ABSTRACT
CD3+CD56+ NKT-like cells play pivotal roles in the anti-tumor immune defense response. However, little is known regarding circulating NKT-like cells in patients with primary hepatocellular carcinoma (HCC). In the present study, we demonstrate that circulating NKT-like cells in HCC patients are functionally impaired and anti-PD-1 blockade improves their anti-tumor potency. Circulating NKT cells were mainly comprised of CD8+ T cells. The frequencies and absolute counts of circulating NKT-like cells were comparable between HCC patents compared to healthy donors. NKT-like cells in HCC patients were impaired in their production of TNF-α and IFN-γ as well as cytotoxicity. The level of activating receptor NKG2D was significantly decreased on NKT-like cells in HCC patients. In contrast, the expression of inhibitory receptors PD-1, Tim-3, and CTLA-4 were markedly increased on NKT-like cells in HCC patients. Meanwhile, the expression of PD-L1 was also upregulated on NKT-like cells in HCC patients. In detail, PD-1+ NKT-like cells expressed lower levels of NKG2D, higher levels of Tim-3, and CTLA-4, and less IFN-γ when compared with PD-1− NKT-like cells. Importantly, PD-1 blocked with anti-PD-1 antibody effectively improved the effector function of NKT-like cells from HCC patients or healthy donors. Our findings unveil the functional characterization of NKT-like cells in HCC patients and provide the potential targets to improve their function, which might benefit the optimization of HCC immunotherapy.
KEYWORDS: Hepatocellular carcinoma, nkt-like cells, pd-1, immune exhaustion, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer mortality worldwide.1The difficulty in early diagnosis and the limited therapeutic options leads to extremely high mortality and low five-year overall survival rates.2 New therapeutic options are urgently needed for patients with advanced HCC. Immune cells play pivotal roles in the development of HCC. In detail, CD8+ T cells and NK cells are involved in the defense against HCC.3,4 In contrast, macrophages build immune-suppressive microenvironments in advanced HCC.5 Currently, immunotherapy is an accepted clinical treatment of HCC. However, only a small proportion of HCC patients benefit from this treatment strategy.6 Therefore, deep understanding of the features and roles of distinct immune cell subsets in HCC might be helpful to optimize HCC immunotherapy and improve clinical efficacy.
CD3+CD56+ NKT-like cells are a unique subpopulation of lymphocytes that not only express NK and T cell markers, but also share functional characteristics from both NK and T cells and possess both innate and adaptive immune functions. NKT-like cells can be activated through T-cell receptor (TCR) ligation.7 Meanwhile, these cells can also been activated to rapidly produce cytotoxic cytokines to kill cancer cells in a non-MHC-restricted manner.8 In addition, the effector function of NKT-like cells can be regulated by activating or inhibitory receptors. For example, tumor-derived soluble NKG2D ligands, MHC class I-related chains (MICs), impair NKT-like cell cytotoxicity in patients with ovarian cancer or prostate cancer.9 NKT-like cells have been found to be a heterogeneous subpopulation of lymphocytes, which comprise γδT cells and mucosal-associated invariant T (MAIT) cells as well as CD4+ cells and CD8+ cells.10 NKT-like cells are widely distributed in the thymus, spleen, and peripheral blood as well as enriched in normal liver tissue, reaching a high proportion of 50% in intrahepatic T cells, but there are relatively few in the peripheral blood, only accounting for 5%–15% of circulating T cells.11,12 Interestingly, NKT-like cells have been found to increase in the peripheral blood with age.13 Of note, NKT-like cells have been revealed to be involved in diverse types of diseases, including cancers, infections, and autoimmune diseases.14–19 Of note, NKT-like cells play important roles in the defense against cancers. The decrease of tumor-infiltrating NKT-like cells with impaired effector function is associated with cancer progression and poor survival of patients with gastric cancer.20 Moreover, the impairment of NKT-like cells is also associated with the progression of chronic lymphocytic leukemia and colorectal cancer,21,22 However, the characteristics and roles of NKT-like cells in HCC remain unclearly elucidated.
To investigate the phenotypic and functional properties of circulating NKT-like cells in HCC patients, we deciphered circulating NKT-like cells, including frequency, absolute count, function, and a detailed analysis of the expression of activating and inhibitory receptors on NKT-like cells from HCC patients. It was discovered that circulating NKT-like cells are functionally exhausted in HCC patients, and are characterized by the down-regulation of NKG2D, the up-regulation of PD-1, Tim-3, and CTLA-4 and impaired functionality. PD-1 blockade effectively improved effector function of NKT-like cells from HCC patients. Our findings might deepen the understanding of the properties of circulating NKT-like cells in HCC and provide new insights for the immunotherapy of HCC.
Materials and methods
Study population and samples
Peripheral blood samples of 52 HCC patients and 80 healthy donors (HD) were collected between 2017 and 2021. None of the patients selected for this study had received chemotherapy or radiotherapy. Additionally, none of the patients selected for this study had pregnancy, multiple sclerosis or history of allergies. Patients’ characteristics are summarized in Table 1. Patients were recruited from the First Affiliated Hospital of Anhui Medical University. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University, the number LLSC20170493, and the protocols were carried out in accordance with the approved guidelines. All participants gave written informed consent to participate in the study.
Table 1.
Clinicopathological correlation of the proportion of CD3+CD56+ cells in HCC
| Variables | No.of patients (%) | % CD3+CD56+ cells | P value |
|---|---|---|---|
| Age, years | 0.5869 | ||
| >57 | 14 (47) | 3.935 (1.843–7.505) | |
| ≤57 | 16 (53) | 4.185 (3.668–6.520) | |
| Gender | 0.1399 | ||
| Male | 25 (83) | 3.990 (2.315–5.915) | |
| Female | 517 | 7.310 (4.305–9.235) | |
| History of Hepatitis B | 0.9084 | ||
| Absent | 620 | 4.110 (2.878–6.863) | |
| Present | 24 (80) | 4.305 (2.248–7.115) | |
| Alpha-fetoprotein | 0.3740 | ||
| >400 μg/L | 1223 | 3.860 (1.070–6.520) | |
| ≤400 μg/L | 18 (60) | 4.420 (2.465–8.263) | |
| GGT | 0.3824 | ||
| >144 U/L | 14 (47) | 3.895 (2.383–5.528) | |
| ≤144 U/L | 16 (53) | 5.465 (2.253–7.895) | |
| ALP | 0.2673 | ||
| >146 U/L | 13 (43) | 3.720 (2.315–5.225) | |
| ≤146 U/L | 17 (57) | 5.400 (2.915–7.700) | |
| Albumin | 0.7053 | ||
| >38 g/L | 15 (50) | 4.300 (3.160–5.920) | |
| ≤38 g/L | 15 (50) | 4.070 (2.180–9.690) | |
| Total bilirubin | 0.2583 | ||
| >20 μmol/L | 924 | 3.160 (2.180–5.965) | |
| ≤20 μmol/L | 21 (70) | 4.540 (3.095–7.700) | |
| PT | 0.7979 | ||
| >12 s | 14 (47) | 4.990 (2.165–6.725) | |
| ≤12 s | 16 (53) | 4.110 (2.455–7.418) | |
| Cirrhosis | 0.7367 | ||
| Absent | 310 | 3.920 (3.720–4.300) | |
| Present | 27 (90) | 4.540 (2.180–7.310) | |
| Portal hypertension | 0.2397 | ||
| Absent | 413 | 3.820 (1.193–4.205) | |
| Present | 26 (87) | 4.775 (2.383–7.505) | |
| PVTT | 0.1466 | ||
| Absent | 21 (67) | 5.010 (3.440–6.920) | |
| Present | 925 | 2.180 (1.475–7.115) | |
| Vascular invasion | 0.7029 | ||
| Absent | 13 (43) | 4.300 (2.815–8.435) | |
| Present | 17 (57) | 4.070 (2.180–6.510) | |
| Intrahepatic metastasis | 0.0831 | ||
| Absent | 19 (63) | 5.910 (2.470–8.090) | |
| Present | 11 (47) | 3.650 (2.180–5.010) | |
| Distant metastasis | 0.1632 | ||
| Absent | 28 (93) | 4.420 (2.455–7.115) | |
| Present | 27 | 2.100 (0.550–3.650) | |
| Tumor number | 0.7475 | ||
| Single | 21 (67) | 4.540 (2.180–7.700) | |
| Multiple | 925 | 4.070 (2.805–5.965) | |
| Maximum tumor size | 0.2527 | ||
| >5 cm | 16 (53) | 3.685 (1.153–7.105) | |
| ≤5 cm | 14 (47) | 4.655 (3.730–6.920) | |
| Child-Pugh class | 0.7704 | ||
| A | 20 (67) | 4.420 (2.628–7.105) | |
| B | 1025 | 3.820 (1.843–7.320) | |
| AJCC TNM | 0.0452 | ||
| I–II | 19 (63) | 5.400 (3.720–7.310) | |
| III–IV | 11 (47) | 2.180 (0.830–4.540) |
Abbreviations: HCC, hepatocellular carcinoma; GGT, gamma-glutamyl-transpeptidase;
ALP, alkaline phosphatase; PT, prothrombin time; PVTT, portal vein tumor thrombus;
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; IQR, interquartile range;
Data are presented as n (%) orMedian(IQR, 25 to 75). Significance was assessed by Mann-Whitney test.
P < 0.05 is considered significant and P > 0.05 is considered non-significant.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of HCC patients or HD by gradient centrifugation with Ficoll-Paque (Solarbio, Beijing, China). Briefly, fresh peripheral blood was diluted with 1× PBS, gently overlaid on Ficoll-Paque, and then centrifuged at 380 × g for 20 min. After centrifugation, the middle layer was harvested and washed with 1× PBS. Lastly, PBMCs were counted in a cell counter Celldrop (DeNovix Inc., DE, USA).
Culture and stimulation of PBMCs
PBMCs were seeded in 96-well round bottom plates at a density of 5 × 105 cells/well in RPMI 1640 containing 10% fetal calf serum (Gibco, Thornton, Australia). To assess intracellular cytokine production, PBMCs were stimulated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL), ionomycin (1 μg/mL), and monensin (5 μg/mL) for 5 hours and monensin (5 μg/mL) was supplemented after the first hour. To assess the effects of anti-PD-1 mAb on NKT-like cells, PBMCs were stimulated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL), ionomycin (1 μg/mL) and monensin (5 μg/mL) for 5 hours in the presence of 20 μg/mL anti-PD-1 neutralizing antibody (JS001) or equal dose of isotype control antibody used as control. Alternatively, PBMCs were stimulated with anti-CD3 (2 μg/mL) and anti-CD28 (1 μg/mL) (Biolegend, San Diego, CA) for 6 hours in the presence of 20 μg/mL anti-PD-1 blocking antibody (J110, Bio-X Cell, Lebanon, USA), equal dose of mouse IgG1, κ isotype antibody as control. Considering the internalization of TCR/CD3 during T cell activation by anti-CD3/CD28, fluorescently-labeled antibody anti-CD3 and anti-CD56 was added and incubated for 20 min in the dark before T cell activation by anti-CD3/anti-CD28.
Flow cytometric analysis of immune cell phenotype
Single-cell suspensions of PBMCs were stained with the fluorescently-labeled antibodies, including FITC anti-human-CD3 (clone OKT3); FITC anti-human-CD3 (clone UCHT1); Brilliant Violet 510™ anti-human-CD3 (clone OKT3); Brilliant Violet 421™ anti-human-CD56 (clone 5.1H11); APC/Cy7 anti-human-CD56 (clone 5.1H11); PE anti-human-CD366 (T cell immunoglobulin and mucin domain-containing protein 3 [TIM-3], clone F38-2E2); APC/Cy7 anti-human-CD366 (TIM-3, clone F38-2E2); PerCP/Cy5.5 anti-human-CD337 (NKp30, clone P30-15); APC anti-human-CD337 (NKp30, clone P30-15); PerCP/Cy5.5 anti-human-CD335 (NKp46, clone 9E2); PE/Cy7-CD335 (NKp46, clone 9E2); PE/Cy7 anti-human-CD314 (NKG2D, clone 1D11); Brilliant Violet 510™ anti-human-CD314 (NKG2D, clone 1D11); PerCP/Cy5.5 anti-human-CD152 (CTLA-4, clone BNI3); PE anti-human-CD274 (PD-L1, clone 29E.2A3). APC anti-human-CD279 (PD-1, clone EH12.2H7); PerCP/Cy5.5 anti-human-CD8(clone RPA-T8); PE/Cy7 anti-human-CD8 (clone RPA-T8); APC anti-human-CD8 (clone HIT8a); PerCP/Cy5.5 anti-human-CD4(clone RPA-T4); PE/Cy7 anti-human-CD4 (clone RPA-T4); APC/Cy7 anti-human-CD4(clone RPA-T4); APC anti-human-TCR α/β (clone IP26); APC anti-human-TCR γ/δ (clone B1); PE anti-human-TCR γ/δ (clone B1); APC anti-human-TCR Vα7.2 (clone 3C10); Brilliant Violet 605™ anti-human-CD161 (clone HP-3G10); PE anti-human FOXP3(eBioscience, clone PCH101). The PE-CD1d-PBS157 tetramer was provided by the tetramer core facility at the National Institutes of Health. For intracellular staining of FoxP3 molecules, cells first were stained with FITC anti-human-CD3, PerCP/Cy5.5 anti-human-CD4 and APC anti-human-CD25 (clone BC96), then permeabilized and fixed using eBioscience fix/perm (eBiosciences) according to the manufacturer’s instructions. PE anti-human FOXP3 was added after permeabilization for 30 minutes. Isotype controls were used to determine cutoff levels for positive staining. Data were acquired using a flow cytometer (Beckman Coulter; Brea, CA, USA) and analyzed using FlowJo software, version 10 (Tree Star; Ashland, OR, USA).
Flow cytometric analysis of immune cell function
The stimulated PBMCs were harvested and labeled with phenotypic markers. Then these cells were intracellularly stained with PE anti-human-tumor necrosis factor (TNF)-α (clone MAb11); and PE/Cy7 anti-human-interferon (IFN)-γ (clone B27) to assess NKT-like cell function after fixation and permeabilization using fixation/permeabilization diluent (eBioscience Company). PerCP/Cy5.5 anti-human-CD107a (clone H4A3) was added into cell culture before stimulation. Isotype controls were used to determine cutoff levels for positive staining. All antibodies and isotypes were purchased from Biolegend (San Diego, CA, USA). Data were acquired using a flow cytometer (Beckman Coulter; Brea, CA, USA) and analyzed using FlowJo software, version 10 (Tree Star; Ashland, OR, USA).
Statistical analysis
Data are shown as median and interquartile ranges (interquartile range, 25 to 75). The difference between groups was analyzed using Mann-Whitney test or Wilcoxon matched-pairs signed rank test. Any potential correlations between variables were analyzed using Pearson’s correlation test. Analyses yielding P-values <0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software Inc., CA,USA).
Results
NKT-like cells were functionally defective in HCC patients
To access the alteration of CD3+CD56+ NKT-like cells in HCC patients, the frequency and absolute count of NKT-like cells in peripheral blood from HCC patients and HD were firstly analyzed. We found that the frequency and absolute count of NKT-like cells in HCC patients were comparable with those in HD (Figure 1(a,b)). NKT-like cells were composed of CD8+ cells and CD4+ cells as well as αβT cells, γδT cells, mucosal-associated invariant T (MAIT) cells and a few invariant NKT (iNKT) cells (sFigure 1(a)). Most of NKT-like cells were CD8+ T cells. The frequencies of these NKT-like cell subsets were comparable between HCC patients and HD (Figure 1(c,d)). However, the frequency of NKT-like cells was lower in patients with stage III/IV HCC than that with stage I/II HCC (Table 1), indicating higher cancer grades were associated with reduced frequency of circulating NKT-like cells. Besides NKT-like cells, the absolute count of lymphocytes as well as the frequencies of lymphocytes, CD3+CD56− T cells and CD3+CD8+CD56− T cells were comparable between HCC patients and HD (sFigure 1(b-f)). However, NK cells were decreased, whereas regulatory T cells were increased in HCC patients (sFigure 1(g-h)), consistent with previous reports.26,27 Interestingly, we found that NKT-like cells displayed stronger effector function than conventional CD3+CD56− T cells (sFigure 2(a)). Moreover, CD8+ NKT-like cells displayed stronger effector function than conventional CD3+CD8+CD56− T cells (sFigure 2(a)).We further access the alteration of effector function of NKT-like cells in HCC patients. The frequencies of TNF-α+ and IFN-γ+ NKT-like cells in HCC patients were lower than those in HD (Figure 1(e-f)), suggesting that the production of cytokines was impaired in NKT-like cells from HCC patients. The frequency of CD107a+ NKT-like cells was lower in HCC patients when compared with HD (Figure 1(g)), suggesting that the cytotoxicity of NKT-like cells was decreased. The functional impairment was observed in CD3+CD56− T cells in HCC patients (sFigure 2(b)). These results reveal that NKT-like cells were functionally impaired in HCC patients.
Figure 1.
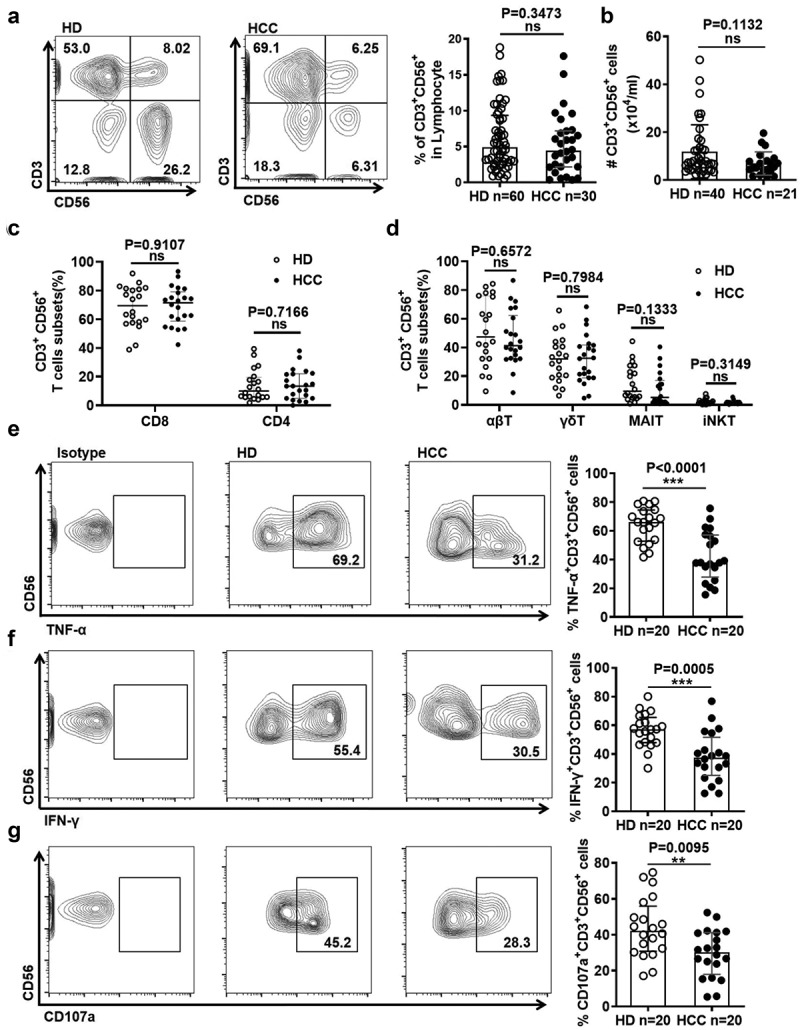
NKT-like cells are functionally impaired in HCC patients
Frequency (A) and absolute count (B) of NKT-like cells in HCC patients and HD. Frequency of CD8+ and CD4+ subsets (C) as well as αβT, γδT, MAIT and iNKT subsets (D) in NKT-like cells in HCC patients and HD. Frequency of (E) TNF-α+ cells, (F) IFN-γ+ cells, and (G) CD107a+ cells in NKT-like cells in HCC patients and HD. Data are shown as means ± standard deviation (s.d.). P-values < 0.05 are considered statistically significant. *P < .05; **P < .01; ***P < .001.
Figure 2.
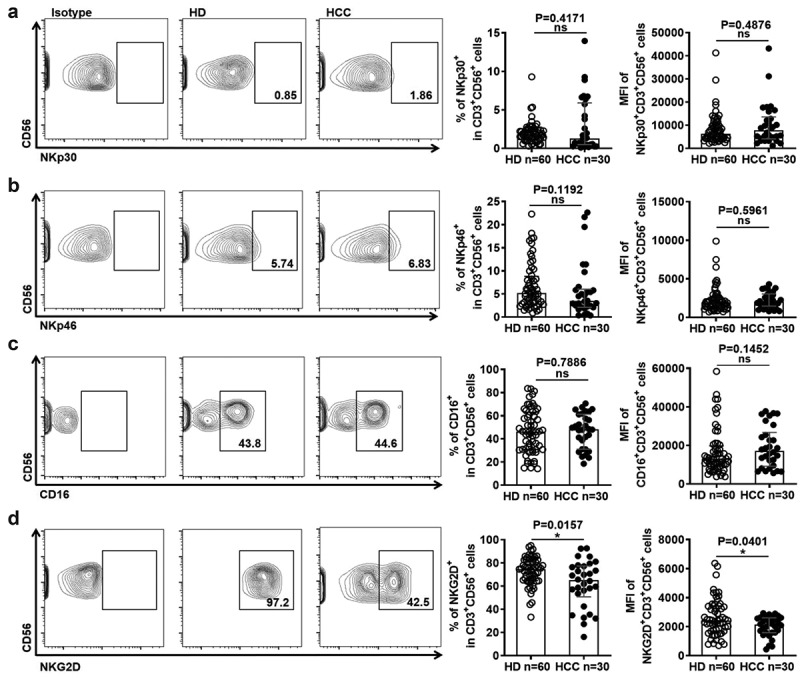
NKT-like cells in HCC patients display lower levels of activating receptors
(A) Frequency of NKp30+ NKT-like cells and MFI of NKp30 on NKT-like cells, (B) frequency of NKp46+ NKT-like cells and MFI of NKp46 on NKT-like cells, (C) frequency of CD16+ NKT-like cells and MFI of CD16 on NKT-like cells, and (D) frequency of NKG2D+ NKT-like cells and MFI of NKG2D on NKT-like cells in HCC patients and HD. Data are shown as means ± standard deviation (s.d.). P-values < 0.05 are considered statistically significant. *P < .05; **P < .01; ***P < .001.
NKT-like cells displayed an exhausted phenotype in HCC patients
Besides effector function, we also examined the alteration of activating receptors and inhibitory receptors on NKT-like cells in HCC patients. As shown in Figure 2, there were no differences in the expression of activating receptors including NKp30, NKp44, and CD16 (Figure 2(a–c)). However, the expression of activating receptors NKG2D on NKT-like cells was significantly reduced in HCC patients (Figure 2(d)). In contrast, the expression of Tim-3, CTLA-4, and PD-1 on NKT-like cell subsets was significantly increased in HCC patients compared with HD (Figure 3(a-c)). More Tim-3+CTLA-4+PD-1+ NKT-like cells appeared in HCC patients (Figure 3(d)), Besides NKT-like cells, the expression of these inhibitory receptors were found to be increased in the CD3+ T cells, CD8+ T cells, and NK cells from HCC patients, compared to HD (sFigure 3(a-c)). Interestingly, the expression of PD-L1 was up-regulated on NKT-like cells in HCC patients (sFigure 4). These results reveal that suggesting the levels of inhibitory receptors were increased increased on NKT cells in HCC patients.
Figure 3.
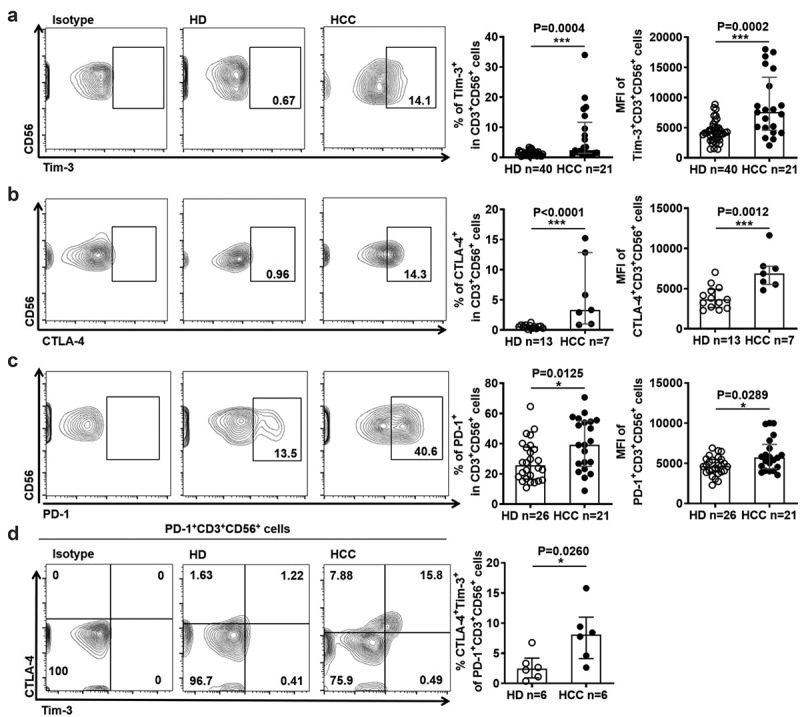
NKT-like cells in HCC patients express higher levels of inhibitory receptors
(A) Frequency of Tim-3+ NKT-like cells and MFI of Tim-3 on NKT-like cells, (B) frequency of CTLA-4+ NKT-like cells and MFI of CTLA-4 on NKT-like cells, (C) frequency of PD-1+ NKT-like cells and MFI of PD-1 on NKT-like cells in HCC patients and HD. (D) Frequency of Tim-3+ CTLA-4+ PD-1+ NKT-like cells in HCC patients and HD. Data are shown as means ± standard deviation (s.d.). P-values < 0.05 are considered statistically significant. *P < .05; **P < .01; ***P < .001.
Figure 4.
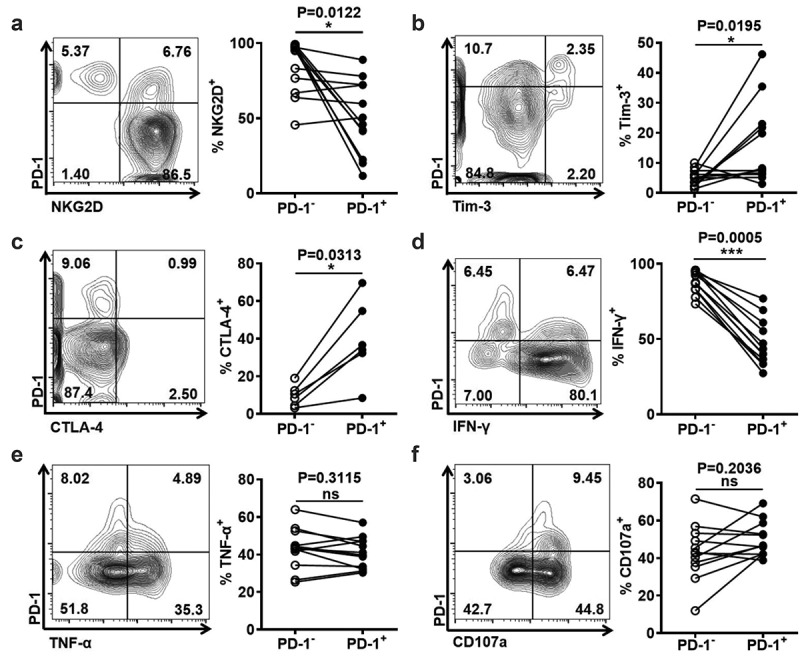
PD-1+ NKT-like cells are more exhausted than PD-1− NKT-like cells
Frequency of (A) NKG2D+ cells, (B) TIM-3+ cells, (C) CTLA-4+ cells, (D) IFN-γ+ cells, (E) TNF-α+ cells, and (F) CD107a+ cells in PD-1+ NKT-like cells and PD-1− NKT-like cells. P-values < 0.05 are considered statistically significant. *P < .05; **P < .01; ***P < .001.
PD-1+ NKT-like cells were more exhausted than PD-1− NKT-like cells
Among these inhibitory receptors, the levels of PD-1 were highest on NKT-like cells in HCC patients. Therefore, we accessed the effect of PD-1 up-regulation on NKT-like cells. We found that PD-1+ NKT-like cells had a lower expression of activating receptor NKG2D and a higher expression of inhibitory receptors Tim-3 and CTLA-4, compared with PD-1− NKT-like cells (Figure 4(a–c)). Moreover, PD-1+ NKT-like cells expressed less IFN-γ, although the levels of TNF-α and CD107a in the PD-1+ NKT-like subset were similar to those in the PD-1− subset (Figure 4(d–f)). These data reveal that PD-1+ NKT-like cells have more exhausted phenotype and more impaired function, suggesting increased PD-1 suppresses the effector function of NKT-like cells in HCC patients.
PD-1 blockade improved function of exhausted NKT-like cells from HCC patients
The up-regulation of PD-1 on NKT-like cells in HCC patients suggested that PD-1 was a potential target for improving NKT-like cell effector function. To evaluate this possibility, anti-PD-1 blocking antibody was used ex vivo before stimulating NKT-like cells from HD. Our data showed that the production of TNF-α and IFN-γ in NKT-like cells was increased in the presence of anti-PD-1 blocking antibody and stimulation with PMA/Ion (Figure 5(a,b)). Similarly, increased levels of TNF-α and IFN-γ and CD107a in NKT-like cells were also observed in the presence of anti-PD-1 mAb when stimulated with anti-CD3/anti-CD28 (Figure 5(c–e)). To confirm the possibility that the blockade of PD-1 improved NKT-like cells in HCC patients, the effector function of NKT-like cells in HCC patients was measured in the presence of anti-PD-1 blocking antibody. As expected, increased levels of TNF-α and IFN-γ in NKT-like cells from HCC patients were observed in the presence of anti-PD-1 mAb and stimulation with PMA/Ion (Figure 6(a,b)). Moreover, levels of TNF-α and IFN-γ and CD107a were also increased in NKT-like cells from HCC patients in the presence of anti-PD-1 mAb when stimulated with anti-CD3/anti-CD28 (Figure 6(c–e)). Of note, the increase in NKT-like cells functionality was conversely correlated with initial PD-1 levels (sFigure 5(a,b)). The short-term PD-1 blockade failed to influence the levels of Tim-3 or CTLA-4 on NKT-like cells (sFigure 5(c,d)). These findings demonstrated that blocking the PD-1/PD-L1 pathway could improve the exhausted effector function of NKT-like cells in HCC patients.
Figure 5.
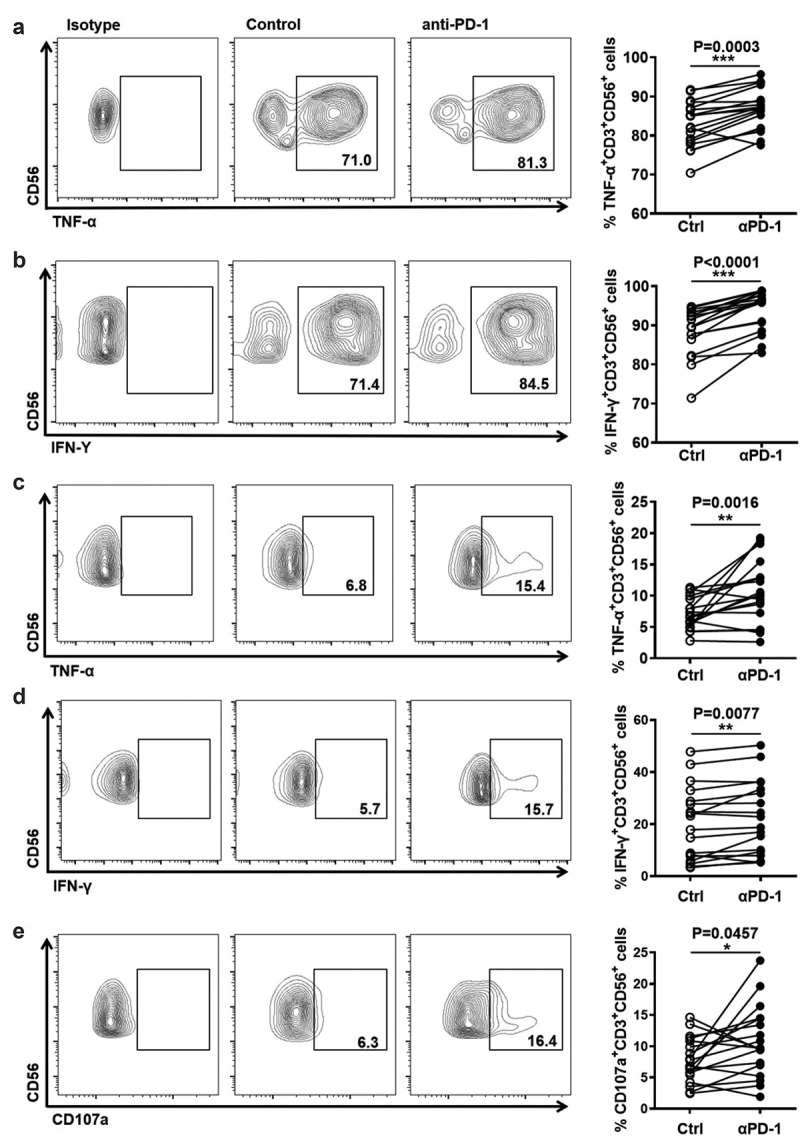
PD-1 blockade improves the function of NKT-like cells from healthy donors
Frequency of (A) TNF-α+ cells in NKT-like cells and (B) IFN-γ+ cells in NKT-like cells from HD in the presence of anti-PD-1 mAb or isotype mAb. PBMCs from healthy donors and were stimulated with PMA (100 ng/mL) and ionomycin (1 μg/mL) for 5 hours in the presence of 20 μg/mL anti-PD-1 mAb or an equal dose of mouse IgG1, κ isotype antibody. Frequency of (C) TNF-α+ cells, (D) IFN-γ+ cells, and (E) CD107a+ cells in NKT-like cells from HD in the presence of anti-PD-1 mAb or isotype mAb. PBMCs from HD were stimulated with anti-CD3 (2 μg/mL)/anti-CD28 (1 μg/mL) for 6 hours in the presence of 20 μg/mL anti-PD-1 mAb or an equal dose of mouse IgG1, κ isotype antibody. P-values < 0.05 are considered statistically significant. *P < .05; **P < .01; ***P < .001.
Figure 6.
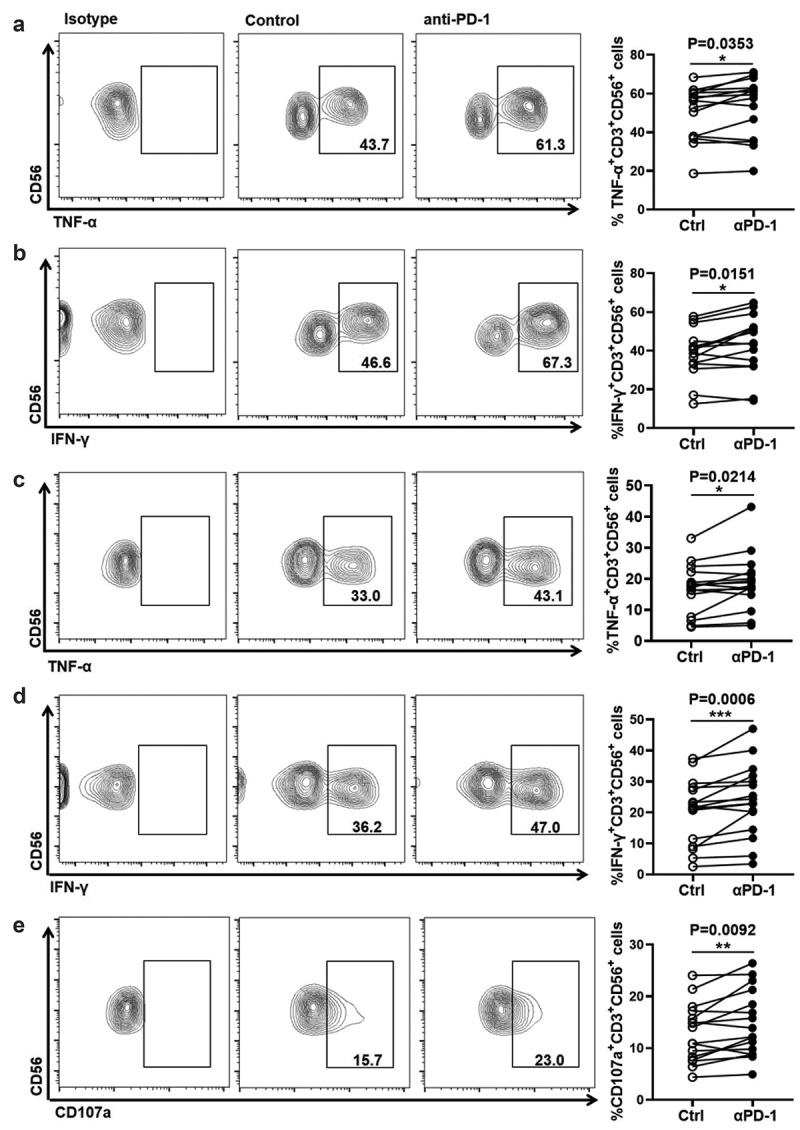
Anti-PD-1 treatment improves the effector function of NKT-like cells from HCC patients
Frequency of (A) TNF-α+ cells and (B) IFN-γ+ cells in NKT-like cells from HCC patients in the presence of anti-PD-1 mAb or isotype mAb. PBMCs from HCC patients were stimulated with PMA (100 ng/mL), ionomycin (1 μg/mL) for 5 hours was in the presence of 20 μg/mL anti-PD-1 mAb or equal dose of mouse IgG1, κ isotype antibody. Frequency of (C) TNF-α+ cells, (D) IFN-γ+ cells, and (E) CD107a+ cells in NKT-like cells from HCC patients in the presence of anti-PD-1 mAb or isotype mAb. PBMCs from HCC patients were stimulated with anti-CD3 (2 μg/mL)/anti-CD28 (1 μg/mL) for 6 hours in the presence of 20 μg/mL anti-PD-1 mAb or an equal dose of mouse IgG1, κ isotype antibody. P-values < 0.05 are considered statistically significant. *P < .05; **P < .01; ***P < .001.
Discussion
The fact that not all HCC patients benefit from immunotherapy, stimulated our investigation of the properties of distinct immune cell subpopulations in HCC. In the present study, we showed that circulating CD3+CD56+ NKT-like cells were exhausted in HCC patients and exposure to anti-PD-1 mAb was capable of improving the function of circulating NKT-like cells, indicating NKT-like cells might be a target for HCC immunotherapy.
NKT-like cells are recognized as a heterogeneous subpopulation of lymphocytes.10 In our study, we confirmed that NKT-like cells comprise αβT cells, γδT cells, MAIT cells and a few iNKT cells as well as CD4+ cells and CD8+ cells. Of note, NKT-like cells are mainly composed of CD8+ cells. Consistent with the finding that CD56 expression is associated with enhanced cytotoxicity and cytokine production of CD3+ T cells,28 we found that NKT-like cells had higher function than CD3+CD56− T cells. Therefore, this population might be important for defense against HCC albeit of TCR heterogeneity.
The number of NKT-like cells infiltrating HCC tissues is much fewer than those present in adjacent tissues.29 Moreover, recent research has suggested that a higher percentage of NKT-like cells was associated with a higher overall survival rate in HCC patients.30 These findings suggest that NKT-like cells play critical roles in HCC incidence and development. However, studies evaluating NKT-like cells in HCC patients is very limited. Our data showed that the percentages and absolute counts of circulating NKT-like cells was comparable between HCC patients and HD. But higher cancer grades were associated with reduced percentage of circulating NKT-like cells, shown by lower percentage of circulating NKT-like cells in patients with stage III/IV HCC when compared with those with stage I/II HCC. In term of function, the production of TNF-α and IFN-γ was decreased in NKT-like cells of HCC patients. Moreover, although no change in KIR2DL and CD94 expression on circulating NKT-like cells in HCC patients was reported,17 the expression of NKG2D on was decreased on NKT-like cells in HCC patients. Inhibitory checkpoint molecules PD-1 and TIM-3 serve as exhaustion markers of T cells and CTLA-4 can suppress anti-tumor immune responses in solid tumors.24,31,32 Our findings showed that Tim-3, CTLA-4, and PD-1 were increased on NKT-like cells in HCC patients compared with HD, which indicated that these inhibitory receptors might contribute to the suppression of NKT-like cell function in HCC patients. However, more studies are needed to investigate the association between circulating NKT-like cells and HCC progression and the role and mechanisms of circulating NKT-like cells in defense against HCC.
PD-1 is an inhibitory receptor involved in regulating host immune responses.33 The increased expression of PD-1 on CD8+ T cells, CD4+ T cells, and NK cells contributes to the impaired function of these cells and correlates with poor prognosis in patients with HCC.25,34,35 PD-L1 has been found to be up-regulated on cancer cells in patients with HCC.36 Increasing evidence shows that PD-L1 on tumor-infiltrating immune cells rather than tumor cells is increased and suppresses anti-tumor immune response.37–39 In the present study, we found the expression of PD-1 was increased on NKT-like cells in HCC patients compared with HD. PD-1+ NKT-like cells produced less IFN-γ and had lower expression of NKG2D and higher expression of Tim-3 and CTLA-4. Meanwhile, PD-L1 was also increased on NKT-like cells in patients with HCC. These results suggested PD-1 could play an important role in mediating functional exhaustion of NKT-like cells in HCC patients. TCR and cytokine-mediated immune activation can induce PD-1 expression on T cells.40 However, the factors mediating the up-regulation of PD-1 and PD-L1 on NKT-like cells dysfunction in HCC patients need further investigation.
The clinical success of anti-PD-1/PD-L1 therapy in a variety of cancers has demonstrated that the PD-1/PD-L1 axis plays an important role in regulating the antitumor immune response. Blocking the PD-1/PD-L1 axis could effectively enhance the function and antitumor efficacy of CD8+ T cells in HCC.23 However, the clinical efficacy of anti-PD-1 therapy is limited to a small subset of HCC patients, with overall response rates of 20% or less.6 More in depth investigation of the PD-1/PD-L1 pathway might aid the discovery and design of new clinically applicable approaches in HCC immunotherapy. In the present study, we found that blocking the PD-1 pathway effectively enhanced TNF-α, IFN-γ, and CD107a levels in NKT-like cells. However, additional studies are required to elucidate the precise mechanisms for improving NKT-like cell function through blocking the PD-1/PD-L1 axis.
In conclusion, our study showed that NKT-like cells displayed an exhausted phenotype and impaired immune activity. Anti-PD-1 drugs could improve the effector function of NKT-like cells. These findings might provide new insights on a NKT-like cell-based therapeutic strategy for the treatment of HCC.
Supplementary Material
Acknowledgments
Publication of this manuscript was supported by the Natural Science Foundation of China (#82070608, #31872741 and #81900539), and the Research Improvement Program of Anhui Medical University (#2019xkjT007).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
J.L. and X.W. conceived the study, designed the experiments and wrote the manuscript. L.T., S.W., and G.K. performed the experiments and analyzed the data. S.J., W.Y. and L.Z. reviewed and revised the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, and Bray F.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3): 209–11. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. The Lancet. 2018;391(10127):1301–12. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 update. Oncology. 2017;93(Suppl. 1):147–159. doi: 10.1159/000481245. [DOI] [PubMed] [Google Scholar]
- 5.Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, and Tang W, . Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18(1):1–12. doi: 10.1186/s12943-019-1047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Galarreta MR, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, and Dhainaut M, et al. β-catenin activation promotes immune escape and resistance to anti–PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, and O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+ CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163(4):2314–2321. [PubMed] [Google Scholar]
- 8.Lepore M, Mori L, De Libero G. The Conventional Nature of Non-MHC-Restricted T Cells. Front Immunol. 2018;9:1365. doi: 10.3389/fimmu.2018.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Yang D, Xu W, Wang Y, Ruan Z, Zhao T, Han J, Wu Y. Tumor-derived soluble MICs impair CD3(+)CD56(+) NKT-like cell cytotoxicity in cancer patients. Immunol Lett. 2008;120(1–2):65–71. doi: 10.1016/j.imlet.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Olmedo AJ, Schulz AR, Huber M, Brehm CU, Chang H-D, Chiarolla CM, Bopp T, Skevaki C, Berberich‐Siebelt F, Radbruch A, et al. Deep phenotypical characterization of human CD3+CD56+T cells by mass cytometry. Eur J Immunol. 2021;51(3):672–681. doi: 10.1002/eji.202048941. [DOI] [PubMed] [Google Scholar]
- 11.Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Vα24-JαQ and γδ T cell receptor bearing cells. Hum Immunol. 1999;60(1):20–31. doi: 10.1016/S0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 12.Golden-Mason L, Castelblanco N, O’Farrelly C, Rosen HR. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol. 2007;81(17):9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peralbo E, Alonso C, Solana R. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42(8):703–708. doi: 10.1016/j.exger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Kawarabayashi N, Seki, S, Hatsuse, K, Ohkawa, T, Koike, Y, Aihara, T, Habu, Y, Nakagawa, R, Ami, K, and Mochizuki, H. Decrease of CD56+ T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32(5):962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava R, Aggarwal R, Bhagat M, Chowdhury A, Naik S. Alterations in natural killer cells and natural killer T cells during acute viral hepatitis E. J Viral Hepat. 2008;15(12):910–916. doi: 10.1111/j.1365-2893.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 16.Okumura A, Ishikawa T, Maeno T, Sato K, Ayada M, Hotta N, Yamauchi T, Fukuzawa Y, Kakumu S. Changes in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol Res. 2005;32(4):213–217. doi: 10.1016/j.hepres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Yuen M-F, Norris S. Expression of inhibitory receptors in natural killer (CD3− CD56+) cells and CD3+ CD56+ cells in the peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Clinical Immunol. 2001;101(3):264–269. doi: 10.1006/clim.2001.5110. [DOI] [PubMed] [Google Scholar]
- 18.Almeida J-S, Couceiro P, López-Sejas N, Alves V, Růžičková L, Tarazona R, Solana R, Freitas-Tavares P, Santos-Rosa M, Rodrigues-Santos P, et al. NKT-Like (CD3+ CD56+) Cells in Chronic Myeloid Leukemia Patients Treated With Tyrosine Kinase Inhibitors. Front Immunol. 2019;10:2493. doi: 10.3389/fimmu.2019.02493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Xu, W, Chen, Z, Zhang, H, Zhang, S, Lian, C, Sun, J, Chen, H, and Zhang, F. Aberrant distribution of CD3+CD56+ NKT-like cells in patients with primary Sjogren’s syndrome. Clin Exp Rheumatol. 2021;39(1):98–104. [DOI] [PubMed] [Google Scholar]
- 20.Peng LS, Mao F-Y, Zhao Y-L, Wang -T-T, Chen N, Zhang J-Y, Cheng P, Li W-H, Lv Y-P, Teng Y-S, et al. Altered phenotypic and functional characteristics of CD3+CD56+ NKT-like cells in human gastric cancer. Oncotarget. 2016;7(34):55222–55230. doi: 10.18632/oncotarget.10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojarska-Junak A, Hus I, Sieklucka M, Wasik-Szczepanek E, Mazurkiewicz T, Polak P, Dmoszynska A, and Rolinski J. Natural killer-like T CD3+/CD16+CD56+ cells in chronic lymphocytic leukemia: intracellular cytokine expression and relationship with clinical outcome. Oncol Rep. 2010;24(3):803–810. doi: 10.3892/or_00000924. [DOI] [PubMed] [Google Scholar]
- 22.Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, Vahrmeijer AL, van de Velde CJH, Heemskerk MHM, Hokland M, et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol, Immunother: CII. 2019;68(6):1011–1024. doi: 10.1007/s00262-019-02343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Song Z, Xiao J, Liu X, Luo Y, Yang Z, Luo R, Li A. Blocking the PD-1/PD-L1 axis in dendritic cell-stimulated cytokine-induced killer cells with pembrolizumab enhances their therapeutic effects against hepatocellular carcinoma. J Cancer. 2019;10(11):2578. doi: 10.7150/jca.26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp & Clin Cancer Res. 2018;37(1):1–12. doi: 10.1186/s13046-018-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, Peng J, Gao L, Liang X, Ma C, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36(44):6143–6153. doi: 10.1038/onc.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 27.Tao L, Wang S, Yang L, Jiang L, Li J, Wang X. Reduced Siglec-7 expression on NK cells predicts NK cell dysfunction in primary hepatocellular carcinoma. Clin Exp Immunol. 2020;201(2):161–170. doi: 10.1111/cei.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the Immune System: more Than a Marker for Cytotoxicity? Front Immunol. 2017;8:892. doi: 10.3389/fimmu.2017.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Peng J, Pang Y, Yu S, Yu X, Chen P, Wang W, Han W, Zhang J, and Yin Y, et al. Identification of a FOXP3+ CD3+ CD56+ population with immunosuppressive function in cancer tissues of human hepatocellular carcinoma. Sci Rep. 2015;5(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li -T-T, Sun J, Wang Q, Li W-G, He W-P, Yang R-C, Duan X-Z. The effects of stereotactic body radiotherapy on peripheral natural killer and CD3+ CD56+ NKT-like cells in patients with hepatocellular carcinoma. Hepatobiliary & Pancreatic Dis Int. 2021;20(3):240–250. doi: 10.1016/j.hbpd.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallois A, Silva I, Osman I, Bhardwaj N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology. 2014;3(12):e946365. doi: 10.4161/21624011.2014.946365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He G, Zhang H, Zhou J, Wang B, Chen Y, Kong Y, Xie X, Wang X, Fei R, Wei L, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp & Clin Cancer Res. 2015;34(1):1–11. doi: 10.1186/s13046-015-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi F, Shi M, Zeng Z, Qi R-Z, Liu Z-W, Zhang J-Y, Yang Y-P, Tien P, Wang F-S. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128(4):887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 36.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani E-S, Laurent A, Azoulay D, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship With clinical and pathological features. Hepatology. 2016;64(6):2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 37.Hartley GP, Chow L, Ammons DT, Wheat WH, Dow SW. Programmed Cell Death Ligand 1 (PD-L1) Signaling Regulates Macrophage Proliferation and Activation. Cancer Immunol Res. 2018;6(10):1260–1273. doi: 10.1158/2326-6066.CIR-17-0537. [DOI] [PubMed] [Google Scholar]
- 38.Diskin B, Adam S, Cassini MF, Sanchez G, Liria M, Aykut B, Buttar C, Li E, Sundberg B, Salas RD, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21(4):442–454. doi: 10.1038/s41590-020-0620-x. [DOI] [PubMed] [Google Scholar]
- 39.Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, Guo J, Peng H, Chen M, Fu Y-X, et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020;11(1):4835. doi: 10.1038/s41467-020-18570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. The J Immunol. 2008;181(10):6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


