ABSTRACT
Cell invasion is associated with numerous patho-physiologic states including cell development and metastatic dissemination. This process couples the activation of cell motility with the capacity to degrade the extracellular matrix, thereby permitting cells to pass through basal membranes. Invasion is sustained by the actions of invadosomes, an ensemble of subcellular structures with high functional homology. Invadosomes are 3D acto-adhesive structures that can also mediate local extracellular matrix degradation through the controlled delivery of proteases. Intracellular RHO GTPases play a central role in the regulation of invadosomes where their complex interplay regulates multiple invadosome functions. This review aims to provide an overview of the synergistic activities of the small GTPases in invadosome biology. This broad-based review also reinforces the importance of the spatiotemporal regulation of small GTPases and the impact of this process on invadosome dynamics.
KEYWORDS: Small GTPase, invadosomes, invadopodia, spatiotemporal signalling, podosomes, linear invadosomes
1. Introduction
The discovery of oncogenes in the 1980s highlighted the importance of cell signalling and its role in regulating physiologic and pathologic transitions. Critical cell signalling events have been identified that contribute to tumour progression. Taken together, these events provide examples of how the rewiring of multiple signalling pathways supports proliferation, senescence, metabolic changes, and metastatic dissemination of tumour cells. The discovery of the small GTPase, RAS, highlighted the role of this class of signalling molecules in the pathophysiology of cancer[1]. In many cases, a primary tumour is not the main cause of death; rather, death ensues as a direct result of associated metastatic cellular spread. Advances in in vivo imaging have highlighted different dissemination strategies that are largely based on the type of cancer and stage of tumour progression. In most cases, cell dissemination is only possible when the basal membrane surrounding the primary tumour site is breached. This activity is promoted by F-actin-dependent subcellular structures known as invadosomes, which are critical for adhesion, protrusion, and local degradation of the extracellular membrane (ECM). Since cytoskeletal remodelling is an essential component of invadosome formation and function, these structures are also regulated by the members of the small GTPases superfamily. Our review aims to summarize the most recent developments that focus on the role of small GTPases and their capacity to regulate the formation and function of invadosomes. We also aim to provide a general overview of the complex downstream signalling pathways that involve several of the small GTPases as well as their role in induction and coordination of invadosome function.
2. The invadosome superfamily
The invadosome superfamily is a group of distinct subcellular structures that include podosomes, invadopodia, and linear invadosomes. Each of the invadasomes presents different morphological features and varying patterns of formation depending on the cell type of origin [2]. Podosomes have been identified in cells that constitutively overexpress active SRC mutations and in non-transformed cells including dendritic cells, macrophages, endothelial cells, vascular smooth muscle cells, megakaryocytes, and osteoclasts [3]. Invadopodia have been identified in cancer cells and can promote tissue degradation [4]. Linear invadosomes are subcellular structures that are formed in direct association with thick bundles of collagen I in both transformed and primary cells [5].
Invadosomes have been detected throughout the biological spectrum, from species extending from the nematode, Caenorhabditis elegans, to humans, and serve to promote numerous functions in vivo, including development [6], immune system scanning, vascular reorganization, and bone remodelling [7,8]. Invadosomes have also been implicated in numerous pathological states including in vivo extravasation in the chick chorioallantoic membrane (CAM assay) and breast cancer metastasis in the MMTV-PyMT mouse model. Invadosomes have also been identified in tumours from surgical specimens from the human pancreas (i.e., pancreatic adenocarcinoma) as well as from other organs [6,9].
Invadosome acto-adhesive and mechanosensitive functions
The invadosome unit is a three-dimensional acto-adhesive structure (since higher than large) that is comprised of a dense F-actin core surrounded by a ring of adhesion molecules that colocalize with radial F-actin filaments extending from the actin core and positioned parallel to the substratum (i.e., the actin cloud) [10–12]. The term ‘acto-adhesive function’ implies the capacity to recognize specific components of the ECM; this is supported by the observed aggregation of numerous ECM receptors including CD44, integrins (β1, β2, β3, and β5), and discoidin domain receptors (DDRs). The specific three-dimensional organization of the invadosome acto-adhesive components suggests the existence of original underlying molecular mechanisms that are specific to invadosomes and not shared with other adhesive structures such as those promoting focal adhesion or cell-cell contacts. In contrast to focal adhesions, invadosomes develop contractile mechanisms, although these are currently not well understood. For example, invadosomes can be formed independently of the Myosin II, but do require other contractile components, such as atypical Myosin I [13]. Invadosomes develop in response to the mechanical properties of their local environments, thereby defining their mechanosensitivity [14,15].
Invadosomes are also characterized by their collective behaviour, as individual units can assemble into large and dynamic meta-structures such as rings or large shafts along collagen fibres. Large invadosome meta structures, including rings (also known as rosettes), can expand in diameter, fuse with one another, and ultimately disappear due to continuous remodelling; the latter feature involves the coordinated assembly of new invadosome units at the outer rim and disassembly of older ones at the inner rim [10,16,17]. By contrast, the precise ultrastructure of the functional unit of linear invadosomes has not yet been characterized, although this structure also depends on the three-dimensional organization of actin organization and is associated with adhesion (both DDR-dependent or independent) and degradative properties [5,18].
The degradative function of invadosomes at the ECM
Invadosomes promote tissue degradation via their capacity for localized secretion of matrix metalloproteinases (MMPs). Two specific types of MMPs have been described in association with invadosomes, including membrane-anchored MMPs (MT1-MMP/MMP14 to MT6-MMP) that are released directly during intracellular trafficking and activation of secretory pathways, and proenzyme MMPs (pro-MMPs; MMP2, MMP9, and others) that are secreted in the pericellular environment and are subsequently activated by a feed-back loop triggered by membrane-anchored MMPs [19]. Thus, control of the MMP exocytosis in the immediate vicinity of an invadosome is a critical step underlying coupling between acto-adhesion and ECM degradation. This overall process has been characterized as degradation-on-demand (DOD) or acto-adhesion and degradation coupling (ADC) [20,21]. Adaptor proteins, including the calmodulin (CaM)-dependent GTPase protein, IQGAP1, are particularly important factors underlying the formation of exocyst complexes that transport MT1-MMP from intracellular vesicles to the plasma membrane. Hic-5, a member of the paxillin adaptor family, can bind IQGAP1 as well as to other regulatory GTPases to form a bridge between the exocyst complexes involved in regulating MT1-MMP release and critical adhesive structures [22,23].
3. Small RHO GTPases and invadosomes
Actin organization and the precise coupling between acto-adhesive and ECM degradative functions suggest that the invadosome is a dynamic structure with the capacity to respond to a finely-tuned and coordinated series of signalling events. There is particular interest in the potential role for small GTPases, as these signalling proteins have been implicated in the regulation of adhesion, intracellular trafficking, and modulation of the cytoskeleton. As such, we will summarize the findings from studies that have highlighted the importance of various small GTPases specifically with respect to the structure and function of invadosomes. There are 20 RHO family proteins under the control of guanine nucleotide exchange factors (GEFS) and GTPase-activating proteins (GAPS) that have been divided into 8 groups; these proteins include RHOA, RAC, and CDC42 as well as atypical family members that are regulated instead by kinases or proteasome-based degradation [24–27].
CDC42
Mutations in a CDC42-binding WAS protein (WASP) result in Wiskott-Aldrich syndrome (WAS), which is a disorder characterized by the phenotypic triad known as ‘immunodeficiency-eczema-thrombopenia’; this finding highlighted the importance of CDC42 and the larger family of RHO GTPases in regulation of invadosome function. WASP includes a small-GTPase binding Domain (GBD) that facilitates interactions with the GTPases, Rac, and CDC4228. Actions of Rac and CDC42 result in two characteristic actin structures, including one that is largely spread with thin protrusions forming lamellipodia, and another with rigid microspikes that form filopodia, respectively. The substantial morphologic homology between filopodia and invadosomes has provided impetus towards identifying a role for CDC42 in invadosomes formation. Interestingly, expression of the constitutively active mutant of CDC42, V12Cdc42, was sufficient stimulus for activation of invasive processes in cancer cells and the formation of invadosomes in endothelial cells [28–30]. Moreover, inhibition of CDC42 activation blocked invadosomes that form in response to phorbol ester or to the administration of transforming growth factor-beta (TGF-beta), that classicaly induces epithelial-mesenchymal transition (EMT); remarkably, CDC42 blockade also prevented the formation of physiologic invadosomes in myeloid cell targets [31–34]. Constitutively active mutants of the other CDC42 sub-family members, including RHOJ (TCL) and RHOQ (TC10) induced invadosome formation in endothelial cells [28,35–37].
As detailed above, there is a strong link between CDC42 and invadosome formation. CDC42 is one of the few molecules (including mutant forms of the tyrosine kinases, v-SRC and v-Fes) that induce the rapid formation of invadosomes in any cells when mutated into its active form [3]. In contrast to results observed with other GTPases, this observation defines CDC42 as one of the few drivers of invadosome formation [38]. CDC42 may also regulate various types of invadosomes, including podosomes, invadopodia, and even linear invadosomes [38,39]. However, the expression of the active mutant of CDC42, V12CDC42, in myeloid cells forming invadosomes resulted in structural disorganization; specifically, expression of V12CDC42 altered the distribution of invadosomes in both dendritic cells and macrophages [33,40]. These findings stand in clear support of hypotheses pointing to the need for optimal or precise control of CDC42 signalling activity to maintain organized invadosome function. Moreover, overexpression of CDC42 can induce degradative functions, but only if the level of expression of the scaffolding regulator, Tks5, remains sufficient [38]. The Tks5/Fish adaptor protein is required to promote the formation and function of all types of invadosomes, as it is involved in the generation of specific protein complexes implicated in ECM degradation and has been associated with the regulation of reactive oxygen species (ROS) [41,42]. Taken together, these results reveal that, while CDC42 is important and notably essential for acto-adhesive activity, it is not the sole critical factor involved in invadosome formation and function.
RHO
Studies that revealed the link between the formation of specific actin structures (filopodia, lamellipodia, stress fibres) and the activity of specific small GTPases [43] and others that focused on the essential role of CDC42 in processes associated with invadosome formation raised questions regarding the relative importance of the other small GTPases. Results from ongoing studies indicate that invadosomes were sites of both local and extended interplay among numerous RHO-family GTPases. Among these findings, invadosome rings formed in cells transformed with the tyrosine kinase, v-Src, accumulated RHOs proteins, as detected by targeting the glutathione-S-transferase (GST)-tagged RHO-specific GTPase binding domain (GST-RBD) [44]. In primary myeloid cells, silencing or inhibition of RHO proteins via specific ribosylation by the exoenzyme C3 induced loss of invadosomes; these results confirmed the functional importance of RHOs in this setting [33,45–47]. Interestingly, mouse macrophages that are capable of forming physiologic invadosomes express mostly RHOA and RHOB. Studies in RHOB−/- cells with exoenzyme C3 (i.e., introduction into the cell via TAT-C3, an enzyme that specifically ribosylates and inactivates RHOs proteins) revealed a specific role for RHOA in both formation and regulation of invadosomes [48]. The overall importance of RHO activity with respect to invadosome formation was confirmed in various cell models in which exoenzyme C3 used to inactivate RHO proteins also prevented the induction of invadosome formation [49,50].
As in the case of CDC42, precise and optimal regulation of RHOA activity is critical for appropriate invadosome dynamics. For example, a constitutively-active RHOA mutant, V14RHOA, cannot induce invadosome formation but can serve to inhibit this response [28,45,51]. Indeed, RHOA activation at the whole-cell level induces an overall increase in Myosin II-dependent cell contractility and induction of stress fibres that serve to negatively regulate invadosome formation. Control of RHO activation is essential for the regulation of the F-actin content and size of activated invadosomes. Treatment with exoenzyme C3 will induce the morphological transition of the invadosome-based structure formed on bone matrix, including the sealing zone and the thinner invadosome belt, in experiments performed in osteoclasts in vitro [52–54].
RHO U activity was associated with invadosome formation in fibroblasts and osteoclasts transformed with v-Src [55,56]. Expression of RHO U is upregulated during the formation of the sealing zone during osteoclast maturation [55,56].
RAC
In addition to RHO and CDC42, invadosomes are also affected by the activity of RAC proteins. Among these findings, cytokine-mediated induction of invadosomes in endothelial cells was more profoundly altered in response to expression of the dominant-negative N17Rac1 mutant than in response to expression of dominant-negative mutants, N19RHOA and N17CDC42[49,50]. Moreover, studies performed in gene-deleted animal models revealed that RAC2 was essential for invadosome formation in myeloid cells while RAC1 was more important with respect to their meta-structural organization as rings [57]. RAC-mediated regulation of invadosome function was supported by results of experiments in which the dominant-positive mutant V12RAC1 stabilized invadosomes and increased invadosome-mediated degradation of the ECM by tumour cells [29,58].
In other studies, silencing of RAC3 and associated modulation of β1 integrin activation was found to have a more profound impact on invadosome-mediated ECM degradation than on their formation in cancer cells [59]. As described above, expression of over-activated RAC mutants also resulted in invadosome disorganization; these results also suggest the importance of an ideal degree of RAC activation required to support the initiation and maturation of invadosomes [33,45]. It is also important to consider the possibility of competition between two RAC proteins and the potential for antagonistic effects. In this light, silencing of the RAC family member, RHOG, promotes disorganization and inhibition of invadosome formation and reveals an essential role for this protein as a negative regulator of invasion of breast cancer cell lines [60]. Taken together, these results suggest the possibility of a functional and dynamic equilibrium between RAC2, a protein that promotes invadosome formation, and RHOG, RAC1, and potentially RAC3 that serve to promote ECM degradation.
Others small GTPases implicated in invadosome
RABs
The important relationships that connect RHO GTPases, the regulation of actin, and the specific ECM degradative activity observed in invadosomes suggested that other small GTPases may play roles in membrane trafficking associated with invadosome function. The RABs represent the largest family of small GTPases. These proteins have been implicated in multiple steps associated with endosomes and membrane trafficking. One major function of RABs involves providing direction to endosomes and introduction into a ‘cell sorting pathway’ via their association with the specific intracellular cargoes. Some studies have highlighted the potential for important interactions with metalloproteases, notably MT1-MMP, which are found in the vicinity of podosomes. As such, RABs could modulate invadosome function via their capacity to regulate intracellular trafficking and to direct specific components and their associated-metalloproteases. Endosomes containing RAB5a, RAB8a, RAB14, RAB21, and RAB22a have been found to contain variable amounts of MT1-MMP at their membranes. Moreover, silencing or expression of dominant-negative mutant forms of these RABs (except for RAB21) results in drastically decreased MT1-MMP exposure of the basement membrane of myeloid cells; as such, these cells are rendered less capable of degrading the ECM [61]. Metalloproteases MMP2 and MMP9 are also involved in invadosome-mediated matrix degradation. RAB40 promotes the extracellular secretion of these enzymes; silencing of this protein reduces the number and size of invadosome rings in breast cancer cells [62]. Likewise, binding interactions between RAB40 and Tks5 (a specific Src substrate that is also a driver of invadosome functions) promote RAB40 accumulation in invadosome.
Other RABs are direct regulators of cell invasion into the ECM; these proteins might also serve to regulate invadosome functions. RAB27 silencing leads to the diminished extracellular release of the protease, cathepsin D. By contrast, RAB27 overexpression facilitates invasion of invasive glioma cells in three-dimensional matrices [63]. In other experiments, RAB27 silencing results in diminished cell proliferation in tumour spheroids and limits their ability to invade a three-dimensional ECM [64]. These findings might explain the successful results of a screening procedure for anti-RAB27 drugs for those that would limit the invasive capacity of breast carcinoma cells [65]. Finally, RAB1 silencing also results in diminished invasiveness of highly invasive MDA-MB-231 cells; conversely, RAB1 overexpression promotes invasive behaviour in otherwise poorly-invasive MCF7 cells [66]. Taken together, these findings suggest that RABs play a major role in directing vesicle-bound factors that promote ECM degradation to sites of developing invadosomes. These actions serve to reinforce invadosome-mediated protrusive and degradative functions.
ARF
The ADP-ribosylation factors (ARFs) are members of another family of small GTPases that have been implicated in the regulation of membrane trafficking via their impact on membrane lipid composition, the recruitment of coat proteins, and their capacity to promote local reorganization of the actin cytoskeleton. The three classes of ARF proteins include Class I (ARF1, ARF2, and ARF3), Class II (ARF4 and ARF5), and Class III (ARF6) [67]. While there is no clear overview of the importance of all ARFs with respect to invadosome regulation, both ARF1 and ARF6 appear to play roles in this process, as modulation of their expression and activity serves to regulate the three-dimensional invasion and ECM degradative activity of invasive cells [68]. Moreover, ARF6 activity has been identified as important for invadosome formation and ECM degradative activity via its control of MT1-MMP trafficking [69–71].
Regulation of GTPases in the invadosome
Regulation of small GTPases by GEFs, GAPs, and guanine nucleotide dissociation
Inhibitors (GDIs)
Signalling activity of small GTPases is associated with cycling between GDP-bound and GTP-bound forms. The GDP-loaded form is generally considered to be inactive, while the GTP-loaded form can interact with a specific set of effectors, thereby leading to activation of downstream signalling pathways. Based on their high affinity for GDP and comparatively slow hydrolysis of GTP, the cycling between GDP-GTP-bound forms of these proteins is regulated by GEFs, which are proteins that promote GDP dissociation, and GAPs, which are proteins that stimulate GTP hydrolysis [72]. Moreover, the activities of lipid-anchored small GTPases are also dependent on direct interactions with GDIs, which are proteins that inhibit ligand dissociation. These interactions promote shielding of their lipid-associated component that lead to membrane extrusion and long term inactivation [73]. As discussed above, invadosome functions are regulated by the activities of various small GTPases, including CDC42, RHO, RAC, RABs, and ARFs. Given the large number of GEFs, GAPs, and GDIs that have been characterized to date, we will limit the discussion to those that have been implicated in invadosome regulation (see Figure 1).
Figure 1.
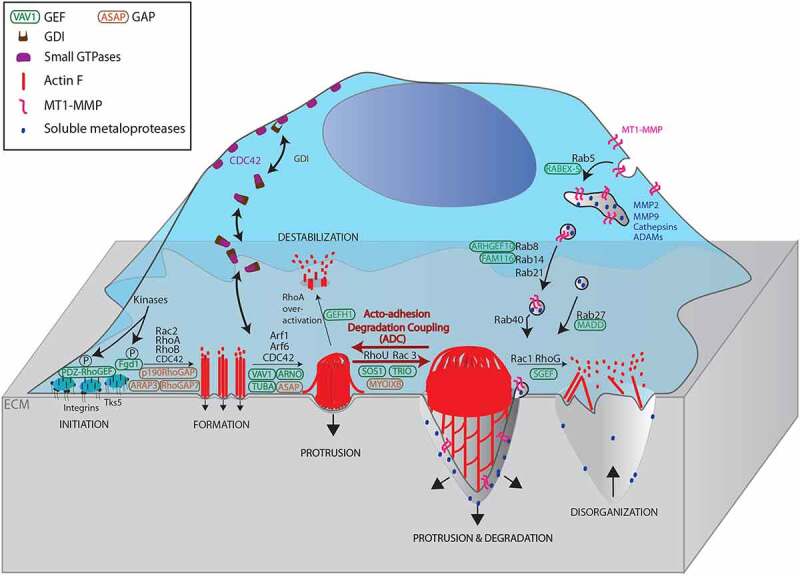
Scheme depicting the actions of various small GTPases and their regulators (GEFs, GAPs, and GDIs) and their role in promoting invadosome formation as well as coupling of its acto-adhesive and ECM degradative activities
Guanine nucleotide exchange factors (GEFs)
GEFs are mainly regulated by transmembrane receptors and can be activated by phosphorylation. This has been described specifically for the CDC42-GEF proteins known as VAVs that are activated by the invadosome driver, SRC. This interaction leads to the activation of CDC42 and the formation of degradative invadosomes [74,75]. Similarly, the CDC42-GEF known as FGD1 binds to Tks5 and is thus recruited to degradative invadosome sites where it promotes local activation of CDC42 and invadosome formation [76,77]. The GEF protein TUBA also sustains CDC42 activation, thereby promoting the formation of linear invadosomes and the organization of invadosome meta structures in breast cancer cell lines [39]. However, it is not yet clear how GEFs regulate CDC42 activity in space and over time.
As discussed previously, regulation of the activation of other GTPases is also important with respect to invadosome function. The regulation of cell contractility by RHO proteins was identified as critical for the formation and maturation of invadosomes. Towards this end, rPDZ-RhoGEF could interact with microtubule-associated proteins such as LC2 through a type I PDZ motif domain; inhibition of this interaction or the disorganization of the microtubule network induced a RhoA-dependent hypercontractile morphology switch within neurons [78]. PDZ-RHOGEF preferentially activates RHOA [79]; expression of a dominant-negative mutant of PDZ-RHOGEF decreases the activity of RHOA and RHOC and thereby promotes the formation of degradative invadosomes [80,81]. Similarly, the microtubule-associated GEF, GEFH1, directly regulates RHOA activation; silencing of RHOA results in the diminished formation of stress fibres and a concomitant increase in the number of invadosomes formed [82,83].
Regulation of RAC sub-family members is also important with respect to the functional regulation of invadosome. Indeed, SH3-containing Guanine Nucleotide Exchange Factor (SGEF) directly controls activation of RHOG in response to phosphorylation of paxillin, thereby promoting the disassembly of invadosomes in breast cancer cells [60]. By contrast, invadosome function and integrity in macrophages is sustained by the activation of Rac1 by the GEF, SOS1, a factor that is recruited by the invadosome driver, Tks5 [84]. Indeed, SOS1 silencing resulted in the disassembly of the invadosomes and diminished macrophage invasiveness in experiments carried out in vitro [85]. Finally, the GEF known as TRIO also regulated RAC-mediated activity in invadosomes and thereby controlled the coupling between acto-adhesion and degradative activities [86].
The GEF, ARNO, can control ARF1 activation specifically in invadosomes to sustain their formation and to promote their degradative activity [87]. Inactivation of ARF1 activity results in increased RHOA activation and Myosin II-dependent cell contractility.
Finally, RAB activity is also finely-tuned by the actions of various GEFs. This is particularly evident in the case of ARHGEF10, a factor that promotes relocalization of RAB8 to exocytic vesicles. ARHGEF10 silencing in breast cancer cells induced the formation of large vesicles containing both RAB6 and RAB8 and diminished invasive potential [88]. RABEX-5, a GEF that regulates RAB5, is overexpressed in breast cancer cells. Silencing of RABEX-5 results in the diminished invasion of cancer cells, an observation that was linked to a decrease in MMP9 expression [89,90]. Furthermore, FAM116, a GEF of RAB14, FAM116, promotes intermediate recycling of ADAM10. Depletion of RAB14 and/or FAM116 resulted in diminished explore of this protease at the plasma membrane [91]. Finally, MADD, the GEF that interacts with RAB27, may also be necessary for cell invasion as shown by the results of silencing experiments [92]. Despite these observations, no clear results are available that demonstrate the impact and implication of these GEFs on invadosome formation and function. Additional systemic studies will be needed to evaluate the precise functions and dynamic localization of all the GEFs in one or more of the various invadosome models.
GTPase-activating proteins (GAPs)
Similar to what has been observed for GEFs, the specific functions of several GAPs have been highlighted with respect to regulation of invadosome structure and function. As previously indicated, the formation of invadosome is balanced with the internal contractility of their cellular components via mechanisms that are controlled by the members of the RHO protein sub-family. An increase in RHOA-GDP has been associated with a concomitant increase in invadosome numbers [93]. Numerous studies have highlighted the importance of p190RHO GAP and its role in controlling of RHOA activation and the functions of invadosomes. Silencing of RHOA activity via the introduction of 190RHOGap promotes invadosome loss [53,94]; these results highlight the importance of RHOA with respect to contractility and invadosome function. The RHO-GAP activity of MYOIXB couples local regulation of RHO with the acto-myosins machinery, thereby regulating invadosome-dependent motility and bone degradative activity of osteoclasts [95]. Moreover, deactivation of RHOGap7 increases RHOA activity in osteoclasts unable to generate invadosomes [51]. Finally, the RHO-GAP protein, ARAP3, is localized in invadosomes; its activity is essential for their formation [96]. As such, fine-tuned control of RHO activity in space and time is essential for the coordination of the invadosomes functions.
GAPs for ARF proteins implicated in invadosome formation have been also identified. The GAP, ASAP1, that regulates ARF1 was identified as essential for invadosome formation and degradative activity [82,97]. This is also the case for ARAP1 (a GAP for ARF1) and ARAP3 (a GAP for ARF6) that both serve as regulators of invadosome formation and functions [96,98]. The GAPs GIT1 and GIT2 that negatively regulate both ARF1 and ARF6 activities are also essential towards sustaining normal invadosome functions [69,99,100].
Guanine nucleotide dissociation inhibitors (GDIs)
GDIs orchestrate regulation by controlling the level of GTPases in the membrane surrounding an invadosome. Specifically, GDIs associate with and retain membrane-associated GTPases in the cytosolic compartment by shielding their conjugated lipids [101]. The protein, GDIα, serves in this role by directing the localization of CDC42 within an invadosome site during cell invasion in vivo [102,103].
As we discussed above, the complex dynamics that regulate the interplay among the multiple invadosome functions are regulated by the optimal activation of a series of GTPase-based switches. As such, and in addition to our inherent interest in this critical subcellular structure, the invadosome may be useful as a model to study basic signalling mechanisms and the interplay between these GTPases and their regulatory factors.
Spatiotemporal coordination of GTPases
The development of biosensors with the capacity to monitor spatiotemporal modulations of RHO GTPase activities has been a key step towards an improved understanding of these highly dynamic signalling nodes. These types of methods have been applied to a limited extent in the study of invadosome biology. Results obtained thus far document spatial confinement and interactions among small RHO GTPases as critical features in the regulation of the acto-adhesive and ECM degradative functions of invadosomes.
Although specific biosensors have been developed for small number of members of the small RHO GTPase superfamily, it has been possible to follow the activity of the members of the main sub-families. This limited use of this experimental strategy has highlighted the complexity of spatiotemporal control at specific signalling nodes. For example, a CDC42-specific biosensor revealed intense but apparently transient activity within the invadosome core in myeloid cells, despite the central role played by CDC42 in invadosome and WASP activation [104]. This strategy also facilitated the detection of active CDC42 in regions between the invadosome core in the adhesion area. Interestingly, the results of experiments performed with specific RHO biosensors revealed a distinct spatial pattern of activation. Specifically, RHOA activity was detected in random locations at the basal levels of cells during the process of invadosome formation, while RHOC activity was geometrically-confined to a region around the invadosome core [105]. A similar, complex pattern of activation was reported for the members of the RAC superfamily. A biosensor detecting activation of RAC1 revealed its potential for regulation of invadosome ring via the promotion of invadosome disorganization. No RAC1 activity was detected in the invadosome cores in cancer cells; photoactivation induced invadosome disorganization [86]. By contrast, RAC3 activity was localized to the invadosome core or the adhesive ring, suggesting a differential requirement that responds to the need to couple acto-adhesion and ECM degradative activity [59]. Further dynamic studies and the development of additional biosensors will facilitate clarification of the mechanisms underlying RHO signalling in invadosomes.
It is important to recognize that we lack a full understanding of the roles of additional extracellular factors even for those cell surface receptors known to promote signal transduction via RHO GTPase activation (e.g., epithelial growth factor receptor [EGFR], integrins, and G-protein coupled receptors [GPCRs]). Among these factors, there is only a limited appreciation of the mechanical, concentration dependent, diffusion, and co-detection properties of both growth factors and the ECM. Furthermore, even small RHO GTPases are strong mediators of phenotypic change; they are also highly pleiotropic. This implies that a single signalling node might have the capacity to control several distinct cellular responses (e.g., actin polymerization, membrane trafficking, and changes in lipid composition to name a few). This also implies that a single type of RHO GTPases might promote different events via their capacity for diffusion through membranes as well as changes in the local concentration of their effectors and cycling rates between active and inactive forms. Indeed, super-resolution and modelling experiments have both highlighted the importance of nanoclusters of small GTPases and their capacity to induce multiple, distinct cellular responses in space and time in this form [106,107]. Small GTPase activity might be considered as controlling events via stable oscillations between ‘on’ and ‘off’ states. However, signalling can be also described as an analog circuit that transmits continuous information via output that is directly proportional to the input stimulus. Theoretical models and in silico simulations have focused on control of the size, distribution, and numbers of RAS nanoclusters that directly support high-fidelity analog signalling activity [107]. As such, a full analysis of the different nanoclusters formed from multiple small GTPases implicated in invadosome biology might explain how individual signalling nodes promote simultaneous activation or antagonism of different pathways (i.e., actin polymerization with adhesion activation and ECM degradation) in response to the same signalling molecule.
A global genetic analysis served to highlight the specificity of action of the small GTPases in the multiple models involving invadosomes in different cell types. This initial effort highlighted future needs that involve a further understanding of the spatiotemporal interplay and the complex regulation of multiples invadosome functions. Further studies will involve the development of new and specific multiplex fluorescence resonance energy transfer (FRET) probes as well as optogenetic methods to facilitate dynamic control of each small GTPase. These directions will be essential towards future investigations and elucidation of the molecular basis of each of these signalling pathways.
Acknowledgments
We apologize to those authors whose important work we were unable to cite due to space limitations. We thank the members of the laboratory for their helpful discussions. P.R. was funded by the Ligue Nationale contre le Cancer. This work was supported by Agence Nationale de la Recherche (ANR) and the Association de la Recherche pour le Cancer. Support for our team was from the “Fond pour la Recherche Médicale” (FRM).
Funding Statement
This work was supported by the Ligue Contre le Cancer [PhD Fellowship]; Fondation recherche médicale
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Aspenström P. Activated rho GTPases in cancer—the beginning of a new paradigm. Int J Mol Sci. 2018;19(12):3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Di Martino J, Henriet E, Ezzoukhry Z, et al. The microenvironment controls invadosome plasticity. J Cell Sci. 2016;129(9):1759–1768. [DOI] [PubMed] [Google Scholar]
- [3].Tarone G, Cirillo D, Giancotti FG, et al. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159(1):141–157. [DOI] [PubMed] [Google Scholar]
- [4].Mueller SC, Chen WT.. Cellular invasion into matrix beads: localization of β1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99 (Pt 2):213–225. [DOI] [PubMed] [Google Scholar]
- [5].Juin A, Billotteta C, Moreau V, et al. Physiological type I collagen organization induces the formation of a novel class of linear invadosomes. Mol Biol Cell. 2012;23(2):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Génot E, Gligorijevic B. Invadosomes in their natural habitat. Eur J Cell Biol. 2014;93(10–12):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leong HS, Robertson AE, Stoletov K, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8(5):1558–1570. [DOI] [PubMed] [Google Scholar]
- [8].Williams KC, Cepeda MA, Javed S, et al. Invadopodia are chemosensing protrusions that guide cancer cell extravasation to promote brain tropism in metastasis. Oncogene. 2019;38(19):3598–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen YC, Baik M, Byers JT, et al. TKS5-positive invadopodia-like structures in human tumor surgical specimens. Exp Mol Pathol. 2019;106:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Destaing O, Saltel F, Géminard JC, et al. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14(2):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jurdic P, Saltel F, Chabadel A, et al. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85(3–4):195–202. [DOI] [PubMed] [Google Scholar]
- [12].Luxenburg C, Geblinger D, Klein E, et al. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2(1):e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee BS. Myosins in osteoclast formation and function. Biomolecules. 2018;8(4):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Collin O, Tracqui P, Stephanou A, et al. Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J Cell Sci. 2006;119(9):1914–1925. [DOI] [PubMed] [Google Scholar]
- [15].Alexander NR, Branch KM, Parekh A, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18(17):1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Albiges-Rizo C, Destaing O, Fourcade B, et al. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122(17):3037–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Destaing O, Planus E, Bouvard D, et al. 1A integrin is a master regulator of invadosome organization and function. Mol Biol Cell. 2010;21(23):4108–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferrari R, Martin G, Tagit O, et al. MT1-MMP directs force-producing proteolytic contacts that drive tumor cell invasion. Nat Commun. 2019;10(1). DOI: 10.1038/s41467-019-12930-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122(17):3015–3024. [DOI] [PubMed] [Google Scholar]
- [20].Destaing O, Petropoulos C, Albiges-Rizo C. Coupling between acto-adhesive machinery and ECM degradation in invadosomes. Cell Adhes Migr. 2014;8(3):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Infante E, Castagnino A, Ferrari R, et al. LINC complex-Lis1 interplay controls MT1-MMP matrix digest-on-demand response for confined tumor cell migration. Nat Commun. 2018;9(1): DOI: 10.1038/s41467-018-04865-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sakurai-Yageta M, Recchi C, Le Dez G, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181(6):985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Petropoulos C, Oddou C, Emadali A, et al. Roles of paxillin family members in adhesion and ECM degradation coupling at invadosomes. J Cell Biol. 2016;213(5):585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aspenström P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313(17):3673–3679. [DOI] [PubMed] [Google Scholar]
- [25].Shutes A, Berzat AC, Chenette EJ, et al. Biochemical analyses of the Wrch atypical Rho family GTPases. Methods Enzymol. 2006. [DOI] [PubMed] [Google Scholar]
- [26].Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7(1):54–62. [DOI] [PubMed] [Google Scholar]
- [27].Fort P. Rho signaling: an historical and evolutionary perspective. In: Rho GTPases: molecular biology in health and disease. 2017. [Google Scholar]
- [28].Moreau V, Tatin F, Varon C, et al. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol. 2003;23(19):6809–6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nakahara H, Otani T, Sasaki T, et al. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8(12):1019–1027. [DOI] [PubMed] [Google Scholar]
- [30].Yamaguchi H, Lorenz M, Kempiak S, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168(3):441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moreau V, Tatin F, Varon C, et al. Cdc42-driven podosome formation in endothelial cells. Eur J Cell Biol. 2006;85(3–4):319–325. [DOI] [PubMed] [Google Scholar]
- [32].Dutartre H, Davoust J, Gorvel JP, et al. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109 (Pt 2):367–377. [DOI] [PubMed] [Google Scholar]
- [33].Burns S, Thrasher AJ, Blundell MP, et al. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98(4):1142–1149. [DOI] [PubMed] [Google Scholar]
- [34].West MA, Prescott AR, Eskelinen EL, et al. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10(14):839–848. [DOI] [PubMed] [Google Scholar]
- [35].Aspenström P, Å F, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377(2):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vignal E, De Toledo M, Comunale F, et al. Characterization of TCL, a new GTPase of the Rho family related to TC10 and Cdc42. J Biol Chem. 2000;275(46):36457–36464. [DOI] [PubMed] [Google Scholar]
- [37].Billottet C, Rottiers P, Tatin F, et al. Regulatory signals for endothelial podosome formation. Eur J Cell Biol. 2008;87(8–9):543–554. [DOI] [PubMed] [Google Scholar]
- [38].Di Martino J, Paysan L, Gest C, et al. Cdc42 and Tks5: A minimal and universal molecular signature for functional invadosomes. Cell Adhes Migr. 2014;8(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Juin A, Di Martino J, Leitinger B, et al. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol. 2014;207(4):517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Linder S, Nelson D, Weiss M, et al. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96(17):9648–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Saini P, Courtneidge SA. Tks adaptor proteins at a glance. J Cell Sci. 2018;131(1):jcs203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Diaz B, Shani G, Pass I, et al. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2(88):ra53-ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. [DOI] [PubMed] [Google Scholar]
- [44].Berdeaux RL, Díaz B, Kim L, et al. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ory S, Munari-Silem Y, Fort P, et al. Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J Cell Sci. 2000;113 (Pt 7):1177–1188. [DOI] [PubMed] [Google Scholar]
- [46].Zhang D, Udagawa N, Nakamura I, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108 (Pt 6):2285–2292. [DOI] [PubMed] [Google Scholar]
- [47].Zhang R, Lee DM, Jimah JR, et al. Dynamin regulates the dynamics and mechanical strength of the actin cytoskeleton as a multifilament actin-bundling protein. Nat Cell Biol. 2020;22(6):674–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wheeler AP, Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp Cell Res. 2007;313(16):3505–3516. [DOI] [PubMed] [Google Scholar]
- [49].Osiak AE, Zenner G, Linder S. Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp Cell Res. 2005;307(2):342–353. [DOI] [PubMed] [Google Scholar]
- [50].Wang J, Taba Y, Pang J, et al. GIT1 mediates vegf-induced podosome formation in endothelial cells. Critical role for PLCγ. Arterioscler Thromb Vasc Biol. 2009;29(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schramp M, Ying O, Tai YK, et al. ERK5 promotes Src-induced podosome formation by limiting Rho activation. J Cell Biol. 2008;181(7):1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Clark K, Langeslag M, Van Leeuwen B, et al. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. Embo J. 2006;25(2):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci. 2004;117(2):223–231. [DOI] [PubMed] [Google Scholar]
- [54].Saltel F, Destaing O, Bard F, et al. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell. 2004;15(12):5231–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ory S, Brazier H, Blangy A. Identification of a bipartite focal adhesion localization signal in RhoU/Wrch-1, a Rho family GTPase that regulates cell adhesion and migration. Biol Cell. 2007;99(12):701–716. [DOI] [PubMed] [Google Scholar]
- [56].Brazier H, Pawlak G, Vives V, et al. The Rho GTPase Wrch1 regulates osteoclast precursor adhesion and migration. Int J Biochem Cell Biol. 2009;41(6):1391–1401. [DOI] [PubMed] [Google Scholar]
- [57].Wheeler AP, Wells CM, Smith SD, et al. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci. 2006;119(13):2749–2757. [DOI] [PubMed] [Google Scholar]
- [58].Furmaniak-Kazmierczak E, Crawley SW, Carter RL, et al. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res. 2007;100(9):1328–1336. [DOI] [PubMed] [Google Scholar]
- [59].Donnelly SK, Cabrera R, Mao SPH, et al. regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J Cell Biol. 2017;216(12):4331–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Goicoechea SM, Zinn A, Awadia SS, et al. A RhoG-mediated signaling pathway that modulates invadopodia dynamics in breast cancer cells. J Cell Sci. 2017;jcs.195552. DOI: 10.1242/jcs.195552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wiesner C, El Azzouzi K, Linder S. A specific subset of RabGTPases controls cell surface exposure of MT1-MMP, extracellular matrix degradation and three-dimensional invasion of macrophages. J Cell Sci. 2013;126(13):2820–2833. [DOI] [PubMed] [Google Scholar]
- [62].Jacob A, Linklater E, Bayless BA, et al. The role and regulation of Rab40b-Tks5 complex during invadopodia formation and cancer cell invasion. J Cell Sci. 2016;129(23):4341–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wu X, Hu A, Zhang M, et al. Effects of Rab27a on proliferation, invasion, and anti-apoptosis in human glioma cell. Tumor Biol. 2013;34(4):2195–2203. [DOI] [PubMed] [Google Scholar]
- [64].Tang L, Wei D, Yan F. MicroRNA-145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple-negative breast cancer. Cancer Gene Ther. 2016;23(8):258–265. [DOI] [PubMed] [Google Scholar]
- [65].Kang SM, Nam KY, Jung SY, et al. Inhibition of cancer cell invasion by new ((3,4-dihydroxy benzylidene)hydrazinyl)pyridine-3-sulfonamide analogs. Bioorganic Med Chem Lett. 2016;26(4):1322–1328. [DOI] [PubMed] [Google Scholar]
- [66].Zhang W, Xu J, Wang K, et al. MIR1393p suppresses the invasion and migration properties of breast cancer cells by targeting RAB1A. Oncol Rep. 2019. DOI: 10.3892/or.2019.7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schlienger S, Campbell S, Claing A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol Biol Cell. 2014;25(1):17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Heckel T, Czupalla C, Santo AIE, et al. Src-dependent repression of ARF6 is required to maintain podosome-rich sealing zones in bone-digesting osteoclasts. Proc Natl Acad Sci U S A. 2009;106(5):1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Marchesin V, Castro-Castro A, Lodillinsky C, et al. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J Cell Biol. 2015;211(2):339–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Marchesin V, Montagnac G, Chavrier P. ARF6 promotes the formation of Rac1 and WAVE-dependent ventral F-Actin rosettes in breast cancer cells in response to epidermal growth factor. PLoS One. 2015;10(3):e0121747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical Elements in the Control of Small G Proteins. Cell. 2007;129(5):865–877. [DOI] [PubMed] [Google Scholar]
- [73].Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93(1):269–309. [DOI] [PubMed] [Google Scholar]
- [74].Razidlo GL, Schroeder B, Chen J, et al. Vav1 as a central regulator of invadopodia assembly. Curr Biol. 2014;24(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Faccio R, Teitelbaum SL, Fujikawa K, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11(3):284–290. [DOI] [PubMed] [Google Scholar]
- [76].Zagryazhskaya-Masson A, Monteiro P, Macé AS, et al. Intersection of TKS5 and FGD1/CDC42 signaling cascades directs the formation of invadopodia. J Cell Biol. 2020;219(9): DOI: 10.1083/jcb.201910132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ayala I, Giacchetti G, Caldieri G, et al. Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 2009;69(3):747–752. [DOI] [PubMed] [Google Scholar]
- [78].Longhurst DM, Watanabe M, Rothstein JD, et al. Interaction of PDZRhoGEF with microtubule-associated protein 1 light chains: link between microtubules, actin cytoskeleton, and neuronal polarity. J Biol Chem. 2006;281(17):12030–12040. [DOI] [PubMed] [Google Scholar]
- [79].Jaiswal M, Gremer L, Dvorsky R, et al. Mechanistic insights into specificity, activity, and regulatory elements of the regulator of G-protein signaling (RGS)-containing Rho-specific guanine nucleotide exchange factors (GEFs) p115, PDZ-RhoGEF (PRG), and leukemia-associated RhoGEF (LARG). J Biol Chem. 2011;286(20):18202–18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nicholas NS, Pipili A, Lesjak MS, et al. PAK4 suppresses PDZ-RhoGEF activity to drive invadopodia maturation in melanoma cells. Oncotarget. 2016;7(43):70881–70897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Semprucci E, Tocci P, Cianfrocca R, et al. Endothelin A receptor drives invadopodia function and cell motility through the β-arrestin/PDZ-RhoGEF pathway in ovarian carcinoma. Oncogene. 2016;35(26):3432–3442. [DOI] [PubMed] [Google Scholar]
- [82].Shiba Y, Randazzo PA. GEFH1 binds ASAP1 and regulates podosome formation. Biochem Biophys Res Commun. 2011;406(4):574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rafiq NBM, Nishimura Y, Plotnikov SV, et al. A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat Mater. 2019; [DOI] [PubMed] [Google Scholar]
- [84].Rufer AC, Rumpf J, von Holleben M, et al. Isoform-Selective Interaction of the Adaptor Protein Tks5/FISH with Sos1 and Dynamins. J Mol Biol. 2009;390(5):939–950. [DOI] [PubMed] [Google Scholar]
- [85].Baruzzi A, Remelli S, Lorenzetto E, et al. Regulates Macrophage Podosome Assembly and Macrophage Invasive Capacity. J Immunol. 2015;195(10):4900–4912. [DOI] [PubMed] [Google Scholar]
- [86].Moshfegh Y, Bravo-Cordero JJ, Miskolci V, et al. A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nat Cell Biol. 2014;16(6):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rafiq NBM, Lieu ZZ, Jiang T, et al. Podosome assembly is controlled by the GTPase ARF1 and its nucleotide exchange factor ARNO. J Cell Biol. 2017;216(1):181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shibata S, Kawanai T, Hara T, et al. ARHGEF10 directs the localization of Rab8 to Rab6-positive executive vesicles. J Cell Sci. 2016;129(19):3620–3634. [DOI] [PubMed] [Google Scholar]
- [89].Zhang X, Min J, Wang Y, et al. Li H. RABEX-5 plays an oncogenic role in breast cancer by activating MMP-9 pathway. J Exp Clin Cancer Res. 2013;32(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang S, Lu A, Chen X, et al. Rabex-5 is upregulated and plays an oncogenic role in gastric cancer development by activating the VEGF signaling pathway. PLoS One. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Linford A, Yoshimura SI, Bastos RN, et al. Rab14 and Its Exchange Factor FAM116 Link Endocytic Recycling and Adherens Junction Stability in Migrating Cells. Dev Cell. 2012;22(5):952–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Saini S, Sripada L, Tulla K, et al. Loss of MADD expression inhibits cellular growth and metastasis in anaplastic thyroid cancer. Cell Death Dis. 2019;10(2). DOI: 10.1038/s41419-019-1351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nakahara H, Mueller SC, Nomizu M, et al. Activation of β1 Integrin Signaling Stimulates Tyrosine Phosphorylation of p190 RhoGAP and Membrane-protrusive Activities at Invadopodia. J Biol Chem. 1998;273(1):9–12. [DOI] [PubMed] [Google Scholar]
- [94].Crimaldi L, Courtneidge SA, Gimona M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp Cell Res. 2009;315(15):2581–2592. [DOI] [PubMed] [Google Scholar]
- [95].McMichael BK, Scherer KF, Franklin NC, et al. The RhoGAP activity of myosin IXB is critical for osteoclast podosome patterning, motility, and resorptive capacity. PLoS One. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yu C, NBM R, Krishnasamy A, et al. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep. 2013;5(5):1456–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Vi LH, Bharti S, Inoue H, et al. ASAP3 is a focal adhesion-associated Arf GAP that functions in cell migration and invasion. J Biol Chem. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Segeletz S, Danglot L, Galli T, et al. ARAP1 Bridges Actin Dynamics and AP-3-Dependent Membrane Traffic in Bone-Digesting Osteoclasts. iScience. 2018;6:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wang Y, Kelber JA, Cao HST, et al. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression. Proc Natl Acad Sci. 2010;107:10920–10925. Internet] ; :. Available from. http://www.pnas.org/cgi/doi/ 10.1073/pnas.0914776107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tanna CE, Goss LB, Ludwig CG, et al. Arf GAPs as regulators of the actin cytoskeleton—An update. Int J Mol Sci. 2019;20(2):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15(7):356–363. [DOI] [PubMed] [Google Scholar]
- [102].MacKeil JL, Brzezinska P, Burke-Kleinman J, et al. A PKA/cdc42 signaling axis restricts angiogenic sprouting by regulating podosome rosette biogenesis and matrix remodeling. Sci Rep. 2019;9(1). DOI: 10.1038/s41598-018-37805-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lohmer LL, Clay MR, Naegeli KM, et al. A sensitized screen for genes promoting invadopodia function in vivo: CDC-42 and Rab GDI-1 direct distinct aspects of invadopodia formation. PLoS Genet. 2016;12(1):e1005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hanna S, Miskolci V, Cox D, et al. A new genetically encoded single-chain biosensor for Cdc42 based on FRET, useful for live-cell imaging. PLoS One. 2014;9(5). DOI: 10.1371/journal.pone.0096469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bravo-Cordero JJ, Oser M, Chen X, et al. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21(8):635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Remorino A, De Beco S, Cayrac F, et al. Gradients of Rac1 nanoclusters support spatial patterns of Rac1 signaling. Cell Rep. 2017;21(7):1922–1935. [DOI] [PubMed] [Google Scholar]
- [107].Zhou Y, Hancock JF. Ras nanoclusters: versatile lipid-based signaling platforms. biochim. Biophys. Acta - Mol. Cell Res. 2015; [DOI] [PubMed] [Google Scholar]


