Abstract
Human monocytic ehrlichiosis is an emerging infectious disease caused by Ehrlichia chaffeensis, a gram-negative obligatory intracellular bacterium closely related to E. canis. The immunoreactive recombinant fusion proteins rP28 and rP30 have become available after cloning and expressing of the 28- and 30-kDa major outer membrane protein genes of E. chaffeensis and E. canis, respectively. Western immunoblotting was performed to analyze the antibody responses of the 37 E. chaffeensis indirect fluorescent-antibody assay (IFA)-positive and 20 IFA-negative serum specimens with purified whole organisms, rP28, and rP30. All IFA-negative sera were negative with purified whole organisms, rP28, or rP30 by Western immunoblot analysis (100% relative diagnostic specificity). Of 37 IFA-positive sera, 34 sera reacted with any native proteins of E. chaffeensis ranging from 44 to 110 kDa, and 30 sera reacted with 44- to 110-kDa native E. canis antigens. The 28-kDa E. chaffeensis and 30-kDa E. canis native proteins were recognized by 25 IFA-positive sera. Fifteen IFA-positive sera reacted with rP28 by Western blot analysis, whereas 34 IFA-positive sera reacted with rP30 (92% relative diagnostic specificity), indicating that rP30 is more sensitive than rP28 for detecting the antibodies in IFA-positive sera. These 34 IFA-positive sera were positive by the dot blot assay with rP30, distinguishing them from IFA-negative sera. Except for three rP30-negative but IFA-positive specimens that instead showed an E. ewingii infection-like profile by Western immunoblotting, the results of Western and dot blot assays with rP30 matched 100% with the IFA test results. Densitometric analysis of dot blot reactions showed a positive correlation between the dot density and the IFA titer. These results suggest that rP30 antigen would provide a simple, consistent, and rapid serodiagnosis for human monocytic ehrlichiosis.
Ehrlichia chaffeensis, a gram-negative obligatory intracellular bacterium of monocytes and macrophages, is the causative agent of human monocytic ehrlichiosis (HME), a tick-borne zoonosis (6). After a 7- to 9-day incubation period following the tick bite, patients develop acute nonspecific clinical signs such as fever, chills, headache, and myalgia. Laboratory tests often indicate thrombocytopenia, leukopenia, and elevated liver enzyme activities (8, 9, 15, 20, 28). The severity of the disease ranges from subclinical seroconversion to fatal illness. Since the discovery of HME in 1986, by identification of ehrlichial inclusions in the leukocytes of a patient's blood and by indirect fluorescent-antibody assay (IFA) tests with E. canis as an antigen (15), the IFA test has been the most frequently used test for diagnosis of HME. The Centers for Disease Control and Prevention had received one or more serum specimens from 754 individuals who tested positive (IFA titer of >64) against E. chaffeensis or E. canis antigen (4), and >1,500 E. chaffeensis IFA-positive sera (IFA titer of >64) were reported from MRL Reference Laboratory (Cypress, Calif.). IFA-positive cases have also been reported from Europe and Africa (10, 11, 16, 21, 27). The incidence of HME may be higher than reported, because HME is not well known among clinicians, the surveillance of HME is not active in most states, and HME became a nationally reportable disease only in 1998.
Direct tests such as culture isolation, PCR, and microscopic observation of morulae (microcolonies of ehrlichiae) are ideal if they are easily applicable and reliable. A PCR test based on the E. chaffeensis-specific partial sequence of a 16S rRNA gene was reported to have a greater sensitivity than an IFA test in detecting infection at an early stage of disease (5). The culture isolation and microscopic observation of morulae in Romanovsky dye-stained peripheral blood monocytes provide definitive proof of ehrlichial infection. However, these tests cannot be used as a single diagnostic test for HME, because negative results from these tests cannot rule out HME, owing to a high false-negative rate caused by conditions of the sample and the assay. False-positive PCR results caused by the carryover DNA are another problem. PCR requires a thermocycler and trained personnel, is relatively expensive, and is time-consuming. Culture isolation requires aseptically collected fresh specimens, extensive time, use of cell culture techniques and facilities, and subsequent 16S rRNA gene sequence analysis or PCR to identify each isolate at the species level. Microscopic observation of morulae is insensitive and inaccurate, owing to low numbers of organisms and infected cells, even at acute stages of the disease, except in immunocompromised patients. An additional test is required to identify the ehrlichia at the species level. These tests have not been developed for use in routine clinical diagnosis.
Since serologic tests are completely dependent on the patient's humoral immune response specific to E. chaffeensis, the tests have fundamental limitations, such as giving false-negative results in immunocompromised patients or in patients treated with antibiotics at very early stages of infection and not being able to distinguish current infection from previous infection or exposure from actual infection. Because ehrlichial infection induces significant antibody titers in nonimmunocompromised patients and since nonexposed people seldom have antibodies reactive to Ehrlichia spp., serologic tests are considered the most reliable tests for ehrlichiosis, especially for ruling out the possibility of HME. Among several serologic tests, IFA with culture-derived E. chaffeensis antigen is the most widely used. Before isolation of E. chaffeensis in the cell culture system, E. canis, which is genetically closely related to E. chaffeensis, had been used as the antigen for IFA testing (7, 15). Advantages of the IFA test are its ease of both specimen handling and assay procedure, high sensitivity and specificity, and low cost. The limitations of the IFA method, however, are the subjective microscopic evaluation of antigen slides and variations in the reaction result, which are caused by variations in the conditions of E. chaffeensis culturing, antigen slide preparation, and slide storage, the fluorescent antibody used, and the model of fluorescence microscope used. Therefore, a more reliable and convenient method with diagnostic sensitivity and specificity comparable or superior to those of the IFA test is still needed for diagnosis of HME.
Dot immunoblot assay has been developed for serodiagnosis of ehrlichial agents or related species, providing an objective evaluation and a convenient, time-saving, and inexpensive method (18, 31, 34). Either the purified whole organism or the purified or recombinant major antigen is used as a dot blot antigen. Several immunodominant major outer membrane proteins of ehrlichial agents have been cloned and expressed as immunoreactive fusion proteins (18, 19, 34). We reported that a dot immunoblot assay of dog and human sera with the recombinant 30-kDa protein (rP30) of E. canis and the 44-kDa protein (rP44) of the human granulocytic ehrlichiosis agent, respectively, gives diagnostic sensitivity and specificity comparable to those of IFA tests, using the infected cells as the antigen (18, 34). Although any serologic assay is not expected to replace the role of PCR or cell culture isolation methods in HME diagnosis, the preparation of the recombinant proteins as an antigen is less labor intensive, easier to standardize, more economical, and less hazardous than handling infected cultures, and therefore, it is expected to greatly improve the serodiagnosis of HME.
Cloning and characterization of immunodominant 28-kDa-range surface proteins of E. chaffeensis and 30-kDa-range surface proteins of E. canis indicated that these proteins are immunologically highly cross-reactive and are encoded by a polymorphic multigene family that is not segregated between E. chaffeensis and E. canis (18, 19, 22), suggesting that one gene product may be superior to the other as a serologic diagnostic antigen. In the present study, E. chaffeensis IFA-positive and -negative patient sera were analyzed by Western blotting to determine the reactive protein compositions of purified E. chaffeensis and E. canis antigens, and the reactivities of the sera to rP28 and rP30 antigens were compared by Western blotting. Last, the patients' sera were evaluated by dot blot assay with rP30.
MATERIALS AND METHODS
Organisms and purification.
E. chaffeensis Arkansas and E. canis Oklahoma were cultivated in DH82 cells, a dog macrophage cell line, and maintained in Dulbecco minimum essential medium supplemented with 10% heat-inactivated fetal bovine serum-1 mM l-glutamine-10 mM N-(2-hydroxyethylpiperazine)-N′-(4-butanesulfonic acid) buffer as previously described. Ehrlichial organisms were purified by Sephacryl S-1000 column chromatography as previously described (24). Protein concentrations of purified E. chaffeensis and E. canis were determined with the bicinchoninic acid protein assay (Pierce, Rockford, Ill.), using bovine serum albumin as the standard.
Serum specimens.
A total of 57 E. chaffeensis IFA-positive and -negative sera were obtained from MRL Reference Laboratory. Sera from three E. ewingii PCR-positive patients and from two E. chaffeensis PCR-positive patients and one serum from an E. ewingii PCR-positive dog were provided by R. B. Buller and G. A. Storch, Washington University, St. Louis, Mo. One serum from a dog experimentally infected with E. ewingii was from our previous study (24), and a serum from a naturally infected dog was kindly provided by Steven L. Stickham, College of Veterinary Medicine, University of Missouri, Columbia.
IFA.
MRL used 1:64 as the lowest and 1:1,024 as the highest IFA titer cutoffs. We retitrated these sera starting at a 1:20 dilution to the end point. IFA was performed by a procedure described elsewhere (24). E. chaffeensis Arkansas- and E. canis Oklahoma-infected DH82 cells were used for the preparation of antigen slides, and fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin G (IgG) (Organon Teknika Co., Durham, N.C.) was used at 1:200 as a secondary antibody.
Purification of recombinant proteins.
The recombinant clone that expresses the rP30 protein of E. canis was cultured, and the recombinant fusion protein was purified by affinity chromatography with a His-Bind buffer kit containing 6 M urea (Novagen, Madison, Wis.) as described previously (18). rP28 protein was affinity purified by using an S-Tag purification kit (Novagen). The rP28 protein, partially purified by the same procedure as that used for rP30 purification (18), was extracted with 1× bind-wash buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl), including 8 M urea. After incubation with S-protein agarose beads on an orbital shaker for 30 min, unbound proteins were removed by washing the beads twice with 1× bind-wash buffer. The recombinant protein was eluted from the beads by incubation with 1× bind-wash buffer supplemented with 2 M guanidine thiocyanate and 6 M urea. Both purified proteins were refolded by dialysis against 20 mM Tris-HCl (pH 7.9) containing 4 and 2 M urea and finally against 20 mM Tris-HCl buffer and were concentrated by being sprinkled with polyethylene glycol (molecular weight, 15,000 to 20,000; Sigma, St. Louis, Mo.), and the protein concentration was determined as previously described (18).
Western immunoblotting.
Western immunoblotting was performed as previously described with a modification (18). Although trypsin was used for purification of E. risticii with Percoll gradients for analysis of l-glutamine metabolism (26), trypsin has never been used for purification of any ehrlichial organisms for Western immunoblot analysis in our laboratory (18, 19, 23, 24, 35). Fifteen micrograms of uninfected DH82 cells and pET29a-transformed Escherichia coli lysates (negative controls), 15 μg of purified E. chaffeensis and E. canis, and 1 μg of affinity-purified rP28 and rP30 proteins were used for Western immunoblotting analysis. All serum samples were preabsorbed three times with pET29a-transformed E. coli at 4°C overnight before use.
A dilution of 1:1,000 or 1:500 was used for human or dog sera, respectively, and peroxidase-conjugated affinity-purified anti-human IgG-IgM-IgA or anti-dog IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was used at a 1:2,000 dilution.
Dot immunoblot assay.
Dot immunoblotting was performed as previously described (18). The affinity-purified rP30 (0.5 μg) was adsorbed onto a nitrocellulose membrane by using a dot blot apparatus. The incubation with primary and secondary antibodies was performed as described for Western immunoblotting. The color intensity was analyzed with image analysis software (ImageQuant; Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
To examine the applicability of the recombinant antigens for serodiagnosis of HME, sera were evaluated based on reactivities to 28- to 30-kDa and the remaining 44- to 110-kDa native antigens of whole organisms and to rP28 and rP30 in Western blot analysis. As mentioned in Materials and Methods, the serum samples were preabsorbed with pET29a-transformed E. coli before use. In order to rule out serum cross-reactions to DH82 cells in which E. chaffeensis and E. canis had been cultivated and to E. coli or the vector, DH82 cells and pET29a-transformed E. coli were used as control antigens in Western blot analysis. None of the sera in Fig. 1 and none of the serum used in this study reacted to the 28- or 30-kDa protein of E. coli or to the DH82 cell control. Reactions to the control antigens, if present was directed to antigens with molecular sizes greater than 28 or 30 kDa, such as 35, 37, 44, 56, or 62 kDa. The reaction to 56-kDa-range proteins in the E. coli control may be due to some antibodies to heat shock proteins still remaining after preabsorption of the sera. Therefore, reactions to 28- to 30-kDa proteins of native antigens or to rP28 and rP30 are specific and are not due to cross-reactivity to DH82 cells or E. coli or vector proteins, respectively.
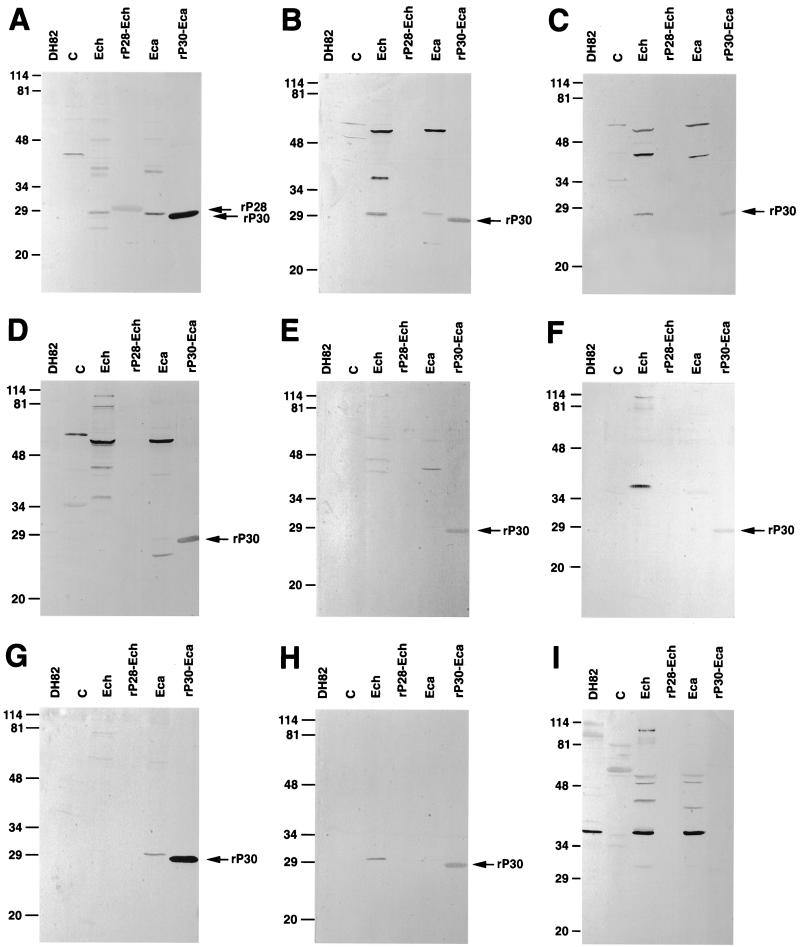
FIG. 1.
Western immunoblotting analysis of representative serum samples from groups A through I in Table 1. Lanes: DH82, dog macrophage cell line DH82 (negative control); C, pET29-transformed E. coli (negative control); Ech, purified E. chaffeensis; rP28-Ech, affinity-purified recombinant fusion protein of E. chaffeensis (31 kDa); Eca, purified E. canis; rP30-Eca, affinity-purified recombinant fusion protein of E. canis (27 kDa). The recombinant proteins rP28 and rP30 are indicated by arrows. The samples subjected to SDS-PAGE consisted of 15 μg each of DH82 cells, E. coli, purified E. chaffeensis, and E. canis and 1 μg each of rP28 and rP30. The numbers on the left of each panel indicate molecular masses in kilodaltons, based on broad-range prestained standards (Bio-Rad Laboratories, Richmond, Calif.).
The reactivities of 57 serum samples can be divided into 10 distinct groups, as shown in Fig. 1 and Table 1. Because obtaining definitive proof of infection through the culture isolation technique is impossible for these specimens and not generally practical, the IFA test was used to serve as the standard of comparison for our new assay (14), and a 1:20 cutoff titer was used to compare with a Western blot cutoff of zero density. All 20 IFA-negative sera did not react with any of the native or recombinant antigens of E. chaffeensis or E. canis (group J). The relative diagnostic sensitivity with any of these antigens is, therefore, 100%. Group A consists of 15 sera (41% of IFA-positive sera) of the highest IFA titer, which reacted to both 28- to 30-kDa and 44- to 110-kDa proteins of whole E. chaffeensis and E. canis and to rP28 and rP30. It is noteworthy that three sera in group I showed reactivities only to 44- to 110-kDa native proteins of E. chaffeensis and E. canis and not to native or recombinant 28- to 30-kDa antigens. Overall, of 37 E. chaffeensis IFA-positive sera, 34 (92%), 25 (68%), 30 (84%), and 25 (68%) sera reacted with 44- to 110-kDa proteins of E. chaffeensis, the 28-kDa protein of E. chaffeensis, 44- to 110-kDa proteins of E. canis, and the 30-kDa protein of E. canis, respectively (Table 2). Fifteen IFA-positive sera (41%) reacted with rP28, and 34 IFA-positive sera (92%) reacted with rP30 (Table 3). The relative diagnostic sensitivity was highest (92%; 34 of 37 sera) with the mixture of 44- to 110-kDa proteins of E. chaffeensis and rP30 antigen compared with the E. chaffeensis IFA result (Table 4). In general, the IFA titers of sera and the densities of reacting bands in Western blotting were proportional, indicating the positive correlation between them. The relative diagnostic sensitivity of rP28 is lowest (41%).
TABLE 1.
Distribution of 57 human sera based on their reactivities to the different ehrlichial antigens by Western immunoblotting
| Group | No. of sera | Reactivity with agenta
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Purified E. chaffeensis antigen
|
rP28 | Purified E. canis antigen
|
rP30 |
E. chaffeensis IFA titer
|
|||||
| 44–110 kDa | 28 kDa | 44–110 kDa | 30 kDa | Range | Geometric mean | ||||
| A | 15 | + | + | + | + | + | + | 1:40–1:1,280 | 1:640 |
| B | 6 | + | + | − | + | + | + | 1:40–1:1,280 | 1:320 |
| C | 3 | + | + | − | + | − | + | 1:40–1:640 | 1:80 |
| D | 2 | + | − | − | + | + | + | 1:40–1:640 | 1:160 |
| E | 1 | + | − | − | + | − | + | 1:320 | 1:320 |
| F | 4 | + | − | − | − | − | + | 1:80–1:320 | 1:160 |
| G | 2 | − | − | − | − | + | + | 1:320–1:640 | 1:320 |
| H | 1 | − | + | − | − | − | + | 1:20 | 1:20 |
| I | 3 | + | − | − | + | − | − | 1:80–1:1,280 | 1:640 |
| J | 20 | − | − | − | − | − | − | <1:20 | <1:20 |
| Total | 57 | ||||||||
+, reaction; −, no reaction.
TABLE 2.
Number of sera reacting to native E. chaffeensis and E. canis antigens
| E. chaffeensis IFA resulta | Total no. of sera | No. of Western blotting-positive sera with:
|
|||
|---|---|---|---|---|---|
|
E. chaffeensis antigen
|
E. canis antigen
|
||||
| 44–110 kDa | 28 kDa | 44–110 kDa | 30 kDa | ||
| + | 37 | 34 | 25 | 30 | 25 |
| − | 20 | 0 | 0 | 0 | 0 |
+, reaction; −, no reaction.
TABLE 3.
Number of sera reacting to E. chaffeensis and E. canis recombinant proteins
| E. chaffeensis IFA resulta | Total no. of sera | No. of Western immunoblotting-positive sera with:
|
|
|---|---|---|---|
| E. chaffeensis rP28 | E. canis rP30 | ||
| + | 37 | 15 | 34 |
| − | 20 | 0 | 0 |
+, reaction; −, no reaction.
TABLE 4.
Diagnostic sensitivity and specificity and positive and negative predictive values of Western immunoblotting relative to the E. chaffeensis IFA
| Protein(s) | Relative diagnostic sensitivity (%) | Relative diagnostic specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| E. chaffeensis 44–110 kDa | 92 | 100 | 100 | 87 |
| E. chaffeensis 28 kDa | 68 | 100 | 100 | 63 |
| E. canis 44–110 kDa | 81 | 100 | 100 | 74 |
| E. canis 30 kDa | 68 | 100 | 100 | 63 |
| E. chaffeensis rP28 | 41 | 100 | 100 | 48 |
| E. canis rP30 | 92 | 100 | 100 | 87 |
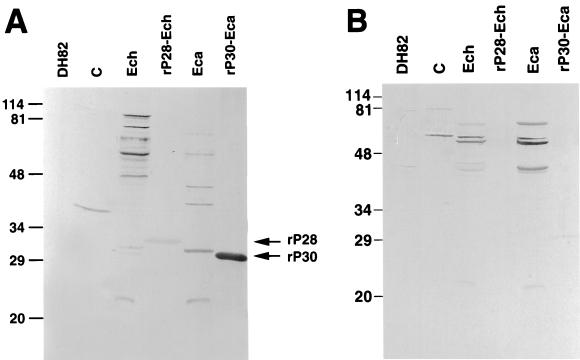
Recently, human infections with an E. ewingii-like agent have been documented (2). Like those of E. ewingii-infected dogs (23), these patients' sera are IFA and Western immunoblot positive for both E. chaffeensis and E. canis. However E. ewingii-infected human sera showed a minimal reaction to the 30-kDa antigen of E. canis or to the 28-kDa antigen of E. chaffeensis. Instead, reactions are limited to the 40- to 110-kDa range of proteins of both E. chaffeensis and E. canis (2). To investigate whether three sera in group I were from patients infected with E. ewingii and whether rP28 and rP30 are useful for detecting this group of sera, the reactivities of sera from three and two patients positive by E. ewingii PCR and E. chaffeensis PCR, respectively, were compared. The sera from two PCR-positive HME patients showed strong reactions to 28- and/or 30-kDa native and recombinant proteins. Although sera from three E. ewingii PCR-positive patients showed clear positive reactions to 40- to 110-kDa antigens of E. chaffeensis and E. canis, these sera showed no or minimal reactivity to E. chaffeensis 28-kDa or E. canis 30-kDa native or recombinant antigens. Because there was no increased reactivity to the latter proteins with the sera collected from the same patients 6 to 30 days later, the lack of reactivity was not caused by the infections being in early stages (data not shown). Sera from two dogs experimentally infected with E. ewingii and the serum from a naturally infected dog (PCR positive) were also tested by Western blotting. They all lacked reactivity or had weak reactivity to 28- or 30-kDa native and recombinant antigens (data not shown). The Western blot results for one E. chaffeensis PCR-positive human serum and one E. ewingii PCR-positive human serum are shown in Fig. 2. Thus, if we do not include the three rP30-negative but IFA-positive specimens (Fig. 1I) which, rather, showed an E. ewingii infection-like profile by Western immunoblotting, the results of the Western and dot blot assays with rP30 exactly matched the IFA test results.
FIG. 2.
Western immunoblotting analysis of human anti-E. chaffeensis (A) and anti-E. ewingii (B) serum samples. Lanes: DH82, dog macrophage cell line DH82 (negative control); C, pET29-transformed E. coli (negative control); Ech, purified E. chaffeensis; rP28-Ech, affinity-purified recombinant fusion protein of E. chaffeensis (31 kDa); Eca, purified E. canis; rP30-Eca, affinity-purified recombinant fusion protein of E. canis (27 kDa). The recombinant proteins rP28 and rP30 are indicated by arrows. The numbers on the left of each panel indicate molecular masses in kilodaltons, based on broad-range prestained standards (Bio-Rad).
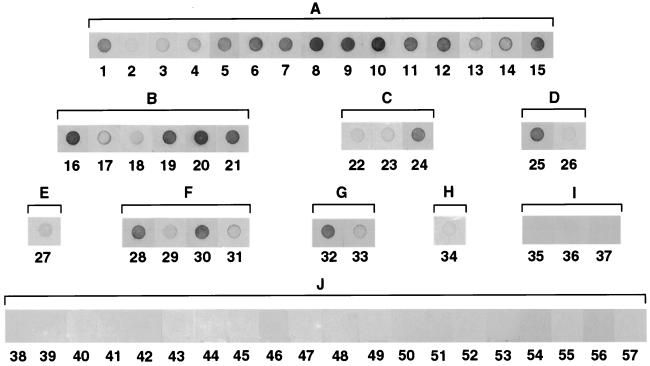
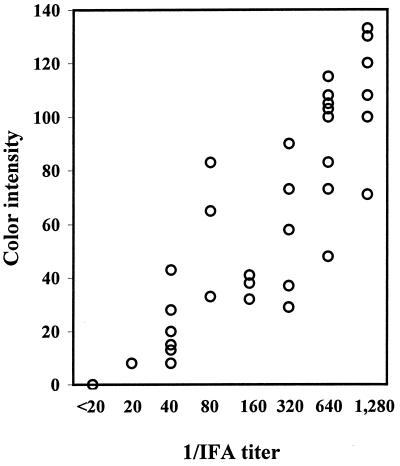
The 57 E. chaffeensis IFA-positive and -negative human serum samples were analyzed by dot immunoblot assay with rP30 which was not subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) denaturing conditions (SDS, β-mercaptoethanol, and boiling) (Fig. 3). In agreement with the Western immunoblotting data, the sera in groups A through H reacted with rP30, although the remaining groups, I and J, did not. Except for three E. ewingii infection-like samples in group I, dot blot reactions of IFA-positive and -negative samples were clearly distinguishable by the naked eye. Sera with IFA titers of 1:20 and 1:40 had significantly greater color intensities than those with titers of <1:20. Therefore, even at this low IFA titer range, there is a concordance between the IFA titer and the color density. Based on the densitometric analysis of the dot reaction (Fig. 4), the color intensities of the dots and the IFA titers were proportional, and the correlation coefficient (r) for these two variables was 0.913 (P < 0.001).
FIG. 3.
Reaction profiles of rP30 antigens (0.5 μg) by dot immunoblot analysis with 57 human serum samples. Serum identification numbers are indicated below the dots. A to I above brackets correspond to group identifications in Table 1.
FIG. 4.
Correlation between IFA titer (reciprocal dilution) and color intensity of dot blot reactions of sera, except for three sera in group I. The color intensities of each dot in Fig. 3 were determined and plotted. Each circle represents one sample. The correlation coefficient was 0.913 (P < 0.001; n = 54).
Table 5 shows a comparison of specificity and sensitivity with an IFA cutoff of 1:20 and a dot density cutoff of 0 versus an IFA cutoff of 1:80 (the currently accepted IFA cutoff) and a dot density cutoff of 23, with group I sera included. The only difference between these different cutoff values is that two specimens with an IFA titer of 1:40 gave a dot density of >23. This may be considered a false positive (lowered specificity relative to the IFA), or the dot blot assay may be more sensitive than the IFA test because the specific antigen is more concentrated in the antigen and gave a significant density value.
TABLE 5.
Diagnostic sensitivity and specificity and positive and negative predictive values of dot blot assay with rP30 relative to the E. chaffeensis IFA
| IFA cutoff | Dot blot density cutoff | Relative diagnostic sensitivity (%) | Relative diagnostic specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| 1:20 | 0 | 92 | 100 | 100 | 87 |
| 1:80 | 23 | 90 | 93 | 93 | 89 |
DISCUSSION
Previous studies indicated that 28- to 30-kDa proteins of E. chaffeensis and E. canis are immunodominant major outer membrane proteins recognized by sera from HME patients (1, 3, 19). After cloning and characterization of genes in a multigene family encoding 28-kDa proteins of E. chaffeensis and 30-kDa proteins of E. canis, the immunoreactive recombinant fusion proteins rP28 and rP30, respectively, became available (18, 19). We previously reported that both native and recombinant 28-kDa proteins of E. chaffeensis and the 30-kDa protein of E. canis are immunologically highly cross-reactive (18, 19). Therefore, rP28 and rP30, products of the homologous multigene family, are the primary recombinant antigen candidates for use in the serodiagnosis of HME.
Based on the comparative reactivities to the native and recombinant E. chaffeensis and E. canis antigens in Western immunoblotting, all sera except those in group G (Table 1) are most likely from E. chaffeensis infections. Although all five human isolates and PCR results for patients in the United States showed 16S rRNA gene sequences identical to those of E. chaffeensis (20), the lack of reactivity of group G samples to E. chaffeensis antigen could indicate infection with an agent closely related to E. canis. Three samples in group I suggest E. ewingii infections. Because E. ewingii has not been isolated from human patients, a strong cross-reactivity of patient sera to E. canis or E. chaffeensis antigen with no or minimal reactivity to 28- or 30-kDa native or recombinant antigen may be used as a tentative criterion for serodiagnosis of E. ewingii infection. E. ewingii or E. canis infection may account for some previously reported cases (3 of 21 cases) with a positive serologic response to E. canis or E. chaffeensis but with negative results in PCR assays for E. chaffeensis (5). Although, based on the 16S rRNA gene sequence, E. ewingii is closely related to E. canis and E. chaffeensis, E. ewingii either lacks the 30-kDa range of antigens or has homologous proteins of very different antigenic composition. This difference may be related to their host cell specificities: both E. chaffeensis and E. canis are monocyte-tropic, but E. ewingii is granulocyte-tropic.
The diagnostic sensitivity relative to E. chaffeensis IFA results may be misleading, because IFA-positive results could include false-positive sera owing to exposure of patients to cross-reactive agents, i.e., proteins such as heat shock proteins present in the infected cells used in antigen slides. Although the relative diagnostic sensitivity with E. chaffeensis whole-organism Western blotting (92%) appears to be high, no specific band could be identified consistently. The use of rP30 provided results as sensitive as those with E. chaffeensis whole organisms. If we consider group I sera to be from E. ewingii infection because of the lack of reactivity to the 28- to 30-kDa native and recombinant proteins, the relative diagnostic sensitivity of rP30 would increase to 100%.
At first glance this result appears to be odd, because the use of rP30 as a Western blot antigen is more sensitive than the use of rP28 for HME serodiagnosis. Although different denaturing conditions and affinity purification tags were used to solubilize and purify these two proteins, which are quite hydrophobic membrane proteins, the urea or guanidine thiocyanate used should not change the primary structures of the proteins. Since urea and guanidine thiocyanate were completely removed through extensive stepwise dialysis and the difference in reactivity between rP28 and rP30 was seen in Western blotting with SDS- and β-mercaptoethanol-solubilized and boiled protein antigens, this difference is due to the difference in the primary structures of these two proteins rather than to secondary or tertiary structures. Although refolding may not be perfect with these proteins even with slow removal of urea, this does not account for the inferior reactivity of rP28 in Western blot analysis.
Our laboratory and others have begun to characterize ehrlichial major outer membrane protein genes. What is unique for ehrlichial major outer membrane proteins is that they are encoded by a multigene family (18, 19, 21, 33–35). Both p28 and p30 belong to the same multigene family, consisting of more than a dozen homologous but not identical polymorphic genes. In other words, the p28 families and p30 families are not segregated between E. chaffeensis and E. canis but overlap each other. We expressed P28 of the E. chaffeensis Arkansas strain, which was the first P28 homologous protein cloned (19), and used it for this study. The overall poor reactivity of patients' sera to rP28 suggests that this protein may not be the one abundantly expressed in patients and recognized by patients' immune systems. But an unknown P28 homologous protein(s) that shares antigenic epitopes with E. canis Oklahoma strain P30, which was the first P30 homologous gene of E. canis cloned and expressed (18), may be expressed in patients. In order to prove this speculation and to obtain a better recombinant protein, one may need to identify P28 homologous proteins abundantly expressed and recognized by the humoral immune system of HME patients. Meanwhile, rP30 of E. canis, which is already available, can serve as a surrogate antigen for serodiagnosis until we are able to come up with a superior E. chaffeensis P28 homologous protein.
Differences in sensitivity between IFA and Western blotting results with recombinant antigens might also be attributed to differences in the method used to prepare the antigen. For IFA, an acetone-denatured native whole infected-cell antigen containing major outer membrane proteins originally associated with membrane phospholipid is utilized. Western immunoblot analysis uses SDS- and β-mercaptoethanol-solubilized and heat-denatured native or recombinant antigens. Our dot blot assay used the recombinant antigen carefully refolded by stepwise dialysis to slowly remove urea. We previously compared native antigen (whole purified organism) and recombinant P30 antigen without any chemical fixation or SDS-PAGE denaturing procedure by dot blot assay with canine anti-E. canis sera (18). There was almost 100% concordance in the dot intensities of reactions between native (membrane-associated, nondenatured) and recombinant antigens. Therefore, an absence of association with phospholipid in the purified recombinant antigen preparation or the denaturing conditions used for SDS-PAGE may not account for the lower diagnostic sensitivity relative to IFA.
Brouqui et al. reported that 10 E. chaffeensis IFA-positive patient sera reacted with 20- to 29-kDa proteins of E. chaffeensis, but not with proteins of similar sizes of E. canis, by Western immunoblot analysis (1). The reactivity of these sera appears to be similar to that of three sera belonging to group C in the present study. Yu et al. developed a dot blot assay with a recombinant partial 120-kDa protein that comprises the first two repeat units and 42 bp upstream of the repeat region and a 36-kDa (by SDS-PAGE) truncated protein (30, 31). Of 28 E. chaffeensis IFA-positive specimens, 24 were positive by the dot blot assay, and 12 IFA-negative specimens were all negative by the dot blot assay. Chen et al. (3) examined 27 E. chaffeensis IFA-positive serum samples by Western blot analysis, using three strains of E. chaffeensis (Arkansas, 91HE17, and Sapulpa), and showed that 16 samples recognized 29- and 28-kDa proteins from one to three of the strains. On the other hand, 22 samples were positive for the 120-kDa protein by Western blot analysis, and 23 were positive for the recombinant 120-kDa protein by the dot blot assay. Based on their study and ours, the denatured native 28-kDa E. chaffeensis protein is not sensitive enough to be used for a serodiagnostic antigen. We recently demonstrated that the human granulocytic ehrlichiosis agent has approximately 18 polymorphic homologous p44 major outer membrane protein genes and that 5 of these genes are expressed in the HL-60 cell culture system (33). Our present data suggests that P28 homologous proteins which contain epitopes that are most commonly recognized by HME patients' immune systems are not abundantly expressed by E. chaffeensis cultivated in the DH82 cell culture system.
The IFA test for ehrlichiosis had been developed in the field of veterinary medicine, long before problems in the diagnosis of human ehrlichiosis were realized. To determine the cutoff value for the IFA for Potomac horse fever or canine ehrlichiosis, we experimentally inoculated animals with E. risticii or E. canis and compared the time courses of development of IgG and IgM antibody titers, PCR, culture isolation, and clinical signs, and we also compared a large number of clinical specimens by IFA and PCR and/or culture isolation (12, 13, 17, 24, 25, 29). Based on these results, we recommended 1:80 as a cutoff value for ehrlichial IFA serodiagnosis to determine the positive or negative status of the current infection. However, there have been very limited studies to determine the best cutoff titer for serodiagnosis for HME. A titer of 1:64 or 1:80 as a cutoff value for IFA has been generally accepted. One laboratory in France uses a 1:20 cutoff. Recently, with nine culture-confirmed HME cases, only 33% were seropositive by the first test, and seroconversion was seen in 88% of cases by using 1:64 as a cutoff titer (4). In the present work we used a 1:20 IFA titer as an assay cutoff value to compare the sensitivities and specificities between the whole organism and the recombinant antigen by Western and dot blot analyses with zero density as a cutoff value. When we used a 1:80 IFA titer and a dot density of 23 as cutoff values, only two specimens with a 1:40 IFA titer became false positive relative to IFA. At least some of these low IFA titers (1:20 and 1:40) may not be nonspecific reactions but rather may be due to early stages of infection, weak immune status (immunosuppression) of the patients, or residual titers from previous exposure to the pathogen.
Overall, higher-IFA-titer sera reacted more strongly and to more proteins, including recombinant antigens; therefore, convalescent-phase sera would provide more reliable results by Western blot or dot blot analysis than acute-phase sera. Of course, paired specimens are desirable. Although IFA is widely used as a primary diagnostic tool for HME, variations in titers and cutoff reactivities among laboratories and individual examiners are common due to subjective visual evaluation of stained slides. Specific equipment and personnel, as well as a tissue culture facility, are required to prepare E. chaffeensis antigen. Depending on the types of fluorescence microscopes, bulbs, and filters, the sensitivity and, consequently, cutoff values may vary among laboratories. Use of whole infected cells or organisms as the antigen may produce inconsistent results owing to nonspecific antibody binding and to batch-to-batch variation of E. chaffeensis antigen preparations. In addition, the serologic cross-reactivity of heat shock proteins, such as HSP60 (32) and other proteins, may cause false-positive reactions in the IFA test (18, 34). The antigen preparation of recombinant proteins is simpler, more consistent, and more economical than purified native antigens from cell culture systems. The rP30 antigen may be a potential antigen candidate for serodiagnosis by immunoblot assay, as well as by other serologic methods such as enzyme-linked immunosorbent plate assays. In addition, E. ewingii-infected serum samples may be distinguished from E. chaffeensis- and E. canis-infected samples by the lack of or weak reactivity to rP30 in immunoblot analysis after IFA testing.
Because serologic methods provide primary diagnosis of human ehrlichiosis, the availability of recombinant immunoreactive proteins such as rP30 is expected to greatly facilitate laboratory diagnosis and increase the availability of serologic tests.
ACKNOWLEDGMENTS
This work was supported by grant AI40934 from the National Institutes of Health. A.U. is a recipient of a scholarship from the National Ministry of Education in Turkey.
REFERENCES
- 1.Brouqui P, Lecam C, Olson J, Raoult D. Serologic diagnosis of human monocytic ehrlichiosis by immunoblot analysis. Clin Diagn Lab Immunol. 1994;1:645–649. doi: 10.1128/cdli.1.6.645-649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buller R S, Arens M, Hmiel S P, Paddock C, Sumner J W, Rikihisa Y, Unver A, Graudreauls-Keener M, Marinian F A, Liddell A M, Schmulewitz N, Storch G. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 3.Chen S M, Cullman L C, Walker D H. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and E. canis. Clin Diagn Lab Immunol. 1997;4:731–735. doi: 10.1128/cdli.4.6.731-735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childs J E, McQuiston J, Sumner J W, Nicholson W L, Comer J A, Massung R E, Standaert S M, Paddock C D. Human monocytic ehrlichiosis due to Ehrlichia chaffeensis: how do we count the cases? In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millenium. Paris, France: Elsevier; 1999. pp. 287–293. [Google Scholar]
- 5.Comer J A, Nicholson W L, Sumner J W, Olson J G, Childs J E. Diagnosis of human ehrlichiosis by PCR assay of acute-phase serum. J Clin Microbiol. 1999;37:31–34. doi: 10.1128/jcm.37.1.31-34.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson J E, Rikihisa Y, Ewing S A, Fishbein D B. Serologic diagnosis of human ehrlichiosis using two E. canis isolates. J Infect Dis. 1991;163:564–567. doi: 10.1093/infdis/163.3.564. [DOI] [PubMed] [Google Scholar]
- 8.Everett E D, Evans K A, Henry R B, McDonald G. Human ehrlichiosis in adults after tick exposure. Ann Intern Med. 1994;120:730–735. doi: 10.7326/0003-4819-120-9-199405010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Fishbein D B, Dawson J E, Robinson L E. Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med. 1994;120:736–743. doi: 10.7326/0003-4819-120-9-199405010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Guarino P D, Meek J I, Kusha B, et al. Statewide surveillance for ehrlichiosis—Connecticut and New York, 1994–1997. Morbid Mortal Weekly Rep. 1998;47:476–480. [PubMed] [Google Scholar]
- 11.Guerrero A, Fishbein D B, Mesa E, Escudero R. Human infection by Ehrlichia canis in Spain? Med Clin (Barcelona) 1991;96:236–237. [PubMed] [Google Scholar]
- 12.Iqbal Z, Chaichanasiriwithaya W, Rikihisa Y. Comparison of PCR and Western immunoblot analysis in early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658–1662. doi: 10.1128/jcm.32.7.1658-1662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal Z, Rikihisa Y. Application of polymerase chain reaction for detection of Ehrlichia canis in tissues of dogs. Vet Microbiol. 1994;42:281–287. doi: 10.1016/0378-1135(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson R H. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech Off Int Epizool. 1998;17:469–486. doi: 10.20506/rst.17.2.1119. [DOI] [PubMed] [Google Scholar]
- 15.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 16.Morais J D, Dawson J E, Greene C, Filipe A R, Galhardas L C, Bacellar F. First European cases of ehrlichiosis. Lancet. 1991;338:633–634. doi: 10.1016/0140-6736(91)90644-5. [DOI] [PubMed] [Google Scholar]
- 17.Mott J, Rikihisa Y, Zhang Y, Reed S M, Yu C Y. Polymerase chain reaction and Southern blot analysis of Ehrlichia risticii in the blood and feces of horses. J Clin Microbiol. 1997;35:2215–2219. doi: 10.1128/jcm.35.9.2215-2219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohashi N, Unver A, Zhi N, Rikihisa Y. Cloning and characterization of multigenes encoding immunodominant 30-kilodalton major outer membrane protein of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol. 1998;36:2671–2680. doi: 10.1128/jcm.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paddock C D, Sumner J W, Shore G M, Bartley D C, Elie R C, McQuade J G, Martin C R, Goldsmith C S, Childs J E. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierard D, Levtchenko E, Dawson J E, Lauwers S. Ehrlichiosis in Belgium. Lancet. 1995;346:1233–1234. doi: 10.1016/s0140-6736(95)92943-6. [DOI] [PubMed] [Google Scholar]
- 22.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 23.Rikihisa Y, Ewing S A, Fox J C. Western blot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infection of dogs and human. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rikihisa Y, Ewing S A, Fox J C, Siregar A G, Pasaribu F H, Malole M B. Enzyme-linked immunosorbent assay and Western immunoblot analysis of Ehrlichia canis and canine granulocytic Ehrlichia. J Clin Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rikihisa Y, Reed S M, Sams R A, Gordon J C, Pretzman C I. Serosurvey of horses with evidence of equine monocytic ehrlichiosis (Potomac horse fever) J Am Vet Med Assoc. 1990;197:1327–1332. [PubMed] [Google Scholar]
- 26.Rikihisa Y, Zhang Y, Park J. Role of Ca2+ and calmodulin on ehrlichial survival in macrophages. Infect Immun. 1995;63:2310–2316. doi: 10.1128/iai.63.6.2310-2316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uhaa I J, MacLean J D, Green C R, Fishbein D B. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46:161–164. doi: 10.4269/ajtmh.1992.46.161. [DOI] [PubMed] [Google Scholar]
- 28.Walker D H, Dumler J S. Emergence of the ehrlichiosis as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen B, Rikihisa Y, Zhi N, Greene R, Bartsch R. Nested polymerase chain reaction for detection of Ehrlichia canis infection in dogs. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu X-J, Crocquet-Valdes P, Walker D H. Cloning and sequencing of the gene for a 120 kDa immunodominant surface protein of Ehrlichia chaffeensis. Gene. 1997;184:149–154. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 31.Yu X-J, Crcoquet-Valdes P, Cullman L C, Walker D H. The recombinant 120-kilodalton protein of Ehrlichia chaffeensis, a potential diagnostic tool. J Clin Microbiol. 1996;34:2853–2855. doi: 10.1128/jcm.34.11.2853-2855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Ohashi N, Lee E H, Tamura A, Rikihisa Y. Ehrlichia sennetsu groEL operon and antigenic properties of the GroEL homolog. FEMS Immunol Med Microbiol. 1997;18:39–46. doi: 10.1111/j.1574-695X.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]
- 34.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of 44-kDa major surface protein gene of human granulocytic ehrlichiosis (HGE) agent and application of recombinant protein to serodiagnosis. J Clin Microbiol. 1998;35:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhi N, Rikihisa Y, Kim H Y, Wormser G P, Horowitz H W. Comparison of major antigenic proteins of six strains of human granulocytic ehrlichiosis agents by Western immunoblot analysis. J Clin Microbiol. 1997;35:2606–2611. doi: 10.1128/jcm.35.10.2606-2611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]