Abstract
A PCR-based study of the incidence of enteropathogenic campylobacter infection in humans was done on the basis of a detection and identification algorithm consisting of screening PCRs and species identification by PCR-enzyme-linked immunosorbent assay. This was applied to DNA extracted from 3,738 fecal samples from patients with sporadic cases of acute gastroenteritis, submitted by seven regional Public Health Laboratories in England and Wales over a 2-year period. The sending laboratories had cultured “Campylobacter spp.” from 464 samples. The PCR methodologies detected 492 Campylobacter-positive samples, and the combination of culture and PCR yielded 543 Campylobacter-positive samples. There was identity (overlap) for 413 samples, but 79 PCR-positive samples were culture negative, and 51 culture-positive samples were PCR negative. While there was no statistically significant difference between PCR and culture in detection of C. jejuni-C. coli (PCR, 478 samples; culture, 461 samples), PCR provided unique data about mixed infections and non-C. jejuni and non- C. coli campylobacters. Mixed infections with C. jejuni and C. coli were found in 19 samples, and mixed infection with C. jejuni and C. upsaliensis was found in one sample; this was not apparent from culture. Eleven cases of gastroenteritis were attributed to C. upsaliensis by PCR, three cases were attributed to C. hyointestinalis, and one case was attributed to C. lari. This represents the highest incidence of C. hyointestinalis yet reported from human gastroenteritis, while the low incidence of C. lari suggests that it is less important in this context.
Gastroenteritis due to Campylobacter jejuni and C. coli is the principal cause of acute bacterial diarrhea (21). Several other species such as C. upsaliensis, C. hyointestinalis, and C. fetus have also been shown to be enteropathogenic for humans, but their significance remains unclear because the procedure for the isolation of C. jejuni and C. coli uses selective media that may inhibit their growth (1, 9, 15). Furthermore, clinical laboratories usually do not identify campylobacter isolates to the species level, since they have relatively fastidious growth requirements and lack easily distinguishable biochemical characteristics (9). Thus, non-C. jejuni and non-C. coli campylobacters may be underreported in human gastrointestinal illness (2, 15).
The target genes used for PCR identification of Campylobacter species from cultured isolates have included 16S rRNA (7, 17), 23S rRNA (4), flaA (flagellin) (29), GTP-binding protein (27), ceuE (iron transport protein) (8), and hip (hippuricase) (16). Flagellin gene and 16S rRNA gene (rDNA) PCR assays have been applied to foodstuffs (7, 30). The flaA (20), hip (16), and 16S rDNA (14) PCRs have been applied directly to small numbers of fecal samples without culture of an isolate. A recent study of 493 fecal samples (28) was based on enrichment culture and 16S rDNA PCR for detection of C. jejuni, C. coli, and C. lari.
To our knowledge, the present study represents the largest PCR-based survey of Campylobacter gastroenteritis yet undertaken and demonstrates the utility of this approach for investigation of the incidence and epidemiology of the full spectrum of enteropathogenic campylobacters.
MATERIALS AND METHODS
Study design.
Clinical samples were collected over a 2-year period by seven Public Health Laboratories (PHLs) in England and Wales, termed PHLs A, B, C, D, E, F, and G. The samples were from patients with acute gastroenteritis submitted from general practices and outpatient departments or collected by environmental health officers. Repeat, follow-up samples and samples from inpatients were not examined.
DNA was extracted from aliquots of fecal samples sent to the Central Public Health Laboratory not later than 10 days after initial receipt and culture at the collaborating laboratory. Samples were simply cultured on a selective medium, and no attempt was made to quantify the Campylobacter cells present; the lag between specimen culture and DNA extraction precluded direct quantitative comparison. Culture data collected by the collaborating laboratories were withheld until completion of blind PCR assays with DNA extracted from the corresponding fecal samples at the Central Public Health Laboratory. PCR data were then compared with the results of conventional selective culture performed by the contributing laboratories.
Bacterial reference strains.
A large range of type and reference strains (19 Campylobacter, 12 Helicobacter, and 4 Arcobacter strains, and 11 strains of other enteropathogenic species) were used as controls as described previously (16).
Bacteriological investigation of clinical samples.
Fecal samples were examined for Campylobacter spp., Clostridium difficile, Escherichia coli O:157, Salmonella spp., Shigella spp., and ova, cysts, and parasites by standard methods. With the exceptions of laboratories A and F, campylobacters were cultured on Campylobacter Blood Free Selective Agar Base (Oxoid CM739) with Charcoal Cefoperazone Desoxycholate Agar supplement (Oxoid SR155). Laboratory A used cefoperazone amphotericin B teicoplanin supplement (Oxoid SR174), while laboratory F used a cefoperazone and amphotericin B supplement (Prolab, Neston, United Kingdom). All plates were incubated for 48 h at 41 to 42°C (except Laboratory A [37°C]) under microaerobic conditions (5% O2, 5% CO2, 2% H2, and 88% N2, by volume). Isolates were identified to the genus level by morphology and Gram staining. Laboratory A further identified isolates by hippurate hydrolysis, indoxyl acetate hydrolysis, and urease production.
Extraction of nucleic acid from feces.
Approximately 200 mg of each clinical fecal sample was homogenized in 2 ml of brucella broth (Life Technologies Ltd., Paisley, United Kingdom). Nucleic acid was extracted from a 100-μl aliquot of the fecal suspension as described previously by Lawson et al. (13). DNA extracts were stored at −20°C prior to screening by PCR.
PCR screening assays.
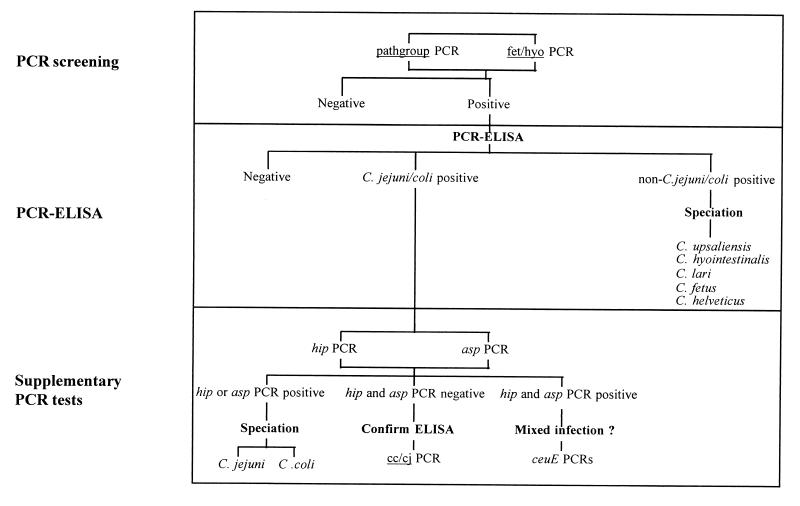
Nucleic acid extracts were screened in batches of 96 samples (including positive and negative controls). Two 16S rDNA PCR assays were used to screen all fecal extracts. The first assay, termed pathgroup, was a newly designed assay specific for the C. jejuni, C. coli, C. lari, C. upsaliensis, and C. helveticus group. The second assay, termed fet/hyo, was a duplex assay specific for C. fetus and C. hyointestinalis (17). The screening step is shown in the algorithm presented in Fig. 1.
FIG. 1.
Algorithm for Campylobacter detection and species identification (speciation) by PCR and PCR-ELISA.
Each 2.5-μl nucleic acid extract obtained from a fecal sample was amplified in a 25-μl reaction volume in a 96-well-format microtiter plate as described previously (14). Amplification conditions were denaturation at 94°C for 1 min, annealing at either 66°C for pathgroup or 65°C for fet/hyo for 1 min, and extension at 72°C for 1 min for 30 cycles in a RoboCycler thermocycler with a hot top assembly (Stratagene, La Jolla, Calif.). The products were analyzed by 96-well-format electrophoresis (on a 1% [wt/vol] agarose gel). The gels were stained with SYBR green I (Flowgen Instruments Ltd., Lichfield, United Kingdom).
Species identification.
Samples positive by the screening PCR assays were identified by PCR-enzyme-linked immunosorbent assay (PCR-ELISA) (18) by using capture probes specific for C. jejuni-C. coli, C. upsaliensis, C. hyointestinalis, C. lari, C. fetus, and C. helveticus. Those samples identified as C. jejuni-C. coli by PCR-ELISA were further examined by supplementary PCR assays specific for the hip gene of C. jejuni and the aspartokinase (asp) gene of C. coli (16). In cases in which these two PCRs proved negative, a more sensitive PCR (cc/cj) for the multicopy 16S rRNA gene (16) was applied to confirm the PCR-ELISA data. In cases of potential mixed culture, two sets of ceuE gene primers (8) capable of distinguishing C. jejuni from C. coli were also used. The procedure used for species identification is summarized in the algorithm (Fig. 1).
Statistical analysis.
The results of Campylobacter detection by the PCR screening assay and PCR-ELISA were compared with those obtained by culture on selective agar at contributing laboratories by McNemar's test (22).
RESULTS
Design and application of primers.
Phylogenetic analysis was conducted with Megalign (Lasergene package; DNASTAR, Inc., Madison, Wis.). Sequences of 16S rDNA from Helicobacter pylori, Bacteroides ureolyticus, and 15 species of Campylobacter were recovered from the GenBank database and were aligned by the Clustal method (23). PCR primer pairs were designed from the alignment with the aid of the program Oligo (version 4.0; National Biosciences, Plymouth, Mass.). A set of pathgroup primers which inclusively detected C. jejuni, C. coli, C. upsaliensis, C. lari, and C. helveticus were designed in this manner. The forward pathgroup primer was 5′-ACA TGC AAG TCG AAC GAT GAA GC-3′ and the reverse pathgroup primer was 5′-TAT AGA TTT GCT CCA CCT CGC GG-3′. These yielded an amplicon of 1,195 bp from DNA prepared from reference strains of the five species mentioned above but not from the remaining type strains of Campylobacter and the other enteropathogenic species listed in Materials and Methods. The pathgroup primers were also tested with a set of 200 fecal samples which included 18 cc/cj-positive samples (14) known to contain C. jejuni or C. coli. With an annealing temperature of 66°C, pathgroup primers detected all 18 previous cc/cj-positive samples. Nonspecific mismatch products could be eliminated by raising the annealing temperature to 68°C, but at the expense of some loss of sensitivity. The lower annealing temperature (66°C) was retained for screening purposes in the full survey. For this and for the fet/hyo assay, any mismatch products were eliminated by the subsequent PCR-ELISA (cf. Fig. 1).
In the full survey of 3,738 samples, the pathgroup PCR was positive for 720 samples, while the fet/hyo duplex PCR was positive for 29 samples.
PCR-ELISA data.
The 749 samples positive by the screening assays described above were subjected to PCR-ELISA according to the algorithm presented in Fig. 1. This identified campylobacters in 492 samples, as follows: C. jejuni-C. coli in 477 samples, C. jejuni-C. coli and C. upsaliensis in 1 sample, C. upsaliensis in 10 samples, C. hyointestinalis in 3 samples, and C. lari in 1 sample. The remaining 257 screening PCR-positive samples were PCR-ELISA negative. In this survey neither C. fetus nor C. helveticus was detected. A breakdown, by sending laboratory, for detection of C. jejuni-C. coli and non-C. jejuni–non-C. coli campylobacters is given in Table 1.
TABLE 1.
Detection of Campylobacter species
| Source | No. of samples examined | No. of samples positive by specific technique/total no. of samples positive by both techniquesa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
C. jejuni-C. coli
|
C. upsaliensis
|
C. hyointestinalis
|
C. lari
|
||||||
| PCR-ELISA | Culture | PCR-ELISA | Culture | PCR-ELISA | Culture | PCR-ELISA | Culture | ||
| Lab A | 1,107 | 106/121 | 108/121 | 3b/3 | 1c/3 | 1/1 | 0/1 | ||
| Lab B | 725 | 63/73 | 65/73 | 1/1 | 0/1 | 1/1 | 1c/1 | ||
| Lab C | 1,178 | 186/203 | 189/203 | 5/5 | 0/5 | 1/1 | 0/1 | ||
| Lab D | 300 | 55/57 | 35/57 | 1/1 | 0/1 | 1/1 | 0/1 | ||
| Lab E | 166 | 20/21 | 12/21 | ||||||
| Lab F | 162 | 31/36 | 35/36 | 1/1 | 1c/1 | ||||
| Lab G | 100 | 17/18 | 17/18 | ||||||
| Total | 3,738 | 478/529 | 461/529 | 11/11 | 2/11 | 3/3 | 1/3 | 1/1 | 0/1 |
For example, in the case of C. jejuni-C. coli, a total of 461 of 3,738 isolates were detected by culture, while 478 of 529 were identified by the PCR algorithm described in Fig. 1 (PCR-ELISA). A total of 529 were found by a combination of methods; i.e., 68 were not detected by culture and 51 were not identified by PCR-ELISA.
Includes one sample with a mixed infection with C. upsaliensis and C. jejuni detected by PCR-ELISA.
For these samples, culture identified the strain only as a “Campylobacter sp.” Subsequent species identifications were by PCR-ELISA.
Identification of C. jejuni and C. coli.
PCR-ELISA detected 478 C. jejuni-C. coli-positive samples. These were subjected to supplementary PCRs, as outlined in Fig. 1 and Materials and Methods. With the hip primers, the campylobacters in 408 samples were positively identified as C. jejuni. With the C. coli-specific asp primers, the campylobacters in 16 samples were positively identified as C. coli. A further 19 samples were positive for both hip and asp, indicative of a mixed infection. These 19 samples were therefore investigated with different sets of ceuE primers, one specific for the C. jejuni sequence and one specific for the C. coli sequence. All 19 were positive by assays with both sets of primers, confirming mixed infections. From the remaining 35 samples positive for C. jejuni-C. coli by PCR-ELISA, no hip or asp amplicon was obtained. We were therefore unable to distinguish the two species in these samples but confirmed the PCR-ELISA result by the equivalent simple cc/cj PCR assay (Fig. 1).
Comparison of PCR-based and culture-based detection.
The combination of results from the PCR screening, PCR-ELISA, and supplementary PCR assays allowed each sample to be assigned an overall molecular identification. This was then compared with the culture data: “Campylobacter spp.” were cultured by the seven contributing laboratories from 464 of the 3,738 samples. Of these, the campylobacters in 413 samples (410 with C. jejuni-C. coli, 2 with C. upsaliensis, and 1 with C. hyointestinalis) had been identified by PCR-ELISA with the corresponding fecal sample. The remaining 51 were culture positive but PCR-ELISA negative (Table 1).
Seventy-nine culture-negative samples were positive by the screening PCR and PCR-ELISA (67 with C. jejuni-C. coli, 1 with a mixture of C. jejuni-C. coli and C. upsaliensis, 8 with C. upsaliensis, 2 with C. hyointestinalis, and 1 with C. lari; cf. Table 1). Thus, the combination of screening PCR and PCR-ELISA detected Campylobacter spp. in 543 of the 3,738 samples (528 with C. jejuni-C. coli, 1 with a mixture of C. jejuni- C. coli and C. upsaliensis, and 14 with other non-C. jejuni-non C. coli campylobacters).
For three of the samples reported by sending laboratories to be “Campylobacter sp. positive,” PCR-ELISA detected C. upsaliensis (two samples) and C. hyointestinalis (one sample). Nine other C. upsaliensis-positive samples and two other C. hyointestinalis-positive samples were detected by PCR-ELISA but not by culture. One of those C. upsaliensis strains was detected by PCR-ELISA in a sample with a mixed infection with C. jejuni. The latter was detected by culture as well as by PCR-ELISA. A single C. lari infection was identified by PCR-ELISA but not by culture (cf. Table 1).
There was no statistical difference between the number of C. jejuni-C. coli-positive samples detected by PCR-ELISA or by culture in the study as a whole (0.5 > P > 0.1). In terms of individual sending laboratories, there was no statistical difference between the culture and PCR-ELISA for laboratories A, B, C, (P > 0.5), F (0.5 > P > 0.1), and G (P = 0.5). However, the detection rate was significantly higher by PCR-ELISA than by culture for laboratories D (P < 0.001) and E (0.02 > P > 0.01).
DISCUSSION
To our knowledge this study is the largest molecular survey of Campylobacter gastroenteritis yet undertaken and represents the first application of a PCR-based protocol to investigate the incidence of the enteropathogenic Campylobacter spp. in an epidemiologically valid context.
A key development in this study was the design of PCR assays specific for groups of enteropathogenic species rather than the use of a series of individual species-specific PCRs as we have described previously (14, 16). This was intended to reduce the number of PCR assays performed in the course of a large-scale survey. The basis for the screening PCRs described in Fig. 1 was phylogenetic trees drawn from alignments of Campylobacter 16S rDNA sequences (12, 24, 26). These trees all contained three distinct clades (species groups). The first contained C. gracilis, C. sputorum, C. curvus, C. concisus, C. rectus, and C. showae, organisms principally associated with niches in the periodontal cavities of humans and animals and which have as yet no association with human gastroenteritis. The second clade consisted of C. fetus, C. hyointestinalis, and C. mucosalis, which are historically associated with disease in farm animals, although the first two have also been occasionally implicated in human disease. The third clade consisted of C. jejuni, C. coli, C. lari, C. upsaliensis, and C. helveticus, species (other than C. helveticus) known to cause gastroenteritis in humans. In accordance with the requirements of this study, we developed primers for the third clade and used existing primers specific for C. fetus and C. hyointestinalis (17), the only other causative agents of human gastroenteritis that have been described.
In the course of the study several key practical issues of importance to any large-scale PCR-based survey were identified. These included the necessity of a dedicated PCR suite to reduce the risk of contamination, the robustness of the thermocyclers, and the regular monitoring of the performance and detection threshold for PCR primers (their titers and qualities could deteriorate over time or could vary between batches and manufacturers).
C. jejuni and C. coli, as expected, represented the largest proportion of PCR-positive samples. There was congruence between PCR and culture for 77.5% of the 529 positive samples, while 12.9% were found to be positive only by PCR and 9.6% were found to be positive only by culture. In any comparison of two detection methods with a sizeable sample number, one would not expect a complete correlation. Nonetheless, we note that in this investigation there are further factors to consider. Culture positive-only samples may have been PCR negative due to degradation of Campylobacter cells and DNA in the period (up to 10 days) between culture and receipt of the fecal sample for DNA extraction. In some cases inhibitory substances present in feces may have reduced the sensitivities of the PCR assays. There is also a possibility that certain wild-type C. jejuni or C. coli strains might have 16S rDNA sequences which are sufficiently divergent that they are not detected by PCR-ELISA, despite its detection of all Penner serotype reference strains (18). Isolates from a proportion of the specimens (laboratory C) which were negative by PCR were all successfully identified as C. jejuni with the PCR algorithm (Fig. 1). This suggests that culture-positive, PCR-negative samples occurred due to sampling factors rather than variations in target DNA sequences.
Culture has been found to be more sensitive than PCR in seeding experiments with logarithmic-phase cultures of laboratory strains of C. jejuni (14). That finding may be due to the amount of fecal material which is sampled when inoculating a selective agar plate, as opposed to the small volume of diluted material (2.5 μl) sampled by PCR.
Nonetheless, our study found more positive samples by PCR-ELISA than by culture alone. This probably reflects the detection of Campylobacter cells in metabolic states that are less amenable to culture on selective media (sublethally damaged cells, viable but nonculturable cells, or even dead cells). A key feature of the PCR algorithm was that it provided both detection and identification.
The 11 C. upsaliensis isolates detected by PCR-ELISA represent an incidence of 0.29%. Only two of these samples had been positive by culture, and for each sample the isolate was reported as a “Campylobacter sp.” The sex/age (in years) distributions for the patients who provided C. upsaliensis-positive samples (m/2, m/5, m/10, f/23, m/25, f/27, f/33, m7/8, m/u, m/u, and u/u, where m is male, f is female, and u is unspecified) showed no evidence of an association with pediatric gastroenteritis, as has been previously reported in the literature (2, 10).
The three cases of C. hyointestinalis infection represent an incidence of 0.08%. Only one of these samples was reported as “Campylobacter sp.” positive by culture. The sex/age distributions were m/39, f/66, and f/u. This represents the highest incidence of C. hyointestinalis yet reported as a cause of human gastroenteritis. The only other incidence figure available, based on culture, was 0.01% (2 of 15,185 cases) (11). Earlier pilot PCR studies by our group had detected C. hyointestinalis in 1 of 25 (16) and 1 of 200 (14) patients with gastroenteritis. Altogether, this would give an incidence of 0.13% (5 of 3,963). Although C. hyointestinalis is associated with proliferative enteritis in pigs (6), it has previously been considered to be only a very rare cause of gastroenteritis in humans (3, 5, 19). Our results suggest that further investigation of C. hyointestinalis as a human enteropathogen would be appropriate.
We detected C. lari (by PCR-ELISA alone) in only one sample (an incidence of 0.03%), although this species is often cited (15) as the third most commonly isolated enteropathogenic campylobacter from humans. The low incidence in our survey suggests that C. lari is less important than C. upsaliensis and C. hyointestinalis in human gastroenteritis.
In 19 samples there was evidence from supplementary PCR assays of a mixed infection with C. jejuni and C. coli not apparent from culture. Seven of these occurred in a laboratory (laboratory A) which had the capacity to identify some isolates to the species level and which reported that five were C. jejuni and two were C. coli. It is likely, therefore, that only the predominant colony type had been selected for identification. It is interesting that there were slightly more mixed C. jejuni and C. coli infections than C. coli infections alone. Mixtures of Campylobacter species (and serotypes) in human infections may be more common than was heretofore assumed. Another mixed Campylobacter infection noted in the survey was of C. upsaliensis and C. jejuni, which were codetected by PCR-ELISA; here, only the C. jejuni component was apparent from culture. There were also instances of coinfections of C. jejuni-C. coli with other enteropathogens detected by culture and/or microscopy at the contributing laboratories. There were mixtures of C. jejuni with both Shigella sonnei and Blastocystis hominis cysts (one sample), with a Salmonella sp. (two samples), and with Cryptosporidium parvum oocysts (three samples). The mixtures of Cryptosporidium and C. jejuni-C. coli are of interest since the presence of a coccidian, which is most frequently associated with waterborne infections (25), suggests that the route of transmission of the C. jejuni may have been water for these samples.
Although PCR is more expensive and labor-intensive than culture, it offers a nonselective way to monitor the incidence of enteropathogenic bacteria. We have shown that it is of value for epidemiological purposes, and it will ultimately be amenable to automation. The use of a broad-specificity screening PCR greatly reduces the number of assays required for a comprehensive survey. We have also demonstrated that our previously published PCR-ELISA (18) can be successfully applied in this context. In summary, the PCR algorithm presented here offers a different perspective on Campylobacter gastroenteritis than that provided by culture, giving information on the identity and occurrence of species that are not detected by culture. PCR-based analysis has a role in large-scale epidemiological surveys of Campylobacter.
ACKNOWLEDGMENTS
We are most grateful to the staff of Ashford PHL, Bangor PHL, Central Middlesex PHL, Chelmsford PHL, Dorchester PHL, Exeter PHL, and Preston PHL for the clinical samples and culture data.
This work was supported by a grant (grant DH220B) from the Department of Health, London, United Kingdom.
REFERENCES
- 1.Advisory Committee on the Microbiological Safety of Food, Department of Health. Interim report on campylobacter. London, United Kingdom: Her Majesty's Stationery Office; 1993. [Google Scholar]
- 2.Bourke B, Chan V L, Sherman P. Campylobacter upsaliensis: waiting in the wings. Clin Microbiol Rev. 1998;11:440–449. doi: 10.1128/cmr.11.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmonds P, Patton C M, Griffin P M, Barrett T J, Schmid G P, Baker C N, Lambert M A, Brenner D J. Campylobacter hyointestinalis associated with human gastrointestinal disease in the United States. J Clin Microbiol. 1987;25:685–691. doi: 10.1128/jcm.25.4.685-691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyers M, Chapelle S, Van Camp G, Goossens H, De Wachter R. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J Clin Microbiol. 1993;31:3340–3343. doi: 10.1128/jcm.31.12.3340-3343.1993. . (Erratum, 32:1623, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fennell C L, Rompalo A M, Totten P A, Bruch K L, Flores B M, Stamm W E. Isolation of “Campylobacter hyointestinalis” from a human. J Clin Microbiol. 1986;24:146–148. doi: 10.1128/jcm.24.1.146-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebhart C J, Edmonds P, Ward G E, Kurtz H J, Brenner D J. “Campylobacter hyointestinalis” sp. nov.: a new species of campylobacter found in the intestines of pigs and other animals. J Clin Microbiol. 1985;21:715–720. doi: 10.1128/jcm.21.5.715-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giesendorf B A, Quint W G. Detection and identification of Campylobacter spp. using the polymerase chain reaction. Cell Mol Biol. 1995;41:625–638. [PubMed] [Google Scholar]
- 8.Gonzalez I, Grant K A, Richardson P T, Park S F, Collins M D. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J Clin Microbiol. 1997;35:759–763. doi: 10.1128/jcm.35.3.759-763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens H, Butzler J P. Isolation and identification of Campylobacter spp. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 93–109. [Google Scholar]
- 10.Goossens H, Pot B, Vlaes L, Van den Borre C, Van den Abbeele R, Van Naelten C, Levy J, Cogniau H, Marbehant P, Verhoef J, Kersters K, Butzler J P, Vandamme P. Characterization and description of “Campylobacter upsaliensis” isolated from human feces. J Clin Microbiol. 1990;28:1039–1046. doi: 10.1128/jcm.28.5.1039-1046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goossens H, Vlaes L, De Boeck M, Pot B, Kersters K, Levy J, De Mol P, Butzler J P, Vandamme P. Is 'Campylobacter upsaliensis' an unrecognised cause of human diarrhoea? Lancet. 1990;335:584–586. doi: 10.1016/0140-6736(90)90359-d. [DOI] [PubMed] [Google Scholar]
- 12.Lawson A J, Linton D, Stanley J. 16S rRNA gene sequences of ‘Candidatus Campylobacter hominis’, a novel uncultivated species, are found in the gastrointestinal tract of healthy humans. Microbiology. 1998;144:2063–2071. doi: 10.1099/00221287-144-8-2063. [DOI] [PubMed] [Google Scholar]
- 13.Lawson A J, Linton D, Stanley J, Owen R J. Polymerase chain reaction detection and speciation of Campylobacter upsaliensis and C. helveticus in human faeces and comparison with culture techniques. J Appl Microbiol. 1997;83:375–380. doi: 10.1046/j.1365-2672.1997.00240.x. [DOI] [PubMed] [Google Scholar]
- 14.Lawson A J, Shafi M S, Pathak K, Stanley J. Detection of Campylobacter in gastroenteritis: comparison of direct PCR assay of faecal samples with selective culture. Epidemiol Infect. 1998;121:547–553. doi: 10.1017/s0950268898001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linton D. Old and new Campylobacters: a review. PHLS Microbiol Digest. 1996;13:10–15. [Google Scholar]
- 16.Linton D, Lawson A J, Owen R J, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J Clin Microbiol. 1997;35:2568–2572. doi: 10.1128/jcm.35.10.2568-2572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linton D, Owen R J, Stanley J. Rapid identification by PCR of the genus Campylobacter and of five Campylobacter species enteropathogenic for man and animals. Res Microbiol. 1996;147:707–718. doi: 10.1016/s0923-2508(97)85118-2. [DOI] [PubMed] [Google Scholar]
- 18.Metherell L A, Logan J M, Stanley J. PCR–enzyme-linked immunosorbent assay for detection and identification of Campylobacter species: application to isolates and stool samples. J Clin Microbiol. 1999;37:433–435. doi: 10.1128/jcm.37.2.433-435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minet J, Grosbois B, Megraud F. Campylobacter hyointestinalis: an opportunistic enteropathogen? J Clin Microbiol. 1988;26:2659–2660. doi: 10.1128/jcm.26.12.2659-2660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyofo B A, Thornton S A, Burr D H, Trust T J, Pavlovskis O R, Guerry P. Specific detection of Campylobacter jejuni and Campylobacter coli by using polymerase chain reaction. J Clin Microbiol. 1992;30:2613–2619. doi: 10.1128/jcm.30.10.2613-2619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skirrow M B. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113–149. doi: 10.1016/s0021-9975(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 22.Swinscow S D V. Statistics at square one. 9th ed. London, United Kingdom: BMJ Publishing Group; 1996. [Google Scholar]
- 23.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson L M I, Smibert R M, Johnson J L, Krieg N R. Phylogenetic study of the genus Campylobacter. Int J Syst Bacteriol. 1988;38:190–200. [Google Scholar]
- 25.Tzipori S, Griffiths J K. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. doi: 10.1016/s0065-308x(08)60116-5. [DOI] [PubMed] [Google Scholar]
- 26.van Camp G, van de Peer Y, Nicolai S, Neefs J M, Vandamme P, de Wachter R. Structure of 16S and 23S ribosomal RNA genes in Campylobacter species: phylogenetic analysis of the genus Campylobacter and presence of internal transcribed spacers. Syst Appl Microbiol. 1993;16:361–368. [Google Scholar]
- 27.van Doorn L J, Giesendorf B A, Bax R, van der Zeijst B A, Vandamme P, Quint W G. Molecular discrimination between Campylobacter jejuni, Campylobacter coli, Campylobacter lari and Campylobacter upsaliensis by polymerase chain reaction based on a novel putative GTPase gene. Mol Cell Probes. 1997;11:177–185. doi: 10.1006/mcpr.1997.0100. [DOI] [PubMed] [Google Scholar]
- 28.Vanniasinkam T, Lanser J A, Barton M D. PCR for the detection of Campylobacter spp. in clinical specimens. Lett Appl Microbiol. 1999;28:52–56. doi: 10.1046/j.1365-2672.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 29.Waegel A, Nachamkin I. Detection and molecular typing of Campylobacter jejuni in fecal samples by polymerase chain reaction. Mol Cell Probes. 1996;10:75–80. doi: 10.1006/mcpr.1996.0011. [DOI] [PubMed] [Google Scholar]
- 30.Wegmuller B, Luthy J, Candrian U. Direct polymerase chain reaction detection of Campylobacter jejuni and Campylobacter coli in raw milk and dairy products. Appl Environ Microbiol. 1993;59:2161–2165. doi: 10.1128/aem.59.7.2161-2165.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]