Abstract
Atherosclerosis research typically focuses on the evolution of intermediate or advanced atherosclerotic lesions rather than on prelesional stages of atherogenesis. Yet these early events may provide decisive leads on the triggers of the pathologic process, before lesions become clinically overt. Thereby, it is mandatory to consider extracellular lipoprotein deposition at this stage as the prerequisite of foam cell formation leading to a remarkable accumulation of LDL (Low Density Lipoproteins). As progression of atherosclerosis displays the characteristic features of a chronic inflammatory process on the one hand and native LDL lacks inflammatory properties on the other hand, the lipoprotein must undergo biochemical modification to become atherogenic. During the last 25 years, evidence was accumulated in support of a different concept on atherogenesis proposing that modification of native LDL occurs through the action of ubiquitous hydrolytic enzymes (enzymatically modified LDL or eLDL) rather than oxidation and contending that the physiological events leading to macrophage uptake and reverse transport of eLDL first occur without inflammation (initiation with reversion). Preventing or reversing initial atherosclerotic lesions would avoid the later stages and therefore prevent clinical manifestations. This concept is in accordance with the response to retention hypothesis directly supporting the strategy of lowering plasma levels of atherogenic lipoproteins as the most successful therapy for atherosclerosis and its sequelae. Apart from but unquestionable closely related to this concept, there are several other hypotheses on atherosclerotic lesion initiation favoring an initiating role of the immune system (‘vascular-associated lymphoid tissue’ (VALT)), defining foam cell formation as a variant of lysosomal storage disease, relating to the concept of the inflammasome with crystalline cholesterol and/or mitochondrial DAMPs (damage-associated molecular patterns) being mandatory in driving arterial inflammation and, last but not least, pointing to miRNAs (micro RNAs) as pivotal players. However, direct anti-inflammatory therapies may prove successful as adjuvant components but will likely never be used in the absence of strategies to lower plasma levels of atherogenic lipoproteins, the key point of the perception that atherosclerosis is not simply an inevitable result of senescence. In particular, given the importance of chemical modifications for lipoprotein atherogenicity, regulation of the enzymes involved might be a tempting target for pharmacological research.
Keywords: initial atherosclerotic lesion, lipoprotein insudation, enzymatically modified LDL (eLDL), oxidized LDL (oxLDL), macrophage, C-reactive protein, complement system
1. Introduction
Over 100 years ago, Klotz and Mannig made the following statement: “It is quite useless to argue the questions concerning the development of intimal sclerosis if we study and discuss the late stages of the disease alone … If we wish to gain a true insight into the complex question of arteriosclerosis, we must attempt to follow the lesion from its earliest beginning” [1]. Also aging is that, even at a very early age, a “normal” patient with an aorta completely free of structural alterations does not exist, i.e., the population cannot be divided into a group that has atherosclerosis and another group that does not [2]. Rather, the contiguous nature of the histologic changes and the time of life at which a specific change predominates indicate that each represents a gradation or stage in a temporal sequence (see below). The mechanisms involved in this long process are not entirely clear. Once symptom-producing lesions are present, lesion composition and architecture are complex and pathogenetic mechanisms are difficult to unravel. Atherogenesis can be better understood, and more successfully influenced, before lesions become clinically overt. Nevertheless, the definition of such initial atherosclerotic lesions inevitably is the result of a classification of the whole spectrum of atherosclerotic lesions from early to late stages (Table 1).
Table 1.
Concepts on initiation and/or progression of human atherosclerotic lesion. Denomination: terminology used to describe the beginning of atherosclerotic lesion development. Features: hallmarks and triggers of lesion initiation. Lesion progression due to…: hallmarks and triggers of lesion progression (provided that lesion progression is considered).
| Denomination | Features | Lesion Progression Due to … | Ref. |
|---|---|---|---|
| fatty streak, minimal sudanophilic intimal deposit | both intra- and extracellular “globules” of lipid, slight increase in interstitial mucinous material | conversion into fibrous plaques | [2] |
| type I (initial) lesion | isolated macrophage foam cells | small pools of lipid droplets and dead cell remnants as a source of extracellular lipid in addition to macrophage foam cells (preatheroma) | [3,4] |
| intimal xanthoma | isolated macrophage foam cells | extracellular lipid accumulation (lipid pools) that are rich in extracellular matrix proteoglycans (pathologic intimal thickening (PIT)) | [5,6,7] |
| grade of lipid deposition 1 | fatty streaks with extracellular lipids colocalizing with biglycan and decorin in the outer layer of the intima | n/a | [8] |
| early lesion | plasma albumin and apolipoprotein B insudation | n/a | [9] |
| early lesion | interstitial lipid deposits resulting from the encrustation or imbibition of fibrin onto or into the intima | n/a | [10] |
| gelatinous lesion | balances of intact LDL/“deposited” cholesterol and of fibrinogen/fibrin | loss of steady state concentrations reflecting rates of egress of macromolecules depending on molecular sieving (immobilization of LDL by fibrin) | [11] |
| n/a | n/a | influx-efflux imbalance in the cell and blood vessel wall | [12] |
| epicardial coronary atherosclerosis | impairment of lymphatic drainage from the coronary arteries (absence of a potential system for removing protein, fluid and lipids from the arterial wall) | impairment of lymphatic drainage from the coronary arteries (absence of a potential system for removing protein, fluid and lipids from the arterial wall) | [13] |
| prelesional stage | ‘inert’ lipoprotein insudation without monocyte/ macrophage infiltration, lipoprotein modification and complement activation | overload of the cholesterol removal machinery, enzymatic modification of LDL, complement activation, persisting macrophages secreting a variety of molecules accelerating lipoprotein retention, plaque instability, and clotting on rupture | [14,15,16,17,18,19,20,21,22,23,24,25] |
| early fatty streak | intracellular lipid accumulation in SMCs | degeneration of lipid-containing cells with extravasation of lipid particles into the extracellular space | [26] |
| early lesion | ionic interaction of positively charged regions of apolipoprotein B with matrix proteins, including proteoglycans, collagen, and fibronectin | n/a | [27] |
| initial lipid deposition | unesterified cholesterol-rich lipid particles | n/a | [28] |
| fatty streak | LDL accumulation and oxidation preceding intimal accumulation of monocytes | n/a | [29] |
| n/a | n/a | cholesterol crystals or clefts in the musculoelastic (deep) layer of the intima or in the tunica media | [30] |
| fatty streak | accumulation of mononuclear cells | n/a | [31,32] |
| type I (initial) lesion | alteration in electron density of the matrix of lysosomal bodies as well as the formation of lamellar bodies in lysosomes | substantial structural changes of lysosomes in the ‘normal intima-initial lesion-fatty streak’ sequence | [33,34] |
| early lesion | unesterified, crystalline cholesterol | n/a | [35,36] |
| initial lesion | miRNAs mediating cellular regulation in endothelial activation and inflammation, differentiation of macrophages and their polarization, having important functional properties in lipoprotein homeostasis and playing a central role in the mechanisms determining SMC phenotype | miRNAs mediating cellular regulation in endothelial activation and inflammation, differentiation of macrophages and their polarization, having important functional properties in lipoprotein homeostasis and playing a central role in the mechanisms determining SMC phenotype | [37,38,39,40,41] |
2. The Evolution of Classification Systems
Previous work considered the “simple” fatty streak the earliest lesion of atherosclerosis [2]. A fully developed classification was first provided by the AHA’s Committee on Vascular Lesions [42,43,44], later on updated [3] and modified [5], respectively. However, a shortcoming of the latter classification systems is that extracellular lipoprotein deposition as the prerequisite of foam cell formation is not considered. Unlike, extracellular lipoprotein deposition was considered in the elegant study of Nakashima et al. [8] on early human coronary atherosclerosis recognizing that fatty streaks develop via extracellular deposition of lipids associated with specific types of proteoglycans in the outer layer of preexisting diffuse or “nonatherosclerotic” intimal thickening (DIT) [5,8]. DIT was strongly expressed from an early age in arteries that are considered to be prone to atherosclerosis. These findings suggest that the development of atherosclerosis depends at least partly on the degree of DIT [45].
3. The Matter of Insudation
Previous observations suggested that plasma albumin and apolipoprotein B insudation is the earliest sign of a focal intimal lesion [9] and even before interstitial lipid deposits were discussed to result from the encrustation or imbibition of fibrin onto or into the intima [10].
Spontaneous insudation of LDL was demonstrated some 40 years ago [46,47]. The latter led to the “response to retention” hypothesis, which states that atherosclerosis develops in response to LDL entrapment [14,48]. This entrapment leads to a remarkable accumulation of LDL [49]. In contrast to other peripheral tissues, in which the concentration of LDL particles in the extracellular fluid is only 10% of that in the circulating blood, in the arterial intima the concentration of LDL-particles was found to be the same as in the circulation, i.e., it is 10-fold higher than in other tissues [11]. The observation that the extracellular fluid in the normal intima and the early developing lesions is not drained by lymphatic capillaries [13] might promote this concentration gradient by virtue of the absence of a potential system for removing protein, fluid and lipids from the arterial wall.
Atherosclerosis research typically focuses on the evolution of intermediate or advanced atherosclerotic lesions rather than on prelesional stages of atherogenesis. Yet these early events may provide decisive leads on the triggers of the pathologic process [15]. For example, apolipoprotein B insudation without macrophages as observed in our previous study was proposed to represent a prelesional stage that has hitherto been neglected and is not contained in current classifications of atherosclerotic lesion development [16]. In line with our results, a study on 38 Japanese autopsied subjects who died between 7 and 49 years of age reported extracellular deposition of lipids associated with specific types of proteoglycans before macrophage infiltration [8]. Both studies may provide a key in settling the chicken-vs.-egg debate of atherosclerotic plaque progression: does lipid come first, or do macrophages?
4. Extra- or Intracellular Lipoproteins?
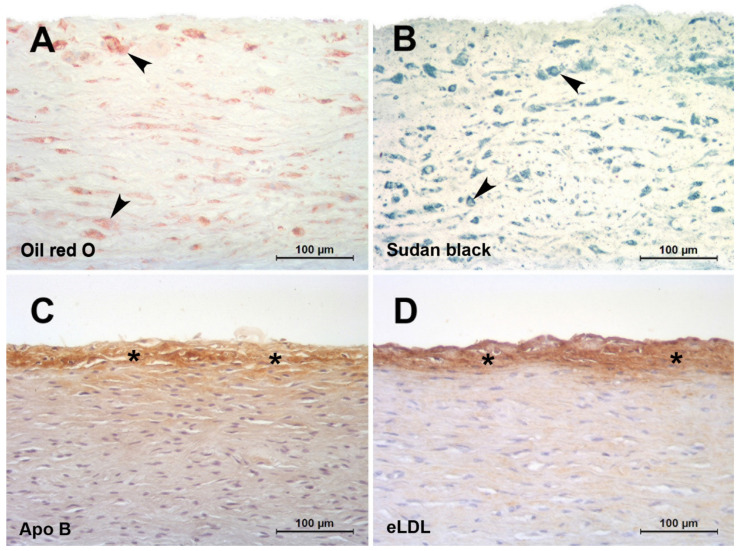
In the classical paper by Geer et al., it was discussed that it is difficult to believe that initial accumulation occurs by phagocytosis of lipid filtered from the blood [26]. It was further stated that the existence of lipid-containing cells with cytoplasmic changes suggesting degeneration provides a possible explanation for the source of extracellular lipid. Moreover, and forward-looking, substantiation of this hypothesis was declared to be impossible until methods can be developed for the positive identification of extracellular lipid [26]. Indeed, lipid-soluble dyes such as Oil red O or Sudan black do not detect extracellular but only intracellular lipids (Figure 1A,B) putting across the latter untenable hypothesis. Not until elaborated staining methods like filipin or immunohistochemical staining (Figure 1C,D) revealed that extracellular particles constituted a significant component of accumulated cholesterol [50]. Accordingly, atherosclerosis is distinguished by the accumulation of lipoproteins within the arterial wall. An ionic interaction of positively charged regions of apolipoprotein B with matrix proteins, including proteoglycans, collagen, and fibronectin, is thought to initiate this process. Proteoglycans are complex glycoproteins containing highly negatively charged carbohydrate chains. These proteins are abundant in atherosclerosis lesions, and they associate with apolipoprotein B-containing lipoproteins [27]. Transgenic mice expressing modified apolipoprotein B that binds poorly to proteoglycans show reduced atheroma development [51]. This highlights the fundamental importance of LDL entrapment for initiating lesion formation rather than any modification of the lipoprotein occurring prior to insudation. It is generally held that progression of atherosclerosis displays the characteristic features of a chronic inflammatory process [52]. Hence, there must be a link between insudation of native LDL and inflammation. Native LDL lacks inflammatory properties, and it follows that the lipoprotein must undergo biochemical alterations to become atherogenic.
Figure 1.
Red herrings and hallmarks of the initial atherosclerotic lesion. (A,B): Initial human aortic atherosclerotic lesions fixed in 4% formaldehyde and infiltrated and embedded in the water soluble plastic Technovit 7100 based on HEMA and GMA [53,54]. The use of alcohol containing fluids was strictly avoided. Staining with lipid-soluble dyes such as oil red O (A) or Sudan black (B) do not detect extracellular but only intracellular lipids (arrowheads). (C,D): Initial human aortic atherosclerotic lesions fixed in 4 % formaldehyde and infiltrated and embedded in paraffin. Staining with a polyclonal antibody against apolipoprotein B (C) [16] or AIL-3 (IgG1) against eLDL (D) [23]. Even after lipid extraction by the dehydration process, extensive extracellular epitopes (asterisks) can be detected in spite of the almost complete absence of macrophages.
5. The Fate of Tissue-Stranded LDL
What happens to tissue-stranded LDL? What kind of modification takes place? Among several other candidates, two different concepts of lipoprotein modification are propagated: the widespread oxidation hypothesis [55] and the less common eLDL hypothesis, which proposes that modification of LDL occurs through the action of ubiquitous hydrolytic enzymes (enzymatically modified LDL or eLDL) rather than oxidation [17,18]. Originally, enzymatic modification of LDL in vitro was performed by sequential treatment with trypsin, cholesterol esterase and neuraminidase [20], the former later on replaced by other proteases at will [56]. As for the latter enzyme, multiple evidence has been provided that desialylation of LDL causes uncontrolled accumulation of lipids within the artery wall [57]. Importantly, both desialylation and proteolysis can sensitize LDL particles to other modifications via a cascade of well-arranged changes starting already in the blood comprising hydrolysis of the core cholesteryl esters and further to cholesterol crystallization [58]. It is well known for a long time that early atherosclerotic lesions contain much free cholesterol [28,35,50,59].
In general, global quantification indicates presence of only small amounts of oxidized lipids [60]. OxLDL detected within early human lesions is localized mainly intracellularly [29,61]. Napoli et al. analyzed fatty streaks in human fetuses. Epitopes of oxLDL were found, but these were localized mainly within macrophages [29]. Calara et al. reported that a single injection of heterologous LDL resulted in its accumulation in the arterial wall, where it became oxidatively modified within hours. However, the lipoprotein was localized mainly intracellulary in SMCs [61]. But the question of localization is exactly of paramount importance, because an extracellular atherogenic modification of LDL is mandatory to cause efficient cellular uptake of LDL. The oxidation hypothesis itself claims that oxidation is a prerequisite for cellular uptake of LDL. Another shortcoming of the oxidation hypothesis is that it does not provide for the fact that extracellular cholesterol in early lesions is mainly unesterified and can form crystals [28,35,50,59].
6. Atherogenesis in Infants and Children?
Arteries of infants and children may provide a model to study mechanisms involved in lesion initiation in the absence of many confounding factors occurring in adults. Several partly very old histological studies on normal and pathological conditions predominantly in carotid and coronary arteries of infants and children have been performed [2,62,63,64,65,66,67,68]. With the exception of our own study (see below) only two studies have addressed the temporal and spatial sequence of LDL insudation, extracellular modification and intimal monocyte accumulation in very early stages of lesion formation in premature fetuses [29] and children aged 1–13 years [69]. However, these studies did not address CRP deposition, eLDL formation or complement activation, important issues that were addressed in our study investigating very early stages of lesion formation in human aortas of infants and children below 1 year of age and up to the age of 15 years [16]. Acting with caution regarding the overall scarcity of lesions, novel insights were obtained into the temporal and spatial sequence of LDL insudation, modification and intimal monocyte accumulation in the earliest detectable stages of atherogenesis. Hitherto undescribed possible prelesional stages of atherogenesis were established. They are characterized by ‘inert’ lipoprotein insudation without monocyte/macrophage infiltration, lipoprotein modification and complement activation in individuals <1 year of age (Figure 1C) and an incipient enzymatic lipoprotein modification in individuals between 6 and 15 years of age [16] (Figure 1D).
7. Lesion Progression Due to…?
From a morphological point of view, repeated suggestions have been made to address lesions that represent the link between foam cell accumulations and atheromas; six of 32 fatty streaks (19%) investigated by Guyton and Klemp contained cholesterol crystals or clefts, which were found in the musculoelastic (deep) layer of the intima or in the tunica media [30]. Their findings suggest that the lipid-rich core region does not originate primarily from the debris of dead foam cells in the superficial intima, but instead arises from lipids accumulating gradually in the extracellular matrix of the deep intima corresponding to Stary’s preatheroma lesion [70,71]. Stary himself, however, claimed that preatheroma lesions contain small pools of lipid droplets and dead cell remnants as a source of extracellular lipid in addition to macrophage foam cells [4]. The earliest feature of a morphological bridge to more advanced plaques (progressive atherosclerosis) as described by the AHA classification is PIT, which is characterized by extracellular lipid accumulation (lipid pools) that are rich in extracellular matrix proteoglycans [5,6,7].
The initial atherosclerotic lesion is reversible and essentially harmless. What tips the scales towards irreversible, advanced lesions, i.e., the point of no return from a mechanistic point of view? In pursuit of an answer, it is important to clearly distinguish between initiation and progression of atherosclerotic lesion development. A factor intimately involved in the advanced stages of atherosclerosis and its sequelae does not necessarily play an important role in the initiation of atherosclerosis. Vice versa, a factor intimately involved in atherogenesis might become less important in the course of lesion progression [18].
First of all, the concept that “whole plasma” crosses endothelium, and the steady state concentrations reflect rates of egress of the macromolecules, which in turn depend on molecular sieving was propagated by Smith in 1977 [11]. Next, investigators have stressed the influx-efflux aspects of lipid metabolism in the arterial wall [12]. As already mentioned above, the absence of lymphatics draining the epicardial coronary arteries was considered a factor predisposing to coronary atherosclerosis by virtue of the absence of a potential system for removing protein, fluid and lipids from the arterial wall [13]. If the amount of insudated LDL exceeds the recycling capacity of the normal intima, or, in other words, the capacity of the system is overburdened, this would lead to an imbalance between lipoprotein and cholesterol deposition and removal, with subsequent accumulation of extracellular LDL particles [17,18]. The macrophages that entered the arterial wall and consumed the retained and modified lipoproteins would then persist rather than simply leave, secreting a variety of molecules that accelerate lipoprotein retention, plaque instability, and clotting on rupture [14].
8. The eLDL Hypothesis (Figure 2)
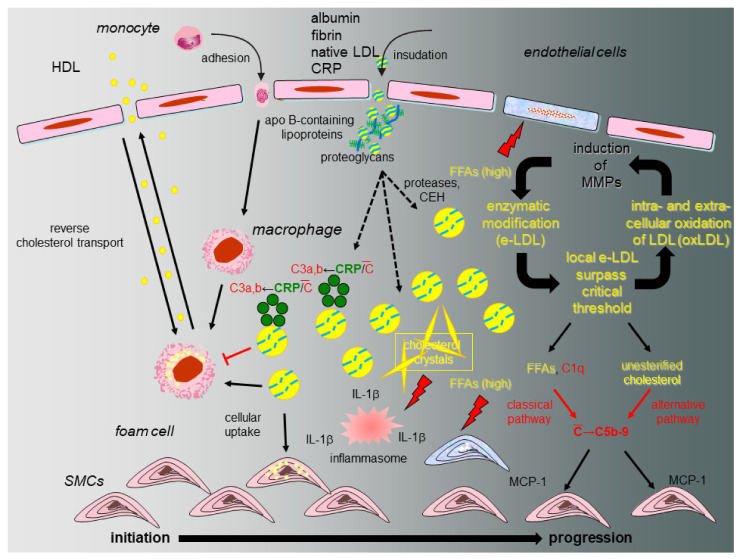
A fundamental question relates to the fate of tissue-stranded native LDL and the nature of the modification that bestows novel properties onto the lipoprotein. As already indicated above, during the last 25 years, we accumulated evidence in support of a different concept on atherogenesis [15,17,18] distinguishing between atherosclerotic lesion initiation with reversion or lesion initiation with progression (Figure 2). Our hypothesis heeds the above-mentioned shortcomings of the oxidation hypothesis and proposes that modification of native LDL occurs through the action of ubiquitous hydrolytic enzymes (enzymatically modified LDL or eLDL) rather than oxidation and contends that the physiological events leading to macrophage uptake and reverse transport of eLDL first occur without inflammation (initiation with reversion). Under hypercholesterolemic conditions, however, the cholesterol removal system is overburdened due to disequilibrium between rates of insudation and reverse transport followed by excessive tissue-stranding of eLDL. Unhalted activation of innate immune effectors, in particular complement and other detrimental effects follow [72]. Overburdening of the physiological eLDL removal machinery is further accompanied by IL-6 production possibly assuming a critical role [19] which, by the way, could explain the slightly elevated CRP levels. LDL additionally activates the alternative complement pathway [17,20] possibly due to the free cholesterol itself. The demonstration of C5b-9 in early human atherosclerotic lesions and its colocalization with SMCs [21], as well as the complement induced release of MCP-1 from human SMCs [22], provided further evidence of a possible role of complement activation in the early stages of atherogenesis. Notably, our work indicated that eLDL might activate complement in atherosclerotic lesions via CRP-dependent and -independent pathways and later on demonstrated that the CRP dependent pathway halts before the proinflammatory terminal sequence, while the CRP-independent pathway proceeds to completion with the generation of C5b-9 complexes [17,21,23,24,25].
Figure 2.
Proposed model of initiation and progression of atherosclerosis with special emphasis on the role of CRP and the complement system. Under normal circumstances (initiation and reversion, normocholesterolemia, left), native LDL entrapped within the arterial intima and associated with specific types of proteoglycans is enzymatically modified (eLDL), leading to a sequence of events that serve to clear the vessel wall of cholesterol. Binding of CRP to eLDL is the first trigger for complement activation (C), but in this early stage the terminal sequence is spared. The physiological sequence of events is concluded by reverse cholesterol transport. If the capacity of the system is overburdened (initiation and progression, hypercholesterolemia, right), this leads to accumulation of eLDL with subsequent generation of potentially harmful C5b-9 complexes by both the classical and alternative pathway as well as accumulation and oxidation of extracellular LDL particles followed by a wealth of well-documented events like MMP production in surrounding cells and subsequent amplification of enzymatic degradation of LDL. Hydrolysis of the core cholesteryl esters and subsequent cholesterol crystallization mediates formation of the inflammasome directly stimulating NLRP3 leading to increased tissue levels of IL-1 β. FFAs play multifaceted roles through their dual capacity to exert stimulatory and cytotoxic effects on neighboring cells (modified from [18]).
In summary, evidence was accumulated in support of a different concept on atherogenesis (summarized in [15,17,18]) proposing that modification of native LDL occurs through the action of ubiquitous hydrolytic enzymes rather than oxidation and contending that the physiological events leading to macrophage uptake and reverse transport of eLDL first occur without inflammation. As already stated above and also by Holman et al. in 1958, the factors responsible for succeeding stages of atherosclerosis may be different from those which initiated the first stage. “Intelligent therapeutic attempts must take cognizance of these different stages, for that treatment which is effective in one stage may be ineffective or even contra-indicated in another stage” [2]. In other words, preventing or reversing initial atherosclerotic lesions would avoid the later stages and therefore prevent clinical manifestations. The response to retention hypothesis directly supports the strategy of lowering plasma levels of atherogenic lipoproteins as the most successful therapy for atherosclerosis and its sequelae [14].
9. Alternative Hypotheses on Atherosclerotic Lesion Initiation and Progression
Apart from but unquestionable closely related to the above-mentioned mechanistic considerations, several other hypotheses on atherosclerotic lesion initiation and progression merit consideration and discussion.
9.1. Vascular-Associated Lymphoid Tissue (VALT)
Wick’s group coined the term VALT for the accumulation of mononuclear cells at regions of the arterial wall in healthy children and adolescents that are predisposed to the development of atherosclerotic lesions later in life if risk factors are present. Accordingly, they claimed to demonstrate that immunological-inflammatory cells are present “in the earliest stages of atherogenesis” in 15–34 year old subjects, arguing in favor of an initiating role of the immune system in atherosclerosis development [31,32]. However, on closer examination fatty streaks are put on a level with “the earliest stages of atherogenesis”. Without challenging the importance of the immune system in atherosclerosis development, fatty streaks cannot be declared as earliest stages of atherogenesis.
9.2. Lysosomal Storage Disease
Following the hypothesis by de Duve et al. defining foam cell formation in atherosclerosis as a variant of lysosomal storage disease [33], Bobryshev et al. investigated parameters of the regulatory system of expression of genes relating to the lysosomal function of intimal cells residing in the initial lesion (Type I lesion) [34]. In addition to altered patterns of CD68 distribution, substantial structural changes of lysosomes in the ‘normal intima-initial lesion-fatty streak’ sequence were observed as well. These changes included the alteration in electron density of the matrix of lysosomal bodies as well as the formation of lamellar bodies in lysosomes which might reflect the summary of a variety of altered metabolic pathways rather than specific gene defects. It is suggested that the observed occurrence of the dysregulation in the expression of the lysosome-relating gene may contribute to the inability of lysosomes to cope with a high intake of lipids in developing fatty streaks [34].
9.3. Inflammasome and Mitochondria
Another approach relates to the concept of the inflammasome termed in 2002 describing multimolecular complexes formed in the cell cytosol upon stimulation [73]. The inflammasome is a complex of distinct proteins which, when brought together, serve to convert inactive IL-1 β to the active proinflammatory cytokine IL-1 β. The formation can be mediated by multiple different signals including cholesterol crystals and oxLDL deposited in vessel walls or mitochondrial DAMPs released from damaged cells (see below) and directly stimulating NLRP3 inflammasome leading to increased tissue levels of IL-1 β and inflammation [36]. Very interestingly, it was demonstrated that minute cholesterol crystals are present already in early diet-induced mice atherosclerotic lesions and that their appearance coincides with the first appearance of inflammatory cells suggesting that crystalline cholesterol acts as an endogenous danger signal and its deposition in arteries is an early cause rather than a late consequence of inflammation [36] (As a comprehensive appreciation of atherosclerotic lesion development in relevant animal models would go beyond the scope of the present review, the reader is referred to an excellent recent presentation of this issue [74]). This assumption is in perfect accordance with the proposed significance of unesterified cholesterol in early human atherosclerotic lesions (see above). Provided that the crystalline form of cholesterol may be mandatory in driving arterial inflammation, this may offer new therapeutic targets, e.g., solubilization of cholesterol crystals and anti-IL-1 β treatment strategies on the one hand [75] and explain the apparent paradox that ACAT inhibitors tested in large clinical trials showed not a decrease but rather an increase in the size of the coronary atheroma [76,77] on the other hand, because the crystalline form of cholesterol would be expected to be increased after inhibition of ACAT.
Mitochondria are another possible candidate for initiation and regulation of NLRP3 (see above). On the one hand, mitochondrial DAMPs are released from damaged cells contributing to the stimulation and/or perpetuating of innate immunity. On the other hand, the presence of certain mtDNA mutations leading to defective mitophagy can prevent the arrest of a proinflammatory response or even increase it upon repeated stimulation [78].
9.4. Micro RNAs
Accumulating evidence indicates that numerous miRNAs play a pivotal role in both atherosclerotic initiation and progression. miRNAs are highly conserved, single-stranded noncoding RNA molecules, exerting post-transcriptional effects on gene expression by promoting degradation of mRNA target and/or inhibiting mRNA translation [37]. Accordingly, they participate in positive as well as negative regulatory loops resulting in a hierarchical network. From both in vitro and in vivo studies, it is evident that miRNAs regulate diverse cell types involved in atherosclerotic disease initiation and progression. They mediate cellular regulation in endothelial activation and inflammation [38], regulate the differentiation of macrophages and their polarization [39], which is a critical component of the inflammatory response and play a central role in the mechanisms determining SMC phenotype [40]. Moreover, circulating miRNAs are promising new candidates as biomarkers for atherosclerosis and pharmacological targeting of dysregulated miRNAs seems to be a promising therapeutic concept [41,79].
10. Conclusions
Direct anti-inflammatory therapies may prove successful as adjuvant components but will likely never be used in the absence of strategies to lower plasma levels of atherogenic lipoproteins, the key point of the perception that atherosclerosis is not simply an inevitable result of senescence [2]. In particular, given the importance of chemical modifications for lipoprotein atherogenicity, regulation of the enzymes involved might be a tempting target for pharmacological research [57].
Abbreviations
| ACAT | acetyl-CoA acetyltransferase |
| AHA | American Heart Association |
| CRP | C-reactive protein |
| DAMPs | damage-associated molecular patterns |
| DIT | diffuse intimal thickening |
| eLDL | enzymatically modified LDL |
| FFA | free fatty acid |
| GMA | glycol methacrylate) |
| HEMA | 2-hydroxyethylmethacrylate |
| IL-1 β | interleukin-1 β |
| IL-6 | interleukin-6 |
| LDL | Low Density Lipoproteins |
| MCP-1 | monocyte chemotactic protein-1 |
| mtDNA | mitochondral DNA |
| miRNAs | micro RNAs |
| MMP | matrix metalloproteinase |
| NLRP3 | NOD-, LRR- and pyrin-domain containing 3 |
| oxLDL | oxidized LDL |
| PIT | pathological intimal thickening |
| SMC | smooth muscle cell |
| VALT | vascular-associated lymphoid tissue |
Funding
This work was supported by the Robert Bosch Foundation, Stuttgart, Germany, grant number P7. The APC was funded by Robert Bosch Gesellschaft für medizinische Forschung mbH.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klotz O., Mannig M.F. Fatty streaks in the intima of arteries. J. Path. Bact. 1911;16:211–220. doi: 10.1002/path.1700160117. [DOI] [Google Scholar]
- 2.Holman R.L., Mc G.H., Jr., Strong J.P., Geer J.C. The natural history of atherosclerosis: The early aortic lesions as seen in New Orleans in the middle of the of the 20th century. Am. J. Pathol. 1958;34:209–235. [PMC free article] [PubMed] [Google Scholar]
- 3.Stary H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000;20:1177–1178. doi: 10.1161/01.ATV.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 4.Stary H.C. Lipid and macrophage accumulations in arteries of children and the development of atherosclerosis. Am. J. Clin. Nutr. 2000;72:1297S–1306S. doi: 10.1093/ajcn/72.5.1297s. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000;20:1262–1275. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 6.Kolodgie F.D., Burke A.P., Nakazawa G., Cheng Q., Xu X., Virmani R. Free cholesterol in atherosclerotic plaques: Where does it come from? Curr. Opin. Lipidol. 2007;18:500–507. doi: 10.1097/MOL.0b013e3282efa35b. [DOI] [PubMed] [Google Scholar]
- 7.Otsuka F., Kramer M.C., Woudstra P., Yahagi K., Ladich E., Finn A.V., de Winter R.J., Kolodgie F.D., Wight T.N., Davis H.R., et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis. 2015;241:772–782. doi: 10.1016/j.atherosclerosis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima Y., Fujii H., Sumiyoshi S., Wight T.N., Sueishi K. Early human atherosclerosis: Accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Cliff W.J., Schoefl G.I., Higgins G. Plasma protein insudation as an index of early coronary atherogenesis. Am. J. Pathol. 1993;143:496–506. [PMC free article] [PubMed] [Google Scholar]
- 10.Duguid J.B. Thrombosis as a factor in the pathogenesis of aortic atherosclerosis. J. Pathol. Bacteriol. 1948;60:57–61. doi: 10.1002/path.1700600107. [DOI] [PubMed] [Google Scholar]
- 11.Smith E.B. Molecular interactions in human atherosclerotic plaques. Am. J. Pathol. 1977;86:665–674. [PMC free article] [PubMed] [Google Scholar]
- 12.Wolinsky H. A proposal linking clearance of circulating lipoproteins to tissue metabolic activity as a basis for understanding atherogenesis. Circ. Res. 1980;47:301–311. doi: 10.1161/01.RES.47.3.301. [DOI] [PubMed] [Google Scholar]
- 13.Eliska O., Eliskova M., Miller A.J. The absence of lymphatics in normal and atherosclerotic coronary arteries in man: A morphologic study. Lymphology. 2006;39:76–83. [PubMed] [Google Scholar]
- 14.Tabas I., Williams K.J., Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 15.Torzewski M., Bhakdi S. Complement and atherosclerosis-united to the point of no return? Clin. Biochem. 2013;46:20–25. doi: 10.1016/j.clinbiochem.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Torzewski M., Navarro B., Cheng F., Canisius A., Schmidt T., Bhakdi S., Urban R., Lackner K.J. Investigation of Sudan IV staining areas in aortas of infants and children: Possible prelesional stages of atherogenesis. Atherosclerosis. 2009;206:159–167. doi: 10.1016/j.atherosclerosis.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Bhakdi S., Lackner K.J., Han S.R., Torzewski M., Husmann M. Beyond cholesterol: The enigma of atherosclerosis revisited. Thromb. Haemost. 2004;91:639–645. doi: 10.1160/TH03-12-0733. [DOI] [PubMed] [Google Scholar]
- 18.Torzewski M. Enzymatically modified LDL, atherosclerosis and beyond: Paving the way to acceptance. Front. Biosci. Landmark. 2018;23:257–271. doi: 10.2741/4642. [DOI] [PubMed] [Google Scholar]
- 19.Klouche M., Gottschling S., Gerl V., Hell W., Husmann M., Dorweiler B., Messner M., Bhakdi S. Atherogenic properties of enzymatically degraded LDL: Selective induction of MCP-1 and cytotoxic effects on human macrophages. Arterioscler. Thromb. Vasc. Biol. 1998;18:1376–1385. doi: 10.1161/01.ATV.18.9.1376. [DOI] [PubMed] [Google Scholar]
- 20.Bhakdi S., Dorweiler B., Kirchmann R., Torzewski J., Weise E., Tranum-Jensen J., Walev I., Wieland E. On the pathogenesis of atherosclerosis: Enzymatic transformation of human low density lipoprotein to an atherogenic moiety. J. Exp. Med. 1995;182:1959–1971. doi: 10.1084/jem.182.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torzewski M., Torzewski J., Bowyer D.E., Waltenberger J., Fitzsimmons C., Hombach V., Gabbert H.E. Immunohistochemical colocalization of the terminal complex of human complement and smooth muscle cell alpha-actin in early atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1997;17:2448–2452. doi: 10.1161/01.ATV.17.11.2448. [DOI] [PubMed] [Google Scholar]
- 22.Torzewski J., Oldroyd R., Lachmann P., Fitzsimmons C., Proudfoot D., Bowyer D. Complement-induced release of monocyte chemotactic protein-1 from human smooth muscle cells. A possible initiating event in atherosclerotic lesion formation. Arterioscler. Thromb. Vasc. Biol. 1996;16:673–677. doi: 10.1161/01.ATV.16.5.673. [DOI] [PubMed] [Google Scholar]
- 23.Torzewski M., Klouche M., Hock J., Messner M., Dorweiler B., Torzewski J., Gabbert H.E., Bhakdi S. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler. Thromb. Vasc. Biol. 1998;18:369–378. doi: 10.1161/01.ATV.18.3.369. [DOI] [PubMed] [Google Scholar]
- 24.Bhakdi S., Torzewski M., Klouche M., Hemmes M. Complement and atherogenesis: Binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler. Thromb. Vasc. Biol. 1999;19:2348–2354. doi: 10.1161/01.ATV.19.10.2348. [DOI] [PubMed] [Google Scholar]
- 25.Torzewski M., Rist C., Mortensen R.F., Zwaka T.P., Bienek M., Waltenberger J., Koenig W., Schmitz G., Hombach V., Torzewski J. C-reactive protein in the arterial intima: Role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2000;20:2094–2099. doi: 10.1161/01.ATV.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 26.Geer J.C., Mc G.H., Jr., Strong J.P. The fine structure of human atherosclerotic lesions. Am. J. Pathol. 1961;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil M.F., Wagner W.D., Goldberg I.J. Molecular interactions leading to lipoprotein retention and the initiation of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:2211–2218. doi: 10.1161/01.ATV.0000147163.54024.70. [DOI] [PubMed] [Google Scholar]
- 28.Chao F.F., Amende L.M., Blanchette-Mackie E.J., Skarlatos S.I., Gamble W., Resau J.H., Mergner W.T., Kruth H.S. Unesterified cholesterol-rich lipid particles in atherosclerotic lesions of human and rabbit aortas. Am. J. Pathol. 1988;131:73–83. [PMC free article] [PubMed] [Google Scholar]
- 29.Napoli C., D‘Armiento F.P., Mancini F.P., Postiglione A., Witztum J.L., Palumbo G., Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Investig. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyton J.R., Klemp K.F. Transitional features in human atherosclerosis. Intimal thickening, cholesterol clefts, and cell loss in human aortic fatty streaks. Am. J. Pathol. 1993;143:1444–1457. [PMC free article] [PubMed] [Google Scholar]
- 31.Millonig G., Schwentner C., Mueller P., Mayerl C., Wick G. The vascular-associated lymphoid tissue: A new site of local immunity. Curr. Opin. Lipidol. 2001;12:547–553. doi: 10.1097/00041433-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Millonig G., Malcom G.T., Wick G. Early inflammatory-immunological lesions in juvenile atherosclerosis from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY)-study. Atherosclerosis. 2002;160:441. doi: 10.1016/S0021-9150(01)00596-2. [DOI] [PubMed] [Google Scholar]
- 33.Haley N.J., Fowler S., de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J. Lipid Res. 1980;21:961–969. doi: 10.1016/S0022-2275(20)34756-8. [DOI] [PubMed] [Google Scholar]
- 34.Bobryshev Y.V., Shchelkunova T.A., Morozov I.A., Rubtsov P.M., Sobenin I.A., Orekhov A.N., Smirnov A.N. Changes of lysosomes in the earliest stages of the development of atherosclerosis. J. Cell. Mol. Med. 2013;17:626–635. doi: 10.1111/jcmm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruth H.S. Subendothelial accumulation of unesterified cholesterol. An early event in atherosclerotic lesion development. Atherosclerosis. 1985;57:337–341. doi: 10.1016/0021-9150(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 36.Grebe A., Hoss F., Latz E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 37.Bezsonov E.E., Sobenin I.A., Orekhov A.N. Immunopathology of Atherosclerosis and Related Diseases: Focus on Molecular Biology. Int. J. Mol. Sci. 2021;22:4080. doi: 10.3390/ijms22084080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreou I., Sun X., Stone P.H., Edelman E.R., Feinberg M.W. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol. Med. 2015;21:307–318. doi: 10.1016/j.molmed.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartmann P., Zhou Z., Natarelli L., Wei Y., Nazari-Jahantigh M., Zhu M., Grommes J., Steffens S., Weber C., Schober A. Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4. Nat. Commun. 2016;7:11907. doi: 10.1038/ncomms11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., He X., Wang J., Wang D., Cong P., Zhu A., Chen W. The Regulation of Exosome-Derived miRNA on Heterogeneity of Macrophages in Atherosclerotic Plaques. Front. Immunol. 2020;11:2175. doi: 10.3389/fimmu.2020.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo C.C., Liu W., Lin X.Y., Dorajoo R., Lee K.W., Richards A.M., Lee C.N., Wongsurawat T., Nookaew I., Sorokin V. The interaction between 30b-5p miRNA and MBNL1 mRNA is involved in vascular smooth muscle cell differentiation in patients with coronary atherosclerosis. Int. J. Mol. Sci. 2019;21:11. doi: 10.3390/ijms21010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stary H.C., Blankenhorn D.H., Chandler A.B., Glagov S., Insull W., Jr., Richardson M., Rosenfeld M.E., Schaffer S.A., Schwartz C.J., Wagner W.D. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992;85:391–405. doi: 10.1161/01.CIR.85.1.391. [DOI] [PubMed] [Google Scholar]
- 43.Stary H.C., Chandler A.B., Glagov S., Guyton J.R., Insull W., Jr., Rosenfeld M.E., Schaffer S.A., Schwartz C.J., Wagner W.D., Wissler R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. 1994;14:840–856. doi: 10.1161/01.ATV.14.5.840. [DOI] [PubMed] [Google Scholar]
- 44.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Jr., Rosenfeld M.E., Schwartz C.J., Wagner W.D., Wissler R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 1995;15:1512–1531. doi: 10.1161/01.ATV.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima Y., Chen Y.X., Kinukawa N., Sueishi K. Distributions of diffuse intimal thickening in human arteries: Preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch. 2002;441:279–288. doi: 10.1007/s00428-002-0605-1. [DOI] [PubMed] [Google Scholar]
- 46.Stender S., Zilversmit D.B. Transfer of plasma lipoprotein components and of plasma proteins into aortas of cholesterol-fed rabbits. Molecular size as a determinant of plasma lipoprotein influx. Arteriosclerosis. 1981;1:38–49. doi: 10.1161/01.ATV.1.1.38. [DOI] [PubMed] [Google Scholar]
- 47.Camejo G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: Its possible role in atherogenesis. Adv. Lipid Res. 1982;19:1–53. doi: 10.1016/b978-0-12-024919-0.50007-2. [DOI] [PubMed] [Google Scholar]
- 48.Williams K.J., Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Öörni K., Kovanen P.T. Aggregation Susceptibility of Low-Density Lipoproteins—A Novel Modifiable Biomarker of Cardiovascular Risk. J. Clin. Med. 2021;10:1769. doi: 10.3390/jcm10081769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kruth H.S. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. Am. J. Pathol. 1984;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- 51.Skalen K., Gustafsson M., Rydberg E.K., Hulten L.M., Wiklund O., Innerarity T.L., Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 52.Ross R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 53.Van Goor H., Gerrits P.O., Grond J. The application of lipid-soluble stains in plastic embedded sections. Histochemistry. 1986;85:251–253. doi: 10.1007/BF00494811. [DOI] [PubMed] [Google Scholar]
- 54.Meyer W., Schmidt J., Busche R., Jacob R., Naim H.Y. Demonstration of free fatty acids in the integument of semi-aquatic and aquatic mammals. Acta Histochem. 2012;114:145–150. doi: 10.1016/j.acthis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 56.Torzewski M., Suriyaphol P., Paprotka K., Spath L., Ochsenhirt V., Schmitt A., Han S.R., Husmann M., Gerl V.B., Bhakdi S., et al. Enzymatic modification of low-density lipoprotein in the arterial wall: A new role for plasmin and matrix metalloproteinases in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:2130–2136. doi: 10.1161/01.ATV.0000144016.85221.66. [DOI] [PubMed] [Google Scholar]
- 57.Mezentsev A., Bezsonov E., Kashirskikh D., Baig M.S., Eid A.H., Orekhov A. Proatherogenic Sialidases and Desialylated Lipoproteins: 35 Years of Research and Current State from Bench to Bedside. Biomedicines. 2021;9:600. doi: 10.3390/biomedicines9060600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehti S., Nguyen S.D., Belevich I., Vihinen H., Heikkilä H.M., Soliymani R., Käkelä R., Saksi J., Jauhiainen M., Grabowski G.A., et al. Extracellular Lipids Accumulate in Human Carotid Arteries as Distinct Three-Dimensional Structures and Have Proinflammatory Properties. Am. J. Pathol. 2018;188:525–538. doi: 10.1016/j.ajpath.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 59.Simionescu N., Vasile E., Lupu F., Popescu G., Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am. J. Pathol. 1986;123:109–125. [PMC free article] [PubMed] [Google Scholar]
- 60.Kühn H., Heydeck D., Hugou I., Gniwotta C. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. J. Clin. Investig. 1997;99:888–893. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calara F., Dimayuga P., Niemann A., Thyberg J., Diczfalusy U., Witztum J.L., Palinski W., Shah P.K., Cercek B., Nilsson J., et al. An animal model to study local oxidation of LDL and its biological effects in the arterial wall. Arterioscler. Thromb. Vasc. Biol. 1998;18:884–893. doi: 10.1161/01.ATV.18.6.884. [DOI] [PubMed] [Google Scholar]
- 62.Zinserling W.D. Untersuchungen über Atherosklerose. I. Über die Aortaverfettung bei Kindern. Virchows Arch. Path. Anat. 1925;255:677–705. doi: 10.1007/BF01891708. [DOI] [Google Scholar]
- 63.Zeek P. Juvenile arteriosclerosis. Arch. Path. 1930;10:417–446. [Google Scholar]
- 64.Albert Z. Die Veränderungen der Aorta bei Kindern und ihr Verhältnis zur Atherosklerose. Virchows Arch. Path. Anat. 1939;303:265–279. doi: 10.1007/BF02595161. [DOI] [Google Scholar]
- 65.Velican C., Velican D. Intimal histolysis as an early stage of atherosclerotic involvement. Observations on the human coronary arteries. Med. Interne. 1976;14:185–190. [PubMed] [Google Scholar]
- 66.Sakurai I., Tosaka A., Yamada T., Kuwahara T., Masubuchi K. Childhood coronary sclerosis. Acta Pathol. Jpn. 1978;28:41–52. doi: 10.1111/j.1440-1827.1978.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 67.Bland J., Skordalaki A., Emery J.L. Early intimal lesions in the common carotid artery. Cardiovasc. Res. 1986;20:863–868. doi: 10.1093/cvr/20.11.863. [DOI] [PubMed] [Google Scholar]
- 68.Stary H.C. Macrophages, macrophage foam cells, and eccentric intimal thickening in the coronary arteries of young children. Atherosclerosis. 1987;64:91–108. doi: 10.1016/0021-9150(87)90234-6. [DOI] [PubMed] [Google Scholar]
- 69.Napoli C., Glass C.K., Witztum J.L., Deutsch R., D‘Armiento F.P., Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 70.Stary H.C. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:I19–I32. [PubMed] [Google Scholar]
- 71.Stary H.C. Composition and classification of human atherosclerotic lesions. Virchows Arch. A Pathol. Anat. Histopathol. 1992;421:277–290. doi: 10.1007/BF01660974. [DOI] [PubMed] [Google Scholar]
- 72.Weber C., Zernecke A., Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat. Rev. Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 73.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 74.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nuñez G., Schnurr M., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vedder V.L., Aherrahrou Z., Erdmann J. Dare to Compare. Development of Atherosclerotic Lesions in Human, Mouse, and Zebrafish. Front. Cardiovasc. Med. 2020;7:109. doi: 10.3389/fcvm.2020.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu X., Kakkar V. Inflammasome and atherogenesis. Curr. Pharm. Des. 2014;20:108–124. doi: 10.2174/13816128113199990586. [DOI] [PubMed] [Google Scholar]
- 77.Nissen S.E., Tuzcu E.M., Brewer H.B., Sipahi I., Nicholls S.J., Ganz P., Schoenhagen P., Waters D.D., Pepine C.J., Crowe T.D., et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N. Engl. J. Med. 2006;354:1253–1263. doi: 10.1056/NEJMoa054699. [DOI] [PubMed] [Google Scholar]
- 78.Meuwese M.C., de Groot E., Duivenvoorden R., Trip M.D., Ose L., Maritz F.J., Basart D.C., Kastelein J.J., Habib R., Davidson M.H., et al. ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: The CAPTIVATE randomized trial. JAMA. 2009;301:1131–1139. doi: 10.1001/jama.301.11.1131. [DOI] [PubMed] [Google Scholar]
- 79.Fichtlscherer S., De Rosa S., Fox H., Schwietz T., Fischer A., Liebetrau C., Weber M., Hamm C.W., Röxe T., Müller-Ardogan M., et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010;107:677. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]