Abstract
Simple Summary
Monoclonal antibody-bearing radionuclides have been under clinical investigation over the last two decades for their use in theranostic (diagnostic and therapeutic) applications in cancer. However, despite the numerous trials that have been conducted, only two radioimmunotherapies (RIT) have been approved by the FDA for the targeted therapy of hematologic tumors expressing CD20 antigens. Moreover, RIT applications for solid cancers faced major issues—such as radiotoxicity due to low antibodies penetrance requiring substantial curative dose—where new discoveries concerning antibody engineering or radionuclides are trying to overcome. Here, we performed an overview of the last 11-year clinical trials involving RIT for solid and non-solid cancers conducted either with full antibodies or antibody fragments. We discussed the low-to-moderate efficiency of RIT compared to conventional therapies and described the last advances in clinic for antibodies carriers (F(ab′)2, Fab′, ScFv). Finally, we discussed about the complexity of RIT as a therapy and depicted both the issues and the prospects of such a strategy.
Abstract
The specific irradiation of tumors with selective radiolabeled antibodies constitutes an attractive therapeutic approach. Consequent preclinical research has been conducted by both biologists to identify pertinent targets and to select corresponding antibodies (mAb) and by radiochemists to radiolabel mAbs. These numerous preclinical investigations have ascertained the therapeutic interest of radioimmunotherapy (RIT) protocols in mice models. Here, we summarize the clinical studies that have been performed the last decade, including clinical trials (phases I, II, and III), prospective and retrospective studies, and cases series. We thereby reported 92 clinical studies. Among them, 62 concern the treatment of hematological malignancies, and 30 concern solid tumors. For hematologic diseases, the analysis was complex due to the high discrepancy of therapeutic strategies (first-line therapy, consolidation, stem cell transplantation conditioning) as well as the high variety of malignancies that were treated. The clinical studies from the last decade failed to expand anti-CD20 RIT indications but confirmed that RIT using radiolabeled anti-CD20 remains a pertinent choice for patients with relapse follicular lymphomas. For solid tumors, the positive benefit of RIT is more mitigated, apart for few malignancies that can be treated locally. Clinical trials also demonstrated the potential of some antibody formats, such as F(ab′)2, which has already been approved by the China State FDA under the trend name Licartin®. Despite disparate results, mAb fragments are an interesting prospect for the improvement of RIT efficiency as well as for pretargeted strategies that delay the injection of radioactive treatments from the mAb ones.
Keywords: RIT, hematologic cancers, solid cancers, antibody fragments, PRIT, radionuclides
1. Introduction
When addressing the role of radionuclides and their action toward tumor antigens, the use of monoclonal antibodies (mAbs) as vectors has been a great challenge since the 1980s. The idea, which was initially conceptualized in 1900 by Erlich (“magic bullet”) [1], combined the therapeutic properties of radioisotopes with specific vectors in order to eradicate tumors, regardless of their location. The proof of concept of radioimmunotherapy (RIT) was then demonstrated in preclinical models since the 1970s thanks to the development of hybridomas by Kohler and Milstein [2]. Several studies performed thereafter with radiolabeled antibodies confirmed the potential of RIT in mice, as RIT decreased tumor growth and/or improved survival [3,4].
One of the main prerequisites for RIT relies on the accessibility of the antigens by their cognate mAbs. Therefore, membrane proteins such as certain specific activating proliferation receptors (HER family members), the hematopoietic cluster of differentiation proteins (CD20 in most cases), or the carcinoma embryonic antigen (CEA) were considered to be antigens of interest for such an approach. However, it should be noted that these antigens are not exclusively found in tumors, so targeting them may induce adverse and unwanted events. Such off-targeted effects mainly depend on the radionuclide toxicity, and they can induce hematopoietic damage due to the long half-life of mAbs in the blood (~7- to 21-day half-life depending on the isotype).
An increasing panel of radionuclides with different properties (half-life, spectra emission, particles or electrons) is currently under evaluation in theranostic approaches (for a review see [5]). However, until now, the choice of radionuclides for RIT in clinical trials was limited to I-131 (8.0 days half-life), Y-90 (2.7 days half-life), Lu-177 (6.7 days half-life), and Re-188 (16.9 hours half-life) for β--emitters and to Bi-213 (45.6 minutes half-life) and At-211 for α-particles (7.2 hours half-life). As therapeutic radionuclides aim to destroy the tumor, the dose that is delivered should induce enough lethal alterations to prevent DNA reparations and escape mechanisms. Dosimetry determination is crucial for RIT and can be calculated by combining the intrinsic radionuclide theoretical properties and radiolabeled mAb pharmacokinetics. In some cases, a personalized dosimetry can be performed using a couple of radionuclides (one for imaging, one for therapy) that are bound to the same binding-site on the mAb or by using therapeutic isotopes that are also able to emit γ-rays for SPECT acquisition, such as I-131 or Lu-177. Even though dosimetry is now well established for external beam radiotherapy, it is still challenge to accurately determine it in RIT [6]. RIT delivers continuous ionizations over time, which are characterized by the linear energy transfer (LET): LET (~0.2 keV/µm) for β-emitters and a high LET (~50–230 keV /µm) for α-particles [7].
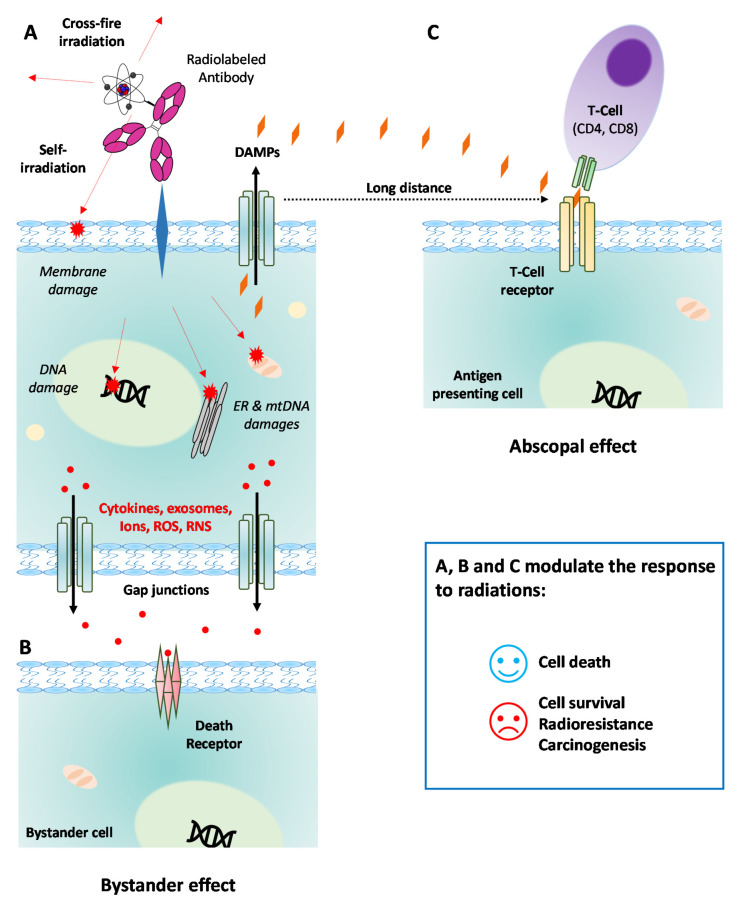
Besides the intrinsic properties of radionuclides, RIT also induces different therapeutic effects depending on the properties of the antibody that is used (Figure 1). A radiolabeled antibody that undergoes internalization leads to more DNA double-strand breaks than a non-internalizing antibody targeting the cell membrane does. A previous review discussed the effects of (1) crossfire irradiation, which induces the irradiation of juxtracrine cells (Figure 1A); (2) bystander effects, which occur through communication between targeted cells and the surrounding cells (Figure 1B); and (3) the abscopal effect, which is characterized by the death of non-targeted cells that are distant from the targeted cells (Figure 1C) [6,8]. These three processes result in oxidative stress followed by cell death following macromolecule alterations. This involves the activation of numerous pathways as well as the immune response due to neoantigen occurrence for abscopal response [8]. RIT thus represents an attractive therapeutic approach for disseminated tumors. In this work, we wanted to provide an overview on the clinical evaluations of RIT that have taken place over the last decade. We focused on the clinical studies that have been reported since the first review by Pouget et al. published in 2011 [7].
Figure 1.
Cell mechanisms underlying radioimmunotherapy (RIT). (A) Targeted effects on tumor cell after binding by a radiolabeled mAb to its cognate membrane antigen. RIT induces direct damages to DNA, mtDNA, membranes, and other cell components (e.g., ER). Surrounding cells are also directly irradiated through the crossfire effect. Damages to the cell lead to the secretion of cytokines, ions, ROS, RNS, or exosomes that are released in the extracellular microenvironment. (B) Off-target bystander effect induced in other cancer cells (close or not). Cytokines and other death effectors released in the microenvironment bind to the cell death receptors. (C) Off-target abscopal effect involving the immune system response far away from the irradiated cells. DAMPs excreted by the irradiated cell can bind the T-cell receptor of an antigen-presenting cell, resulting in the activation of the immune system through the binding of the CD4 or CD8 T lymphocytes. NB: Similar mechanisms occur if RIT is performed with a radiolabeled mAb that can be internalized into the cell. DAMPs: damage-associated molecular patterns; ER: endoplasmic reticulum; mAb: monoclonal antibody; mtDNA: mitochondrial DNA; RNS: reactive nitrogen species; ROS: reactive oxygen species.
The web search concerning publication from the last 10 years was performed on PubMed using the terms “Radioimmunotherapy” and both the filters “Clinical Trials” and “10 years”. Over the 129 publications that were retrieved, we removed 35 that were irrelevant (i.e., imaging studies only, dosimetry calculations only, or external radiotherapy combined with immunotherapy). For the ongoing clinical trials, we searched the terms “Radioimmunotherapy” and “radiolabeled antibody” on government websites from the US, Europe, China, and Japan as well as the WHO ICTRP. Here, we report and discuss RIT efficacy for both hematological and solid tumors based on clinical trial data as well as prospects based on the use of fragments or pretargeting strategies.
2. Last Ten Years Publications Involving RIT Protocols
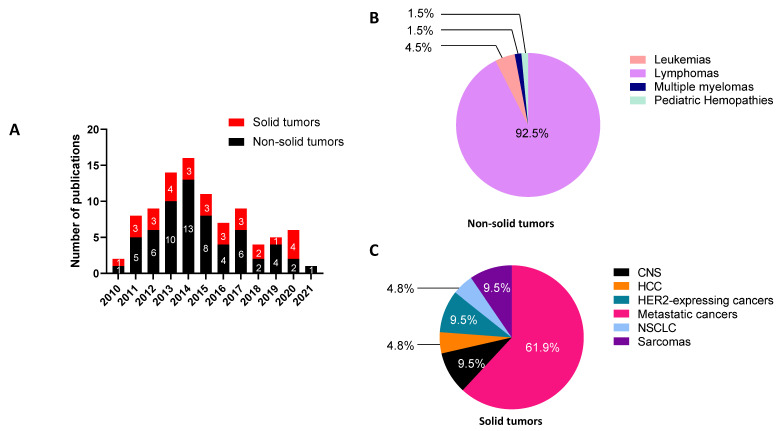
We identified 92 publications describing RIT in humans that have been published in the last 10 years (Figure 2). Among them, we observed that 67% concerned RIT for non-solid tumors (62 studies) and that only 33% concerned the use of RIT for solid tumors (30 studies), as detailed in Figure 2A. The years 2013 and 2014 were the most prolific, with 30 out of the 92 publications being published during the last decade. The repartition of non-solid cancer RIT (Figure 2B) showed that studies on lymphomas (encompassing follicular lymphomas, mantle-cell lymphomas, Burkitt lymphomas, diffuse large B-cell lymphomas, marginal zone lymphomas, and Hodgkin lymphomas) were highly preponderant, thus representing 92.5% of the investigations. In contrast, the RIT of solid tumors has been assessed on a higher variety of targets and tumors (Figure 2C), with metastatic cancers representing 61.9% of the reported studies. In the following parts, we will discuss the selection of the RIT strategy depending on whether it is for the treatment of solid or non-solid cancers, antigen targets, association protocols, and the choice of mAbs or radionuclides.
Figure 2.
Publications about RIT clinical trials from 2010 to 2021. (A) Number of publications per year reporting clinical trials with RIT protocols for solid and non-solid tumors. (B) Repartition of clinical trials for non-solid cancer for the entire period of 2010–2021. Leukemias encompass acute myeloid leukemias and acute lymphoblastic leukemias; lymphomas encompass follicular lymphomas, mantle cell lymphomas, Burkitt lymphomas, diffuse large B-cell lymphomas, marginal zone lymphomas, and Hodgkin lymphomas. (C) Repartition of clinical trials for solid cancers for the entire 2010–2021 period. HER2-expressing cancers encompass breast cancer, peritoneal carcinomatosis, and gastric cancer; metastatic cancers were from colorectal cancers, prostate cancers, melanoma, pancreatic carcinomas, and renal cell carcinomas. CNS: central nervous system tumors (medulloblastomas and neuroblastomas); HCC: hepatocellular carcinoma; NSCLC: non-small cell-lung cancer.
2.1. Combination for Hematologic Malignancies: Modest Outcomes-Based RIT?
During the first decade of the 2000s, positive clinical results led to the subsequent FDA-approval of two radioimmunoconjugates, 90Y-ibritumomab tiuxetan (Zevalin®, Bayer, Leverkusen, Germany) in 2002 and 131I-tositumomab (Bexxar ®, GSK, Brentford, UK) in 2003, both of which are based on murine anti-CD20. This progress then opened the door widely for RIT for hematologic malignancies [9]. Both were initially indicated for the treatment of patients with relapsed or refractory (R/R), low-grade or follicular B-cell non-Hodgkin lymphoma (B-NHL), including patients with rituximab refractory follicular NHL. In 2009, 90Y-ibritumomab tiuxetan obtained an indication for the consolidation strategy for patients with previously untreated follicular NHL who had achieved a partial or complete response to first-line chemotherapy.
After the first approval, a significant number of clinical trials (62 studies over the 2010–2021 period, summarized in Table 1 for anti-CD-20 RIT and in Table 2 for other approaches, for details see Supplementary Table S1) evaluated the interest of RIT in various hematologic malignancies and therapeutic strategies. Most studies (~90%) assessed the efficacy of anti-CD-20 radioimmunoconjugates (90Y-ibritumomab tiuxetan: n = 36, 131I-tositumomab: n = 12, radiolabeled rituximab: n = 6). Among them, the majority concerned B-NHL patients, including one-third with a follicular lymphoma (FL). Diffuse large B-cell lymphomas (DLBCL), mantle-cell lymphoma (MCL), marginal-zone lymphoma (MZL), and Burkitt lymphoma were less represented. Only five studies reported other hematologic diseases such as acute lymphoblastic leukemia (ALL, n = 1), acute myeloid leukemia (AML, n = 2), Hodgkin lymphoma (n = 1), pediatric hematologic malignancies and non-malignancies (n = 1), and multiple myeloma (MM, n = 1). Interestingly, phase II trials represented 50% of the studies, phase I and I/II trials represented 22% of the studies, and phase III trials only represented 10%. The rest of the reported studies were prospective, retrospective, or cases series.
Table 1.
Overview of anti-CD20 RIT for non-solid cancers in clinical trials from 2010 to 2021. (For details, see Supplementary Table S1).
| Target/ | Isotope | Cancer Type 1 | n 2 | Type of Studies | Line of Treatment | [Ref] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vector | I | I/II | II | III | Others 3 | 1st Line | Conditioning | Consolidation | R/R 4 | ||||
|
Ibritumomab
tiuxetan |
Y-90 | FL | 12 | 1 | 1 | 9 | 1 | − | 4 | 1 | 6 | 1 | [10,11,12,13,14,15,16,17,18,19,20,21] |
| MZL | 4 | − | − | 3 | − | 1 (P) | 2 | − | 1 | 1 | [13,17,22,23] | ||
| MCL | 7 | 1 | − | 4 | − | 1(R)/1(P) | − | 1 | 5 | 1 | [24,25,26,27,28,29,30] | ||
| DLBCL | 4 | − | − | 3 | − | 1(R) | − | 2 | 2 | − | [31,32,33,34] | ||
| Burkitt Lymphoma | 1 | − | − | − | − | 1(R) | − | − | 1 | − | [35] | ||
| B-NHL | 9 | 2 | − | 5 | − | 1(R)/1(CS) | − | 5 | 1 | 3 | [36,37,38,39,40,41,42,43,44] | ||
| MM | 1 | 1 | − | − | − | − | − | 1 | − | − | [45] | ||
| Rituximab | I-131 | FL | 1 | − | − | 1 | − | − | 1 | − | − | − | [46] |
| B-NHL | 3 | − | 1 | 1 | − | 1(R) | − | 1 | − | 2 | [47,48,49] | ||
| Lu-177 | Low-grade B-NHL | 1 | − | 1 | − | − | − | − | − | − | 1 | [50] | |
| B-NHL | 1 | − | − | − | − | 1(P) | − | − | − | 1 | [51] | ||
| Y-90 | B-NHL | 1 | − | − | − | − | 1(CS) | − | − | − | 1 | [52] | |
| Tositumomab | I-131 | FL | 2 | − | − | − | 2 | − | − | − | 2 | − | [53,54] |
| Low-grade B-NHL | 2 | − | − | 1 | 1 | − | − | − | − | 2 | [55,56] | ||
| DLBCL | 3 | − | − | 2 | 1 | − | − | 1 | 2 | − | [57,58,59] | ||
| B-NHL | 2 | 2 | − | 1 | − | 1(CS) | 2 | − | 2 | [60,61,62,63] | |||
| HL | 1 | 1 | − | − | − | − | − | − | − | 1 | [64] | ||
1 B-NHL: B-cell non-Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HL: Hodgkin lymphoma; MCL: mantle cell lymphoma; MM: multiple myeloma; MZL: marginal zone lymphoma. 2 n: number of studies. 3 CS: case series; P: prospective; R: retrospective. 4 R/R: relapsed/refractor.
Table 2.
Overview of other RIT approaches for non-solid cancers in clinical trials from 2010 to 2021.
| Target/ Vector |
Isotope | Cancer Type 1 | Phase | n 2 | Line of Treatment 3 | Association 4 (+/−) | SCT (Auto/Allo/−) |
Cold mAb (+/−) |
Fraction-Ation * (+/−) |
PFS Median (Months) or X Years PF 5 (%) |
OS Median (Months) or X Years OS (%) |
ORR (%) | CR (%) |
NCT (or eq.), [Ref] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD22 | ||||||||||||||
| Epratuzumab tetraxetan | Y-90 | ALL | I | 17 | R/R | − | − | + | − | 6.1 | 3.6 | − | − | NCT01354457, [65] |
| DLBCL | II | 71 | Consolidation | + (C) |
− | + (anti-CD20 mAb) |
− | NR 2 y: 82% |
NR 2 y: 89% |
− | 77 | NCT00906841, [66] | ||
| B-NHL | I | 18 | R/R | − | − | + (anti-CD20 mAb) |
− | 6.2 | − | 53 | 18 | - [67] | ||
| CD33 | ||||||||||||||
| Lintuzumab | Bi-213 | AML | I/II | 31 | 1st line or R/R | + (C) | − | − | + | − | 4.6 | 19 | 10 | - [68] |
| CD66 | ||||||||||||||
| Anti-CD66 | Re-188 | AML | II | 58 | Conditioning | + (C+I) | Allo | − | − | 2 y DFS: 38% | 2 y: 38% | − | − | - [69] |
| BW250-183 | Y-90 | Pediatric malignant (Mal.) and non-maligant (Non-Mal.) hemopathies | II | 30 | Conditioning | + (C) | Auto Allo |
− | − | Mal.: 12 Non-Mal.: NR |
Mal.: NR Non-Mal.: NR |
− | − | - [70] |
1 ALL: Acute lymphoblastic leukemia; AML: acute myeloid leukemia; B-NHL: B-cell non-Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma. 2 n: number of patients. 3 R/R: relapsed/refractory; SCT: stem cell transplantation. 4 (C): chemotherapy; (I): immunotherapy. 5 CR: complete response; DFS: disease-free survival; NR: non-reached; ORR: overall response rate; OS: overall survival. * Fractionation concerns RIT protocol.
2.1.1. Therapeutic Strategies Studied
Concerning B-NHL, the clinical trials evaluated RIT efficacy for relapsed/refractory (R/R) malignancies (28%) and in consolidation (32%) or conditioning (27%) strategies, and only 12% presented evaluations of RIT as a first-line therapy. One study evaluated RIT as a late intensification strategy. RIT was mostly used in combination with the corresponding cold mAb (85%) in order to saturate non-neoplastic CD-20 receptor sites and to limit toxicities. When used as a consolidation treatment, either in first-line or in R/R patients, RIT was associated with cold rituximab (R-) and with chemotherapy 89% of the time. Chemotherapy protocols were fludarabine/mitoxantrone/rituximab (FMR) for FL or MZL [10,11,12,13]; cyclophosphamide/doxorubicine/vincristine/predisolone (CHOP) [24,25,31,53,54,57,66]; cyclophosphamide/vincristine/predisone (CVP) [26]; prednisolone/etoposide/chlorambucil/lomustine (PECC) [32]; or etoposide/prednisolone/vincristine/cyclophosphamide/doxorubicine (EPOCH) [58] for more aggressive lymphomas. These chemotherapy protocols corresponded to protocols that are commonly used in clinical practice, and in most cases, RIT was an adjuvant therapeutic in these protocols. It must be noted that one case series showed that RIT using 90Y-ibritumomab tiuxetan could be beneficial in a consolidation protocol for patients with aggressive NHL who did not fully respond to autologous stem cell transplantation (Auto-SCT) (RIA 2011). In the last decade, RIT has also been evaluated as a part of the conditioning protocol before auto-SCT (n = 13) or allo-SCT (n = 5) in patients presenting untreated [69,70] or R/R [14,27,28,33,36,37,38,39,40,45,47,59,60] or transformed [34] B-NHL. Conditioning protocols use high-dose carmustine/etoposide/cytarabine/melphalan (BEAM) [27,28,33,34,38,59] or reduced-intensity conditioning (fludarabine alone [60], fludarabine/melphalan/alemtuzumab [36], or fludarabine/melphalan/thiothepa [37]) without or with total-body-irradiation [39,40]. In 75% of cases, the conditioning protocols were associated with anti-CD20 cold mAb. As a first-line strategy, anti-CD20 RIT was used without combination with CT but was systemically associated with a cold anti-CD20 mAb [12,15,16,17,22,46].
2.1.2. Low-Grade B-NHL
The efficacy and good tolerance of 90Y-ibritumomab tiuxetan [12,15,16] and 131I-rituximab [46] have been demonstrated in first-line therapy for advanced FL. However, to the best of our knowledge, this was not followed by further phase III evaluation. Phase II evaluations with 90Y-ibritumomab tiuxetan for first-line consolidation protocols in FL [10,11] showed promising results and were followed by a phase III study [18]. A clear advantage in progression-free survival (PFS) compared with no consolidation strategy was thereby demonstrated. However, phase III first-line consolidation studies using 131I-tositumomab failed to demonstrate a PFS advantage compared to cold rituximab consolidation strategies [53,54]. In the relapse/refractory consolidation strategy, RIT was also able to provide a clinical benefit, as suggested for 90Y-ibritumomab tiuxetan in a phase II trial [19,20].
Concerning R/R FL, the safety and efficacy of 90Y-ibritumomab tiuxetan has been demonstrated in a phase I study [21]. Phase III trials otherwise assessed RIT with 131I-tositumomab. However, this trial has been early terminated due to a lack of feasibility, as only 14 patients have been included, thereby failing to draw significant conclusions [55].
In MZL patients, three Phase II studies suggest that 90Y-ibritumomab tiuxetan could also have benefits in first-line [17,22] or consolidation therapy [13].
2.1.3. Aggressive B-NHL
In aggressive B-NHL encompassing transformed low-grade B-NHL, DLBCL, and MCL, anti-CD20 RIT was evaluated either for conditioning protocols before SCT or for response consolidation in untreated or R/R patients. Phase II and retrospective studies performed during a conditioning regimen followed by Auto-SCT or Allo-SCT showed that anti-CD20 RIT was well-tolerated [27,33,34,36,37,39]. A significant benefit in survival was highlighted in most of the studies evaluating anti-CD20 RIT regardless to the type of aggressive B-NHL [33,34,38,39,40,47,60]. However, in MCL, one study failed to show a significant improvement in survival compared to protocols involving cold rituximab [27]. Despite those promising results, the Phase III trial comparing conditioning 131I-tositumomab-associated BEAM or cold rituximab did not show any differences neither in terms of PFS nor in the overall survival (OS) rates for patients with chemotherapy-sensitive relapsed DLBCL [59].
Consolidation Phase II trials showed mitigated results. Some studies suggest an interest for anti-CD20 RIT in untreated [57] or R/R patients [41], whereas others alerted researchers to the potential toxicities of such a regimen [58]. In MCL, it was suggested that radiolabeled anti-CD20 could be an effective therapeutic strategy for untreated [24,25,26] or R/R patients [29]. In DLBCL, a pilot study suggested that tandem consolidation therapy using 90Y-ibritumomab tiuxetan followed by HDT with Auto-SCT is not feasible for the treatment of high-risk patients with DLBCL who are in remission after R-CHOP, and the pilot study failed to provide any beneficial effects [31]. Conversely, a recent study showed interesting response rates for relapsed DLBCL patients, especially in terms of long-term response as well as an acceptable safety profile [32].
2.1.4. Other Hematologic Diseases
In pathologies other than B-NHL, Phase I anti-CD20 RIT showed good outcomes and tolerance for a conditioning regimen followed by auto-SCT for MM (ORR: 73% and CR: 23%) [45] or for R/R Hodgkin lymphomas (2 CR/12 patients) [64]. RIT for hematologic malignancies mainly rely on anti-CD20 approaches. However, other strategies have been explored and have demonstrated interesting results, e.g., with Y-90 anti-CD22 RIT in R/R ALL [65], in DLBCL [66], and in B-NHL [67] with Bi-213 anti-CD33 in AML [68], and for conditioning with Re-188 or Y-90 anti-CD66 for AML [69] or pediatric hematologic diseases [70].
2.1.5. Modest Efficacy?
Even though the clinical interest and good RIT tolerance were undeniable for some hematologic malignancies such as FL, which has led to the approval of the two main radiolabeled anti-CD20 mAb, the last decade failed to extend the use of RIT in other indications. Indeed, multiple promising Phase II clinical trials have explored RIT in front-line consolidation or conditioning strategies. Furthermore, the rare subsequent Phase III trials failed to highlight a significant difference in anti-CD20 RIT compared to cold rituximab. Because it is well-tolerated globally, anti-CD20 RIT might be of interest for specific fragile populations such as the elderly. However, other modalities of anti-CD20 RIT, such as multi-fractionation, should be explored to determine an optimal RIT regimen for aggressive B-NHL, as their therapeutic response differs from indolent B-NHL [49]. Otherwise, RIT also faces major drawbacks, such as the economic discontinuation of 131I-tositumomab in 2014, 90Y-ibritumomab tiuxetan supply difficulties, some complex practical limitations due to the need of specialized centers, the cost of treatment, and the emergence of new treatments such as ibrutinib, which highly restrict the expansion of RIT in hematologic malignancies.
2.2. RIT for Solid Tumors: A Viable Strategy?
RIT of solid tumors represents a smaller part among the total number of studies that were identified (around 35% of the total) (Figure 2). In this section, we will first discuss RIT performed with full mAbs as the targeting vectors and then those investigating antibody fragments, such as F(ab′)2, Fab′, or ScFv. Finally, alternative RIT strategies, such as the two-step pretargeting approach, will also be described to investigate their advantage in terms of efficacy compared to the radiolabeled mAbs.
2.2.1. Overview of the Last Ten Years of RIT Involving Full-Length Antibodies
Despite improvements in radiochemistry processes and RIT protocols, the expansion of RIT for solid tumors has remained quite limited. In addition, there are two-times less clinical trials with radiolabeled full mAbs (phases I to III) for solid tumors that have been conducted over the last ten years, other than those reported by Pouget et al. in 2011 (20 vs. 48) (Table 3). We observed a lack of Phase III trials among all of the published RIT clinical trials with full mAbs as vectors, with ~50% being Phase I trials, ~40% being Phase I /II assessments, and two studies being retrospective or unspecified. Most of them assessed feasibility and toxicity, thereby making it complicated to clearly determine the therapeutic benefits of RIT. Table 2 also indicates the administration routes other than intravenous injection used in the clinical studies. Clinical trials involving local RIT injections demonstrated an absence of significant hematotoxicity and favored a higher dose delivered in situ. In addition, a retrospective study showed a dose delivered to the cerebral spinal fluid of 32.1 Gy in patients after the intraventricular injection of 131I-radiolabeled mAbs targeting GD2 or B7H3, which is neither associated with radionecrosis (<1%) nor neurologic defects [71]. With those encouraging results, intracerebroventricular RIT is currently being assessed in new clinical trials involving patients with central nervous system tumors (NCT 03275402, NCT 04022213, NCT04315246, NCT04167618, NCT03276572, and NCT04743661/EudraCT 2020-000670-22). Most of the clinical trials reported for RIT on solid tumors involved a limited number of patients who were already in the metastatic phase and who were resistant to first-line or second-line treatments. We can however discuss the target, mAb or radionuclide, as well as the addition of cold mAb or its combination with an active molecule such as chemotherapy.
Table 3.
Overview of RIT of solid tumors in clinical trials, from 2010 to 2021.
| Target/Vector | Isotope | Clinical Phase 1 | n 2 | Association 3 | Cold mAb (+/−) | Fractionation (+/−) | PFS (Months) or X-Year PFS (%) | OS (Months) or X-Year OS (%) | NCT | Year of Publication | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2-expressing breast cancer, peritoneal carcinomatosis or gastric cancer | |||||||||||
| HER2/Trastuzumab | Pb-212 | I | 16 | − | + | − | − | − | − | 2014 | [73] |
| Lu-177 | I | 10 | − | + | − | − | − | − | 2017 | [74] | |
| Medulloblastoma and neuroblastoma | |||||||||||
| B7H3/Omburtamab | I-131 | Retro | 94 | (R) | − | + | − | − |
NCT00445965 NCT00089245 |
2015 | [71] |
| GD2 /3F8 | II | 43 | - | − | + | 11 | 24.9 | − | 2018 | [75] | |
| Metastatic colorectal cancer | |||||||||||
| A33/huA33 | I-131 | I | 19 | (C) | − | − | 5 | 28.7 | NCT00291486 | 2014 | [76] |
| CEA/cT84.66 | Y-90 | I/II | 16 | (C) | − | − | 9.6 | 41.2 | − | 2017 | [77] |
| CEA/Labetuzumab | I-131 | II | 63 | - | − | − | 16 | 55 | NCT27763687 | 2017 | [78] |
| Metastatic melanoma | |||||||||||
| MSCP/cDTPA-9.2.27 | Bi-213 | I | 38 | - | − | + | 20.4 | − | − | 2011 | [79] |
| Metastatic pancreatic cancer | |||||||||||
| hPAM4 (MUC-1)/Clivatuzumab tetraxetan | Y-90 | I | 21 | - | − | + | 1.3 | 4.3 | NCT00603863 | 2011 | [80] |
| I | 42 | (C) | − | + | − | 7.7 | NCT01956812 | 2012 | [81] | ||
| Ib | 58 | (C) | − | + | − | 7.9 (A) vs. 3.4 (B) | NCT01956812 | 2015 | [82] | ||
| Metastatic prostate cancer | |||||||||||
| PSMA/J591 | Lu-177 | II | 47 | - | + | − | − | 22.2 vs. 11.4 | NCT00195039 | 2013 | [83] |
| I/II | 49 | - | − | − | − | 42.3 | NCT00538668 | 2019 | [84] | ||
| I | 15 | (C) | − | − | − | − | NCT00916123 | 2020 | [85] | ||
| I/II | 6 | - | − | + | − | − | NCT00538668 | 2020 | [86] | ||
| Metastatic renal cell carcinomaz | |||||||||||
| CAIX/Girentuximab | Lu-177 | I | 23 | - | − | + | 11.1 | 25.3 | − | 2013 | [87] |
| II | 14 | (C + R) | − | + | − | − | NCT02002312 | 2016 | [88] | ||
| Non-small cell lung cancer | |||||||||||
| DNA/chTNT | I-131 | 96 | (C + R+Pmc) | − | − | − | 23–29.1 | − | 2016 | [89] | |
| Sarcoma | |||||||||||
| FZD10/OTSA-101 | Y-90 | I | 20 | - | − | + | PR in 3/8 | − | NCT01469975 | 2018 | [90] |
| B7H3/Omburtamab | I-131 | I/II | 52 | - | − | − | − | − | NCT01099644 | 2020 | [91] |
1 Retro: retrospective. 2 n: number of patients. 3 (C): chemotherapy; (R): external-beam radiotherapy; Pmc: percutaneous microwave coagulation therapy. PFS: progression-free survival; OS: overall survival.
2.2.2. Combination RIT
In contrast to RIT for hematologic tumors, 63% of the clinical studies conducted on solid tumors were not associated with any other additional treatment or cold antibody. Some trials used Omburtamab, Girentixumab, J591, Labetuzumab, (the majority being IgG1), which possess antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and/or antibody-dependent cell-mediated phagocytosis (ADCP) mechanisms, but a only few of the studies investigated the association of RIT and the cold antibody [72]. Among them, two studies, based on trastuzumab reported the concomitant administration of cold mAb during RIT protocols [73,74].
However, both were pilot trials that were only assessing the radiolabeling and radiotoxicity of the radiolabeled trastuzumab. Interestingly, targeting PSMA with a mixture of 177Lu-J591 and cold J591 mAbs was associated with a decrease in the circulating tumor cells in 82% of patients (14 out of 17) bearing detectable circulating tumor cells [84]. It has also been recently demonstrated that the co-administration of unconjugated mAb with its cognate antibody–dye conjugate increases its penetration into solid tumors [92]. The impact of this antibody concentration on radiolabeled antibody biodistribution remains undemonstrated and should be confirmed—if possible—at the microscopic level. Another study related a synergistic association of the antibody hu33, an antibody directed towards the A33 antigen, and chemotherapy (gemcitabine) in patients with CRC [76]. No other combination RIT has been reported in previously clinical trials, thereby highlighting that idea that RIT was expected to be effective alone.
2.2.3. Choice of the Targets/mAbs/Radionuclides
If RIT of hematologic cancers has focused on four different antigen targets (i.e., CD20, CD22, CD33 and CD66) until now we have identified 12 different antigens for RIT of solid tumors, with each one only being assessed once or twice in clinical trials. The multiplication of targets makes it difficult to compare different studies to determine whether RIT is effective. Considering CRC RIT, the A33 target is of more interest than the canonic CEA target. Indeed, this protein is retained on the membrane and is not released in the blood, thereby limiting hematotoxicity and enhancing bioavailability [77,78]. Alternatively, targeting antigens on circulating tumor cells (CTC) is of interest for metastatic cancers, as demonstrated with RIT using radiolabeled J591, which is associated with a CTC decrease [84]. One of the reasons why it is difficult for RIT to be successful in the treatment of solid tumors is because it relies on the penetration of full mAbs and the subsequent low dose that is delivered. Tumor vasculature is indeed abnormal, with the leaky vessels and the presence of a high complex extracellular matrix impeding tumor cell access to mAbs. Furthermore, hypoxia, which is present in most of solid tumors, reduces radiation efficiency, except for that of alpha particles, which do not need oxygen. Solid tumor features that modulate RIT efficiency were clearly described in a recent review [93]. To circumvent these limitations, one solution could be to perform repeated treatment cycles to limit radiotoxicity. This approach was tested in different trials, with substantial results in patients with metastatic renal cell carcinoma [88], neuroblastoma or medulloblastoma [71], melanomas [79], pancreatic cancer [81,82], or sarcomas [90,91] (Table 3). It is important to note that in patients with mCRPC, the targeting of PSMA with 177Lu-J591 through fractionated doses did not provide any benefits [86]. A recent study reported the association of 177Lu-J591 and docetaxel/prednisone in a cohort of 15 patients with mCRPC without inducing additional toxicity, with taxanes being assumed to be radio sensitizers [85]. It is well known that PSMA is highly specific to prostate, cancer and further clinical trials will thereby consider the efficacy of J591 radiolabeled with the highly potent Ac-225, which gives rise to alpha particles (NCT03276572). Alpha particles (e.g., Pb-212 or Bi-213) have thereby been evaluated in two clinical trials (either on melanoma or peritoneal carcinomatosis from HER2+ primary lesions). These radionuclides-generating alpha particles with a high LET have a very short half-life and do not induce any radiotoxicity [73,79]. One problem of this very attractive strategy is the difficulty of acquiring radionuclides [94]. For instance, the production of Actinium-225 in 2018 would have only supported the treatment for several hundred patients [93].
3. Alternatives to Conventional RIT and Prospects
IgGs are excellent vectors for carrying a payload towards a membrane antigen. However, their size (~150 kDa) impedes their filtration via the kidneys—which possess a glomeruli threshold at around 70 kDa—thus resulting in major hepatocellular uptake. In addition, the Fc region of IgGs contains a FcRn sequence (i.e., neonatal Fc receptor, or Brambell receptor), a heterodimer derived from the major histocompatibility complex class I receptor that is involved in folding, transport, and FcRn functions [95]. The ability of the Fc region of IgG to bind FcRn protects them from lysosomal degradation and induces their translocation back to the cell surface. While IgG recycling is highly dependent on the IgG subclass, it still results in a serum half-life of 7 to 21 days (for IgG3 or IgG1,2,4, respectively). The long half-life of IgGs represents a major drawback underlying RIT of solid tumors, as it causes hematotoxicity and resistance phenomena due to the slow penetration of mAbs into the center of the tumor [3]. Thereby, two main strategies have been explored to circumvent these issues, both of which are based on improving the pharmacokinetic of the vector carrying the radionuclide. Firstly, the antibody fragments have been considered as a vector instead of as full-sized IgGs; secondly, RIT can be performed in two steps using pretargeting approaches.
3.1. Fragments-Based RIT
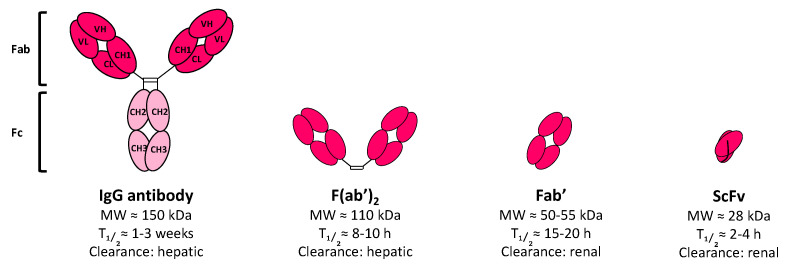
IgG fragments possess a smaller size, resulting in faster biodistribution (Figure 3). Their characteristics promote penetration in tissue as well as quicker clearance from the blood via the kidneys, and these properties are very useful for a wide variety of diagnosis or therapeutic applications [96,97,98]. In addition, fragments lack an Fc domain, thereby impeding both FcRn recycling and immune response-mediated Fc (i.e., CDC and ADCC). The main antibody fragments that have been assessed for oncology applications are schematized in Figure 3. Among them, antigen-binding antibody fragments, such as F(ab′)2 or Fab′ can be produced directly via the proteolytic cleavage of natural antibodies or can be designed by genetic engineering methods, while single chain variable fragments (ScFv) are only genetically engineered. Fragments are low costs compared to full mAbs because they can be easily produced in microbial, yeast, or human cells. All of them are designed to retain at least the same antigen specificity as full-length mAbs and can be used either as diagnosis tools or to carry a therapeutic payload to the tumor target.
Figure 3.
IgG fragment formats assessed in clinic for RIT. The molecular weight (MW), blood half-life (T), and main clearance route are given for each format.
3.1.1. Fab′
Fab′ is the oldest fragment to be assessed in clinic, with its first FDA approval being received in 1994 to prevent blood clot formation in angioplasty (Abciximab, ReoPro®, Centocor/Eli Lilly) [98]. To date, several Fab′ fragments have been also approved by the US FDA, EMA, and Chinese State FDA agencies for a wide range of applications, including for the treatment of cancers (e.g., Ranibizumab, Lucentis® derived from Bevacizumab, Avastin®, both anti-VEGF) [96]. However, none have been approved for RIT as of yet. A divalent Fab′ maleimide fragment derived from the humanized A5B7 antibody radiolabeled with I-131 was assessed for RIT in 2002 in patients bearing CRC tumors in a very small cohort of pilot study [99]. The imaging follow-up demonstrated a specific signal in patients bearing CEA-expressing tumors. However, the quantification of the scan failed to demonstrate a significant uptake in tumors compared to in the other organs. Moreover, MIRD assessment dosimetry was unfavorable for the divalent Fab′ maleimide due to a high uptake in the kidneys. Aggregates were also observed for three patients (of a total of ten) at later time points (up to 29% at 24 h), which was correlated to the amount of CEA antigens measured in patients’ serum [99].
Aggregation is one of the most well-known major drawbacks of antibody fragments, which can vary according to fragment type [100]. While the feasibility was clearly demonstrated, to the best of our knowledge, no other clinical assessments involving this Fab′ fragment have been conducted in the last ten years (Table 4).
Table 4.
Overview of fragment-based RIT in clinical trials.
| Target | Vector | n 1 | Phase | Isotope | Application 2 | Year of Publication | NCT or eq. | Ref |
|---|---|---|---|---|---|---|---|---|
| Non-solid cancer | ||||||||
| Tenascin C | F16SIP F(ab′)2 | 8 | I/II | I-131 | HL | 2014 | EudraCT2007-007259-15 | [101] |
| Solid cancers | ||||||||
| CEA | CIGB-M3 ScFv | 17 | I | I-131 | CRC * | 2011 | – | [102] |
| CD147 | HAb18 metuximab F(ab′)2 (Licartin®) |
110 | III | I-131 | HCC * | 2010 | NCT00829465 | [103] |
| 68 | P | I-131 | HCC * | 2012 | ChiCTR-TRC-08000250 | [104] | ||
| 60 | II | I-131 | HCC * | 2013 | – | [105] | ||
| 127 | III | I-131 | HCC * | 2014 | ChiCTR-TRC-10000837 | [106] | ||
| 156 | II | I-131 | HCC * | 2020 | NCT00819650 | [107] | ||
1n: number of patients. 2 CRC: colorectal cancer; HCC: hepatocellular carcinoma; HL: Hodgkin lymphoma. * Small metastases derived from those primary origins. P: prospective.
It is interesting to note that Fab′ fragments have however demonstrated some potential for diagnosis. For example, the 99mTc-nofetumimab merpentan Fab′ fragment (Verluma®, Boehringer Ingelheim Pharma KG, Ingelheim, Germany) targeting the pancarcinoma antigen expressed in cancer cells has been FDA-approved in 1992 for the diagnosis of small-cell lung cancer but was discontinued in 2013 [108]. The 99mTc-sulesomab Fab′ (LeukoScan®, Immunomedics, Morris Plains, NJ, USA) received EMA-approval in 1997 for the SPECT imaging of activated granulocytes in patients with osteomyelitis, but it was also discontinued in 2018 [109].
3.1.2. F(ab′)2
In 2011, the centruroides (scorpion) immune F(ab′)2 (equine) (Anascorp®, Rare Disease Therapeutics, Inc., Franklin, TN, USA) has been the first F(ab′)2 to be FDA-approved, for the treatment of clinical signs of scorpion envenomation [110]. This was followed by the FDA-approval of crotalidae immune F(ab′)2 (equine) (Anavip®, Rare Disease Therapeutics, Inc., Franklin, TN, USA) in 2015 for the management of coagulopathic effects in adults or in pediatric patients with North American Pit Viper envenomation (from cottonmouth, copperhead or rattlesnake) [111]. The phase III clinical assessments demonstrated a similar safety but also a better efficacy in treating venom-caused hematologic toxicity and a higher stability over time (3 years) than the Fab′ antivenom version that was approved in 2007 (CroFab®, BTG, UK). However, in oncology, the efficacy of F(ab′)2 is more contrasted. Concerning RIT, F(ab′)2 has been the most common fragment type assessed in clinical trials and still represent five studies out of the seven conducted over the last ten years that are listed in Table 4. Different antigens have been evaluated as potential targets for RIT with F(ab′)2 fragments [3], but only two have remained under investigation since 2010, CD147 [112] for metastatic solid cancers and tenascin C [101] for Hodgkin lymphoma. With the exception of CD147/metuximab, the targeting of tenascin C and CEA are still at early stage clinical assessments (Phase I/II) and only involve a small number of patients. In most cases, radiolabeling has been achieved with the conventional I-131 β-emitter (8 day half-life, mean LET of 0.25 keV/µm).
Several of the clinical trials listed in Table 4 have been performed with the 131I-metuximab HAb18G/CD147 F(ab′)2 fragment (Licartin®, Chengdu Hoist Hitech Co. Ltd, Chengdu, China), as a post-surgery treatment, or it has been combined with radiofrequency ablation in patients with recurrent metastatic hepatocellular carcinoma (HCC) [103,104,105,106,107,112,113]. RIT with metuximab was associated with mild to moderate hematologic/hepatic toxicities, ≤Grade 3, especially for injected activities ≥27.75 MBq/kg; however, this amount of activity is considered to be the most appropriate amount in order to obtain therapeutic efficacy in most studies. In addition, no damages in thyroid function nor significant positive human anti-murine antibody (HAMA) response were observed in most of the patients after two cycles of treatment. The median OS was found between 20 to 60 months for the most recent study, which was associated with a 5-year PFS of around 43.4% for the treated group compared to 21.7% for the control group [107]. In April 2015, Licartin® 131I-metuximab HAb18G/CD147 became the first radioconjugates F(ab′)2 to receive approval by the China State FDA (n°S20050039) [113,114].
While fragments have mainly been assessed for RIT of solid tumors, one phase I/II study in 2014 reported the feasibility of such an approach to treat chemorefractory Hodgkin lymphoma [101]. 131I-tenarad F16SIP, an 80-kDa F(ab′)2, recognizes the extra-domain A1 of tenascin C, which is highly expressed in extracellular matrix of tumors. Eight patients received 2.05 GBq/m2 after a median of three previous lines of chemotherapy, and seven out of the eight patients had undergone bone marrow transplantation. All of the patients showed moderate to severe thrombocytopenia (up to Grade 3) plus Grade 2/3 neutropenia and lymphopenia, and one patient suffered from severe multilineage hematological toxicity (Grade 4) 5 weeks after the administration of the first dose of Tenarad (4.18 GBq). While this single study demonstrated the proof-of-concept of tenascin C targeting for RIT, the high related toxicity implies mandatory protocol modifications for the administration of the most effective and the safest therapeutic option.
Despite encouraging clinical trials, RIT with the F(ab′)2 fragment is still not approved by the US and European agencies. Furthermore, to the best of our knowledge, there is no F(ab′)2 that has been approved by the US FDA or EMA for use in oncology.
3.1.3. ScFv
Single-chain antibody fragments (ScFv) are genetically engineered fragments of about 28 kDa and are composed of the antibody VH and VL domains connected by a flexible polypeptide linker (Figure 3). Their small size allows for their quick clearance via kidney glomeruli filtration after a few hours. In oncology, ScFv fragments have been widely used in chimeric antigen receptor (CAR) T-cell-based immunotherapy to engineer T-cells with a recombinant receptor in order to redirect them toward tumor-associated antigens [115]. In 2017/2018, two CD19-targeted CAR T-cells were approved by the US FDA for the treatment of hematologic tumors: R/R B-cell ALL and/or R/R DLBCL (Tisagenlecleucel, Kymriah®, Novartis, Cambridge, MA, USA; Axicabtagene ciloleucel, Yescarta®, Kite Pharma, Los Angeles, CA, US) [115]. Moreover, ScFv possessing an antigen-binding capacity have also been used for the engineering of natural killer (NK) cell immune checkpoint inhibitors to improve their anti-cancer activity [116]. However, in both cases, the ScFv is used for ex vivo engineering construction and is not administered alone in patients as a therapeutic protein. To the best of our knowledge, the only FDA approval for ScFv antibody fragments has concerned applications outside of the oncology field, such as Brolucizumab (Beovu®, Novartis, Cambridge, MA, USA), a humanized 26-kDa ScFv inhibiting VEGF-A that was US FDA-approved in October 2019 for the treatment of neovascular (wet) age-related macular degeneration [117].
In RIT clinical trials (Table 4), the only study describing the use of radiolabeled ScFv was performed in 2011 through a phase I trial using 131I-CIGB-M3 trivalent ScFv that is specific for CEA targeting in patients bearing colorectal metastasis [102]. The feasibility was assessed on a small cohort of 17 patients who were divided into two groups that received either 0.3 mg or 1 mg of CIGB-M3 ScFv for similar injected activities of about 185–259 MBq of I-131. Toxicity evaluations demonstrated the low off-target toxicity of the ScFv fragment in both groups and was associated with lower HAMA responses compared to patients receiving a single 1 mg dose of the parental CB-CEA-1 full antibody. While trivalent CIGB-M3 ScFv demonstrated interesting pharmacokinetic outcomes and quite favorable dosimetry (0.07 ± 0.02 to 0.08 ± 0.02 mGy/MBq in the whole body, 1.1 ± 0.6 to 2.0 ± 1.3 mGy/MBq in kidneys, it has however not been pursued clinical development so far.
3.1.4. Limitations and Prospects of Antibody Fragments
The investigations summarized in Table 4 illustrate the difficulty of engineering robust antibody fragments with high affinity towards antigens, favorable pharmacokinetic profiles, low toxicity, and good stability over time. Fragments must also be able to undergo harsh radiochemical processes without the induction of deleterious effects on the previously mentioned properties. Concerning small antibody fragments, such as ScFv or single-domain antibodies, which are also called nanobodies, 15-kDa fragments derived from Camelidae antibodies, radiochemical processes are known to dramatically affect their affinity towards antigens [98,118]. These small fragments are also more prone to aggregation and possess a lower thermostability than full IgGs or bigger fragments. The scare and high disparity of clinical studies also makes it difficult to conclude about the efficiency of antibody fragments for RIT application. Indeed, the low number of studies, the low number of enrolled patients, and the high variety of fragment formats or targets is quite disappointing, resulting in little evidence representing the efficacy of RIT with fragments. However, such strategies remain of interest for RIT or nuclear imaging applications, but these strategies still require optimization. Recent advances in phage-display technology, chemical and chemoenzymatic engineering, and radiolabeling protocols have introduced new insights to circumvent most of these drawbacks and have shown promising outcomes for antibody fragments in preclinical studies [5,119].
3.2. Pretargeted Radioimmunotherapy (PRIT)
The other potential strategy for improving clearance and to reduce off-target toxicity is to delay the administration of the radionuclide from one of the mAbs, delaying administration by a few hours to several days, through the so-called pretargeting (or PRIT) approach (Figure 4) [120,121].
Figure 4.
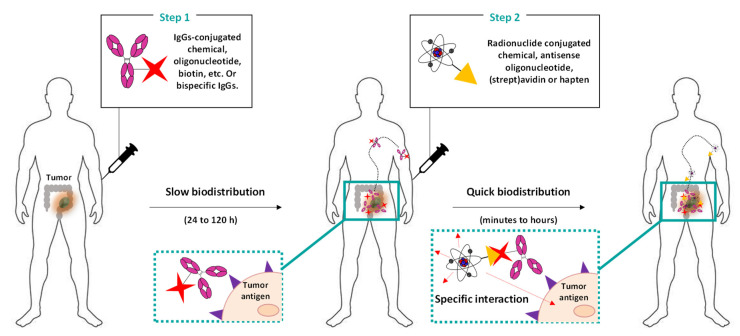
Pretargeted radioimmunotherapy (PRIT): general strategy. Antibodies or fragments are first functionalized with biotin, avidin, and oligonucleotides (e.g., phosphorodiamidate morpholinos, peptide nucleic acids) and are then chemically engineered to be bispecific. Then, the conjugated mAbs are administered (in most cases intravenously). After a delay of 24–72 h (to allow for the sufficient clearance of unbounded conjugated mAbs), radionuclides functionalized with a specific counterpart that is only able to recognize the conjugates attached to the mAbs (such as biotin, (strept)avidin, countersense oligonucleotide, chemical of haptens) are administered (intravenously, intraperitoneally, or locally). Due to the small size of the radioligand, the biodistribution and clearance are very quick (few hours), thereby limiting the off-target irradiation of healthy tissues.
Full mAbs or fragments are conjugated with a biological or chemical moiety and are first administered to have time to accumulate into the tumor before the administration of the radioactive component (delayed from few hours to several days) [121]. The radioligand is designed to carry a reactive payload that is highly specific to one of the conjugated mAbs. In addition, a high affinity is required between the two reactive species in order to obtain a quick reaction (k2 = 101–107 M−1s−1). Due to the small size of the radioligand, there is a quick biodistribution and a short half-life in the blood. The PRIT approach is thereby particularly interesting to reduce the off-target toxicity of heathy tissues.
Different PRIT strategies have been developed in the last 30 years. The oldest strategy involves a very high affinity between the biotin and (strept)avidin proteins. Despite interesting outcomes in preclinical trials, clinical trials phase I/II demonstrated the important immunogenicity of (strept)avidin and the off-target binding of endogenous biotin. Regarding those drawbacks, biotin-(strep)avidin pretargeting systems have still not yet been introduced to the market. Another PRIT strategy based on bispecific (or multispecific) antibodies (bsAbs) or fragments was developed soon after. This strategy required genetic and chemical engineering to design bsAbs with one (or two) antigen binding domain directed against a tumor antigen plus another one that was specific to a small, radiolabeled peptide. The engineering process led to the development and clinical trials of several bsAbs formats for oncology; their advantages/drawbacks and applications are detailed in specific reviews such as [122,123]. To date, only three bispecific formats have been marketed: (i) blinatumomab (Blincyto®, Amgen, Thousand Oaks, CA, USA), a bispecific tandem ScFv anti-CD3 × CD19 that became FDA-approved in 2015 for the treatment of ALL and B-ALL; (ii) emicizumab (Hemlibra®, Roche, Basel, Switzerland), a bispecific IgG4 anti-FIX × FX that was EMA-approved in 2018 for the restoration of the missing FVIIIa function in patients with haemophilia A [124]; and (iii) catumaxomab (Removab®, Trion Pharma GmbH, Munich, Germany), a chimeric bispecific rat/mouse IgG1 anti-EpCAM × anti-CD3 that became EMA-approved in 2009 for the treatment of patients with malignant ascites and that was withdrawn for economic reasons in 2013 (US) and 2017 (EU).
In PRIT clinical assays, bsAbs have been developed to target CEA (Table 5). In 2012, a phase II study was performed with a chimeric bispecific human/mouse (hMN-14 × m734) F(ab′)2, an anti-CEA × anti-diethylenetriaminepentaacetic acid (DTPA) radiolabeled with I-131 for the treatment of patients with medullary thyroid carcinoma [125]. The administration of bsAbs (40 mg/m2) and 131I-DTPA (1.8 GBq/m2) were delayed from 4 to 6 days. The results showed that PRIT was well tolerated in most of the patients and that it was associated with low increases of HAMA and human anti-human antibody (HAHA) responses (2.3% and 26.2%, respectively). In addition, the efficacy of PRIT was demonstrated to have disease control in 76% of patients and was associated with a median PFS of 13.6 months and a median OS of 43.9 months. Recently, the chimeric (hMN-14 × m734) F(ab′)2 has also demonstrated interesting outcomes for the treatment of metastatic colorectal cancer [78].
Table 5.
Overview of PRIT in clinical trials over the last ten years.
| Target | Antibody | Vector | n | Phase | Isotope | Application 1 | Year of Publication |
NCT | Ref |
|---|---|---|---|---|---|---|---|---|---|
| CEA | hMN14 × m734 F(ab′)2 |
Di-DTPA | 42 | II | I-131 | MTC | 2012 | NCT00467506 | [125] |
| 63 | II | I-131 | mCRC | 2016 | - | [78] | |||
| TF2 | IMP-288 | 21 | I | Lu-177 | mCRC | 2013 | NCT00860860 | [126] | |
| 18 | I/II | Lu-177 | SCLC and NSCLC | 2015 |
NCT01221675, EudraCT 200800603096 |
[127] |
1 MTC: medullary thyroid carcinoma; mCRC: metastatic colorectal cancer; NSCLC: non-small cell lung carcinoma.
A new generation of bsAbs formats have then been assessed to decrease the observed immunogenicity of the chimeric F(ab′)2. Trispecific humanized TF2 Fab′ is composed of an anti- histamine-succinyl-glutamine (HSG) fragment that is derived from the 679 anti-HSG IgG1 and 2 fragments of the humanized anti-CEA derived from hMN-14 IgG1 (labetuzumab®, CEA-CIDE, Immunomedics, Inc., Morris Plains, NJ, USA) [128]. The first-in-man study of Schoffelen et al. (NCT00860860), which was published in 2013, demonstrated the feasibility and safety of PRIT with TF2 and 177Lu-IMP-288 (a 1.5 kDa HSG peptide) in patients bearing metastatic CRC [126]. A previous imaging study with TF2/111In-IMP-288 determined that an administration interval of 24 h between the administration of both treatments was the most suitable. Patients received a mean injected activity of 5.6 GBq (dose escalation from 2.5 to 7.4 GBq), with no significant differences being observed between cohorts. However, the HAHA response toward TF2 was measured to be quite high (mean 386 ng/mL−1) in 11 of the 21 patients 1 week following the administration, and this response gradually increased over the 8-week follow-up period, suggesting that TF2 was surprisingly immunogenic despite being humanized and lacking an Fc portion. PRIT with bsAbs thereby represents a viable alternative for metastatic cancers but still requires further study to optimize the appropriate medication and to reduce immunogenicity.
More recent pretargeting systems involving oligonucleotides or chemical moieties have demonstrated promising outcomes in preclinical studies. While the low stability of oligonucleotides still impedes its clinical transfer, systems employing bioorthogonal chemistry only entered clinical trials testing their application as antibody-drug conjugate (NCT04106492) last October. In such strategies, mAbs are functionalized with the trans-cyclooctene (TCO), and the radioligand is conjugated to tetrazines (Tz), with both entities being non-immunogenic and highly reactive (k2 up to 106 m’1s’1) and able to specifically interact in vivo to form a covalent bond through the inverse-electron demand [4 + 2] Diels–Alder cycloaddition [121]. The application of the TCO/Tz bioorthogonal reaction is however still challenging, especially when considering the isomerization risks of the TCO ring, which is photosensitive but that still represents a potential prospect for the PRIT of solid tumors.
4. Conclusions
Despite numerous clinical trials assessing the FDA-authorized anti-CD20 radiopharmaceuticals 90Y-ibritumomab-tiuxetan and 131I-tositumumab through different strategies (first-line, consolidation, SCT-conditioning), the last ten years have failed to expand anti-CD20 RIT indications but have confirmed that RIT using radiolabeled anti-CD20 remains a pertinent choice for patients with follicular lymphomas, especially after relapse. These radiolabeled mAbs can indeed enhance survival in low-grade B-NHL. However, even though the use of the cold anti-CD20 mAb, rituximab, showed excellent results when applied generally, it may have limited the use of RIT in hematologic malignancies. In contrast to the last decade, there are, to our knowledge, only 10 ongoing clinical trials of RIT for hematologic malignancies, and among them, only one targets CD20 (Table 6). This may traduce a decline of interest for this therapeutic strategy in hematologic malignancies.
Table 6.
Ongoing RIT in clinical trials, either for solid or non-solid tumors.
| NCT | Starting Date | Phase | Target | Vector | Isotope | Application 1 | Administration Route 2 | Association 3 (+/−) | Cold mAb (+/−) |
|---|---|---|---|---|---|---|---|---|---|
| Non-solid tumors | |||||||||
| NCT01796171 | 2012 | I/II | CD37 | Betalutin | Lu-177 | NHL/FL | I.V | - | + |
| NCT02320292 | 2015 | III | CD20 | Ibritumomab tiuxetan | Y-90 | FL | I.V | - | + |
| NCT04082286 | 2016 | I | CD66 | Anti-CD66 | Y-90 | AL/AML | I.V | + (T) | - |
| NCT03128034 | 2017 | I/II | CD45 | BC8-B10 | At-211 | AML | I.V | + (C + R) | - |
| NCT02658968 | 2017 | I | CD37 | Betalutin | Lu-177 | DLBCL | I.V | - | + |
| NCT03806179 | 2018 | I | CD37 | Betalutin | Lu-177 | NHL/FL | I.V | - | + |
| NCT04466475 | 2020 | I | CD38 | OK10-B10 | At-211 | MM | I.V | + (C + T) | |
| NCT04083183 | 2020 | I/II | CD45 | BC8-B10 | At-211 | Non-mal. hemopathies | I.V | + (C + T + R) | + |
| NCT04856215 | 2021 | II | CD66 | Anti-CD66 | Y-90 | Leukemia | I.V. | + (T + R) | - |
| NCT04871607 | 2021 | II | CD25 | Basiliximab | Y-90 | R/R HL | I.V. | + (C + R + T) | + |
| Solid tumors | |||||||||
| NCT02454010 | 2015 | I | CDH3 | FF21101 | Y-90 | Adv. Solid T. | Not. Spec. | - | - |
| ChiCTR-IPR-17011206 | 2017 | III | CD147 | Metuximab * | I-131 | HCC | I.V. | – | – |
| NCT03724747 | 2018 | I | Mesothelin | BAY2315497 | Th-227 | mCRPC | Not Spec. | + (H) | - |
| NCT03507452 | 2018 | I | BAY2287411 | Th-227 | OC, Adv. Solid T. | Not Spec. | - | - | |
| NCT03275402 | 2018 | II/III | B7H3 | Omburtamab | I-131 | CNS meta. | I.C | - | - |
| NCT04022213 | 2019 | II | I-131 | SRCT/PC | + (R) | - | |||
| NCT04644770 | 2020 | I | hK21 | h11B6 | Ac-225 | mCRPC | I.V. | - | - |
| NCT04674722 | 2020 | I | HER2 | NM02 ** | Re-188 | Breast cancer | I.V. | - | - |
| NCT04315246 | 2020 | I/II | B7H3 | Omburtamab | Lu-177 | LP meta. | I.V. | - | - |
| NCT04167618 | 2020 | I/II | Lu-177 | MB | - | - | |||
|
NCT04743661 Eudra CT 2020-000670-22 |
2021 | II | I-131 | MB | + (C) | - | |||
| NCT03276572 | 2021 | I | PSMA | J591 | Ac-225 | mCRPC | I.V | - | - |
1 Adv solid T.: advanced solid tumors; AL: acute lymphoma; AML: acute myeloid leukemia; CNS meta.: metastases from central nervous system tumors (neuroblastoma + leptomeningeal metastasis); DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HCC: hepatocellular carcinoma; hK2: human kallikrein-2; LP meta.: leptomeningeal metastasis; MB: medulloblastoma; mCRPC: metastatic castration-resistant prostate cancer; MM: multiple myeloma; NHL: non-Hodgkin lymphoma; NSCLC: non-small cell lung carcinoma; Not Spec.: not specified; SLCL: small-cell lung cancer; OC: ovarian cancer; PC: peritoneal cancers; R/R HL: relapsed/refractory Hodgkin lymphoma; RSRCT: small-round cell tumors. 2 I.V.: intravenous; I.C.: intracerebroventricular. 3 (C): chemotherapy; (H): hormone therapy; (R): external-beam radiotherapy; (T): autologous hematopoietic stem cell transplantation. * F(ab′)2; ** single-domain antibody (nanobody).
Moreover, in solid tumors, there is no RIT that has been validated by the FDA nor the EMA as of yet. Metuximab only received China State FDA approval in 2005, and the data from clinical trials are quite disappointing. Despite encouraging results highlighting the interest of several different potential targets, there are still few data available, and most of the studies did not reach the phase III investigation step. However, it is interesting to note that the amount of RIT in solid tumors has increased significantly and has recently reached a peak, thereby representing more than 50% of the on-going RIT clinical trials (Table 6). The high variety of newly discovered targets/antibodies combined with the use of potent α-emitters suggests new promising prospects for RIT in solid tumors, especially for metastatic malignancies. In addition, recent advances in small mAbs fragment bioconjugation as well as pretargeted strategies represent assets for improving the efficacy and for expanding RIT for solid tumors in the next decade.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13215570/s1, Table S1. Details of anti-CD20 RIT for non-solid cancers in clinical trials, from 2010 to 2021.
Author Contributions
Conceptualization, A.R. and F.D.; methodology, A.R.; software, A.R. and J.R.; validation, A.R., J.R. and F.D.; formal analysis, A.R., J.R. and F.D.; investigation, A.R., J.R. and F.D.; resources, A.R. and F.D.; data curation, A.R.; writing−original draft preparation, A.R. and F.D.; writing−review and editing, A.R., J.R. and F.D.; visualization, A.R.; supervision, F.D.; funding acquisition, F.D. All authors have read and agreed to the published version of the manuscript.
Funding
F. Degoul received financial support from the Ligue Régionale Contre le Cancer (Comité de l’Allier, de la Haute-Loire et du Puy de Dôme) for a research project involving RIT.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bosch F., Rosich L. The Contributions of Paul Ehrlich to Pharmacology: A Tribute on the Occasion of the Centenary of His Nobel Prize. Pharmacology. 2008;82:171–179. doi: 10.1159/000149583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Teulon I., Lozza C., Pelegrin A., Vives E., Pouget J.P. General overview of radioimmunotherapy of solid tumors. Immunotherapy. 2013;5:467–487. doi: 10.2217/imt.13.34. [DOI] [PubMed] [Google Scholar]
- 4.Larson S.M., Carrasquillo J.A., Cheung N.K., Press O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer. 2015;15:347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrero Alvarez N., Bauer D., Hernandez-Gil J., Lewis J.S. Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem. 2021;16:2909–2941. doi: 10.1002/cmdc.202100135. [DOI] [PubMed] [Google Scholar]
- 6.Pouget J.P., Lozza C., Deshayes E., Boudousq V., Navarro-Teulon I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015;2:12. doi: 10.3389/fmed.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pouget J.P., Navarro-Teulon I., Bardies M., Chouin N., Cartron G., Pelegrin A., Azria D. Clinical radioimmunotherapy—The role of radiobiology. Nat. Rev. Clin. Oncol. 2011;8:720–734. doi: 10.1038/nrclinonc.2011.160. [DOI] [PubMed] [Google Scholar]
- 8.Pouget J.P., Georgakilas A.G., Ravanat J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid. Redox Signal. 2018;29:1447–1487. doi: 10.1089/ars.2017.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kersten M.J. Radioimmunotherapy in follicular lymphoma: Some like it hot. Transfus Apher Sci. 2011;44:173–178. doi: 10.1016/j.transci.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Zinzani P.L., Derenzini E., Pellegrini C., Rigacci L., Fabbri A., Gandolfi L., Argnani L., Casadei B., Pulsoni A., Gobbi M., et al. Long-term efficacy and toxicity results of the FLUMIZ trial (fludarabine and mitoxantrone followed by yttrium-90 ibritumomab tiuxetan in untreated follicular lymphoma) Ann. Oncol. 2012;23:805–807. doi: 10.1093/annonc/mdr633. [DOI] [PubMed] [Google Scholar]
- 11.Zinzani P.L., Tani M., Pulsoni A., De Renzo A., Stefoni V., Broccoli A., Montini G.C., Fina M., Pellegrini C., Gandolfi L., et al. A phase II trial of short course fludarabine, mitoxantrone, rituximab followed by (9)(0)Y-ibritumomab tiuxetan in untreated intermediate/high-risk follicular lymphoma. Ann. Oncol. 2012;23:415–420. doi: 10.1093/annonc/mdr145. [DOI] [PubMed] [Google Scholar]
- 12.Scholz C.W., Pinto A., Linkesch W., Linden O., Viardot A., Keller U., Hess G., Lastoria S., Lerch K., Frigeri F., et al. (90)Yttrium-ibritumomab-tiuxetan as first-line treatment for follicular lymphoma: 30 months of follow-up data from an international multicenter phase II clinical trial. J. Clin. Oncol. 2013;31:308–313. doi: 10.1200/JCO.2011.41.1553. [DOI] [PubMed] [Google Scholar]
- 13.Karmali R., Kassar M., Venugopal P., Shammo J.M., Fung H.C., Bayer R., O’Brien T., Gregory S.A. Safety and efficacy of combination therapy with fludarabine, mitoxantrone, and rituximab followed by yttrium-90 ibritumomab tiuxetan and maintenance rituximab as front-line therapy for patients with follicular or marginal zone lymphoma. Clin. Lymphoma Myeloma Leuk. 2011;11:467–474. doi: 10.1016/j.clml.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Khouri I.F., Saliba R.M., Erwin W.D., Samuels B.I., Korbling M., Medeiros L.J., Valverde R., Alousi A.M., Anderlini P., Bashir Q., et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012;119:6373–6378. doi: 10.1182/blood-2012-03-417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illidge T.M., Mayes S., Pettengell R., Bates A.T., Bayne M., Radford J.A., Ryder W.D., Le Gouill S., Jardin F., Tipping J., et al. Fractionated (9)(0)Y-ibritumomab tiuxetan radioimmunotherapy as an initial therapy of follicular lymphoma: An international phase II study in patients requiring treatment according to GELF/BNLI criteria. J. Clin. Oncol. 2014;32:212–218. doi: 10.1200/JCO.2013.50.3110. [DOI] [PubMed] [Google Scholar]
- 16.Ibatici A., Pica G.M., Nati S., Vitolo U., Botto B., Ciochetto C., Petrini M., Galimberti S., Ciabatti E., Orciuolo E., et al. Safety and efficacy of (90) yttrium-ibritumomab-tiuxetan for untreated follicular lymphoma patients. An Italian cooperative study. Br. J. Haematol. 2014;164:710–716. doi: 10.1111/bjh.12695. [DOI] [PubMed] [Google Scholar]
- 17.Samaniego F., Berkova Z., Romaguera J.E., Fowler N., Fanale M.A., Pro B., Shah J.J., McLaughlin P., Sehgal L., Selvaraj V., et al. 90Y-ibritumomab tiuxetan radiotherapy as first-line therapy for early stage low-grade B-cell lymphomas, including bulky disease. Br. J. Haematol. 2014;167:207–213. doi: 10.1111/bjh.13021. [DOI] [PubMed] [Google Scholar]
- 18.Morschhauser F., Radford J., Van Hoof A., Botto B., Rohatiner A.Z., Salles G., Soubeyran P., Tilly H., Bischof-Delaloye A., van Putten W.L., et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: Updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J. Clin. Oncol. 2013;31:1977–1983. doi: 10.1200/JCO.2012.45.6400. [DOI] [PubMed] [Google Scholar]
- 19.Illidge T.M., McKenzie H.S., Mayes S., Bates A., Davies A.J., Pettengell R., Stanton L., Cozens K., Hampson G., Dive C., et al. Short duration immunochemotherapy followed by radioimmunotherapy consolidation is effective and well tolerated in relapsed follicular lymphoma: 5-year results from a UK National Cancer Research Institute Lymphoma Group study. Br. J. Haematol. 2016;173:274–282. doi: 10.1111/bjh.13954. [DOI] [PubMed] [Google Scholar]
- 20.Miura K., Tsujimura H., Masaki Y., Iino M., Takizawa J., Maeda Y., Yamamoto K., Tamura S., Yoshida A., Yagi H., et al. Consolidation with (90) Yttrium-ibritumomab tiuxetan after bendamustine and rituximab for relapsed follicular lymphoma. Hematol. Oncol. 2021;39:51–59. doi: 10.1002/hon.2809. [DOI] [PubMed] [Google Scholar]
- 21.Roy R., Evens A.M., Patton D., Gallot L., Larson A., Rademaker A., Cilley J., Spies S., Variakojis D., Gordon L.I., et al. Bortezomib may be safely combined with Y-90-ibritumomab tiuxetan in patients with relapsed/refractory follicular non-Hodgkin lymphoma: A phase I trial of combined induction therapy and bortezomib consolidation. Leuk. Lymphoma. 2013;54:497–502. doi: 10.3109/10428194.2012.722215. [DOI] [PubMed] [Google Scholar]
- 22.Lossos I.S., Fabregas J.C., Koru-Sengul T., Miao F., Goodman D., Serafini A.N., Hosein P.J., Stefanovic A., Rosenblatt J.D., Hoffman J.E. Phase II study of (90)Y Ibritumomab tiuxetan (Zevalin) in patients with previously untreated marginal zone lymphoma. Leuk. Lymphoma. 2015;56:1750–1755. doi: 10.3109/10428194.2014.975801. [DOI] [PubMed] [Google Scholar]
- 23.Vanazzi A., Grana C., Crosta C., Pruneri G., Rizzo S., Radice D., Pinto A., Calabrese L., Paganelli G., Martinelli G. Efficacy of (9)(0)Yttrium-ibritumomab tiuxetan in relapsed/refractory extranodal marginal-zone lymphoma. Hematol. Oncol. 2014;32:10–15. doi: 10.1002/hon.2078. [DOI] [PubMed] [Google Scholar]
- 24.Smith M.R., Hong F., Li H., Gordon L.I., Gascoyne R.D., Paietta E.M., Advani R.H., Forero-Torres A., Horning S.J., Kahl B.S. Mantle cell lymphoma initial therapy with abbreviated R-CHOP followed by (90)Y-ibritumomab tiuxetan: 10-year follow-up of the phase 2 ECOG-ACRIN study E1499. Leukemia. 2017;31:517–519. doi: 10.1038/leu.2016.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith M.R., Li H., Gordon L., Gascoyne R.D., Paietta E., Forero-Torres A., Kahl B.S., Advani R., Hong F., Horning S.J. Phase II study of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy followed by yttrium-90-ibritumomab tiuxetan in untreated mantle-cell lymphoma: Eastern Cooperative Oncology Group Study E1499. J. Clin. Oncol. 2012;30:3119–3126. doi: 10.1200/JCO.2012.42.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurczak W., Gruszka A.M., Sowa Staszczak A., Dlugosz-Danecka M., Szostek M., Zimowska-Curylo D., Giza A., Krawczyk K., Jakobczyk M., Hubalewska-Dydejczyk A., et al. Consolidation with (90)Y ibritumomab tiuxetan radioimmunotherapy in mantle cell lymphoma patients ineligible for high dose therapy: Results of the phase II multicentre Polish Lymphoma Research Group trial, after 8-year long follow-up. Leuk. Lymphoma. 2019;60:2689–2696. doi: 10.1080/10428194.2019.1602261. [DOI] [PubMed] [Google Scholar]
- 27.Berger M.D., Branger G., Klaeser B., Taleghani B.M., Novak U., Banz Y., Mueller B.U., Pabst T. Zevalin and BEAM (Z-BEAM) versus rituximab and BEAM (R-BEAM) conditioning chemotherapy prior to autologous stem cell transplantation in patients with mantle cell lymphoma. Hematol. Oncol. 2016;34:133–139. doi: 10.1002/hon.2197. [DOI] [PubMed] [Google Scholar]
- 28.Kolstad A., Laurell A., Jerkeman M., Gronbaek K., Elonen E., Raty R., Pedersen L.B., Loft A., Bogsrud T.V., Kimby E., et al. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood. 2014;123:2953–2959. doi: 10.1182/blood-2013-12-541953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohloch K., Windemuth-Kieselbach C., Zinzani P.L., Cacchione R., Jurczak W., Suh C., Trumper L., Scholz C.W. Radioimmunotherapy for mantle cell lymphoma: 5-year follow-up of 90 patients from the international RIT registry. Ann. Hematol. 2020;99:1073–1079. doi: 10.1007/s00277-020-03956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaven A.W., Shea T.C., Moore D.T., Feldman T., Ivanova A., Ferraro M., Ford P., Smith J., Goy A. A phase I study evaluating ibritumomab tiuxetan (Zevalin(R)) in combination with bortezomib (Velcade(R)) in relapsed/refractory mantle cell and low grade B-cell non-Hodgkin lymphoma. Leuk. Lymphoma. 2012;53:254–258. doi: 10.3109/10428194.2011.608445. [DOI] [PubMed] [Google Scholar]
- 31.Han E.J., Lee S.E., Kim S.H., Sohn H.S., Jung S.E., Park G., Choi B.O., Lee S.N., Yang S.W., Han K., et al. Clinical outcomes of post-remission therapy using (90)yttrium ibritumomab tiuxetan (Zevalin(R)) for high-risk patients with diffuse large B-cell lymphoma. Ann. Hematol. 2011;90:1075–1082. doi: 10.1007/s00277-011-1191-6. [DOI] [PubMed] [Google Scholar]
- 32.Lugtenburg P.J., Zijlstra J.M., Doorduijn J.K., Bohmer L.H., Hoogendoorn M., Berenschot H.W., Beeker A., van der Burg-de Graauw N.C., Schouten H.C., Bilgin Y.M., et al. Rituximab-PECC induction followed by (90) Y-ibritumomab tiuxetan consolidation in relapsed or refractory DLBCL patients who are ineligible for or have failed ASCT: Results from a phase II HOVON study. Br. J. Haematol. 2019;187:347–355. doi: 10.1111/bjh.16087. [DOI] [PubMed] [Google Scholar]
- 33.Briones J., Novelli S., Garcia-Marco J.A., Tomas J.F., Bernal T., Grande C., Canales M.A., Torres A., Moraleda J.M., Panizo C., et al. Autologous stem cell transplantation after conditioning with yttrium-90 ibritumomab tiuxetan plus BEAM in refractory non-Hodgkin diffuse large B-cell lymphoma: Results of a prospective, multicenter, phase II clinical trial. Haematologica. 2014;99:505–510. doi: 10.3324/haematol.2013.093450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei M., Wondergem M.J., Palmer J.M., Shimoni A., Hasenkamp J., Tsai N.C., Simpson J., Nademanee A., Raubitschek A., Forman S.J., et al. Autologous transplantation for transformed non-Hodgkin lymphoma using an yttrium-90 ibritumomab tiuxetan conditioning regimen. Biol. Blood Marrow Transplant. 2014;20:2072–2075. doi: 10.1016/j.bbmt.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Koechli V., Klaeser B., Banz Y., Mueller B.U., Pabst T. Consolidation of first remission using radioimmunotherapy with yttrium-90-ibritumomab-tiuxetan in adult patients with Burkitt lymphoma. Leuk. Res. 2015;39:307–310. doi: 10.1016/j.leukres.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Bethge W.A., von Harsdorf S., Bornhauser M., Federmann B., Stelljes M., Trenschel R., Baurmann H., Dittmann H., Faul C., Vogel W., et al. Dose-escalated radioimmunotherapy as part of reduced intensity conditioning for allogeneic transplantation in patients with advanced high-grade non-Hodgkin lymphoma. Bone Marrow Transplant. 2012;47:1397–1402. doi: 10.1038/bmt.2012.62. [DOI] [PubMed] [Google Scholar]
- 37.Cabrero M., Martin A., Briones J., Gayoso J., Jarque I., Lopez J., Grande C., Heras I., Arranz R., Bernal T., et al. Phase II Study of Yttrium-90-Ibritumomab Tiuxetan as Part of Reduced-Intensity Conditioning (with Melphalan, Fludarabine +/− Thiotepa) for Allogeneic Transplantation in Relapsed or Refractory Aggressive B Cell Lymphoma: A GELTAMO Trial. Biol. Blood Marrow Transplant. 2017;23:53–59. doi: 10.1016/j.bbmt.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan A.Y., Palmer J., Nademanee A.P., Chen R., Popplewell L.L., Tsai N.C., Sanchez J.F., Simpson J., Spielberger R., Yamauchi D., et al. Phase II Study of Yttrium-90 Ibritumomab Tiuxetan Plus High-Dose BCNU, Etoposide, Cytarabine, and Melphalan for Non-Hodgkin Lymphoma: The Role of Histology. Biol. Blood Marrow Transplant. 2017;23:922–929. doi: 10.1016/j.bbmt.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopal A.K., Guthrie K.A., Rajendran J., Pagel J.M., Oliveira G., Maloney D.G., Matesan M.C., Storb R.F., Press O.W. (9)(0)Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118:1132–1139. doi: 10.1182/blood-2010-12-324392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassaday R.D., Storer B.E., Sorror M.L., Sandmaier B.M., Guthrie K.A., Maloney D.G., Rajendran J.G., Pagel J.M., Flowers M.E., Green D.J., et al. Long-term outcomes of patients with persistent indolent B cell malignancies undergoing nonmyeloablative allogeneic transplantation. Biol. Blood Marrow Transplant. 2015;21:281–287. doi: 10.1016/j.bbmt.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ria R., Musto P., Reale A., Guariglia R., Iodice G., Dammacco F., Vacca A. 90Y-ibritumomab tiuxetan as consolidation therapy after autologous stem cell transplantation in aggressive non-Hodgkin lymphoma. J. Nucl. Med. 2011;52:891–895. doi: 10.2967/jnumed.110.084376. [DOI] [PubMed] [Google Scholar]
- 42.Witzig T.E., Wiseman G.A., Maurer M.J., Habermann T.M., Micallef I.N., Nowakowski G.S., Ansell S.M., Colgan J.P., Inwards D.J., Porrata L.F., et al. A phase I trial of immunostimulatory CpG 7909 oligodeoxynucleotide and 90 yttrium ibritumomab tiuxetan radioimmunotherapy for relapsed B-cell non-Hodgkin lymphoma. Am. J. Hematol. 2013;88:589–593. doi: 10.1002/ajh.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puvvada S.D., Guillen-Rodriguez J.M., Yan J., Inclan L., Heard K., Rivera X.I., Anwer F., Mahadevan D., Schatz J.H., Persky D.O. Yttrium-90-Ibritumomab Tiuxetan (Zevalin(R)) Radioimmunotherapy after Cytoreduction with ESHAP Chemotherapy in Patients with Relapsed Follicular Non-Hodgkin Lymphoma: Final Results of a Phase II Study. Oncology. 2018;94:274–280. doi: 10.1159/000486788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaklavas C., Meredith R.F., Shen S., Knox S.J., Micallef I.N., Shah J.J., LoBuglio A.F., Forero-Torres A. Phase I study of a modified regimen of (9)(0)Yttrium-ibritumomab tiuxetan for relapsed or refractory follicular or transformed CD20+ non-Hodgkin lymphoma. Cancer Biother. Radiopharm. 2013;28:370–379. doi: 10.1089/cbr.2012.1387. [DOI] [PubMed] [Google Scholar]
- 45.Dispenzieri A., D’Souza A., Gertz M.A., Laumann K., Wiseman G., Lacy M.Q., LaPlant B., Buadi F., Hayman S.R., Kumar S.K., et al. A phase 1 trial of (90)Y-Zevalin radioimmunotherapy with autologous stem cell transplant for multiple myeloma. Bone Marrow Transplant. 2017;52:1372–1377. doi: 10.1038/bmt.2017.164. [DOI] [PubMed] [Google Scholar]
- 46.McQuillan A.D., Macdonald W.B., Turner J.H. Phase II study of first-line (131)I-rituximab radioimmunotherapy in follicular non-Hodgkin lymphoma and prognostic (18)F-fluorodeoxyglucose positron emission tomography. Leuk. Lymphoma. 2015;56:1271–1277. doi: 10.3109/10428194.2014.949260. [DOI] [PubMed] [Google Scholar]
- 47.Wagner J.Y., Schwarz K., Schreiber S., Schmidt B., Wester H.J., Schwaiger M., Peschel C., von Schilling C., Scheidhauer K., Keller U. Myeloablative anti-CD20 radioimmunotherapy +/− high-dose chemotherapy followed by autologous stem cell support for relapsed/refractory B-cell lymphoma results in excellent long-term survival. Oncotarget. 2013;4:899–910. doi: 10.18632/oncotarget.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang H.J., Lee S.S., Byun B.H., Kim K.M., Lim I., Choi C.W., Suh C., Kim W.S., Nam S.H., Lee S.I., et al. Repeated radioimmunotherapy with 131I-rituximab for patients with low-grade and aggressive relapsed or refractory B cell non-Hodgkin lymphoma. Cancer Chemother. Pharmacol. 2013;71:945–953. doi: 10.1007/s00280-013-2087-z. [DOI] [PubMed] [Google Scholar]
- 49.Lee I., Byun B.H., Lim I., Kim B.I., Choi C.W., Kim K.M., Shin D.Y., Kang H.J., Lim S.M. Comparisons of (131)I-rituximab treatment responses in patients with aggressive lymphoma and indolent lymphoma. Ann. Nucl. Med. 2019;33:881–890. doi: 10.1007/s12149-019-01401-5. [DOI] [PubMed] [Google Scholar]
- 50.Forrer F., Oechslin-Oberholzer C., Campana B., Herrmann R., Maecke H.R., Mueller-Brand J., Lohri A. Radioimmunotherapy with 177Lu-DOTA-rituximab: Final results of a phase I/II Study in 31 patients with relapsing follicular, mantle cell, and other indolent B-cell lymphomas. J. Nucl. Med. 2013;54:1045–1052. doi: 10.2967/jnumed.112.115170. [DOI] [PubMed] [Google Scholar]
- 51.Yadav M.P., Singla S., Thakral P., Ballal S., Bal C. Dosimetric analysis of 177Lu-DOTA-rituximab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Nucl. Med. Commun. 2016;37:735–742. doi: 10.1097/MNM.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 52.Thakral P., Singla S., Vashist A., Yadav M.P., Gupta S.K., Tyagi J.S., Sharma A., Bal C.S., Snehlata E.Y., Malhotra A. Preliminary Experience with Yttrium-90-labelled Rituximab (Chimeric Anti CD-20 Antibody) in Patients with Relapsed and Refractory B Cell Non-Hodgkins Lymphoma. Curr. Radiopharm. 2016;9:160–168. doi: 10.2174/1874471009999160625110400. [DOI] [PubMed] [Google Scholar]
- 53.Press O.W., Unger J.M., Rimsza L.M., Friedberg J.W., LeBlanc M., Czuczman M.S., Kaminski M., Braziel R.M., Spier C., Gopal A.K., et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J. Clin. Oncol. 2013;31:314–320. doi: 10.1200/JCO.2012.42.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shadman M., Li H., Rimsza L., Leonard J.P., Kaminski M.S., Braziel R.M., Spier C.M., Gopal A.K., Maloney D.G., Cheson B.D., et al. Continued Excellent Outcomes in Previously Untreated Patients With Follicular Lymphoma After Treatment With CHOP Plus Rituximab or CHOP Plus (131)I-Tositumomab: Long-Term Follow-Up of Phase III Randomized Study SWOG-S0016. J. Clin. Oncol. 2018;36:697–703. doi: 10.1200/JCO.2017.74.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quackenbush R.C., Horner T.J., Williams V.C., Giampietro P., Lin T.S. Patients with relapsed follicular lymphoma treated with rituximab versus tositumomab and iodine I-131 tositumomab. Leuk. Lymphoma. 2015;56:779–781. doi: 10.3109/10428194.2014.927461. [DOI] [PubMed] [Google Scholar]
- 56.Olney H.J., Freeman M.A., Stewart D.A., Mangel J.E., White D.J., Elia-Pacitti J.O. Prolonged progression-free survival and preserved quality of life in the Canadian prospective study of tositumomab and iodine(131)-tositumomab for previously treated, rituximab-exposed, indolent non-Hodgkin lymphoma. Leuk. Lymphoma. 2014;55:2754–2760. doi: 10.3109/10428194.2014.894190. [DOI] [PubMed] [Google Scholar]
- 57.Friedberg J.W., Unger J.M., Burack W.R., Gopal A.K., Raju R.N., Nademanee A.P., Kaminski M.S., Li H., Press O.W., Miller T.P., et al. R-CHOP with iodine-131 tositumomab consolidation for advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0433. Br. J. Haematol. 2014;166:382–389. doi: 10.1111/bjh.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hudson K.E., Rizzieri D., Thomas S.M., LeBlanc T.W., Powell Z., Diehl L., Moore J.O., DeCastro C., Beaven A.W. Dose-intense chemoimmunotherapy plus radioimmunotherapy in high-risk diffuse large B-cell lymphoma and mantle cell lymphoma: A phase II study. Br. J. Haematol. 2019;184:647–650. doi: 10.1111/bjh.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vose J.M., Carter S., Burns L.J., Ayala E., Press O.W., Moskowitz C.H., Stadtmauer E.A., Mineshi S., Ambinder R., Fenske T., et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: Results from the BMT CTN 0401 trial. J. Clin. Oncol. 2013;31:1662–1668. doi: 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chow V.A., Rajendran J.G., Fisher D.R., Appelbaum F.R., Cassaday R.D., Martin P.S., Holmberg L.A., Gooley T.A., Stevenson P.A., Pagel J.M., et al. A phase II trial evaluating the efficacy of high-dose Radioiodinated Tositumomab (Anti-CD20) antibody, etoposide and cyclophosphamide followed by autologous transplantation, for high-risk relapsed or refractory non-hodgkin lymphoma. Am. J. Hematol. 2020;95:775–783. doi: 10.1002/ajh.25818. [DOI] [PubMed] [Google Scholar]
- 61.Gopal A.K., Gooley T.A., Rajendran J.G., Pagel J.M., Fisher D.R., Maloney D.G., Appelbaum F.R., Cassaday R.D., Shields A., Press O.W. Myeloablative I-131-tositumomab with escalating doses of fludarabine and autologous hematopoietic transplantation for adults age >/= 60 years with B cell lymphoma. Biol. Blood Marrow Transplant. 2014;20:770–775. doi: 10.1016/j.bbmt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dewaraja Y.K., Schipper M.J., Shen J., Smith L.B., Murgic J., Savas H., Youssef E., Regan D., Wilderman S.J., Roberson P.L., et al. Tumor-Absorbed Dose Predicts Progression-Free Survival Following (131)I-Tositumomab Radioimmunotherapy. J. Nucl. Med. 2014;55:1047–1053. doi: 10.2967/jnumed.113.136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elstrom R.L., Ruan J., Christos P.J., Martin P., Lebovic D., Osborne J., Goldsmith S., Greenberg J., Furman R.R., Avram A., et al. Phase 1 study of radiosensitization using bortezomib in patients with relapsed non-Hodgkin lymphoma receiving radioimmunotherapy with 131I-tositumomab. Leuk. Lymphoma. 2015;56:342–346. doi: 10.3109/10428194.2014.914195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacene H., Crandall J., Kasamon Y.L., Ambinder R.F., Piantadosi S., Serena D., Kasecamp W., Wahl R.L. Initial Experience with Tositumomab and I-131-Labeled Tositumomab for Treatment of Relapsed/Refractory Hodgkin Lymphoma. Mol. Imaging Biol. 2017;19:429–436. doi: 10.1007/s11307-016-1019-9. [DOI] [PubMed] [Google Scholar]
- 65.Chevallier P., Eugene T., Robillard N., Isnard F., Nicolini F., Escoffre-Barbe M., Huguet F., Hunault M., Marcais A., Gaschet J., et al. (90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: A phase 1 dose-escalation study. Lancet Haematol. 2015;2:e108–e117. doi: 10.1016/S2352-3026(15)00020-4. [DOI] [PubMed] [Google Scholar]
- 66.Kraeber-Bodere F., Pallardy A., Maisonneuve H., Campion L., Moreau A., Soubeyran I., Le Gouill S., Tournilhac O., Daguindau E., Jardel H., et al. Consolidation anti-CD22 fractionated radioimmunotherapy with (90)Y-epratuzumab tetraxetan following R-CHOP in elderly patients with diffuse large B-cell lymphoma: A prospective, single group, phase 2 trial. Lancet Haematol. 2017;4:e35–e45. doi: 10.1016/S2352-3026(16)30168-5. [DOI] [PubMed] [Google Scholar]
- 67.Witzig T.E., Tomblyn M.B., Misleh J.G., Kio E.A., Sharkey R.M., Wegener W.A., Goldenberg D.M. Anti-CD22 90Y-epratuzumab tetraxetan combined with anti-CD20 veltuzumab: A phase I study in patients with relapsed/refractory, aggressive non-Hodgkin lymphoma. Haematologica. 2014;99:1738–1745. doi: 10.3324/haematol.2014.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenblat T.L., McDevitt M.R., Mulford D.A., Pandit-Taskar N., Divgi C.R., Panageas K.S., Heaney M.L., Chanel S., Morgenstern A., Sgouros G., et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010;16:5303–5311. doi: 10.1158/1078-0432.CCR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider S., Strumpf A., Schetelig J., Wunderlich G., Ehninger G., Kotzerke J., Bornhauser M. Reduced-Intensity Conditioning Combined with (188)Rhenium Radioimmunotherapy before Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: The Role of In Vivo T Cell Depletion. Biol. Blood Marrow Transplant. 2015;21:1754–1760. doi: 10.1016/j.bbmt.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 70.Schulz A.S., Glatting G., Hoenig M., Schuetz C., Gatz S.A., Grewendorf S., Sparber-Sauer M., Muche R., Blumstein N., Kropshofer G., et al. Radioimmunotherapy-based conditioning for hematopoietic cell transplantation in children with malignant and nonmalignant diseases. Blood. 2011;117:4642–4650. doi: 10.1182/blood-2010-06-284349. [DOI] [PubMed] [Google Scholar]
- 71.Kramer K., Pandit-Taskar N., Zanzonico P., Wolden S.L., Humm J.L., DeSelm C., Souweidane M.M., Lewis J.S., Cheung N.-K.V. Low incidence of radionecrosis in children treated with conventional radiation therapy and intrathecal radioimmunotherapy. J. Neuro-Oncol. 2015;123:245–249. doi: 10.1007/s11060-015-1788-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petricevic B., Laengle J., Singer J., Sachet M., Fazekas J., Steger G., Bartsch R., Jensen-Jarolim E., Bergmann M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013;11:307. doi: 10.1186/1479-5876-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meredith R., Torgue J., Shen S., Fisher D.R., Banaga E., Bunch P., Morgan D., Fan J., Straughn J.M., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014;55:1636–1642. doi: 10.2967/jnumed.114.143842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhusari P., Vatsa R., Singh G., Parmar M., Bal A., Dhawan D.K., Mittal B.R., Shukla J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer. 2017;140:938–947. doi: 10.1002/ijc.30500. [DOI] [PubMed] [Google Scholar]
- 75.Kramer K., Pandit-Taskar N., Humm J.L., Zanzonico P.B., Haque S., Dunkel I.J., Wolden S.L., Donzelli M., Goldman D.A., Lewis J.S., et al. A phase II study of radioimmunotherapy with intraventricular (131) I-3F8 for medulloblastoma. Pediatr. Blood Cancer. 2018;65 doi: 10.1002/pbc.26754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herbertson R.A., Tebbutt N.C., Lee F.T., Gill S., Chappell B., Cavicchiolo T., Saunder T., O’Keefe G.J., Poon A., Lee S.T., et al. Targeted chemoradiation in metastatic colorectal cancer: A phase I trial of 131I-huA33 with concurrent capecitabine. J. Nucl. Med. 2014;55:534–539. doi: 10.2967/jnumed.113.132761. [DOI] [PubMed] [Google Scholar]
- 77.Cahan B., Leong L., Wagman L., Yamauchi D., Shibata S., Wilzcynski S., Williams L.E., Yazaki P., Colcher D., Frankel P., et al. Phase I/II Trial of Anticarcinoembryonic Antigen Radioimmunotherapy, Gemcitabine, and Hepatic Arterial Infusion of Fluorodeoxyuridine Postresection of Liver Metastasis for Colorectal Carcinoma. Cancer Biother. Radiopharm. 2017;32:258–265. doi: 10.1089/cbr.2017.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahlmann C.O., Homayounfar K., Niessner M., Dyczkowski J., Conradi L.C., Braulke F., Meller B., Beissbarth T., Ghadimi B.M., Meller J., et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer. 2017;123:638–649. doi: 10.1002/cncr.30390. [DOI] [PubMed] [Google Scholar]
- 79.Allen B.J., Singla A.A., Rizvi S.M., Graham P., Bruchertseifer F., Apostolidis C., Morgenstern A. Analysis of patient survival in a Phase I trial of systemic targeted alpha-therapy for metastatic melanoma. Immunotherapy. 2011;3:1041–1050. doi: 10.2217/imt.11.97. [DOI] [PubMed] [Google Scholar]
- 80.Gulec S.A., Cohen S.J., Pennington K.L., Zuckier L.S., Hauke R.J., Horne H., Wegener W.A., Teoh N., Gold D.V., Sharkey R.M., et al. Treatment of advanced pancreatic carcinoma with 90Y-Clivatuzumab Tetraxetan: A phase I single-dose escalation trial. Clin. Cancer Res. 2011;17:4091–4100. doi: 10.1158/1078-0432.CCR-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ocean A.J., Pennington K.L., Guarino M.J., Sheikh A., Bekaii-Saab T., Serafini A.N., Lee D., Sung M.W., Gulec S.A., Goldsmith S.J., et al. Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer. 2012;118:5497–5506. doi: 10.1002/cncr.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Picozzi V.J., Ramanathan R.K., Lowery M.A., Ocean A.J., Mitchel E.P., O’Neil B.H., Guarino M.J., Conkling P.R., Cohen S.J., Bahary N., et al. (90)Y-clivatuzumab tetraxetan with or without low-dose gemcitabine: A phase Ib study in patients with metastatic pancreatic cancer after two or more prior therapies. Eur. J. Cancer. 2015;51:1857–1864. doi: 10.1016/j.ejca.2015.06.119. [DOI] [PubMed] [Google Scholar]
- 83.Tagawa S.T., Milowsky M.I., Morris M., Vallabhajosula S., Christos P., Akhtar N.H., Osborne J., Goldsmith S.J., Larson S., Taskar N.P., et al. Phase II Study of Lutetium-177–Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013;19:5182–5191. doi: 10.1158/1078-0432.CCR-13-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tagawa S.T., Vallabhajosula S., Christos P.J., Jhanwar Y.S., Batra J.S., Lam L., Osborne J., Beltran H., Molina A.M., Goldsmith S.J., et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 ((177) Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125:2561–2569. doi: 10.1002/cncr.32072. [DOI] [PubMed] [Google Scholar]
- 85.Batra J.S., Niaz M.J., Whang Y.E., Sheikh A., Thomas C., Christos P., Vallabhajosula S., Jhanwar Y.S., Molina A.M., Nanus D.M., et al. Phase I trial of docetaxel plus lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 ((177)Lu-J591) for metastatic castration-resistant prostate cancer. Urol. Oncol. 2020;38:848.e9–848.e16. doi: 10.1016/j.urolonc.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 86.Niaz M.J., Batra J.S., Walsh R.D., Ramirez-Fort M.K., Vallabhajosula S., Jhanwar Y.S., Molina A.M., Nanus D.M., Osborne J.R., Bander N.H., et al. Pilot Study of Hyperfractionated Dosing of Lutetium-177-Labeled Antiprostate-Specific Membrane Antigen Monoclonal Antibody J591 ((177) Lu-J591) for Metastatic Castration-Resistant Prostate Cancer. Oncologist. 2020;25:477-e895. doi: 10.1634/theoncologist.2020-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stillebroer A.B., Boerman O.C., Desar I.M., Boers-Sonderen M.J., van Herpen C.M., Langenhuijsen J.F., Smith-Jones P.M., Oosterwijk E., Oyen W.J., Mulders P.F. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol. 2013;64:478–485. doi: 10.1016/j.eururo.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 88.Muselaers C.H., Boers-Sonderen M.J., van Oostenbrugge T.J., Boerman O.C., Desar I.M., Stillebroer A.B., Mulder S.F., van Herpen C.M., Langenhuijsen J.F., Oosterwijk E., et al. Phase 2 Study of Lutetium 177-Labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. 2016;69:767–770. doi: 10.1016/j.eururo.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Z., Su Z., Zhang W., Luo M., Wang H., Huang L. A randomized study comparing the effectiveness of microwave ablation radioimmunotherapy and postoperative adjuvant chemoradiation in the treatment of non-small cell lung cancer. J. BUON. 2016;21:326–332. [PubMed] [Google Scholar]
- 90.Giraudet A.L., Cassier P.A., Iwao-Fukukawa C., Garin G., Badel J.N., Kryza D., Chabaud S., Gilles-Afchain L., Clapisson G., Desuzinges C., et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18:646. doi: 10.1186/s12885-018-4544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Modak S., Zanzonico P., Grkovski M., Slotkin E.K., Carrasquillo J.A., Lyashchenko S.K., Lewis J.S., Cheung I.Y., Heaton T., LaQuaglia M.P., et al. B7H3-Directed Intraperitoneal Radioimmunotherapy With Radioiodinated Omburtamab for Desmoplastic Small Round Cell Tumor and Other Peritoneal Tumors: Results of a Phase I Study. J. Clin. Oncol. 2020;38:4283–4291. doi: 10.1200/JCO.20.01974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu G., Nishio N., van den Berg N.S., Martin B.A., Fakurnejad S., van Keulen S., Colevas A.D., Thurber G.M., Rosenthal E.L. Co-administered antibody improves penetration of antibody-dye conjugate into human cancers with implications for antibody-drug conjugates. Nat. Commun. 2020;11:5667. doi: 10.1038/s41467-020-19498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.White J.M., Escorcia F.E., Viola N.T. Perspectives on metals-based radioimmunotherapy (RIT): Moving forward. Theranostics. 2021;11:6293–6314. doi: 10.7150/thno.57177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nelson B.J.B., Andersson J.D., Wuest F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics. 2020;13:49. doi: 10.3390/pharmaceutics13010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rondon A., Mahri S., Morales-Yanez F., Dumoulin M., Vanbever R. Protein Engineering Strategies for Improved Pharmacokinetics. Adv. Funct. Mater. 2021;31:2101633. doi: 10.1002/adfm.202101633. [DOI] [Google Scholar]
- 96.Kholodenko R.V., Kalinovsky D.V., Doronin I.I., Ponomarev E.D., Kholodenko I.V. Antibody Fragments as Potential Biopharmaceuticals for Cancer Therapy: Success and Limitations. Curr. Med. Chem. 2019;26:396–426. doi: 10.2174/0929867324666170817152554. [DOI] [PubMed] [Google Scholar]
- 97.Nelson A.L. Antibody fragments: Hope and hype. MAbs. 2010;2:77–83. doi: 10.4161/mabs.2.1.10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nelson A.L., Reichert J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 2009;27:331–337. doi: 10.1038/nbt0409-331. [DOI] [PubMed] [Google Scholar]
- 99.Casey J.L., Napier M.P., King D.J., Pedley R.B., Chaplin L.C., Weir N., Skelton L., Green A.J., Hope-Stone L.D., Yarranton G.T., et al. Tumour targeting of humanised cross-linked divalent-Fab′ antibody fragments: A clinical phase I/II study. Br. J. Cancer. 2002;86:1401–1410. doi: 10.1038/sj.bjc.6600198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vermeer A.W.P., Norde W. The Thermal Stability of Immunoglobulin: Unfolding and Aggregation of a Multi-Domain Protein. Biophys. J. 2000;78:394–404. doi: 10.1016/S0006-3495(00)76602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aloj L., D’Ambrosio L., Aurilio M., Morisco A., Frigeri F., Caraco C., Di Gennaro F., Capobianco G., Giovannoni L., Menssen H.D., et al. Radioimmunotherapy with Tenarad, a 131I-labelled antibody fragment targeting the extra-domain A1 of tenascin-C, in patients with refractory Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:867–877. doi: 10.1007/s00259-013-2658-6. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez G.P., Garcia I.G., Gonzalez J.G., Sanchez L.P., Mirabal M.V., Marin C.C., Ruiz F.L., Iglesias E.G., de Queralta R.L., Toirac R.R., et al. Phase I clinical trial of the (131)I-labeled anticarcinoembryonic antigen CIGB-M3 multivalent antibody fragment. Cancer Biother. Radiopharm. 2011;26:353–363. doi: 10.1089/cbr.2010.0899. [DOI] [PubMed] [Google Scholar]
- 103.Wu L., Yang Y.F., Ge N.J., Shen S.Q., Liang J., Wang Y., Zhou W.P., Shen F., Wu M.C. Hepatic arterial iodine-131-labeled metuximab injection combined with chemoembolization for unresectable hepatocellular carcinoma: Interim safety and survival data from 110 patients. Cancer Biother. Radiopharm. 2010;25:657–663. doi: 10.1089/cbr.2010.0801. [DOI] [PubMed] [Google Scholar]
- 104.Wu L., Yang Y.F., Ge N.J., Shen S.Q., Liang J., Wang Y., Zhou W.P., Shen F., Wu M.C. Hepatic artery injection of (1)(3)(1)I-labelled metuximab combined with chemoembolization for intermediate hepatocellular carcinoma: A prospective nonrandomized study. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:1306–1315. doi: 10.1007/s00259-012-2145-5. [DOI] [PubMed] [Google Scholar]
- 105.Li Z., Zhou J.X., Ren J.Z., Zhang W.J., Han X.W. Clinical value of iodine [131I] metuximab infusion combined with TACE for treatment of patients with post-intervention relapse of mid or advanced stage hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2013;21:728–733. doi: 10.3760/cma.j.issn.1007-3418.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 106.Bian H., Zheng J.S., Nan G., Li R., Chen C., Hu C.X., Zhang Y., Sun B., Wang X.L., Cui S.C., et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju239. [DOI] [PubMed] [Google Scholar]
- 107.Li J., Xing J., Yang Y., Liu J., Wang W., Xia Y., Yan Z., Wang K., Wu D., Wu L., et al. Adjuvant (131)I-metuximab for hepatocellular carcinoma after liver resection: A randomised, controlled, multicentre, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2020;5:548–560. doi: 10.1016/S2468-1253(19)30422-4. [DOI] [PubMed] [Google Scholar]
- 108.Breitz H.B., Tyler A., Bjorn M.J., Lesley T., Weiden P.L. Clinical experience with Tc-99m nofetumomab merpentan (Verluma) radioimmunoscintigraphy. Clin. Nucl. Med. 1997;22:615–620. doi: 10.1097/00003072-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Quigley A.M., Gnanasegaran G., Buscombe J.R., Hilson A.J. Technetium-99m-labelled sulesomab (LeukoScan) in the evaluation of soft tissue infections. Med. Princ. Pract. 2008;17:447–452. doi: 10.1159/000151565. [DOI] [PubMed] [Google Scholar]
- 110.Klotz S.A., Yates S., Smith S.L., Dudley S., Jr., Schmidt J.O., Shirazi F.M. Scorpion Stings and Antivenom Use in Arizona. Am. J. Med. 2021;134:1034–1038. doi: 10.1016/j.amjmed.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 111.Cocchio C., Johnson J., Clifton S. Review of North American pit viper antivenoms. Am. J. Health Syst. Pharm. 2020;77:175–187. doi: 10.1093/ajhp/zxz278. [DOI] [PubMed] [Google Scholar]
- 112.Chen Z.N., Mi L., Xu J., Song F., Zhang Q., Zhang Z., Xing J.L., Bian H.J., Jiang J.L., Wang X.H., et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: Clinical phase I/II trials. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:435–444. doi: 10.1016/j.ijrobp.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 113.Xu J., Shen Z.-Y., Chen X.-G., Zhang Q., Bian H.-J., Zhu P., Xu H.-Y., Song F., Yang X.-M., Mi L., et al. A randomized controlled trial of licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45:269–276. doi: 10.1002/hep.21465. [DOI] [PubMed] [Google Scholar]
- 114.Xu J., Xu H.-Y., Zhang Q., Song F., Jiang J.-L., Yang X.-M., Mi L., Wen N., Tian R., Wang L., et al. HAb18G/CD147 Functions in Invasion and Metastasis of Hepatocellular Carcinoma. Mol. Cancer Res. 2007;5:605–614. doi: 10.1158/1541-7786.MCR-06-0286. [DOI] [PubMed] [Google Scholar]
- 115.Chen W., Yuan Y., Jiang X. Antibody and antibody fragments for cancer immunotherapy. J. Control. Release. 2020;328:395–406. doi: 10.1016/j.jconrel.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 116.Kamiya T., Seow S.V., Wong D., Robinson M., Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019;129:2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Markham A. Brolucizumab: First Approval. Drugs. 2019;79:1997–2000. doi: 10.1007/s40265-019-01231-9. [DOI] [PubMed] [Google Scholar]
- 118.Massa S., Xavier C., Muyldermans S., Devoogdt N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opin. Drug Deliv. 2016;13:1149–1163. doi: 10.1080/17425247.2016.1178235. [DOI] [PubMed] [Google Scholar]
- 119.Alfaleh M.A., Alsaab H.O., Mahmoud A.B., Alkayyal A.A., Jones M.L., Mahler S.M., Hashem A.M. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front. Immunol. 2020;11:1986. doi: 10.3389/fimmu.2020.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharkey R.M., Karacay H., Cardillo T.M., Chang C.H., McBride W.J., Rossi E.A., Horak I.D., Goldenberg D.M. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin. Cancer Res. 2005;11:7109s–7121s. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 121.Rondon A., Degoul F. Antibody Pretargeting Based on Bioorthogonal Click Chemistry for Cancer Imaging and Targeted Radionuclide Therapy. Bioconjug Chem. 2020;31:159–173. doi: 10.1021/acs.bioconjchem.9b00761. [DOI] [PubMed] [Google Scholar]
- 122.Labrijn A.F., Janmaat M.L., Reichert J.M., Parren P. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 123.Shim H. Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules. 2020;10:360. doi: 10.3390/biom10030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scott L.J., Kim E.S. Emicizumab-kxwh: First Global Approval. Drugs. 2018;78:269–274. doi: 10.1007/s40265-018-0861-2. [DOI] [PubMed] [Google Scholar]
- 125.Salaun P.Y., Campion L., Bournaud C., Faivre-Chauvet A., Vuillez J.P., Taieb D., Ansquer C., Rousseau C., Borson-Chazot F., Bardet S., et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: Biomarker response and survival improvement. J. Nucl. Med. 2012;53:1185–1192. doi: 10.2967/jnumed.111.101865. [DOI] [PubMed] [Google Scholar]
- 126.Schoffelen R., Boerman O.C., Goldenberg D.M., Sharkey R.M., van Herpen C.M., Franssen G.M., McBride W.J., Chang C.H., Rossi E.A., van der Graaf W.T., et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: First clinical results. Br. J. Cancer. 2013;109:934–942. doi: 10.1038/bjc.2013.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bodet-Milin C., Ferrer L., Rauscher A., Masson D., Rbah-Vidal L., Faivre-Chauvet A., Cerato E., Rousseau C., Hureaux J., Couturier O., et al. Pharmacokinetics and Dosimetry Studies for Optimization of Pretargeted Radioimmunotherapy in CEA-Expressing Advanced Lung Cancer Patients. Front. Med. 2015;2:84. doi: 10.3389/fmed.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rossi E.A., Goldenberg D.M., Cardillo T.M., McBride W.J., Sharkey R.M., Chang C.H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc. Natl. Acad. Sci. USA. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.