Abstract
The p38 group of kinases belongs to the mitogen-activated protein (MAP) kinase superfamily with structural and functional characteristics distinguishable from those of the ERK, JNK (SAPK), and BMK (ERK5) kinases. Although there is a high degree of similarity among members of the p38 group in terms of structure and activation, each member appears to have a unique function. Here we show that activation of p38γ (also known as ERK6 or SAPK3), but not the other p38 isoforms, is required for γ-irradiation-induced G2 arrest. Activation of the MKK6-p38γ cascade is sufficient to induce G2 arrest in cells, and expression of dominant negative alleles of MKK6 or p38γ allows cells to escape the DNA damage-induce G2 delay. Activation of p38γ is dependent on ATM and leads to activation of Cds1 (also known as Chk2). These data suggest a model in which activation of ATM by γ irradiation leads to the activation of MKK6, p38γ, and Cds1 and that activation of both MKK6 and p38γ is essential for the proper regulation of the G2 checkpoint in mammalian cells.
Cell cycle arrest is a common response to stress stimuli that can cause DNA damage. It is believed that cell cycle arrest has an important role in minimizing the consequences of DNA damage to cells (22). It is generally believed that G2 arrest provides cells with time to repair DNA damage before moving into mitosis (48). DNA damage and its effect on cell cycle progression have been intensively studied in yeast and Xenopus oocyte extracts as well as in mammalian cells. Such studies show that the kinase activity of Cdc2-cyclin B complex is required for the G2-to-M-phase (G2/M) transition in the normal cell cycle and that tyrosine phosphorylation of Cdc2 inhibits its kinase activity (47). Both inhibition of Cdc2 kinase activity and enhanced phosphorylation of Cdc2 have been observed following DNA damage (6, 27, 36). The phosphorylation state of Cdc2 is maintained by the kinases Wee1 and Myt1 and by the phosphatase Cdc25 (10, 39, 41, 43). Although checkpoint regulation of both sides exists, it is thought regulation of Cdc25 activity is an important factor in the maintenance of a G2 arrest after DNA damage (53). In mammalian cells, two kinases, Chk1 and Cds1 (also known as Chk2), have been identified (5, 38, 54) and shown to phosphorylate Cdc25C and prevent it from dephosphorylating and activating Cdc2 (5, 9, 16, 38, 52, 54). It is thought that phosphorylation on Cdc25 facilitates association with 14-3-3 protein, resulting in its export from the nucleus (37); however, the mechanism of Chk1 and Cds1 activation following irradiation is not clear. The response of Cds1 to DNA damage has been shown to be dependent on the activity of ATM, the gene of which is mutated in patients with ataxia telangiectasia (AT) (5, 8, 9, 38). ATM is thus upstream of the signaling pathway. Consistent with the notion that G2 arrest has a protective effect against DNA damage, patients with an ATM defect suffer enhanced sensitivity to irradiation (32). Pharmacological agents which override the G2/M block often sensitize the cells to γ radiation (30, 49); γ-radiation-induced DNA damage followed by cell death is considered to be the mechanism for cancer cell elimination by radiotherapy.
The p38 mitogen-activated protein (MAP) kinase pathway is a primary signaling pathway that is activated by stressful events such as UV irradiation. The p38 group of MAP kinases belongs to a subfamily of the MAP kinase superfamily. The prototypic member of this group, p38α (also known as p38, CSBP, or RK), was discovered as being tyrosine phosphorylated in macrophages upon treatment with bacterial lipopolysaccharide (20, 21). This protein was also identified as a specific target of a series of anti-inflammatory compounds of which SB203580 is the prototypic member (34). p38α and p38β are sensitive to inhibition by SB203580, while the activities of p38γ and p38δ are not affected by this compound (17). While the activation profiles of different p38 isoforms in response to stress are similar, an increasing body of evidence suggests that individual p38 isoforms have distinct biological functions. For example, p38α and p38β antagonize each other in cardiomyocyte hypertrophy, and p38γ has been implicated in muscle differentiation and in the response to hypoxia (33, 35, 40). Several proteins have been identified as substrates for p38α, including transcription factors such as CHOP10, MEF2C, and Sap1 in TCF, enzymes such as cPLA2, and downstream protein kinases such as MAPKAPK2/3, MNK1/2, and PRAK (for reviews, see references 46 and 50). All four members of the p38 kinase family can phosphorylate the transcription factor activating factor 2 (ATF2) in vitro (17). Like other MAP kinases, activation of p38 group MAP kinases requires specific phosphorylation by their upstream kinases. Two MAP kinase kinases, MKK3 and MKK6, have been identified as immediate upstream activators of the p38 family (13).
The involvement of MAP kinases in cell cycle arrest has been studied in a number of organisms. Perhaps due to differences in substrate specificity and regulation among the MAP kinases, their roles in cell cycle regulation appear to be different. Activation of p42MAPK is required for the G2/M transition in the maturation of Xenopus oocytes (51), and inactivation of this MAP kinase releases G2 arrest at the time of Xenopus oocyte fertilization (2). MEK2 activity was recently reported to be necessary for G2 arrest in mammalian cells (1), while MEK1 activity is required for the G2/M transition (60). BMK1 (ERK5) was reported to be required for epidermal growth factor-induced progression through S phase (29). p38α has been reported to be involved in Cdc42-induced G1 arrest as well as the spindle assembly checkpoint (42, 56).
To address the role of p38 and its isoforms in response to γ radiation, we examined activation of the components of the p38 pathway in γ-irradiated cells. The function of these signaling molecules was determined by introducing constitutively active and/or dominant negative alleles of these molecules. We report here that MKK6 and all p38 isoforms are activated by γ irradiation, that the active form of MKK6 is sufficient to arrest cells in G2, and that inhibiting MKK6 or a specific p38 isoform, p38γ, disrupts the DNA damage checkpoint. Furthermore, we find that the activation of p38γ in γ-irradiated cells is ATM dependent and that the MKK6-p38γ cascade is involved in regulating Cds1 activity. Activation of the p38 pathway ultimately leads to Cdc2 phosphorylation and subsequent G2 arrest. These data support an important interplay between the p38 pathway and G2 cell cycle checkpoint control.
MATERIALS AND METHODS
Cell culture.
Normal (GM637G; ATM+/+) and AT-deficient (GM5849C; ATM−/−) simian virus 40-transformed human fibroblasts were obtained from Coriell Institute for Medical Research, Camden, N.J. Other cell lines were obtained from the American Type Culture Collection. Cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 50 U of penicillin/ml, 50 mg of streptomycin/ml, and 1% nonessential amino acids.
γ irradiation.
Cells in six-well plates were irradiated with a single dose from a 137Cs source in a Gamma Cell 40 at a rate of 65 rads/min (18).
Adenovirus infection.
Recombinant adenoviruses were generated as described elsewhere (25). Cells were seeded into six-well plates at 2 × 105 cells per well 24 h before infection with recombinant adenovirus (103 inclusion-forming units [IFU]/cell). After the indicated time postinfection, the cells were harvested and subjected to further analysis.
Cell cycle analysis.
Cells were harvested, washed, resuspended in phosphate-buffered saline (PBS), and fixed for >18 h after the stepwise addition of ice-cold absolute ethanol. Cells were resuspended in RNase A (1 mg/ml) for 30 min at 37°C and then stained with propidium iodide (PI; 0.05 mg/ml) for 1 h on ice. Fluorescence-activated cell sorting (FACS) analysis was performed with a FACS IV flow cytometer (Becton Dickinson, San Jose, Calif.). Cells were excited at 488 nm, and the emission was collected above 590 nm.
Mitototic index assay.
Immediately after γ irradiation or adenovirus infection, cells were treated with nocodazole (100 ng/ml), Calbiochem, Calif. for 14 h. Then cells were fixed in 70% ethanol and stored at 4°C for 24 h before staining with Hoechst DNA stain (Molecular Probes). Mitotic index was scored under a fluorescence microscope.
Pulse-chase labeling.
Ninety percent confluent cultures of HeLa and U2OS cells in six-well plates were labeled with Trans-[35S]Label ([35S]Met-Cys; Amersham) for 1 h before exposure to radiation. The cells were then chased for the times indicated by culture in medium containing excess unlabeled Met-Cys but no [35S]Met-Cys, and lysed Cdc25C was immunoprecipitated with anti-Cdc25C antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) and protein G resin and analyzed by polyacrylamide gel electrophoresis (PAGE).
Clonogenic survival assay.
Cells were detached, suspended as single-cell suspensions, and counted. Clonogenic survival was determined by plating cells in growth medium plus 0.3% Noble agar. After 2 weeks, plates were fixed and stained with 0.01% crystal violet in 1.5% acetic acid, and colonies larger than 50 cells were counted. The number of survival colonies in nonirradiated samples is taken as 100%.
In vitro kinase assay.
Kinase reactions were performed in vitro, using [γ-32P]ATP as described previously (13, 26). Recombinant fusion proteins of activating transcription factor 2 (glutathione S-transferase [GST]–ATF2) and an inactive form of p38 [GST-p38(KM)] were used as the substrates for p38 and MKK6, respectively. A synthetic peptide corresponding to residues 208 to 225 of Cdc25C was used as the substrate for human Cds1. The levels of peptide phosphorylation were determined as follows. The reaction mixture was applied onto Whatman P-81 phosphocellulose paper. The filters were washed twice in 1 mM acetic acid followed by 4 mM sodium pyrophosphate. The remaining radioactivity was quantified by liquid scintillation counting. The phosphorylation of protein substrates was analyzed by sodium dodecyl sulfate (SDS)-PAGE as described elsewhere (13); enzyme activity was determined by autoradiography or phosphorimaging.
Immunocomplex kinase assay.
Kinases were immunoprecipitated from cells lysed in buffer containing 20 mM Tris-HCl, 120 mM NaCl, 10% glycerol, 1 mM Na3VO4, 2 mM EDTA, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride (pH 7.5) for 15 min at 4°C (13, 26). Insoluble material was removed by centrifugation at 13,000 × g for 10 min at 4°C. Then 3 to 5 μl of antibody for MAPK or MKK or 1 μl for Cds1 was added, and the mixture was incubated overnight at 4°C; 40 μl of protein A-Sepharose (1:1 slurry in PBS) was added, and the tube was rotated for 2 h at 4°C to allow the protein A to bind to the antibody. The immunoprecipitate was then washed five times with PBS containing 1% Triton X-100 and used as enzyme in the in vitro kinase assay described above.
Recombinant proteins.
GST-ATF2 and GST-Cds1 fusion proteins were expressed in Escherichia coli BL21(DE3) and purified using glutathione-Sepharose 4B beads (Pharmacia Biotech, Uppsala, Sweden). All of the His6-tagged recombinant proteins were expressed in E. coli BL21(DE3) and purified using a Ni-nitrilotriacetic acid purification system (Qiagen). His6-Cds1 generated from recombinant baculovirus-infected Sf9 cells has been described previously (5). Western blotting was performed as described elsewhere (20). Anti-Cdc2 and anti-phospho-Cdc2 antibodies were from New England Biolabs (NEB), Beverly, Mass. Anti-Cdc25C antibody was from Santa Cruz Biotechnology. Antiactin antibody was from Sigma, St. Louis, Mo.
Northern blotting.
Total RNA was extracted from U2OS cells using Trizol reagent from Gibco BRL, Gaithersburg, Md. Northern blotting and hybridization were performed by standard methods. cDNA against human Cdc25C mRNA was generated from the coding region of human Cdc25C cDNA by PCR.
Western blotting.
The cell lysates or immunoprecipitates were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. Polyclonal antibodies raised in rabbits were used. Dilutions of the first antibodies are as follow: anti-p38α/β, 1:5,000; anti-p38γ, 1:2,000; anti-p38δ, 1:1,000; antiactin (Sigma), 1:1,000; anti-phospho-Cdc2 (NEB, Beverly, Mass.), 1:1,000; and anti-Cdc2 (NEB), 1:1,000.
Reporter gene assay.
The GAL4-responsive plasmid pG5E1bLuc contains five GAL4 sites cloned upstream of a minimal promoter driving a luciferase gene. Expression plasmids encoding the GAL4 DNA binding domain fused with the activation domain of MEF2C, ATF2, ELK1, or c-Jun were described previously (61). Cells were grown on 35-mm-diameter multiwell plates and transiently transfected with 1 μg of total plasmid DNA using Lipofectamine reagent (Gibco BRL). A β-galactosidase expression plasmid (pCMV-β-gal; Clontech, Palo Alto, Calif.) was used to control for transfection efficiency. The total amount of DNA for each transfection was kept constant by using the empty vector pcDNA3. Cell extracts were prepared 36 h later, and β-galactosidase and luciferase activities were measured. In the experiments to evaluate the inhibitory effect of inactive MKKs, the cells were infected with recombinant adenovirus 12 h before the transfection. A stimulation with serum or tumor necrosis factor was applied 24 h after transfection for 12 h.
RESULTS
Activation of the p38 MAP kinase pathway by γ irradiation.
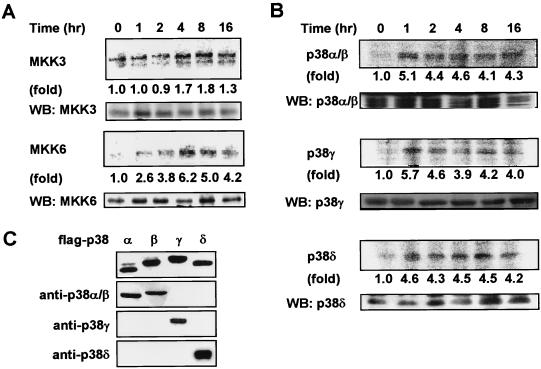
To evaluate the role of the p38 pathway in γ-irradiation-stimulated cells, we initially examined the activation of different components of the p38 pathway. The activation of the MAP kinase kinases in this cascade, MKK3 and MKK6, was determined in U2OS cells (a human osteosarcoma cell line) exposed to γ radiation. The cells were treated with 6 Gy of γ radiation and harvested at different time points postirradiation. MKK3 or MKK6 was immunoprecipitated by specific antibodies as described elsewhere (25). The immunoprecipitates were used to phosphorylate p38(KM) in in vitro kinase assays. As shown in Fig. 1A, the activation of MKK6 was detected 1 h following irradiation and was maintained for 16 h. In contrast, only moderate activation of MKK3 was observed under the same conditions, and the activation was much delayed. A similar approach was used to study the activation of the p38 isoforms by γ radiation. p38 isoforms were immunoprecipitated with isoform-specific antibodies after irradiation, and a kinase assay was performed with ATF2 as substrate. Figure 1B shows thatp38α/β, p38γ, and p38δ are activated by γ radiation in similar time frames. The activation was seen 1 h following irradiation and remained elevated for at least 16 h, correlating with the kinetics of MKK6 activation. The specificity of the antibodies used in the experiments is shown in Fig. 1C. Similar activation of MKK6 and p38 isoforms was seen when HeLa cells were used (data not shown). The activation of p38 isoforms observed here is most likely to be downstream of MKK6 and MKK3, while MKK6 appears to be primarily responsible for the activation. The prolonged activation of p38s seen in response to γ radiation contrasts with that produced by other stimuli, such as cytokines, in which case p38 activation is transient (17, 46).
FIG. 1.
γ irradiation activates the MKK6-p38 pathway. (A) U2OS cells were treated with 6 Gy of γ irradiation, and MKK3 and MKK6 were immunoprecipitated at the indicated times postirradiation. Kinase activities of MKK3 and MKK6 were determined by kinase assay using GST-p38(KM) as substrate. Western blotting (WB) was used to ensure that equivalent amounts of kinase were used in all samples (lower panel). (B) Time course of γ-irradiation-induced activation of p38α/β, -γ, and -δ by immunocomplex kinase assay. The kinase activities of p38s isolated by immunoprecipitation with p38 isoform-specific antiserum were measured with GST-ATF2(1-109) as substrate. Again, equal loading of kinase was ensured by Western blotting (lower panel). Fold activation was calculated by dividing the radioactive intensity of phosphorylated p38(KM) (A) or ATF2 (B) at different time points by that at time zero. Similar results were obtained in two experiments. (C) Specificity of isoform-specific antibodies was determined as follows. Flag-tagged p38α, p38β, p38γ, and p38δ were expressed in 293 cells. The expression of these epitope-tagged proteins was determined by Western blotting using anti-Flag monoclonal antibody M2 (top panel). p38α/β, p38γ, or p38δ was immunoprecipitated using the isoform-specific antibodies from the cell lysates of 293 cells that had been transiently transfected with different Flag-tagged p38 isoforms. The immunoprecipitates were analyzed by Western blotting using anti-Flag monoclonal antibody M2.
Constitutive activation of MKK6 leads to G2 arrest.
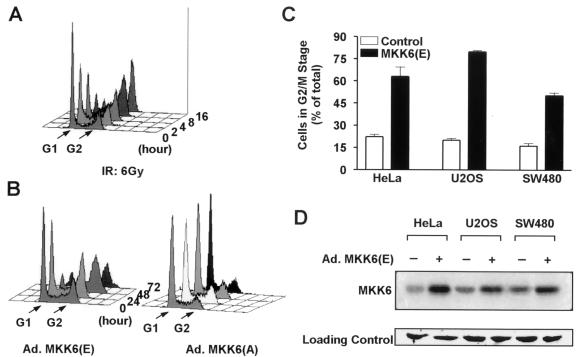
Since the p38 pathway is activated by γ irradiating cells and γ radiation can cause G2 arrest of many different cells, it is possible that γ radiation induced activation of the p38 pathway contributes to G2/M checkpoint control. We therefore tested whether activation of the p38 pathway can mimic γ radiation in inducing a G2 arrest. We used Ad.MKK6(E), the constitutively active form of MKK6 encoded by recombinant adenovirus, to activate the p38 pathway without extracellular stimuli (25). The adenovirus gene delivery system reached >95% infection efficiency without influencing cell viability or the cell cycle in our experimental cell lines (data not shown). U2OS cells were cultured in six-well plates and treated with γ radiation, Ad.MKK6(E), or Ad.MKK6(A) (adenovirus-encoded inactive MKK6). The cell cycle phase distribution of cells was determined by FACS analysis; as shown in Fig. 2A, cells accumulated with a 4N DNA content after γ irradiation. Cells infected with Ad.MKK6(E) also accumulated with a 4N DNA content (Fig. 2B). The number of cells with 4N DNA started to increase at 24 h after Ad.MKK6(E) infection, and concomitant reduction of cells in G1 phase was seen. The increase in cells with a 4N DNA content followed expression of MKK6(E), which was detectable at 16 h postinfection (data not shown). Cell morphology was examined by light microscopy. Cells infected with Ad.MKK6(E) showed morphological changes similar to those of cells γ irradiated and were typically enlarged and flattened with almost no dividing cells. Thus, the accumulation of cells with 4N DNA is unlikely to be due to an increase in M-phase cells. This was further confirmed using the mitotic index assay (data not shown). Infection with Ad.MKK6(A) (Fig. 2B) or adenoviruses encoding green fluorescent protein (Ad.GFP) (data not shown) had no effect on the cell cycle profile or on cell morphology (data not shown). Thus, both γ radiation and MKK6(E) expression induce G2 cell cycle arrest.
FIG. 2.
Both γ irradiation and MKK6(E) expression induce G2 arrest. (A) U2OS cells were collected and fixed at the indicated times after 6 Gy of γ irradiation (IR), and the cell cycle profile was determined by FACS analysis using PI staining. The graph was generated using CellQuest software. (B) U2OS cells were seeded in six-well plates at a density of 2 × 105 cells/well and infected with Ad.MKK6(E) or Ad.MKK6(A) at 1,000 IFU/cell 24 h after plating. Cells were then collected for cell cycle analysis at the indicated times postinfection. (C) HeLa, U2OS, and SW480 cells were infected with either Ad.GFP (control) or Ad.MKK6(E), and cell cycle analysis was performed 48 h postinfection. The percentage of cells in the G2/M stage was calculated using MetLight software. Data are means ± standard errors of three independent observations. (D) Western blotting analysis of adenovirus-mediated MKK6 gene expression. HeLa, U2OS, or SW480 cells were infected with Ad.MKK6(E) at 1,000 IFU/cell 24 h after plating. The cells were lysed 16 h postinfection, and a Western analysis was performed using anti-MKK6 polyclonal antibody. The expression of adenovirus-encoded MKK6 was evidenced by the increase of total MKK6 protein. Equal loading of samples was shown by staining the membrane with Ponceau S. A portion of the membrane is shown at the bottom.
Ad.MKK6(E) infection of HeLa cells and SW480 cells (Fig. 2C) also leads to an increase in cells with a 4N DNA content. Figure 2D shows the expression of Ad.MKK6(E) detected by Western blotting. The tendency of cells to arrest in G2 differed slightly between cell lines, although all three lines demonstrated significant G2 arrest after MKK6(E) expression. While U2OS cells express wild-type p53 (24), HeLa cells and SW480 colon cancer cells are p53 deficient. Curiously, U2OS showed the highest percentage of G2-arrested cells. Whether p53 status plays a role in determining the level of MKK6-mediated G2 arrest is unknown. Similar experiments were performed with Ad.MKK3(E), which can cause G2 arrest but to a much lesser extent compared to MKK6(E)-treated cells (data not shown). Since γ irradiation and MKK6(E) expression induce G2 arrest (Fig. 2), and since the p38 pathway is activated by γ radiation (Fig. 1), it is likely that MKK6 is an intermediate component in the signal transduction pathway by which γ irradiation induces a G2 arrest.
The MKK6-p38γ cascade is required for G2 arrest in response to DNA damage.
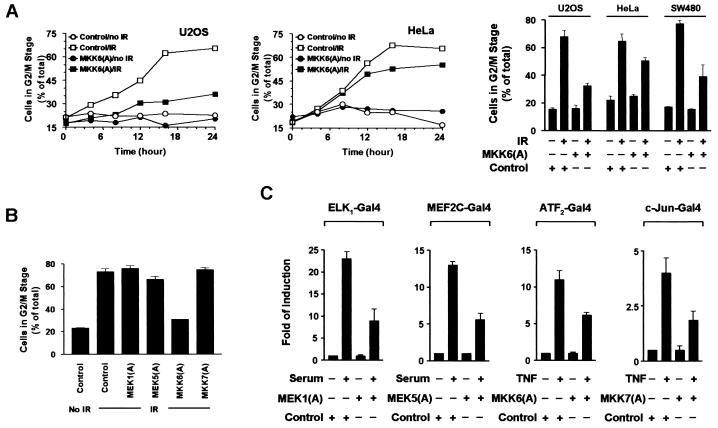
Activation of the p38 pathway is sufficient for G2 arrest (Fig. 2). We therefore tested whether activation of the p38 pathway is required for the G2 arrest induced by γ irradiation. We first determined whether MKK6 activation is required for G2 arrest. U2OS or HeLa cells were infected with Ad.MKK6(A) and treated 24 h later with 6 Gy of γ radiation. The cell cycle profile was analyzed at different time points as shown in Fig. 3A. γ radiation induces a time-dependent increase of cells with a 4N content of DNA in U2OS, SW480, and HeLa cultures. Expression of MKK6(A) prior to γ irradiation significantly reduced the extent of G2 arrest seen in U2OS cells (left panel); the inhibitory effect of MKK6(A) on G2 arrest was also seen in HeLa cells (middle panel) but less than in U2OS cells. A similar experiment was performed using U2OS, HeLa, and SW480 cells, except that triplicate samples were analyzed at a single time point (right panel). MKK6(A) expression interfered with γ-irradiation-induced G2 arrest in all three cell lines. The inhibitory effect of MKK6(A) on γ-irradiation-induced G2 arrest was further supported by the observation that MKK6(A) expression did not have a noticeable effect on the normal cell cycle without γ irradiation (Fig. 3A), and infection of cells with Ad.GFP had no influence on the cell cycle progress of either γ-irradiation-treated or untreated cells. The effect of MKK3(A) expression on γ-irradiation-induced G2 arrest was tested. Despite comparable expression of MKK3(A) and MKK6(A), MKK3(A) expression had no detectable effect on γ-irradiation-induced G2 arrest (data not shown). These data suggest that MKK6 activity is necessary for G2 arrest induced by γ irradiation.
FIG. 3.
MKK6 is necessary for γ-irradiation-induced G2 arrest. (A) U2OS or HeLa cells were seeded at 2 × 105/well in six-well plates and infected with Ad.GFP (control) or Ad.MKK6(A) for 24 h before being treated with 6 Gy of γ radiation. Cells were then collected at the indicated time points, and the cycle profile was analyzed using PI staining and flow cytometry (left and middle panels). U2OS, HeLa, or SW480 cells were seeded at 2 × 105/well in six-well plates and infected with Ad.GFP (control) or Ad.MKK6(A) for 24 h before being treated with 6 Gy of γ radiation (IR). Cells were then collected at 16 h after γ irradiation, and the cycle profile was analyzed using PI staining and flow cytometry (right panel). Data with error bars are means ± standard errors of triplicate samples. (B) U2OS cells were infected with the indicated forms of Ad.MKK(A) for 24 h and then treated with 6 Gy of γ radiation; 24 h after irradiation, G2 arrest was analyzed. (C) Inhibitory effects of different inactive MKK(A)s on endogenous MKKs. U2OS cells were infected with recombinant adenovirus encoding GFP (control), MEK1(A), MEK5(A), MKK6(A), or MKK7(A). The cells were replaced with serum-free medium 12 h after infection. The infected cells were then cotransfected with β-galactosidase expression vector pCMVβ, reporter plasmid pG5E1bluc, or an expression vector for the GAL4 DNA binding domain fused with the transactivation domain of ELK1, MEF2C, ATF2, or c-Jun as indicated. Empty vector pcDNA3 was used to normalize the total DNA to 1 μg per transfection. Cells were stimulated with 20% fetal bovine serum or tumor necrosis factor alpha (100 ng/ml) 24 h following transfection for 12 h. Luciferase and β-galactosidase activities in the cell lysates were measured. The ratio of luciferase activity to β-galactosidase activity is presented as mean ± standard deviation (n = 3).
To test whether accumulation of G2-arrested cells is a general functional feature of all MAP kinase pathways, we examined the effect of inhibiting the ERK, JNK, and ERK5/BMK pathways on γ-radiation-induced G2 arrest. Adenoviruses encoding inactive forms of MEK1, MEK5, and MKK7 were used to impede the ERK, BMK, and JNK pathways, respectively. U2OS cells were irradiated with 6 Gy of γ radiation 24 h after virus infection and harvested 24 h later for cell cycle analysis. As shown in Fig. 3B, expression of MEK1(A), MKK7(A), or MEK5(A) did not influence the G2 arrest induced by γ radiation. The expression of these mutants and their ability to interfere with their respective MAP kinase pathways has previously been demonstrated (12, 28). Analysis of reporter genes transcription was used to demonstrate that ∼50% inhibition of endogenous genes was obtained in these experiments (Fig. 3D). Thus, our data suggested no requirement for MEK1, MKK7, or MEK5 in γ-radiation-induced G2 cell cycle arrest and support the hypothesis that the inhibitory effect of MKK6(A) on G2 arrest is specific.
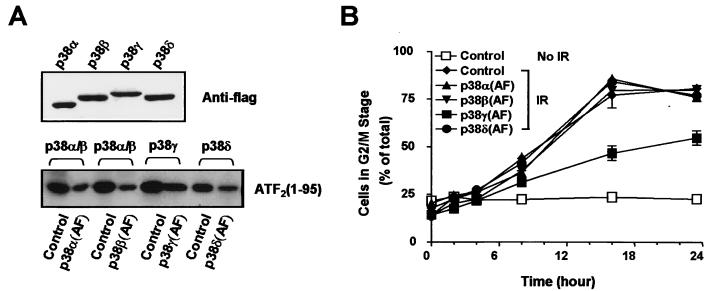
Four isoforms of p38 were activated by γ irradiation (Fig. 1). We therefore examined which p38 isoforms are required for γ-irradiation-induced G2 arrest. Inactive forms of the different p38 isoforms, in which the phosphorylation sites TGY were mutated to AGF (AF mutation), were expressed as described above using an adenovirus-mediated delivery system. Expression of these proteins was determined to be comparable by Western blotting (Fig. 4A, top panel). The ability of these mutants to inhibit endogenous p38 MAP kinases was determined by immunocomplex kinase assay as shown in Fig. 4A. Approximately 50% inhibition of endogenous p38s activity was observed in U2OS cells. Cells were irradiated with 6 Gy of γ radiation 24 h after virus infection, and the DNA contents were analyzed at different times after γ irradiation. As shown in Fig. 4B, only Ad.p38γ(AF) was able to attenuate the γ-irradiation-induced G2 arrest. Infection with control virus expressing GFP or virus expressing the AF mutant of p38α, -β, or -δ had no effect. We also used SB203580, an inhibitor of p38α/β, to test the role of these two p38 isoforms in γ-irradiation-induced G2 arrest. SB203580 had no influence on G2 arrest even at a very high concentration (50 μM). Collectively, our data place p38γ as a downstream kinase of MKK6 in mediating the G2 arrest.
FIG. 4.
p38γ is involved in γ-radiation-induced G2 arrest. (A) U2OS cells were infected with Ad.GFP (control) or adenoviruses expressing an inactive form of p38 as indicated and were γ irradiated 24 h later. The percentage of cells in the G2/M stage was analyzed at the indicated times postirradiation. Data with error bars are means ± standard errors of triplicate observations; data from two experiments with similar results were obtained. (B) Inhibitory effect of p38α(AF), p38β(AF), p38γ(AF), or p38δ(AF) expression on endogenous p38 activity. U2OS cells were infected with Ad.GFP (control) or Ad.p38 as indicated; 24 h later, the cells were treated with γ irradiation (IR; 6 Gy). The expression of p38(AF) mutant was determined by a Western blotting analysis using anti-Flag antibody M2 (top panel). The kinase activities of p38α/β in Ad.GFP (control)- or Ad.p38α(AF)-infected cells, p38α/β in Ad.GFP (control)- or Ad.p38β(AF)-infected cells, p38γ in Ad.GFP (control)- or Ad.p38γ(AF)-infected cells, and p38δ in Ad.GFP (control)- or Ad.p38δ(AF)-infected cells were determined by immunocomplex kinase assay 6 h after irradiation using GST-ATF2(1-109) as substrate.
Activation of p38γ is ATM dependent.
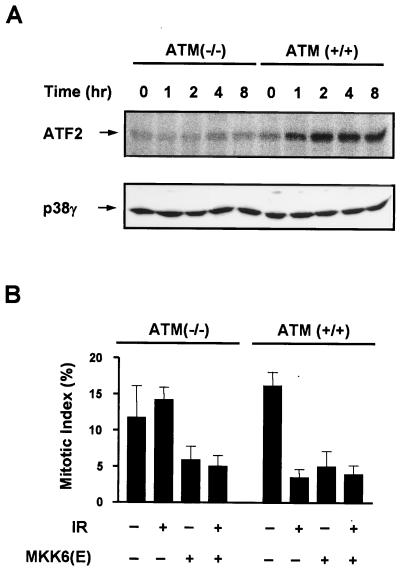
It is believed that DNA damage is a key initiator of γ-irradiation-induced cellular responses. ATM is thought to promote cell survival in the face of DNA damage by activating a number of cellular functions including a G2 cell cycle arrest. Therefore, we tested whether the activation of p38γ has any relationship with ATM. ATM+/+ or ATM−/− human fibroblasts were irradiated with 6 Gy of γ radiation, and the activity of p38γ was measured by immunocomplex kinase assay as described above. The p38γ isoform was activated in ATM+/+ cells but not in ATM−/− cells (Fig. 5A). UV-induced activation of p38γ was normal in ATM−/− cells (data not shown), which is similar to the JNK activation by γ irradiation and UV observed in ATM+/+ and ATM−/− cells (55). Thus, activation of the p38 pathway by γ irradiation is downstream of ATM.
FIG. 5.
p38γ activation by γ irradiation is ATM dependent. (A) Both ATM+/+ and ATM−/− human fibroblasts were seeded into 6-cm-diameter dishes and treated with 6 Gy of γ radiation. p38γ was immunoprecipitated from the cell lysates collected at the indicated time points after irradiation. p38γ activities were measured in kinase assays using GST-ATF2(1-109) as substrate. Equivalent loading of p38γ in the γ-irradiated samples was confirmed by Western blotting using anti-p38γ antibody. (B) Both ATM+/+ and ATM−/− human fibroblasts were seeded into 6-cm-diameter dishes and infected with Ad.GFP (control) or Ad.MKK6(E) at 10,000 IFU/cell 24 h after plating. The cells were treated with or without γ radiation (IR) 24 h after infection. Mitotic index was determined 24 h postirradiation as described in Materials and Methods. Data with error bars are means ± standard errors of triplicate samples.
Since it appears that the MKK6-p38γ cascade is downstream of ATM following γ irradiation, we tested whether G2 arrest can be induced in ATM−/− cells by MKK6(E) expression. ATM+/+ and ATM−/− human fibroblasts were irradiated with 6 Gy of γ radiation with or without preinfection with Ad.MKK6(E). G2 arrest was determined by mitotic index assay as shown in Fig. 5B. γ irradiation induced G2 arrest in ATM+/+ cells but not ATM−/− cells, while MKK6(E) caused cell cycle arrest in both cell types. This result supported the idea that MKK6-p38γ is downstream of ATM in mediating a G2 arrest.
Activation of MKK6-p38γ cascade influences Cds1 activity indirectly.
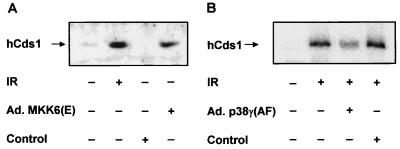
There are several reports regarding the function of p38γ (11, 23, 33, 35, 40). However, available data on p38γ provide little information regarding the mechanisms by which p38γ regulates G2 arrest. Genetic studies on yeast cell cycle checkpoint control identified Chk1 as a necessary component of γ-irradiation-induced G2 arrest (57), and Cds1 is required for a DNA replication checkpoint (44). In mammalian cells, both Chk1 and Cds1 are thought to be involved in G2 arrest after γ irradiation (5, 38, 54). To determine whether Cds1 is a downstream target of activated p38γ, we examined the activation of Cds1 in cells that had been infected with constitutively active MKK6 or dominant inactive p38γ. Cds1 autophosphorylation is activated in response to γ irradiation (5, 38), and we used this assay system to determine if Cds1 is activated by MKK6(E). Cds1 was immunoprecipitated from U2OS cells 24 h after infection with either Ad.MKK6(E) or control virus Ad.GFP, or from cells 1 h after γ irradiation. As shown in Fig. 6A, enhanced autophosphorylation of Cds1 shows that both γ irradiation and MKK6(E) activate Cds1. Similar results were obtained from HeLa cells (data not shown). When U2OS cells were preinfected with Ad.p38γ(AF) for 24 h prior to γ irradiation, Cds1 activation was significantly attenuated (Fig. 6B). Control virus expressing GFP had no effect. These data suggest that the MKK6-p38γ cascade participates in Cds1 activation by γ irradiation.
FIG. 6.
MKK6(E) activates Cds1 in vivo. (A) U2OS cells were collected and lysed 24 h after adenovirus infection as indicated or 1 h after treatment with 6 Gy of γ irradiation (IR). (B) U2OS cells were first infected with adenovirus as indicated for 24 h before γ irradiation. Human Cds1 (hCds1) was immunoprecipitated, autophosphorylated in vitro using [γ-32P]ATP, and visualized by SDS-PAGE. Similar results were obtained from three independent experiments.
γ irradiation induces a modification of human Chk1 that can be detected as an increase in electrophoretic mobility on SDS-PAGE (54). To determine if Chk1 is also regulated by MKK6(E) expression, U2OS cells were transfected with hemagglutinin-tagged human Chk1 cDNA and then infected with Ad.MKK6(E) or Ad.GFP. Recombinant Chk1 protein was visualized by Western blotting. No Chk1 band shift was observed on MKK6(E) expression (data not shown), suggesting that Chk1 may not be regulated by the MKK6-p38γ cascade.
Since dominant active MKK6 expression produces upregulation of Cds1 activity, it is possible that Cds1 lies directly downstream of the MKK6-p38γ pathway. To examine this possibility, we tested whether p38γ can directly regulate Cds1 activity. Recombinant His-tagged human Cds1, p38γ, nd MKK6(E) were prepared, and coupled kinase assays with MKK6(E), p38γ, Cds1 and a synthetic peptide corresponding to residues 208 to 225 of Cdc25C were performed (7). MKK6(E) was included in the reaction mixture to activate p38γ, with the peptide being a substrate for Cds1. No significant increase in Cds1 activity was detected despite strong p38γ activity (measured using GST-ATF2 as a substrate [data not shown]). Similar results were obtained both with bacterially and baculovirally expressed Cds1. Since recombinant Cds1 had a measurable basal activity toward Cdc25C, the negative result was probably not due to misfolding of recombinant protein. Thus, in vivo activation of Cds1 by p38γ may be indirect.
MKK6 activation leads to enhanced phosphorylation on Cdc2.
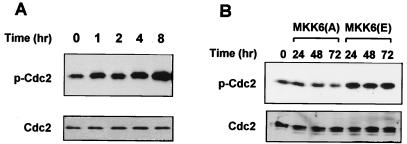
Activation of the Cdc2-cyclin B complex is a key event controlling the G2/M transition. G2 arrest requires inhibitory tyrosine phosphorylation of Cdc2 (6, 27). Therefore, the phosphorylation state of Cdc2 in the cells subject to γ irradiation or Ad.MKK6(A) or Ad.MKK6(E) infection was determined. Both γ irradiation (Fig. 7A) and MKK6(E) (Fig. 7B) increased the phosphorylation levels of Cdc2 as determined by antibody specific to phosphorylated Cdc2. Constitutive activity of MKK6(E) can maintain the phosphorylation stage of Cdc2 for several days (Fig. 7B). In contrast, no increase in Cdc2 phosphorylation was observed upon expression of MKK6(A) (Fig. 7B).
FIG. 7.
Both γ radiation and MKK6(E) lead to Cdc2 phosphorylation. U2OS cells were subjected to 6 Gy of γ radiation (A) or infected with either Ad.MKK(E) or Ad.MKK6(A) (B). Cells were collected at the indicated times after treatment, and the level of phosphorylation on Cdc2 was detected by Western blotting with anti-phospho (p)-Cdc2 antibody. Blots were stripped and reprobed with anti-Cdc2 antibody to confirm equal protein loading.
γ irradiation and MKK6 activation downregulate Cdc25C levels.
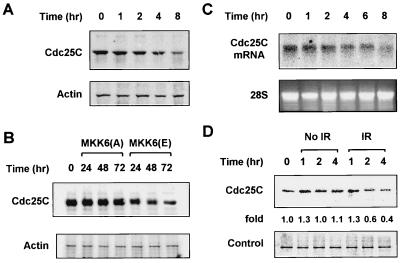
To evaluate any relationship between MKK6 activation and the mitotic inducer Cdc25C, Cdc25C protein levels were measured by Western blotting. Following γ irradiation, a significant reduction in Cdc25C protein level was observed (Fig. 8A and B). The kinetics of Cdc25C reduction following γ irradiation correlated with the enhanced phosphorylation of Cdc2 (Fig. 7A) and the G2 arrest (Fig. 2A). This was also true for cells expressing MKK6(E). Expression of MKK6(A) had no effect on the Cdc25C level. Therefore, the reduction of the Cdc25C level is linked to activation of MKK6-p38 or γ radiation and is not a consequence of nonspecific metabolic changes in infected cells. To determine the cause of the reduction of Cdc25C, we measured Cdc25C mRNA levels and protein stability. As shown in Fig. 8C, Northern blotting revealed a time-dependent reduction of steady-state Cdc25C mRNA levels after γ irradiation, which may account for the change seen in protein expression. The stability of Cdc25C protein was also decreased in cells treated with γ radiation based on the pulse-chase labeling experiments (Fig. 8D), suggesting that a decrease in Cdc25C stability may also contribute to the reduction of Cdc25C caused by activation of the MKK6-p38γ cascade. The decrease in Cdc25C levels was seen 4 h postirradiation. It may therefore contribute to the maintenance of the G2 arrest in cells which have arrested initially due to inactivation or sequestration of Cdc25 (5, 52).
FIG. 8.
Reduction of Cdc25C level after γ irradiation or MKK6(E) expression. U2OS cells were treated with 6 Gy of γ radiation (A) or Ad.MKK6(E) (B), and Cdc25C levels were analyzed by Western blotting at the indicated time points after treatment. The blots were stripped and reprobed with antiactin antibody to show even loading. (C) A 20-μg aliquot of total RNA from U2OS cells treated with 6 Gy of γ radiation was separated on an agarose gel, and Cdc25C mRNA levels were detected by Northern blotting. Levels of 28S rRNA were shown to indicate even loading. (D) U2OS cells were prelabeled with [35S]Cys-Met for 1 h and γ irradiated. Cells were then chased with excess unlabeled Met-Cys for the indicated times before labeled Cdc25C was immunoprecipitated from the cell lysate and visualized by SDS-PAGE. A band that was nonspecifically pulled down was used as a loading control. The experiment was repeated three times with similar results.
Blocking the MKK6-p38γ cascade sensitizes cells to radiation-induced cell killing.
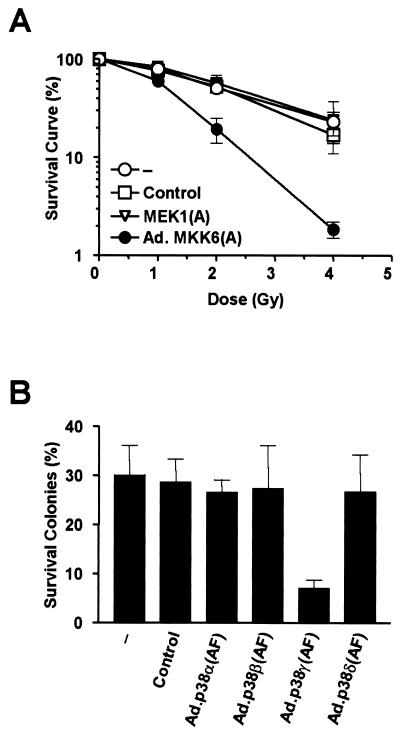
Since we have shown that blocking the MKK6-p38γ cascade impedes cell cycle arrest induced by γ irradiation, we tested whether inhibiting MKK6 or p38γ would sensitize cells to γ radiation. Cells that fail to repair damaged DNA before going into mitosis may have chromosomal strand breaks and encounter disruption in subsequent cell cycles. This is reflected by their inability to form colonies. A colony survival assay was performed using cells infected with either Ad.GFP, Ad.MKK6(A), or Ad.MEK1(A) before γ irradiation. Serial dilutions of cells were cultured until colonies were large enough to be counted (>50 cells per colony). γ radiation reduced the number of colonies formed in a dose-dependent manner, demonstrating its cytotoxic effect. However, cells infected with Ad.MKK6(A) dramatically reduced the number of colonies formed after γ irradiation in comparison with mock-infected cells or cells with Ad.GFP or Ad.MEK1(A) (Fig. 9A). No effect of adenovirus infection was observed on colony formation without γ irradiation. Blocking p38γ activity in infecting cells with Ad.p38γ(AF) also sensitized cells to the killing effects of γ radiation, as shown in Fig. 9B; 4 Gy of γ radiation had a significantly higher cytotoxic effect on Ad.p38γ(AF)-infected cells than on cells infected with Ad.p38α(AF), Ad.p38β(AF), Ad.p38δ(AF), or Ad.GFP. These data clearly demonstrate that interfering with the MKK6-p38γ cascade sensitizes tumor cells to γ radiation.
FIG. 9.
Inhibition of the MKK6-p38γ pathway enhances γ-radiation-induced cell death. U2OS cells were seeded in serial dilution and treated with nothing (−), Ad.GFP (control), Ad.MKK6(A), or Ad.MEK1(A) (A) or with Ad.p38α(AF), Ad.p38β(AF), Ad.p38γ(AF), or Ad.p38δ(AF) (B) for 24 h before being exposed to the indicated doses of γ radiation (4 Gy in panel B). Cells were cultured, and colonies larger than 50 cells were counted. Survival was calculated by comparison to the survival of nonirradiated cells (taken as 100%). Data shown are means ± standard errors of triplicate observations.
DISCUSSION
Essential role of the MKK6-p38γ cascade in signaling G2 arrest.
In this report we have shown that the MKK6-p38γ cascade is activated by γ irradiation (Fig. 1) and that this activation is not only necessary (Fig. 3 and 4) but also sufficient (Fig. 2) for G2 arrest following DNA damage. Thus, the MKK6-p38γ cascade forms an essential part of the signaling pathway leading to G2 arrest. Although MKK6 appears to be the primary MKK for activating p38s in γ-irradiated cells, the modest activation of MKK3 may have an additive effect on the activation of p38 group members. It was recently reported that expression of dominant negative MEK2, but not MEK1, leads to slightly delayed G2/M arrest upon γ irradiation and a substantial inability to progress through the G2 arrest upon recovery from the radiation exposure (1). We have also shown that MEK1 is not required for G2 arrest (Fig. 3B). However, MEK1 was reported to be required for the G2/M transition in normal cell cycle progression (60). MEK1 and MEK2 are closely related homologues. Functional differences between MEK1 and MEK2 in cell cycle regulation are analogous to the respective roles of p38α and p38γ in spindle assembly and G2/M checkpoint control. Although there are no data indicating whether MEK2 activation is sufficient for G2 arrest, the influence of dominant negative MEK2 on γ-radiation-induced G2 arrest suggests the involvement of other MAP kinases in this process. It would be useful to investigate the relationship between the MKK6-p38γ cascade and MEK2. The ERK cascade is independent of the p38 pathway; therefore, it is unlikely that MKK6-p38γ and MEK2 are in the same cascade. However, the possibility of cross talk cannot be formally excluded.
The ability of MKK6 activation to produce G2 arrest in p53-deficient cells suggests that the p53 is not essential for the MKK6-p38γ-mediated G2 arrest. However, the possibility that p53 contributes to the MKK6-p38γ G2 response cannot be excluded. Although there is a paucity of information concerning activation of the MKK6-p38γ cascade under different physiological conditions, there is evidence that this cascade can be activated by a variety of stimuli. However, not all of the stimuli that activate MKK6-p38γ can cause cells to arrest at G2/M phase. Prolonged activation of the MKK6-p38γ cascade may be a feature that distinguishes γ-radiation-induced activation from other stimuli.
Interaction of the MKK6-p38γ cascade with the DNA damage checkpoint machinery.
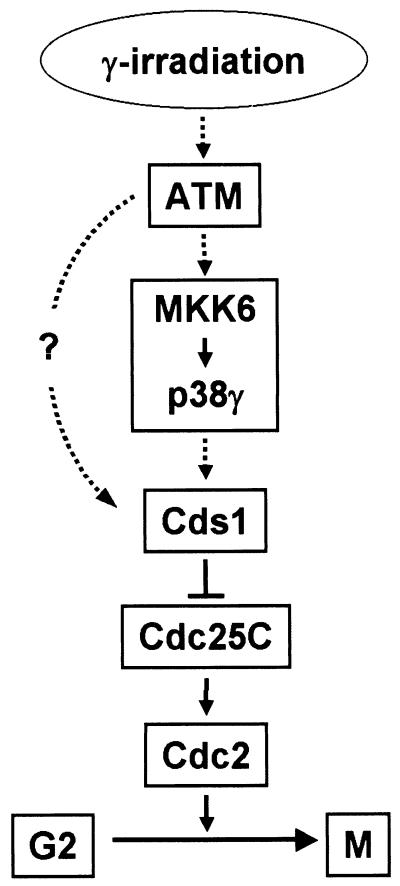
Several proteins are required for the DNA damage checkpoint control machinery in mammalian cells. ATM is required for G2 arrest after γ irradiation and other checkpoint components, such as Cds1, lie downstream of ATM. Since p38γ activation is ATM dependent and the activation of the MKK6-p38γ cascade leads to Cds1 activation, we propose that the MKK6-p38γ response is located between ATM and Cds1. A schematic diagram is shown in Fig. 10. MKK6-p38γ lies downstream of ATM in response to γ-radiation-induced DNA damage. Cds1 is activated in a MKK6-p38γ- and ATM-dependent manner and, together with Chk1, downregulates Cdc25C activity by direct phosphorylation. Reduced activity of Cdc25C and a subsequent increase in Cdc2 phosphorylation lead to cell cycle arrest at G2. Although our data suggest that MKK6-p38γ lies upstream of Cds1 activation, this model does not exclude the possibility that other pathways also regulate Cds1 activity.
FIG. 10.
The MKK6-p38γ pathway participates in the DNA damage checkpoint. See text for details.
Cdc25C, as a core regulator in DNA damage checkpoint control, is a target of both Cds1 and Chk1 (5, 16, 38, 52, 54). The current model for Cdc25C regulation in response to DNA damage is that phosphorylation of Cdc25C by Chk1 and/or Cds1 allows 14-3-3 binding, which facilitates export of Cdc25C from the nucleus (37). Inactivation of Cdc25C by Cds1 and Chk1 phosphorylation has also been reported (5, 15). We have found a decrease in Cdc25C levels after γ irradiation or MKK6-p38γ activation (Fig. 7). This change in Cdc25C protein levels correlated with increased Cdc2 phosphorylation (Fig. 5), suggesting that loss of this protein also contributes to the control of Cdc2 activity. Cdc25 degradation has been reported in mammalian cells and fission yeast (4, 45) and implicated as an important regulatory mechanism for cell cycle progression. A role for changes in Cdc25C protein level in G2/M arrest has not previously been reported. Although the immediate events that lead to Cdc2 inactivation would be Cdc25C phosphorylation and inactivation, it is possible that protein reduction of Cdc25C is an additive mechanism that reinforces the cell cycle checkpoint.
Different functions of p38 group MAP kinase members in cell cycle regulation.
Published data have implicated a member of the p38 group in cell cycle progression. p38α has been reported to be important in the spindle assembly checkpoint of somatic cell cycles (56). Microinjection of p38α has also been reported to prevent the G1/S transition (42). Here we provide evidence for a role for p38γ in γ-radiation-induced G2 arrest. Although all p38 isoforms were activated in γ-irradiated cells, the effect of p38γ appears to be significant because only activation of p38γ was required to induce a G2-phase arrest independent of the other p38 group members. However, p38α's effects in the G1 and/or spindle assembling checkpoint cannot be excluded from γ-irradiated cells because some cells inevitably escape the G2 checkpoint. Therefore, other p38 group members may also participate in γ-irradiated cells to protect cells at multiple checkpoints. The different members of the p38 kinase group may function differently in normal or stressful situations. It was observed that inhibition of p38α/β by SB203580 significantly retarded cell proliferation (unpublished observation) and overexpression of p38α inhibited cell growth (21), suggesting an involvement of p38α in normal cell cycle progression. However, there is no evidence to suggest a role for p38γ in the normal progression of the cell cycle. Inhibiting p38γ activity by overexpression of p38γ(AF) had no detectable effect on cell proliferation determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (data not shown), indicating that p38γ may be primarily involved in transmitting stressful stimuli to cell cycle machinery.
The MKK6-p38γ cascade is a potential target for sensitizers of radiotherapy.
Radiotherapy is widely used in cancer treatment but is often unsuccessful because of tumor cell radioresistance (58, 59). Cell death induced by ionizing radiation has been studied in many different types of cells, and its mechanism and time course appear to be variable. The length of G2/M arrest positively correlates with radioresistance of the cell (3). Pharmacological agents such as caffeine that force cells to pass the G2/M block after DNA damage are useful because they increase cell death (31). We have shown in this report that interfering with the MKK6-p38γ cascade dramatically enhances γ-irradiation-mediated cell killing (Fig. 9). Because p38γ is inactive under normal conditions and inhibition of p38γ did not influence normal cell proliferation, the molecules in this pathway can be considered potential targets for radiation sensitization. Since there has been great success in finding specific pharmacological inhibitors for different kinases (14, 34), the development of specific inhibitor for p38γ or another kinase in this pathway is possible. Thus, understanding the mechanism by which p38γ regulates G2 arrest may help to develop new strategies for the improvement of radiation therapy.
ACKNOWLEDGMENTS
We thank J. V. Kuhns for excellent secretarial assistance.
This work was supported by grants from the California Cancer Research Program (J. Han) and the PEW Scholar Program in Biomedical Science (S. Huang) and by a California Breast Cancer Research Program postdoctoral fellowship (X. Wang).
Footnotes
Publication no. 12814-IMM from the Department of Immunology, The Scripps Research Institute, La Jolla, Calif.
REFERENCES
- 1.Abbott D W, Holt J T. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732–2742. doi: 10.1074/jbc.274.5.2732. [DOI] [PubMed] [Google Scholar]
- 2.Abrieu A, Fisher D, Simon M N, Doree M, Picard A. MAPK inactivation is required for the G2 to M-phase transition of the first mitotic cell cycle. EMBO J. 1997;16:6407–6413. doi: 10.1093/emboj/16.21.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge D R, Radford I R. Explaining differences in sensitivity to killing by ionizing radiation between human lymphoid cell lines. Cancer Res. 1998;58:2817–2824. [PubMed] [Google Scholar]
- 4.Baldin V, Cans C, Knibiehler M, Ducommun B. Phosphorylation of human CDC25B phosphatase by CDK1-cyclin A triggers its proteasome-dependent degradation. J Biol Chem. 1997;272:32731–32734. doi: 10.1074/jbc.272.52.32731. [DOI] [PubMed] [Google Scholar]
- 5.Blasina A, de Weyer I V, Laus M C, Luyten W H, Parker A E, McGowan C H. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 6.Blasina A, Paegle E S, McGowan C H. The role of inhibitory phosphorylation of CDC2 following DNA replication block and radiation-induced damage in human cells. Mol Biol Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasina A, Price B D, Turenne G A, McGowan C H. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 1999;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- 8.Brown A L, Lee C H, Schwarz J K, Mitiku N, Piwnica-Worms H, Chung J H. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi P, Eng W K, Zhu Y, Mattern M R, Mishra R, Hurle M R, Zhang X, Annan R S, Lu Q, Faucette L F, Scott G F, Li X, Carr S A, Johnson R K, Winkler J D, Zhou B B. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 10.Coleman T R, Dunphy W G. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 11.Conrad P W, Rust R T, Han J, Millhorn D E, Beitner-Johnson D. Selective activation of p38alpha and p38gamma by hypoxia. Role in regulation of cyclin D1 by hypoxia in PC12 cells. J Biol Chem. 1999;274:23570–23576. doi: 10.1074/jbc.274.33.23570. [DOI] [PubMed] [Google Scholar]
- 12.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch R J, Davis R J. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 14.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furnari B, Blasina A, Boddy M N, McGowan C H, Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol Biol Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 17.Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hain J, Weller E, Jung T, Burkart W. Effects of ionizing- and UV B-radiation on proteins controlling cell cycle progression in human cells: comparison of the MCF-7 adenocarcinoma and the SCL-2 squamous cell carcinoma cell line. Int J Radiat Biol. 1996;70:261–271. doi: 10.1080/095530096144996. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Jiang Y, Li Z, Kravchenko V V, Ulevitch R T. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Lee J D, Tobias P S, Ulevitch R J. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 21.Han J, Lee J-D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 22.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa M, Cuenda A, Spillantini M G, Thomas G M, Buee-Scherrer V, Cohen P, Goedert M. Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J Biol Chem. 1999;274:12626–12631. doi: 10.1074/jbc.274.18.12626. [DOI] [PubMed] [Google Scholar]
- 24.He L, Yu J X, Liu L, Buyse I M, Wang M S, Yang Q C, Nakagawara A, Brodeur G M, Shi Y E, Huang S. RIZ1, but not the alternative RIZ2 product of the same gene, is underexpressed in breast cancer, and forced RIZ1 expression causes G2-M cell cycle arrest and/or apoptosis. Cancer Res. 1998;58:4238–4244. [PubMed] [Google Scholar]
- 25.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Chen C, Li Z, Guo W, Gegner J A, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 27.Jin P, Gu Y, Morgan D O. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato Y, Kravchenko V, Tapping R, Han J, Ulevitch R, Lee J. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato Y, Tapping R I, Huang S, Watson M H, Ulevitch R J, Lee J D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 30.Kohn K, Jackman J, O'Connor P. Cell cycle control and cancer chemotherapy. J Cell Biochem. 1994;54:440–452. doi: 10.1002/jcb.240540411. [DOI] [PubMed] [Google Scholar]
- 31.Lau C C, Pardee A B. Mechanism by which caffeine potentiates lethality of nitrogen mustard. Proc Natl Acad Sci USA. 1982;79:2942–2946. doi: 10.1073/pnas.79.9.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavin M F, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 33.Lechner C, Zahalka M A, Giot J-F, Moler N P H, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heyes R J, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I, Fisher S, Livi G P, White J R, Adams J L, Young P R. Identification and characterization of a novel protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Jiang Y, Ulevitch R J, Han J. The primary structure of p38gamma: a new member of p38 group of MAP kinase. Biochem Biophys Res Commun. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 36.Lock R B, Ross W E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990;50:3761–3766. [PubMed] [Google Scholar]
- 37.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 38.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 39.McGowan C H, Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mertens S, Craxton M, Goedert M. SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Lett. 1996;383:273–276. doi: 10.1016/0014-5793(96)00255-4. [DOI] [PubMed] [Google Scholar]
- 41.Millar J B, Russell P. The cdc25 M-phase inducer: an unconventional protein phosphatase. Cell. 1992;68:407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- 42.Molnar A, Theodoras A M, Zon L I, Kyriakis J M. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–13235. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 43.Mueller P R, Coleman T R, Kumagai A, Dunphy W G. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 44.Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:871–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- 45.Nefsky B, Beach D. Pub1 acts as an E6-AP-like protein ubiquitin ligasse in the degradation of Cdc25. EMBO J. 1996;15:1301–1312. [PMC free article] [PubMed] [Google Scholar]
- 46.New L, Han J. The p38 MAP kinase pathway and it function. Trends Cardiovasc Med. 1998;8:220–228. doi: 10.1016/s1050-1738(98)00012-7. [DOI] [PubMed] [Google Scholar]
- 47.Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10:3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 49.O'Connor P, Kohn K. A fundamental role for cell cycle regulation in the chemosensitivity of cancer cells? Semin Cancer Biol. 1992;3:409–416. [PubMed] [Google Scholar]
- 50.Ono K, Han J. The p38 signaling transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 51.Palmer A, Gavin A C, Nebreda A R. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 53.Poon R Y, Chau M S, Yamashita K, Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- 54.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 55.Shafman T D, Saleem A, Kyriakis J, Weichselbaum R, Kharbanda S, Kufe D W. Defective induction of stress-activated protein kinase activity in ataxia-telangiectasia cells exposed to ionizing radiation. Cancer Res. 1995;55:3242–3245. [PubMed] [Google Scholar]
- 56.Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- 57.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 58.Weichselbaum R R, Beckett M A, Dahlberg W, Dritschilo A. Heterogeneity of radiation response of a parent human epidermoid carcinoma cell line and four clones. Int J Radiat Oncol Biol Phys. 1988;14:907–912. doi: 10.1016/0360-3016(88)90013-2. [DOI] [PubMed] [Google Scholar]
- 59.Weichselbaum R R, Dahlberg W, Beckett M, Karrison T, Miller D, Clark J, Ervin T J. Radiation-resistant and repair-proficient human tumor cells may be associated with radiotherapy failure in head- and neck-cancer patients. Proc Natl Acad Sci USA. 1986;83:2684–2688. doi: 10.1073/pnas.83.8.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright J H, Munar E, Jameson D R, Andreassen P R, Margolis R L, Seger R, Krebs E G. Mitogen-activated protein kinase kinase activity is required for the G2/M transition of the cell cycle in mammalian fibroblasts. Proc Natl Acad Sci USA. 1999;96:11335–11340. doi: 10.1073/pnas.96.20.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao M, New L, Kravchenko V V, Kato Y, Gram H, Di Padova F, Olson E N, Ulevitch R J, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]