Abstract
Introduction
Awake craniotomy (AC) has emerged as a better modality for resection of intra-axial brain tumors. The advantages are not just related to the preservation of neurological function, but also include early recovery, short hospital stay and possibly lower costs. However, data on AC for meningioma resection is deficient, likely because of concerns related to intra-operative pain and blood loss.
Methods
All patients who underwent AC, using awake through-out technique for resection of meningioma, during the last five years, were included in the study. Non-probability consecutive sampling technique was employed. Variables for demographics, and details of diagnosis and surgical procedure were recorded. The outcomes measured were length of hospital stay, worsening of neurological function during surgery and significant intra-operative or post-operative pain.
Results
Seventeen patients underwent AC for resection of meningioma during the study period. Eleven of these were grade I meningioma, and six were grade II meningioma. The mean age was 45.8 ± 10.5 years. Presenting complaints were variable, with seizures being the most common (n = 7; 41.2%). The mean duration of surgery was 180.8 ± 36.2 minutes and median estimated blood loss was 450 ml (IQR: 225 ml - 737.5 ml). The mean length of stay in the hospital was 3.1 ± 1.3 days. Only one patient had a prolonged hospital stay of seven days, because of post-operative seizures. Eleven patients (58.3%) had convexity meningioma, 4 (33.3%) had parasagittal meningioma and 1 each had a parafalcine and anterior skull-base meningioma. Simpson grade I resection was performed in 6 (41.7%) patients, grade II resection in 10 (50%) patients, and grade III resection in 1 (5.9%) patient. None of our patients had deterioration in their neurological deficits after surgery and no one required emergency intubation, conversion of surgery to general anesthesia, or redo exploration.
Conclusion
AC may be considered a safe modality for surgical resection of convexity and parasagittal meningioma, with no significant risk of intra-operative or post-operative pain, although it requires more evidence. It can be offered to patients who are at higher risk, or are not willing to undergo general anesthesia. Ultimately, it might also be beneficial in terms of reducing overall costs.
Keywords: scalp block, tumors, meningioma, extra-axial lesions, awake craniotomy
Introduction
Surgical resection of brain tumors under awake craniotomy (AC) has gained popularity during the last two decades [1]. The technique was initially introduced for epilepsy surgery more than two hundred years ago. Nowadays, it is frequently being opted for resection of tumors near eloquent cortex [2,3]. It is considered the gold standard tool for real-time localization of functionally important areas in the brain during surgery, thereby decreasing dependence on often inconsistent anatomic landmarks for brain mapping [4].
Literature review, however, suggests that the advantages of AC are not just limited to safe tumor resection. AC for brain tumor surgery not only allows for continuous monitoring of patients’ neurological function, but also saves them from the risks of general anesthesia. It results in saving resources in low- and middle-income countries by decreasing hospital length of stay and critical care admissions [5,6]. Recent data also suggests that AC has a significant role in promoting faster recovery, limiting the need for extensive post-operative care [7]. Although most of the published literature supports AC for resection of intra-axial brain tumors, there have been some evidence highlighting its importance for extra-axial tumors as well [8-10].
Meningiomas are the most common extra-axial brain tumors in the brain. These are dural-based lesions, and it has been an assumption that performing AC for meningioma would be associated with significant intra-operative pain on dural manipulation. There have been few case reports and case series, reporting successful AC for resection of meningioma and other extra-axial tumors [8-10]. Considering the dearth of literature on the subject, our objective was to report technique employed at our health care setup for resection of meningioma using AC.
Materials and methods
Patient population
All patients who underwent AC for resection of meningioma between January 2016 to January 2021 were included. The detail of these patients was retrieved from operating room database. Ethical review board’s exemption was also taken. The sampling technique was non-probability consecutive sampling. Data was collected on a pre-designed data collection form, which had details about demographics, diagnosis and intraoperative and postoperative management. Statistical analysis was conducted using SPSS Inc. Version 21.0. Continuous data has been represented as means and standard deviation, while categorical data has been represented as frequencies and proportions. All cases were performed by the same surgeon (the senior author), and the same anesthetic technique was used to maintain uniformity and to avoid variability.
The outcomes included length of hospital stay, worsening of neurological examination during surgery, which was present in the neurological examination on first follow-up in clinic (10 days from surgery), and significant intra-operative or post-operative pain. Neurological worsening was defined as any impairment in patients’ function of speech, gross motor power in the limbs, sensations in the limbs, or vision in any eye at the time of clinic follow-up (10 days from surgery). This information was extracted from the patients’ pre-operative and first follow-up in clinic neurological examinations, mentioned in their medical record files. Since it was a retrospective review, pain was assessed according to the patients’ analgesic requirement during or within 24 hours after surgery. Extent of tumor resection was graded according to the Simpson grading system [11].
Surgical technique and anesthesia for AC
As per our routine practice, all patients planned for AC had initial preoperative assessment and counselling by neuro-anesthetist. The dedicated neuro-anesthesia team managed the perioperative care of these cases. It included protocol-based anesthetic care, comprising of handover of AC-related educational brochure to the patient, preoperative urinary catheterization on the night before surgery and scheduling of these cases as first on the list. Patients were also reassured about possible discomforts like bone drilling and feeling of pain during dural retraction. An awake throughout the approach of AC was employed for all study patients. This included institution of scalp block and providing conscious sedation using dexmedetomidine infusion. There were no absolute contraindications to Dexmedetomidine, however, patients were observed for possible adverse effects including bradycardia, hypotension and hypertension. For scalp block, Ropivacaine 0.5 % (30-40 ml) along with adrenaline and 8 mg dexamethasone was used.
Routine ASA (American Society of Anesthesiologists) specified monitoring was done during surgery, including heart rate, non-invasive/invasive blood pressure, saturation and end-tidal CO2. The depth of sedation was titrated according to Bispectral Index (BIS) monitoring, level of which was kept between 75-80 during tumor resection phase. Additional doses of fentanyl were occasionally required during dural retraction. We gave Oxygen to all patients via nasal prongs at 2 L/min, which has got in-built end-tidal CO2 monitoring port. Patients were also administered intravenous Paracetamol 1 gm, at the start of operation. In all cases, once craniotomy was done, 2% xylocaine with adrenaline-soaked gauze was placed on the dura for 1 minute for local absorption, before durotomy. Double antiemetic prophylaxis was given using dexamethasone and ondansetron (0.1 mg/kg). Levetiracetam 1 gm stat was prescribed for perioperative seizure prophylaxis. All procedures were performed under microscope, and Cavitron ultrasonic aspirator (CUSA) was also used in some cases for tumor resection. Most of the patients were not administered Mannitol at the beginning or during surgery.
Post-operative management
Post-operative management included the transfer of patient to post-anaesthesia care unit and then to a high dependency unit for overnight observation. Postoperative pain management was done with intermittent doses of Paracetamol 1 gm Q6H, and tramadol 50-60 mg Q 6-8 hourly. Antiemetic and Seizure prophylaxis was also continued for 48 hours postoperatively.
Results
Seventeen patients underwent AC for resection of extra-axial lesions during the study period, all of which were later diagnosed to have meningioma on histopathology. Most of the patients were young, with the mean age of these patients being 45.8 ± 10.5 years. Presenting complains were variable, with seizures being the most common (n = 7; 41.2%). The mean duration of surgery was 180.8 ± 36.2 minutes and the median estimated blood loss (EBL) was 450 ml (IQR: 225 ml - 737.5 ml). The mean length of stay in the hospital was 3.1 ± 1.3 days. Only one patient had a prolonged hospital stay of seven days because he had developed seizures which were controlled by escalating antiepileptic drug dosage. Details of demographics are shown in Table 1.
Table 1. Demographics.
HTN: hypertension; IHD: ischemic heart disease; DM: diabetes mellitus.
| Case | Gender | Age (years) | Co-morbids | Presenting complains | Duration of surgery (mins) | Estimated blood loss (ml) | Length of hospital stay (days) |
| 1 | Male | 25 | None | Seizure | 150 | 750 | 3 |
| 2 | Male | 45 | None | Seizure | 215 | 800 | 3 |
| 3 | Male | 41 | HTN | Impaired sensory function | 160 | 200 | 3 |
| 4 | Male | 35 | None | Impaired sensory function | 230 | 1500 | 3 |
| 5 | Female | 30 | None | Visual impairment | 173 | 700 | 3 |
| 6 | Female | 48 | None | Impaired motor function | 201 | 350 | 2 |
| 7 | Male | 49 | None | Seizure | 125 | 50 | 2 |
| 8 | Male | 55 | None | Seizure & Impaired motor function | 175 | 500 | 4 |
| 9 | Male | 39 | IHD | Headache | 140 | 200 | 2 |
| 10 | Female | 42 | HTN | Speech impairment | 217 | 400 | 3 |
| 11 | Male | 50 | HTN & DM | Impaired sensory function | 155 | 500 | 7 |
| 12 | Male | 47 | None | Impaired motor function | 165 | 300 | 2 |
| 13 | Male | 62 | HTN | Seizure | 213 | 600 | 3 |
| 14 | Male | 48 | None | Seizure | 125 | 250 | 3 |
| 15 | Female | 56 | None | Visual impairment | 240 | 1150 | 5 |
| 16 | Female | 65 | HTN | Speech impairment | 210 | 900 | 2 |
| 17 | Male | 42 | DM | Seizure | 180 | 100 | 3 |
Only one patient had the lesion located at skull-base (lateral sphenoid-wing); more than half of the patients (64.7%) had their lesions located at the convexity. Figures 1 to 4 are pre-operative and post-operative MRIs of some of the cases.
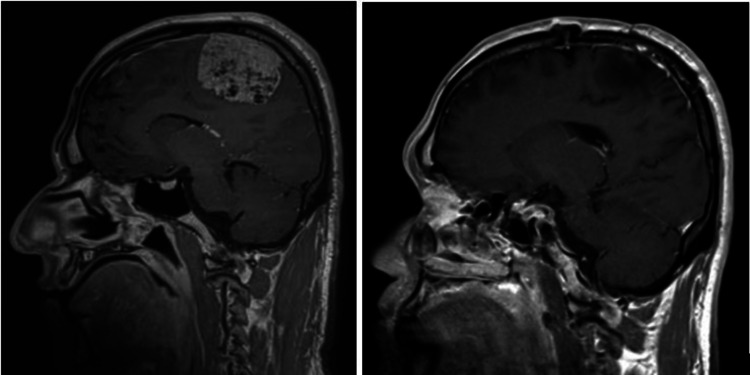
Figure 1. MRI brain T1 post-contrast, sagittal section, pre-operative (left) and post-operative (right) images. The parasagittal meningioma was located in the middle of the superior sagittal sinus, and a small part of the lesion adherent to the sinus wall was left behind.
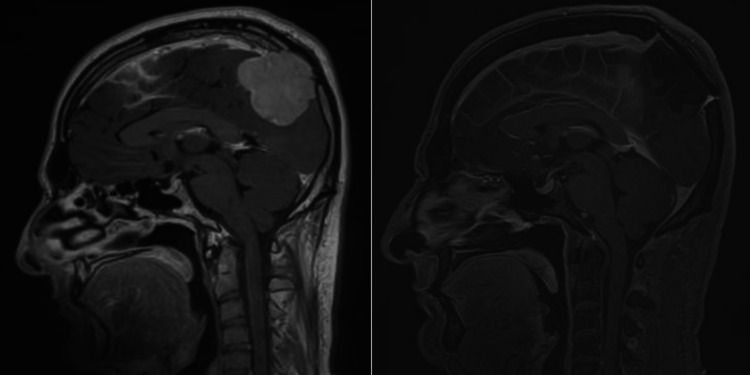
Figure 2. MRI brain T1 post-contrast, sagittal section, pre-operative (left) and post-operative (right) images. The posteriorly located parasagittal meningioma had thrombosed superior sagittal sinus, and was completely resected with duroplasty and cranioplasty with titanium mesh as seen in post-operative scan on the right.
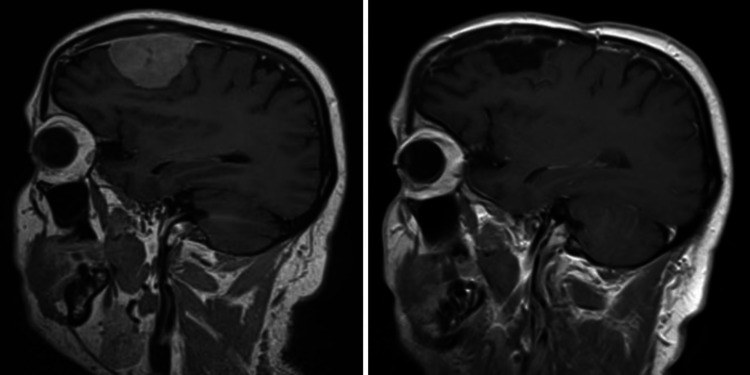
Figure 3. MRI brain T1 post-contrast, sagittal section, pre-operative (left) and post-operative (right) images. The frontal convexity meningioma was completely resected as seen in post-operative scan on the right.
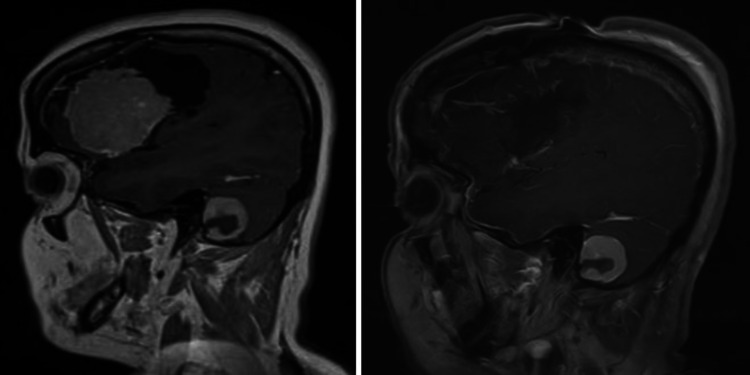
Figure 4. MRI brain T1 post-contrast, sagittal section, pre-operative (left) and post-operative (right) images. The lateral sphenoid-wing meningioma was completely resected as seen in post-operative scan on the right. Another homogenously enhancing lesion with a small hypo-intense central part, can be seen in the posterior fossa, which was not causing symptoms, was not resected.
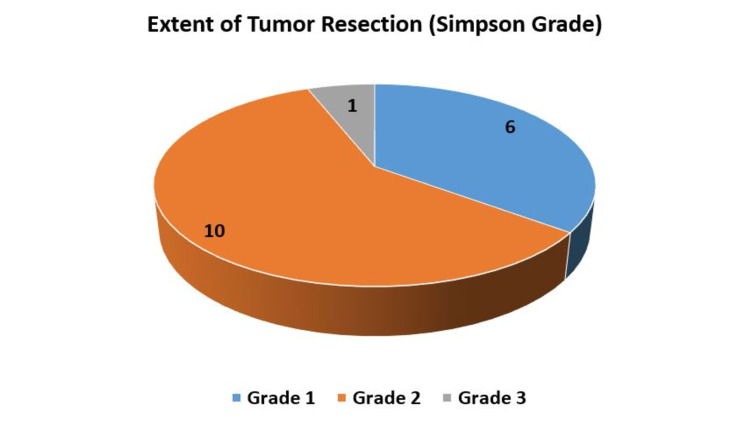
Nine patients (52.9%) showed improvement in their functional status at follow-up. Two patients (case numbers 8 and 12) had a significantly poor functional status before surgery because of right hemiparesis which remained stable after surgery in one case and improved in the other. None of the patients required additional oral or intravenous analgesics, apart from the standard regime. All patients had received prophylactic antiemetics post-operatively (intravenous ondansetron 8 mg Q8H or intravenous metoclopramide 8 mg Q8H) for 48 hours, and none of them reported nausea or vomiting during or after surgery. The details of surgery and functional outcome are described in Table 2, and the extent of tumor resection is depicted in Figure 5.
Table 2. Details of diagnosis and surgery.
KPS: Karnofsky performance score.
| Case | Histopathological diagnosis | Site of Lesion | Side of Lesion | Extent of Resection (Simpson grade) | Admission KPS | KPS at last follow-up | Post-op changes in functional status |
| 1 | Meningioma grade II | Convexity | Right | 2 | 90 | 90 | No change |
| 2 | Meningioma grade II | Parasagittal | Left | 2 | 70 | 90 | Improvement |
| 3 | Meningioma grade II | Convexity | Left | 2 | 90 | 100 | Improvement |
| 4 | Meningioma grade I | Parasagittal | Left | 1 | 80 | 100 | Improvement |
| 5 | Meningioma grade I | Convexity | Right | 2 | 90 | 100 | Improvement |
| 6 | Meningioma grade I | Convexity | Left | 1 | 70 | 80 | Improvement |
| 7 | Meningioma grade II | Convexity | Right | 1 | 90 | 100 | Improvement |
| 8 | Meningioma grade II | Parasagittal | Right | 1 | 50 | 50 | No change |
| 9 | Meningioma grade I | Convexity | Right | 1 | 90 | 100 | Improvement |
| 10 | Meningioma grade I | Sphenoid wing | Left | 2 | 80 | 100 | Improvement |
| 11 | Meningioma grade I | Convexity | Right | 3 | 90 | 90 | No change |
| 12 | Meningioma grade II | Parasagittal | Left | 2 | 60 | 70 | Improvement |
| 13 | Meningioma grade I | Falcine | Right | 2 | 80 | 80 | No change |
| 14 | Meningioma grade I | Convexity | Left | 2 | 90 | 90 | No change |
| 15 | Meningioma grade I | Convexity | Left | 2 | 80 | 80 | No change |
| 16 | Meningioma grade I | Convexity | Left | 2 | 80 | 80 | No change |
| 17 | Meningioma grade I | Convexity | Right | 1 | 90 | 90 | No change |
Figure 5. Extent of tumor resection according to Simpson grading.
Discussion
AC has been mainly employed in neuro-oncology for resection of intra-axial brain tumors. Coupled with cortical mapping, it has proved to be an important tool for the removal of tumors located in eloquent regions, particularly low-grade gliomas, with less surgical morbidity [12]. It allows a better chance of preservation of neurological function, notwithstanding the lesion’s size, primary pathology or patients’ history [13,14]. Lately, it has been shown in studies that AC can be utilized for resection of most supratentorial lesions including extra-axial tumors, cerebral abscess and arterio-venous malformations [15-17]. Many neurosurgeons now recommend AC to avoid risks associated with general anesthesia, and to decrease complication rates. Surveys have also revealed increased patient satisfaction when they undergo awake cranial surgery [17-19]. The only absolute contraindication to AC in literature is patient refusal, as compiled by Kaiying et al [20]. They had also reported mental health disorders, obstructive sleep apnea, inability to lay still for long periods, highly vascular large lesions and tumors at skull-base as relative contraindications to AC [20].
Although AC has been in practice for a long time, recent advancements in neuro-anesthesia and pain management have caused a resurgence and increased acceptance of this modality for neurosurgeons, as well as the patients [21]. Serlatis et al. had published a large prospective series of 610 patients who had undergone AC for supratentorial lesions, irrespective of the histopathology [5]. Eleven (1.8%) patients in their data had successful uneventful meningioma resection, and they had proposed this technique for resection of all kinds of intracranial lesions [5]. In a case report, Kumar et al. reported a 29 years old, 13 weeks pregnant patient who successfully underwent awake resection of a large meningioma [9]. In another case report, a 72 years old male patient had successfully undergone AC, far lateral approach, for resection of foramen magnum meningioma, with the patient in a three-quarter prone position [8].
A possible reason for underutilization of AC for excision of dural-based lesions is the fear of inadequate pain control. During surgery, the steps of bone flap elevation, durotomy and separation of tumor from dura or dural coagulation are the most painful steps. Inadequate pain relief at these steps can potentially result in the patient becoming restless and agitated, and might cause insufficient tumor resection [5]. Earlier studies had labelled lesions with significant dural involvement and high vascularity to be contraindications for AC [22,23]. The protocol-based anesthetic and surgical care worked very well for our study population. The fact that 50% of our patients underwent Simpson grade II resections where dura was coagulated, with no adverse event during surgery, strongly advocates for the use of this technique for resection of extra-axial tumors. Although the median EBL was 450 ml, five patients had an EBL of more than 700 ml which was managed with intra-operative blood transfusion and did not pose any significant challenge intra-operatively.
Another major limitation that should be considered while planning an AC, is the risk of emergency intubation to convert the procedure to general anesthesia, in patients who develop severe pain, threatened airway or seizures. None of our patients required conversion of the procedure to general anesthesia, although there is a 16% risk reported in the literature [24]. This might be related to the effective use of scalp block and BIS-guided sedation using Dexmedetomidine. There is around 5% risk of intra-operative seizures during AC, more with resection of intra-axial lesions, and may even result from cortical stimulation [5]. None of the patients in our series had intra-operative seizures, and only one patient had post-operative seizures that prolonged his hospital stay.
Studies have also reported less occurrence of post-operative nausea and vomiting after AC as compared to patients who receive general anesthesia [25]. We had prescribed intravenous ondansetron and dexamethasone to all of our patients post-operatively, so that may be a reason for no significant nausea and vomiting reported by our patients. One patient (5.9%) in our study had post-operative seizures. None of our patients developed post-operative surgical site hematoma, CSF leak or required re-operation or re-admission after discharge. Our complication rate is much less than that reported in the literature (approximately 15%), mostly for intra-axial tumors [5]. However, because of smaller number of cases, and the absence of comparison group, we recommend large cohort studies comparing AC and general anesthesia for meningioma resection, to draw more generally acceptable results.
Conclusions
Based on the results from this case series, and the surgical technique described, we propose that AC may be safely used for resection of extra-axial tumors, particularly meningioma located at convexity or parasagittal sites, and intraoperative pain and blood loss are not a major concern. It can be offered as a possible alternate to patients who are afraid, unwilling or high risk to undergo general anesthesia. Even though cortical mapping is not of much benefit for these lesions, AC does prevent complications that can arise from general anesthesia, and can lead to early discharge from the hospital in most cases. More evidence is, however, needed to substantiate our findings, and to study the possibly lower costs of treatment and shorter hospital stay as compared to resection under GA.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Review Board exemption issued approval Not applicable. The study was exempted from ethical review approval as it was a retrospective chart review with no interaction with the patients.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. J Neurosurg. 2004;100:369–375. doi: 10.3171/jns.2004.100.3.0369. [DOI] [PubMed] [Google Scholar]
- 2.The history of awake craniotomy for brain tumor and its spread into Asia. July J, Manninen P, Lai J, Yao Z, Bernstein M. Surg Neurol. 2009;71:621–624. doi: 10.1016/j.surneu.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Marshall C. Hafner Publishing Co. Vol. 288. New York: Hafner Publishing Co; 1967. Surgery of epilepsy and motor disorders. A history of neurological surgery; p. 305. [Google Scholar]
- 4.The reliability of neuroanatomy as a predictor of eloquence: a review. Pouratian N, Bookheimer SY. Neurosurg Focus. 2010;28:0. doi: 10.3171/2009.11.FOCUS09239. [DOI] [PubMed] [Google Scholar]
- 5.Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. Serletis D, Bernstein M. J Neurosurg. 2007;107:1–6. doi: 10.3171/JNS-07/07/0001. [DOI] [PubMed] [Google Scholar]
- 6.Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. Taylor MD, Bernstein M. J Neurosurg. 1999;90:35–41. doi: 10.3171/jns.1999.90.1.0035. [DOI] [PubMed] [Google Scholar]
- 7.Teaching and sustainably implementing awake craniotomy in resource-poor settings. Howe KL, Zhou G, July J, et al. World Neurosurg. 2013;80:0–4. doi: 10.1016/j.wneu.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Awake far lateral craniotomy for resection of foramen magnum meningioma in a patient with tenuous motor and somatosensory evoked potentials. deipolyi AR, Han SJ, Sughrue ME, Litt L, Parsa AT. J Clin Neurosci. 2011;18:1254–1256. doi: 10.1016/j.jocn.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Utilization of awake craniotomy for supra-tentorial tumor resection during pregnancy: A technique useful for fetal-maternal wellbeing. Kumar D, Siraj S, Ahsan K, Shafiq F. Pak J Med Sci. 2020;36:293–295. doi: 10.12669/pjms.36.2.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awake craniotomy for brain lesions within and near the primary motor area: a retrospective analysis of factors associated with worsened paresis in 102 consecutive patients. Shinoura N, Midorikawa A, Yamada R, et al. Surg Neurol Int. 2013;4:149. doi: 10.4103/2152-7806.122003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B. J Neurosurg. 2017;126:201–211. doi: 10.3171/2016.1.JNS151842. [DOI] [PubMed] [Google Scholar]
- 12.World's first magnetic resonance imaging/x-ray/operating room suite: a significant milestone in the improvement of neurosurgical diagnosis and treatment. Matsumae M, Koizumi J, Fukuyama H, et al. J Neurosurg. 2007;107:266–273. doi: 10.3171/JNS-07/08/0266. [DOI] [PubMed] [Google Scholar]
- 13.Failed awake craniotomy: a retrospective analysis in 424 patients undergoing craniotomy for brain tumor. Nossek E, Matot I, Shahar T, et al. J Neurosurg. 2013;118:243–249. doi: 10.3171/2012.10.JNS12511. [DOI] [PubMed] [Google Scholar]
- 14.Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L, Berger MS. J Neurosurg. 2015;123:325–339. doi: 10.3171/2014.10.JNS141520. [DOI] [PubMed] [Google Scholar]
- 15.Case report: emergency awake craniotomy for cerebral abscess in a patient with unrepaired cyanotic congenital heart disease. D'Antico C, Hofer A, Fassl J, Tobler D, Zumofen D, Steiner LA, Goettel N. F1000Res. 2016;5:2521. doi: 10.12688/f1000research.9722.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awake craniotomy in arteriovenous malformation surgery: the usefulness of cortical and subcortical mapping of language function in selected patients. Gamble AJ, Schaffer SG, Nardi DJ, Chalif DJ, Katz J, Dehdashti AR. World Neurosurg. 2015;84:1394–1401. doi: 10.1016/j.wneu.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 17.The experience of patients undergoing awake craniotomy for intracranial masses: expectations, recall, satisfaction and functional outcome. Manchella S, Khurana VG, Duke D, Brussel T, French J, Zuccherelli L. Br J Neurosurg. 2011;25:391–400. doi: 10.3109/02688697.2011.568640. [DOI] [PubMed] [Google Scholar]
- 18.Patients' perceptions of awake and outpatient craniotomy for brain tumor: a qualitative study. Khu KJ, Doglietto F, Radovanovic I, Taleb F, Mendelsohn D, Zadeh G, Bernstein M. J Neurosurg. 2010;112:1056–1060. doi: 10.3171/2009.6.JNS09716. [DOI] [PubMed] [Google Scholar]
- 19.Awake craniotomy. A patient`s perspective. Bajunaid KM, Ajlan AM. Neurosciences. 2015;20:248–252. doi: 10.17712/nsj.2015.3.20140548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awake craniotomy: indications, benefits, and techniques. Zhang K, Gelb AW. Revista Colombiana de Anestesiología. 2018;46:46–51. [Google Scholar]
- 21."Scalp block" during craniotomy: a classic technique revisited. Osborn I, Sebeo J. J Neurosurg Anesthesiol. 2010;22:187–194. doi: 10.1097/ANA.0b013e3181d48846. [DOI] [PubMed] [Google Scholar]
- 22.Awake craniotomy. Jones H, Smith M. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4:189–192. [Google Scholar]
- 23.Anaesthesia for awake craniotomy. Rath GP, Mahajan C, Bithal PK. J Neuroanaesthesiol Crit Care. 2014;1:173–177. [Google Scholar]
- 24.Multimodal protocol for awake craniotomy in language cortex tumour surgery. Picht T, Kombos T, Gramm HJ, Brock M, Suess O. Acta Neurochir. 2006;148:127–128. doi: 10.1007/s00701-005-0706-0. [DOI] [PubMed] [Google Scholar]
- 25.Postoperative nausea and vomiting after craniotomy for tumor surgery: a comparison between awake craniotomy and general anesthesia. Manninen PH, Tan TK. J Clin Anesth. 2002;14:279–283. doi: 10.1016/s0952-8180(02)00354-9. [DOI] [PubMed] [Google Scholar]