Kδ inhibitors in the context of clinical trials enrolling patients with lymphoma.
| PI3Kδ Inhibitor | Phase | Lymphoma Subtypes | Any Grade, AE (%) | Grade ≥ 3, AE (%) |
|---|---|---|---|---|
| Copanlisib | 2 [108] | FL, MZL, SLL, WM/LPL, DLBCL | Diarrhea (35.2), colitis (0.7), hyperglycemia (50.0), hypertension (29.6), neutropenia (28.9), pneumonitis (6.3) | Diarrhea (8.5), colitis (0.7), hyperglycemia (40.1), hypertension (23.9), neutropenia (24.0), pneumonitis (1.4) |
| Umbralisib | 2 [109] | MZL, FL, SLL | Neutropenia (15.9), diarrhea (59.1), colitis (1.9), fatigue (30.8), increased ALT (20.2), increased AST (18.8) | Neutropenia (11.5), diarrhea (10.1), colitis (0.5), fatigue (3.4), increased ALT (6.7), increased AST (7.2) |
| Duvelisib | 2 [113] | SLL, FL, MZL | Diarrhea (48.8), neutropenia (28.7), throbocytopenia (18.6), anemia (26.4), febrile neutropenia (9.3), increased ALT (14.0), increased lipase (9.3), pneumonia (7.8), colitis (7.8) | Diarrhea (14.7), neutropenia (24.8), throbocytopenia (11.6), anemia (14.7), febrile neutropenia (9.3), increased ALT (5.4), increased lipase (7.0), pneumonia (5.4), colitis (5.4) |
| Idelalisib | 2 [114] | FL, SLL, MZL, WM/LPL | Diarrhea (43.0), pneumonia (11.0), increased ALT (47.0), increased AST (35.0) | Diarrhea (13.0), pneumonia (7.0), increased ALT (13.0), increased AST (8.0) |
| Tenalisib | 1 [115] | DLBCL, MCL, PTCL, CLL, HL | Anemia (29.0), neutropenia (20.0), thrombocytopenia (26.0), pyrexia (37.0), cough (43.0), dyspnea (26.0) | Anemia (11.0), neutropenia (17.0), thrombocytopenia (17.0), pyrexia (3.0) |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate eminotransferase; BL, Burkitt lymphoma; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LPL, Lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; PTCL, peripheral T-cell lymphoma; SLL, small lymphocytic lymphoma; HL, Hodgkin lymphoma. WM, Waldenström’s macroglobulinemia.
No significant changes in Pb T-cells subsets were seen in the phase I and II studies evaluating idelalisib in patients with relapsed indolent lymphoma [122,123] and in the phase I for CLL [114]. Conversely, a decrease of the Treg percentage was described in the Pb of 13/19 relapsed/refractory CLL patients treated for one month of idelalisib in a separate phase I study evaluating the small molecule as single agent followed by 6 months of combination therapy with the anti-CD20 antibody ofatumumab [117]. Importantly, the decrease of Tregs in the Pb was stronger in patients that experienced toxicity [117].
A reduction in the Pb Tregs was also observed in 14/19 relapsed/refractory CLL patients exposed to the PI3Kδ inhibitor ACP-319 in the phase I study [121].
Serial biopsies were obtained in 30 patients with relapsed/refractory solid tumors or lymphoma enrolled in a phase I study of copanlisib, a pan PI3K inhibitor, preferentially targeting the PI3Kα/PI3Kδ isoforms [108]. There was a reduction in the proportion of CD4+T-cells in tumors after 14 days of treatment in 14 of patients treated at 0.8 mg/Kg but not at 0.4 mg mg/Kg (n = 16), with no changes in the CD8+ cells [108]. The reduction in the CD4+ cells suggests that Tregs were affected; however no additional staining was performed.
In the phase I study, exploring the dual PI3K/BRAF inhibitor sonolisib in patients with advanced solid tumors bearing the BRAF V600 mutation, biopsies were performed at baseline and at day 8 of the first cycle in six patients [124]. An increase in CD8+ cells at immunohistochemistry was observed in 5/6 patients [124]. This was paired with higher PD-L1 staining in the two cases with a partial response and not in patients with stable or progressive disease [124]. Additionally, here, no data are available for Tregs.
Finally, since PI3Kδ is also downstream to FcεRI, activated by IgE binding in mast cells and basophils, idelalisib has been evaluated in patients with allergic rhinitis [125]. In a phase 1 study the PI3Kδ inhibitor decreased plasma levels of CD631/CCR31 basophils, and inhibited ex vivo basophil activation in response to allergen stimulation [125]. A similar effect has also been reported in relapsed/refractory lymphoma patients enrolled in a phase I with the PI3Kδ inhibitor dezapelisib [126].
6. Conclusions
PI3Kδ inhibitors are active anti-cancer compounds in lymphomas. Their mechanism of action is promiscuous, and it is mediated via a direct inhibition of PI3Kδ in the lymphoma cells but also due to an inhibitory activity in multiple non-neoplastic cells. In particular, the data we have summarized highlight that the pharmacological inhibition of PI3Kδ in Tregs is clearly effective in boosting anti-tumor immune system. Further studies are needed to exploit this therapeutic option, avoiding the possible insurgence of autoimmune disorders. Discovery of other pathways and molecules that preferentially inhibit PI3K signaling specifically in Tregs is needed.
Abbreviations
| APC | antigen presenting cells |
| CLL | chronic lymphocytic leukemia |
| CTL | cytotoxic T-lymphocyte |
| CTLA-4 | Cytotoxic T-Lymphocyte Associated Protein 4 |
| DLBCL | diffuse large B-cell lymphoma |
| ECM | extracellular matrix |
| NK | natural killer |
| PD-1 | Programmed Cell Death 1 |
| PI3K | phosphoinositide 3-kinases |
| TAM | tumor-associated macrophages |
| Tconvs | conventional T-cells |
| TFH | T follicular helper cells |
| TME | tumor microenvironment |
| Tregs | regulatory CD4+ T-cells |
Author Contributions
Conceptualization, C.T., L.A., P.L.Z., F.B.; writing—original draft preparation, C.T.; writing—review and editing, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Lisa Argnani: consultancy fee from Servier Italia. Pierluigi Zinzani: honoraria from Abbvie, ADC Therapeutics, Astellas, Astrazeneca, Autolus, Bayer, Beigene, BMS, Celltrion, Debiopharma, Eusapharma, Gilead, Hikma, Incyte, Janssen, Karyopharm, Kirin Kyowa, Mei Pharma, Merck, Portola, Roche, Servier, Takeda, TG Therapeuticals, Verastem; speakers Bureau for Abbvie, Astellas, Beigene, BMS, Celltrion, Eusapharma, Gilead, Incyte, Janssen, Kirin Kyowa, Merck, Portola, Roche, Servier, Takeda, Verastem; membership on an entity’s Board of Directors or advisory committees of Abbvie, ADC Therapeutics, Astrazeneca, Autolus, Bayer, Beigene, Eusapharma, Gilead, Hikma, Janssen, Karyopharm, Kirin Kyowa, MEI Pharma, Merck, Roche, Servier, Takeda, TG Therapeuticals, Verastem. Francesco Bertoni: institutional research funds from Acerta, ADC Therapeutics, Bayer AG, Cellestia, CTI Life Sciences, EMD Serono, Helsinn, ImmunoGen, Menarini Ricerche, NEOMED Therapeutics 1, Nordic Nanovector ASA, Oncology Therapeutic Development, PIQUR Therapeutics AG; consultancy fee from Helsinn, Menarini; expert statements provided to HTG; travel grants from Amgen, Astra Zeneca, Jazz Pharmaceuticals, PIQUR Therapeutics AG.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thorpe L.M., Yuzugullu H., Zhao J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer. 2015;15:7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lampson B.L., Brown J.R. PI3Kdelta-selective and PI3Kalpha/delta-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma. Expert Opin. Investig. Drugs. 2017;26:1267–1279. doi: 10.1080/13543784.2017.1384815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broccoli A., Zinzani P.L. Phosphatidyl-inositol 3-kinase inhibitors in the treatment of T-cell lymphomas. Ann. Lymphoma. 2018;2 doi: 10.21037/aol.2017.12.01. [DOI] [Google Scholar]
- 4.Kienle D.L., Stilgenbauer S. Approved and emerging PI3K inhibitors for the treatment of chronic lymphocytic leukemia and non-Hodgkin lymphoma. Expert Opin. Pharmacother. 2020;21:917–929. doi: 10.1080/14656566.2020.1737010. [DOI] [PubMed] [Google Scholar]
- 5.Visentin A., Frezzato F., Severin F., Imbergamo S., Pravato S., Gargarella L.R., Manni S., Pizzo S., Ruggieri E., Facco M., et al. Lights and Shade of Next-Generation Pi3k Inhibitors in Chronic Lymphocytic Leukemia. OncoTargets Ther. 2020;13:9679–9688. doi: 10.2147/OTT.S268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips T.J., Michot J.M., Ribrag V. Can Next-Generation PI3K Inhibitors Unlock the Full Potential of the Class in Patients With B-Cell Lymphoma? Clin. Lymphoma Myeloma Leuk. 2021;21:8–20. doi: 10.1016/j.clml.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Cheah C.Y., Fowler N.H. Idelalisib in the management of lymphoma. Blood. 2016;128:331–336. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berning P., Lenz G. The role of PI3K inhibitors in the treatment of malignant lymphomas. Leuk. Lymphoma. 2021;62:517–527. doi: 10.1080/10428194.2020.1839654. [DOI] [PubMed] [Google Scholar]
- 9.Okkenhaug K., Graupera M., Vanhaesebroeck B. Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer Discov. 2016;6:1090–1105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Xu Y., Zhou Q., Chen M., Zhang Y., Liang H., Zhao J., Zhong W., Wang M. PI3K in cancer: Its structure, activation modes and role in shaping tumor microenvironment. Future Oncol. 2018;14:665–674. doi: 10.2217/fon-2017-0588. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell J.S., Massi D., Teng M.W.L., Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin. Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Beielstein A.C., Pallasch C.P. Tumor Metabolism as a Regulator of Tumor-Host Interactions in the B-Cell Lymphoma Microenvironment-Fueling Progression and Novel Brakes for Therapy. Int. J. Mol. Sci. 2019;20:4158. doi: 10.3390/ijms20174158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petroni G., Buqué A., Zitvogel L., Kroemer G., Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2020 doi: 10.1016/j.ccell.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Caforio M., de Billy E., De Angelis B., Iacovelli S., Quintarelli C., Paganelli V., Folgiero V. PI3K/Akt Pathway: The Indestructible Role of a Vintage Target as a Support to the Most Recent Immunotherapeutic Approaches. Cancers. 2021;13:4040. doi: 10.3390/cancers13164040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter T., Tzankov A. Lymphomas and Their Microenvironment: A Multifaceted Relationship. Pathobiology. 2019;86:225–236. doi: 10.1159/000502912. [DOI] [PubMed] [Google Scholar]
- 16.Menter T., Tzankov A., Dirnhofer S. The tumor microenvironment of lymphomas: Insights into the potential role and modes of actions of checkpoint inhibitors. Hematol. Oncol. 2021;39:3–10. doi: 10.1002/hon.2821. [DOI] [PubMed] [Google Scholar]
- 17.Pizzi M., Boi M., Bertoni F., Inghirami G. Emerging therapies provide new opportunities to reshape the multifaceted interactions between the immune system and lymphoma cells. Leukemia. 2016;30:1805–1815. doi: 10.1038/leu.2016.161. [DOI] [PubMed] [Google Scholar]
- 18.Shugg R.P., Thomson A., Tanabe N., Kashishian A., Steiner B.H., Puri K.D., Pereverzev A., Lannutti B.J., Jirik F.R., Dixon S.J., et al. Effects of isoform-selective phosphatidylinositol 3-kinase inhibitors on osteoclasts: Actions on cytoskeletal organization, survival, and resorption. J. Biol. Chem. 2013;288:35346–35357. doi: 10.1074/jbc.M113.507525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue E.W., Li Y.-L., Douty B., He C., Mei S., Wayland B., Maduskuie T., Falahatpisheh N., Sparks R.B., Polam P., et al. INCB050465 (Parsaclisib), a Novel Next-Generation Inhibitor of Phosphoinositide 3-Kinase Delta (PI3Kδ) ACS Med. Chem. Lett. 2019;10:1554–1560. doi: 10.1021/acsmedchemlett.9b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lannutti B.J., Meadows S.A., Herman S.E., Kashishian A., Steiner B., Johnson A.J., Byrd J.C., Tyner J.W., Loriaux M.M., Deininger M., et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoegenauer K., Soldermann N., Zécri F., Strang R.S., Graveleau N., Wolf R.M., Cooke N.G., Smith A.B., Hollingworth G.J., Blanz J., et al. Discovery of CDZ173 (Leniolisib), Representing a Structurally Novel Class of PI3K Delta-Selective Inhibitors. ACS Med. Chem. Lett. 2017;8:975–980. doi: 10.1021/acsmedchemlett.7b00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z., Lou Y., Tan J., Wang C., Ge X., Gu Y., Zhou H. Abstract B048: A novel PI3K delta inhibitor suppresses tumor progression by immune modulation. Cancer Immunol. Res. 2016;4:B048. [Google Scholar]
- 23.Down K., Amour A., Baldwin I.R., Cooper A.W., Deakin A.M., Felton L.M., Guntrip S.B., Hardy C., Harrison Z.A., Jones K.L., et al. Optimization of Novel Indazoles as Highly Potent and Selective Inhibitors of Phosphoinositide 3-Kinase δ for the Treatment of Respiratory Disease. J. Med. Chem. 2015;58:7381–7399. doi: 10.1021/acs.jmedchem.5b00767. [DOI] [PubMed] [Google Scholar]
- 24.Xie C., He Y., Zhen M., Wang Y., Xu Y., Lou L. Puquitinib, a novel orally available PI3Kdelta inhibitor, exhibits potent antitumor efficacy against acute myeloid leukemia. Cancer Sci. 2017;108:1476–1484. doi: 10.1111/cas.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen R.A., Brookings D.C., Powell M.J., Delgado J., Shuttleworth L.K., Merriman M., Fahy I.J., Tewari R., Silva J.P., Healy L.J., et al. Seletalisib: Characterization of a Novel, Potent, and Selective Inhibitor of PI3Kδ. J. Pharmacol. Exp. Ther. 2017;361:429–440. doi: 10.1124/jpet.116.237347. [DOI] [PubMed] [Google Scholar]
- 26.O’Farrell M., Ventura R., Tai A., Tyner J.W., Loriaux M.M., Mahadevan D., Morales C., Brown S.D., Matthews D.J. Preclinical Characterization of PWT143, a Novel Selective and Potent Phosphatidylinositol 3-kinase Delta (PI3K delta) Inhibitor with Ex-Vivo Activity in Hematologic Malignancies. Blood. 2012;120:2907. doi: 10.1182/blood.V120.21.2907.2907. [DOI] [Google Scholar]
- 27.Cushing T.D., Hao X., Shin Y., Andrews K., Brown M., Cardozo M., Chen Y., Duquette J., Fisher B., de Turiso F.G.-L., et al. Discovery and in vivo evaluation of (S)-N-(1-(7-fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H-purin-6-amine (AMG319) and related PI3Kδ inhibitors for inflammation and autoimmune disease. J. Med. Chem. 2015;58:480–511. doi: 10.1021/jm501624r. [DOI] [PubMed] [Google Scholar]
- 28.Yang X., Yang X., Cui X., Su D., Wu Y., Sun X., Wang J., Bai H., Wei W., Li J., et al. Abstract 664: BGB-10188, a highly selective PI3Kδ inhibitor with improved safety profile and superior anti-tumor activities in vivo. Cancer Res. 2020;80:664. [Google Scholar]
- 29.Patel L., Chandrasekhar J., Evarts J., Forseth K., Haran A.C., Ip C., Kashishian A., Kim M., Koditek D., Koppenol S., et al. Discovery of Orally Efficacious Phosphoinositide 3-Kinase δ Inhibitors with Improved Metabolic Stability. J. Med. Chem. 2016;59:9228–9242. doi: 10.1021/acs.jmedchem.6b01169. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence T., Khullar A., Yang W., Hahka-Kemppinen M., Kania M., Su W. Abstract 5234: A phase I study of HMPL-689, a small molecule selective inhibitor of phosphoinositide 3-kinase-delta, inpatients with relapsed or refractory lymphoma. Cancer Res. 2020;80:5234. [Google Scholar]
- 31.Ewings K., MacQueen A., Shah P., Tsapara A., Papakonstanti E., Veen L.V.D., Lahn M., Johnson Z. Abstract 2692: Preclinical development of a novel, highly selective PI3Kδ inhibitor, IOA-244, for the treatment of solid malignancies. Cancer Res. 2019;79:2692. [Google Scholar]
- 32.Norman P. Evaluation of WO2013136076: Two crystalline forms of the phosphatidylinositol 3-kinase-delta inhibitor RV-1729. Expert Opin. Ther. Pat. 2014;24:471–475. doi: 10.1517/13543776.2014.865725. [DOI] [PubMed] [Google Scholar]
- 33.Fan L., Wang C., Wang Z., Zhang X., Cao L., Miao Y., Du X., Xu W., Li J. SHC014748M, a Novel Selective Inhibitor of PI3Kδ, Demonstrates Promising Pre-Clinical Antitumor Activity in B Cell Lymphomas and CLL. Blood. 2019;134:5306. doi: 10.1182/blood-2019-129469. [DOI] [Google Scholar]
- 34.Scott W.J., Hentemann M.F., Rowley R.B., Bull C.O., Jenkins S., Bullion A.M., Johnson J., Redman A., Robbins A.H., Esler W., et al. Discovery and SAR of Novel 2,3-Dihydroimidazo[1,2-c]quinazoline PI3K Inhibitors: Identification of Copanlisib (BAY 80-6946) ChemMedChem. 2016;11:1517–1530. doi: 10.1002/cmdc.201600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folkes A.J., Ahmadi K., Alderton W.K., Alix S., Baker S.J., Box G., Chuckowree I.S., Clarke P.A., Depledge P., Eccles S.A., et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Jiang W., Li S., Wang Y., Sun P., Zhou P., Zheng Y., Zhan J., Li Z. Safety and efficacy of TQ-B3525, a novel and selective oral PI3K α/δ inhibitor, in Chinese patients with advanced malignancies: A phase I dose-escalation and expansion trial. J. Clin. Oncol. 2020;38:8058. doi: 10.1200/JCO.2020.38.15_suppl.8058. [DOI] [Google Scholar]
- 37.Hancox U., Cosulich S., Hanson L., Trigwell C., Lenaghan C., Ellston R., Dry H., Crafter C., Barlaam B., Fitzek M., et al. Inhibition of PI3Kbeta signaling with AZD8186 inhibits growth of PTEN-deficient breast and prostate tumors alone and in combination with docetaxel. Mol. Cancer Ther. 2015;14:48–58. doi: 10.1158/1535-7163.MCT-14-0406. [DOI] [PubMed] [Google Scholar]
- 38.Nastoupil L.J., Neelapu S.S., Davis R.E., Samaniego F., Fowler N.H., Westin J., Lee H.J., Wang M., Hagemeister F., Cecil A.R.L., et al. Preclinical and phase I studies of KA2237, a selective and potent inhibitor of PI3K β/δ in relapsed refractory B cell lymphoma. Leuk. Lymphoma. 2021:1–11. doi: 10.1080/10428194.2021.1957874. [DOI] [PubMed] [Google Scholar]
- 39.Ndubaku C.O., Heffron T.P., Staben S.T., Baumgardner M., Blaquiere N., Bradley E., Bull R., Do S., Dotson J., Dudley D., et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): A β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J. Med. Chem. 2013;56:4597–4610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 40.Ihle N.T., Paine-Murrieta G., Berggren M.I., Baker A., Tate W.R., Wipf P., Abraham R.T., Kirkpatrick D.L., Powis G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol. Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler D.G., Faia K.L., DiNitto J.P., Ali J.A., White K.F., Brophy E.E., Pink M.M., Proctor J.L., Lussier J., Martin C.M., et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem. Biol. 2013;20:1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Vakkalanka S., Viswanadha S., Gaudio E., Zucca E., Bertoni F., Bernasconi E., Rossi D., Stathis A. Dual PI3Kδ/γ Inhibition By RP6530 Induces Apoptosis and Cytotoxicity In B-Lymphoma Cells. Blood. 2013;122:4411. doi: 10.1182/blood.V122.21.4411.4411. [DOI] [Google Scholar]
- 43.Thompson S.K., Jaleel M., Nyavanandi V.K., Ramachandra M., Subramanya H., Basavaraju A., Sihorkar V., Smith R.A., Rao N., Gupta S., et al. Abstract B100: ASN003, a unique B-RAF inhibitor with additional selective activity against PI3K and mTOR kinases, shows strong antitumor activity in multiple xenograft models. Mol. Cancer Ther. 2015;14:B100. [Google Scholar]
- 44.Deng C., Lipstein M.R., Scotto L., Jirau Serrano X.O., Mangone M.A., Li S., Vendome J., Hao Y., Xu X., Deng S.X., et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kδ and CK1ε in hematological malignancies. Blood. 2017;129:88–99. doi: 10.1182/blood-2016-08-731240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian C., Lai C.J., Bao R., Wang D.G., Wang J., Xu G.X., Atoyan R., Qu H., Yin L., Samson M., et al. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin. Cancer Res. 2012;18:4104–4113. doi: 10.1158/1078-0432.CCR-12-0055. [DOI] [PubMed] [Google Scholar]
- 46.Zydelig (Idelalisib): Highlights of Prescribing Information. Revised: October 2020. Gilead Sciences Inc. [(accessed on 15 September 2021)];2020 Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 47.Aliqopa (Copanlisib): Highlights of Prescribing Information. Bayer HealthCare Pharmaceuticals Inc. Revised: February 2020. [(accessed on 15 September 2021)];2020 Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 48.Copiktra (Duvelisib): Highlights of Prescribing Information. Verastem, Inc. Revised: July 2019. [(accessed on 15 September 2021)];2019 Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 49.Ukoniq (Umbralisib): Highlights of Prescribing Information. TG Therapeutics, Inc. Revised: February 2021. [(accessed on 15 September 2021)];2021 Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 50.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teng M.W., Swann J.B., Koebel C.M., Schreiber R.D., Smyth M.J. Immune-mediated dormancy: An equilibrium with cancer. J. Leukoc. Biol. 2008;84:988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 53.Speiser D.E., Ho P.-C., Verdeil G. Regulatory circuits of T cell function in cancer. Nat. Rev. Immunol. 2016;16:599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 54.Teng M.W., Galon J., Fridman W.H., Smyth M.J. From mice to humans: Developments in cancer immunoediting. J. Clin. Invest. 2015;125:3338–3346. doi: 10.1172/JCI80004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 56.Dieu-Nosjean M.C., Antoine M., Danel C., Heudes D., Wislez M., Poulot V., Rabbe N., Laurans L., Tartour E., de Chaisemartin L., et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 57.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Hanson H.L., Donermeyer D.L., Ikeda H., White J.M., Shankaran V., Old L.J., Shiku H., Schreiber R.D., Allen P.M. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/S1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 61.Kalams S.A., Walker B.D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J., Schreiber R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 63.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 65.Matsushita H., Vesely M.D., Koboldt D.C., Rickert C.G., Uppaluri R., Magrini V.J., Arthur C.D., White J.M., Chen Y.S., Shea L.K., et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palucka A.K., Coussens L.M. The Basis of Oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward-Hartstonge K.A., Kemp R.A. Regulatory T-cell heterogeneity and the cancer immune response. Clin. Transl. Immunol. 2017;6:e154. doi: 10.1038/cti.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patton D.T., Garden O.A., Pearce W.P., Clough L.E., Monk C.R., Leung E., Rowan W.C., Sancho S., Walker L.S.K., Vanhaesebroeck B., et al. Cutting edge: The phosphoinositide 3-kinase p110δ is critical for the function of CD4+ CD25+ Foxp3+ regulatory T cells. J. Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 71.Poli A., Fiume R., Mongiorgi S., Zaurito A., Sheth B., Vidalle M.C., Hamid S.A., Kimber S., Campagnoli F., Ratti S., et al. Exploring the controversial role of PI3K signalling in CD4+ regulatory T (T-Reg) cells. Adv. Biol. Regul. 2020;76:100722. doi: 10.1016/j.jbior.2020.100722. [DOI] [PubMed] [Google Scholar]
- 72.Lindqvist C.A., Loskog A.S. T regulatory cells in B-cell malignancy—Tumour support or kiss of death? Immunology. 2012;135:255–260. doi: 10.1111/j.1365-2567.2011.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell D.J. Control of Regulatory T Cell Migration, Function, and Homeostasis. J. Immunol. 2015;195:2507–2513. doi: 10.4049/jimmunol.1500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holmgaard R.B., Zamarin D., Li Y., Gasmi B., Munn D.H., Allison J.P., Merghoub T., Wolchok J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015;13:412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eales K.L., Hollinshead K.E., Tennant D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190. doi: 10.1038/oncsis.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagarsheth N., Wicha M.S., Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan C.L., Kuchroo J.R., Sage P.T., Liang D., Francisco L.M., Buck J., Thaker Y.R., Zhang Q., McArdel S.L., Juneja V.R., et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 2021;218:e20182232. doi: 10.1084/jem.20182232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mittal S., Marshall N.A., Duncan L., Culligan D.J., Barker R.N., Vickers M.A. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–5370. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 79.Wu W., Wan J., Xia R., Huang Z., Ni J., Yang M. Functional role of regulatory T cells in B cell lymphoma and related mechanisms. Int. J. Clin. Exp. Pathol. 2015;8:9133–9139. [PMC free article] [PubMed] [Google Scholar]
- 80.De Charette M., Houot R. Hide or defend, the two strategies of lymphoma immune evasion: Potential implications for immunotherapy. Haematologica. 2018;103:1256–1268. doi: 10.3324/haematol.2017.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang C., Wu S.Y., Kang Y.W., Lin K.P., Chen T.Y., Medeiros L.J., Chang K.C. High levels of regulatory T cells in blood are a poor prognostic factor in patients with diffuse large B-cell lymphoma. Am. J. Clin. Pathol. 2015;144:935–944. doi: 10.1309/AJCPUJGMVV6ZF4GG. [DOI] [PubMed] [Google Scholar]
- 82.Ali K., Soond D.R., Pineiro R., Hagemann T., Pearce W., Lim E.L., Bouabe H., Scudamore C.L., Hancox T., Maecker H., et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chellappa S., Kushekhar K., Munthe L.A., Tjønnfjord G.E., Aandahl E.M., Okkenhaug K., Taskén K. The PI3K p110δ Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function. J. Immunol. 2019;202:1397–1405. doi: 10.4049/jimmunol.1701703. [DOI] [PubMed] [Google Scholar]
- 84.Stark A.K., Davenport E.C.M., Patton D.T., Scudamore C.L., Vanhaesebroeck B., Veldhoen M., Garden O.A., Okkenhaug K. Loss of Phosphatidylinositol 3-Kinase Activity in Regulatory T Cells Leads to Neuronal Inflammation. J. Immunol. 2020;205:78–89. doi: 10.4049/jimmunol.2000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong S., Harrington B.K., Hu E.Y., Greene J.T., Lehman A.M., Tran M., Wasmuth R.L., Long M., Muthusamy N., Brown J.R., et al. PI3K p110δ inactivation antagonizes chronic lymphocytic leukemia and reverses T cell immune suppression. J. Clin. Invest. 2019;129:122–136. doi: 10.1172/JCI99386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmad S., Abu-Eid R., Shrimali R., Webb M., Verma V., Doroodchi A., Berrong Z., Samara R., Rodriguez P.C., Mkrtichyan M., et al. Differential PI3Kdelta Signaling in CD4(+) T-cell Subsets Enables Selective Targeting of T Regulatory Cells to Enhance Cancer Immunotherapy. Cancer Res. 2017;77:1892–1904. doi: 10.1158/0008-5472.CAN-16-1839. [DOI] [PubMed] [Google Scholar]
- 87.Luo C.T., Liao W., Dadi S., Toure A., Li M.O. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529:532–536. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roychoudhuri R., Clever D., Li P., Wakabayashi Y., Quinn K.M., Klebanoff C.A., Ji Y., Sukumar M., Eil R.L., Yu Z., et al. BACH2 regulates CD8(+) T cell differentiation by controlling access of AP-1 factors to enhancers. Nat. Immunol. 2016;17:851–860. doi: 10.1038/ni.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roychoudhuri R., Eil R.L., Clever D., Klebanoff C.A., Sukumar M., Grant F.M., Yu Z., Mehta G., Liu H., Jin P., et al. The transcription factor BACH2 promotes tumor immunosuppression. J. Clin. Invest. 2016;126:599–604. doi: 10.1172/JCI82884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma M.D., Shinde R., McGaha T.L., Huang L., Holmgaard R.B., Wolchok J.D., Mautino M.R., Celis E., Sharpe A.H., Francisco L.M., et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci. Adv. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ten Hacken E., Burger J.A. Microenvironment interactions and B-cell receptor signaling in Chronic Lymphocytic Leukemia: Implications for disease pathogenesis and treatment. Biochim. Biophys. Acta. 2016;1863:401–413. doi: 10.1016/j.bbamcr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu N., Haike K., Glaeske S., Paul J., Mumberg D., Kreft B., Ziegelbauer K. Copanlisib in combination with anti-PD-1 induces regression in animal tumor models insensitive or resistant to the monotherapies of PI3K and checkpoint inhibitors. Hematol. Oncol. 2017;35:257–258. doi: 10.1002/hon.2438_123. [DOI] [Google Scholar]
- 93.Martinelli S., Maffei R., Fiorcari S., Quadrelli C., Zucchini P., Benatti S., Potenza L., Luppi M., Marasca R. Idelalisib impairs T-cell-mediated immunity in chronic lymphocytic leukemia. Haematologica. 2018;103:e598–e601. doi: 10.3324/haematol.2017.187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanna B.S., Roessner P.M., Scheffold A., Jebaraj B.M.C., Demerdash Y., Öztürk S., Lichter P., Stilgenbauer S., Seiffert M. PI3Kδ inhibition modulates regulatory and effector T-cell differentiation and function in chronic lymphocytic leukemia. Leukemia. 2019;33:1427–1438. doi: 10.1038/s41375-018-0318-3. [DOI] [PubMed] [Google Scholar]
- 95.Maharaj K., Powers J.J., Achille A., Mediavilla-Varela M., Gamal W., Burger K.L., Fonseca R., Jiang K., Miskin H.P., Maryanski D., et al. The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 2020;4:3072–3084. doi: 10.1182/bloodadvances.2020001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin N., Stubbs M., Koblish H., Yue E.W., Soloviev M., Douty B., Wang K.H., Wang Q., Gao M., Feldman P., et al. Parsaclisib Is a Next-Generation Phosphoinositide 3-Kinase δ Inhibitor with Reduced Hepatotoxicity and Potent Antitumor and Immunomodulatory Activities in Models of B-Cell Malignancy. J. Pharmacol. Exp. Ther. 2020;374:211–222. doi: 10.1124/jpet.120.265538. [DOI] [PubMed] [Google Scholar]
- 97.Lauder S.N., Smart K., Kersemans V., Allen D., Scott J., Pires A., Milutinovic S., Somerville M., Smart S., Kinchesh P., et al. Enhanced antitumor immunity through sequential targeting of PI3Kdelta and LAG3. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Serrat N., Guerrero-Hernandez M., Matas-Cespedes A., Yahiaoui A., Valero J.G., Nadeu F., Clot G., Di Re M., Corbera-Bellalta M., Magnano L., et al. PI3Kdelta inhibition reshapes follicular lymphoma-immune microenvironment cross talk and unleashes the activity of venetoclax. Blood Adv. 2020;4:4217–4231. doi: 10.1182/bloodadvances.2020001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rolf J., Bell S.E., Kovesdi D., Janas M.L., Soond D.R., Webb L.M.C., Santinelli S., Saunders T., Hebeis B., Killeen N., et al. Phosphoinositide 3-Kinase Activity in T Cells Regulates the Magnitude of the Germinal Center Reaction. J. Immunol. 2010;185:4042–4052. doi: 10.4049/jimmunol.1001730. [DOI] [PubMed] [Google Scholar]
- 100.Lim E.L., Cugliandolo F.M., Rosner D.R., Gyori D., Roychoudhuri R., Okkenhaug K. Phosphoinositide 3-kinase δ inhibition promotes antitumor responses but antagonizes checkpoint inhibitors. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Brien S.M., Lamanna N., Kipps T.J., Flinn I., Zelenetz A.D., Burger J.A., Keating M., Mitra S., Holes L., Yu A.S., et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126:2686–2694. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Curigliano G., Shah R.R. Safety and Tolerability of Phosphatidylinositol-3-Kinase (PI3K) Inhibitors in Oncology. Drug Saf. 2019;42:247–262. doi: 10.1007/s40264-018-0778-4. [DOI] [PubMed] [Google Scholar]
- 103.Hanlon A., Brander D.M. Managing toxicities of phosphatidylinositol-3-kinase (PI3K) inhibitors. Hematol. Am. Soc. Hematol. Educ. Program. 2020;2020:346–356. doi: 10.1182/hematology.2020000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lampson B.L., Kim H.T., Davids M.S., Abramson J.S., Freedman A.S., Jacobson C.A., Armand P.A., Joyce R.M., Arnason J.E., Rassenti L.Z., et al. Efficacy results of a phase 2 trial of first-line idelalisib plus ofatumumab in chronic lymphocytic leukemia. Blood Adv. 2019;3:1167–1174. doi: 10.1182/bloodadvances.2018030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wagner-Johnston N.D., Sharman J., Furman R.R., Salles G., Brown J.R., Robak T., Gu L., Xing G., Chan R.J., Rajakumaraswamy N., et al. Idelalisib immune-related toxicity is associated with improved treatment response. Leuk. Lymphoma. 2021:1–6. doi: 10.1080/10428194.2021.1948038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodgers T.D., Williams A.M., Baran A., Reagan P.M., Casulo C., Zent C.S., Evans A., Friedberg J.W., Barr P.M. Toxicity patterns of novel PI3K combinations in patients with non-Hodgkin lymphoma. Leuk. Lymphoma. 2021;62:598–605. doi: 10.1080/10428194.2020.1837796. [DOI] [PubMed] [Google Scholar]
- 107.Cuneo A., Barosi G., Danesi R., Fagiuoli S., Ghia P., Marzano A., Montillo M., Poletti V., Viale P., Zinzani P.L. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper. Hematol. Oncol. 2019;37:3–14. doi: 10.1002/hon.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morschhauser F., Machiels J.P., Salles G., Rottey S., Rule S.A.J., Cunningham D., Peyrade F., Fruchart C., Arkenau H.T., Genvresse I., et al. On-Target Pharmacodynamic Activity of the PI3K Inhibitor Copanlisib in Paired Biopsies from Patients with Malignant Lymphoma and Advanced Solid Tumors. Mol. Cancer Ther. 2020;19:468–478. doi: 10.1158/1535-7163.MCT-19-0466. [DOI] [PubMed] [Google Scholar]
- 109.Flinn I.W., Patel M., Oki Y., Horwitz S., Foss F.F., Allen K., Douglas M., Stern H., Sweeney J., Kharidia J., et al. Duvelisib, an oral dual PI3K-delta, gamma inhibitor, shows clinical activity in indolent non-Hodgkin lymphoma in a phase 1 study. Am. J. Hematol. 2018;93:1311–1317. doi: 10.1002/ajh.25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flinn I.W., Hillmen P., Montillo M., Nagy Z., Illes A., Etienne G., Delgado J., Kuss B.J., Tam C.S., Gasztonyi Z., et al. The phase 3 DUO trial: Duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132:2446–2455. doi: 10.1182/blood-2018-05-850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Locatelli S.L., Careddu G., Serio S., Consonni F.M., Maeda A., Viswanadha S., Vakkalanka S., Castagna L., Santoro A., Allavena P., et al. Targeting Cancer Cells and Tumor Microenvironment in Preclinical and Clinical Models of Hodgkin Lymphoma Using the Dual PI3Kdelta/gamma Inhibitor RP6530. Clin. Cancer Res. 2019;25:1098–1112. doi: 10.1158/1078-0432.CCR-18-1133. [DOI] [PubMed] [Google Scholar]
- 112.O’Brien S., Patel M., Kahl B.S., Horwitz S.M., Foss F.M., Porcu P., Jones J., Burger J., Jain N., Allen K., et al. Duvelisib, an oral dual PI3K-delta,gamma inhibitor, shows clinical and pharmacodynamic activity in chronic lymphocytic leukemia and small lymphocytic lymphoma in a phase 1 study. Am. J. Hematol. 2018;93:1318–1326. doi: 10.1002/ajh.25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Horwitz S.M., Koch R., Porcu P., Oki Y., Moskowitz A., Perez M., Myskowski P., Officer A., Jaffe J.D., Morrow S.N., et al. Activity of the PI3K-delta,gamma inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood. 2018;131:888–898. doi: 10.1182/blood-2017-08-802470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown J.R., Byrd J.C., Coutre S.E., Benson D.M., Flinn I.W., Wagner-Johnston N.D., Spurgeon S.E., Kahl B.S., Bello C., Webb H.K., et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flinn I.W., O’Brien S., Kahl B., Patel M., Oki Y., Foss F.F., Porcu P., Jones J., Burger J.A., Jain N., et al. Duvelisib, a novel oral dual inhibitor of PI3K-delta,gamma, is clinically active in advanced hematologic malignancies. Blood. 2018;131:877–887. doi: 10.1182/blood-2017-05-786566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei B., Velazquez P., Turovskaya O., Spricher K., Aranda R., Kronenberg M., Birnbaumer L., Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc. Natl. Acad. Sci. USA. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lampson B.L., Kasar S.N., Matos T.R., Morgan E.A., Rassenti L., Davids M.S., Fisher D.C., Freedman A.S., Jacobson C.A., Armand P., et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016;128:195–203. doi: 10.1182/blood-2016-03-707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weidner A.S., Panarelli N.C., Geyer J.T., Bhavsar E.B., Furman R.R., Leonard J.P., Jessurun J., Yantiss R.K. Idelalisib-associated Colitis: Histologic Findings in 14 Patients. Am. J. Surg. Pathol. 2015;39:1661–1667. doi: 10.1097/PAS.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 119.Louie C.Y., DiMaio M.A., Matsukuma K.E., Coutre S.E., Berry G.J., Longacre T.A. Idelalisib-associated Enterocolitis: Clinicopathologic Features and Distinction From Other Enterocolitides. Am. J. Surg. Pathol. 2015;39:1653–1660. doi: 10.1097/PAS.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 120.Foukas L.C., Bilanges B., Bettedi L., Pearce W., Ali K., Sancho S., Withers D.J., Vanhaesebroeck B. Long-term p110α PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol. Med. 2013;5:563–571. doi: 10.1002/emmm.201201953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lanasa M.C., Glenn M., Mato A.R., Allgood S.D., Wong S., Amore B., Means G., Stevens E., Yan C., Friberg G., et al. First-In-Human Study Of AMG 319, a Highly Selective, Small Molecule Inhibitor Of PI3Kδ, In Adult Patients With Relapsed Or Refractory Lymphoid Malignancies. Blood. 2013;122:678. doi: 10.1182/blood.V122.21.678.678. [DOI] [Google Scholar]
- 122.Gopal A.K., Kahl B.S., de Vos S., Wagner-Johnston N.D., Schuster S.J., Jurczak W.J., Flinn I.W., Flowers C.R., Martin P., Viardot A., et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flinn I.W., Kahl B.S., Leonard J.P., Furman R.R., Brown J.R., Byrd J.C., Wagner-Johnston N.D., Coutre S.E., Benson D.M., Peterman S., et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123:3406–3413. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yam C., Xu X., Davies M.A., Gimotty P.A., Morrissette J.J.D., Tetzlaff M.T., Wani K.M., Liu S., Deng W., Buckley M., et al. A Multicenter Phase I Study Evaluating Dual PI3K and BRAF Inhibition with PX-866 and Vemurafenib in Patients with Advanced BRAF V600-Mutant Solid Tumors. Clin. Cancer Res. 2018;24:22–32. doi: 10.1158/1078-0432.CCR-17-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horak F., Puri K.D., Steiner B.H., Holes L., Xing G., Zieglmayer P., Zieglmayer R., Lemell P., Yu A. Randomized phase 1 study of the phosphatidylinositol 3-kinase δ inhibitor idelalisib in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2016;137:1733–1741. doi: 10.1016/j.jaci.2015.12.1313. [DOI] [PubMed] [Google Scholar]
- 126.Phillips T.J., Forero-Torres A., Sher T., Diefenbach C.S., Johnston P., Talpaz M., Pulini J., Zhou L., Scherle P., Chen X., et al. Phase 1 study of the PI3Kδ inhibitor INCB040093 ± JAK1 inhibitor itacitinib in relapsed/refractory B-cell lymphoma. Blood. 2018;132:293–306. doi: 10.1182/blood-2017-10-812701. [DOI] [PMC free article] [PubMed] [Google Scholar]