Abstract
Chronic osteomyelitis is a challenging disease due to its serious rates of mortality and morbidity while the currently available treatment strategies are suboptimal. In contrast to the adopted systemic treatment approaches after surgical debridement in chronic osteomyelitis, local drug delivery systems are receiving great attention in the recent decades. Local drug delivery systems using special carriers have the pros of enhancing the feasibility of penetration of antimicrobial agents to bone tissues, providing sustained release and localized concentrations of the antimicrobial agents in the infected area while avoiding the systemic side effects and toxicity. Most important, the incorporation of osteoinductive and osteoconductive materials in these systems assists bones proliferation and differentiation, hence the generation of new bone materials is enhanced. Some of these systems can also provide mechanical support for the long bones during the healing process. Most important, if the local systems are designed to be injectable to the affected site and biodegradable, they will reduce the level of invasion required for implantation and can win the patients’ compliance and reduce the healing period. They will also allow multiple injections during the course of therapy to guard against the side effect of the long-term systemic therapy. The current review presents different available approaches for delivering antimicrobial agents for the treatment of osteomyelitis focusing on the recent advances in researches for local delivery of antibiotics.
HIGHLIGHTS
Chronic osteomyelitis is a challenging disease due to its serious mortality and morbidity rates and limited effective treatment options.
Local drug delivery systems are receiving great attention in the recent decades.
Osteoinductive and osteoconductive materials in the local systems assists bones proliferation and differentiation
Local systems can be designed to provide mechanical support for the long bones during the healing process.
Designing the local system to be injectable to the affected site and biodegradable will reduces the level of invasion and win the patients’ compliance.
Keywords: Osteomyelitis, bone scaffolds, tissue engineering, nanoparticles, 3 D printing, bone grafts, local drug delivery
1. Introduction
Osteomyelitis, a term indicating infection in the bone, is accompanied by acute or chronic inflammatory events. Bone infection usually arises from pyogenic organisms including bacteria, fungi and mycobacteria. In cases such as ischemia, trauma, surgery or those associated with incorporation of artificial joint devices, susceptibility of the bone and its structures to catch the causative microorganism is high (Momodu & Savaliya, 2020).
The opportunistic gram positive Staphylococcus aureus was found to be the most common causative microorganism in bone infection, which calls for approximately 75% of cases of osteomyelitis (Walter et al., 2012; Kavanagh et al., 2018). Other causative microorganisms include enterococcus species, streptococcus species, pseudomonas aeruginosa and enterobacteriaceae (Fraimow, 2009).
The tendency of bacteria to form biofilm of high density of microbial content made of condensed matrix of DNA, proteins and polysaccharides is considered an obstacle in the treatment of osteomyelitis (Zimmerli & Moser, 2012). Formation of biofilm can hinder the accessibility of the antimicrobial agents into the infected site resulting in resistance of the bacteria to the antimicrobial agents and increasing the rates of failure of treatment (Høiby et al., 2011).
Etiologically, three clinical categories of osteomyelitis are identified; (1) A bone infection that is derived or transported through the systemic circulation called hematogenous osteomyelitis, (2) Osteomyelitis associated with a contiguous source such as surgical procedure, incorporation of artificial joint or trauma and (3) Osteomyelitis due to contiguous source associated with vascular insufficiency which is most commonly seen in patients with peripheral vascular diseases and diabetes mellitus (Ford & Cassat, 2017).
Osteomyelitis has more prevalence in the developing countries than in the United States (U.S) (Uskokovic, 2015). Reports from the U.S hospitals admissions in 1999 showed that the overall incidence of osteomyelitis was found to be about 50,000 cases per year or as high as 1 in 675 hospital admissions annually (Rubin et al., 1999). A comprehensive epidemiological study describing the entire spectrum of osteomyelitis in the U.S over 41-year from 1969 to 2009, showed that there was an increased incidence of osteomyelitis due to the increase in the prevalence of risk factors such as diabetes. The major incidence of osteomyelitis among older individuals was mainly because of the higher frequency of disorders such as diabetes mellitus and orthopedic surgeries that lead to infections. The most common etiology in children was hematogenous infections (Kremers et al., 2015).
The three categories of osteomyelitis can occur in acute or chronic phases. Acute osteomyelitis develops over several days or weeks in contrast to the chronic one, which is known to evolve over a span of months or even years. The mechanism of acute infection involves the entry of the microorganisms, which release proteolytic enzymes and toxic free radicals causing inflammation and destruction of the surrounding tissues. This leads to unreachable blood flow to the area of infection, which causes poor vascularized area. The poor vascularization enhances the chance of forming sequestrum that results from the destruction of bone and hence boosts the impediment of penetration of the antimicrobial agents, which accounts later for the chronicity of the infection. Chronic osteomyelitis has different stages demonstrating the progression of the disease as well as the degree of deterioration of bones. Surgery is usually needed to remove the infection focus for the complete resolution of infection.
As it is very important to identify the clinical stage of patients with chronic osteomyelitis before treatment, many classification systems were described by several authors. The most widely used classification system is the one developed by Cierny and colleagues in 1985 (Cierny et al., 1985). This classification provides guiding for surgical or antibiotic therapy approaches (Calhoun et al., 2009). It was developed based on several parameters namely; the anatomical position of the infection, the extent of bone damage and the patient status. According to this classification, there are four anatomic types of osteomyelitis based on the degree of tissues involvement. These four types are: (1) medullary osteomyelitis when the affected part of the bone is restricted only to the intramedullary cavity of the cortical bone and is usually caused by single pathogen, (2) superficial osteomyelitis when the bone surface is damaged, (3) localized osteomyelitis which involves both the cortical and medullary areas in the damaged bone and is usually caused by multiple pathogens and (4) diffuse osteomyelitis when the entire thickness of the bone is damaged and extend to multiple bones and soft tissues structures that leads to bone destruction. These anatomic types are combined with other physiologic factors to define the clinical stage (stages 1–4) of the patient with their corresponding medical intervention (Cierny et al., 2003) as presented in Table 1.
Table 1.
Clinical stages and suggested medical intervention of chronic osteomyelitis according to Cierny –Mader (Cierny et al., 2003) classification system.
| Clinical stage | Required medical intervention |
|---|---|
| Stage I |
|
| Stage II |
|
| Stage III |
|
| Stage IV |
|
2. Background on the current treatment strategies
Due to the increased morbidity rates in patients suffering from osteomyelitis and the challenging treatment plan for complete eradication of the infection, extensive efforts are being spent and researches are being devoted that improved the therapeutic strategies to include:
2.1. Surgical procedures
Surgical procedures that involve complete debridement of the formed biofilm and necrotic tissues is a functional and effective approach in the eradication of the infection in complicated chronic osteomyelitis (Inzana et al., 2016). However, the critical problem of developing what is called dead space necessitates the effective management to reduce the chance of re-infection. This involves the implementation of bone grafts in the debrided tissues or the implementation of biomaterial spacers loaded with antibiotics and located in the bone tissue to deliver them locally in the area (Nandi et al., 2009). In a retrospective review study, Segreto et al. (2018) reported that early surgical intervention showed better outcomes than delayed one.
2.2. Systemic antibiotic therapy
Systemic antibiotic therapy is an effective strategy for the eradication of acute infections, and if combined with surgical treatment, it can be used in the treatment of chronic osteomyelitis for the complete eradication of the infectious microorganisms (Lew & Waldvogel, 2004). Antimicrobial therapy could be initiated according to culture media data of collected specimens. According to causative microorganisms, a set of antimicrobial agents were reported as effective therapy for most cases of osteomyelitis (Howard‐Jones & Isaacs, 2013; Senneville & Nguyen, 2013) either alone or as an adjunctive therapy after surgery. Table 2 presents examples of the officially approved products for systemic treatments with their date of approval. The treatment course usually starts with intravenous (IV) antimicrobials to improve the drug concentration in bone. Following, shifting to oral anti-bacterial agents might be considered in patients with good clinical response (Peters & Lipsky, 2013; Peltola & Pääkkönen, 2014).
Table 2.
Examples of the currently available antimicrobial therapy targeting osteomyelitis causative pathogens with the systemic route of administration.
| Microorganism | Antimicrobial agent | Route | Products approved by the FDA | Date of approval |
|---|---|---|---|---|
| Methicillin- resistant staphylococcus aureus (MRSA) | Vancomycin | IV | Firvanq® | 2018 |
| Vancomycin® | 1999 | |||
| Tedizolid | IV/ Oral | Sivextro® | 2014 | |
| Daptomycin | IV | Cubicin® | 2003 | |
| Linezolid | IV/ Oral | Zyvox® | 2000 | |
| Trimethoprim/sulfamethoxazole (+oral Rifampin) |
IV | Sulfamethoxazole and trimethoprim® | 1991 | |
| Oral | Sulfamethoxazole and trimethoprim® | 1986 | ||
| Clindamycin | IV | Cleocin phosphate® | 1972 | |
| Oral | Cleocin hydrochloride ® | 1970 | ||
| Methicillin- sensitive staphylococcus aureus (MSSA) | Cefazolin | IV | Cefazolin sodium® | 1988 |
| Nafcillin/oxacillin | IV | Unipen® | 1970 | |
| P. aeruginosa | Levofloxacin | Oral | Levofloxacin® | 2011 |
| IV | Levofloxacin® | 2005 | ||
| Ciprofloxacin | IV | Ciprofloxacin® | 2009 | |
| Oral | Ciprofloxacin® | 2004 | ||
| Meropenem | IV | Merrem® | 1996 | |
| Cefepime | IV | Maxipime® | 1996 | |
| Piperacillin/tazobactam | IV | Zosyn® | 1993 | |
| Ceftazidime | IV | Fortaz® | 1985 | |
| Imipenem/cilastin | IV | Primaxin® | 1985 | |
| Enterobacteriaceae | Moxifloxacin (+antipseudomonal agents) |
IV/oral | Moxifloxacin hydrochloride® | 2015 |
| Ceftriaxone | IV | Cftriaxone® | 2003 | |
| Cefotaxime | IV | Cefotaxime sodium® | 2002 | |
| Enterobacteriaceae (Anaerobes) |
Clindamycin | IV/oral | Cleocin phosphate® | 1999 |
| Cleocin HCL® | 1998 | |||
| Metronidazole | Oral | Flagyl® | 1995 | |
| IV | Flagyl® | 1981 | ||
| Enterococcus spp. (Ampicillin resistant) |
Vancomycin | IV | Firvanq® | 2018 |
| Vancomycin® | 1999 | |||
| Enterococcus spp. (Ampicillin sensitive) |
Ampicillin/ sulbactam | IV | Unasyn® | 1986 |
| Enterococcus spp. (Vancomycin resistant) |
Tedizolid | IV/oral | Sivextro® | 2014 |
| Linezolid | IV/ oral | Zyvox® | 2007 | |
| Daptomycin | IV | Cubcin® | 2003 | |
| Streptococcus spp. | Penicillin G | IV | Bicillin L-A® | 1952 |
Empiric antimicrobial therapy that covers all possible microorganisms is vital when the isolation of the causative pathogens from the infection site is not possible. Empiric therapy should target likely the causative pathogen(s) based on patient-specific risk factors and route of infection. The activity of empiric antimicrobial therapy against S. aureus should be taken into consideration in the treatment strategy in all the classifications of osteomyelitis (Conrad, 2010). Nosocomial infections that got from the hospitals are usually derived from methicillin-resistant S. aureus (MRSA), while infections got from somewhere else are often polymicrobial involving Gram-negative bacteria in some cases (Conrad, 2010). The utilization of anti-MRSA antimicrobials ought to be considered as the first-line therapy for empiric inclusion of suspected staphylococcal osteomyelitis, then antimicrobial therapy ought to be modified depending on culture and sensitivity data of appropriately collected specimens (Howell & Goulston, 2011).
Customarily, anti-infection treatment of osteomyelitis is comprised of 2–6 week course of IV administration of antibiotics, followed by oral antibiotics for 6-months course (Calhoun & Manring, 2005). Oral antimicrobial agents should possess such attributes as high bioavailability, good bone accessibility and long half-life (Senneville & Nguyen, 2013). Oral antibiotics that were concluded as being successful include clindamycin, rifampin, trimethoprim-sulfamethoxazole, linezolid and fluoroquinolones (Chihara & Segreti, 2010; Harik & Smeltzer, 2010; Liu et al., 2011; Senneville & Nguyen, 2013).
For parenteral route, Vancomycin, a member of the glycopeptide family, is a broad-spectrum antibiotic, active against Gram-positive bacteria including MRSA and is considered the first line therapy unless the minimum inhibitory concentration is greater than 2 mcg/mL (Senneville & Nguyen, 2013). Clindamycin is active against most Gram-positive bacteria, including staphylococci. It has excellent bioavailability and is administered by IV route for 1–2 weeks followed by oral administration (Liu et al., 2011). Linezolid is active against MRSA and vancomycin-resistant Enterococcus. It can penetrate the bone very well and can be administered via IV or oral routes (Liu et al., 2011). Tedizolid, a new oxazolidinone compound, has fewer side effects compared to linezolid and is effective against MRSA. It was reported that tedizolid monotherapy as well as tedizolid combination therapy with rifampin are active against MRSA in experimental foreign body-associated osteomyelitis (Park et al., 2017).
Oral quinolones are often prescribed for adults suffering from Gram-negative organisms. Ciprofloxacin, moxifloxacin and levofloxacin are used most commonly among the oral quinolones’ family owing to their kinetics and good distribution in bone tissues (Rimmele et al., 2004; Metallidis et al., 2006). Rifampin is active against MRSA. It can be combined with other antibiotics to have synergistic effect and to avoid rapid occurrence of resistant strains (Liu et al., 2011). Moreover, the utilization of rifampin in combination with other antibiotics has more efficiency than monotherapy in treating infections related to surgical hardware implants (Euba et al., 2009; Vergidis et al., 2011). Trimethoprim-sulfamethoxazole was indicated to be successful in the treatment of osteomyelitis in patients with MRSA infected orthopedic implants either alone or in combination with other antibiotics (Sato et al., 2019).
However, systemically administered antimicrobial agents suffer from several drawbacks in the treatment of chronic osteomyelitis including prolonged duration of treatment, increased incidence of side effects, restriction to penetration due to the sequestrum formed in the infection site and increasing the chance of development of resistance to the antimicrobial agent. Resistance develops when the delivery of antibiotics became obstructed. Some bacteria, such as S. epidermidis in prosthesis contaminations, adhere to the formed biofilm which protects the organism from phagocytosis in addition to the obstruction of the antibiotic penetration caused by the biofilm/ sequestrum, hence treatment fails. Similar conclusions were reported by many clinics (Zimmerli, 2014; Khatoon et al., 2018; Álvarez et al., 2019; Shipitsyna et al., 2020).
Thereby, the utilization of local antimicrobial therapy in the management of chronic osteomyelitis in long bones offers the advantage of localizing the therapeutic agent at the infection site in much higher concentration, lowering the incidence of side effects associated with the systemic route and offering the ability to increase the infection control rate and enhance the regeneration of bone tissues using tissue engendering techniques and biomaterial agents.
2.3. Clinically applied topical cements and scaffolds containing antimicrobial agents for managing osteomyelitis
From the surgeons’ point of view, as drawn from clinical investigations and studies conducted in groups of patients over several years, the use of bone cements mixed with antibiotics provides a promising and very efficient strategy for the management of infectious osteomyelitis. This has been routinely used strategy in orthopedic surgeries either applying a readymade delivery system (available in the market) or in-situ mixed dug and cements during the surgery (at operation sit). Clinical studies on local delivery of antibiotics along with the delivery vehicle that contains bone filling materials for the treatment of long bone infections are also available. They have been proven very effective in: (1) early suppression of infection, (2) showing several-folds reduction in the recurrent infection incidence, (3) minimizing the risk of pathological fracture due to internal reinforcement, (4) providing early recovery of extremity’s function and (5) creation of favorable conditions for bone structures restoration (Dzyuba et al., 2016; Mauffrey et al., 2016; Wang et al., 2021). The highlighted advantages of some cements being biodegradable and their ability to sustain the release of antibiotics over weeks of recovery period have greatly encouraged its widespread.
The oldest most widely used non-biodegradable material is Poly (methyl methacrylate) PMMA beads which have been the gold standard for local delivery of the therapeutic agents since decades (Evans & Nelson, 1993; Mohanty et al., 2003). PMMA beads can also be mixed with the antimicrobial agent just before the surgery (Gogia et al., 2009). PMMA beads offer the advantages of the lack of hypersensitivity reactions in the host in addition to maintaining high concentrations of the drug in-situ due to wide surface area for the release of the therapeutic agents (Lalidou et al., 2014).
In a study conducted by Dzyuba et al. (2016), scaffolds made intraoperatively from PMMA was implanted during operations to 30 patients suffering from chronic osteomyelitis. The data collected were compared with those of 30 patients treated with the conventional systemic therapy. Results showed that suppression of infection was better controlled in the group treated with local implant which also showed significant decrease in the recurrent infection incidence.
In a clinical study published by Wang et al. (2021), 19 patients diagnosed with chronic osteomyelitis in long bones underwent debridement procedure to remove the dead tissues followed by implanting a 3 D printed intramedullary titanium nail coated with PMMA impregnated with antimicrobial agents. Procedure involved formation of the 3 D molds that simulates the defect areas followed by using titanium nails coated by PMMA and vancomycin/imipenem to fit the molds. The titanium nails were inserted into the defect areas of the patients’ bones. The infection control was evaluated both clinically and radiographically, which showed that all cases had good infection control and confirmed the effectiveness of the used strategy for the treatment of defected infected bone along with bone healing.
However, PMMA beads have the shortcomings of requiring secondary surgery for removal, being non-biodegradable. In addition to heat production during the process of polymerization for the preparation of the beads, which makes it unsuitable for most antimicrobial agents as heat generation affects their stability. Finally, the in situ developed system suffers from unpredicted release profiles of the drugs, being prepared and inserted during the operation without pre-evaluation (Nandi et al., 2009).
Therefore, attempts have been made to encourage the use of biodegradable and bioabsorbable materials namely; hydroxyapatite, calcium sulfate, calcium phosphate, collagen and others incorporated into different forms (Maier et al., 2013; Anugraha et al., 2019). A local antibiotic delivery system with biodegradable drug carrier can be considered as therapeutically efficient platform for the treatment of osteomyelitis. Scaffolds made from biodegradable materials greatly affect the release rate of the incorporated drug. Therefore, when reasonably controlled drug release is desired, scaffold degradation rate has to be set properly (Dorati et al., 2017b).
In a retrospective investigation, 51 patients suffering from chronic osteomyelitis of lower extremities were distributed into two gropes either treated with combination therapy of vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA as test group or vancomycin-loaded PMMA as a control grope. The combination therapy of both biomaterials achieved synergistic effect in controlling the infection compared to the group given PMMA loaded with vancomycin only (Luo et al., 2016).
The available commercial products for local antibiotic delivery and their pharmaceutical forms are listed in Table 3.
Table 3.
commercially available systems for local antibiotic delivery to the affected sites of bones.
| Carrier | Antimicrobial agent | Form | Commercial Products | Official Approval |
|---|---|---|---|---|
| PMMA | Gentamicin | Beads impregnated with the antibiotic | Palacos G® | FDA, 2003 |
| Tobramycin | Bone cement incorporating the antibiotic | Simplex P® | FDA, 2007 | |
| Gentamicin and clindamycin | Bone cement incorporating the antibiotics | Copal® G + C | FDA, 2019 | |
| Gentamicin and vancomycin | Bone cement incorporating the antibiotics | Copal® G + V | FDA, 2019 | |
| Gentamicin | Beads impregnated with the antibiotic | Septopal® | The Therapeutic Goods Administration, Australia 2020 | |
| Hydroxyapatite | — | Xenograft granules that can be preloaded with suitable antibiotic | Endobon® | FDA, 2011 |
| Hydoxyapatite/ Calcium sulfate | Gentamicin. | Synthetic bone substitute incorporated with the antibiotic | Cerament® G | Health Canada, 2018 |
| Calcium sulfate | Tobramycin. | Bone graft substitute incorporating the antibiotic | Osteoset® | FDA, 2004 |
| — | Synthetic bone substitute that can be preloaded with suitable antibiotic. | Stimulan® | FDA, 2015 | |
| Calcium phosphate | — | Synthetic bone substitute that can be preloaded with suitable antibiotic. | Cerasorb® M | FDA, 2012 |
| — | Synthetic bone cement that can be preloaded with suitable antibiotic. | Biopex-R® | The Pharmaceutical and medical devices agency, Japan 2000 | |
| Calcium phosphate/ collagen | … | Synthetic bone substitute that can be preloaded with suitable antibiotic. | Cerasorb® Ortho Foam | FDA, 2020 |
| Collagen | Gentamicin. | Matrix impregnated with the antibiotic | Collatamp® | Health Canada, 2008 |
3. Systems under investigation for the local delivery of antibiotic for osteomyelitis management
Locally administered drug delivery systems to the site of infections are still under extensive investigations to improve the drug delivery to the infected bone. Using appropriate carriers, specific amount of the antimicrobial agents and controlling the released rate of the drug can help in the infection control and limit the recurrence rate. Additionally, if the delivery system made osteogenic in nature, they can exert dual function of eradicating the pathogens and assisting the bone regeneration after surgical debridement. Furthermore, these systems can be designed for in situ gelling after being injected to the close vicinity of the infected bone which allow the administration of a support therapy during the course of treatment to guard against a second surgical procedure for implant application or the drawbacks of the systemic therapy. Some clinical trials proved that in some cases, locally administered systems can minimize, the surgical intervention for removing the affected bone (Dzyuba et al., 2016; Luo et al., 2016).
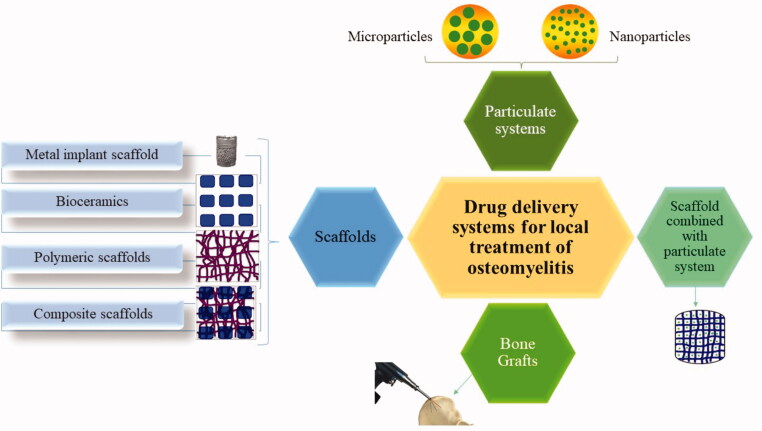
Biodegradable materials that have been investigated for the local application of the antimicrobial agents include natural (Boles et al., 2018) and synthetic polymers (Tsiolis et al., 2011), ceramics (Cao et al., 2016), bioactive glasses (Jia et al., 2015), allograft bone (Kim et al., 2011) and demineralized bone graft (Turner et al., 2001). These materials are designed in many forms or systems for the local delivery to the affected parts as presented in Figure 1.
Figure 1.
Local drug delivery systems under investigation for the management of osteomyelitis.
3.1. Scaffolds
Tissue regeneration is a process that happens following an acute injury. Enhancing the tissue regeneration process can occur in the defective site through rebuilding or repairing the tissue structure by providing a scaffold that simulate the body extracellular matrix (ECM). The use of scaffolds combined with therapeutic agents have been investigated and widely applied for the eradication of infections, revitalizing the damaged bones and restoring the loss of functionality of an organ as a result of tissue damages (Patzakis et al., 1993; Rasyid et al., 2009; Bhattacharya et al., 2013). Some other components are widely incorporated in the fabrication of bone tissue scaffold for the tissue engineering purpose including biomaterials, bioactive factors and cells (Oryan et al., 2018; Elkasabgy & Mahmoud, 2019; Morsi et al., 2019; Abdel-Salam et al., 2020).
Scaffold facilitates the processes of attachment, proliferation and differentiation of cells. Being biocompatible, neighboring cells are likely to be infiltrated into the scaffold and proliferate. Biomaterials, whether biodegradable or non-biodegradable, are better to have some properties including: (1) integrating with the biological molecules and cells, (2) promoting cells migration, proliferation, differentiation and revitalization of tissues, (3) exerting osteoinductive property, where osteogenesis is induced through stimulation of immature cells to develop into pre-osteoblasts as well as osteoconductive property, where bone tissue is allowed to grow on their surfaces and (4) providing mechanical structure to the affected bone (Sarigol-Calamak & Hascicek, 2018).
The bioengineered scaffolds are advantageous when applied in such cases of pathological conditions where, tissues do not have sufficient self-healing power, thus, promoting quicker tissue regeneration and rapid healing become mandatory (Porter et al., 2009). Over time, the implanted scaffold supports and promotes tissue regeneration accompanied with local delivery of certain dose of antibiotic for the desired period with minimization of its release to sites other than the targeted one. From this viewpoint, tissue engineering can be considered as a unique type of controlled drug delivery combined with scaffolding biomaterials (Dorati et al., 2017b).
Bone tissue scaffolds would be ideal if they fulfill the following criteria: (1) degradation in the same rate as the rate of bone regeneration, (2) leaving nontoxic and non-immunological degradation products, (3) having the ability to adhere to bone cells and provide mechanical support, (4) having interconnected porous structure of average diameter of 100 µm or greater where 50% or more are open in nature to facilitate cell infiltration and migration into the scaffold and finally (5) Their porous structure can provide satisfactory nutrients and oxygen diffusion (Sarigol-Calamak & Hascicek, 2018). In order to fulfill the previously-mentioned criteria, scaffolds made from bioceramics, polymers (natural and synthetic) and their composites are the most frequently studied types of scaffolds in the management of osteomyelitis.
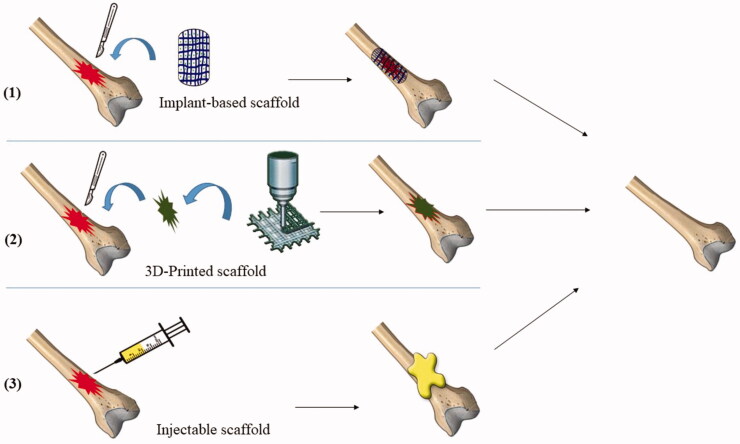
Different techniques were employed for scaffold fabrication and application to the affected parts of bones either in animal studies and/or in clinical trials, including topical implantation of the compounded system (Singh et al., 2020; Fang et al., 2021), in situ forming implant of an injectable system (Wasupalli & Verma, 2020; Moeinzadeh et al., 2021) and three-dimensional (3 D) printing of a fitting system (Sun et al., 2021; Wang et al., 2021). Graphical presentation of the application methods of scaffolds loaded with antibiotics into the infected bone tissues are shown in Figure 2.
Figure 2.
Representation of the methods used for the application of scaffolds loaded with antibiotics into the infected bone tissues.
The implant-based scaffold requires surgical procedure to be applied to the infected bone. They are prepared by several techniques including lyophilization (Nair et al., 2015; Narayan et al., 2017), solvent casting (Siemann, 2005; Choudhury et al., 2015), particulate leaching (Phull et al., 2013; Sabzi et al., 2021) gas foaming (Teng et al., 2007; Diaz-Gomez et al., 2017), or electrospinning techniques (Frohbergh et al., 2012; Su et al., 2012). In a study by Zhou et al. (2018) a bone tissue scaffold made of gelatin and β tricalcium phosphate was prepared by lyophilization technique and loaded with vancomycin. The surgical procedure for applying scaffolds to the rabbits’ tibias after osteomyelitis induction was accompanied by debridement of the necrotic bone tissues for enhancing the therapeutic efficacy. Results showed that scaffolds were able to sustain vancomycin release for eight weeks with good infection control and ability to repair the bone defects.
Another technique of scaffold formation is the in-situ forming of implants after injecting natural and/or synthetic polymer dispersion to the affected site. The most important parameters to control the formation of in-situ forming implant-scaffold is the ability of the polymers to undergo self-assembly transformation from a liquid monomeric phase into a solid or semi-solid polymeric network when affected by various factors such as temperature and pH Changes at the site of injection (Chaudhari et al., 2016). Injectable systems offer several advantages including patients comfort with a less invasive application technique, shorter recovery period and significant cost reduction with effective treatment of bone infections (Mahmoudian & Ganji, 2017). Hydrogel was found to be an ideal bulk platform scaffold for bone regeneration and for the localized release of antibiotics for the treatment of bone infections (Xing et al., 2013; Wan et al., 2015; Morsi et al., 2019). Furthermore, a novel injectable chitosan-based in-situ forming implant loaded with bioactive glass nanoparticles and raloxifene was prepared for the repair of noninfectious bone defect which can be extended for application in cases of bone infections. The injectable formulation had the ability to transform into hydrogel upon being exposed to body temperature (37 °C) within 20 min and offered sustained release of the drug for 56 days. The in vivo studies showed accelerated bone formation in induced non-critical injuries in rats tibias when compared to the untreated bone defect (Abdel-Salam et al., 2020).
Among the advanced technologies, utilizing computer-aided design (CAD) is the three-dimensional (3 D) printing. 3 D printing technologies has been characterized as an effective fabrication technique for customized scaffolds based on tissue engineering and antibiotic therapy (Mills et al., 2018; Elkasabgy et al., 2020; El-Habashy et al., 2021; Shamma et al., 2021). They showed promising potential in construction scaffolds fitting to the shape and site of the bone defect through controlling the cornerstones of 3 D printing such as accuracy and speed of fabrication (Xu et al., 2010; Seol et al., 2012; Lee et al., 2013). Additionally, the porous interconnected inner structure of 3 D printed scaffolds helps in enhancing the process of regeneration of new tissues. this can be useful in bone defects associated with bone infection (Lee et al., 2010, 2011; Shim et al., 2012; Sa & Kim, 2015).
3 D printed scaffolds loaded with tobramycin have been fabricated for the treatment of osteomyelitis where tobramycin was mixed with molten blend polymers of ploy-caprolactone (PCL) and poly (lactic-co-glycolic) acid (PLGA), dispensed using multi-head disposition system and exposed to 500 kPa pneumatic pressure to distribute the mixture through a nozzle made of steel. The bactericidal activity of the 3 D printed scaffolds against S. aureus and E. coli was successfully maintained after the process of thermal printing in an in-vitro study. The in-vivo study after implanting the scaffold in a rat femur model, confirmed its efficacy in developing new bone at the end of eighth week (Shim et al., 2015).
Additionally, Wu et al. revealed that 3 D printed multilayered concentric cylinder construction implant incorporating levofloxacin and tobramycin was able to enhance the antibiotics pharmacodynamic action through a sustained and programmed release pattern. The implanted scaffolds completely eradicated the infectious microorganisms and treated osteomyelitis in animal model with no recurrences after follow up for 2 months (Wu et al., 2016).
3.1.1. Metal implant
Some approaches are developed to prevent implants from bacterial infections as a result of the tremendous number of patients requiring orthopedic implants. Local delivery of bioactive agents through implant coating is one of the effective approaches to deal with infections. Long-term controlled drug release from the implant can be guaranteed without the existence of systemic side effects or development of bacterial biofilms. Among the metals used as orthopedic implants due to their satisfactory characteristics (excellent mechanical strength, good corrosion resistance and biocompatibility) are titanium (Ti) (Liu et al., 2017), cobalt (Co) (Lin et al., 2019) and stainless steel (Gimeno et al., 2013). However, the use of metallic implants suffers from the disadvantages of non-biodegradability and limited processability in the biological environment (Long & Rack, 1998; Chen et al., 2017). The most commonly used metal in implants for osteomyelitis is titanium.
3.1.1.1. Titanium implants
Ti-based alloys have great widespread to be used as metal implants and they are commercially available as load-bearing components in total bone or joint replacement in the form of nails, pedicle screws and fracture plates. However, Ti-based implants have the drawbacks of incidence of implant loosening, in addition to the probability of inhibition of subsequent osseointegration due to formation of a fibrous layer that is non-adherent to the metal implant (Chan et al., 2017). They can also induce immune reactions due to sensitization and activation of pro-inflammatory and anti-inflammatory cytokines which may lead to allergic reactions (Fage et al., 2016). Although titanium alloys have inherent antimicrobial properties, infection may still arise, which requires the application of antimicrobial coat on the implant’s surface in order to reduce infection and colonization of bacteria (Zhao et al., 2009). Furthermore, It is worthy to mention that metal implants are not osteoinductive nor osteoconductive and they do not enhance bone regeneration. Therefore, modification of the surface of an implant for the optimization of its osteoinductive and osteoconductive properties are critical for the implant to maximize its biological function while interacting with bone tissues (Asri et al., 2017). The following represents the most important surface modifications of Ti alloys:
3.1.1.1.1. Titanium implant doped with heavy metals
For the purpose of improving the antimicrobial effect of Ti alloys implants, they were supplemented (doped) with heavy metals that have antibacterial activity resulting in better outcome of treatment. Norambuena et al. (2017) in 2017 added copper (Cu) to the surface of Ti alloy to form titanium-copper oxide at different strengths of Cu. The in-vitro studies demonstrated that high doses of Cu (reaching 80% of the implant weight) had the most reliable effect in diminishing biofilm and bacterial growth of S. epidermidis. Moreover, in an in-vivo study in mice comparing the antibacterial effect of titanium implant coated with silver to the uncoated one, it was found that the number of S. epidermidis bacteria has been diminished by greater than 500-fold in case of the silver-coated implant compared to the uncoated 1 after 2 weeks of treatment (Tîlmaciu et al., 2015). Doping the titanium implant with metals can be serious if the local release of metal ions in the site of implant is high, which may lead to necrosis of cells. furthermore, the systemic exposure to these metal ions can be fatal if they distribute into brain, spleen, liver and other organs. Therefore, the toxicity of the doped metal ions should be under control (Gulati et al., 2021).
3.1.1.1.2. Titanium implants with antibiotics coated surface
In a study carried out by Sharma et al. in 2016 (Sharma et al., 2016), gentamicin loaded into the natural polymeric material, silk fibroin, nanoparticles were prepared and deposited over the Ti implant surface in order to achieve sustained release of gentamicin. The fibroin nanoparticle offered the advantage of changing the surface topography of the bare metal implant, which became rougher and more hydrophilic. Thus, the nanoparticles coated implants were found to be unrivaled for the osteoinduction and osteogenesis through improving osteoblast adhesion, proliferation and differentiation if compared with the uncoated Ti surface. This approach was considered as one of the cost-effective approaches for the treatment of osteomyelitis.
In another study, Ti alloy underwent surface modification through coating with chitosan/lysine biopolymers as a carrier for gentamicin. This modification did not only eradicate the infection through sustained drug release after the initial burst release from the implant, but also improved the adhesion of cells followed by proliferation when tested in simulated body fluid. Results showed that the coat had the capability of bioactivity through improving bone growth and new bone tissue formation. This reinforced the idea of the favored environment of the modified implant to encourage bone regeneration (Raj et al., 2018).
David and colleague (2018) adopted surface modification of Ti metal using chitosan-gelatin polyelectrolyte complex as a carrier for vancomycin. The complex-loaded with vancomycin was incorporated into scaffold formed of gelatin: stronium: hydroxyapatite (HA) simulating the chemical composition of bone. The fabricated scaffold achieved its antibacterial activity through growth inhibition of MRSA and MSSA strains with sustained and controlled release of vancomycin for an adequate period to resolve the infection.
Additionally, modifying the surface of Ti scaffold for providing antibacterial properties was achieved successfully by introducing a biodegradable system made of gentamicin loaded microparticles linked to the pore walls of the Ti scaffolds by cross-linked sodium alginate. The fabricated system provided the required doses of gentamicin to combat the infection after implementation of the scaffold. The system was fully cytocompatible when tested against osteoblast-like cells (Rumian et al., 2016).
Creating and using an intramedullary nail to be implanted in the intramedullary cavity coated with antibiotic and growth factors can be a successful approach for eradicating the infection and encouraging bone regeneration according to Berebichez-Fridman et al. (2017). The placement of nanoparticles on intramedullary nail at the area of infection can guarantee local application of the antimicrobial agents restricted to the contact sites. After certain time, the nanoparticles were able to kill the pathogens and invigorate the formation of new healthy bone, besides providing constant immobilization and stability of the affected bone by the intramedullary nail, which improved bone healing.
3.1.1.1.3. Titanium implants with surfaces’ nanopatterning
Nanoscale dimensions are substantial in the design of surfaces for tissue engineering, as cells can recognize the topographical changes in the extracellular matrices where they can adhere to and proliferate (Jeong et al., 2012). Moreover, nanopatterned surfaces can facilitate the development of new functional materials with increased resistance to bacterial contamination and infection in addition to offering a new platform for tuning implant properties. Nanopatterned surfaces can be of natural source such as cicada wings and dragonfly wings with nanopillar patterns (Diu et al., 2014) or of an artificial source such as Ti (Sengstock et al., 2014), silicon (Ivanova et al., 2013) and polymers (Dickson et al., 2015).
A suggested mechanism for the bactericidal activity of the nanopatterned surfaces when tested on a cicada wing-like nanopatterned surface is that the nanopatterned surfaces have a bactericidal activity arising from physical interactions between the nano-surfaces structures and the bacterial cells because of the remarkable increase in the contact adhesion area (Li, 2016).
Nanpaterterning of Ti implant is made through direct changing the surface of implant at the nanoscopic level. Nanopatterning technology can provide biologically optimized surfaces for implantable materials where cells can adhere to, since bone tissues are composed of nanostructures such as hydroxyapatite crystals, fibrillar collagen and non-collagenous organic proteins (Tan et al., 2014). An in-vitro study using mesenchymal stem cells revealed that the fabricated three-dimensional (3 D) nanopatterned surfaces of titanium induced the osteogenic differentiation with cells on these surfaces (Sjöström et al., 2013). In another in-vitro study, Variola et al. (2014) proved that the Ti nanopatterned surfaces had resistance against the adhesion of candida albicans yeast, S.aureus and E.coli aggregation which proves their antimicrobial activity. More investigations on metal scaffolds are listed in Table 4.
Table 4.
More investigations on the types of scaffolds as delivery systems of antimicrobial agents for the management of osteomyelitis.
| Types of Scaffolds | Antimicrobial agents | Carriers | Achievements | Study type/ assayed model | References |
|---|---|---|---|---|---|
| Metal implants | Gentamicin | Titanium implant | High prophylaxis against implant-related osteomyelitis | In-vivo/Rats | (Diefenbeck et al., 2016) |
| Vancomycin | Zeolitic imidazolate nanocrystals |
|
In-vitro | (Karakeçili et al., 2019) | |
| 3D printed Titanium implant |
|
In-vivo/ Rabbits | (Zhang et al., 2020) | ||
| Clindamycin | 3D printed coatings on titanium and stainless steel implant |
|
In-vitro | (Maver et al., 2021) | |
| Bioceramics | Vancomycin | Hydroxyapatite/ Calcium phosphate | Successful management of diabetic foot infection. | Clinical/ Patients | (Karr, 2011) |
| Hydroxyapatite/ Calcium phosphate | Controlled antibiotic release pattern over a 12-day period. | In-vitro | (Thanyaphoo & Kaewsrichan, 2012) | ||
| Hydroxyapatite/ Poly amino acid |
|
In-vivo/Rabbits | (Cao et al., 2016) | ||
| Hydroxyapatite |
|
In-vitro | (Hess et al., 2016) | ||
| Hydroxyapatite |
|
In-vitro | (Parent et al., 2016) | ||
| Calcium polyphosphate | Sustained release of the antibiotic. | In-vitro | (Comeau & Filiaggi, 2017) | ||
| Calcium phosphate/ calcium sulfate |
|
Clinical/ Patients | (Zhao et al., 2020) | ||
| Gentamicin | Calcium sulfate | Significant bactericidal activity of the scaffold. | In-vitro | (Thein et al., 2013) | |
| Hydroxyapatite/ Calcium phosphate | Clinical cure of the heel ulcers in diabetic foot patients after 16 weeks without need of amputation. | Clinical/ Patients | (Drampalos et al., 2018) | ||
| Vancomycin/gentamicin | Calcium sulfate | Full recovery in 4 months in a patient with diabetic foot infection. | Clinical/ Patients | (Morley et al., 2016) | |
| Calcium sulfate/ hydroxyapatite | Prevention of biofilm formation | In-vitro | (Bidossi et al., 2020) | ||
| Ceftriaxone/sulbactam | Bioactive glass |
|
In-vivo/Rabbits | (Kundu et al., 2011) | |
| Gatifloxacin/ fluconazole | Bioactive glass | Sustained antibiotic release for up to 6 weeks. | In-vitro | (Soundrapandian et al., 2011) | |
| Vancomycin/ rhBMP-2 | Calcium sulfate |
|
In-vivo/Rabbits | (Wang et al., 2011) | |
| Ceftriaxone/sulbactam | Hydroxyapatite | New bone formation
|
In-vivo/Rabbits Clinical/ Patients |
(Bhattacharya et al., 2013) | |
| Linezolid | Calcium deficient apatite (CDA) | Enhanced efficacy of treatment with IV treatment. | In-vivo/Rabbits | (Gaudin et al., 2013) | |
| Amphotericin B/ voriconazole | Hydroxyapatite/ Calcium sulfate | Maintained effective antifungal concentrations over 96 hours. | In-vitro | (Karr & Lauretta, 2015) | |
| Levofloxacin | Bioactive glass/ HA nanoparticles | Sustained with pH-dependent release of drug at the infection site. | In-vitro | (Cicuéndez et al., 2018) | |
| Sitafloxacin/ rifampin | Calcium phosphate | Decreased bacterial colonization
|
In-vivo/ Mice | (Trombetta et al., 2019) | |
| Rifampicin | Nanohydroxyapatite /calcium sulfate |
|
In-vivo/Rats | (Qayoom et al., 2020) | |
| Polymeric | Gentamicin | PMMA | Effective treatment of patients with infected nonunioun of the long bones. | Clinical/ Patients | (Selhi et al., 2012) |
| Gentamicin/silver ion | Silk fibrin |
|
In-vivo/Rats | (Zhang et al., 2019b) | |
| Vancomycin/rifampin | Polydioxanone | Inhibition of biofilm formation. | In-vitro | (Waeiss et al., 2014) | |
| Vancomycin/cefuroxime | PMMA | Successful treatment of the infection in 7 cases with chronic osteomyelitis. | Clinical/ Patients | (Bharti et al., 2016) | |
| Gentamicin,vancomycin, amikacin, ceftriaxone | PMMA | Effective inhibition of MRSA growth for 42 days | In-vitro | (Noor et al., 2016) | |
| Vancomycin | PMMA | Prolonged release of the antibiotic from the bone cement over 6 weeks in femoral osteomyelitis model. | In-vivo/Rats | (Oh et al., 2016) | |
| Chitosan |
|
In-vivo/Rabbits | (Tao et al., 2020) | ||
| Fosfomycin | Chitosan |
|
In-vitro | (Tucker et al., 2021) | |
| Linezolid/daptomycin /vancomycin | PMMA/ PLGA microparticles | Synergistic effect of the antibiotics
|
In-vitro | (Parra-Ruíz et al., 2017) | |
| Vancomycin/amikacin | Chitosan sponge | Prevention and clearance of polymicrobial implant associated-biofilm. | In-vivo/ Mice | (Boles et al., 2018) | |
| Ciprofloxacin | Poly (hydroxyethyl methacrylate) |
|
In-vitro | (Sreeja et al., 2020) | |
| Rifampicin | PCL | Sustained antibiotic release for 14 days | In-vitro | (Lee et al., 2020) | |
| Composite | Vancomycin | PLLA/ β TCP |
|
In-vivo/Rats | (Kankilic et al., 2014) |
| Hydroxyapatite/ collagen |
|
In-vitro | (Coelho et al., 2015) | ||
| Calcium sulfate / PMMA |
|
Clinical/ Patients | (Luo et al., 2016) | ||
| Gelatin/ β TCP |
|
In-vivo/Rabbits | (Zhou et al., 2018) | ||
| PLA/ nanohydroxyapatite |
|
In-vitro | (Zhao et al., 2019) | ||
| Nano- hydroxyapatite / Gelatin / PLA |
|
In-vivo/Rats | (Krishnan et al., 2020) | ||
| Heparinized nanohydroxyapatite/collagen |
|
In-vitro | (Padrão et al., 2021) | ||
| Polyurethane/ hydroxyapatite |
|
In-vivo/Rabbits | (Beenken et al., 2021) | ||
| Hydroxyapatite/ Sodium alginate/ chitosan |
|
In-vitro | (Liu et al., 2021) | ||
| Gentamicin | Hydroxyapatite/ collagen |
|
In-vitro | (Oshima et al., 2020) | |
| Ceftriaxone | Hydroxyapatite/ β TCP /chitosan | Prolonged release pattern for more than 5 weeks. | In-vitro | (Kundu et al., 2010) | |
| Moxifloxacin | Chitosan/ calcium phosphate |
|
In-vivo/Rabbits | (Radwan et al., 2020) | |
| Poly-lactide-co-ε-caprolactone/calcium phosphate |
|
In-vivo/Rabbits | (Radwan et al., 2021) | ||
| Gatifloxacine | β TCP/ PLGA | Osteoconductive scaffolds with efficacy in local treatment of osteomyelitis. | In-vivo/Rabbits | (Tamazawa et al., 2011) | |
| Rifampicin/ ciprofloxacin | PCL/ β TCP |
|
In-vitro | (Ahola et al., 2012) | |
| Gentamicin/ vancomycin | PMMA/ β TCP | Eradication of infection through custom made devices in femoral osteomyelitis model. | In-vivo/Rabbits | (Giavaresi et al., 2012) | |
| Daptomycin | Calcium phosphate / chitosan |
|
In-vivo/Rabbits | (Beenken et al., 2014) | |
| Ciprofloxacin | Gelatin/ hydroxyapatite |
|
In-vitro | (Krishnan et al., 2015) | |
| Hydroxyapatite/ PCL | Controlled the release of antibiotic in implant. | In-vitro | (Nithya & Sundaram, 2015) | ||
| Rifapentine | Hydroxyapatite/ Poly amino acid |
|
In-vivo/Rabbits | (Yan et al., 2015) | |
| Tobramycin | PCL/ PEG/ Calcium phosphate/ Hydroxyapatite | Osteoconductivity and resorbtion with sustained antibiotic release. | In-vivo/Rabbits | (Jones et al., 2016) | |
| Doxycycline | Bioactive glass/ mesoporous silica |
|
In-vitro | (Szewczyk et al., 2021) | |
| Silver ion | Nano- hydroxyapatite / polyurethane |
|
In-vivo/Rabbits | (Zhang et al., 2019a) |
3.1.2. Bioceramics scaffolds
Enhancing bone regeneration, while promoting controlled delivery of therapeutic agents is one of the chief goals for the development of bioceramic-based scaffolds. Bioceramic scaffolds are types of inorganic biomaterials such as calcium phosphate derivatives and bioactive glass. Hydroxyapatite (HA) and tricalciumphosphate (TCP) are among the most widely used derivatives of calcium phosphate biomaterials and the oldest applied ones are bone substitute (Lew et al., 2012). Bioceramic scaffolds are chemically and structurally similar to the inorganic component of bone, therefore, they have been widely used for bone regeneration purposes (Stevens, 2008).
3.1.2.1. Calcium phosphate bioceramics
Depending on the ratio of calcium to phosphate, the temperature of synthesis of the ceramics and the existence of impurities and water, calcium phosphate can differ in crystal pattern. They can be classified according to their crystal pattern into mono-, di-, tri- (including both α-tricalcium phosphate (α-TCP) and β-tricalcium phosphate (β-TCP)), tetra- calcium phosphate and HA (Lew et al., 2012). Calcium phosphate bioceramics demonstrate promising biological and functional properties as bone scaffold material due to their biocompatibility, high loading capacities, nontoxicity, non-immunogenicity, bioresorbability and osteoinductive features (Kalita et al., 2007). They are perfect and natural candidates as bone-filling drug carriers as they resemble the mineral phase of the bone tissues. Additionally, they participate in stimulating osteoblastic differentiation and proliferation, so they aid in the formation of new bone when these compounds degrade and release phosphate and calcium ions, which can be used as ionic ingredients for bone regeneration (Uskokovic, 2015).
Hence, calcium phosphate is an excellent platform for the incorporation of antibacterial agents to control the release kinetics and to stimulate osteogenesis. The use of injectable calcium phosphate as an experimental biomaterials systems was evaluated for treating induced S. aureus osteomyelitis in an animal models (Inzana et al., 2016). These cements are generally formed when the soluble calcium phosphate is combined with water or another aqueous solution, where this solution will precipitate calcium phosphate when being exposed to body environment. The release of antibiotic from the scaffold is controlled by diffusion rather than the degradation of the matrix (Inzana et al., 2016). Injectable calcium phosphate cements offers many advantages namely; localizing the therapeutic agent at the site of action, minimizing invasion, providing perfect fit to the defect site as they are moldable materials and offering superior biological reactivity due to their porous structure (Ginebra et al., 2018).
Ghosh et al. (2016) successfully incorporated ciprofloxacin and vancomycin into nanoparticulate hydroxyapatite to act as an injectable cement with tunable drug release kinetics, which was found to be less invasive and more affordable. In a more recent study, the application of low frequency pulsed ultrasound resulted in increasing the extent of vancomycin release from vancomycin-loaded calcium phosphate cement, together with prolonging the duration of vancomycin release and accelerating the degradation rate of the applied cement (Shi et al., 2018). Additionally, Inzana et al. (2015) fabricated rifampin- and vancomycin-laden calcium phosphate scaffolds (CPS) by three-dimensional (3 D) printing to treat an implant-associated Staphylococcus aureus bone infection in a murine model. All vancomycin- and rifampin-laden CPS treatments significantly reduced the bacterial burden compared with vancomycin-laden PMMA.
3.1.2.2. Bioactive glasses
Bioactive glass is a type of bioceramics that has been widely applied in experimental models and clinical trials for the treatment of osteomyelitis along with its applications in other bone tissue engineering (Lindfors et al., 2010; Zhang et al., 2010; Drago et al., 2013; Rahaman et al., 2014; Ryan et al., 2019; Abdel-Salam et al., 2020). Bioactive glass is structurally composed of calcium, silicon and phosphate ions. Its action in bone tissue engineering is related to their biocompatibility, osteoconductivity and its ability to release calcium, sodium, silicon and phosphate ions during its dissolution process. These ions are biologically active when released in a rate favorable for the bone-cells proliferation and differentiation (Hupa, 2018). Bioactive glass binds to the damaged bone tissue through chemical bond as a result of the formation of a hydrocarbonate apatite layer on the glass surface which, has similarity to the bone components, thus enhancing the interaction with the damaged bone and promoting the osteogenesis process (Rahaman et al., 2011).
Bioactive glass materials have shown promise for application as antibiotic carrier in the local treatment of osteomyelitis (Rahaman et al., 2014). In a study carried out on rabbit model, the local teicoplanin delivery from borate bioactive-glass implant was found to be more effective in the treatment of MRSA-induced osteomyelitis compared to teicoplanin intravenous administration (Jia et al., 2015). A clinical study on 27 patients suffering from osteomyelitis in long bones was performed by Drago et al. (Drago et al., 2013) using bioactive glass granules. The granules were implanted at the site of the osteomyelitic lesion after complete debridement of the dead tissues. Resulted showed that the bioactive glass granules had antibacterial activity against the causative bacteria without addition of antibiotics. The main downsides of bioactive glasses are the increased concentrations of ions at the site of application of the implant, the slow rate of degradation of the glass and the difficulties encountered in fabrication processes of the scaffold (Mancuso et al., 2017). More investigations on bioceramics scaffolds are listed in Table 4.
3.1.3. Polymeric scaffolds
In the field of polymeric scaffold fabrication, it is important to use biomaterials adaptable to the bone tissues. The ideal polymeric materials for this purpose are those simulating the natural ECM of bone and possessing the essential biochemical and mechanical characteristics (Bose et al., 2012). Natural polymeric materials or synthetic ones offer great potential in designing the scaffolds due to their biocompatibility, biodegradability and being able to interact with cells. Natural polymers including, collagen chitosan, silk and alginate as well as synthetic polymers including poly-lactic acid (PLA), polycaprolactone (PCL) and poly (lactic-co-glycolic) acid (PLGA) have been investigated as bone scaffolds with different morphologies for bone tissue engineering and as drug carriers. The use of synthetic polymers offered easier modification of the mechanical and physicochemical properties in addition to adjusting the biodegradation rates (Bose et al., 2012; Sarigol-Calamak & Hascicek, 2018; Elkasabgy et al., 2019).
3.1.3.1. Natural polymeric scaffolds
Collagen is a naturally occurring protein in the ECM of bone. The high biocompatibility of collagen makes it ideal biomaterial to be used as scaffolding material (Meyer, 2019). The application of collagen implant loaded with antibacterial agents has been investigated for the treatment of acute and chronic osteomyelitis in clinical studies since decades and the composite system made of the drug and collagen was found to be biodegradable and bioresorbable, hence does not need second surgery for the removal of the implant when compared to PMMA (Feil et al., 1990; Ipsen et al., 1991).
Chitosan is another natural polymer produced by deacetylation of chitin, which is a primary component of cell wall in some living organisms (Di Martino et al., 2005). Being of chemical nature that offer modification besides its biological characteristics including biodegradability, biocompatibility, tissue engineering capability and antibacterial activity, it has been widely investigated both in vitro and in animal models as local drug delivery vehicle for the treatment of osteomyelitis (Noel et al., 2008; Wells et al., 2018; Boles et al., 2018; Sarwar et al., 2020). Chitosan suffers from poor mechanical properties if used as a sole component in the implanted scaffold, so it is usually combined with other synthetic polymer such as PCL and PEG to allow tailoring its properties as well as expanding its applications (Malheiro et al., 2010; Lima et al., 2018; Pawar & Srivastava, 2019).
Another example of the naturally occurring polymers is alginate. It is being widely used in the pharmaceutical industry owing to its gelling and stabilizing properties. It has been used also as drug delivery systems for bone through acting as a biodegradable carrier for antibacterial agents and bone cells (Ueng et al., 2007).
3.1.3.2. Synthetic polymeric scaffolds
PLGA, a group of synthetic, biodegradable and biocompatible polymers. They were extensively investigated as building materials for scaffolds and as drug delivery systems. They show minimal toxicity since they degrade into lactic and glycolic acids in the human body. They also offer great ability to sustain drug release which is an important feature for a bone scaffolds (Danhier et al., 2012; Shamma et al., 2017). According to Ueng et al. (2011) it was possible to sustain the release of vancomycin from the polymeric composite system of PLGA and collagen. The composite system of the two polymers revealed adequate osteogenic differentiation of mesenchymal stem cells in a rabbit model (Ueng et al., 2011). Further in-vitro and in-vivo studies applying the biodegradable PLGA-vancomycin beads confirmed their success in eradicating the induced infection in the damaged bone of rabbits model (Ueng et al., 2016).
PLA is also used as scaffolding material due to its biocompatibility and biodegradability (Sarigol-Calamak & Hascicek, 2018). In a study conducted by Tsiolis et al. (2011) linezolid was mixed with PLA to form an implant for application at the bone site where MRSA infection was induced in experimental rabbit model. They proved the inhibition of the induced infection during the first six weeks after the implant application. The system composed of the drug and the polymer succeeded in reducing the microbial count continuously as well as showing good histopathological results.
Another example of the synthetic polymers mostly used for bone regeneration purposes is PCL. It offers many important features namely; biocompatibility, biodegradability, processability and good mechanical characteristics (Waknis & Jonnalagadda, 2011). PCL can exert their action in tissue engineering through contribution to adhesion and proliferation of cells and its ability to interact with the biological aqueous fluids after modifying its hydrophobic properties by blending with natural polymers (Zhang et al., 2013; Siddiqui et al., 2018). Injectable and cytocompatible implants made from physical blends of poloxamine and PCL by hot melting solvent-free method and incorporating ciprofloxacin hydrochloride showed tunable bioerosion rate, osteoconductive features and sustained drug release (Puga et al., 2012). More investigations on polymeric scaffolds are listed in Table 4.
3.1.4. Composite scaffolds
Composite scaffolds are systems where two or more materials are combined into the same system to combine the advantages of both materials. Composite scaffolds offers good load bearing and strong mechanical properties for bone tissue engineering applications (Wei & Ma, 2004; Abdel-Salam et al., 2020). A good example is the combination of bioceramics with polymers in scaffold fabrication. Although bioceramics can provide high structural support and compressive strength, however, they are very brittle. Therefore, when polymers are integrated with bioceramic materials, the composite system acquire mechanical strength and processability in addition to controlling the drug release from the scaffold (Perez et al., 2012; Abdel-Salam et al., 2020).
In a study by Mostafa et al. (2017) ciprofloxacin was incorporated into a scaffold composed of chitosan and bioactive glasses in a ratio of 1–2. The composite carrier was biocompatible and offered optimal adhesion, cell migration and growth as well as sustained drug release profile when compared to the sole chitosan scaffolds (Mostafa et al., 2017). More investigations on composite scaffolds are listed in Table 4.
3.2. Particulate systems
Particulate systems have been investigated as suitable systems for the local delivery of antibacterial agents in the treatment of bone infections (Elkheshen et al., 2013; Mobarak et al., 2014; Hassani Besheli et al., 2017; Cobb et al., 2020). Injectable particulate systems exhibit the advantage of being easier to be delivered to the affected part when compared with the implanted tissue scaffolds. However, they do not usually fully fit in the dead space due to their relative fluidity in contrast with the tissue scaffolds, which fills the dead space ensuring the adhesion and proliferation of the newly formed cells. Various particulate systems have been identified in the local antibiotics treatment of osteomyelitis namely; nanoparticles/nanospheres and microparticles/microspheres.
3.2.1. Nanoparticles/nanospheres
The delivery of drugs in the nano-form have been developed and gained much interest since decades with the aim of improving the bioavailability and therapeutic efficacy of medicinal agents. These nano-formulated drug-carriers have high surface area relative to their mass, which enable them to solve the problems of drugs suffering from delivery obstacles as low solubility. They also minimize the drugs systemic side effects or even help in targeting them to certain organ in the body through adding a tag molecule to the surface of the particles which recognize the required site (Nikezić et al., 2020). Controlled release polymeric nanoparticles (NPs) exhibit biodegradability, which allow the gradual degradation of the polymer with controlling the release of drugs. The rate of drug release from polymeric NPs can be controlled by controlling the particle size as well as the stability and chemical structure of their components (Cong et al., 2015). NPs also offer high protection of drugs against degradation. The therapeutic agents are loaded to these systems through wrapping with polymeric or non-polymeric materials, dissolving in the bulk of the particles or adsorption to the their surface (Han et al., 2018).
Polymeric NPs as delivery systems have been widely investigated in the field of osteomyelitis local treatment aiming to deliver the antibiotics to the infected site with controlled release manner. This could strongly aid the efficient eradication of infection while avoiding the systemic therapy (Posadowska et al., 2015; Hassani Besheli et al., 2017; Zhang et al., 2017, 2018). Gentamicin sulfate was encapsulated in PLGA NPs using double emulsification (water/oil/water) technique. Agar-diffusion tests using S. aureus and S. epidermidis confirmed the antibacterial activity of gentamicin after loading to nanoparticles and the in-vitro release studies revealed sustained gentamicin release for 35 days (Posadowska et al., 2015).
Aragonite is a biocompatible form of calcium carbonate, which proved its potential application in the evolution of advanced and novel drug delivery platforms for bone tissue engineering (Islam et al., 2013). Utilizing cost effective and easy approach to design biodegradable antibiotic system in the nano-form, Saidykhan et al., loaded vancomycin into aragonite NPs. The Nano-system showed biocompatability, good bone resorbability and optimal antibacterial efficacy. The prepared NPs loaded with vancomycin displayed high antibacterial activity against MRSA, confirming that vancomycin retained its potency after loading into aragonite NPs (Saidykhan et al., 2016).
In a study conducted by Zhang et al. (2017) a cationic derivative of chitosan namely; N,N,N-trimethyl chitosan (TMC) formed a complex, in a nano-form, with the anionic vancomycin through ionotropic gelation technique. TMC-NPs exhibited initial burst release along with an ideal sustained release kinetics of vancomycin besides enhancing the product biocompatibility. The beads also promoted new bone formation in rabbit model as a result of the unique degradation behavior.
Gelatin nanospheres acquire inherent advantages including biocompatibility, biodegradability and cost effectiveness, which encouraged their use as efficient carriers for the local delivery of antibiotics. Moreover, gelatin nanospheres, being positively charged, can bind to oppositely charged molecules upon cross-linking, which can be adjusted to control the drug release through controlling their degradation rate (Santoro et al., 2014; Song & Leeuwenburgh, 2014). In-vitro and in-vivo studies by Zhang et al. (2018) showed that gelatin nanospheres loaded with vancomycin had good internalization of vancomycin by macrophages compared to vancomycin delivered systemically which had poor internalization. The in-vivo model using zebrafish larvae confirmed that the local application of vancomycin-loaded gelatin nanospheres into the macrophages had significantly higher survival rate of zebrafish larvae compared to the survival rate after systemic administration of the drug over time.
3.2.2. Microparticles/microspheres
Microparticles having size range in the micrometer scale have been utilized as drug carriers to enhance the delivery of drugs since decades (Ogay et al., 2020). Differences in the size range is the principal differentiating the nanoparticle carriers and the microparticle ones. Microspheres are type of microparticles composed of a polymer shell that surrounds the drug. Microspheres have several advantages such as enhancing the solubility of poorly soluble drugs in aqueous environment or controlling the drug’s half-life (Freiberg & Zhu, 2004). A biodegradable drug delivery system of (PLGA-PEG) microspheres containing gentamicin was successfully prepared using two different techniques; single emulsion and double emulsion solvent evaporation (Dorati et al., 2016). A composite in-situ forming gel using chitosan solution mixed with glycerol phosphate cross-linked with a natural bone substitute and incorporating gentamicin-microspheres was further developed to study the local release of the drug in a simulated environment of the bone. The gel retained the antibiotic activity and showed suitable properties to be applied as bone scaffolds offering prolonged release of the drug and enhanced cellular penetration (Dorati et al., 2017a).
In attempt to solve the problem associated with the High IV doses of Hydrochloric norvancomycin (HNV), HNV-loaded PLGA microspheres were successfully developed using double emulsion solvent evaporation method. HNV-PLGA microspheres showed maintained controlled release, lacking burst effect, over a period of 7 days. The antimicrobial studies demonstrated that the encapsulation of HNV enhanced its antibacterial action and effectively enabled the inhibition of the growth of microorganisms. The authors concluded that the use of HNV-loaded PLGA microspheres met the requirements for local anti-infection to prevent bone destruction (Yang et al., 2017).
In another study, Poly (ε-Caprolactone) microparticles loaded with vancomycin were prepared. The prepared microparticles showed prolonged release of vancomycin from the formulations where the local delivery to the affected site allowed massive reduction of the dose compared to the systemic one and limited the side effects. The in-vivo study was performed in a rabbit model where chronic osteomyelitis was induced and confirmed a significant killing of the causing bacteria after 11 days (Le Ray et al., 2005).
3.3. Scaffolds combined with particulate system
Combining the bulk scaffold with particulate system can offer the advantages of both systems. Scaffold can be osteoconductive by occupying the dead space in the bone structure and enhancing the process of osteogenesis, while the particulate system can deliver the antibiotic with tunable release kinetics without being dependent on the degradation of the scaffold (Posadowska et al., 2016; Mahmoudian & Ganji, 2017).
A biodegradable scaffold was developed by Dorati and coworkers (2017a) in order to induce prolonged local release of gentamicin at the infected bone tissue for the treatment of osteomyelitis. The prepared scaffold was an injectable, thermosetting composite scaffold based on a polymeric solution of chitosan supplemented with bovine bone substitute particulate. Chitosan solution can form thermosetting at the physiological environment of 37 ºC in the presence of β-glycerophosphate, which acts as inducer to stimulate gel formation in addition to being able to enhance bone regeneration. The supplementation of bovine bone substitute particulate affected the physicochemical properties of the prepared scaffolds namely; its mechanical properties, interconnectivity, porosity, and biological activity. After lyophilization of the prepared scaffolds, solid composite 3D scaffolds were obtained having high water retention affinity and hydrophilic nature, which was able to enhance cell attachment and infiltration. The polymeric components of the composite scaffolds showed no remarkable toxicity caused by degradation products during in-vitro incubation in simulated physiological conditions. Additionally, the scaffolds had excellent physical stability in-vitro.
Vancomycin was incorporated into composite system based on hydroxypropyl methylcellulose (HPMC) microparticles and chitosan thermosensitive hydrogel for the local treatment of osteomyelitis. Mixtures of vancomycin hydrochloride and HPMC were spray dried to prepare vancomycin-loaded HPMC microparticles in the first step of preparation. Later, the microparticles were incorporated in an injectable chitosan/glycerophosphate solution (free-flowing at room temperature) which form a semi-solid gel at body temperature. The system was able to retard the release of vancomycin (Mahmoudian & Ganji, 2017). In another study, injectable system composed of gentamicin-loaded PLGA NPs, embedded in the polysaccharide gellan gum hydrogel was fabricated. This system ensured less-invasive administration technique compared to the surgical intervention required for the implants. The NPs ensured sustained and controlled drug release, while the hydrogel enabled localized drug release by keeping the NPs at the infected area. The system was evaluated regarding injectability and handling which proved good performance (Posadowska et al., 2016).
3.4. Bone grafts loaded with antibiotics
One of the potential procedures used in the treatment of chronic osteomyelitis is utilizing a bone graft loaded with antibiotics to be implanted in the defect area. That graft can provide a high and effective concentration of antibiotics at the site of defect with the avoidance of systemic side effects in addition to facilitating bone formation through acting as scaffold (Fölsch et al., 2020). Bone grafts can either be autologous if a bone from the donor is transported to another location in the same donor (Miller & Chiodo, 2016) or allogenous if a bone from the donor is transported to different receiver (Kumar et al., 2013). Either autologous or allogenous bone grafts may be used depending on the location of the infection, the size of the bone defect and the availability of an internal bone bank. Generally, three types of bone autologous grafts are identified; fresh/fresh-frozen, freeze-dried and demineralized freeze-dried (Anagnostakos & Schröder, 2012).
It was previously reported in the literature that incorporation of antibiotics may be made by mixing with the bone graft manually, or by immersing the bone grafts into solutions containing the antibiotic for various time periods (Winkler et al., 2000; Ahmed et al., 2018). In a study performed by Fang et al. (2021), a new antibacterial matrix was designed through crosslinking demineralized extracellular cancellous bone with vancomycin. The in-vitro results showed that the matrix had sustained release of vancomycin over 6 weeks accompanied with enhancing osteogenesis in the tested mesenchymal stem cells and osteoblasts. The in-vivo studies in rat model confirmed its antibacterial activity in addition to enhancing the osteogenesis in the defect area. In another study, bone grafts were obtained from fibrous tissue, cartilage and cortical layer that were extracted from the femoral heads of patients. The grafts were saturated with vancomycin and the in-vitro study showed that the release of the drug was controlled over a prolonged period, which helped in preventing the development of resistance to vancomycin (Melicherčík et al., 2012).
4. Challenges facing the development of local drug delivery systems for managing osteomyelitis
However, there are some commercially available cements, either incorporating antimicrobial agents or lacing them to allow surgeons to impregnate them with appropriate antimicrobial agents during operation, they still do not justify the tremendous amount of research-work and time invested in this area including developing different categories of products and delivery systems, controlling their drug release patterns and testing them in animal models neither they satisfy all patients’ needs.
From clinical point of view, the commercially available cements incorporating antimicrobial agents do not satisfy the variety of personalized conditions facing surgeons namely, the patients’ ages and their general health conditions, the severity/chronicity of their infection-conditions, the type and volume of the involved bones’ tissues which affect the required dose of the antimicrobial agent that can eradicated the infection and do not deteriorate the patients general health conditions if absorbed to the system through the close muscular tissues. This is further complicated by the diversity of the infectious microorganism causing bone infections and the continuous development of resistance and the needs for diversity of antimicrobial to be incorporated in delivery products besides the continuous introduction of new chemical moieties.
On the other hand, however, the commercially available blank cements allow surgeons to incorporate the proven effective antimicrobial agents in the required strengths during the operation, the in-situ developed scaffolds are not standardized with respect to the rate of drug release which does not guarantee the delivery of sufficient amount of the drug to keep its concentration above the minimum inhibitory concentration (MIC) for the specified infectious organism during the whole treatment course. They also are not tested for being not dose dumbing for the specific antimicrobial agent to avoid fast depletion of the scaffold and/or the toxicity effect on patients. The previous analysis leads to continuous personalized research studies in many orthopedic departments of hospitals and clinics seeking beneficiary treatments for the wellbeing of their patients (Weinrauch et al., 2007; Kammerlander et al., 2011; Cazzato et al., 2014; Dzyuba et al., 2016; Luo et al., 2016).
Furthermore, all the commercially available scaffolding material, whether medicated or blank, require surgical procedures for application into the infection site. If surgical debridement is not part of the treatment strategy, applying the local implant may be unfavorable option for many patients due to significant extra costs, the required recovery period and the probability of development of infection recurrences if the scaffold did not completely eradicate the infection which dictate reoperation. Moreover, the continuous developed resistance against potential antibiotics may impede their use with the biomaterials to achieve the required minimum inhibitory concentration (MIC) in the infected bone (Kavanagh et al., 2018).
From the industrial perspective, personalized medicine based on developing customized scaffolds for filling the dead spaces in patients’ infected bones are not feasible neither will be creating the huge variety of required strengths of different antimicrobial agents per each scaffolding materials. Creating the personalized scaffold shape and volume is better achieved using the advanced technology of 3 D printing (Ford & Cassat, 2017) in hospitals and clinics. From the industrial point of view this can be solved by the blank scaffolding cements which, in the same time, allow incorporating different variety of antimicrobials and different strengths. This leads again to the shortcoming of the lack of standardization of the developed scaffolds.
From the researchers’ overview, to be able to create a multipurpose scaffolding materials for managing different stages of osteomyelitis, many areas has to be tackled. The most important of which is creating reliable osteomyelitis models that imitates features of osteomyelitis conditions in humans’ bones which, continues to be a challenge to scientists (Roux et al., 2021). The current animal models employed to assess the efficacy of scaffolds for elimination of infection and regeneration of bone should be fully detailed and reproducible, with well-defined gold standard for the diagnosis of osteomyelitis, there originating sources and stage of infection in order to be able to simulate the clinical scenarios (Reizner et al., 2014). Furthermore, scaffolding materials have to offer multipurpose including eradication of infections, assisting the proliferation and healing of the bone tissues besides acting as internal mechanical supports.
5. Future perspectives
New strategies are highly encouraged to develop and commercially distribute antibiotics-loaded delivery systems that meet different patients’ needs for the local treatment of osteomyelitis whether surgical debridement is part of the treatment strategy or not. Currently, there are plenty of biomaterials with different characteristics that can be adjusted to achieve controlled-release of antibiotics and keep the concentration above the MIC yet do not dump the loaded amount of the drug in a much higher rate than required to allow the delivery system to be effective for longer period of time (Elkasabgy et al., 2019; Abdel-Salam et al., 2020). Among those biomaterials namely; ploy (lactic acid), poly (lactic co-glycolic) and chitosan either alone or in combination with bioactive glass can offer many advantages including: (1) being biocompatible, they do not initiate hypersensitivity reactions after application, (2) being biodegradable, they do not need postoperative surgery to remove the empty scaffold besides the fact that with research effort their rate of degradation can be adjusted to simulate the rate of bone regeneration (3) being polymeric in nature they can be designed to undergo in-situ gelling upon injection to the affected bone site or in its close vicinity which will be advantageous if surgical debridement is not required, (4) being injectable will allow surgeons to give multiple injection of the controlled release delivery system to the operated site of infection during the course of treatment to adjust the in-situ concentration above the MIC without having to re-operate for the re-application of the cement or to continue the course of antimicrobial therapy, systemically with all the risks of the systemic therapy on the general health condition of patients specially most of them are elderly, (5) having the nano-size bioactive glass (NBAG) will allow them to acquire more stiffness upon the in-situ setting of the polymeric materials which act as internal mechanical support specially in cases when long bones are involved. Besides the osteoinductive and osteoconductive properties of NBAG, these characteristics can be improved by incorporating antiresorptive drugs in a combination therapy to promote bone healing. With the financial support of industrial companies a well-designed researches specially in the field of developing animal models for osteomyelitis, more studies including the application of the injectable scaffolding materials loaded with different antimicrobials on multiple animal species so that a good reflections on possible human manifestations and responses to therapies may be drown which allow for clinical experimentation and finally commercial production.
6. Conclusion
Different local drug-delivery systems for the treatment of osteomyelitis were discussed in the current review. As a conclusion of the above discussion, it is very important while designing a new local antibiotic delivery system for the management of osteomyelitis, either in its early stages or after surgical debridement of the dead tissues, that these systems should have as many desirable features as possible. These features include being biodegradable, biocompatible, can enhance the regeneration of new bone tissues, being able to provide mechanical support to the affected long bone during the healing period and can offer sustained drug release. Finally, if made injectable it would provide two major advantages namely; reducing the level of invasion and wins patients’ compliance and allowing multiple injection to the affected part after surgical debridement over the treatment period to avoid the systemic therapy for long time which leads to many side effects and toxicity.
Disclosure statement
The authors of the current review declare that there is no financial, ethical, copyright or any other conflict of interest in the current work.
References
- Abdel-Salam FS, Elkheshen SA, Mahmoud AA, et al. (2020). In-situ forming chitosan implant-loaded with raloxifene hydrochloride and bioactive glass nanoparticles for treatment of bone injuries: Formulation and biological evaluation in animal model. Int J Pharm 580:119213. [DOI] [PubMed] [Google Scholar]
- Ahmed G, Ishaque B, Rickert M, Fölsch C. (2018). Allogeneic bone transplantation in hip revision surgery : indications and potential for reconstruction. Orthopade 47:52–66. [DOI] [PubMed] [Google Scholar]
- Ahola N, Veiranto M, Männistö N, et al. (2012). Processing and sustained in vitro release of rifampicin containing composites to enhance the treatment of osteomyelitis. Biomatter 2:213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez A, Fernández L, Gutiérrez D, et al. (2019). Methicillin-resistant staphylococcus aureus in hospitals: Latest trends and treatments based on bacteriophages. J Clin Microbiol 57:e01006–019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostakos K, Schröder K. (2012). Antibiotic-impregnated bone grafts in orthopaedic and trauma surgery: a systematic review of the literature. Int J Biomater 2012:538061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anugraha A, Hughes LD, Pillai A. (2019). A novel technique for fabricating antibiotic-coated intramedullary nails using an antibiotic-loaded calcium sulphate hydroxyapatite bio-composite, Cerament-V. J Surg Case Rep 2019:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asri R, Harun W, Samykano M, et al. (2017). Corrosion and surface modification on biocompatible metals: a review. Mater Sci Eng C 77:1261–74. [DOI] [PubMed] [Google Scholar]
- Beenken KE, Campbell MJ, Campbell MJ, et al. (2021). Evaluation of a bone filler scaffold for local antibiotic delivery to prevent Staphylococcus aureus infection in a contaminated bone defect. Sci Rep 11:10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken KE, Smith JK, Skinner RA, et al. (2014). Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J Biomater Appl 29:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berebichez-Fridman R, Montero-Olvera P, Gómez-García R, Berebichez-Fastlicht E. (2017). An intramedullary nail coated with antibiotic and growth factor nanoparticles: an individualized state-of-the-art treatment for chronic osteomyelitis with bone defects. Med Hypotheses 105:63–8. [DOI] [PubMed] [Google Scholar]
- Bharti A, Saroj UK, Kumar V, et al. (2016). A simple method for fashioning an antibiotic impregnated cemented rod for intramedullary placement in infected non-union of long bones. J Clin Orthop Trauma 7:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R, Kundu B, Nandi SK, Basu D. (2013). Systematic approach to treat chronic osteomyelitis through localized drug delivery system: bench to bed side. Mat Sci Eng C 33:3986–93. [DOI] [PubMed] [Google Scholar]
- Bidossi A, Bottagisio M, Logoluso N, De Vecchi E. (2020). In vitro evaluation of gentamicin or vancomycin containing bone graft substitute in the prevention of orthopedic implant-related infections. IJMS 21:9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles LR, Awais R, Beenken KE, et al. (2018). Local delivery of amikacin and vancomycin from chitosan sponges prevent polymicrobial implant-associated biofilm. Mil Med 183:459–65. [DOI] [PubMed] [Google Scholar]
- Bose S, Roy M, Bandyopadhyay A. (2012). Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JH, Manring MM. (2005). Adult osteomyelitis. Infect Dis Clin 19:765–86. [DOI] [PubMed] [Google Scholar]
- Calhoun JH, Manring M, Shirtliff M.. 2009. Osteomyelitis of the long bones. Semin Plast Surg. 23:059–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Jiang D, Yan L, Wu J. (2016). In vitro and in vivo osteogenic activity of the novel vancomycin-loaded bone-like hydroxyapatite/poly(amino acid) scaffold. J Biomater Appl 30:1566–77. [DOI] [PubMed] [Google Scholar]
- Cazzato RL, Buy X, Eker O, et al. (2014). Percutaneous long bone cementoplasty of the limbs: experience with fifty-one non-surgical patients. Eur Radiol 24:3059–68. [DOI] [PubMed] [Google Scholar]
- Chan C-W, Carson L, Smith GC, et al. (2017). Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl Surf Sci 404:67–81. [Google Scholar]
- Chaudhari A, Vig K, Baganizi D, et al. (2016). Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. IJMS 17:1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Frith JE, Dehghan-Manshadi A, et al. (2017). Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. J Mech Behav Biomed Mater 75:169–74. [DOI] [PubMed] [Google Scholar]
- Chihara S, Segreti J. (2010). Osteomyelitis. Dis Mon 56:5–31. [DOI] [PubMed] [Google Scholar]
- Choudhury M, Mohanty S, Nayak S. (2015). Effect of different solvents in solvent casting of porous PLA scaffolds—in biomedical and tissue engineering applications. J Biomater Tissue Eng 5:1–9. [Google Scholar]
- Cicuéndez M, Doadrio JC, Hernández A, et al. (2018). Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater 65:450–61. [DOI] [PubMed] [Google Scholar]
- Cierny G, Mader JT, Penninck JJ. (1985). A clinical staging system for adult osteomyelitis. Contemporary Orthopaedics 10:17–37. [DOI] [PubMed] [Google Scholar]
- Cierny G, Mader JT, Penninck JJ. (2003). The classic: a clinical staging system for adult osteomyelitis. Clin Orthop Relat Res 414:7–24. [DOI] [PubMed] [Google Scholar]
- Cobb LH, McCabe EM, Priddy LB. (2020). Therapeutics and delivery vehicles for local treatment of osteomyelitis. J Orthop Res 38:2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CC, Sousa SR, Monteiro FJ. (2015). Heparinized nanohydroxyapatite/collagen granules for controlled release of vancomycin. J Biomed Mater Res A 103:3128–38. [DOI] [PubMed] [Google Scholar]
- Comeau P, Filiaggi M. (2017). A two-stage cold isostatic pressing and gelling approach for fabricating a therapeutically loaded amorphous calcium polyphosphate local delivery system. J Biomater Appl 32:126–36. [DOI] [PubMed] [Google Scholar]
- Cong Y, Quan C, Liu M, et al. (2015). Alendronate-decorated biodegradable polymeric micelles for potential bone-targeted delivery of vancomycin. J Biomater Sci Polym Ed 26:629–43. [DOI] [PubMed] [Google Scholar]
- Conrad DA. (2010). Acute hematogenous osteomyelitis. Pediatr Rev 31:464–71. [DOI] [PubMed] [Google Scholar]
- Danhier F, Ansorena E, Silva JM, et al. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–22. [DOI] [PubMed] [Google Scholar]
- David N, Nallaiyan R. (2018). Biologically anchored chitosan/gelatin-SrHAP scaffold fabricated on Titanium against chronic osteomyelitis infection. Int J Biol Macromol 110:206–14. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Sittinger M, Risbud MV. (2005). Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983–90. [DOI] [PubMed] [Google Scholar]
- Diaz-Gomez L, García-González CA, Wang J, et al. (2017). Biodegradable PCL/fibroin/hydroxyapatite porous scaffolds prepared by supercritical foaming for bone regeneration. Int J Pharm 527:115–25. [DOI] [PubMed] [Google Scholar]
- Dickson MN, Liang EI, Rodriguez LA, et al. (2015). Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 10:021010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbeck M, Schrader C, Gras F, et al. (2016). Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 101:156–64. [DOI] [PubMed] [Google Scholar]
- Diu T, Faruqui N, Sjöström T, et al. (2014). Cicada-inspired cell-instructive nanopatterned arrays. Sci Rep 4:7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorati R, De Trizio A, Genta I, et al. (2017a). Gentamicin-loaded thermosetting hydrogel and moldable composite scaffold: formulation study and biologic evaluation. J Pharm Sci 106:1596–607. [DOI] [PubMed] [Google Scholar]
- Dorati R, DeTrizio A, Genta I, et al. (2016). An experimental design approach to the preparation of pegylated polylactide-co-glicolide gentamicin loaded microparticles for local antibiotic delivery. Mater Sci Eng C 58:909–17. [DOI] [PubMed] [Google Scholar]
- Dorati R, DeTrizio A, Modena T, et al. (2017b). Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago L, Romanò D, De Vecchi E, et al. (2013). Bioactive glass BAG-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones: an in vitro and prospective clinical study. BMC Infect Dis 13:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drampalos E, Mohammad HR, Kosmidis C, et al. (2018). Single stage treatment of diabetic calcaneal osteomyelitis with an absorbable gentamicin-loaded calcium sulphate/hydroxyapatite biocomposite: The Silo technique. Foot (Edinb) 34:40–4. [DOI] [PubMed] [Google Scholar]
- Dzyuba G, Reznik L, Erofeev S, Odarchenko D. (2016). Efficiency of local cement reinforcing antibacterial implants in surgical treatment of long bones chronic osteomyelitis. Khirurgiia 5:31–6. [DOI] [PubMed] [Google Scholar]
- El-Habashy SE, El-Kamel AH, Essawy MM, et al. (2021). Engineering 3D-printed core-shell hydrogel scaffolds reinforced with hybrid hydroxyapatite/polycaprolactone nanoparticles for in vivo bone regeneration. Biomater Sci 9:4019–39. [DOI] [PubMed] [Google Scholar]
- Elkasabgy NA, Abdel-Salam FS, Mahmoud AA, et al. (2019). Long lasting in-situ forming implant loaded with raloxifene HCl: an injectable delivery system for treatment of bone injuries. Int J Pharm 571:118703. [DOI] [PubMed] [Google Scholar]
- Elkasabgy NA, Mahmoud AA. (2019). Fabrication strategies of scaffolds for delivering active ingredients for tissue engineering. AAPS PharmSciTech 20:256. [DOI] [PubMed] [Google Scholar]
- Elkasabgy NA, Mahmoud AA, Maged A. (2020). 3D printing: an appealing route for customized drug delivery systems. Int J Pharm 588:119732. [DOI] [PubMed] [Google Scholar]
- Elkheshen S, Mobarak D, Salah S, Essam T. (2013). Formulation of ciprofloxacin hydrochloride loaded biodegradable nanoparticles: optimization of the formulation variables. J Pharm Res Opin 3:72–81. [DOI] [PubMed] [Google Scholar]
- Euba G, Murillo O, Fernandez-Sabe N, et al. (2009). Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother 53:2672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RP, Nelson CL. (1993). Gentamicin-impregnated polymethylmethacrylate beads compared with systemic antibiotic therapy in the treatment of chronic osteomyelitis. Clin Orthop Relat Res 295:37–42. [PubMed] [Google Scholar]
- Fage SW, Muris J, Jakobsen SS, Thyssen JP. (2016). Titanium: a review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis 74:323–45. [DOI] [PubMed] [Google Scholar]
- Fang B, Qiu P, Xia C, et al. (2021). Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy. Biomaterials 268:120603. [DOI] [PubMed] [Google Scholar]
- Feil J, Bohnet S, Neugebauer R, Rübenacker S. (1990). Bioresorbable collagen-gentamicin compound as local antibiotic therapy. Aktuelle Probl Chir Orthop 34:94–103. [PubMed] [Google Scholar]
- Fölsch C, Bok J, Krombach G, et al. (2020). Influence of antibiotic pellets on pore size and shear stress resistance of impacted native and thermodisinfected cancellous bone: An in vitro femoral impaction bone grafting model. J Orthop 22:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CA, Cassat JE. (2017). Advances in the local and targeted delivery of anti-infective agents for management of osteomyelitis. Expert Rev anti Infect Ther 15:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraimow HS. 2009. Systemic antimicrobial therapy in osteomyelitis. In Seminars in plastic surgery. Stuttgart: Thieme Medical Publishers, 90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg S, Zhu X. (2004). Polymer microspheres for controlled drug release. Int J Pharm 282:1–18. [DOI] [PubMed] [Google Scholar]
- Frohbergh ME, Katsman A, Botta GP, et al. (2012). Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 33:9167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin A, Jacqueline C, Gautier H, et al. (2013). A delivery system of linezolid to enhance the MRSA osteomyelitis prognosis: in vivo experimental assessment. Eur J Clin Microbiol Infect Dis 32:195–8. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Wu V, Pernal S, Uskoković V. (2016). Self-setting calcium phosphate cements with tunable antibiotic release rates for advanced antimicrobial applications. ACS Appl Mater Interfaces 8:7691–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavaresi G, Minelli EB, Sartori M, et al. (2012). New PMMA-based composites for preparing spacer devices in prosthetic infections. J Mater Sci Mater Med 23:1247–57. [DOI] [PubMed] [Google Scholar]
- Gimeno M, Pinczowski P, Vázquez FJ, et al. (2013). Porous orthopedic steel implant as an antibiotic eluting device: prevention of post-surgical infection on an ovine model. Int J Pharm 452:166–72. [DOI] [PubMed] [Google Scholar]
- Ginebra M-P, Espanol M, Maazouz Y, et al. (2018). Bioceramics and bone healing. EFORT Open Rev 3:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. (2009). Local antibiotic therapy in osteomyelitis. Semin Plast Surg 23:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati K, Scimeca J-C, Ivanovski S, Verron E. (2021). Double-edged sword: therapeutic efficacy versus toxicity evaluations of doped titanium implants. Drug Discovery Today. doi: 10.1016/j.drudis.2021.07.004 [DOI] [PubMed] [Google Scholar]
- Han J, Zhao D, Li D, et al. (2018). Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harik NS, Smeltzer MS. (2010). Management of acute hematogenous osteomyelitis in children. Expert Rev anti Infect Ther 8:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani Besheli N, Mottaghitalab F, Eslami M, et al. (2017). Sustainable release of vancomycin from silk fibroin nanoparticles for treating severe bone infection in rat tibia osteomyelitis model. ACS Appl Mater Interfaces 9:5128–38. [DOI] [PubMed] [Google Scholar]
- Hess U, Mikolajczyk G, Treccani L, et al. (2016). Multi-loaded ceramic beads/matrix scaffolds obtained by combining ionotropic and freeze gelation for sustained and tuneable vancomycin release. Mater Sci Eng C 67:542–53. [DOI] [PubMed] [Google Scholar]
- Høiby N, Ciofu O, Johansen HK, et al. (2011). The clinical impact of bacterial biofilms. Int J Oral Sci 3:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard‐Jones AR, Isaacs D. (2013). Systematic review of duration and choice of systemic antibiotic therapy for acute haematogenous bacterial osteomyelitis in children. J Paediatr Child Health 49:760–8. [DOI] [PubMed] [Google Scholar]
- Howell WR, Goulston C. (2011). Osteomyelitis: an update for hospitalists. Hosp Pract (1995) 39:153–60. [DOI] [PubMed] [Google Scholar]
- Hupa L. 2018. Composition-property relations of bioactive silicate glasses. In: Ylänen H, ed. Bioactive glasses. Cambridge: Woodhead Publishing, 2–4. [Google Scholar]
- Inzana JA, Schwarz EM, Kates SL, Awad HA. (2016). Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 81:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana JA, Trombetta RP, Schwarz EM, et al. (2015). 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur Cell Mater 30:232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen T, Jørgensen PS, Damholt V, Tørholm C. (1991). Gentamicin-collagen sponge for local applications. 10 cases of chronic osteomyelitis followed for 1 year. Acta Orthop Scand 62:592–4. [DOI] [PubMed] [Google Scholar]
- Islam KN, Ali ME, Bakar MZBA, et al. (2013). A novel catalytic method for the synthesis of spherical aragonite nanoparticles from cockle shells. Powder Technol 246:434–40. [Google Scholar]
- Ivanova EP, Hasan J, Webb HK, et al. (2013). Bactericidal activity of black silicon. Nat Commun 4:2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, EJ, Lee JW, Yeon SJ, et al. (2012). Fabrication of nanopatterned surfaces for tissue engineering. In: 2012 International conference on biomedical engineering and biotechnology. Macao: Institute of Electrical and Electronics Engineers, 1030–3. [Google Scholar]
- Jia W-T, Fu Q, Huang W-H, et al. (2015). Comparison of borate bioactive glass and calcium sulfate as implants for the local delivery of teicoplanin in the treatment of methicillin-resistant Staphylococcus aureus-induced osteomyelitis in a rabbit model. Antimicrob Agents Chemother 59:7571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Z, Brooks AE, Ferrell Z, et al. (2016). A resorbable antibiotic eluting bone void filler for periprosthetic joint infection prevention. J Biomed Mater Res B Appl Biomater 104:1632–42. [DOI] [PubMed] [Google Scholar]
- Kalita SJ, Bhardwaj A, Bhatt H, C E. (2007). Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater Sci Eng C 27:441–9. [Google Scholar]
- Kammerlander C, Gebhard F, Meier C, et al. (2011). Standardised cement augmentation of the PFNA using a perforated blade: a new technique and preliminary clinical results. A prospective multicentre trial. Injury 42:1484–90. [DOI] [PubMed] [Google Scholar]
- Kankilic B, Bilgic E, Korkusuz P, Korkusuz F. (2014). Vancomycin containing PLLA/β-TCP controls experimental osteomyelitis in vivo. J Orthop Surg Res 9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakeçili A, Topuz B, Korpayev S, Erdek M. (2019). Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater Sci Eng C 105:110098. [DOI] [PubMed] [Google Scholar]
- Karr JC. (2011). Management in the wound-care center outpatient setting of a diabetic patient with forefoot osteomyelitis using Cerament Bone Void Filler impregnated with vancomycin: off-label use. J Am Podiatr Med Assoc 101:259–64. [DOI] [PubMed] [Google Scholar]
- Karr JC, Lauretta J. (2015). In vitro activity of calcium sulfate and hydroxyapatite antifungal disks loaded with amphotericin B or voriconazole in consideration for adjunctive osteomyelitis management. J Am Podiatr Med Assoc 105:104–10. [DOI] [PubMed] [Google Scholar]
- Kavanagh N, Ryan EJ, Widaa A, et al. (2018). Staphylococcal osteomyelitis: disease progression, treatment challenges, and future directions. Clin Microbiol Rev 31:e00084–00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon Z, McTiernan CD, Suuronen EJ, et al. (2018). Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Ryu J-i, Bak KH. (2011). The safety and efficacy of cadaveric allografts and titanium cage as a fusion substitutes in pyogenic osteomyelitis. J Korean Neurosurg Soc 50:348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers HM, Nwojo ME, Ransom JE, et al. (2015). Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg 97:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan AG, Biswas R, Menon D, Nair MB. (2020). Biodegradable nanocomposite fibrous scaffold mediated local delivery of vancomycin for the treatment of MRSA infected experimental osteomyelitis. Biomater Sci 8:2653–65. [DOI] [PubMed] [Google Scholar]
- Krishnan AG, Jayaram L, Biswas R, Nair M. (2015). Evaluation of antibacterial activity and cytocompatibility of ciprofloxacin loaded gelatin-hydroxyapatite scaffolds as a local drug delivery system for osteomyelitis treatment. Tissue Eng Part A 21:1422–31. [DOI] [PubMed] [Google Scholar]
- Kumar P, Vinitha B, Fathima G. (2013). Bone grafts in dentistry. J Pharm Bioallied Sci 5:S125–S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B, Lemos A, Soundrapandian C, et al. (2010). Development of porous HAp and β-TCP scaffolds by starch consolidation with foaming method and drug-chitosan bilayered scaffold based drug delivery system. J Mater Sci Mater Med 21:2955–69. [DOI] [PubMed] [Google Scholar]
- Kundu B, Nandi SK, Dasgupta S, et al. (2011). Macro-to-micro porous special bioactive glass and ceftriaxone-sulbactam composite drug delivery system for treatment of chronic osteomyelitis: an investigation through in vitro and in vivo animal trial. J Mater Sci Mater Med 22:705–20. [DOI] [PubMed] [Google Scholar]
- Lalidou F, Kolios G, Drosos G. (2014). Bone infections and bone graft substitutes for local antibiotic therapy. Surg Technol Int 24:353–62. [PubMed] [Google Scholar]
- Le Ray A-M, Gautier H, Laty M-K, et al. (2005). In vitro and in vivo bactericidal activities of vancomycin dispersed in porous biodegradable poly(epsilon-caprolactone) microparticles. Antimicrob Agents Chemother 49:3025–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Ahn G, Kim JY, Cho D-W. (2010). Evaluating cell proliferation based on internal pore size and 3D scaffold architecture fabricated using solid freeform fabrication technology. J Mater Sci Mater Med 21:3195–205. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Baik J-M, Yu Y-S, et al. (2020). Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci Rep 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Choi B, Wu B, Lee M. (2013). Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 5:045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Kang KS, Lee SH, et al. (2011). Bone regeneration using a microstereolithography-produced customized poly (propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials 32:744–52. [DOI] [PubMed] [Google Scholar]
- Lew K-S, Othman R, Ishikawa K, Yeoh F-Y. (2012). Macroporous bioceramics: a remarkable material for bone regeneration. J Biomater Appl 27:345–58. [DOI] [PubMed] [Google Scholar]
- Lew DP, Waldvogel FA. (2004). Osteomyelitis. Lancet 364:369–79. [DOI] [PubMed] [Google Scholar]
- Li X. (2016). Bactericidal mechanism of nanopatterned surfaces. Phys Chem Chem Phys 18:1311–6. [DOI] [PubMed] [Google Scholar]
- Lima DB, Almeida RD, Pasquali M, et al. (2018). Physical characterization and modeling of chitosan/PEG blends for injectable scaffolds. Carbohydr Polym 189:238–49. [DOI] [PubMed] [Google Scholar]
- Lin W-C, Yao C, Huang T-Y, et al. (2019). Long-term in vitro degradation behavior and biocompatibility of polycaprolactone/cobalt-substituted hydroxyapatite composite for bone tissue engineering. Dent Mater 35:751–62. [DOI] [PubMed] [Google Scholar]
- Lindfors N, Hyvönen P, Nyyssönen M, et al. (2010). Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 47:212–8. [DOI] [PubMed] [Google Scholar]
- Liu C, Bayer A, Cosgrove SE, et al. (2011). Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. [DOI] [PubMed] [Google Scholar]
- Liu D, He C, Liu Z, Xu W. (2017). Gentamicin coating of nanotubular anodized titanium implant reduces implant-related osteomyelitis and enhances bone biocompatibility in rabbits. IJN 12:5461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liu Z, Zou J, et al. (2021). Synthesis and characterization of a hydroxyapatite-sodium alginate-chitosan scaffold for bone regeneration. Front Mater 8:69. [Google Scholar]
- Long M, Rack H. (1998). Titanium alloys in total joint replacement—a materials science perspective. Biomaterials 19:1621–39. [DOI] [PubMed] [Google Scholar]
- Luo S, Jiang T, Yang Y, et al. (2016). Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskelet Disord 17:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudian M, Ganji F. (2017). Vancomycin-loaded HPMC microparticles embedded within injectable thermosensitive chitosan hydrogels. Prog Biomater 6:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier GS, Roth KE, Andereya S, et al. (2013). In vitro elution characteristics of gentamicin and vancomycin from synthetic bone graft substitutes. Open Orthop J 7:624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malheiro VN, Caridade SG, Alves NM, Mano JF. (2010). New poly(epsilon-caprolactone)/chitosan blend fibers for tissue engineering applications. Acta Biomater 6:418–28. [DOI] [PubMed] [Google Scholar]
- Mancuso E, Bretcanu OA, Marshall M, et al. (2017). Novel bioglasses for bone tissue repair and regeneration: Effect of glass design on sintering ability, ion release and biocompatibility. Mater Des 129:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauffrey C, Butler N, Hake ME. (2016). Fabrication of an interlocked antibiotic/cement-coated carbon fiber nail for the treatment of long bone osteomyelitis. J Orthop Trauma 30:S23–S24. [DOI] [PubMed] [Google Scholar]
- Maver T, Mastnak T, Mihelič M, et al. (2021). Clindamycin-based 3D-printed and electrospun coatings for treatment of implant-related infections. Materials 14:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melicherčík P, Jahoda D, Nyč O, et al. (2012). Bone grafts as vancomycin carriers in local therapy of resistant infections. Folia Microbiol (Praha) 57:459–62. [DOI] [PubMed] [Google Scholar]
- Metallidis S, Charokopos N, Nikolaidis J, et al. (2006). Penetration of moxifloxacin into sternal bone of patients undergoing routine cardiopulmonary bypass surgery. Int J Antimicrob Agents 28:428–32. [DOI] [PubMed] [Google Scholar]
- Meyer M. (2019). Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online 18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CP, Chiodo CP. (2016). Autologous bone graft in foot and ankle surgery. Foot Ankle Clin 21:825–37. [DOI] [PubMed] [Google Scholar]
- Mills DK, Jammalamadaka U, Tappa K, Weisman J. (2018). Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads. Bioact Mater 3:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarak DH, Salah S, Elkheshen SA. (2014). Formulation of ciprofloxacin hydrochloride loaded biodegradable nanoparticles: optimization of technique and process variables. Pharm Dev Technol 19:891–900. [DOI] [PubMed] [Google Scholar]
- Moeinzadeh S, Park Y, Lin S, Yang YP. (2021). In-situ stable injectable collagen-based hydrogels for cell and growth factor delivery. Materialia 15:100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty S, Kumar M, Murthy N. (2003). Use of antibiotic-loaded polymethyl methacrylate beads in the management of musculoskeletal sepsis-a retrospective study. J Orthop Surg (Hong Kong) 11:73–9. [DOI] [PubMed] [Google Scholar]
- Momodu II, Savaliya V. (2020). Osteomyelitis. Treasure Island (FL): StatPearls Publishing. [PubMed] [Google Scholar]
- Morley R, Lopez F, Webb F. (2016). Calcium sulphate as a drug delivery system in a deep diabetic foot infection. The Foot 27:36–40. [DOI] [PubMed] [Google Scholar]
- Morsi NM, Shamma RN, Eladawy NO, Abdelkhalek AA. (2019). Risedronate-loaded macroporous gel foam enriched with nanohydroxyapatite: preparation, characterization, and osteogenic activity evaluation using Saos-2 cells. AAPS PharmSciTech 20:104. [DOI] [PubMed] [Google Scholar]
- Mostafa AA, El-Sayed MM, Mahmoud AA, Gamal-Eldeen AM. (2017). Bioactive/natural polymeric scaffolds loaded with ciprofloxacin for treatment of osteomyelitis. AAPS PharmSciTech 18:1056–69. [DOI] [PubMed] [Google Scholar]
- Nair BP, Gangadharan D, Mohan N, et al. (2015). Hybrid scaffold bearing polymer-siloxane Schiff base linkage for bone tissue engineering. Mater Sci Eng C 52:333–42. [DOI] [PubMed] [Google Scholar]
- Nandi SK, Mukherjee P, Roy S, et al. (2009). Local antibiotic delivery systems for the treatment of osteomyelitis–a review. Mater Sci Eng C 29:2478–85. [Google Scholar]
- Narayan R, Agarwal T, Mishra D, et al. (2017). Ectopic vascularized bone formation by human mesenchymal stem cell microtissues in a biocomposite scaffold. Colloids Surf B 160:661–70. [DOI] [PubMed] [Google Scholar]
- Nikezić AVV, , Bondžić AM, and , Vasić VM. (2020). Drug delivery systems based on nanoparticles and related nanostructures. European Journal of Pharmaceutical Sciences 151:105412. [DOI] [PubMed] [Google Scholar]
- Nithya R, Sundaram NM. (2015). Biodegradation and cytotoxicity of ciprofloxacin-loaded hydroxyapatite-polycaprolactone nanocomposite film for sustainable bone implants. Int J Nanomed 10:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SP, Courtney H, Bumgardner JD, Haggard WO. (2008). Chitosan films: a potential local drug delivery system for antibiotics. Clin Orthop Relat Res 466:1377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor S, Gilson A, Kennedy K, et al. (2016). Pre-packing of cost effective antibiotic cement beads for the treatment of traumatic osteomyelitis in the developing world–an in-vitro study based in Cambodia. Injury 47:805–10. [DOI] [PubMed] [Google Scholar]
- Norambuena GA, Patel R, Karau M, et al. (2017). Antibacterial and biocompatible titanium-copper oxide coating may be a potential strategy to reduce periprosthetic infection: an in vitro study. Clin Orthop Relat Res 475:722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogay V, Mun EA, Kudaibergen G, et al. (2020). Progress and prospects of polymer-based drug delivery systems for bone tissue regeneration. Polymers 12:2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EJ, Oh SH, Lee IS, et al. (2016). Antibiotic-eluting hydrophilized PMMA bone cement with prolonged bactericidal effect for the treatment of osteomyelitis. J Biomater Appl 30:1534–44. [DOI] [PubMed] [Google Scholar]
- Oryan A, Eslaminejad MB, Kamali A, et al. (2018). Mesenchymal stem cells seeded onto tissue-engineered osteoinductive scaffolds enhance the healing process of critical-sized radial bone defects in rat. Cell Tissue Res 374:63–81. [DOI] [PubMed] [Google Scholar]
- Oshima S, Sato T, Honda M, et al. (2020). Fabrication of gentamicin-loaded hydroxyapatite/collagen bone-like nanocomposite for anti-infection bone void fillers. IJMS 21:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrão T, Coelho CC, Costa P, et al. (2021). Combining local antibiotic delivery with heparinized nanohydroxyapatite/collagen bone substitute: a novel strategy for osteomyelitis treatment. Mater Sci Eng C 119:111329. [DOI] [PubMed] [Google Scholar]
- Parent M, Magnaudeix A, Delebassée S, et al. (2016). Hydroxyapatite microporous bioceramics as vancomycin reservoir: antibacterial efficiency and biocompatibility investigation. J Biomater Appl 31:488–98. [DOI] [PubMed] [Google Scholar]
- Park K-H, Greenwood-Quaintance KE, Schuetz AN, et al. (2017). Activity of tedizolid in methicillin-resistant Staphylococcus epidermidis experimental foreign body-associated osteomyelitis. Antimicrob Agents Chemother 61:e01644–01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Ruíz FJ, González-Gómez A, Fernández-Gutiérrez M, et al. (2017). Development of advanced biantibiotic loaded bone cement spacers for arthroplasty associated infections. Int J Pharm 522:11–20. [DOI] [PubMed] [Google Scholar]
- Patzakis MJ, Mazur K, Wilkins J, et al. (1993). Septopal beads and autogenous bone grafting for bone defects in patients with chronic osteomyelitis. Clin Orthop Relat Res 295: 112–8. [PubMed] [Google Scholar]
- Pawar V, Srivastava R. (2019). Chitosan-polycaprolactone blend sponges for management of chronic osteomyelitis: a preliminary characterization and in vitro evaluation. Int J Pharm 568:118553. [DOI] [PubMed] [Google Scholar]
- Peltola H, Pääkkönen M. (2014). Acute osteomyelitis in children. N Engl J Med 370:352–60. [DOI] [PubMed] [Google Scholar]
- Perez RA, Kim H-W, Ginebra M-P. (2012). Polymeric additives to enhance the functional properties of calcium phosphate cements. J Tissue Eng 3:2041731412439555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EJ, Lipsky BA. (2013). Diagnosis and management of infection in the diabetic foot. Med Clin North Am 97:911–46. [DOI] [PubMed] [Google Scholar]
- Phull MK, Eydmann T, Roxburgh J, et al. (2013). Novel macro-microporous gelatin scaffold fabricated by particulate leaching for soft tissue reconstruction with adipose-derived stem cells. J Mater Sci Mater Med 24:461–7. [DOI] [PubMed] [Google Scholar]
- Porter JR, Ruckh TT, Popat KC. (2009). Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog 25:1539–60. [DOI] [PubMed] [Google Scholar]
- Posadowska U, Brzychczy-Włoch M, Drożdż A, et al. (2016). Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Expert Opin Drug Deliv 13:613–20. [DOI] [PubMed] [Google Scholar]
- Posadowska U, Brzychczy-Włoch M, Pamuła E. (2015). Gentamicin loaded PLGA nanoparticles as local drug delivery system for the osteomyelitis treatment. Acta Bioeng Biomech 17:41–8. [PubMed] [Google Scholar]
- Puga AM, Rey-Rico A, Magariños B, et al. (2012). Hot melt poly-ε-caprolactone/poloxamine implantable matrices for sustained delivery of ciprofloxacin. Acta Biomater 8:1507–18. [DOI] [PubMed] [Google Scholar]
- Qayoom I, Teotia AK, Panjla A, et al. (2020). Local and sustained delivery of rifampicin from a bioactive ceramic carrier treats bone infection in rat tibia. ACS Infect Dis 6:2938–49. [DOI] [PubMed] [Google Scholar]
- Radwan NH, Nasr M, Ishak RA, et al. (2020). Chitosan-calcium phosphate composite scaffolds for control of post-operative osteomyelitis: fabrication, characterization, and in vitro-in vivo evaluation. Carbohydr Polym 244:116482. [DOI] [PubMed] [Google Scholar]
- Radwan NH, Nasr M, Ishak RA, Awad GA. (2021). Moxifloxacin-loaded in situ Synthesized Bioceramic/Poly (L-lactide-co-ε-caprolactone) Composite Scaffolds for Treatment of Osteomyelitis and Orthopedic regeneration. Int J Pharm 602:120662. [DOI] [PubMed] [Google Scholar]
- Rahaman MN, Bal BS, Huang W. (2014). Emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater Sci Eng C 41:224–31. [DOI] [PubMed] [Google Scholar]
- Rahaman MN, Day DE, Bal BS, et al. (2011). Bioactive glass in tissue engineering. Acta Biomater 7:2355–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj RM, Priya P, Raj V. (2018). Gentamicin-loaded ceramic-biopolymer dual layer coatings on the Ti with improved bioactive and corrosion resistance properties for orthopedic applications. J Mech Behav Biomed Mater 82:299–309. [DOI] [PubMed] [Google Scholar]
- Rasyid HN, van der Mei HC, Frijlink HW, et al. (2009). Concepts for increasing gentamicin release from handmade bone cement beads. Acta Orthop 80:508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizner W, Hunter JG, O'Malley NT, et al. (2014). A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur Cell Mater 27:196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele T, Boselli E, Breilh D, et al. (2004). Diffusion of levofloxacin into bone and synovial tissues. J Antimicrob Chemother 53:533–5. [DOI] [PubMed] [Google Scholar]
- Roux KM, Cobb LH, Seitz MA, Priddy LB. (2021). Innovations in osteomyelitis research: A review of animal models. Animal Model Exp Med 4:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RJ, Harrington CA, Poon A, et al. (1999). The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg Infect Dis 5:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumian Ł, Tiainen H, Cibor U, et al. (2016). Ceramic scaffolds enriched with gentamicin loaded poly (lactide-co-glycolide) microparticles for prevention and treatment of bone tissue infections. Mater Sci Eng C 69:856–64. [DOI] [PubMed] [Google Scholar]
- Ryan EJ, Ryan AJ, González-Vázquez A, et al. (2019). Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 197:405–16. [DOI] [PubMed] [Google Scholar]
- Sa M-W, Kim JY. (2015). Comparison analysis and fabrication of hollow shaft scaffolds using polymer deposition system. Tissue Eng Regen Med 12:46–52. [Google Scholar]
- Sabzi E, Abbasi F, Ghaleh H. (2021). Interconnected porous nanofibrous gelatin scaffolds prepared via a combined thermally induced phase separation/particulate leaching method. J Biomater Sci Polym Ed 32:488–503. [DOI] [PubMed] [Google Scholar]
- Saidykhan L, Bakar MZBA, Rukayadi Y, et al. (2016). Development of nanoantibiotic delivery system using cockle shell-derived aragonite nanoparticles for treatment of osteomyelitis. Int J Nanomed 11:661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Tatara AM, Mikos AG. (2014). Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release 190:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarigol-Calamak E, Hascicek C. (2018). Tissue scaffolds as a local drug delivery system for bone regeneration. In: Chun H, Park CH, Kwon K, Khang G. (Ed.), Cutting-edge enabling technologies for regenerative medicine. Advances in experimental medicine and biology, 1078. Singapore: Springer, 475–93. [DOI] [PubMed] [Google Scholar]
- Sarwar MS, Huang Q, Ghaffar A, et al. (2020). A Smart drug delivery system based on biodegradable chitosan/poly (allylamine hydrochloride) blend films. Pharmaceutics 12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yazawa H, Ikuma D, et al. (2019). Osteomyelitis due to methicillin-resistant Staphylococcus aureus successfully treated by an oral combination of minocycline and trimethoprim–sulfamethoxazole. SAGE Open Med Case Rep 7:2050313X1984146–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreto FA, Beyer GA, Grieco P, et al. (2018). Vertebral osteomyelitis: a comparison of associated outcomes in early versus delayed surgical treatment. Int J Spine Surg 12:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhi HS, Mahindra P, Yamin M, et al. (2012). Outcome in patients with an infected nonunion of the long bones treated with a reinforced antibiotic bone cement rod. J Orthop Trauma 26:184–8. [DOI] [PubMed] [Google Scholar]
- Sengstock C, Lopian M, Motemani Y, et al. (2014). Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 25:195101. [DOI] [PubMed] [Google Scholar]
- Senneville E, Nguyen S. (2013). Current pharmacotherapy options for osteomyelitis: convergences, divergences and lessons to be drawn. Expert Opin Pharmacother 14:723–34. [DOI] [PubMed] [Google Scholar]
- Seol Y-J, Kang T-Y, Cho D-W. (2012). Solid freeform fabrication technology applied to tissue engineering with various biomaterials. Soft Matter 8:1730–5. [Google Scholar]
- Shamma RN, Elkasabgy NA, Mahmoud AA, et al. (2017). Design of novel injectable in-situ forming scaffolds for non-surgical treatment of periapical lesions: in-vitro and in-vivo evaluation. Int J Pharm 521:306–17. [DOI] [PubMed] [Google Scholar]
- Shamma RN, Sayed RH, Madry H, et al. (2021). Triblock copolymer bioinks in hydrogel 3D printing for regenerative medicine-a focus on PF127. Tissue Eng B Rev. doi: 10.1089/ten.teb.2021.0026 [DOI] [PubMed] [Google Scholar]
- Sharma S, Bano S, Ghosh AS, et al. (2016). Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomed Nanotechnol Biol Med 12:1193–204. [DOI] [PubMed] [Google Scholar]
- Shi M, Chen L, Wang Y, et al. (2018). Effect of low-frequency pulsed ultrasound on drug delivery, antibacterial efficacy, and bone cement degradation in vancomycin-loaded calcium phosphate cement. Med Sci Monit 24:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J-H, Kim M-J, Park JY, et al. (2015). Three-dimensional printing of antibiotics-loaded poly-ε-caprolactone/poly (lactic-co-glycolic acid) scaffolds for treatment of chronic osteomyelitis. Tissue Eng Regen Med 12:283–93. [Google Scholar]
- Shim J-H, Lee J-S, Kim JY. (2012). Fabrication of solid freeform fabrication based 3D scaffold and its in-vitro characteristic evaluation for bone tissue engineering. Tissue Eng Regen Med 9:A16–23. [Google Scholar]
- Shipitsyna I, Osipova E, Ovchinnikov E, Leonchuk D. (2020). [Dependence of biofilm-forming ability on the antibiotic sensitivity of Pseudomonas aeruginosa clinical strains isolated from patients with chronic osteomyelitis.]. Klin Lab Diagn 65:37–41. [DOI] [PubMed] [Google Scholar]
- Siddiqui N, Asawa S, Birru B, et al. (2018). PCL-based composite scaffold matrices for tissue engineering applications. Mol Biotechnol 60:506–32. [DOI] [PubMed] [Google Scholar]
- Siemann U. 2005. Solvent cast technology–a versatile tool for thin film production. In: Scattering methods and the properties of polymer materials. Berlin/Heidelberg: Springer, 1–14. [Google Scholar]
- Singh BN, Veeresh V, Mallick SP, et al. (2020). Generation of scaffold incorporated with nanobioglass encapsulated in chitosan/chondroitin sulfate complex for bone tissue engineering. Int J Biol Macromol 153:1–16. [DOI] [PubMed] [Google Scholar]
- Sjöström T, McNamara LE, Meek RD, et al. (2013). 2D and 3D nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Adv Healthc Mater 2:1285–93. [DOI] [PubMed] [Google Scholar]
- Song J, Leeuwenburgh SC. (2014). Sustained delivery of biomolecules from gelatin carriers for applications in bone regeneration. Ther Deliv 5:943–58. [DOI] [PubMed] [Google Scholar]
- Soundrapandian C, Basu D, Sa B, Datta S. (2011). Local drug delivery system for the treatment of osteomyelitis: in vitro evaluation. Drug Dev Ind Pharm 37:538–46. [DOI] [PubMed] [Google Scholar]
- Sreeja S, Muraleedharan C, Varma PH, Sailaja G. (2020). Surface-transformed osteoinductive polyethylene terephthalate scaffold as a dual system for bone tissue regeneration with localized antibiotic delivery. Mater Sci Eng C 109:110491. [DOI] [PubMed] [Google Scholar]
- Stevens MM. (2008). Biomaterials for bone tissue engineering. Mater Today 11:18–25. [Google Scholar]
- Su Y, Su Q, Liu W, et al. (2012). Controlled release of bone morphogenetic protein 2 and dexamethasone loaded in core-shell PLLACL-collagen fibers for use in bone tissue engineering. Acta Biomater 8:763–71. [DOI] [PubMed] [Google Scholar]
- Sun X, Ma Z, Zhao X, et al. (2021). Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact Mater 6:757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk A, Skwira A, Konopacka A, et al. (2021). Mesoporous silica-bioglass composite pellets as bone drug delivery system with mineralization potential. IJMS 22:4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamazawa G, Ito A, Miyai T, et al. (2011). Gatifloxacine-loaded PLGA and β-tricalcium phosphate composite for treating osteomyelitis. Dent Mater J 30:264–73. [DOI] [PubMed] [Google Scholar]
- Tan G, Tan Y, Ni G, et al. (2014). Controlled oxidative nanopatterning of microrough titanium surfaces for improving osteogenic activity. J Mater Sci Mater Med 25:1875–84. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhang Y, Shen A, et al. (2020). Injectable chitosan-based thermosensitive hydrogel/nanoparticle-loaded system for local delivery of vancomycin in the treatment of osteomyelitis. Int J Nanomed 15:5855–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Ren J, Gu S. (2007). Preparation and characterization of porous PDLLA/HA composite foams by supercritical carbon dioxide technology. J Biomed Mater Res B Appl Biomater 81:185–93. [DOI] [PubMed] [Google Scholar]
- Thanyaphoo S, Kaewsrichan J. (2012). Synthesis and evaluation of novel glass ceramics as drug delivery systems in osteomyelitis. J Pharm Sci 101:2870–82. [DOI] [PubMed] [Google Scholar]
- Thein E, Tafin U, Betrisey B, et al. (2013). In vitro activity of gentamicin-loaded bioabsorbable beads against different microorganisms. Materials (Basel) 6:3284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tîlmaciu C-M, Mathieu M, Lavigne J-P, et al. (2015). In vitro and in vivo characterization of antibacterial activity and biocompatibility: a study on silver-containing phosphonate monolayers on titanium. Acta Biomater 15:266–77. [DOI] [PubMed] [Google Scholar]
- Trombetta RP, Ninomiya MJ, El-Atawneh IM, et al. (2019). Calcium phosphate spacers for the local delivery of sitafloxacin and rifampin to treat orthopedic infections: efficacy and proof of concept in a mouse model of single-stage revision of device-associated osteomyelitis. Pharmaceutics 11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiolis P, Giamarellos-Bourboulis EJ, Mavrogenis AF, et al. (2011). Experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus treated with a polylactide carrier releasing linezolid. Surg Infect (Larchmt) 12:131–5. [DOI] [PubMed] [Google Scholar]
- Tucker LJ, Grant CS, Gautreaux MA, et al. (2021). Physicochemical and antimicrobial properties of thermosensitive chitosan hydrogel loaded with fosfomycin. Mar Drugs 19:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TM, Urban RM, Gitelis S, et al. (2001). Radiographic and histologic assessment of calcium sulfate in experimental animal models and clinical use as a resorbable bone-graft substitute, a bone-graft expander, and a method for local antibiotic delivery: one institution’s experience. J Bone Joint Surg 83:8–18. [DOI] [PubMed] [Google Scholar]
- Ueng SW, Lee MS, Lin SS, et al. (2007). Development of a biodegradable alginate carrier system for antibiotics and bone cells. J Orthop Res 25:62–72. [DOI] [PubMed] [Google Scholar]
- Ueng SW, Lin S-S, Wang I-C, et al. (2016). Efficacy of vancomycin-releasing biodegradable poly(lactide-co-glycolide) antibiotics beads for treatment of experimental bone infection due to Staphylococcus aureus. J Orthop Surg Res 11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueng SW, Yuan L-J, Lin S-S, et al. (2011). In vitro and in vivo analysis of a biodegradable poly (lactide-co-glycolide) copolymer capsule and collagen composite system for antibiotics and bone cells delivery. J Trauma Acute Care Surg 70:1503–9. [DOI] [PubMed] [Google Scholar]
- Uskokovic V. (2015). Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis. Crit Rev Ther Drug Carrier Syst 32:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Variola F, Zalzal SF, Leduc A, et al. (2014). Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int J Nanomed 9:2319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergidis P, Rouse MS, Euba G, et al. (2011). Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob Agents Chemother 55:1182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeiss RA, Negrini TC, Arthur RA, Bottino MC. (2014). Antimicrobial effects of drug-containing electrospun matrices on osteomyelitis-associated pathogens. J Oral Maxillofac Surg 72:1310–9. [DOI] [PubMed] [Google Scholar]
- Waknis V, Jonnalagadda S. (2011). Novel poly-DL-lactide-polycaprolactone copolymer based flexible drug delivery system for sustained release of ciprofloxacin. Drug Deliv 18:236–45. [DOI] [PubMed] [Google Scholar]
- Walter G, Kemmerer M, Kappler C, Hoffmann R. (2012). Treatment algorithms for chronic osteomyelitis. Dtsch Arztebl Int 109:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan T, Stylios GK, Giannoudi M, Giannoudis PV. (2015). Investigating a new drug delivery nano composite membrane system based on PVA/PCL and PVA/HA (PEG) for the controlled release of biopharmaceuticals for bone infections. Injury 46:S39–S43. [DOI] [PubMed] [Google Scholar]
- Wang G, Luo W, Zhou Y, et al. (2021). Custom-made antibiotic cement-coated nail for the treatment of infected bone defect. BioMed Res Int 2021:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Li H, et al. (2011). Assessing the character of the rhBMP-2- and vancomycin-loaded calcium sulphate composites in vitro and in vivo. Arch Orthop Trauma Surg 131:991–1001. [DOI] [PubMed] [Google Scholar]
- Wasupalli GK, Verma D. (2020). Injectable and thermosensitive nanofibrous hydrogel for bone tissue engineering. Mater Sci Eng C 107:110343. [DOI] [PubMed] [Google Scholar]
- Wei G, Ma PX. (2004). Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 25:4749–57. [DOI] [PubMed] [Google Scholar]
- Weinrauch PC, Bell C, Wilson L, et al. (2007). Shear properties of bilaminar polymethylmethacrylate cement mantles in revision hip joint arthroplasty. J Arthroplasty 22:394–403. [DOI] [PubMed] [Google Scholar]
- Wells CM, Beenken KE, Smeltzer MS, et al. (2018). Ciprofloxacin and rifampin dual antibiotic-loaded biopolymer chitosan sponge for bacterial inhibition. Mil Med 183:433–44. [DOI] [PubMed] [Google Scholar]
- Winkler H, Janata O, Berger C, et al. (2000). In vitro release of vancomycin and tobramycin from impregnated human and bovine bone grafts. J Antimicrob Chemother 46:423–8. [DOI] [PubMed] [Google Scholar]
- Wu W, Ye C, Zheng Q, et al. (2016). A therapeutic delivery system for chronic osteomyelitis via a multi-drug implant based on three-dimensional printing technology. J Biomater Appl 31:250–60. [DOI] [PubMed] [Google Scholar]
- Xing J, Hou T, Luobu B, et al. (2013). Anti-infection tissue engineering construct treating osteomyelitis in rabbit tibia. Tissue Eng Part A 19:255–63. [DOI] [PubMed] [Google Scholar]
- Xu H, Han D, Dong JS, et al. (2010). Rapid prototyped PGA/PLA scaffolds in the reconstruction of mandibular condyle bone defects. Int J Med Robotics Comput Assist Surg 6:66–72. [DOI] [PubMed] [Google Scholar]
- Yan L, Jiang D-M, Cao Z-D, et al. (2015). Treatment of Staphylococcus aureus-induced chronic osteomyelitis with bone-like hydroxyapatite/poly amino acid loaded with rifapentine microspheres. Drug Design Dev Ther 9:3665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hao Y, Liu Q, et al. (2017). Preparation and in vitro study of hydrochloric norvancomycin encapsulated poly (d,l-lactide-co-glycolide, PLGA) microspheres for potential use in osteomyelitis. Artif Cells Nanomed Biotechnol 45:1326–30. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jia W, Gu Y, et al. (2010). Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 31:5865–74. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jiang Y, Zhang Y, et al. (2013). Effect of porosity on long-term degradation of poly (ε-caprolactone) scaffolds and their cellular response. Polym Degrad Stab 98:209–18. [Google Scholar]
- Zhang Y, Liang R-j, Xu J-j, et al. (2017). Efficient induction of antimicrobial activity with vancomycin nanoparticle-loaded poly(trimethylene carbonate) localized drug delivery system. Int J Nanomed 12:1201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu W, Wu X-D, et al. (2019a). Efficacy of novel nano-hydroxyapatite/polyurethane composite scaffolds with silver phosphate particles in chronic osteomyelitis. J Mater Sci Mater Med 30:59. [DOI] [PubMed] [Google Scholar]
- Zhang P, Qin J, Zhang B, et al. (2019b). Gentamicin-loaded silk/nanosilver composite scaffolds for MRSA-induced chronic osteomyelitis. R Soc Open Sci 6:182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Song J, Klymov A, et al. (2018). Monitoring local delivery of vancomycin from gelatin nanospheres in zebrafish larvae. Int J Nanomed 13:5377–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wei Q, Zhou H, et al. (2020). Sustainable release of vancomycin from micro-arc oxidised 3D-printed porous Ti6Al4V for treating methicillin-resistant Staphylococcus aureus bone infection and enhancing osteogenesis in a rabbit tibia osteomyelitis model. Biomater Sci 8:3106–15. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chu PK, Zhang Y, Wu Z. (2009). Antibacterial coatings on titanium implants. J Biomed Mater Res Part B 91:470–80. [DOI] [PubMed] [Google Scholar]
- Zhao X, Han Y, Zhu T, et al. (2019). Electrospun polylactide-Nano-hydroxyapatiteVancomycin composite scaffolds for advanced Osteomyelitis therapy. J Biomed Nanotechnol 15:1213–22. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Wang G, Zhang Y, et al. (2020). The effect of calcium sulfate/calcium phosphate composite for the treatment of chronic osteomyelitis compared with calcium sulfate. Ann Palliat Med 9:1821–33. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhou X, Wang J, et al. (2018). Treatment of osteomyelitis defects by a vancomycin-loaded gelatin/β-tricalcium phosphate composite scaffold. Bone Joint Res 7:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W. (2014). Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 276:111–9. [DOI] [PubMed] [Google Scholar]
- Zimmerli W, Moser C. (2012). Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 65:158–68. [DOI] [PubMed] [Google Scholar]