Abstract
Introduction: Sleep disorders, especially insomnia, are very common in different kinds of cancers, but their prevalence and incidence are not well-known. Disturbed sleep in cancer is caused by different reasons and usually appears as a comorbid disorder to different somatic and psychiatric diagnoses, psychological disturbances and treatment methods. There can be many different predictors for sleep disturbances in these vulnerable groups, such as pre-existing sleep disorders, caused by the mental status in cancer or as side effect of the cancer treatment. Methods: A systematic literature review of 8073 studies was conducted on the topic of sleep and sleep disorders in cancer patients. The articles were identified though PubMed, PsycInfo and Web of Knowledge, and a total number of 89 publications were qualified for analysis. Results: The identified eighty-nine studies were analyzed on the topic of sleep and sleep disorders in cancer, twenty-six studies on sleep and fatigue in cancer and sixty-one studies on the topic of sleep disorders in cancer. The prevalence of sleep disturbences and/or sleep disorders in cancer was up to 95%. Discussion: Sleep disturbances and sleep disorders (such as insomnia, OSAS, narcolepsy and RLS; REM-SBD) in cancer patients can be associated with different conditions. Side effects of cancer treatment and cancer-related psychological dysfunctions can be instigated by sleep disturbances and sleep disorders in these patients, especially insomnia and OSAS are common. An evidence-based treatment is necessary for concomitant mental and/or physical states.
Keywords: sleep, sleep disorders, sleep disturbances, insomnia, sleep-related breathing disorder (SRBD)/obstructive sleep apnea syndrome (OSAS), narcolepsy and restless legs syndrome (RLS), REM-sleep behavior disorder (REM-SBD), cancer, fatigue
1. Introduction
Sleep disturbances and different sleep disorders (e.g., insomnia and sleep-related breathing disorder (SRBD)/obstructive sleep apnea syndrome (OSAS)) are common and considerable complaints of cancer patients. Narcolepsy, restless legs syndrome (RLS) and REM-sleep behavior disorder (REM-SBD) are rarely found. Up to 95% of cancer patients complain of sleep disturbances/disorders during diagnosis, treatment and after 10 years of survivorship. Sleep disturbances/disorders and excessive daytime sleepiness (EDS) have been reported to influence fatigue [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] and its perceptions. Savard et al. studied cancer survivors and showed that 52% of them reported sleeping difficulties, and 58% reported that cancer either caused or aggravated their sleeping problems [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88], especially [58].
Disturbed sleep appears before, while and after cancer diseases. The personalized treatment of the most frequent sleep disorders, e.g., insomnia or sleep-related breathing disorder, could improve both their mental and physical health, specifically for diseases such as cancer. The analyses for this review were very challenging, specifically with regards to systematizing the complex and nonhomogeneous literature about sleep, sleep disturbances and different sleep disorders, their prevalence and the severity of sleep complaints in cancer patients, especially because the cancer population is very heterogenous.
The aim of this systematic review was to evaluate critically the prevalence, severity and efficacy of treatments in cancer-related sleep disorders (CRSD).
2. State-of-the-Art
2.1. Sleep Disturbances in the Case of Cancer-Related Fatigue (CrF)
In spite of severe cancer-related fatigue (CrF) [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] and its perceptions [43,54,58,63,64,67] in cancer patients, there is often also a high prevalence of sleep disturbances (30–50%) in which the proportion of poor sleep or bad sleep quality is significantly higher than in the general population [6,21,23,58,64] (Table 2). Due to frequent “naps” during the day caused by CrF, an additional increase in nocturnal problems can observed [1].
For the research of sleep and quality of sleep, the easy-to-use actigraphy is commonly used [89,90]. Actigraphy data from various studies have shown that there is a strong correlation between the changes in subjectively experienced CrF and sleep quality [2,10,16]. Therefore, CrF-induced sleep disorders can be used as a well-quantifiable CrF-induced event to diagnose and control the course of CrF. Table 1 shows the four sleep-specific phenotypes according to which patients with chronic fatigue syndrome can be classified by means of the more elaborate, but more informative, polysomnography [11].
Table 1.
Sleep-specific phenotypes of fatigue (according to Reference [11]).
| First Phenotype |
|
| Second Phenotype |
|
| Third Phenotype |
|
| Fourth Phenotype |
|
2.2. Insomnia in Cancer
Insomnia is a very common and frequent comorbidity in cancer patients. The cancer-related insomnia rate is nearly three times higher than that in the general population. Different analyses have shown that 30–50% (up to 95%) of cancer patients have severe sleep difficulties, such as insomnia symptoms or insomnia syndromes (Tables 3–5). Cancer-related insomnia is characterized by a delayed sleep onset, sleep maintenance disorders, reduced total sleep time and/or early-morning awakenings and is associated with excessive daytime sleepiness, fatigue, impaired performance and daytime wellbeing. Furthermore, we established a connection between insomnia and pain, depression, anxiety and/or a reduced quality of life [27,43,53,54,58,63,64,65]. Various types of treatments for insomnia include pharmacological therapies (e.g., hypnotica, sedativa, antidrepressiva, neuroleptics, antihistamine, hormones (melatonin) and herbal extracts) [28,30,42,44,48,57] and nonpharmacological therapies (like Psychoeducational intervention, Cognitive Behavior Therapy (CBT), Professionally administered CBT (PCBT), Video-based CBT (VCBT), Behavioral Therapy (BT), Individualized Sleep Promotion Plan (ISPP), Mindfulness-Based Stress Reduction (MBSR), Valencia model of Waking hypnosis, Internet intervention/Sleep Healthy Using The internet (SHUTi), Progressive Muscle Relaxation (PMR), Autogenic Training (AT), (Electro)Acupuncture (EA), Tai Chi Chih (TCC), Cool Pad Pillow Topper (CPPT), Combined multimodal-aerobic Treatment (CT), Multimodal Treatment (MT) and Aerobic Treatment (AeT)) [29,31,32,33,34,35,36,37,38,39,40,41,44,46,47,49,50,51,52,55,56,57,59,61,62,66,67,68,69,70,71]. Most of the patients with comorbid cancer-related insomnia (that means around 25–50%) are treated pharmacologically [31]. Especially, cancer patients have many side effects and sevaral physical problems from this kind of treatment, so there are numerous limitations that emerge from these pharmacological treatments. Such side effects generally include headaches, dizziness, fatigue, excessive daytime sleepiness and residual daytime sedation and could be potentiated in cancer patients [31]. There is a need and use of complementary and alternative medical methods in cancer patients with cancer-related insomnia. Recent research has shown that complementary and alternative treatments may provide a clinically relevant benefit in cancer-related insomnia [29,31,32,33,34,35,36,37,38,39,40,41,44,46,47,49,50,51,52,55,56,57,59,61,62,66,67,68,69,70,71].
2.3. Sleep-Related Breathing Disorder (SRBD)/Obstructive Sleep Apnea Syndrome (OSAS) in Cancer
Sleep-related breathing disorders (SRBD), especially obstructive sleep apnea syndrome, (OSAS) are common disorders that are characterised by repetitive interruptions of ventilation during sleep. They are caused by recurrent (upper) airway collapses and follwed by sleep fragmentation, intermitted hypoxia and oxidative stress. Systemic and vascular inflammations with endothelial dysfunctions cause diverse multiorgan chronic morbidities and mortalities that affect the cerebrovascular, cardiovascular and metabolic systems in the progress to cancer. Sleep-related breathing disorders are an independent risk factor for cerebrovascular diseases, cardiovascular diseases, metabolic diseases and cognitive decline and are associated with high rates of morbidity and mortality [72,73,74,75,76,77,78,79,80,81,82].
Chronic and intermittent hypoxias seem to play a key role in the regulation of various stages of tumor formation and their progressions. In recent years, some important studies have shown that OSAS patients tend to have a higher prevalence and incidence of cancer and even a higher prevalence of cancer-related mortality [72,73,74,75,76,77,78,79,80,81,82]. One article was able to show that early CPAP treatment can reduce these prevalences: In vitro studies have shown that, in OSAS, there are pro-oncogenic hypoxia properties that are mediate mainly by enhanced posttranslational HIF effects. Intermittant hypoxia results in the increased expression of vascular endothelial growth factor (VEGF) and in tumor growth and metastasis. An effective OSAS treatment coud prevent cancer, its growth and/or metastasis [74] (Tables 3 and 6).
2.4. Narcolepsy in Cancer
The cancer risk as a comorbidity profile of narcoleptic patients has been rarely analyzed [83,84,85] (Tables 3 and 7). There exist only two case studies, and one evaluated the Taiwan nationwide database. Tseng et al. researched the risk of cancer (incidence) among adult narcoleptics [85]. They found that adult narcoleptic patients have a higher risk for developing cancer, but the study was not able to describe the underlying mechanisms for this [83,84,85]. Further research is needed to understand the association between narcolepsy and the development of cancer.
2.5. Restless Legs Syndrome (RLS) in Cancer
Decreased sleep quality, sleep disturbences and/or sleep disruption are very common in cancer patients, especially when they receive chemotherapy [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] (Tables 3 and 7). Until now the processes and their pathophysiology have not been completely understood, but most likely, they are multifactorial [86]. Additionally, disturbed sleep and sleep disorders like insomnia and OSAS as disorders and/or diseases with pain, fatigue and mood disturbances often occur in clusters. These clusters can negatively impact the quality of life and the outcome of diseases [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Sleep disturbance, fatigue and mood disorders (like depression and anxiety) can be based on distinct biologic processes. These processes could be the trigger for inflammatory signaling as a contributing factor of restless legs syndrome (RLS) [86].
The prevalence and/or incidence of restless legs syndrome in cancer is insufficiently researched. A recent study of Saini et al. showed that RLS is frequent in patients with cancer during chemotherapy. They demonstrated that the prevalence is approximately double compared to the normal population (around 18%). In most cases, restless legs syndrome was correlated with depression, anxiety and a decreased quality of life [86].
2.6. REM Sleep Behavior Disorder in Cancer
Rapid Eye Movement Sleep Behavior Disorders (REM-SBD) and cancer are very seldom reported [83,87,88] (Tables 3 and 9). REM-SBD are forms of parasomnias. They are characterised by severe dream-related behavior and increased abnormal electromyographic activity during REM sleep. Sometimes, they are associated with nightmares and parvor nocturnus [83,87,88]. The excessive electromyographic activity during REM sleep reflects the dysfunction of the brainstem structures in REM-SBD patients [87]; acutely, they can be caused by different medications, such as antidepressants or anticholinergic drugs [88].
3. Method
3.1. Data Sources
This review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting process where applicable [91].
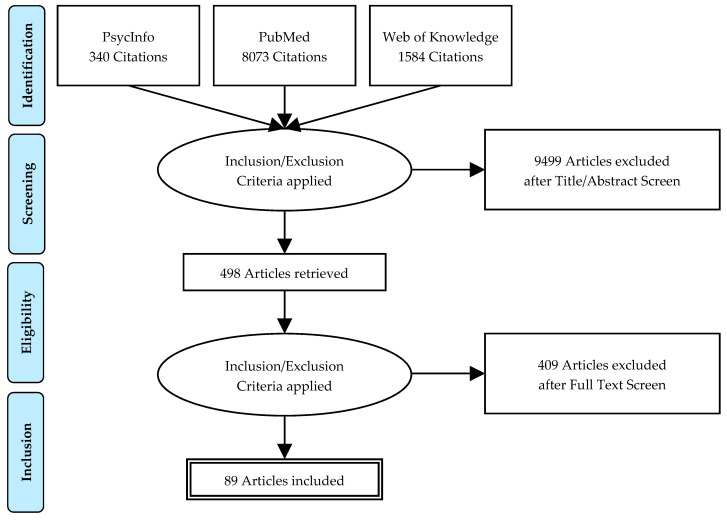
A systematic literature search was carried out on January 2019 of the databases PubMed, PsycInfo and Web of Knowledge (Figure 1).
Figure 1.
PRISMA flowchart of a systematic review of sleep andand fatigue in cancer.
The search terms included the following keywords and keyword combinations (sleep OR sleep quality OR sleep disorders OR insomnia OR sleep-related breathing disorder OR obstructive sleep apnea syndrome OR narcolepsy OR restless legs syndrome) OR REM sleep behavior disorder (REM-SBD) AND (cancer) (AND (fatigue)) in English. The keywords were combined as pairs, e.g., sleep disorders AND cancer.
In addition, the reference lists of all of the obtained studies were evaluated. Hard copies of all of the articles were obtained, and they were fully read.
For the analyses of sleep disorders in cancer, only studies from the period 1999/2000–2018 were included in the review, with three exceptions: two studies about sleep and cancer-related fatigue (CrF) in cancer from 1983 to 1993 and a study about OSAS and cancer from 1988. For the analysis of sleep and fatigue in cancer, we even included some older ones.
The 8073 publications were found in the three databases—498 articles were read, and a total number of 89 publications were included in the final analysis.
3.2. Types of Studies
Randomized controlled trials (RCTs) and quasi-randomized controlled trials (qRCTs), prospective and retrospective studies, cross-sectional surveys, uncontrolled studies and controlled trials without randomization methods, a special article and case studies were included in this systematic review, because important literature was very rare and inconsistent. We only excluded any forms of qualitative studies.
3.3. Types of Participants
Participants who were diagnosed with a sleep disorder (insomnia, sleep-related breathing disorder (SRBD)/obstructive sleep apnea syndrome (OSAS), narcolepsy, restless legs syndrome (RLS) and REM-sleep behavior disorder (REM-SBD)) due to cancer (regardless of gender and age) were included.
3.4. Types of Intervention
The review included studies that evaluated different types of insomnia interventions: nonpharmacological interventions—Psychoeducational intervention, Cognitive Behavior Therapy (CBT), Professionally administered CBT (PCBT), Video-based CBT (VCBT), Behavioral Therapy (BT), Individualized Sleep Promotion Plan (ISPP)), Mindfulness-Based Stress Reduction (MBSR), Valencia model of Waking hypnosis, Internet intervention/Sleep Healthy Using The internet (SHUTi), Progressive Muscle Relaxation (PMR), Autogenic Training (AT), (Electro)Acupuncture (EA), Tai Chi Chih (TCC), Cool Pad Pillow Topper (CPPT), Combined multimodal-aerobic Treatment (CT), Multimodal Treatment (MT) and Aerobic Treatment (AeT) and pharmacological interventions, for example, —melatonin (hormone), mirtazapine (hypnoticum); herbal extracts—valerian.
3.5. Types of Outcomes
3.5.1. Primary Outcomes
-
1.
The prevalence and/or the incidence of sleep disturbences and/or sleep disorders in cancer were evaluated firstly by objective measurements—polysomnography (PSG)/gold standard and polygraphy (PG) for OSAS or actigraphy. The important sleep parameters included total sleep time (TST), time in bed (TIB), sleep efficiency (SE), sleep quality (SQ), sleep onset latency (SOL), wake after sleep onset or total waking time (WASO).
-
2.
The prevalence and/or the incidence of sleep disturbences and/or sleep disorders in cancer are measured secondly by subjective measurements—by scales or indices for the sleep quality (e.g., the Pittsburgh Sleep Quality Index (PSQI)) or special sleep disorders: insomnia (e.g., Insomnia Severity Index (ISI), Athens Insomnia Scale (ASI)), OSAS (e.g., Berlin questionnaire), Narcolepsy (e.g., Narcolepsy Symptom Questionnaire (NSQ)) or RLS (International Restless Legs Syndrome Study Group rating scale (IRLS)).
3.5.2. Secondary Outcomes
The effectiveness of insomnia treatments are measured with sleep diaries. Generally, they include various subjective approaches or several items for reflecting the subjective assessment of daily night’s sleep, including the total sleep time (TST), time in bed (TIB), sleep efficiency (SE), sleep quality (SQ), satisfaction of sleep onset latency (SOL), wake after sleep onset, total waking time (WASO), number of awakenings and morning woken-up time.
3.6. Selection of Studies and Data Extraction
The databases PubMed, PsycInfo and Web of Knowledge were searched and potentially studies screened: After the initial screening with checking the titles and abstracts, all the full-text articles were read. The articles that were included in the review were identified, and the data, according to predefined criteria, were extracted. Information such as samples (e.g., kind and number of participants), interventions (in the case of insomnia), measuring instruments, measuring times, methods, outcomes and results were obtained and documented from each study.
4. Results
Twenty-six studies for the topic of sleep and fatigue in cancer and sixty-one studies for the topic of sleep disorders in cancer were analyzed, one for sleep disorders generally, forty-four studies for the topic “Insomnia in Cancer” (eight for the “Prevalence of Insomnia in Cancer” and thrirty-six for the “Treatment of Insomnia in Cancer”), twelve studies for the topic “Sleep-Related Breathing Disorder (SRBD)/Obstructive Sleep Apnea Syndrome (OSAS) in Cancer”, three studies for the topic “Narcolepsy in Cancer”and one study for the topic “Restless Legs Syndrome (RLS) in Cancer” (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9).
Table 2.
Studies on Sleep and Cancer-related Fatigue (CrF) in cancer/Connection between sleep and fatigue in oncological diseases.
| Author | Sample | Fatigue-Measurement | Sleep-/Rhythm-Measurement | Results |
|---|---|---|---|---|
| Ancoli-Israel et al., Eur J Cancer Care 2001; 10 (4): 245–255 [1] | Metaanalysis of existing research: Sleep & Fatigue | |||
| Ancoli-Israel et al., Support
Care Cancer 2006; 14 (3): 201–209 [2] |
85 Mamma CA/stages I–IIIA | Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
Actigraphy; Sleep diary PSQI; FOSQ |
|
| Ancoli-Israel et al., Support Care Cancer 2014; 22: 2535–2545 [3] |
68 Mamma CA/stages I–III 60 Controls Data collection at three times: (1) baseline/before chemo (2) end of cycle 4 (3) 1 year post-chemo |
Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
Actigraphy; PSQI |
|
| Banthia et al., Psychol
Health 2009; 24 (8): 965–980 [4] |
70 Mamma CA/stages I-IV | 5 dimensions CrF (general, physical, mental, emotional fatigue + vigor) Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
PSQI; CES-D |
|
| Berger & Farr, Oncol Nurs Forum 1999; 26 (10): 1663–1671 [5] | 72 Mamma CA/stages I-II | Piper Fatigue Scale | Actigraphy |
|
| Chang et al., Cancer 2000; 88 (5): 1175–1183 [6] |
240 CA | Memorial Symptom Assessment Scale (MSAS)/Item “Lack of Energy” |
Functional Assessment Cancer Therapy (FACT-G)/Skala GF/Item Sleep | ↑ Fatigue leads to ↑ sleep problems ↑ pain |
| Cimprich, Cancer Nurs
1999; 22 (3): 185–194 [7] |
74 Mamma CA | Symptom Distress Scale; POMS |
no |
|
| Clevenger et al., Brain
Behav Immun 2012; 26 (7): 1037–1044 [8] |
136 Ovarian CA – Interleukin-6 (IL-6) |
POMS-SF; Multidimen. Fatigue Symptom Inventory (MFSI); Fatigue Symptom Inventory (FSI) |
PSQI; Sleep diary |
before surgery higher IL-6 → significant relationship (↑ Sleep disorders & ↑ Fatigue) after surgery lower IL-6 → significant relationship (↓ Sleep disorders & ↓ Fatigue) |
| Engstrom et al., Cancer Nurs 1999; 22(2): 143–148 [9] |
150 CA – Phase I 42 CA – Phase II | no | Telefon Interview | Phase I: Report—44% poorly sleep Phase II: Report—45% sleep problems (1/2 severe; main problems: nightly awake, ↓ TST, difficulty to fall asleep) |
| Fiorentino et al., Drug
Discov Today Dis Models 2011; 8 (4): 167–173 [10] |
40 Mamma CA/stages I–III | Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
Actigraphy | later sleep time & later morning awakening (rhythm shift) leads to ↑ Fatigue |
| Illi et al., Cytokine 2012; 58 (3): 437–447 [12] |
168 CA Patients 85 Caring relatives – Interleukin-4 (IL-4) |
Lee Fatigue Scale | General Sleep Disturbance Scale (GSDS) | phenotype for disease behaviour → role of IL-4 in symptom clusters → 3 classes |
| Kaye et al., 1983; 114: 107–113 [13] | 30 CA28 Cardiological Patients24 Controls | no | Sleep Behaviour Questionnaire |
|
| Liu et al., Psychooncology 2009; 18 (2): 187–194 [14] | 76 Mamma CA /stages I–III | Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
PSQI | significant correlation between Fatigue & Sleep parameters |
| Liu et al., Sleep 2012a; 35 (2): 237–245 [15] |
97 Mamma CA/stages I–III | Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
Actigraphy; PSQI |
comparison T0 & Chemotherapy: → Fatigue ↑ & ↓ SQ → Relationship: + CrF & subjective sleep (PSQI) + CrF & objective sleep (Actigraphy)/TST - CrF & objective sleep (Actigraphy) /Wake daytime |
| Liu et al., Brain Behav
Immun 2012b; 26 (5): 706–713 [16] |
53 Mamma CA/stages I–III – Interleukin-6 (IL-6) – Interleukin-1 Receptor Antagonist (IL-1RA) – C-Reactive Protein (CRP) |
Multidimen. Fatigue Symptom Inventory (MFSI-SF) |
PSQI | comparison T0 & Chemotherapie:
underlie biochemical mechanism |
| Miaskowski & Lee, Journal of Pain and Symptom Management 1999; 17 (5): 320–332 [17] | 24 Bone metastases patients | Lee Fatigue Scale | Actigraphy | Fatigue: ↑ at evening & ↓ at morning; Sleep: ↓ SE; Fatigue associated with
|
| Mormont et al., Pathol Biol 1996; 44(3): 165–171 [18] | 30 Colorectal CA | no | Actigraphy | < difference in rest/activity between day & night |
| Mormont et al., Clin Cancer Res 2000; 6 (8): 3038–3045 [19] | 200 Colorectal CA | no | Actigraphy | 2-years-survivors 5x higher than those with changes in activity rhythms |
| Morrow, G.R. et al., ???, 1999 (look: at Roscoe et al., Support Care Cancer 2002; 10: 329–336) [20] |
78 Mamma CA | Multidimentional Assessment of Fatigue; Fatigue Symptom Checklist; POMS |
Actigraphy | robust & consistent Circadian rhythms associated with ↓ Fatigue (even after depression) |
| Mustian et al., Oncol
Hematol Rev 2012; 8 (2): 81–88 [21] |
Overview Prevalence: i.e., Fatigue & Sleep | |||
| Owen et al., Oncol Nurs
Forum 1999; 26 (10): 1649–1651 [22] |
15 CA | no | Self-Report; PSQI | CA Patients significant ↓ SQ, ↓ SE & ↑ SOL |
| Palesh et al., J Clin Oncol 2009; 28 (2): 292–298 [23] | 823 CA (after Chemo) |
POMS/Fatigue Inactivity; POMS/Energy; Fatigue Symptom Checklist; Multidimen. Assessment of Fatigue |
Hamilton Depression Inventory (HDI) |
|
| Reyes-Gibby et al., Lancet Oncol 2008; 9 (8): 777–785 [24] |
Overview: Cytokines as markers for Cancer-Related Symptoms |
Memorial Symptom Assessment Scale (MSAS)/Item “Lack of Energy” |
Functional Assessment Cancer Therapy (FACT-G)/Scale GF/ Item Sleep | Polymorphism in different Cytokine Genes = Potential markers for genetic susceptibility
|
| Roscoe et al., The Oncologist
2007; 12 (suppl 1): 35–42 [25] |
Review: Cancer-Related Fatigue and Sleep Disorders | |||
| Silberfarb et al., J. Clin
Oncol 1993; 11 (5): 997- 1004 [26] |
15 Mamma CA 17 Lung CA 32 Insomnics 32 Controls |
no | PSG (SE, SOL, WASO) |
Lung CA ↓ SE, ↑ SOL & ↑ WASO compared with Mamma CA & Controls |
Notes: ???: unclear; ↑: increase; ↓: decrease.
Table 3.
Study on Sleep Disorders in cancer.
| Author | Sample | Measuring Instrument | Measuring Time | Results |
|---|---|---|---|---|
| Davidson et al., Social
Science & Medicine 2002; 54: 1309–1321 [27] |
982 Cancer patients 303 Breast 108 Gastrointestinal (GI) 155 Genitourinary (GU) 180 Gynecologic (GYN) 114 Lung 123 Skin |
Sleep Survey Questionnaire
|
3 months cross-sectional survey study | analyses of sleep disorders pevalence
|
|
Methods: (1) prevalence of reported sleep problems/six clinics (2) sleep problem prevalence in relation to cancer treatment (3) nature of insomnia (type, duration & associated factors) |
Table 4.
Studies on Insomnia in cancer.
| Author | Sample | Measuring Instrument(s) | Measuring Time(s) | Results |
|---|---|---|---|---|
| Graci, J Support Oncol 2005; 3 (5): 349–359 [43] | Review: Pathogenesis & Management of Cancer-Related Insomnia | |||
| Howell et al., Annals
of Oncology 2014; 25: 791–800 [45] |
Review: grey literature data sources and empirical databases from 2004 to 2012 |
Review includes:
|
||
| Minton & Stone, BMJ S&P Care 2012; 2: 231–238 [53] | 114 Mamma CA - 69 Controls - 45 CRFS |
Actigraphy; Insomnia Severity Index (ISI) |
between 3 months and 2 years after cancer therapy |
Insomnia prevalence significant > in CRFS < in Controls (effect ISI > Actigraphy !) |
| Park et al., Sleep Med Res 2016; 7(2): 48–54 [54] |
1248,914 patients analyzed 33,262 were diagnosed with cancer |
ICD-10 | 1-year cross-sectional study | Insomnia was prevalent in 8.21%: 15.2% lung cancer 9.2% non-Hodgkin’s lymphoma 8.8% bladder cancer 8.6% colorectal cancer 8.0% stomach cancer 7.8% prostate, breast & cervix cancer 6.6% liver cancer 5.8% thyroid cancer |
| Savard et al., Sleep 2001; 24 (5): 583–590 [58] |
300 Mamma CA |
Insomnia Interview Schedule (IIS)—Revised | one time | 19% Insomnia syndrome 95% chronic 33% onset of insomnia followed by breast cancer diagnosis 58% cancer either caused or aggravated the sleep difficulties factors associated with an increased risk for insomnia were:
|
| Savard et al., J Clin
Oncol 2009; 27: 5233–5239 [63] |
991 CA 466 Mamma 269 Prostata 118 Gynecological |
Self-Report Scales; Insomnia Diagnostic Interview |
T1—Baseline T2—2 months Tx—6, 10, 14 & 18 months |
total: 59.5% 28.5% Insomnia 31.0% Insomnia symptoms; Mamma & Gynecological > Prostata; Insomnia ↓ Therapy course |
| Savard et al., J Clin
Oncol 2011; 29: 3580–3586 [64] |
856 CA 426 Mamma 235 Prostata 96 Gynecological |
Insomnia Interview Schedule (IIS) | T1—Baseline Tx—2, 6, 10 & 14 months T6—18 months |
total: 59% 28% Insomnia 31% Insomnia symptoms; Mamma & Gynecological > Prostata; Insomnia ↓ Therapy course |
| Savard & Savard, Sleep Med Clin 2013; 8: 373–387 [67] | Review: Insomnia – Cancer – Prevalence – Risk factors – Nonpharmacologic treatment | |||
Table 5.
Studies on Insomnia Treatment in cancer.
| Author | Sample | Measuring Instrument(s) | Measuring Time(s) & Method(s) |
Results |
|---|---|---|---|---|
| Barton et al., J Sup- port Oncol 2011; 9 (1) 24–31 [28] |
227 (202) Cancer patients 130 Breast 14 Colon 4 Prostate 52 Other |
PSQI; FOSQ (Functional Outcomes of Sleep Questionnaire); BFI (Brief Fatigue Inventory); POMS (Profile of Mood States); TNAS (Toxicity Numeric Analogue Scale); CTCAE (Common Terminology Criteria for Adverse Events) |
T1—Baseline T2—Follow-up (4 weeks) T3—Follow-up (8 weeks) |
|
|
Methods: - RCT, dopple-blind - 450 mg Valerian (Herbal Medicine versus Placebo) | ||||
| Berger et al., Psycho-oncology 2009; 18 (6): 634–646 [29] | 219 Cancer patients/ stages I–III |
Actigraphy; PSQI; Sleep Diary (SOL, WASO, TIB, TST, SE) |
T1—Baseline T2—Follow-up (within 7 days) T3—Follow-up (30 days) |
|
|
Methods: - RCT - BT versus Controls (Behavioural Therapy [Individualized Sleep Promotion Plan (ISPP)]) | ||||
| Chen et al., Breast
Cancer Res Treat 2014; 145 (2): 381–388 [30] |
95 Postmenopausal Breast CA/stages 0–III |
PSQI; CES-D (Center for Epidemiologic Studies – Depression Scale); NCCTG (North Central Cancer Treatment Group) |
T1—Baseline T2—Follow-up (4 months) |
|
|
Methods: - RCT, dopple-blind - 3 mg Melatonin versus Placebo | ||||
| Choi et al., Integrative Cancer Therapies 2017; 16 (2) 135–146 [31] | A Systematic Review of Randomized Clinical Trials: Acupuncture for Managing Cancer-Related Insomnia | |||
| Dupont et al., Health Psychol 2014; 33 (2): 155–163 [32] | 558 Mamma CA | SF-36 (partly); IES-R (Revised Impact of Event Scale); CES-D (Center for Epidemiologic Studies—Depression Scale); PANAS (Positive and Negative Affect Scale) FSI (Fatigue Symptom Inventory); MOS (Medical Outcomes Study); BCPT (Breast Cancer Prevention Trial) |
T1—Baseline T2—Post-Treatment (4 weeks) T3—Follow-up (2 months) T4—Follow-up (6 months) T5—Follow-up (12 months) |
|
|
Methods: three types of information: (1) print material (2) print material & peer- modeling videotape (3) print material, videotape, 2 education sessions & information workbook | ||||
| Epstein & Dirksen, Oncology Nursing
Forum 2007; 34 (5); 51–59 [33] |
81 Mamma CA - 40 Controls - 41 CBT-I |
Actigraphy; Sleep Diary (SOL, WASO, TIB, TST, SE) PFS (Piper Fatigue Scale) |
T1—Baseline T2—Post-CBT-I (6 weeks) T3—Follow-up (12 weeks) |
|
Methods:
| ||||
| Espie et al., J of
Clinical Oncology 2008; 26: 4651–4658 [34] |
150 CA 87 Mamma 34 Prostate 24 Colorectal 5 Gynecological (110 CBT/50 TAU) |
PSQI; ESS; Sleep Diary (SOL, WASO, TST, SE); HADS; FSI (Fatigue Symptom Inventory); CrQoL (Cancer-Related Quality of Life); FACT-G (Functional Assessment of Cancer Therapy Scale – General) |
T1—Baseline T2—Post-Treatment T3—Follow-up (6 months) |
|
Methods:
| ||||
| Fiorentino et al., Nature and Science of Sleep 2010; 2: 1–8 [35] |
21 Mamma CA - 11 IND-CBT-I - 10 Controls |
Actigraphy; PSQI; Insomnia Severity Index (ISI); Sleep Diary (SOL, WASO, TIB, TST, SE) |
T1—Baseline T2—Post-CBT-I (6 weeks) T3—Follow-up (12 weeks) |
|
Methods:
| ||||
| Fleming (Espie) et al., Psychooncology 2014; 23 (6): 679–684 [36] |
113 Cancer patients with Insomnia - 73 CBT-I - 40 Controls |
PSQI; Sleep Diary (SOL, WASO, TIB, TST, SE) HADS; FSI (Fatigue Symptom Inventory) |
T1—Baseline T2—Post-Treatment T3—Follow-up (6 months) |
|
Methods:
| ||||
| Garland et al., Contemporary Clinical Trials 2011; 32 (5): 747–754 [37] |
??? | Actigraphy; Sleep Diary (SOL, WASO, TST, SE) ??? |
T1—Baseline T2—Post-Treatment (2 months) T3—Follow-up (3 months) |
|
|
Methods: CBT-I versus MBSR (Mindfulness-Based Stress Reduction) | ||||
| Garland et al., J
Clin Oncol 2014; 32: 1–9 [38] |
327 screened CA 111 randomly assigned 53 Breast 12 Prostate 11 Blood/lymph 10 Female Genitourinary 9 Head & Neck 7 Colon/GI 7 Lung 2 Skin CBT-I: n = 47 MBSR: n = 64 |
Actigraphy; PSQI; Insomnia Severity Index (ISI); Sleep Diary (SOL, WASO, TST, SE) |
T1—Baseline T2—Post-Treatment (2 months) T3—Follow-up (5 months) |
|
|
Methods: CBT-I versus MBSR (Mindfulness-Based Stress Reduction) | ||||
| Garland et al., Neuropsychiatric Disease and Treatment 2014; 10: 1113–1124 [39] |
Review: Efficency of CBT-I in cancer Inclusion of 4 studies |
|
→ clinically improvements in subjective sleep outcomes improved sleep → Improvement in:
|
|
| Garland et al., Explore (N.Y.) 2015; 11 (6): 445–454 [40] | 72 Cancer patients MBCR: n = 32 CBT-I: n = 40 |
??? | T1—Baseline T2—Post-Treatment (? months) T3—Follow-up (3 months) |
|
|
Methods: CBT-I versus MBCR (Mindfulness-Based Cancer Recovery) | ||||
| Garland et al., Contemporary Clinical Trials 2016; 47: 349- 355 [41] |
160 Cancer patients with Insomnia |
??? | T1—Baseline T2—Mid-Treatment (4 weeks) T3—Post-Treatment (8 weeks) T4—Follow-up (3 months) |
??? |
Methods:
| ||||
| Garland et al., Sleep Medicine 2016; 20: 18–24 [42] | 88 Cancer patients with Insomnia |
ESS; Sleep Diary (SOL, WASO, TST, SE) |
T1—Baseline T2—Post-Treatment (7 weeks) T3—Follow-up (3 months) |
|
|
Methods: (RCT) (1) CBT-I + P (CBT-I and Placebo) (2) CBT-I + A (CBT-I and Armodafinil) (3) ARM (Armodafinil alone) (4) PLA (Placebo alone) | ||||
| Heckler (Garland) et al., Supportive Care in Cancer 2016; 24 (5): 2059–2066 [44] | 96 Cancer patients with Insomnia |
Insomnia Severity Index (ISI); BFI (Brief Fatigue Inventory); FACIT-Fatigue scale |
T1—Baseline T2—Post-Treatment (7 weeks) T3—Follow-up (3 months) |
|
|
Methods: (RCT) (1) CBT-I + P (CBT-I and Placebo) (2) CBT-I + A (CBT-I and Armodafinil) (3) ARM (Armodafinil alone) (4) PLA (Placebo alone) | ||||
| Irwin et al., JNCIM 2014; No. 50; 295–301 [46] |
90 Mamma CA random subsample (n = 48) |
Blood samples: - C-Reactive Protein (CRP) - Interleukin-6 (IL-6) - Tumor Necrosis Factor-α (TNF) subsample analyzed by genome-wide transcriptional profiling |
T1—Baseline T2—Post-Treatment (3 months) |
|
|
Methods: CBT-I versus TCC (Tai Chi Chih) | ||||
| Kim M. et al., BMJ open, 2017; 7 (8): 1- 10 [47] |
45 Cancer patients |
Actigraphy; Insomnia Severity Inventory (ISI); PSQI; Sleep Diary (SOL, WASO, TIB, TST, SE) BDSS (Blood Deficiency Scoring System); EA (Electroacupuncture); FACT-F (Functional Assessment of Cancer Therapy- Fatigue); MoCA (Montreal Cognitive Assessment) |
T1—Baseline T2—Treatment (3 weeks) T2—Post-Treatment (5 weeks) T2—Post-Treatment (9 weeks) |
Without results !!!
„The result of this study will be published in peer-reviewed journals or presented at academic conferences.“ |
|
Methods: (4 weeks) EA versus Sham-EA (Electroaccupuncture) versus TAU (Treatment As Usual) | ||||
| Kim S.W. et al., Psychiatry and Clinical Neurosciences 2008; 62: 75–83 [48] |
45 Cancer patients 25 Lung 5 Breast 6 Gastrointestinal tract 3 Hepatobiliary tract 3 Other malignancy |
C-LSEQ (Chonnam National University Hospital- Leeds Sleep Evaluation Questionnaire) SF-36; MADRS (Montgomery-Asberg Depression Rating Scale); EuroQoL (EQ) -5D |
T1—Baseline T2—Post-Treatment (4 weeks) |
mirtazapine rapidly improved sleep disturbance, nausea, pain and quality of life, as well as depression in cancer patients Sleep ↑: ↑ TST, ↓ SOL, ↓ SQ |
Methods:
| ||||
| Kröz et al., BMC
cancer 2017; 17 (1), 166: 1–13 [49] |
126 Mamma CA | PSQI; CFS-D (Cancer Fatigue Scale) |
T1—Baseline T2—Post-Treatment (10 weeks) T3—Follow-up (6 months) |
|
|
Methods: (RCT) (a) MT (Multimodal Treatment) (b) CT (MT + AeT) (Combined Treatment) (c) AeT (Aerobic Training) | ||||
| Lengacher et al., Psychooncology 24 (4): 424–432 [50] |
79 Mamma CA /stages 0-III |
OSP (Objective Sleep Parameters): - Actigraphy SSP (Subjective Sleep Parameters): - PSQI; - Sleep diary |
T1—Baseline T2—Treatment (6 weeks) T2—Post-Treatment (12 weeks) |
|
|
Methods: (RCT) MBSR (BC) vs. UC (Mindfulness-Based Stress Reduction [Breast Cancer]) (Usual Care) | ||||
| Marshall-McKenna et al., Supportive Care in Cancer 2016; 24 (4): 1821–1829 [51] | 74 Mamma CA with Insomnia - 68.9 % pre-menopausal - 31.1% post-menopausal |
HADS; FACT-B (Functional Assessment of Cancer Therapy - Breast) sleep/hot flush diaries (over 2-week periods) |
T1—Baseline T2—Treatment (x weeks) T3—Post-Treatment (x weeks) |
|
|
Methods: (RCT) - Intervention Arm: CPPT + SC (Cool Pad Pillow Topper + Standard Care) vs. - Control Arm: SC (Standard Care) | ||||
| Mendoza et al., Psychooncology 2017; 26 (11): 1832–1838 [52] | 44 Cancer patients | MOOS (Medical Outcomes Survey Sleep); PROMIS (Fatigue) (Problem Index Patient-reported Outcomes Measurement Information System); NRS (Pain intensity) (Numerical Rating Scales) |
T1—Baseline T2—Treatment (3 weeks) T3—Post-Treatment (3 months) |
VMWH-CBT vs. Controls → beneficial effects of the VMWH-CBT - sleep problems - fatigue - average pain intensity |
|
Methods: - RCT, cross-over - VMWH-CBT vs. Controls (Valencia model of Waking Hypnosis with Cognitive-Behavioural Therapy) | ||||
| Peoples (Garland) et al., Journal of Cancer Survivorship 2017; 11 (3): 401–409 [55] |
95 Cancer patients with Insomnia |
Insomnia Severity Index (ISI); FACT-G (QoL) (Functional Assessment of Cancer Therapy - General) |
T1—Baseline T2—Post-Treatment (7 weeks) T3—Follow-up (3 months) |
|
|
Methods: (RCT) (1) CBT-I + P (CBT-I and Placebo) (2) CBT-I + A (CBT-I and Armodafinil) (3) ARM (Armodafinil alone) (4) PLA (Placebo alone) | ||||
| Ritterband et al., Psychooncology 2012; 21 (7): 695–705 [56] | 28 Cancer patients with Insomnia |
Insomnia Severity Index (ISI); Sleep Diary (SOL, WASO, TIB, TST, SE) MFSI-SF (Multidimensional Fatigue Symptom Inventory - Short Form); UQ (Internet Intervention Utility Questionnaire); HADS; SF-12 |
T1—Baseline T2—Post-Treatment (3 months) |
SHUTi vs. Controls → beneficial effects of the SHUTi - ↓ ISI - ↓ HADS - ↑ SF-12 - Sleep Diary: ↑ SE, ↑ TST, ↓ SOL & ↓ WASO (Controls improved a little too: SE & WASO) |
Methods:
| ||||
| Roscoe, J.A., (Garland, Sh.N.) et al., Journal of Clinical Oncology 2015; 33 (2): 165–171 [57] | 96 Cancer patients with Insomnia |
PSQI; Insomnia Severity Index (ISI) | T1—Baseline T2—Post-Treatment (7 weeks) T3—Follow-up (3 months) |
CBT-I + A & CBT-I + P: → CBT-I improves Insomnia Severity (ISI) → CBT-I improves Sleep Quality (PSQI) → Armodafinil no effect on Insomnia & SQ |
|
Methods: (RCT) (1) CBT-I + P (CBT-I and Placebo) (2) CBT-I + A (CBT-I and Armodafinil) (3) ARM (Armodafinil alone) (4) PLA (Placebo alone) | ||||
| Savard (Quesnel) et al., JCCP 2003; 71 (1): 189–200 [59] | 10 Mamma CA | PSG; Insomnia Severity Inventory (ISI); Sleep Diary (SOL, WASO, TIB, TST, SE) MFI (Multidimensional Fatigue Inventory); BDI & STAI; QLQ-C30+ 3 (European Organization for Research & Treatment of Ca. Quality of Life Questionnaire) |
T1—Baseline T2—Post-Treatment (3 months) T3—Follow-up (6 months) |
CBT was associated with - ↓ ISI: = ↓ Insomnia severity - ↑ PSG & ↑ Sleep Diary: = ↑ SE, ↑ TST, ↓ SOL & ↓ WASO |
| Savard et al., Journal
of Pain and Symptom Management 2004; 27 (6): 513–522 [60] |
24 Mamma CA | PSG; Skin conductance |
??? |
|
|
Methods: CBT | ||||
| Savard et al., JCO
2005 I & II; 23 (25): 6083–6096 & 6097- 6106 [61] |
57 women with insomnia caused or aggravated by breast cancer |
PSG; Insomnia Severity Inventory (ISI); Sleep Diary (SOL, WASO, TIB, TST, SE) MFI (Multidimensional Fatigue Inventory); HADS; QLQ-C30+ 3 (European Organization for Research & Treatment of Ca. Quality of Life Questionnaire); Immune measures: enumeration of blood cell counts (i.e., WBCs, monocytes, lymphocytes, CD3, CD4, CD8, CD16/CD56) & cytokine product. (Interleukin-1-beta [IL-1β], Interferon gamma [IFN-γ]) |
T0—Pre-Waiting T1—Baseline T2—Post-Treatment T3—Follow-up (3 months) T4—Follow-up (6 months) T5—Follow-up (12 months) |
CBT was associated with (post-treatment vs. control patients) - ↓ ISI: = ↓ Insomnia severity - ↑ PSG & ↑ Sleep Diary: = ↑ SE, ↑ TST, ↓ SOL & ↓ WASO - higher secretion and/or level of IFN-γ & IL-1β - lower increase of lymphocytes |
|
Methods: CBT versus WLC (Waiting-List Control) | ||||
| Savard (Tremblay) et al., JCCP 2009; 77 (4): 742–750 [62] | 57 Mamma CA | PSG; Insomnia Severity Inventory (ISI); Sleep Diary (SOL, WASO, TIB, TST, SE) DBAS (Dysfunctional Beliefs and Attitudes about Sleep Scale); ABS (Adherence to Behavioural Strategies) TEPCQ (Treatment Expectancies and Perceived Credibility Questionnaire); TAPQ (Therapeutic Alliance Perception Questionnaire); HADS |
T1—Baseline T2—Post-Treatment (2 months) T3—Follow-up (6 months) |
|
|
Methods: CBT versus WLC (Waiting-List Control) | ||||
| Savard et al., Psycho-Oncology 2013; 22 (6): 1381–1388 [65] | 60 Prostate CA | Insomnia Severity Index (ISI); PSQ (Physical Symptoms Questionnaire) |
T1—Baseline Tx—1, 2, 4, 6, 8 & 12 months T8—16 months |
|
|
Methods: ADT (Androgen Deprivation Therapy) RTH (Radiation therapy) | ||||
| Savard (Casault) et al., Behaviour Research and Therapy 2013; 67: 45–54 [66] |
83 Cancer patients |
??? | T1—Baseline T2—Post-Treatment T3—Follow-up (3 months) T4—Follow-up (6 months) |
|
|
Methods: mCBT versus no Treatment (minimal CBT) | ||||
| Savard et al., Sleep 2014; 37 (8): 1305- 1314 [68] |
242 Mamma CA | Actigraphy; Insomnia Severity Index (ISI); Sleep Diary (SOL, WASO, TIB, TST, SE) |
T1—Baseline T2—Post-Treatment (6 weeks) |
- ↓ Insomnia severity - ↓ Early Morning Awakenings (EMA) - ↓ depression - ↓ fatigue - ↓ dysfunctional beliefs about sleep
- 71.3% vs. 44.3%, p < 0.005 |
|
Methods: (RCT) (1) Professionally administered CBT-I (PCBT-I; n = 81) (2) Video-based CBT-I (VCBT-I; n = 80) (3) no treatment (CTL; n = 81) | ||||
| Savard et al., Sleep 2016; 39 (4): 813–823 [69] |
242 Mamma CA | Insomnia Severity Index (ISI); Insomnia Interview Schedule (IIS); Sleep Diary (SOL, WASO, TIB, TST, SE) MFI (Multidimensional Fatigue Inventory) EORTC QLQ-C30; HADS; DBAS-16 (Dysfunctional Beliefs & Attitudes about Sleep Scale – Abbreviated version); |
T1—Baseline T2—Post-Treatment (6 weeks) T3—Follow-up (3 months) T4—Follow-up (6 months) T5—Follow-up (12 months) |
- ↓ Insomnia severity (ISI, IIS) - ↓ Early Morning Awakenings (EMA) - ↓ depression - ↓ anxiety - ↓ dysfunctional beliefs about sleep - ↑ QoL
VCBT-I and CTL: e.g., 12 month FU - 67% vs. 59% vs. 48%, p < 0.100 |
|
Methods: (RCT) (1) Professionally administered CBT-I (PCBT-I; n = 81) (2) Video-based CBT-I (VCBT-I; n = 80) (3) no treatment (CTL; n = 81) | ||||
| Simeit et al., Suppor-tive Care in Cancer 2004; 12 (3): 176–183 [70] |
229 Cancer patients (breast, kidney or prostate) |
??? | T1—Baseline T2—Post-Treatment (3-4 weeks) T3—Follow-up (6 months) |
- sleep latency (p < 0.001) - sleep duration (p < 0.001) - sleep efficiency (p < 0.001) - sleep quality (p < 0.001) - sleep medication (p < 0.050) - daytime dysfunction (p < 0.050) - quality-of-life
|
|
Methods: (RCT) (1) Progressive Muscle Relaxation (PMR; n = 80) (2) Autogenic Training (AT; n = 71) (3) Control Group (CG; n = 78) | ||||
| Zhou et al., Behavioral Sleep Medicine 2017; 15 (4): 288–301 [71] |
10 (12) Cancer patients | Insomnia Severity Index (ISI); PSQI; Sleep logs [SL]; (SOL, WASO, TIB, TST, SE); SF-12 |
T1—Baseline T2—Post-Treatment (20 days) T3—Follow-up (2 months after T2) |
↑ SE, ↓ SOL, ↓ WASO & ↓ EMA - ↓ Insomnia severity (ISI) - ↓ Sleep Quality (PSQI)
- QoL |
|
Methods: - Adapted CBT-I 3 x intervention in person (6) and via videoconference (6) | ||||
Notes: ???: unclear; ↑: increase; ↓: decrease.
Table 6.
Studies on Sleep-Related Breathing Disorder (SRBD)/Obstructive Sleep Apnea Syndrome (OSAS) in Cancer.
| Author | Sample | Measuring Instrument(s) | Measuring Time(s) | Results |
|---|---|---|---|---|
| Campos-Rodrigues et al., Am J Respir Crit Care Med 2013; 187 (1): 99–105 [72] |
4910 Patients (Multicentric Cohort Study) |
PSG/PG | T—Baseline T2—4.5 years |
↓ TSat<90% vs. ↑ CA Incidence ↑ AHI vs. ↑ CA Incidence → higher Risk: 1. < 65 years; 2. ♂; 3. no CPAP |
| Cao et al., Sleep
Breath 2015; 19 (2): 453–457 [73] |
Obstructive Sleep Apnea promotes Cancer development and progression (Animal studies) |
OSAS = Risk factor
→ activation of HIF-1 & VEGF pathways → tumor growth → aggressive cancer behaviour |
||
| Dewan et al., Chest 2015; 147 (1): 266–274 [74] |
Intermittent hypoxemia and OSA: Implications for comorbidities (Animal & Human studies) |
Intermittent hypoxemia promotes → Oxidative stress → Inflammation → Increased sympathetic activation → Progression of cancer → Effect of CPAP !!! |
||
| Faiz et al., The
Oncologist 2014; 19: 1200–1206 [75] |
56 Patients with tumors in the head and neck region | PSG | Retrospective review from 2006 to 2011 |
1. SRBD = common in patients with tumors in the head/ neck region → caused by sleep disruption 2. Architectural changes from tumor and/or therapy lead to OSA |
| Gomez-Merino et al., Respiration 2003; 70: 107–109 [76] | Case study: 55 years old man non-Hodgkin-Lymphoma |
Symptom development:
|
||
| Kendzerska et al., CMAJ 2014; 186 (13): 985–992 [77] |
10,149 Patients | PSG all patients AHI ≥ 5 or suspected OSAS (but AHI < 5) |
A) from 1994 to 2010 B) from 1991 to 2013 |
Methods:
link between OSA & Cancer development or progression through chronic hypoxemia |
| Marshall et al., JCSM 2014; 10 (4): 355–362 [78] |
400 OSAS-Patients | PG/MESAM IV | T1—Baseline 1990 T2—20 years 2010 (Follow-up) |
Follow-up: 397 people removed n = 4 with a previous stroke from the mortality/ CVD/CHD/stroke analyses (n = 393) n = 7 with cancer history from the cancer analyses (n = 390) 20 years Follow-up
1. moderate-severe OSA was significantly associated with - all-cause mortality (HR = 4.2; 95% CI: 1.9, 9.2) - cancer mortality (HR = 3.4; 95% CI: 1.1, 10.2) - incident cancer (HR = 2.5; 95% CI: 1.2, 5.0) - stroke (HR = 3.7; 95% CI: 1.2, 11.8) but not significantly with - CVD incidence (HR = 1.9; 95% CI: 0.75, 4.6) - CHD incidence (HR = 1.1; 95% CI: 0.24, 4.6) 2. mild OSA was associated with a halving in - mortality (HR = 0.5; 95% CI: 0.27, 0.99) |
| Martinez-Garcia et al., Eur Respir J 2012; 40: 1315–1317 [79] |
--- Special Article |
current insights and perspectives:
→ Cerbrovakular diseases → Metabolic diseases → Systemic inflammatory diseases important role in regulating the various stages of tumor development and progression |
||
| Nieto et al., Am J Respir Crit Care Med 2012; 186 (2): 190–194 [80] |
1522 Patients (Background: Wisconsin Sleep Cohort Study) |
PSG | T1—Baseline T2—22 years |
SRBD = associat. with ↑ CA Mortality → higher Risk in ↑ SRBD: 1. ↑ AHI; 2. ↓ Tsat<90% |
| Partinen et al., Chest 1988: 94 (6): 1200–1204 [81] |
198 OSAS Patients (Tracheostomy vs. Weight loss) |
PG | Retrospective review from 1972 to 1980 |
↑ BMI & ↑ AHI → lead to Vascular death
→ no answer !!! |
| Seidell, Eur J of
Clinical Nutrition 2010; 64: 35–41 [82] |
Review: Waist circumference and Waist/Hip ratio in relation to all-cause Mortality, Cancer and Sleep Apnea (Human studies) |
BMI ↑ → Risk of Cancer ↑ → Risk of OSA ↑ Waist circumference & Waist/Hip ratio → better indicator of all-cause mortality than BMI Relationship Cancer & OSA ??? → no answer !!! |
Notes: ???: unclear; ↑: increase; ↓: decrease; ♂: male.
Table 7.
Studies on Narcolepsy in cancer.
| Author | Sample | Measuring Instrument(s) | Measuring Time(s) | Results |
|---|---|---|---|---|
| Adams et al., Arch Neurol 2011; 68 (4): 521–524 [83] |
Case study: 35 years old man Testicular cancer |
Symptom development:
2.2 min. sleep latency with 5 episodes REM*Onset |
||
| Landolfi & Nadkarni, Neuro-Oncology 2003; 5: 214–216 [84] | Case study: 55 years old man Tonsil cancer |
Symptom development:
MSLT: 9 min. sleep latency with 2 episodes REM*Onset |
||
| Tseng et al., Cancer
Epidemiol 2015; 39 (6): 793–797 [85] |
2,833 Narcoleptics | ??? | from 2000 to 2009/National Health Insurance Research Database |
adult narcoleptic patients → higher cancer risk (74 Cancer/SIR 1.32; 95% CI, 1.04–1.66, p = 0.0248) → ♀ = higher Risk: (SIR 1.52; 95% CI, 1.05–2.13, p = 0.026) 1. ↑ Head & Neck CA (SIR 6.17; 95% CI, 1.66–15.80, p = 0.009) 2. ↑ Gastric CA (SIR 4.87; 95% CI, 1.31–12.48, p = 0.020) → underlying mechanism unclear |
Notes: ???: unclear; ↓: decrease; ♀: female.
Table 8.
Studies on Restless Legs Syndrome (RLS) in cancer.
| Author | Sample | Measuring Instrument(s) | Measuring Time(s) | Results |
|---|---|---|---|---|
| Saini et al., J Pain Symptom Manage 2013; 46: 56–64 [86] | 173 CA different entities 32.4% Colorectal 17.3% Mamma 7.5% Prostata 6.4% Ovary 5.8% - Bladder - Gastroenteropancreatic Neuroendocrine 3.5 % - Pancreas - Testis - Stomach 2.9% - Lung - Adrenal cortical 2.3% - Uterus - Kidney 1.7% Head & Neck 1.2% Thymus 0.6% - Esophagus - Thyroid |
Pittsburgh Sleep Quality Index (PSQI); International Restless Legs Syndrome Study Group rating scale (IRLS); Functional Assessment of Cancer Therapy-General (FACT-G); Hospital Anxiety and Depression Scale (HADS) |
T0—before Chemotherapy T1—after Chemotherapy |
58.8% Sleep problems (PSQI > 5) 20.0% RLS positive screened
|
Table 9.
Studies on REM Sleep Behaviour Disorder (REM-SBD) in cancer.
| Author | Sample | Measuring Instrument(s) | Measuring Time(s) | Results |
|---|---|---|---|---|
| Adams et al., Arch
Neurol 2011; 68 (4): 521–524 [83] |
Case study: 35 years old man Testicular cancer |
PSG |
Polysomnogram:
|
|
| Jianhua, Ch. et al., Intern Med 2013; 52: 617–621 [87] |
Case study: 30 years old man Brainstem lymphoma (diffuse large B-cell) |
PSG; MRI |
Polysomnogram (Sleep Rhythm Disorder):
|
|
| Shinno, H. et al., J
Pain Symptom Manage 2010; 40 (3): 449–452 [88] |
Case study: 3 cases 70–76 years old patients 2 males & 1 femal Advanced cancer (1 x kidney; 2 x stomach) |
PSG |
Polysomnogram:
|
5. Discussion
Sleep disturbances and sleep disorders in cancer patients are very common and have different backgrounds compared with sleep difficulties in normal populations because of the differences in the risk factors, vulnerability and cancer-specific life events.
A personalized treatment of sleep disorders in patients with cancer could improve both their mental and physical health.
The goal of this review was to illuminate approaches that might influence sleep, sleep quality and sleep disorders in cancer patients and treatment possibilities in cancer-related insomnia. However, before treatment trials in different sleep disorders (insomnia, OSAS, narcolepsy, RLS and REM-SBD) can be started, prospective and objective studies are needed to unterstand the baseline levels of sleep, sleep difficulties and circadian rhythm in cancer. Sleep disruption in cancer can be caused by many different reasons, such as stress, mental disorders (like depression and anxiety), pain and treatment side effects.
Bad sleep quality, the degree of sleep disruption and sleep disorders have a very important impact on cancer and can used as predictors. Sleep disruptions and disruptions in the circadian rhythms affecting the sleep quality and the circadian rhythm themselves can result in a variety of psychological and physiological mechanisms, which can foster the developent and persistance of cancer-related fatigue. The role of naps in fatigued cancer patients is unclear; it could be that naps are not helpful to decrease cancer-related fatigue—they could have the opposite effect [17]. In noncancer patients, it is known that daytime naps reduce the nightly sleep quality and total sleep time.
Although the relationship between fatigue, sleep and circadian rhythms in cancer is known, there is a very small quantity of scientific reseach about this topic, and the quality is mostly very poor. The existing literature and research is inhomogeneous, and there are many methodological limitations: the types of studies (e.g., randomized controlled trials, quasi-randomized controlled trials, prospective and retrospective studies, cross-sectional surveys, uncontrolled studies and controlled trials without randomization methods, a special article and case studies); participants (different kinds of cancer patients—e.g., with or without treatment and with different entities); interventions (in the case of insomnia: nonpharmacological and pharmacological interventions); outcomes (objective and/or subjective measurements) are not comparable and the sample sizes are mostly very small.
Davidson et al. found in a big sample size with nearly a thousand patients that the total prevalence scores of RLS were present in nearly half of the researched cancer patients, of overly sleepy and of insomnia in around one-third of the patients, of sleeping more than usual and repetitive leg movements in almost one-fifth of them and of breathing interruptions in approximately ten percent [27].
The causes of decreased sleep quality; chronic sleep difficulties and the different sleep disorders (insomnia, OSAS, narcolepsy, RLS and REM-SBD) are multifaceted, and in recent studies, the attention that was paid this problem was too insufficient. Until now, the pathogenesis of cancer-related sleep disorders and the development such as the progression of cancer based on sleep disorders has been unclear. More research about these topics is needed to understand the nature, duration and severity of the different sleep disorders in cancer or their relationship with it.
The prevention of sleep disorders generally and in cancer patients especially and an early personalized treatment can contribute to reducing cancer-related fatigue and severe mental disorders (like depression and anxiety) and can possibily prevent the development, preservation and/or aggravation of cancer.
5.1. Expert Recommendations
Sleep disturbances; disruptions of the circadian rhythms and different sleep disorders (e.g., insomnia and sleep-related breathing disorder (SRBD)/obstructive sleep apnea syndrome (OSAS)) could be predictors of cancer development and treatment success (look above). Due to that, cancer patients should be screened by sleep anamnesis and/or by sleep diaries, including the structured exploration of predisposing and precipitating cancer factors, and should be diagnosed—in the case of any kind of sleep-wake difficulties—by polysomnography.
Screening should explore unrefreshing sleep: prolonged sleep latency, frequent awakening and reduced sleep efficiency; daytime sleepiness and fatigue; loud snoring; inadequate nightly behavior and/or nightmares.
Both screening and/or the diagnosis of sleep disturbances; disruptions of the circadian rhythm and/or sleep disorders, as well as adequate sleep health education (including sleep hygiene, rules for good sleep quality and information about the consequences of unhealthy and/or untreated sleep disorders for mental and physical health) should be implemented to minimize the health risks caused by sleep disorders.
Tailored programs are needed and could be helpful to reduce cancer-related fatigue and/or severe mental disorders (like depression and anxiety) to support the outcome of the treatment of patients with cancer and comorbid sleep disorders.
Currently, sleep–wake solutions in cancer are mostly aimed only by responding to emergency reasons and based on isolated and/or fragmented interventions, e.g., the treatment of insomnia: cognitive behavioral therapy for insomnia, nightmares: rehearsal therapy and SRBD: CPAP adherence.
Peronalized medical services for cancer patients should include integrated coaching or the early treatment of the most common sleep disorders and web-based telehealth programs [92] to reduce the preservation and/or aggravation of cancer an/or serious implications, including increased cerebrovascular, cardiovascular and/or metabolic diseases; excessive daytime sleepiness and/or cancer-related fatigue.
5.2. References Classification
Studies on Sleep and Cancer-related Fatigue (CrF) in cancer/Connection between sleep and fatigue in oncological diseases [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]; Sleep Disorders (generally) [27]; Insomnia (total) [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]; Sleep-Related Breathing Disorder (SRBD)/Obstructive Sleep Apnea Syndrome (OSAS) [72,73,74,75,76,77,78,79,80,81,82]; Narcolepsy [83,84,85]; Restless Legs Syndrome (RLS) [86]; REM Sleep Behaviour Disorder (REM-SBD) [83,87,88]; Others (Devices & Methods) [89,90,91,92].
6. Conclusions
Cancer patients can suffer under different sleep disturbances and sleep disorders, and these difficulties can be associated with different mental and/or physical problems. Side effects of cancer treatment and cancer-related psychological dysfunctions can be triggered it. Especially insomnia and OSAS are very common in cancer. Because of it, an evidence-based and tailored treatment is necessary.
Abbreviations
| Disorders | |
| CA | Carcinoma |
| CrF | Cancer-related Fatigue |
| CRFS | Cancer-related Fatigue Syndrome |
| CRSD | Cancer-Related Sleep Disorders |
| OSA(S) | Obstructive Sleep Apnea (Syndrome) |
| REM-SBD | REM-Sleep Behavior Disorder |
| RLS | Restless Legs Syndrome |
| SRBD | Sleep-Related Breathing Disorder |
| Measurements | |
| MESAM | Madaus Electronic Sleep Apnea Monitor |
| MRI | Magnetic Resonance Imaging |
| OSP | Objective Sleep Parameters |
| PG | Polygraphy |
| PSG | Polysomnography |
| SSP | Subjective Sleep Parameters |
| Anthropomeric and Clinical Data | |
| CRP | C-Reactive Protein |
| IFN-γ | Interferon gamma |
| IL-6/-1β | Interleukin-6/Interleukin-1-beta |
| IL-1RA | Interleukin-1 Receptor Antagonist |
| QoL | Quality of Life |
| TNF | Tumor Necrosis Factor |
| Therapies | |
| AeT | Aerobic Treatment |
| AT | Autogenic Training |
| BT | Behavioral Therapy |
| CBT | Cognitive Behavior Therapy |
| CPPT | Cool Pad Pillow Topper |
| CPAP | Continuous Positive Airway Pressure |
| CT | Combined Multimodal-Aerobic Treatment |
| EA | Electro-Acupuncture |
| ISPP | Individualized Sleep Promotion Plan |
| MBSR | Mindfulness-Based Stress Reduction |
| MT | Multimodal Treatment |
| PMR | Progressive Muscle Relaxation |
| PCBT | Professionally administered CBT |
| SHUTi | Sleep Healthy Using the Internet |
| SC | Standard Care |
| TCC | Tai Chi Chi |
| VCBT | Video-based CBT |
| Sleep Parameters | |
| TST | Total Sleep Time |
| TIB | Time in Bed |
| SE | Sleep Efficiency |
| SQ | Sleep Quality |
| SOL | Sleep*Onset*Latency |
| SWS | Slow Wave Sleep |
| WASO | Wake after Sleep*Onset or Total Waking Time |
| Questionnaires | |
| ABS | Adherence to Behavioral Strategies |
| ASI | Athens Insomnia Scale |
| BCPT | Breast Cancer Prevention Trial |
| BDI | Becks Depression Inventory |
| BDSS | Blood Deficiency Scoring System |
| BFI | Brief Fatigue Inventory |
| CES-D | Center for Epidemiologic Studies—Depression Scale |
| CFS-D | Cancer Fatigue Scale |
| C-LSEQ | Chonnam National University Hospital—Leeds Sleep Evaluation Questionnaire |
| CrQoL | Cancer-Related Quality of Life |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DBAS | Dysfunctional Beliefs and Attitudes about Sleep Scale |
| ESS | Epworth Sleepiness Scale |
| EQ-5D | Euro QoL |
| FACT | Functional Assessment Cancer Therapy |
| FOSQ | Functional Outcomes of Sleep Questionnaire |
| FSI | Fatigue Symptom Inventory |
| GSDS | General Sleep Disturbance Scale |
| HADS | Hospital Anxiety and Depression Scale |
| HDI | Hamilton Depression Inventory |
| NCCTG | North Central Cancer Treatment Group |
| PSQI | Pittsburgh Sleep Quality Index |
| IES-R | Revised Impact of Event Scale |
| IIS | Insomnia Interview Schedule |
| ISI | Insomnia Severity Index |
| IRLS | International Restless Legs Syndrome Study Group rating scale |
| MADRS | Montgomery-Asberg Depression Rating Scale |
| MFSI-SF | Multidimensional Fatigue Symptom Inventory |
| MoCA | Montreal Cognitive Assessment |
| MOS | Medical Outcomes Study |
| MOOS | Medical Outcomes Survey Sleep |
| MSAS | Memorial Symptom Assessment Scale |
| NRS | Numerical Rating Scales (Pain intensity) |
| PANAS | Positive and Negative Affect Scale |
| POMS | Profile of Mood States |
| PROMIS | Problem Index Patient-reported Outcomes Measurement Information System |
| PSQ | Physical Symptoms Questionnaire |
| QLQ-C30+ 3 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire |
| SF-12/-36 | Short Form of Health Survey |
| STAI | State Trait Anxiety Inventory |
| TAPQ | Therapeutic Alliance Perception Questionnaire |
| TEPCQ | Treatment Expectancies and Perceived Credibility Questionnaire |
| TNAS | Toxicity Numeric Analogue Scale |
| UQ | Internet Intervention Utility Questionnaire |
Author Contributions
Literature research, discussion, evaluation and conceptualization: A.B.-T., Y.-T.K. and K.R.; writing—original draft: A.B.-T. and writing—review and editing: A.B.-T., K.R. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
All the authors state that they do not have any conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ancoli-Israel S., Moore P.J., Jones V. The relationship between fatigue and sleep in cancer patients: A review. Eur. J. Cancer Care. 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S., Liu L., Marler M.R., Parker B.A., Jones V., Sadler G.R., Dimsdale J., Cohen-Zion M., Fiorentino L. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support. Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S., Liu L., Rissling M., Natarajan L., Neikrug A.B., Palmer B., Mills P.J., Parker B.A., Sadler G.R., Maglione J. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support. Care Cancer. 2014;22:2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banthia R., Malcarne V.L., Ko C.M., Varni J.W., Sadler G.R. Fatigued breast cancer survivors: The role of sleep quality, depressed mood, stage and age. Psychol. Heal. 2009;24:965–980. doi: 10.1080/08870440802110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger A.M., Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol. Nurs. Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 6.Chang V.T., Hwang S.S., Feuerman M., Kasimis B.S. Symptom and quality of life survey of medical oncology patients at a Veterans Affairs medical center. Cancer. 2000;88:1175–1183. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1175::AID-CNCR30>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22:185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Clevenger L., Schrepf A., Christensen D., DeGeest K., Bender D., Ahmed A., Goodheart M.J., Penedo F., Lubaroff D.M., Sood A.K., et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav. Immun. 2012;26:1037–1044. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engstrom C.A., Strohl R.A., Rose L., Lewandowski L., Stefanek M.E. Sleep alterations in cancer patients. Cancer Nurs. 1999;22:143–148. doi: 10.1097/00002820-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino L., Rissling M., Liu L., Ancoli-Israel S. The symptom cluster of sleep, fatigue and depressive symptoms in breast cancer patients: Severity of the problem and treatment options. Drug Discov. Today Dis. Model. 2011;8:167–173. doi: 10.1016/j.ddmod.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotts Z.M., Deary V., Newton J., Van der Dussen D., De Roy P., Ellis J.G. Are there sleep-specific phenotypes in patients with chronic fatigue syndrome? A cross-sectional poly-somnography analysis. BMJ Open. 2013;3:1–8. doi: 10.1136/bmjopen-2013-002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Illi J., Miaskowski C., Cooper B., Levine J.D., Dunn L., West C., Dodd M., Dhruva A., Paul S.M., Baggott C., et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye J., Kaye K., Madow L. Sleep pattern in patients with cancer and cardiac disease. J. Psychol. 1983;114:107–113. doi: 10.1080/00223980.1983.9915403. [DOI] [PubMed] [Google Scholar]
- 14.Liu L., Fiorentino L., Natarajan L., Parker B.A., Mills P.J., Sadler G.R., Dimsdale J.E., Rissling M., He F., Ancoli-Israel S. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psycho Oncol. 2009;18:187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L., Rissling M., Natarajan L., Fiorentino L., Mills P.J., Dimsdale J.E., Sadler G.R., Parker B.A., Ancoli-Israel S. The Longitudinal Relationship between Fatigue and Sleep in Breast Cancer Patients Undergoing Chemothera-py. Sleep. 2012;35:237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L., Mills P.J., Rissling M., Fiorentino L., Natarajan L., Dimsdale J.E., Sadler G.R., Parker B.A., Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav. Immun. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miaskowski C.h., Lee K.A. Pain, Fatigue, and Sleep Disturbances in Oncology Outpatients Receiving Radiation Therapy for Bone Metastasis: A Pilot Study. J. Pain Symptom Manag. 1999;17:320–332. doi: 10.1016/S0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 18.Mormont M.C., De Prins J., Levi F. Study of circadian rhythms of activity by actometry: Preliminary results in 30 patients with metastat-ic colorectal cancer. Pathol. Biol. (Paris) 1996;44:165–171. [PubMed] [Google Scholar]
- 19.Mormont M.C., Waterhouse J., Bleuzen P., Giacchetti S., Jami A., Bogdan A., Lellouch J., Misset J.L., Touitou Y., Lévi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 20.Roscoe J.A., Morrow G.R., Hickok J.T., Bushunow P., Matteson S., Rakita D., Andrews P.L. Temporal interrelationships among fatigue, circadian rhythm and de-pression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 21.Mustian K.M., Sprod L.K., Janelsins M., Peppone L.J., Mohile S. Exercise Recommendations for Cancer-Related Fatigue, Cognitive Impairment, Sleep problems, Depression, Pain, Anxiety, and Physical Dysfunction: A Review. Oncol. Hematol. Rev. (US) 2012;8:81–88. doi: 10.17925/OHR.2012.08.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen D.C., Parker K.P., McGuire D.B. Comparison of subjective sleep quality in patients with cancer and healthy subjects. Oncol. Nurs. Forum. 1999;26:1649–1651. [PubMed] [Google Scholar]
- 23.Palesh O.G., Roscoe J.A., Mustian K.M., Roth T., Savard J., Ancoli-Israel S., Heckler C., Purnell J., Janelsins M.C., Morrow G.R. Prevalence, Demographics, and Psychological Associations of Sleep Disruption in Patients with Cancer: University of Rochester Cancer Center–Community Clinical Oncology Program. J. Clin. Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes-Gibby C.C., Wu X., Spitz M., Kurzrock R., Fisch M., Bruera E., Shete S. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9:777–785. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roscoe J.A., Kaufman M.E., Matteson-Rusby S.E., Palesh O.G., Ryan J.L., Kohli S., Perlis M.L., Morrow G.R. Cancer-Related Fatigue and Sleep Disorders. Oncol. 2007;12:35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 26.Silberfarb P.M., Hauri P.J., Oxman E.T., Schnurr P. Assessment of sleep in patients with lung cancer and breast cancer. J. Clin. Oncol. 1993;11:997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- 27.Davidson J.R., MacLean A.W., Brundage M.D., Schulze K. Sleep disturbance in cancer patients. Soc. Sci. Med. 2002;54:1309–1321. doi: 10.1016/S0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 28.Barton D.L., Atherton P.J., Bauer B.A., Moore D.F., Jr., Mattar B.I., LaVasseur B.I., Rowland K.M., Jr., Zon R.T., Lelindqwister N.A., Nagargoje G.G., et al. The Use of Valeriana Officinalis (Valerian) in Improving Sleep in Patients Who Are Undergoing Treatment for Cancer: A Phase III Randomized, Placebo-Controlled, Double-Blind Study: NCCTG Trial, N01C5. J. Support. Oncol. 2011;9:24–31. doi: 10.1016/j.suponc.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger A.M., Kuhn B.R., Farr L.A., Lynch J.C., Agrawal S., Chamberlain J., Von Essen S.G. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psycho Oncol. 2008;18:634–646. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 30.Chen W.Y., Giobbie-Hurder A., Gantman K., Savoie J., Scheib R., Parker L.M., Schernhammer E.S. A randomized, placebo-controlled trial of melatonin on breastcancer survivors: Impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145:381–388. doi: 10.1007/s10549-014-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi T.-Y., Kim J.I., Lim H.-J., Lee M.S. Acupuncture for Managing Cancer-Related Insomnia: A Systematic Review of Randomized Clinical Trials. Integr. Cancer Ther. 2017;16:135–146. doi: 10.1177/1534735416664172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupont A., Bower J.E., Stanton A.L., Ganz P.A. Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Heal. Psychol. 2014;33:155–163. doi: 10.1037/a0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein D.R., Dirksen S.R. Randomized Trial of a Cognitive-Behavioral Intervention for Insomnia in Breast Cancer Survivors. Oncol. Nurs. Forum. 2007;34:51–59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 34.Espie C.A., Fleming L., Cassidy J., Samuel L., Taylor L.M., White C.A., Douglas N.J., Engleman H.M., Kelly H.L., Paul J., et al. Randomized Controlled Clinical Effectiveness Trial of Cognitive Behavior Therapy Compared with Treat-ment As Usual for Persistent Insomnia in Patients with Cancer. J. Clin. Oncol. 2008;26:4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 35.Fiorentino L., McQuaid J.R., Liu L., Natarajan L., He F., Cornejo M., Lawton S., Parker B.A., Sadler G.R., Ancoli-Israel S., et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: A randomized con-trolled crossover pilot study. Nature and Science of Sleep. 2010;2:1–8. doi: 10.2147/NSS.S8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming L., Randell K., Harvey C.-J., Espie C.A. Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psycho Oncol. 2014;23:679–684. doi: 10.1002/pon.3468. [DOI] [PubMed] [Google Scholar]
- 37.Garland S.N., Carlson L., Antle M.C. I-CAN SLEEP: Rationale and design of a non-inferiority RCT of Mindfulness-based Stress Reduction and Cognitive Behavioral Therapy for the treatment of Insomnia in CANcer survivors. Contemp. Clin. Trials. 2011;32:747–754. doi: 10.1016/j.cct.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Garland S.N., Carlson L.E., Stephens A.J., Antle M.C., Samuels C., Campbell T.S. Mindfulness-Based Stress Reduction Compared with Cognitive Behavioral Therapy for the Treatment of Insomnia Comorbid with Cancer: A Randomized, Partially Blinded, Noninferiority Trial. J. Clin. Oncol. 2014;32:1–9. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 39.Garland S.N., Johnson J.A., Savard J., Gehrman P., Perlis M., Carlson L., Campbell T. Sleeping well with cancer: A systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr. Dis. Treat. 2014;10:1113–1124. doi: 10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garland S.N., Rouleau C.R., Campbell T., Samuels C., Carlson L.E. The Comparative Impact of Mindfulness-Based Cancer Recovery (MBCR) and Cognitive Behavior Therapy for Insomnia (CBT-I) on Sleep and Mindfulness in Cancer Patients. Explore (N Y) 2015;11:445–454. doi: 10.1016/j.explore.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Garland S.N., Gehrman P., Barg F.K., Xie S.X., Mao J.J. CHoosing Options for Insomnia in Cancer Effectively (CHOICE): Design of a patient centered com-parative effectiveness trial of acupuncture and cognitive behavior therapy for insomnia. Contemp. Clin. Trials. 2016;47:349–355. doi: 10.1016/j.cct.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Garland S.N., Roscoe J.A., Heckler C.E., Barilla H., Gehrman P., Findley J.C., Peoples A.R., Morrow G.R., Kamen C., Perlis M.L., et al. Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors. Sleep Med. 2016;20:18–24. doi: 10.1016/j.sleep.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graci G. Pathogenesis and management of cancer-related insomnia. J. Support. Oncol. 2005;3:349–359. [PubMed] [Google Scholar]
- 44.Heckler C.E., Garland S.N., Peoples A.R., Perlis M.L., Shayne M., Morrow G.R., Kamen C., Hoefler J., Roscoe J.A. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: A randomized placebo-controlled trial. Supportive Care Cancer. 2016;24:2059–2066. doi: 10.1007/s00520-015-2996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howell D., Oliver T.K., Keller-Olaman S., Davidson J.R., Garland S., Samuels C., Savard J., Harris C., Aubin M., Olson K., et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann. Oncol. 2014;25:791–800. doi: 10.1093/annonc/mdt506. [DOI] [PubMed] [Google Scholar]
- 46.Irwin M.R., Olmstead R., Breen E.C., Witarama T., Carrillo C., Sadeghi N., Arevalo J.M.G., Ma J., Nicassio P., Ganz P.A., et al. Tai Chi, Cellular Inflammation, and Transcriptome Dynamics in Breast Cancer Survivors with Insomnia: A Randomized Controlled Trial. J. Natl. Cancer Inst. Monogr. 2014;2014:295–301. doi: 10.1093/jncimonographs/lgu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M., Kim J.-E., Lee H.-Y., Kim A.-R., Park H.-J., Kwon O.-J., Kim B.-K., Cho J.H., Kim J.-H. Electroacupuncture for treating insomnia in patients with cancer: A study protocol for a randomised pilot clinical trial. BMJ Open. 2017;7:e016269. doi: 10.1136/bmjopen-2017-016269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S.-W., Shin I.-S., Kim J.-M., Kim Y.-C., Kim K.-S., Kim K.-M., Yang S.-J., Yoon J.-S. Effectiveness of mirtazapine for nausea and insomnia in cancer patients with depression. Psychiatry Clin. Neurosci. 2008;62:75–83. doi: 10.1111/j.1440-1819.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 49.Kröz M., Reif M., Glinz A., Berger B., Nikolaou A., Zerm R., Brinkhaus B., Girke M., Büssing A., Gutenbrunner C., et al. Impact of a combined multimodal-aerobic and multimodal intervention compared to standard aerobic treatment in breast cancer survivors with chronic cancer-related fatigue—Results of a three-armed pragmatic trial in a com-prehensive cohort design. BMC Cancer. 2017;17:166. doi: 10.1186/s12885-017-3142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lengacher C.A., Reich R.R., Paterson C.L., Jim H.S., Ramesar S., Alinat C.B., Budhrani-Shani P., Farias J.R., Shelton M.M., Moscoso M.S., et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: A randomized controlled trial. Psycho Oncol. 2014;24:424–432. doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marshall-McKenna R., Morrison A., Stirling L., Hutchison C., Rice A.M., Hewitt C., Paul J., Rodger M., MacPherson I.R., McCartney E. A randomised trial of the cool pad pillow topper versus standard care for sleep disturbance and hot flushes in women on endocrine therapy for breast cancer. Support. Care Cancer. 2015;24:1821–1829. doi: 10.1007/s00520-015-2967-3. [DOI] [PubMed] [Google Scholar]
- 52.Mendoza M., Capafons A., Gralow J., Syrjala K., Suárez-Rodríguez J., Fann J., Jensen M. Randomized controlled trial of the Valencia model of waking hypnosis plus CBT for pain, fatigue, and sleep management in patients with cancer and cancer survivors. Psycho-Oncology. 2016;26:1832–1838. doi: 10.1002/pon.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minton O., Stone P. A comparison of cognitive function, sleep and activity levels in disease-free breast cancer patients with or without cancer-related fatigue syndrome. BMJ Support. Palliat. Care. 2012;2:231–238. doi: 10.1136/bmjspcare-2011-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park B., Youn S., Hann C.W.C., Yi K., Lee S., Lee J.S., Chung S. Prevalence of Insomnia among Patients with the Ten Most Common Cancers in South Korea: Health Insur-ance Review and Assessment Service-National Patient Sample. Sleep Med. Res. 2016;7:48–54. doi: 10.17241/smr.2016.00073. [DOI] [Google Scholar]
- 55.Peoples A.R., Garland S., Perlis M.L., Savard J., Heckler C.E., Kamen C.S., Ryan J.L., Mustian K.M., Janelsins M.C., Peppone L.J., et al. Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: A randomized placebo-controlled trial. J. Cancer Surviv. 2017;11:401–409. doi: 10.1007/s11764-017-0597-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritterband L.M., Bailey E.T., Thorndike F.P., Lord H.R., Farrell-Carnahan L., Baum L.D. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psycho Oncol. 2012;21:695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roscoe J.A., Garland S., Heckler C.E., Perlis M.L., Peoples A.R., Shayne M., Savard J., Daniels N.P., Morrow G.R. Randomized Placebo-Controlled Trial of Cognitive Behavioral Therapy and Armodafinil for Insomnia After Cancer Treatment. J. Clin. Oncol. 2015;33:165–171. doi: 10.1200/JCO.2014.57.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savard J., Simard S., Blanchet J., Ivers H., Morin C.M. Prevalence, Clinical Characteristics, and Risk Factors for Insomnia in the Context of Breast Cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 59.Quesnel C., Savard J., Simard S., Ivers H., Morin C.M. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J. Consult. Clin. Psychol. 2003;71:189–200. doi: 10.1037/0022-006X.71.1.189. [DOI] [PubMed] [Google Scholar]
- 60.Savard J., Davidson J.R., Ivers H., Quesnel C., Rioux D., Dupéré V., Lasnier M., Simard S., Morin C.M. The association between nocturnal hot flashes and sleep in breast cancer survivors. J. Pain Symptom Manag. 2004;27:513–522. doi: 10.1016/j.jpainsymman.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Savard J., Simard S., Ivers H., Morin C.M. Randomized Study on the Efficacy of Cognitive-Behavioral Therapy for Insomnia Secondary to Breast Cancer, Part I: Sleep and Psychological Effects & Part II: Immunologic Effects. JCO. 2005;23:6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- 62.Tremblay V., Savard J., Ivers H. Predictors of the effect of cognitive behavioral therapy for chronic insomnia comorbid with breast cancer. J. Consult. Clin. Psychol. 2009;77:742–750. doi: 10.1037/a0015492. [DOI] [PubMed] [Google Scholar]
- 63.Savard J., Villa J., Ivers H., Simard S., Morin C.M. Prevalence, Natural Course, and Risk Factors of Insomnia Comorbid with Cancer Over a 2-Month Period. J. Clin. Oncol. 2009;27:5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 64.Savard J., Ivers H., Villa J., Caplette-Gingras A., Morin C.M. Natural Course of Insomnia Comorbid with Cancer: An 18-Month Longitudinal Study. J. Clin. Oncol. 2011;29:3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 65.Savard J., Hervouet S., Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psycho-Oncology. 2012;22:1381–1388. doi: 10.1002/pon.3150. [DOI] [PubMed] [Google Scholar]
- 66.Casault L., Savard J., Ivers H., Savard M.-H. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behav. Res. Ther. 2013;67:45–54. doi: 10.1016/j.brat.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Savard J., Savard H.-M. Insomnia and Cancer. Prevalence, Nature, and Nonpharmacologic Treatment. Sleep Med. Clin. 2013;8:373–387. doi: 10.1016/j.jsmc.2013.04.006. [DOI] [Google Scholar]
- 68.Savard J., Ivers H., Savard M.H., Morin C.M. Is a Video-Based Cognitive Behavioral Therapy for Insomnia as Efficacious as a Professionally Adminis-tered Treatment in Breast Cancer? Results of a Randomized Controlled Trial. Sleep. 2014;37:1305–1314. doi: 10.5665/sleep.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savard J., Ivers H., Savard M.-H., Morin C.M. Long-Term Effects of Two Formats of Cognitive Behavioral Therapy for Insomnia Comorbid with Breast Cancer. Sleep. 2016;39:813–823. doi: 10.5665/sleep.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simeit R., Deck R., Conta-Marx B. Sleep management training for cancer patients with insomnia support care. Support Care Cancer. 2004;12:176–183. doi: 10.1007/s00520-004-0594-5. [DOI] [PubMed] [Google Scholar]
- 71.Zhou E.S., Vrooman L.M., Manley P.E., Crabtree V.M., Recklitis C.J. Adapted Delivery of Cognitive-Behavioral Treatment for Insomnia in Adolescent and Young Adult Cancer Survivors: A Pilot Study. Behav. Sleep Med. 2016;15:1–14. doi: 10.1080/15402002.2015.1126597. [DOI] [PubMed] [Google Scholar]
- 72.Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M., Duran-Cantolla J., Peña M.D.L., Masdeu M.J., Gonzalez M., Campo F.D., Gallego I., Marin J.M., et al. Association between Obstructive Sleep Apnea and Cancer Incidence in a Large Multicenter Spanish Cohort. Am. J. Respir. Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 73.Cao J., Feng J., Li L., Chen B. Obstructive sleep apnea promotes cancer development and progression: A concise review. Sleep Breath. 2015;19:453–457. doi: 10.1007/s11325-015-1126-x. [DOI] [PubMed] [Google Scholar]
- 74.Dewan N.A., Nieto F.J., Somers V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest. 2015;147:266–274. doi: 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faiz S.A., Balachandran D., Hessel A.C., Lei X., Beadle B.M., William N.W., Bashoura L. Sleep-Related Breathing Disorders in Patients with Tumors in the Head and Neck Region. Oncologist. 2014;19:1200–1206. doi: 10.1634/theoncologist.2014-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gómez-Merino E., Arriero J.M., Chiner E., Signes-Costa J., Marco J. Obstructive Sleep Apnea Syndrome as First Manifestation of Pharyngeal Non-Hodgkin’s Lym-phoma. Respiration. 2003;70:107–109. doi: 10.1159/000068423. [DOI] [PubMed] [Google Scholar]
- 77.Kendzerska T., Leung R.S., Hawker G., Tomlinson G., Gershon A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. Can. Med. Assoc. J. 2014;186:985–992. doi: 10.1503/cmaj.140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marshall N.S., Wong K.K., Cullen S.R., Knuiman M.W., Grunstein R.R. Sleep Apnea and 20-Year Follow-Up for All-Cause Mortality, Stroke, and Cancer Incidence and Mortal-ity in the Busselton Health Study Cohort. JCSM. 2014;10:355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martínez-García M.Á., Campos-Rodriguez F., Farre R. Sleep apnoea and cancer: Current insights and future perspectives. Eur. Respir. J. 2012;40:1315–1317. doi: 10.1183/09031936.00127912. [DOI] [PubMed] [Google Scholar]
- 80.Nieto F.J., Peppard P.E., Young T., Finn L., Hla K.M., Farré R. Sleep-disordered Breathing and Cancer Mortality Results from the Wisconsin Sleep Cohort Study. Am. J. Respir Crit Care Med. 2012;186:190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Partinen M., Jamieson A., Guilleminault C. Long-term Outcome for Obstructive Sleep Apnea Syndrome Patients. Chest. 1988;94:1200–1204. doi: 10.1378/chest.94.6.1200. [DOI] [PubMed] [Google Scholar]
- 82.Seidell J. Waist circumference and waist/hip ratio in relation to all-cause mortality, cancer and sleep apnea. Eur. J. Clin. Nutr. 2009;64:35–41. doi: 10.1038/ejcn.2009.71. [DOI] [PubMed] [Google Scholar]
- 83.Adams C., McKeon A., Silber M.H., Kumar R. Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch. Neurol. 2011;68:521–524. doi: 10.1001/archneurol.2011.56. [DOI] [PubMed] [Google Scholar]
- 84.Landolfi J.C., Nadkarni M. Paraneoplastic limbic encephalitis and possible narcolepsy in a patient with testicular cancer: Case study. Neuro Oncol. 2003;5:214–216. doi: 10.1215/S1152851702000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tseng C.-M., Chen Y.-T., Tao C.-W., Ou S.-M., Hsiao Y.-H., Li S.-Y., Chen T.-J., Perng D.-W., Chou K.-T. Adult narcoleptic patients have increased risk of cancer: A nationwide population-based study. Cancer Epidemiology. 2015;39:793–797. doi: 10.1016/j.canep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 86.Saini A., Berruti A., Strambi L.F., Castronovo V., Rametti E., Giuliano P.L., Ramassotto B., Picci R.L., Negro M., Campagna S., et al. Restless Legs Syndrome as a Cause of Sleep Disturbances in Cancer Patients Receiving Chemotherapy. J. Pain Symptom Manag. 2013;46:56–64. doi: 10.1016/j.jpainsymman.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 87.Jianhua C., Xiuqin L., Quancai C., Heyang S., Yan H. Rapid Eye Movement Sleep Behavior Disorder in a Patient with Brainstem Lymphoma. Intern. Med. 2013;52:617–621. doi: 10.2169/internalmedicine.52.8786. [DOI] [PubMed] [Google Scholar]
- 88.Shinno H., Kamei M., Maegawa T., Satake A., Inami Y., Horiguchi J., Nakamura Y. Three Patients with Cancer Who Developed Rapid-Eye-Movement Sleep Behavior Disorder. J. Pain Symptom Manag. 2010;40:449–452. doi: 10.1016/j.jpainsymman.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 89.Acker J.G., Becker-Carus C., Büttner-Teleaga A., Cassel W., Danker-Hopfe H., Dück A., Frohn C., Hein H., Penzel T., Rodenbeck A., et al. The role of actigraphy in sleep medicine. Somnologie Schlafforschung Schlafmed. 2021;25:89–98. doi: 10.1007/s11818-021-00306-8. [DOI] [Google Scholar]
- 90.Acker J., Golubnitschaja O., Büttner-Teleaga A., Richter K. Wrist actigraphic approach in primary, secondary and tertiary care based on the principles of predictive, preventive and personalised (3P) medicine. EPMA J. 2021;12:349–363. doi: 10.1007/s13167-021-00250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Translator Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peter L., Reindl R., Zauter S., Hillemacher T., Richter K. Effectiveness of an Online CBT-I Intervention and a Face-to-Face Treatment for Shift Work Sleep Disorder: A Comparison of Sleep Diary Data. Int. J. Environ. Res. Public Heal. 2019;16:3081. doi: 10.3390/ijerph16173081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.