Abstract
Diabetic foot infection is the leading cause of non-traumatic lower limb amputations worldwide. In addition, diabetes mellitus and sequela of the disease are increasing in prevalence. In 2017, 9.4% of Americans were diagnosed with diabetes mellitus (DM). The growing pervasiveness and financial implications of diabetic foot infection (DFI) indicate an acute need for improved clinical assessment and treatment. Complex pathophysiology and suboptimal specificity of current non-invasive imaging modalities have made diagnosis and treatment response challenging. Current anatomical and molecular clinical imaging strategies have mainly targeted the host’s immune responses rather than the unique metabolism of the invading microorganism. Advances in imaging have the potential to reduce the impact of these problems and improve the assessment of DFI, particularly in distinguishing infection of soft tissue alone from osteomyelitis (OM). This review presents a summary of the known pathophysiology of DFI, the molecular basis of current and emerging diagnostic imaging techniques, and the mechanistic links of these imaging techniques to the pathophysiology of diabetic foot infections.
Keywords: diabetic foot infection, molecular imaging, test predictive value, X-ray, optical tomography, DWI, SPECT
1. Introduction
In the past 30 years, diabetic foot infections (DFI) have become increasingly prevalent due to the rising incidence of diabetes mellitus (DM). According to the most recent National Diabetes Statistics Report by the Centers for Disease Control and Prevention, 30 million U.S. adults were reported to have diabetes, and 84.1 million were considered prediabetic [1]. Twenty percent of diabetes-related hospital admissions in the U.S. are from DFI [2], which is typically introduced by direct inoculation through a traumatic entry site in an insensate foot. Cellulitis, myositis, tendinitis, or OM occur in over half of all cases of diabetic foot ulceration (DFU) [3]. The climbing rate of diabetes is accompanied by an increased incidence of DFU, deep tissue infections, and amputations.
A global meta-analysis by Zhang et al. reported that DFU is prevalent in 6.3% of the population, with North America having the highest prevalence of DFU in the world (13.0%). This is followed by Africa (7.2%), Asia (5.5%), Europe (5.1%), and Oceania (3%), although a lack of studies led South America to be excluded from the meta-analysis. Countries with the highest prevalence of DFU included Belgium (16.6%), Canada (14.8%), and the United States (13.0%) [4]. The authors of this meta-analysis chose to exclude any studies that stated ulcer recurrence, suggesting that the overall occurrence of DFU is higher. Furthermore, these numbers are expected to continue rising due to widespread obesity and an aging population.
According to the same meta-analysis, the population most at risk for developing DFU were males with type 2 diabetes and low body mass index (mean BMI of 23.8 ± 1.7 in diabetic patients with DFU versus 24.4 ± 1.7 in diabetic patients without DFU), long diabetic duration (mean 11.3 ± 2.5 years in diabetic patients with DFU versus 7.4 ± 2.2 years in diabetic patients without DFU), diabetic retinopathy, and a history of smoking and hypertension [4]. A retrospective study by Wang et al. found a similar trend in Chinese patients: DFI was more common in men, and the disease prognosis worsened with age. On average, men experienced greater infection severity and underwent amputation 10 years earlier than women, who showed improved wound healing possibly attributable to higher estrogen levels [5,6].
Major amputations have also been associated with decreased life expectancy [7]. While this increased mortality is real, patients who undergo major amputation often die from complications of cardiovascular disease (myocardial infarction, heart failure, and cerebrovascular accidents). Prevention of major amputation is paramount because this can preserve ambulation and, consequently, cardiovascular fitness. Amputees often experience drastic lifestyle changes, including loss of independence and psychological distress. These factors are compounded in developing countries such as Ghana, where access to resources such as prosthetic services or mental health care is limited. [8]. Amputations are life-altering, as well as expensive. Studies have found that, in the United States, a lower limb amputation costs anywhere from $43,800 to $66,215 for the surgical procedure and aftercare such as rehabilitation services, nursing homes, and internal medicine costs [9]. Additionally, lower limb amputation places the contralateral limb at risk for complications due to increased functional demand.
The financial ramifications of DFI are also far-reaching. Collectively, $9 billion is spent annually on diabetic foot care in the U.S., which amounts to more than the yearly cost of breast or colorectal cancers [10]. Ulcers that culminate in DFI alone cost upwards of $1 billion every year in the U.S. [11]. The cost of medical treatment is tremendous, but does not account for indirect expenses related to loss of income, employment, or opportunity. Unfortunately, these secondary costs are often difficult to measure, and the true economic impact of DFI is likely to be much steeper than presently reported. Altogether, this information suggests that investing in the prevention of DFU and early detection of DFI would be economically advantageous.
Given the high prevalence, morbidity, and financial burden of diabetic foot infections, diagnostic methods that efficiently and accurately distinguish soft tissue infection from OM and monitor the therapeutic response are important for optimizing clinical outcomes. Distinguishing soft tissue infection from osteomyelitis may be challenging in clinical practice. Without these diagnostic features, diabetic patients are likely to experience exacerbated infection severity and poorer recovery prognosis. Herein, we review the current understanding of DFI pathogenesis and the corresponding mechanisms of applied imaging modalities.
2. Pathophysiology and Clinical Assessment of DFI
2.1. Diabetic Susceptibility to DFI
Several pathological factors place diabetic patients at increased risk for foot infections, including diabetic neuropathy, vascular insufficiency, and immunological dysfunction [12]. A strong link has been reported between diabetic neuropathy and foot ulceration [13]. Diabetic neuropathy is a complex polyneuropathy consisting of peripheral, motor, and autonomic neuronal damage [14].
Peripheral neuropathy is prevalent in 10.9–32.7% of diabetic patients in the U.S. [15,16]. Minor foot trauma from sources such as ill-fitting shoes or injury goes unnoticed from lack of sensation [17]. When repeated or left unattended, this trauma may lead to ulceration (Figure 1) and subsequent infection. Muscle atrophy, foot deformity (Charcot arthropathy, claw foot, pes cavus, hallux valgus), and gait abnormalities are caused by motor neuropathy resulting from the loss of myelinated fibers [18]. These biomechanical changes predispose neuropathic patients to foot ulceration due to increased pressure and shearing. Additionally, a study by Lung et al. suggests that a moderate-to-fast walking intensity decreases plantar stiffness and increases risk of foot ulceration compared to walking at slower speeds [19]. Charcot arthropathy develops in 13% of patients with neuropathy. It can result in fractures, dislocations, and fracture dislocations causing profound deformity and a rocker-bottom appearance of the plantar foot, due to bone breakdown and joint collapse [20,21]. Autonomic neuropathy in the lower limb causes vasodilation and excess warmth in the foot. Moreover, impaired neuronal control of the sweat glands reduces perspiration (anhidrosis), leading to dry skin that is prone to fissure or callus and increasing the risk of developing a foot wound [22,23]. Diabetic patients may also experience vascular insufficiency (micro and macrovascular) and immunological dysfunction, which further predisposes them to ulceration, impaired healing, and infection (Figure 1).
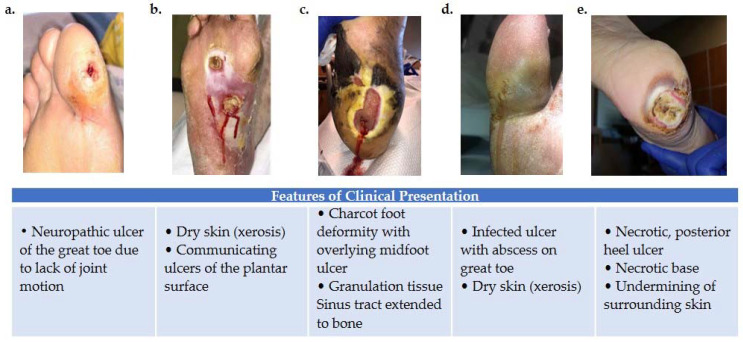
Figure 1.
Variations in foot wound location, presentation, and severity as illustrated by images (a–e). (a) Patient presenting with neuropathic ulcer under the proximal interphalangeal joint of the hallux. The ulcer is related to lack of joint motion at the first metatarsophalangeal joint. (b) Patient presenting with an infected ulcer following the flexor tendons of the foot. Notice a blow lesion at the plantar arch. Dry skin (xerosis) is a sign of autonomic neuropathy. (c) Patient presenting with Charcot foot deformity and overlying midfoot ulcer. Macerated skin around the edges of the ulcer and a sinus tract that extends to the bone is also seen. (d) Patient with an infected ulcer with abscess on the great toe and xerosis suggesting autonomic neuropathy. (e) Patient with a posterior heel ulcer containing a necrotic base and undermining of surrounding skin.
2.2. Clinical Diagnostic Tests
Several diagnostic tests are available to help with the clinical assessment of DFI, including the probe-to-bone test, biopsy, and assessment of inflammatory markers (ESR and CRP). The probe-to-bone test is routinely used during physical examination of the patient to evaluate the potential for OM [24]. In this procedure, a sterile tip probe is introduced through the ulcer to determine if bone is palpable. A solid/gritty end-point is considered positive for OM [25]. Suspected infections may be evaluated for microorganisms by sampling the wound site with an ulcer swab, soft tissue biopsy, or bone biopsy.
Many have argued that the results of these tests should not be independently used for diagnosis and should be only used as a screening method [26]. A negative test result in any of these cases does not exclude the diagnosis of DFI and a positive result may be misleading. For instance, soft tissue biopsies or swab cultures only assess the superficial flora and may not reflect the full extent of infection [27]. Bone biopsies are also limited because the technique requires trained personnel and it is prone to sampling errors. Moreover, there is no standardized definition of a positive bone biopsy and many classifications exist, each with its own merits and weaknesses—further convoluting the problem [27,28]. Historically, classification systems did not include the presence of OM as an indication of infection severity, and it is well known that the presence of OM negatively impacts the outcomes of diabetic foot infections [29]. Without a universal classification system, determinants of infection severity may vary, leading to a diagnostic disagreement among physicians. The accuracy of tests such as the probe-to-bone test relies on the prevalence of OM in the patient cohort being examined. For example, a group of hospitalized patients with severe infection may have a high positive predicative value using these methods, while a group of outpatients with low likelihood of OM may have a low positive predicative value [30]. These factors, as well as others, have encouraged advancements in understanding of the molecular mechanisms in DFI.
2.3. Molecular Mechanisms of DFI Features
All of the currently available diagnostic tests rely on the assessment of changes in DFI at a tissue level (Figure 2); however, such changes are only detectable when damage has already been inflicted. A strong understanding of the molecular changes that precede visual tissue damage is required to advance the diagnosis and treatment of DFI.
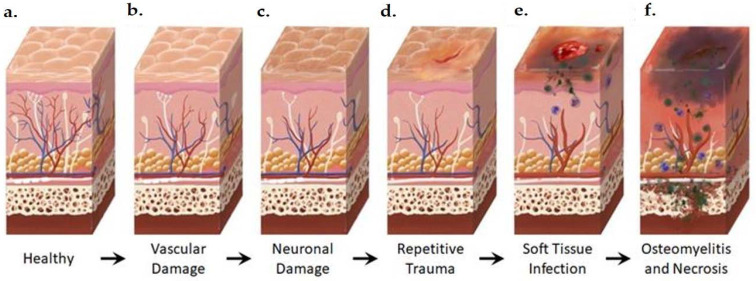
Figure 2.
Anatomical Changes Associated with Diabetic Foot Pathophysiology. Diabetic neuropathy is a multi-faceted polyneuropathy related to an increased risk of ulceration, infection, and amputation. Sustained hyperglycemia damages the endothelial lining of the blood vessels in previously healthy tissue (a), leading to impaired circulation (b). Without sufficient vascular support, nerves die off and the skin may become dry and cracked as sweat secretions decrease (c). In the event of injury, numbness in the foot due to neuronal ischemia may mean that insults go undetected for some time (d). Fissures in the dried skin can harbor microorganisms, increasing the likelihood of wound infection. Initial microbial invasion of the trauma site leads to inflammation, vasodilation, and soft tissue necrosis (e). Decreased vascularization compromises immune response to infection and prolongs healing time. If the infection persists, usually because of delayed care or ineffective treatment, microbes may invade bone tissue, leading to osteomyelitis and bone deformation (f). White: neurons, red: arteries, blue: veins, purple: polymorphonuclear lymphocytes, green: microorganisms.
Bone deformities in the foot such as Charcot arthropathy occur when bone destruction from osteoclastic resorption exceeds bone building from osteoblastic recruitment. This breakdown, radiographically visualized as osteolysis, is mediated through the inflammatory RANKL (receptor activator of the nuclear factor-kappa B ligand) pathway and has been identified as an important pathway in the pathogenesis of Charcot foot. When RANKL concentration is higher than its competitive antagonist, OPG (osteoprotegerin), osteoclastogenesis is stimulated, affecting increased osteoclast activity, osteolysis, and deformity [31,32,33].
Arterial atherosclerosis, excessive leukocyte adhesion, increased vascular permeability, impaired hemostasis, and altered proliferation and apoptosis of vascular cells are all factors contributing to diabetic vascular insufficiency [34]. Poorly controlled glucose levels activate the polyol glucose metabolism pathway in which glucose is reduced to sorbitol and subsequently oxidized to fructose. Excess fructose and sorbitol lead to increased osmotic molality and ultimately osmotic stress [35]. Competitive consumption of NADPH and decreased nitric oxide synthetase activity also contribute to this challenge, as low nitric oxide concentration stimulates vessel constriction [36].
In addition to diminished perfusion, immune dysfunction has also been reported [37]. Diabetic patients frequently present with defective neutrophil function irrespective of glycemic status, including disrupted migration patterns associated with reduced production of chemotactic factors, amplified generation of reactive oxygen species, and abated phagocytosis from complement system dysfunction. Collectively, these changes facilitate pathogen invasion and infection development [38]. One retrospective clinical study demonstrated that the ratio of lymphocytes to neutrophils, platelets, and monocytes could predict the need for amputation due to DFI [39].
The innate immune system is a major source of cytokines. Macrophages and dendritic cells may produce cytokines in response to damage-associated molecular patterns (DAMPS) through the activation of pattern recognition receptors (PRRs). These inflammatory cytokines (such as TNFα, IL-1, IL-6, and interferon-ϒ) trigger osteoblast upregulation of RANKL, facilitating osteoclastogenesis [40]. Mitochondrial antiviral signal proteins (MAVS) also stimulate infected cells to secrete cytokines, activating the NF-κB and IRF3 pathways, which regulate the expression of type-I interferons. When bound to type-1 interferon receptors, these cytokines activate the JAK-STAT pathway, inhibiting pathogen replication and assembly due to a heightened expression of interferon-stimulated genes [41]. Type-I interferons such as INF-β are expressed in stromal cells, which are capable of differentiating into mature osteoblasts [42].
The combination of diabetic neuropathy, impaired perfusion, neutrophil dysfunction, and cytokine imbalance encourages infection development and progression, ischemic ulcers, or gangrene, which may culminate in amputation [22]. To avoid amputation and disease progression, a deeper understanding of pathogen involvement is necessary.
A wide variety of microorganisms may cause DFI (Figure 3), the most common of which is Staphylococcus aureus. The methicillin-resistant Staphylococcus aureus strain (MRSA) occurs in 16.78–30% of DFI cases, although this value varies geographically [43,44]. MRSA infection has been shown to have no impact on mortality, but is correlated with an increased rate of hospitalization and higher risk of limb amputation [45]. Amputation is effective in preventing the spread of infection, and studies in the United Kingdom and Germany have found that the procedure increased the life expectancy by 2 years in 50% of the diabetic subjects studied [46,47]. Even so, only 56% of diabetic patients with ulcerative infections were found to survive 5 years after initial onset of the ulcer [47]. Altogether, this information highlights the need for improved ulcer prevention and the prompt diagnosis of DFI. Imaging may define the precise location of the infected bone and establish the border for amputation. Advanced imaging methods may allow for early detection and verify the adequate response to therapy to avoid amputation.
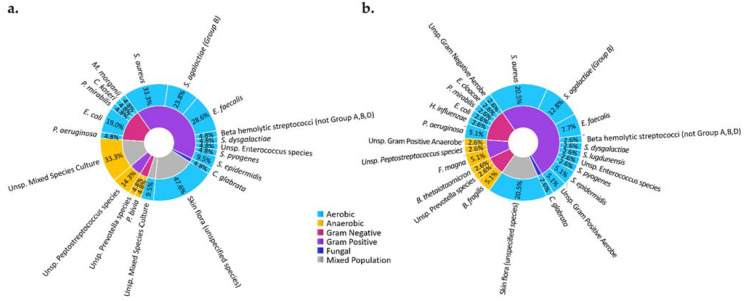
Figure 3.
Microbiota of DFI of observed in subjects of an ongoing clinical study in a tertiary care facility in our medical center at the University of Texas Southwestern Medical Center. Microbiota of DFI in soft tissue biopsies (a) and bone biopsies (b) show that most microbes are aerobic (blue) and gram positive (purple), with Staphylococcus aureus being the most identified microorganism. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Texas Southwestern Medical Center (IRB: 112016-043, approved November 2016). Informed consent was obtained from all subjects involved in the study.
3. Imaging Modalities
Imaging plays an important role in the diagnosis and treatment of DFI. Compared to the probe-to-bone test and biopsy, imaging offers non-invasive methods of detection that characterize many features associated with DFI. Advantages of imaging include the ability to specify infection sites, distinguish soft tissue infection from OM, identify bone abnormalities of Charcot arthropathy, evaluate neural or vascular disease, and characterize DFI pathology in extensive detail. A vital contribution of dual modality molecular and anatomic imaging is the ability to delineate the margin of infected bone from healthy bone, which can be used to determine the appropriate treatment course, including helping to define resection margins [48]. The American College of Radiology (ACR) has issued guidelines for musculoskeletal infections including DFI, soft tissue, or suspected DFO. The practice guidelines suggest screening with radiography, followed by Magnetic Resonance Imaging (MRI), and, finally, radionuclide-based cellular and molecular imaging methods as needed [49]. In this section, we discuss imaging modalities (Table 1) that are currently used to assess DFI, including radiography, MRI, computed tomography (CT), ultrasound, and angiography, with particular focus on molecular techniques.
Table 1.
Diagnostic Imaging Modalities for Diabetic Foot Infections.
| Imaging Method/Radionuclide | Sensitivity (%) | Specificity (%) | Function | Cost | Accessibility | Radiation | References | |
|---|---|---|---|---|---|---|---|---|
| Radiography | 43–86 | 27–83 | +++ | + | +++++ | ☢ | [50,51,52,53,54] | |
| CT | 67–80 | 50–70 | +++ | +++ | +++ | ☢ | [50,55] | |
| Ultrasound | 79 | 80 | ++ | + | +++++ | O | [56] | |
| MRI | 87–100 | 37–83.8 | ++++ | +++ | +++ | O | [52,53,54,57,58,59,60,61,62] | |
| Planar Bone Scintigraphy | 99mTc-MDP | 81.0–84.2 | 28.0–67.7 | +++ | ++ | ++ | ☢☢☢ | [52,54] |
| Planar WBC Scan | 99mTc-besilesomab | 74.8 | 71.8 | +++ | +++ | ++ | ☢☢☢ | [63] |
| 99mTc-HMPAO | 59.0–91 | 79.5–92 | +++ | ++ | ++ | ☢☢☢ | [63,64] | |
| 99mTc-exametazime | 86.0 | 100 | +++ | ++ | ++ | ☢☢☢ | [65] | |
| WBC SPECT/CT | 99mTc-WBC | 87.5–100 | 35–92 | ++++ | ++++ | + | ☢☢☢☢ | [58,62,66,67,68,69] |
| 99mTc-sulesomab | 67–72.0 | 85–88.0 | +++ | +++ | ++ | ☢☢☢☢ | [70,71] | |
| 111In-WBC | 74–92 | 68–75 | ++++ | ++++ | + | ☢☢☢☢ | [54,58] | |
| PET | 18F-FDG | 81–92.3 | 92.0–93 | +++ | ++++ | + | ☢☢☢ | [72,73] |
| PET/CT | 18F-FDG | 43–89 | 67–100 | ++++ | ++++ | + | ☢☢☢☢ | [58,65] |
| 67Ga-citrate | 44–100 | 45–77 | ++ | ++ | ++ | ☢☢☢ | [70,74] | |
| 68Ga-citrate | 100 | 76 | ++ | ++ | ++ | ☢☢☢ | [75] | |
Note: Relative radiation level ratings for effective adult dose: O = 0 mSv, ☢ < 0.1 mSv, ☢☢☢ = 1–10 mSv, ☢☢☢☢ = 10–30 mSv. Utility of each technique was adapted from Noguerol et al. and is graded from most useful (+++++) to least useful (+) [76]. Radiation ratings are adapted from the 2019 ACR appropriateness criteria [49].
3.1. Anatomical Modalities
3.1.1. Radiography
Radiographs are the first-line modality for imaging DFI because of their low cost, high accessibility, and versatility. Radiography facilitates identification of bone changes associated with infection as well as changes in soft tissue (Figure 4).
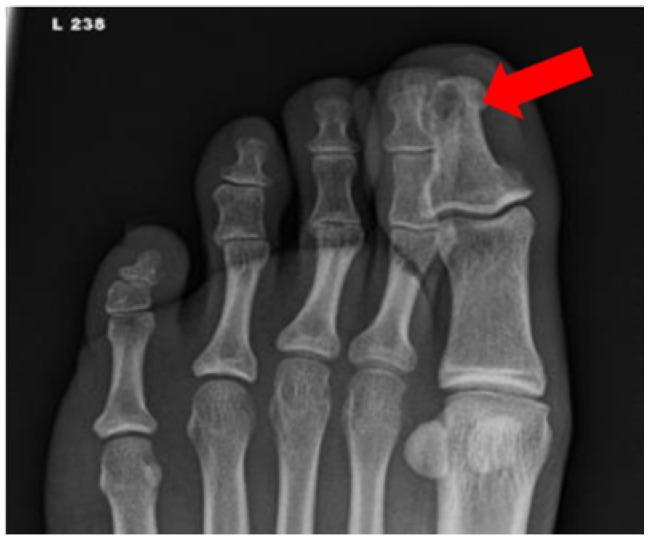
Figure 4.
Radiograph of a foot in a diabetic patient with a history of trauma to the great toe. Anteroposterior view of the left foot demonstrates soft tissue swelling and focal osteolysis to the distal phalanx of the great toe (arrow) with periostitis.
Although conventional radiography is inexpensive, convenient, and fast, there is a delay in reaching the threshold of detection for visualizing OM. Clear evidence of bone erosion may not occur until 10–14 days after infection onset [50]. The radiographic soft tissue abnormality of DFI varies based on the type of infection and tissue response. Soft tissue infections involving gas gangrene or necrotizing fasciitis may be visible as radiolucent patchy gas bubbles in the fascial planes. Cellulitis is seen as the obliteration of fascial planes with stranding in the subcutaneous tissues, while abscess is seen as a focal dense lesion, however, the findings can be non-specific [77]. Visualization of radiolucent foreign bodies (e.g., wooden) can also be challenging on routine radiography [78].
Changes in bone architecture that occur in OM include regional osteopenia, periostitis, cortical bone loss, endosteal scalloping, structural changes in trabecular bone (demineralization), peripheral sclerosis, or peripheral new bone formation [22]. A study by Alvaro-Afonso et al. compared these imaging signs’ radiographic images to bone biopsy. Periosteal reaction, cortical disruption, affected bone marrow (defined as focal loss of trabecular pattern or marrow radiolucency), and sequestrum were evaluated. The most reliable and accurate feature was cortical disruption (sensitivity = 0.70–0.82, specificity = 0.38–0.57) and the least accurate and least agreed upon parameter among clinicians was periosteal reaction (sensitivity = 0.30–0.43, specificity = 0.67–0.84). When at least one of these four parameters was considered an indicator of OM, a pooled sensitivity of 0.86 and specificity of 0.27 was observed (PPV = 0.65–0.76, NPV = 0.36–0.62, PLR = 1.04–1.34, NLR = 0.33–0.80) [51]. Low specificity in radiography often leads to false positive diagnoses of OM, especially in patients with neuroarthropathic changes, such as Charcot’s foot [77]. A false positive diagnosis can result in prolonged antibiotic therapy and aggressive surgical resection of bone.
If a radiograph is strongly suggestive of OM, such as a foot ulcer with adjacent cortical erosion, further imaging may not be warranted. However, OM cannot be excluded on a negative radiograph given the limited sensitivity of radiography compared to other anatomic imaging modalities, such as MRI [79]. Despite this limitation, radiographs are helpful in detecting key features associated with DFI and can guide clinical decision making on further work-up.
3.1.2. Ultrasonography
Ultrasound (US) is a fast, inexpensive, safe, and highly accessible imaging technique that provides real-time images without the use of ionizing radiation [22]. After interacting with a tissue, the amplitude of reflected ultrasonic waves specific to a given tissue are computer analyzed and an image is produced. For DFI, frequencies very between 3–18 MHz; however, higher frequencies have limited tissue penetration and may not reach deeper tissues. Doppler US can be used to assess arterial blood flow velocity to peripheral extremities. Anechoic areas, such as fluid in abscesses, appear dark on US while hyperechoic tissues such as cortical bone are highly reflective and produce a bright signal. The range of gray scale helps identify bone, soft tissue, and fluid [79].
US is often used for draining collections of fluid and assessing arterial occlusions or lesions. Tenosynovitis and joint effusion can also be identified using US. The utility of US is limited by its low sensitivity (78%) and specificity (80%) for OM as well as shadowing caused by cortical bone [52,56]. Instead, the advancement of other imaging modalities such as CT, MRI, and Positron Emission Tomography (PET) has provided new opportunities in the assessment of DFO.
3.1.3. Computed Tomography (CT)
CT imaging capitalizes on the concept that tissues of variable density differentially attenuate X-ray beams. This characteristic is captured in a parameter called the attenuation coefficient, expressed as the Hounsfield unit. CT is particularly useful in the assessment of bone and can detect osseous erosion sooner than radiography. Following image acquisition, computer algorithms generate multiplanar cross-sectional images. Bone images can be separated into the marrow compartment and the cortical compartment. Quantitative CT (qCT) provides compartment-specific bone mass and mineral density measurements. In a study by Commean et al., these qCT parameters were used to monitor changes in the midfoot bones of healthy control patients and type II diabetic patients with peripheral neuropathy. Volumetric qCT was able to detect osteolytic and osteopenic changes in the diabetic foot with comparable precision to that of dual X-ray absorptiometry (bone volume precision: 0.1–0.9%, BMD precision: 0.6–1.9%). Minor osteolytic or osteopenic changes were also detectable, rendering qCT a potentially useful tool in tracking the treatment response in DFI or neuropathic arthropathy with the development of appropriate biomarkers [80].
Clinically, CT provides rapid, high resolution image acquisition of a large anatomic region as well as quantifiable parameters, rendering it an excellent candidate for emergent diagnostic use [81]. Dual energy CT (DECT) enables the identification of bone marrow edema similar MRI. CT is often used to guide biopsy procedures. Some studies have reported that CT is a significant predictor of bone culture positivity [82], although there is a lack of consensus about this relationship in the literature [57,83]. DFI therapies such as wound debridement and abscess drainage can also be guided by CT. In neuropathic arthropathy, CT may be used for surgery planning and treatment monitoring [84].
Several features of OM are detectable by CT. These include soft tissue swelling from pro-inflammatory cytokine release by monocytes and other immune cells, cortical erosion from bacterial breakdown of bone, sinus tracts from the bone to the skin surface, decreased attenuation of the medullary space or bone marrow edema on DECT due to bone marrow inflammation, sequestrum of necrotic bone within the marrow, or gas accumulation from incomplete oxidation by anaerobic or facultative aerobic bacteria [85,86]. Contrast agents, which increase the mass attenuation coefficient of the tissue, can be coupled with CT imaging to enhance the visualization of soft tissue inflammation, and outline the wall of soft tissue or intra-osseous abscess. The small, iodinated molecules in the contrast dyes possess an X-ray absorbing capability, but also display nonspecific distribution and fast clearance; however, in patients with a contrast agent allergy, adverse events may occur, and a different technique may be needed.
In the diagnosis of DFI, CT is limited by a beam-hardening effect in the presence of implants or metal, which obstructs visibility and causes low soft tissue contrast. Moreover, CT uses ionizing radiation whereas MRI does not [79], and MRI possesses superior soft tissue contrast, sensitivity, and image resolution, making it a better candidate for the assessment of DFI [49,79,81].
3.1.4. Magnetic Resonance
Following radiographic evaluation, MRI is used to further evaluate the presence, extent, and severity of DFI. MRI includes sequences of radiofrequency pulses and magnetic field gradients to evaluate tissue features. An MR exam may range in scanning duration, typically lasting 30–45 min, and spares the patient exposure to ionizing radiation. MRI is generally considered an anatomic method because it uses predominantly morphological sequences in clinical practice. Image contrast on morphological sequences depends on the density of proton spins at T1 and T2 relaxation times. T1 relaxation, also called the spin–lattice, is the time for longitudinal magnetization to return to the magnetic field axis (z) after radiofrequency pulses are applied. The spin–spin relaxation time (T2) refers to the time it takes for transverse magnetization to return to the transverse plane. By varying the contrast weighting toward T1 or T2, images can be generated with significant contrast between water and fat. For example, tissues with higher water content such as areas of edema appear dark on T1-weighted images and bright on T2-weighted images, compared to surrounding tissues. Tissues such as fat have the opposite appearance and appear bright on T1-weighted images and less bright on T2-weighted images [87]. However, morphological sequences exhibit limited characterization of soft tissue and bone pathology [88]. Molecular advancements such as dynamic contrast enhancement (DCE), diffusion weighted imaging (DWI), and Dixon-based fat suppression have improved the sensitivity and specificity of MR for the detection and characterization of neuropathic arthropathy and OM—unlike radiography, CT, or ultrasound [76,89].
Bone marrow edema is present in both neuropathic arthropathy and OM and is one pathologic feature that can be exploited by imaging applications. Increased white blood cell accumulation, inflammation, and higher marrow space permeability are characteristics associated with marrow edema in OM. Whereas, in neuropathic arthropathy, marrow edema tends to have lower cellularity and cell variety in comparison to OM [90]. Cortical erosion, ‘ghost sign’ (relative disappearance of bone on T1-weighted image as compared to T2-weighted image), ‘penumbra sign’ (high signal rim of abscess on T1-weighted image) are excellent MRI signs of OM versus cysts and well-defined margins of a neuropathic bone. In DCE-MR, a gadolinium-based contrast agent is injected after a baseline assessment, and multiple images are acquired over a few minutes to evaluate the microvascular environment (Figure 5i). The concentration of the contrast agent within tissues and blood vessels affects the signal intensity of MR. Ultimately, a time–concentration curve is generated which can be quantitatively analyzed to assess the physiological parameters of tissues and surrounding vasculature such as vessel permeability and perfusion [87,91,92]. In a prospective study by Liao et al., receiver–operating characteristic curves (ROCs) were used to assess the accuracy of several DCE-MR parameters in 30 diabetic foot patients with bone marrow edema caused by OM or neuropathic arthropathy. They found that patients with OM had higher contrast agent wash-in from the vascular to interstitial space (Ktrans), higher gadodiamide washout from the interstitial space back into the vascular space (Kep), and higher extra-axial extracellular space volume (Ve) than patients with neuropathic arthropathy. ROC curves revealed that, of these parameters, Ktrans and Ve were the best parameters for distinguishing OM from neuropathic arthropathy [90].
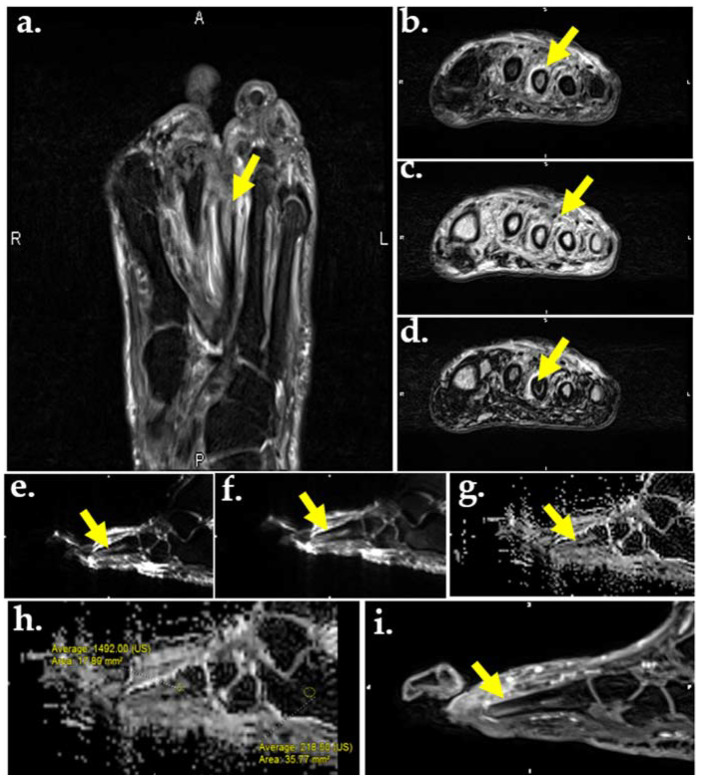
Figure 5.
MR images of the foot in a 56-year-old male with DM and plantar ulcer with plantar ulcer below the 3rd metatarsal and suspected OM. Prior fracture deformity of the second metatarsal head and surgical resection of the third metatarsal are noted. T2-weighted fat suppressed image (a) and T2 Dixon water map (b) show marrow edema of the third metatarsal stump (arrows). In-phase T2 Dixon map (c) shows muscle fatty replacement from DM denervation change (arrow). Opposed-phase T2 Dixon map (d) shows marrow involvement by OM. DWI images (e,f) show marrow replacement by OM on low (a), high (b), and apparent diffusion coefficient (ADC) maps (g,h). Quantification of the marrow abnormality by ADC (h) measures 1.49 compared to normal marrow of 0.21, indicating no intra-osseous abscess, which would appear dark on ADC and bright on DWI. (i) Contrast-enhanced MR shows enhancement of the metatarsal stump.
Another molecular technique used in the assessment of bone marrow edema is DWI, which uses the random (Brownian) movement of free water to generate an indirect estimation of cellularity and cell membrane integrity (Figure 5e,f). As the degree of diffusion weighting—also referred to as the b-value or diffusion moment—increases, the accuracy of DWI improves. The apparent diffusion coefficient (ADC) is the exponential decay of a single component of the diffusion signal which is often used to characterize lesions quantitatively (Figure 5g,h). In OM and soft tissue infection, inflammatory cells, pus, and cellular waste decrease free water diffusion, leading to high signal intensity and moderate-to-low ADC values in abscesses and nonviable tissue. In cases of pure edema—such as in neuropathic arthropathy—high signal intensity and high ADC values are seen due to the T2-shine through phenomenon [76,93]. A majority of the appendicular skeleton contains yellow marrow with mostly large adipocytes, resulting in reduced perfusion and lowered ADC values [94]. Since adipocyte content in the marrow space increases with age and can affect ADC values, fat suppression techniques should be used to further assess the bone marrow compartment [76].
One example of a fat suppression technique is chemical shift. Chemical shift separates fat and water signals according to their echo times (TEs) since they resonate at two different frequencies. The external magnetic field (B0) induces an electron current surrounding the nucleus, which leads to a locally produced magnetic field. [95]. When the locally produced field vector is opposite to that of the external field, it is termed opposed-phase; when the locally produced field vector is in the same direction as that of the external field, it is termed in-phase. The Dixon sequence combines the in-phase and out-of-phase images with decreased sensitivity to inhomogeneities of B0 and B1, resulting in more homogenous fat suppression and creation of multiple maps. Up to four contrasts may be acquired from one image, including in-phase (Figure 5c), opposed-phase (Figure 5d), water only (Figure 5b), and fat only. The Dixon sequence can also be used with other sequences such as gradient or fast spin echo and signal weighting [96]. Because of the homogenous fat suppression achieved by the Dixon technique, it is particularly useful in distinguishing bone marrow edema of OM from that of neuropathic arthropathy. The Dixon sequence is also used to aid in the identification of other features of OM including intraosseous sequestrums, fistulas, cortical destruction, and periostitis [97]. If both OM and neuropathic arthropathy are present, bone contours relatively disappear on T1-weighted images and reappear on fat-suppressed images (also known as the ghost sign). If only neuropathic arthropathy is present, true destruction of the bone occurs and the contours will not re-appear on fat-suppressed images [98,99]. A study by Fujii et al. assessed the efficacy of MR for DFO diagnosis and surgical margin selection. Using histopathology as the gold standard, and T1-weighted and fat-suppressed T2-weighted images, they found that MR was effective in diagnosing neuropathic ulcers (sensitivity and specificity 100%); however, it was not effective in diagnosing ischemic ulcers (sensitivity 29.6%). Bone marrow edema was not well visualized in cases of severe infection and ischemia, rendering MR ineffective in determining surgical margin selection [100].
Several MR techniques are used in the evaluation of peripheral nerves, which can be damaged by diabetes mellitus. Morphologic neurography includes T1-weighted, short-TI inversion recovery (STIR), and fat-suppressed T2-weighted sequences (Figure 5a). This series of images allows for morphological characterization of the nerves, soft tissue, and bone [101,102]. DWI neurographic sequences have also been used; however, DWI has limited spatial resolution, rendering the small nerves of the distal foot difficult to visualize. Diffusion tensor imaging (DTI) neurography is another technique used in the assessment of peripheral nerves and has been found to detect diabetic peripheral neuropathy in both type I and type II diabetics [103,104,105,106]. DTI uses the sensitivity of DWI to the anisotropic water movement within myelinated axons to generate high resolution images that can even provide information regarding myelin sheath damage caused by diabetes. Diabetic neuropathy treatment progression can be tracked using DTI neurography through changes in quantitative biomarkers such as fractional anisotropy, mean diffusivity, and radial diffusivity [76,107]. MR neurography can also aid in pre-surgical planning. One retrospective study used a combination of the techniques mentioned above, including T2-weighted images, T2-weighted non-fat-saturated images, and DWI, to determine the role of MR neurography in preoperative assessment. MR neurography was found to lead to a change in diagnosis or surgical decision in 21.4% of cases [108]. Even though the study was not exclusively performed on diabetics, it highlighted the range of cases that MR neurography can be applied to.
Although these molecular applications of MR (Table 2) may be useful in the differentiation of OM from neuropathic arthropathy, nuclear imaging modalities such as 18F-FDG PET/CT have been reported to have higher diagnostic accuracy [109]. Moreover, MR cannot be used in patients with electronic or ferromagnetic implants, and patient movement can sometimes be problematic. Most importantly, although MR is an excellent anatomical method for imaging soft tissues, it does not provide direct information about molecular changes in the tissue.
Table 2.
Summary of Molecular MR Imaging Techniques in the Assessment of the Diabetic Foot.
| MR Technique | Molecular Basis | Demonstrated Imaging Feature of DFI |
|---|---|---|
| Dynamic Contrast Enhancement (DCE) | Contrast agent alters MR signal intensity in a concentration dependent manner | Bone marrow edema Pattern of Soft tissue involvement Joint impairment Vascular involvement |
| Diffusion Weighted Imaging (DWI) | Takes advantage of restricted diffusion in certain anatomical features such as abscesses and compares this to free water to provide an enhanced image with excellent background suppression. | Bone marrow edema Soft tissue involvement Joint impairment Nerve damage |
| Dixon Sequence | Combines in-phase and out-of-phase images produced through chemical-shift with decreased sensitivity to inhomogeneities of B0 and B1, resulting in homogenous fat suppression. Cortical margins and cysts are best seen on out-of-phase image, marrow edema on water-image, muscle fatty replacement and marrow fat replacement on in-phase and fat-images. | Bone marrow edema Bone lesion identification Nerve damage |
| Diffusion Tensor Imaging (DTI) | Uses the sensitivity of DWI to the anisotropic water movement within myelinated axons to generate high resolution images that can provide information regarding myelin sheath and axonal damage | Nerve damage |
3.2. Molecular Imaging
Radiotracers are chemical compounds containing a radioactive atom that may be injected alone or conjugated to a targeting moiety. The total concentration is so low that the underlying physiology is not disturbed by the radioimaging probe. After administration, molecular interactions with the radiotracer at sites of interest allow the metabolism of a tissue to be detected and quantified. Scintigraphy, single-photon emission computed tomography (SPECT), and PET imaging are molecular imaging modalities used for DFI assessment [110]. They possess higher sensitivity and specificity than anatomical modalities, translating to improved distinction between the infection of soft tissue and bone. Although molecular imaging modalities independently show promise in the evaluation of DFI, the combination of molecular imaging and anatomical studies may possess even greater sensitivity and specificity than either modality alone. For example, as mentioned above, the American College of Radiology listed MR as a first-line modality for cases of suspected OM because of its ability to produce high quality anatomical images [49]. However, the diagnostic accuracy of MRI may significantly improve when combined with molecular imaging assays such as PET, SPECT, or CT. Combined modality approaches provide direct information regarding molecular and tissue level activity.
3.2.1. Bone Scintigraphy
Planar bone scintigraphy involves intravenous injection of a radiotracer—typically 99mTc-methylene diphosphonate or hydroxymethylene diphosphonate (99mTc-MDP/HDP). Other radiotracers such as 18F-sodium fluoride (18F-NaF) may be used for PET imaging of bone. The diphosphonates are retained in bone by binding to hydroxyapatite crystals. Areas of greater radiotracer incorporation by osteoblasts indicate areas of high bone turnover and are often the focal point of infection. Triple phase bone scintigraphy involves capturing images at three stages: immediately after radiotracer injection, 10 min after radiotracer injection, and 2–4 h after radiotracer injection. Each image is used to assess different aspects of DFI. The first image provides information regarding blood flow to the region of interest. The second image, also called the tissue or blood pool phase, assesses the soft tissues and increased retention is generally associated with inflammation or infection. In the third phase, the amount of radiotracer uptake indicates the rate of new bone formation by osteoblasts (Figure 6).
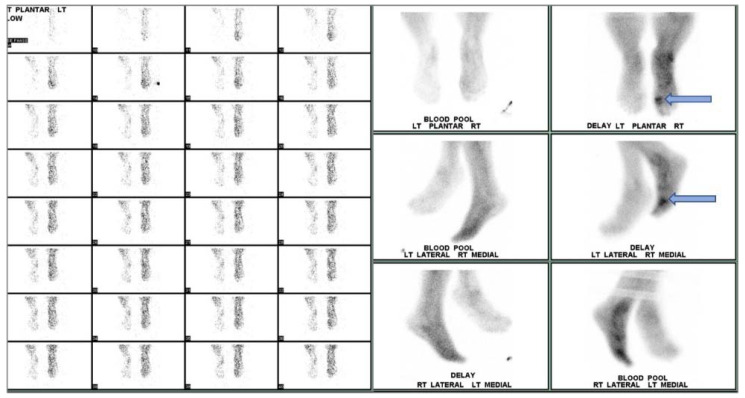
Figure 6.
Three-phase bone scan of an individual with confirmed DFI. Cellulitis is visualized in the right foot with OM in the first metatarsophalangeal region (arrow).
When assessing diabetic foot infection by three-phase bone scintigraphy, variation in radiotracer uptake is observed between phases. In cases of cellulitis and other soft tissue infections, increased radiotracer uptake is only seen during the first two phases, whereas, in OM, increased radiotracer uptake is seen in all three phases identifying focal hyperperfusion, hyperemia, and bone uptake. Moreover, because the third phase of bone scintigraphy reflects general bone turnover, bone deformities associated with degenerative joint disease, fracture, or orthopedic hardware can significantly reduce specificity [79,111]. A meta-analysis by Llewellyn et al. reported an average sensitivity of 84.2% with a relatively low specificity of 67.7% [52]. Although bone scintigraphy is highly sensitive, arterial occlusion or nonspecific inflammatory conditions such as Charcot joint may render the method susceptible to misdiagnosis in cases of DFI [22,52]. Impaired peripheral perfusion may lead to false negatives when assessing the diabetic foot. Sterile inflammation, fracture healing, or Charcot joint may cause increased radiotracer uptake during the third phase, which could be misidentified as a sign of increased bone turnover associated with OM, resulting in falsely positive interpretations. By utilizing a second scintigraphic modality, such as bone marrow imaging with a 99mTc-labeled colloidal preparation and a tomographic method such as SPECT or SPECT/CT, specificity and anatomic localization are improved. This approach provides improved localization of areas with radiotracer uptake that may have been previously obstructed [79].
3.2.2. Radiolabeled WBC and Bone Marrow Planar Scintigraphy, SPECT, and SPECT/CT
While planar bone scintigraphy uses radiotracers to directly detect areas of high bone turnover, leukocyte scans trace localization of radiolabeled white blood cells (WBC) that migrate to the site of infection. In this case, the WBCs act as carriers of the molecular imaging agent. The majority of the WBCs labeled by conventional methods are neutrophils, which are the first and most abundant leukocytes to arrive at the locus of infection [112]. As a result, WBC scans are better for acute infections and can generate highly specific images which can be further improved for spatial localization using SPECT or SPECT/CT.
WBC radiolabeling is performed by in vitro or in vivo methods. In vitro WBC labeling involves the isolation of WBCs from fractionated blood, direct labeling with a radiotracer such as 111In-oxine or 99mTc-HMPAO (exametazine), and reinjection into the subject. In vitro labeling can be limited by the concentration of recovered leukocytes, as optimal labeling requires at least 2000 leukocytes/μL [113]. 111In-oxine displays a normal distribution in the spleen, liver, and bone marrow and because it is highly stable, delayed imaging can be performed to allow labeled cells more time to localize to sites of infection (Figure 7). Importantly, there is no uptake in the uninfected foot. Most importantly, 111In-oxine-labeled cells can be combined with 99mTc radiolabeled agents to target activated bone marrow in dual tracer studies. Such studies are useful in distinguishing OM from Charcot neuropathic changes (Figure 8).
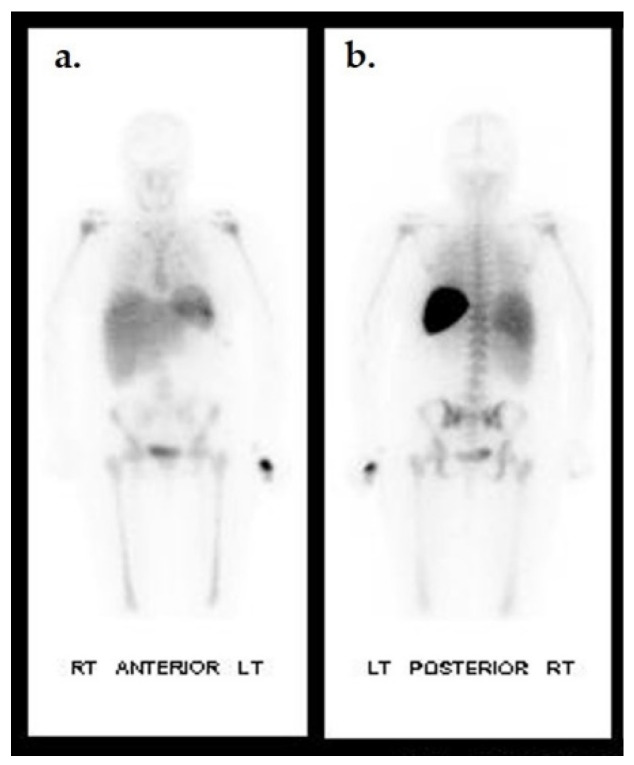
Figure 7.
Biodistribution of 111In-oxine WBC in a non-infected individual. Anterior (a) and posterior (b) planar images of a healthy patient 24 h after 111In-oxine-labeled WBC injection. Uptake is seen in the spleen, liver, and bone marrow.
Figure 8.
Dual tracer imaging using 111 n-WBC and 99mTc-sulfur colloid. Planar 111In-WBC (a,b) and 99mTc-sulfur colloid (c,d) images from a 62-year-old male with diabetes with a left foot abscess. There is spatial and intensity discordance in activity from the radionuclides. Anterior (a) and lateral (b) 111In-WBC images show focus of increased activity in the left mid foot. Anterior (c) and lateral (d) 99mTc-sulfur colloid images show diffuse activity throughout the mid and hind foot, suggesting the development of Charcot foot. Axial and sagittal 111In-WBC SPECT/CT (e) localized activity to an abscess in the plantar aspect of the left mid foot. Osteomyelitis was excluded.
Unfortunately, 111In-oxine WBC scans produce low-resolution images and require a long interval between patient injection and image acquisition (16–30 h); however, 99mTc-HMPAO (Figure 9) overcomes these problems by producing relatively high-quality images and requiring only a short interval between injection and image acquisition. Previous analyses have found that 99mTc-HMPAO WBC scans are highly sensitive and specific in the detection of OM (91% and 92%, respectively) [58]. At present dual-tracer scans are not performed with 99mTc WBC scans because no suitable marrow-targeting agents are commercially available for simultaneous imaging.
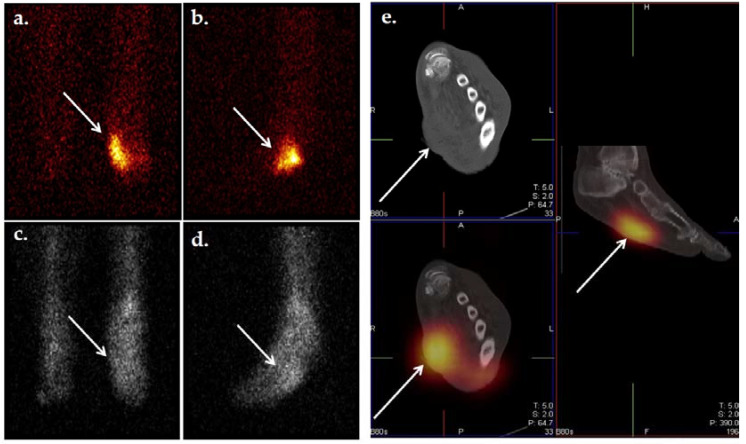
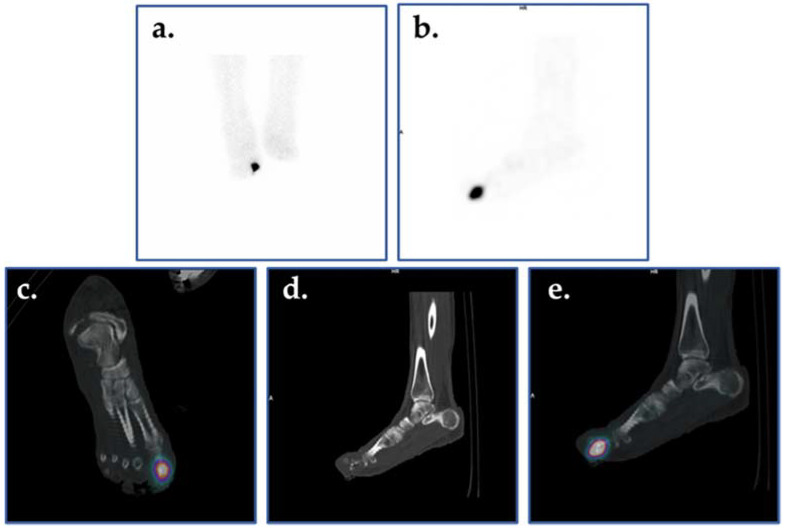
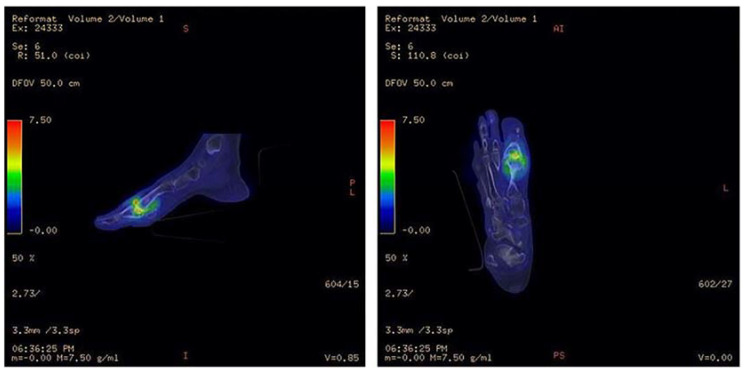
Figure 9.
Planar scintigraphic and SPECT/CT 99mTc-WBC images in an individual with DFI. The patient’s presentation was suspicious for OM involving the great toe. Planar images (a,b) demonstrate increased radiolabeled WBCs in the right forefoot, perhaps in the region of the toes. SPECT/CT (c–e) allows for precise localization of infection to the proximal phalanx of the right great toe.
WBC scans have also been used to assess the efficacy of antibiotic treatment in patients with DFO. Vouillarmet et al. [66] followed 45 patients with newly diagnosed DFO for either remission or treatment failure at one year. All patients were initially treated with appropriate antibiotics for six weeks, after which WBC-SPECT/CT was performed after infusion of 99mTc-HMPAO-labeled leukocytes. In the 23 with negative scans, antibiotics were discontinued; whereas, in the 22 with positive scans, antibiotic therapy was continued for six more weeks after which a second scan was obtained. At the one-year clinical follow-up, 22 of the 23 with negative scans at 6 weeks remained in remission. The remaining 22 were re-scanned at 12 weeks after completion of the second course of antibiotics. At their one-year follow-up, all 9 with a negative re-scan remained in remission; whereas, of the 13 with positive scans at 12 weeks, 7 were in remission at 1 year, and 6 had failed treatment. Overall, in this two-stage testing and treating study, the sensitivity and specificity of the 99mTc-HMPAO-labeled WBC-SPECT/CT were 82% and 86%, respectively, while the predictive value of a negative scan was 97% and the predictive value of a positive scan was 46% (Table 3). These results suggest that a 99mTc-HMPAO-labeled leukocyte scan will accurately predict remission near the end of antibiotic treatment. Moreover, based on reports using 111In-labeled WBCs, WBC scans appear well suited for monitoring the response of DFO patients to antibiotic therapy over time because the abnormal scan findings revert to normal within two–eight weeks after the start of successful antibiotic therapy [114].
Table 3.
Common Molecular Imaging Radiotracers.
| Radiotracer | Imaging Technique | Cellular Parameter | Mechanism of Localization |
Strengths | Weaknesses |
|---|---|---|---|---|---|
| 99mTc-MDP | Bone Scintigraphy | Osteoblastic bone formation | Chemiabsorption onto hydroxyapatite crystals of the bone matrix | Inexpensive High sensitivity |
Low specificity, dependent on area of exposed bone surface |
| 18F-NaF | Bone Scintigraphy or PET | Osteoblastic bone formation | Binds to and engages exchange reaction with hydroxyapatite crystals to form hydroxyfluoroapatite and fluoroapatite in the bone matrix | Smaller molecule than MDP with faster uptake, fast renal clearance, less background | Dependent on area of exposed bone surface |
| 99mTc-HMPAO | Labeled WBC (in vitro) | WBC migration to site of infection | WBC response to infection | Specificity, same day diagnosis of DFI | Simultaneous dual-tracer approach is not possible. Intensive preparation Expensive |
| 111I-oxine | Labeled WBC (in vitro) | WBC migration to site of infection | WBC response to infection | Simultaneous dual-tracer approach is possible | Intensive preparation Expensive Low image quality |
| 18F-FDG | PET/CT | Glucose uptake and metabolism | Taken up by WBC or immune cells with increased glucose disposal | Sensitivity, inherently tomographic | Specificity, Availability, and cost |
| 67/68Ga-Citrate | Scintigraphy/SPECT/CT (67Ga) or PET/CT (68Ga) | WBC activity at site of infection | Iron mimetic. Binds to transferrin in circulating plasma. Binds to lactoferrin released from dying WBCs and bacterial siderophores at the site of infection | Detects low grade infection Low toxicity, Bone/soft tissue distinction |
Delayed imaging High radiation dose, Non-specific accumulation in sterile inflammation or osteoblasts in healing bone |
The dual radiopharmaceutical/radiotracer method is a unique strength of planar and SPECT molecular imaging. In the typical application in DFI, one radiotracer labels WBCs while the other targets bone marrow (Figure 8). This approach is particularly powerful in the evaluation of suspected infection superimposed on confounding pathology such as Charcot neuropathic changes. Sixteen to thirty hours after the initial scan, delayed scans of the bone marrow are often performed using a longer-lived tracer if OM is suspected; however, bone deformities such as fracture or abnormal marrow distribution from atypical hematopoietic activity may lead to false positives [114]. To overcome this, visualization of the bone marrow using a second radiotracer (dual-tracer approach) may be adopted. Depending on the tracers used, some dual-tracer approaches allow for simultaneous visualization, while others such as 99mTc-HMPAO require clearance of the first tracer. 99mTc-labeled sulfur colloid is commonly used for this application because the molecule is phagocytized and sequestered by endothelial cells lining the bone marrow, reticular cells, macrophages, and macrophage precursors [115]. A positive diagnosis is indicated by greater or spatially discordant radiotracer uptake on the leukocyte scan compared with the bone marrow scan. Not only does this combined technique improve sensitivity and specificity, but it is also convenient and can be performed within one scan because of the gamma camera’s ability to distinguish the different energies of 99mTc-sulfur colloid and radionuclides such as 111In.
Although the dual-tracer approach is highly effective, it has limitations; principally, localizing the anatomic compartment of both tracers [79]. To improve this technique, WBC-labeled SPECT may be used in conjunction with CT to better visualize anatomically complex areas of the foot. This combined modality approach has been associated with improved distinction between soft tissue infection and OM as well as fewer false positives, resulting in fewer amputations as well as shorter hospital stays compared to anatomical imaging techniques alone [116]. Still, SPECT/CT in combination with labeled-WBC or labeled-WBC and sulfur colloid scan can be expensive, and in vitro leukocyte labelling is limited by the concentration of leukocytes obtained. Similar to planar imaging, radiotracer uptake—and therefore image quality—depends on the number and type of cells labeled as well as the host response to infection.
3.2.3. 18F-FDG PET and PET/CT
18F-Fluorodeoxyglucose (18F-FDG) is a radiolabeled glucose analogue which accumulates in cells that utilize glucose as an energy source. Facilitative glucose transporter proteins bring 18F-FDG across the cell membrane to the cytoplasm where it is subsequently phosphorylated by hexokinase. 18F-FDG is then trapped in the cell until it is dephosphorylated by glucose-6-phosphatase. The time between retention and clearance reflects the relative activity of glucose transporters, hexokinase, and glucose-6-phosphatase. In comparison to background activity, areas of decreased or absent uptake reflect low or absent glucose metabolism. Regions of increased 18F-FDG uptake reflect greater than normal rates of glucose metabolism [117]. Cells with many glucose transporters and a high metabolic rate, such as active inflammatory cells, display high FDG uptake. In addition, the 18F-FDG molecule is small and can easily penetrate poorly perfused areas, making it useful for imaging patients with vascular insufficiency [111]. When a dual modality approach of 18F-FDG-PET/CT (Figure 10) is adopted, simultaneous CT and PET images are acquired, and a hybrid three-dimensional image is generated that aids in the visualization of infection foci, similar to SPECT/CT imaging. PET-CT is a dual modality that uses the strength of CT and the power of PET tracers to quantify infection and inflammatory processes, and assess many other features of DFI [118]. From its high image resolution and radiotracer sensitivity and specificity, 18F-FDG PET/CT is useful in evaluating patients with characteristics that might otherwise compromise the accuracy of MRI or CT, including prior surgery, trauma, or orthopedic hardware [119,120,121]. One concern may be that 18F-FDG PET imaging could be compromised in patients with increased blood glucose and concomitant diabetic foot infection. A study by Yang et al. found otherwise. In a clinical evaluation of 21 patients with healthy serum glucose levels (<150 mg/dL) and 27 patients with elevated glucose levels (150–250 mg/dL), 18F-FDG PET demonstrated comparable sensitivity for detecting pedal OM between subjects with healthy glucose levels (sensitivity = 87.5%) and subjects with high serum glucose (sensitivity = 88.9%). Impressive levels of specificity of 96.8%, sensitivity of 88.3%, and accuracy of 93.8% were reported for both groups combined [122]. However, DFI patients in a clinical setting often have poorly regulated insulin levels and may have much higher glucose levels. In a retrospective study by Kagna et al., investigators addressed a potential issue that 18F-FDG activity may be altered in patients who received antibiotic therapy for a previous infection. The study assessed 18F-FDG PET in 176 patients who had received between 1–73 days of antibiotic therapy for an infectious process. They reported no false negative cases, correct identification of infectious foci in 61% of the study population, and true negatives in 29% of the study population; however, the study did not specifically target DFI [123].
Figure 10.
18F-FDG PET/CT illustrating infection of the metatarsophalangeal joint. Increased 18F-FDG uptake in the first metatarsophalangeal joint is seen in the bone and soft tissues. Signal uptake in this region is much greater than that of the surrounding healthy tissue, indicating pathologically increased glucose metabolism. Image has been reproduced with permission from Iyengar et al., J Clin Orthop Trauma, published by Elsevier, 2021 [117].
Although 18F-FDG imaging boasts an impressive sensitivity and specificity, it is prone to false positives. Patients with a recent surgical history exhibit increased 18F-FDG uptake in the healing tissue because of the increased cellular metabolic rate. Since FDG localizes to sites of increased metabolic activity rather than directly labelling bacteria, 18F-FDG results are skewed by the human immune system and cannot differentiate among infection, neoplasia, and sterile inflammation that is seen in Charcot [110].
3.2.4. Gallium Scan
Two isotopes of gallium are in clinical use, namely 67Ga and 68Ga. Gallium, as an iron mimetic, may be recruited into several processes that localize the isotope to sites of infection. First, gallium creates a complex with the ferric ion delivering glycoprotein transferrin. During uptake of 67/68Ga, much of the tracer is bound to circulating plasma transferrin. The increased blood flow and vascular permeability to the site of infection then facilitate the delivery and accumulation of 67/68Ga to infectious foci. Any 67/68Ga which is not bound to transferrin can also be bound to lactoferrin and circulating leukocytes, be directly taken up by bacteria, or form a complex with bacterial-produced siderophores, which are then transported into the bacterium and phagocytized by macrophages [111,124,125].
Historically, 67Ga scans were widely used in institutions with limited access to ex vivo WBC labeling or MRI [79]; however, as the availability of these methods has improved over time, 67Ga scans are now mainly used for the imaging of spinal infections. Studies have found other imaging modalities such as Sulesomab-labeled WBC scans (sensitivity = 67%, specificity = 85%) superior to 67Ga bone scintigraphy (sensitivity = 44%, specificity = 77%) [70]. 68Ga is more commonplace now compared to 67Ga. One study assessing the diagnostic potential of 68Ga-citrate PET/CT for osteomyelitis reported a sensitivity of 100%, a specificity of 76%, PPV of 85%, NPV 100%, and overall accuracy of 90%; however, this was not categorically evaluated in a DFI model [75]. Another study compared the kinetics of 18F-FDG, 68Ga-citrate, 11C-methionine, and 11C-donepezil in a porcine OM model. They found that 68Ga-citrate uptake was slow, irreversible, and limited by diffusion whereas the 18F-FDG uptake rate is determined by perfusion [126].
Because 68Ga possesses a shorter half-life and is a positron emitter, it yields higher quality images than those acquired with 67Ga. In addition, 68Ga may be produced by a generator or cyclotron, while 67Ga production requires the use of a cyclotron. As a result, 68Ga has displaced 67Ga as the gallium isotope of choice in some molecular imaging applications, such as neuroendocrine tumor imaging [127]. Although 68Ga retention in infectious sites is, in part, a bacteria-specific localization mechanism (siderophore binding), 18F-FDG PET/CT may still have superior sensitivity and specificity due to favorable emission features of 18F and glucose utilization by WBCs. However, a recent single institution study found that, for identifying infectious foci in patients with Staphylococcus aureus bacteremia, 68Ga citrate was comparable to 18F-FDG in the detection of osteomyelitis, whereas 18F-FDG resulted in a higher signal for detection of soft tissue infection [128].
4. Emerging Radiotracers in Infection Imaging
With the advancement of existing imaging technologies and the development of new ones, the diagnosis of DFI is becoming increasingly less invasive [129]. Although there have been advances in molecular imaging, further development is needed for targeting the infectious agent(s) rather than the host immune response, as many of the clinically available molecular imaging radiotracers do. Those that do target the microorganism localize to the site of infection by nonspecific mechanisms or are restricted by radiotracer production, availability, or cost. Several developments in the molecular imaging of infections target microorganism-specific metabolism and activity using the imaging modalities discussed in this review [130]. By targeting substances that are presented by or released from the pathogenic microorganism or microorganism-specific metabolic pathways, more radiotracers can be developed whose localization mechanism is independent of the host immune response. Some approaches that are being taken in the development of more specific radiotracers include utilizing radiolabeled antibiotics, antimicrobial peptides, and bacteria-specific agents such as radiolabeled sugars.
Radiolabeled antibiotics are relatively well-studied in the context of DFI imaging. Previously studied radiolabeled antibiotics target crucial features required for bacterial pathogenicity and survival, including the synthesis of folic acid required for nucleic acid synthesis, cell wall construction, cell membrane structure and function, transcription, and translation [131]. Antibiotic-resistant bacteria pose a major problem for radiolabeled antibiotics. Depending on the uptake mechanism, some bacteria may demonstrate radiotracer accumulation, while others may not. 99mTc-ciprofloxacin is the most studied antibiotic imaging agent and, although previous studies originally demonstrated its high specificity, subsequent studies have not been able to reproduce the result [132,133,134,135]. In vivo studies of its ability to differentiate gram-positive from gram-negative bacteria have reached contradictory conclusions. The main drawback to 99mTc-ciprofloxacin is its accumulation in dead bacteria [132], which leads to false positives, as suggested by a large multicenter clinical trial that also reported a sensitivity of 66.7%, a specificity of 85.7%, and an accuracy of 72% for both soft tissue infection and OM [136]. Several radiolabeled antibiotics have been studied to date (Table 4); however, the least well-studied are bacterial cell wall synthesis inhibitors such as vancomycin. The SPECT tracer 99 Tc-vancomycin, which was directly labeled, has demonstrated an affinity for S. aureus infectious foci. 99mTc-vancomycin labeled through 99mTc-Tc-HYNIC tetrazine click chemistry corroborated this observation with a three-fold uptake increase in a S. aureus infection site compared to controls [137]. Studies using fluorescently labeled vancomycin have also reported promising results [138], although isotopic-labeled vancomycin has shown greater uptake [139]. Although the 99mTc-vancomycin SPECT tracer may have a valuable place in the clinical management of DFI, the development of a vancomycin PET tracer may provide better image resolution.
Table 4.
Emerging Investigational Radiotracers of Infection Imaging.
| Radiotracer | Clinical Trials | Parameter | Mechanism of Localization | Strength/Weakness | References | |
|---|---|---|---|---|---|---|
| Radiolabeled Antibiotics | 99mTc-ciprofloxacin | Yes | Inhibition of DNA Synthesis | Bacterial DNA gyrase | High sensitivity (85.4–97.2%), ciprofloxacin already used in DFI treatment | [133,134,135,136] |
| Low specificity (66.7–81.7%), antibiotic resistant bacteria | ||||||
| 18F-fluoropropyl-trimethoprim | No | Inhibition of Folic Acid Synthesis | Inhibition of thymidine biosynthesis | Low background, high uptake in bacteria, detect inflammation from soft tissue infection vs sterile inflammation | [167] | |
| Antibiotic resistant bacteria | ||||||
| 99mTc-sulfonamides (pertechnetate, sulfadiazine) | No | Inhibition of Folic Acid Synthesis | Broad spectrum antibiotics, uptake in bacterial and fungal infections | [155] | ||
| Antibiotic resistant bacteria | ||||||
| 99mTc-vancomycin | No | Inhibition of bacterial cell wall synthesis | Binds to D-ala-D-ala lipid moiety | Specific for gram positive organisms | [137,138,139] | |
| Not specific for gram negative organisms Antibiotic resistant bacteria | ||||||
| Radiolabeled Sugars | 18F-FDS | Yes | Bacteria-Specific Glucose Sources for Carbohydrate Metabolism | Bacterial Metabolic Substrate | Antibiotic treatment monitoring, used in humans | [140,141] |
| Uptake by Enterobacteriaceae in the human gut | ||||||
| 18F-FAG | No | Sorbitol analogue utilized only by bacteria | Selective accumulation in E. coli, rapid accumulation, can differentiate infection from sterile inflammation, shows promise for monitoring response to treatment, small molecule | [142] | ||
| Not applied clinically | ||||||
| 18F-maltohexose | No | Bacterial-specific maltodextrin transporter | Can discriminate between live bacteria, metabolically inactive bacteria, and sterile inflammation | [143] | ||
| Poor signal-to-noise ratios, Not applied clinically | ||||||
| 6′′-18F-fluoromaltotriose | No | Bacterial-specific maltodextrin transporter | 2nd Gen, improved signal-to-noise ratio, bacterial-selective uptake in vitro and in vivo | [144,145] | ||
| Not applied clinically | ||||||
| Amino Acid Uptake | D-[methyl-11C] methionine | No | Bacterial Cell Wall Synthesis | Incorporation into the peptidoglycan | Distinguish sterile inflammation from infection in both gram—and gram +, broad sensitivity | [146,147] |
| Not applied clinically | ||||||
| D-5-[11C] glutamine | No | Incorporation into the peptidoglycan | Highly specific, high sensitivity for gram +, no uptake in sterile inflammation, fast clearance | [148] | ||
| Corroborating studies needed, not yet applied clinically | ||||||
| Vitamin Uptake | 124I-fialuridine (FIAU) | Yes | Endogenous TK enzyme of pathogenic bacteria | Trapped in the cell after phosphorylation | Reduced uptake in the presence of metal artifacts, | [154,168,169] |
| More clinical studies needed to assess clinical efficacy | ||||||
| 111In-biotin | No | Production of Fatty Acid | Bacterial growth factor | Essential growth factor for S. aureus | [149] | |
| Corroborating in vivo studies needed to assess clinical relevance | ||||||
| 99mTc-PAMA | No | Vitamin B12 Metabolism | Vitamin B12 derivative that accumulates in rapidly proliferating cells | High uptake in Gram + and Gram - | [150] | |
| Not applied clinically | ||||||
| 18F or 3H-PABA | No | Folic Acid Synthesis | Inhibition of Thymidine Synthesis | Accumulation in MRSA and other resistant organisms | [151] | |
| In vivo studies needed | ||||||
| Polyclonal Antibodies | 64Cu-NODAGA | No | Membrane protein binding of polyclonal antibody | Microbe-specific membrane polyclonal antibody binding | Particular to a specific microbe | [164,165] |
| Slow accumulation time | ||||||
| Siderophores | 68Ga-FOXE | No | Iron Transport | Accumulation of Siderophores in the cell | High uptake in S. aureus and fungi | [160,161,162] |
| Not used in DFI model | ||||||
| Immunoscintigraphy | 99mTc-sulesomab | Yes | WBC migration to infectious foci | Binds to antigen-90 on WBC membranes | Ease of preparation? Not sure about this | [63,71] |
| Dependent upon host response, expensive, limited availability | ||||||
| 99mTc-Besilesomab | Binds to antigen-95 on granulocytes and their precursors | Ease of preparation, good sensitivity and specificity | [63] | |||
| Dependent upon host response, expensive, limited availability |
Table adapted from Ankrah et al. (2018) [131].
Perhaps the most promising approach is to target the metabolism of the microorganism itself. One approach targets bacteria-specific transport systems for sugars. For example, sorbitol is used by gram-negative bacteria and has been developed as a tracer and been successfully studied in humans. One study, using 18F-fluorodeoxysorbitol (FDS) in humans, reported selective uptake in infected lesions, low background uptake, and fast plasma clearance [140]. However, uptake by Enterobacteriaceae of the gut was also seen. A more recent study of 18F-FDS demonstrated the sorbitol-specific pathway by observing uptake in clinically relevant strains of Enterobacterales. Following the in vitro study and a previous in vivo study in murine models, they acquired 18F-FDS PET/CT images in humans, demonstrating the safety and ability of 18F-FDS to differentiate infection from sterile inflammation [141]. In addition, it was used to monitor antibiotic treatment [140]. Bacterial cell wall amino sugars, present in gram positive as well as gram negative bacteria, have also been targeted for imaging. One study in rats used 2-deoxy-2-[18F]fluoroacetamido-D-glucopyranose (18F-FAG) to successfully identify E. coli infection from sterile inflammation by imaging with confirmation by histology [142]. Perhaps the most effective of the previously discussed radiolabeled sugars are maltodextrin-based imaging probes. The maltodextrin transport pathway is bacteria-specific and can discriminate between live bacteria, metabolically inactive bacteria, and inflammation induced by lipopolysaccharides. PET imaging performed with 18F-maltohexose demonstrated uptake in both gram positive and gram negative bacteria [143], although poor signal-to-noise ratios limit this tracer’s clinical application. The second generation tracer 6′′-18F-fluoromaltotriose has improved signal-to-noise ratios while maintaining bacterial-selective uptake in vitro and in vivo [144,145].
Amino acid uptake by bacteria has also been targeted for radiotracer development. D-stereoisomers of amino acids are found in bacterial cell walls but are not present in humans, making these amino acid isoforms promising pathogen-specific imaging targets. One analog of D-methionine, D-[methyl-11C]methionine, has been used to differentiate sterile inflammation from E. coli and S. aureus infections in a murine model, reporting an SUVmax 8–10 times higher (SUVmax = 0.8% ID/cc) in E.coli and S. aureus compared to [11C]L-methionine (SUVmax = 0.1% ID/cc) [146]. An in vitro study demonstrated the broad sensitivity of D-[methyl 11C] methionine across a panel of clinically relevant DFI pathogens, including E. coli, P. aeruginosa, P. mirabilis, S. aureus, S. epidermidis, and E. faecalis, with the greatest uptake occurring in S. aureus and P. aeruginosa [147]. A recent study compared the uptake of D-5-[11C]glutamine and L-5-[11C]glutamine isomers in a murine dual pathogen myositis model with localized MRSA and E.coli infections. Uptake of L-5-[11C]glutamine was higher in the heat-killed control site when compared to D-5-[11C]glutamine, indicating that the D-stereoisomer had better pathogen specificity. When D-5-[11C]glutamine was compared to 18F-FDG in a sterile inflammation model, 18F-FDG uptake was significantly higher, further demonstrating the specificity of D-5-[11C]glutamine for bacterial metabolism [148]. Although 11C-labeled amino acid-based imaging shows specificity, widespread adoption for clinical use may be limited because of the 20-min half-life of 11C.
Microorganisms may also be targeted based on their vitamin uptake. Since biotin is used in the production of fatty acids and is an essential growth factor for S. aureus and other bacteria, it has been explored for infection imaging. Although little data are currently available, an in vitro study using [111In]-In-DTPA-biotin reported selective uptake in S. aureus cultures [149]. In addition to targeting biotin, a radiolabeled vitamin B12 derivative, 99mTc-PAMA has also been used for infection imaging. Since PAMA accumulates in rapidly proliferating cells, the imaging agent has shown high uptake in S. aureus as well as E. coli in vitro and in vivo [150]. Microorganisms commonly use a compound called PABA to synthesize folic acid. A study using 3H-PABA reported accumulation in MRSA as well as other therapy resistant microorganisms. Uptake was 100× greater in the microorganisms than in mammalian cells. When labeled with 18F, uptake was seen in S. aureus [151].
Other targets for bacterial imaging include nucleoside analogs, peptidoglycan targeting aptamers, siderophores, and radiolabeled antibodies. The nucleoside analog 124I-fialuridine (124I-FIAU) is a bacterial tyrosine kinase substrate trapped in the cell following phosphorylation. Although it has been previously used to detect prosthetic joint infections [152], a more recent study found that tracer uptake was reduced in the presence of metal artifacts [153]. A clinical study of 16 patients with suspected musculoskeletal infection, who underwent 18F-FDG and 124I-FIAU PET/CT, reported a range of specificity of 51.7–72.7% and a maximum sensitivity of 100%; however, the minimum sensitivity was incalculable. The authors were also unable to come to a definitive conclusion on its diagnostic efficacy because of equivocal clinical findings in a large number of patients [154]. Aptamers are oligonucleotides that have been used to target the peptidoglycan cell wall. One group that used 99mTc-Antibac1 demonstrated its ability to distinguish between bacterial and fungal infections in vivo and in vitro [155].
Siderophores are small molecules with a high affinity for Fe(III) and are produced primarily by bacteria, but are also produced by virtually all microorganisms—including prokaryotes and eukaryotes [156]. Mammalian macrophages have been reported as sources of siderophores [157]. Organisms use these metal-seeking molecules for extra- or intra-cellular metal transport and storage. Biological processes such as DNA replication, transcription, oxidative stress responses, and respiration all require transition metals. Iron and zinc are among the most abundant in living organisms. Iron is an essential cofactor for respiration and central metabolism. Zinc is the catalytic center for many enzymes and is required for 5–6% of all protein functions [158]. Siderophores are often categorized into the following groups according to the chemical moiety that binds iron: catecholates, hydroxamates, and carboxylates. Mixed type siderophores have two or more of these moieties. Siderophores may actively alter their affinity for iron in order to bind other metals by increasing their expression of metal transporters [158]. Desferricoprogen—a natural siderophore produced by Penicillium chrysogenum and Neurospora crass—reportedly bound to both trivalent (Al(III) and In(III)) or divalent (Cu(II), Ni(II), Zn(II), and Fe(II) metal ions [159]. Transport systems and siderophore production are unregulated during infection and can accumulate in bacteria as well as fungi. An in vitro study which used 68Ga-ferrioxamine (68Ga-FOXE) showed high uptake in S. aureus; however, it was not corroborated in vivo [160]. In fungi, Aspergillus fumigatus-specific fluorescent siderophore conjugates were used to assess pulmonary aspergillosis in vitro [161]; however, recent in vivo studies have only been performed to assess biodistribution in pulmonary aspergillosis [162]. Further research regarding specificity, sensitivity, and use in additional infection models is needed.
In addition to in vitro labeling, WBCs may also be localized in vivo by using radiolabeled antibodies which target WBC antigens—a technique called immunoscintigraphy. For example, 99mTc-besilesomab uses the monoclonal antibody besilesomab to bind to the granulocyte-specific antigen-95. Antigen-95 is present on the membranes of granulocytes and their precursors. Within 45 min of initial injection, 10% of the 99mTc-besilesomab is bound to neutrophils, while 20% is unbound but localizes to the site of infection by nonspecific mechanisms [163]. A recent study of 119 patients with suspected OM compared planar 99mTc-HMPAO WBC and 99mTc-besilesomab imaging in the diagnosis of peripheral OM. They found that 99mTc-HMPAO had a lower sensitivity than 99mTc-besilesomab (59.0% and 74.8%, respectively); however, 99mTc-HMPAO had a higher specificity than 99mTc-besilesomab (79.5% and 71.8%, respectively) [63]. 99mTc-sulesomab also binds to an antigen found on leukocyte membranes—antigen-90—but only 3–6% of 99mTc-sulesomab is bound to circulating neutrophils, and 35% is found in bone marrow 24 h post-injection [111]. The results of a study of combat-related infections suggest that 99mTc-sulesomab may not possess sufficient accuracy (78% accuracy) for the identification of the foci of infections, but may have otherwise comparable diagnostic values (93% PPV, 62% NPV, 72% sensitivity, 88% specificity) [71]. One study of Yersinia enterocolitica infections used immunoPET with 64Cu-NODAGA-labeled Yersinia-specific polyclonal antibodies to target the membrane protein YadA and reported colocalization in a dose-dependent manner with bacterial lesions in a murine model [164]. Another study that used the [64Cu]NODAGA-IgG3 monoclonal antibody from mice to examine C. albicans infections demonstrated its accuracy in diagnosing infection in vitro as well as in vivo [165]. A meta-analysis found a sensitivity of 81% and specificity of 86% for anti-granulocyte scintigraphy with monoclonal antibodies [166]. The use of intact antibodies takes up to several days to accumulate near infectious foci. Clinically, this could be a limitation because of the need for rapid identification of the infection site. However, the use of antibody fragments may lead to faster accumulation time. Overall, it appears that radiolabeled antibodies do not have the ability to replace existing methods in a clinical setting because of the relatively low diagnostic values reported by the studies discussed above.
One recent therapeutic application of US used in DFI is cavitation, in which ultrasonic waves are used to create microbubbles which form from the dissolved gas that accumulates in the wound. The molecular purpose of this treatment is to induce compression and movement of the wound cells and small ions to increase protein synthesis and the permeability of vascular walls and cell membranes [133]. Ultimately, reduced inflammation, angiogenesis, and increased cell proliferation and recruitment are expected at the site of infection [134]. Currently, only three studies have used this technique and two of them demonstrated either complete wound closure or a significant reduction in wound area [135,136,137].
Although these emerging imaging probes are targeting the microorganism rather than the host response, the quantitative measures of probe retention are generally much lower than the clinically available tracers that target the host response. Further, while most of these have not been used in DFI specifically, this is an opportunity for the expansion of molecular imaging approaches to DFI.
5. Conclusions
Currently, the clinical diagnosis of diabetic foot soft tissue infection or OM relies on corroboration by multiple clinical tests, including the probe-to-bone test, pathology, and microbiology. Many of these techniques require special training or are prone to low inter-rater reliability [170]. While imaging modalities such as plain film radiography and MR have demonstrated reasonable agreement (62%) [170], the area of infection is only identified after morphological alteration. In general, molecular imaging approaches possess higher sensitivity and specificity and can indicate pathological changes well before morphological changes occur. Although radiotracers, such as 18F-FDG or those that are used in cellular carriers such as WBCs, are useful in assessing diabetic foot infection, the development of radiotracers that are microorganism-specific is of strong interest. This may have utility in assessing complex cases or treatment efficacy. A novel focus of interest is directly targeting osteoblasts and osteoclasts, both of which can be infected by microorganisms. As a result, changes have been observed in the production of proteins for immune modulation, bone resorption, and intra-cellular signaling. Undoubtedly, the infected cells must have an altered cell surface protein milieu that can be targeted for a new kind of molecular imaging assay.
Acknowledgments
We would like to thank the UT Southwestern librarians for assisting with the literature search for this review.
Author Contributions
Conceptualization, O.K.Ö.; writing—original draft preparation, K.R. and O.K.Ö.; writing—review and editing, K.R., A.S., A.P.C., K.B., R.W.H., D.K.W., L.C., H.H., J.L.F., A.C., L.L. and O.K.Ö.; graphics—A.S., K.R. and O.K.Ö.; supervision—O.K.Ö.; Contribution of clinical cases—J.L.F., A.C. and O.K.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge support of the American Diabetes Association grant number I-17-ICTS-056. OKO is supported in part by the Wechun Pak Professorship in Bone Biophysics.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the University of Texas Southwestern Medical Center (IRB: 112016-043, approved 26 April 2017). Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report. [(accessed on 8 October 2019)];2017 Available online: cdc.gov/media/releases/2017/p0718-diabetes-report.html.
- 2.Frykberg R.G., Wittmayer B., Zgonis T. Surgical management of diabetic foot infections and osteomyelitis. Clin. Podiatr. Med. Surg. 2007;24:469–482. doi: 10.1016/j.cpm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky B.A. Bone of contention: Diagnosing diabetic foot osteomyelitis. Clin. Infect. Dis. 2008;47:528–530. doi: 10.1086/590012. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P., Lu J., Jing Y., Tang S., Zhu D., Bi Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017;49:106–116. doi: 10.1080/07853890.2016.1231932. [DOI] [PubMed] [Google Scholar]
- 5.Wang A., Sun X., Wang W., Jiang K. A study of prognostic factors in Chinese patients with diabetic foot ulcers. Diabet. Foot Ankle. 2014;5:22936. doi: 10.3402/dfa.v5.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilliver S.C., Ashcroft G.S. Sex steroids and cutaneous wound healing: The contrasting influences of estrogens and androgens. Climacteric. 2007;10:276–288. doi: 10.1080/13697130701456630. [DOI] [PubMed] [Google Scholar]
- 7.Vuorlaakso M., Kiiski J., Salonen T., Karppelin M., Helminen M., Kaartinen I. Major Amputation Profoundly Increases Mortality in Patients with Diabetic Foot Infection. Front. Surg. 2021;8:655902. doi: 10.3389/fsurg.2021.655902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amoah V.M.K., Anokye R., Acheampong E., Dadson H.R., Osei M., Nadutey A. The experiences of people with diabetes-related lower limb amputation at the Komfo Anokye Teaching Hospital (KATH) in Ghana. BMC Res. Notes. 2018;11:66. doi: 10.1186/s13104-018-3176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghav A., Khan Z.A., Labala R.K., Ahmad J., Noor S., Mishra B.K. Financial burden of diabetic foot ulcers to world: A progressive topic to discuss always. Ther. Adv. Endocrinol. Metab. 2018;9:29–31. doi: 10.1177/2042018817744513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barshes N.R., Sigireddi M., Wrobel J.S., Mahankali A., Robbins J.M., Kougias P., Armstrong D.G. The system of care for the diabetic foot: Objectives, outcomes, and opportunities. Diabet. Foot Ankle. 2013;4:21847. doi: 10.3402/dfa.v4i0.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks C.W., Selvarajah S., Mathioudakis N., Sherman R.E., Hines K.F., Black J.H., Abularrage C.J. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs. Ann. Vasc. Surg. 2016;33:149–158. doi: 10.1016/j.avsg.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsky B.A., Berendt A.R., Deery H.G., Embil J.M., Joseph W.S., Karchmer A.W., LeFrock J.L., Lew D.P., Mader J.T., Norden C., et al. Diagnosis and treatment of diabetic foot infections. Plast. Reconstr. Surg. 2006;117:212S–238S. doi: 10.1097/01.prs.0000222737.09322.77. [DOI] [PubMed] [Google Scholar]
- 13.Ponirakis G., Elhadd T., Chinnaiyan S., Dabbous Z., Siddiqui M., Al-Muhannadi H., Petropoulos I.N., Khan A., Ashawesh K.A.E., Dukhan K.M.O., et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab. Res. Rev. 2020;36:e3286. doi: 10.1002/dmrr.3286. [DOI] [PubMed] [Google Scholar]
- 14.Juster-Switlyk K., Smith A.G. Updates in diabetic peripheral neuropathy. F1000Research. 2016;5:738. doi: 10.12688/f1000research.7898.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregg E.W., Gu Q., Williams D., de Rekeneire N., Cheng Y.J., Geiss L., Engelgau M. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res. Clin. Pract. 2007;77:485–488. doi: 10.1016/j.diabres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Candrilli S.D., Davis K.L., Kan H.J., Lucero M.A., Rousculp M.D. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J. Diabetes Complicat. 2007;21:306–314. doi: 10.1016/j.jdiacomp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Bader M.S. Diabetic foot infection. Am. Fam. Physician. 2008;78:71–79. [PubMed] [Google Scholar]
- 18.Ababneh A., Bakri F.G., Khader Y., Lazzarini P., Ajlouni K. Prevalence and Associates of Foot Deformities among Patients with Diabetes in Jordan. Curr. Diabetes Rev. 2020;16:471–482. doi: 10.2174/1573399815666191001101910. [DOI] [PubMed] [Google Scholar]
- 19.Lung C.W., Wu F.L., Zhang K., Liau B.Y., Townsend R., Jan Y.K. Using elastographic ultrasound to assess plantar tissue stiffness after waking at different speeds and durations. Appl. Sci. 2020;10:7498. doi: 10.3390/app10217498. [DOI] [Google Scholar]
- 20.Wanzou J.P.V., Sekimpi P., Komagum J.O., Nakwagala F., Mwaka E.S. Charcot arthropathy of the diabetic foot in a sub-Saharan tertiary hospital: A cross-sectional study. J. Foot Ankle Res. 2019;12:33. doi: 10.1186/s13047-019-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wukich D.K., Sung W. Charcot arthropathy of the foot and ankle: Modern concepts and management review. J. Diabetes Complicat. 2009;23:409–426. doi: 10.1016/j.jdiacomp.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Bandyk D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018;31:43–48. doi: 10.1053/j.semvascsurg.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Boulton A.J.M. Diabetes and the Nervous System. Volume 126. Elsevier; Amsterdam, The Netherlands: 2014. Diabetic neuropathy and foot complications; pp. 97–107. [DOI] [PubMed] [Google Scholar]
- 24.Giurato L., Meloni M., Izzo V., Uccioli L. Osteomyelitis in diabetic foot: A comprehensive overview. World J. Diabetes. 2017;8:135. doi: 10.4239/wjd.v8.i4.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulton A.J.M., Kirsner R.S., Vileikyte L. Neuropathic diabetic foot ulcers. N. Engl. J. Med. 2004;351:48–55. doi: 10.1056/NEJMcp032966. [DOI] [PubMed] [Google Scholar]
- 26.Lázaro-Martínez J.L., Tardáguila-García A., García-Klepzig J.L. Diagnostic and therapeutic update on diabetic foot osteomyelitis. Endocrinol. Diabetes Nutr. 2017;64:100–108. doi: 10.1016/j.endinu.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Lavery L.A., Crisologo P.A., La Fontaine J., Bhavan K., Oz O.K., Davis K.E. Are We Misdiagnosing Diabetic Foot Osteomyelitis? Is the Gold Standard Gold? J. Foot Ankle Surg. 2019;58:713–716. doi: 10.1053/j.jfas.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipsky B.A., Berendt A.R., Cornia P.B., Pile J.C., Peters E.J.G., Armstrong D.G., Deery H.G., Embil J.M., Joseph W.S., Karchmer A.W., et al. Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012;54:1679–1684. doi: 10.1093/cid/cis460. [DOI] [PubMed] [Google Scholar]
- 29.Lavery L.A., Ryan E.C., Ahn J., Crisologo P.A., Oz O.K., La Fontaine J., Wukich D.K. The Infected Diabetic Foot: Re-Evaluating the IDSA Diabetic Foot Infection Classification. Clin. Infect. Dis. 2019;70:1573–1579. doi: 10.1093/cid/ciz489. [DOI] [PubMed] [Google Scholar]
- 30.Lavery L.A., Armstrong D.G., Peters E.J.G., Lipsky B.A. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: Reliable or relic? Diabetes Care. 2007;30:270–274. doi: 10.2337/dc06-1572. [DOI] [PubMed] [Google Scholar]
- 31.Walton D.M., Minton S.D., Cook A.D. The potential of transdermal nitric oxide treatment for diabetic peripheral neuropathy and diabetic foot ulcers. Diabetes Metab. Syndr. 2019;13:3053–3056. doi: 10.1016/j.dsx.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Petrova N.L., Petrov P.K., Edmonds M.E., Shanahan C.M. Novel use of a Dektak 150 surface profiler unmasks differences in resorption pit profiles between control and Charcot patient osteoclasts. Calcif. Tissue Int. 2014;94:403–411. doi: 10.1007/s00223-013-9820-9. [DOI] [PubMed] [Google Scholar]
- 33.Ndip A., Williams A., Jude E.B., Serracino-Inglott F., Richardson S., Smyth J.V., Boulton A.J.M., Alexander M.Y. The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathy. Diabetes. 2011;60:2187–2196. doi: 10.2337/db10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rask-Madsen C., King G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013;17:20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burg M.B., Kador P.F. Sorbitol, osmoregulation, and the complications of diabetes. J. Clin. Investig. 1988;81:635–640. doi: 10.1172/JCI113366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanenberg R.J., Donofrio P.D. Neuropathic Problems of the Lower Limbs in Diabetic Patients. In: Bowker J.H., Pfeifer M.A., editors. Levin and O’Neal’s The Diabetic Foot. Elsevier; Amsterdam, The Netherlands: 2007. pp. 33–74. [Google Scholar]
- 37.Hobizal K.B., Wukich D.K. Diabetic foot infections: Current concept review. Diabet. Foot Ankle. 2012;3:1849. doi: 10.3402/dfa.v3i0.18409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insuela D., Coutinho D., Martins M., Ferrero M., Carvalho V. Cells of the Immune System. IntechOpen; London, UK: 2020. Neutrophil Function Impairment Is a Host Susceptibility Factor to Bacterial Infection in Diabetes. [Google Scholar]
- 39.Demirdal T., Sen P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res. Clin. Pract. 2018;144:118–125. doi: 10.1016/j.diabres.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Charles J.F., Nakamura M.C. Bone and the innate immune system. Curr. Osteoporos. Rep. 2014;12:1–8. doi: 10.1007/s11914-014-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seth R.B., Sun L., Ea C.-K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Gao L., Liesveld J., Anolik J., Mcdavid A., Looney R.J. IFNβ signaling inhibits osteogenesis in human SLE bone marrow. Lupus. 2020;29:1040–1049. doi: 10.1177/0961203320930088. [DOI] [PubMed] [Google Scholar]
- 43.Stacey H.J., Clements C.S., Welburn S.C., Jones J.D. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: A meta-analysis. Acta Diabetol. 2019;56:907–921. doi: 10.1007/s00592-019-01301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavery L.A., La Fontaine J., Bhavan K., Kim P.J., Williams J.R., Hunt N.A. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet. Foot Ankle. 2014;5 doi: 10.3402/dfa.v5.23575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saltoglu N., Ergonul O., Tulek N., Yemisen M., Kadanali A., Karagoz G., Batirel A., Ak O., Sonmezer C., Eraksoy H., et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int. J. Infect. Dis. 2018;70:10–14. doi: 10.1016/j.ijid.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Icks A., Scheer M., Morbach S., Genz J., Haastert B., Giani G., Glaeske G., Hoffmann F. Time-dependent impact of diabetes on mortality in patients after major lower extremity amputation: Survival in a population-based 5-year cohort in Germany. Diabetes Care. 2011;34:1350–1354. doi: 10.2337/dc10-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerr M. Supporting, Improving, Caring. Insight Health Economics; London, UK: 2012. Foot Care for People with Diabetes: The Economic Case for Change. [Google Scholar]
- 48.Lauri C., Leone A., Cavallini M., Signore A., Giurato L., Uccioli L. Diabetic Foot Infections: The Diagnostic Challenges. J. Clin. Med. 2020;9:1779. doi: 10.3390/jcm9061779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker E.A., Beaman F.D., Wessell D.E., Cassidy R.C., Czuczman G.J., Demertzis J.L., Lenchik L., Motamedi K., Pierce J.L., Sharma A., et al. ACR Appropriateness Criteria® Suspected Osteomyelitis of the Foot in Patients With Diabetes Mellitus. J. Am. Coll. Radiol. 2019;16:S440–S450. doi: 10.1016/j.jacr.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 50.Pineda C., Espinosa R., Pena A. Radiographic imaging in osteomyelitis: The role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin. Plast. Surg. 2009;23:80–89. doi: 10.1055/s-0029-1214160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Álvaro-Afonso F.J., Lázaro-Martínez J.L., García-Morales E., García-Álvarez Y., Sanz-Corbalán I., Molines-Barroso R.J. Cortical disruption is the most reliable and accurate plain radiographic sign in the diagnosis of diabetic foot osteomyelitis. Diabet. Med. 2019;36:258–259. doi: 10.1111/dme.13824. [DOI] [PubMed] [Google Scholar]
- 52.Llewellyn A., Kraft J., Holton C., Harden M., Simmonds M. Imaging for detection of osteomyelitis in people with diabetic foot ulcers: A systematic review and meta-analysis. Eur. J. Radiol. 2020;131:109215. doi: 10.1016/j.ejrad.2020.109215. [DOI] [PubMed] [Google Scholar]
- 53.Llewellyn A., Jones-Diette J., Kraft J., Holton C., Harden M., Simmonds M. Imaging tests for the detection of osteomyelitis: A systematic review. Health Technol. Assess. 2019;23:1–128. doi: 10.3310/hta23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinh M.T., Abad C.L., Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: Meta-analysis. Clin. Infect. Dis. 2008;47:519–527. doi: 10.1086/590011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Termaat M.F., Raijmakers P.G.H.M., Scholten H.J., Bakker F.C., Patka P., Haarman H.J.T.M. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: A systematic review and meta-analysis. J. Bone Joint Surg. Am. 2005;87:2464–2471. doi: 10.2106/00004623-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Enderle M.D., Coerper S., Schweizer H.P., Kopp A.E., Thelen M.H., Meisner C., Pressler H., Becker H.D., Claussen C., Häring H.U., et al. Correlation of imaging techniques to histopathology in patients with diabetic foot syndrome and clinical suspicion of chronic osteomyelitis. The role of high-resolution ultrasound. Diabetes Care. 1999;22:294–299. doi: 10.2337/diacare.22.2.294. [DOI] [PubMed] [Google Scholar]
- 57.Hoang D., Fisher S., Oz O.K., La Fontaine J., Chhabra A. Percutaneous CT guided bone biopsy for suspected osteomyelitis: Diagnostic yield and impact on patient’s treatment change and recovery. Eur. J. Radiol. 2019;114:85–91. doi: 10.1016/j.ejrad.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Lauri C., Tamminga M., Glaudemans A.W.J.M., Juárez Orozco L.E., Erba P.A., Jutte P.C., Lipsky B.A., IJzerman M.J., Signore A., Slart R.H.J.A. Detection of Osteomyelitis in the Diabetic Foot by Imaging Techniques: A Systematic Review and Meta-analysis Comparing MRI, White Blood Cell Scintigraphy, and FDG-PET. Diabetes Care. 2017;40:1111–1120. doi: 10.2337/dc17-0532. [DOI] [PubMed] [Google Scholar]
- 59.Al-Khawari H.A., Al-Saeed O.M., Jumaa T.H., Chishti F. Evaluating diabetic foot infection with magnetic resonance imaging: Kuwait experience. Med. Princ. Pract. 2005;14:165–172. doi: 10.1159/000084634. [DOI] [PubMed] [Google Scholar]
- 60.Lauri C., Glaudemans A.W.J.M., Campagna G., Keidar Z., Muchnik Kurash M., Georga S., Arsos G., Noriega-Álvarez E., Argento G., Kwee T.C., et al. Comparison of White Blood Cell Scintigraphy, FDG PET/CT and MRI in Suspected Diabetic Foot Infection: Results of a Large Retrospective Multicenter Study. J. Clin. Med. 2020;9:1645. doi: 10.3390/jcm9061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ledermann H.P., Morrison W.B., Schweitzer M.E. MR image analysis of pedal osteomyelitis: Distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223:747–755. doi: 10.1148/radiol.2233011279. [DOI] [PubMed] [Google Scholar]
- 62.La Fontaine J., Bhavan K., Lam K., Van Asten S., Erdman W., Lavery L.A., Öz O.K. Comparison Between Tc-99m WBC SPECT/CT and MRI for the Diagnosis of Biopsy-proven Diabetic Foot Osteomyelitis. Wounds Compend. Clin. Res. Pract. 2016;28:271–278. [PubMed] [Google Scholar]
- 63.Richter W.S., Ivancevic V., Meller J., Lang O., Le Guludec D., Szilvazi I., Amthauer H., Chossat F., Dahmane A., Schwenke C., et al. 99mTc-besilesomab (Scintimun) in peripheral osteomyelitis: Comparison with 99mTc-labelled white blood cells. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:899–910. doi: 10.1007/s00259-011-1731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parsons L.M. Pitfalls in the diagnosis of chronic osteomyelitis in the presence of a contiguous neuropathic ulcer. J. Cutan. Med. Surg. 2009;13((Suppl. 1)):S35–S37. doi: 10.2310/7750.2009.00008. [DOI] [PubMed] [Google Scholar]
- 65.Familiari D., Glaudemans A.W.J.M., Vitale V., Prosperi D., Bagni O., Lenza A., Cavallini M., Scopinaro F., Signore A. Can sequential 18F-FDG PET/CT replace WBC imaging in the diabetic foot? J. Nucl. Med. 2011;52:1012–1019. doi: 10.2967/jnumed.110.082222. [DOI] [PubMed] [Google Scholar]
- 66.Vouillarmet J., Moret M., Morelec I., Michon P., Dubreuil J. Application of white blood cell SPECT/CT to predict remission after a 6 or 12 week course of antibiotic treatment for diabetic foot osteomyelitis. Diabetologia. 2017;60:2486–2494. doi: 10.1007/s00125-017-4417-x. [DOI] [PubMed] [Google Scholar]
- 67.Lazaga F., Van Asten S.A., Nichols A., Bhavan K., La Fontaine J., Oz O.K., Lavery L.A. Hybrid imaging with 99mTc-WBC SPECT/CT to monitor the effect of therapy in diabetic foot osteomyelitis. Int. Wound J. 2016;13:1158–1160. doi: 10.1111/iwj.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erdman W.A., Buethe J., Bhore R., Ghayee H.K., Thompson C., Maewal P., Anderson J., Klemow S., Oz O.K. Indexing severity of diabetic foot infection with 99mTc-WBC SPECT/CT hybrid imaging. Diabetes Care. 2012;35:1826–1831. doi: 10.2337/dc11-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Przybylski M.M., Holloway S., Vyce S.D., Obando A. Diagnosing osteomyelitis in the diabetic foot: A pilot study to examine the sensitivity and specificity of Tc(99m) white blood cell-labelled single photon emission computed tomography/computed tomography. Int. Wound J. 2016;13:382–389. doi: 10.1111/iwj.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delcourt A., Huglo D., Prangere T., Benticha H., Devemy F., Tsirtsikoulou D., Lepeut M., Fontaine P., Steinling M. Comparison between Leukoscan (Sulesomab) and Gallium-67 for the diagnosis of osteomyelitis in the diabetic foot. Diabetes Metab. 2005;31:125–133. doi: 10.1016/S1262-3636(07)70178-7. [DOI] [PubMed] [Google Scholar]
- 71.Loessel C., Mai A., Starke M., Vogt D., Stichling M., Willy C. Value of antigranulocyte scintigraphy with Tc-99m-sulesomab in diagnosing combat-related infections of the musculoskeletal system. BMJ Mil. Health. 2021;167:8–17. doi: 10.1136/jramc-2019-001172. [DOI] [PubMed] [Google Scholar]
- 72.Wang G., Zhao K., Liu Z., Dong M., Yang S. A meta-analysis of fluorodeoxyglucose-positron emission tomography versus scintigraphy in the evaluation of suspected osteomyelitis. Nucl. Med. Commun. 2011;32:1134–1142. doi: 10.1097/MNM.0b013e32834b455c. [DOI] [PubMed] [Google Scholar]
- 73.Nawaz A., Torigian D.A., Siegelman E.S., Basu S., Chryssikos T., Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol. Imaging Biol. 2010;12:335–342. doi: 10.1007/s11307-009-0268-2. [DOI] [PubMed] [Google Scholar]
- 74.Aslangul E., M’bemba J., Caillat-Vigneron N., Coignard S., Larger E., Boitard C., Lipsky B.A. Diagnosing diabetic foot osteomyelitis in patients without signs of soft tissue infection by coupling hybrid 67Ga SPECT/CT with bedside percutaneous bone puncture. Diabetes Care. 2013;36:2203–2210. doi: 10.2337/dc12-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanni C., Errani C., Boriani L., Fantini L., Ambrosini V., Boschi S., Rubello D., Pettinato C., Mercuri M., Gasbarrini A., et al. 68Ga-citrate PET/CT for evaluating patients with infections of the bone: Preliminary results. J. Nucl. Med. 2010;51:1932–1936. doi: 10.2967/jnumed.110.080184. [DOI] [PubMed] [Google Scholar]
- 76.Martín Noguerol T., Luna Alcalá A., Beltrán L.S., Gómez Cabrera M., Broncano Cabrero J., Vilanova J.C. Advanced MR Imaging Techniques for Differentiation of Neuropathic Arthropathy and Osteomyelitis in the Diabetic Foot. Radiographics. 2017;37:1161–1180. doi: 10.1148/rg.2017160101. [DOI] [PubMed] [Google Scholar]
- 77.Fridman R., Bar-David T., Kamen S., Staron R.B., Leung D.K., Rasiej M.J. Imaging of diabetic foot infections. Clin. Podiatr. Med. Surg. 2014;31:43–56. doi: 10.1016/j.cpm.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Kavitha K.V., Tathare S., Kumbhar V., Bidaye A., Panse R., Maheshwari S., Gavankar A., Unnikrishnan A.G. Role of Radiology in the Management of Diabetic Foot Infections: A Report of Three Cases. Clin. Res. Foot Ankle. 2018;6:267. doi: 10.4172/2329-910X.1000267. [DOI] [Google Scholar]
- 79.Hochman M.G., Connolly C. The Diabetic Foot. Contemporary Diabetes. Humana; Cham, Switzerland: 2018. Imaging of Infection in the Diabetic Foot; pp. 55–94. [Google Scholar]
- 80.Commean P.K., Kennedy J.A., Bahow K.A., Hildebolt C.F., Liu L., Smith K.E., Hastings M.K., Ju T., Prior F.W., Sinacore D.R. Volumetric quantitative computed tomography measurement precision for volumes and densities of tarsal and metatarsal bones. J. Clin. Densitom. 2011;14:313–320. doi: 10.1016/j.jocd.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fayad L.M., Carrino J.A., Fishman E.K. Musculoskeletal infection: Role of CT in the emergency department. Radiographics. 2007;27:1723–1736. doi: 10.1148/rg.276075033. [DOI] [PubMed] [Google Scholar]
- 82.Beroukhim G., Shah R., Bucknor M.D. Factors Predicting Positive Culture in CT-Guided Bone Biopsy Performed for Suspected Osteomyelitis. Am. J. Roentgenol. 2019;212:620–624. doi: 10.2214/AJR.18.20125. [DOI] [PubMed] [Google Scholar]
- 83.Lee H.T., Sebro R. Predictors of positive bone cultures from CT-guided bone biopsies performed for suspected osteomyelitis. J. Med. Imaging Radiat. Oncol. 2020;64:313–318. doi: 10.1111/1754-9485.13012. [DOI] [PubMed] [Google Scholar]
- 84.Ergen F.B., Sanverdi S.E., Oznur A. Charcot foot in diabetes and an update on imaging. Diabet. Foot Ankle. 2013;4:21884. doi: 10.3402/dfa.v4i0.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mader J.T., Calhoun J. In: Bone, Joint, and Necrotizing Soft Tissue Infections. 4th ed. Baron S., editor. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 86.Patel S., Srivastava S., Singh M.R., Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019;112:108615. doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 87.Delikatny E.J., Poptani H. MR techniques for in vivo molecular and cellular imaging. Radiol. Clin. N. Am. 2005;43:205–220. doi: 10.1016/j.rcl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 88.Ahmadi M.E., Morrison W.B., Carrino J.A., Schweitzer M.E., Raikin S.M., Ledermann H.P. Neuropathic arthropathy of the foot with and without superimposed osteomyelitis: MR imaging characteristics. Radiology. 2006;238:622–631. doi: 10.1148/radiol.2382041393. [DOI] [PubMed] [Google Scholar]
- 89.Kumar Y., Khaleel M., Boothe E., Awdeh H., Wadhwa V., Chhabra A. Role of Diffusion Weighted Imaging in Musculoskeletal Infections: Current Perspectives. Eur. Radiol. 2017;27:414–423. doi: 10.1007/s00330-016-4372-9. [DOI] [PubMed] [Google Scholar]
- 90.Liao D., Xie L., Han Y., Du S., Wang H., Zeng C., Li Y. Dynamic contrast-enhanced magnetic resonance imaging for differentiating osteomyelitis from acute neuropathic arthropathy in the complicated diabetic foot. Skeletal Radiol. 2018;47:1337–1347. doi: 10.1007/s00256-018-2942-4. [DOI] [PubMed] [Google Scholar]
- 91.O’Connor J.P.B., Tofts P.S., Miles K.A., Parkes L.M., Thompson G., Jackson A. Dynamic contrast-enhanced imaging techniques: CT and MRI. Br. J. Radiol. 2011;84:S112–S120. doi: 10.1259/bjr/55166688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gordon Y., Partovi S., Müller-Eschner M., Amarteifio E., Bäuerle T., Weber M.-A., Kauczor H., Rengier F. Dynamic contrast-enhanced magnetic resonance imaging: Fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc. Diagn. Ther. 2014;4:147–164. doi: 10.3978/j.issn.2223-3652.2014.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdel Razek A.A.K., Samir S. Diagnostic performance of diffusion-weighted MR imaging in differentiation of diabetic osteoarthropathy and osteomyelitis in diabetic foot. Eur. J. Radiol. 2017;89:221–225. doi: 10.1016/j.ejrad.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 94.Padhani A.R., van Ree K., Collins D.J., D’Sa S., Makris A. Assessing the relation between bone marrow signal intensity and apparent diffusion coefficient in diffusion-weighted MRI. Am. J. Roentgenol. 2013;200:163–170. doi: 10.2214/AJR.11.8185. [DOI] [PubMed] [Google Scholar]
- 95.Mahesh M. The Essential Physics of Medical Imaging, Third Edition. Med. Phys. 2013;40:077301. doi: 10.1118/1.4811156. [DOI] [PubMed] [Google Scholar]
- 96.Van Vucht N., Santiago R., Lottmann B., Pressney I., Harder D., Sheikh A., Saifuddin A. The Dixon technique for MRI of the bone marrow. Skeletal Radiol. 2019;48:1861–1874. doi: 10.1007/s00256-019-03271-4. [DOI] [PubMed] [Google Scholar]
- 97.Komarraju A., Chhabra A. The Foot in Diabetes. Wiley; Hoboken, NJ, USA: 2020. Advanced Cross-Sectional Radiology-Ultrasound, Computed Tomography and Magnetic Resonance Imaging of the Diabetic Foot; pp. 169–185. [Google Scholar]
- 98.Donovan A., Schweitzer M.E. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics. 2010;30:723–736. doi: 10.1148/rg.303095111. [DOI] [PubMed] [Google Scholar]
- 99.Tan P.L., Teh J. MRI of the diabetic foot: Differentiation of infection from neuropathic change. Br. J. Radiol. 2007;80:939–948. doi: 10.1259/bjr/30036666. [DOI] [PubMed] [Google Scholar]
- 100.Fujii M., Armstrong D.G., Armsrong D.G., Terashi H. Efficacy of magnetic resonance imaging in diagnosing diabetic foot osteomyelitis in the presence of ischemia. J. Foot Ankle Surg. 2015;52:717–723. doi: 10.1053/j.jfas.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Donovan A., Rosenberg Z.S., Cavalcanti C.F. MR imaging of entrapment neuropathies of the lower extremity. Part 2. The knee, leg, ankle, and foot. Radiographics. 2010;30:1001–1019. doi: 10.1148/rg.304095188. [DOI] [PubMed] [Google Scholar]
- 102.Thakkar R.S., Del Grande F., Thawait G.K., Andreisek G., Carrino J.A., Chhabra A. Spectrum of high-resolution MRI findings in diabetic neuropathy. Am. J. Roentgenol. 2012;199:407–412. doi: 10.2214/AJR.11.7893. [DOI] [PubMed] [Google Scholar]
- 103.Vaeggemose M., Haakma W., Pham M., Ringgaard S., Tankisi H., Ejskjaer N., Heiland S., Poulsen P.L., Andersen H. Diffusion tensor imaging MR Neurography detects polyneuropathy in type 2 diabetes. J. Diabetes Complicat. 2020;34:107439. doi: 10.1016/j.jdiacomp.2019.107439. [DOI] [PubMed] [Google Scholar]
- 104.Vaeggemose M., Pham M., Ringgaard S., Tankisi H., Ejskjaer N., Heiland S., Poulsen P.L., Andersen H. Diffusion tensor imaging MR neurography for the detection of polyneuropathy in type 1 diabetes. J. Magn. Reson. Imaging. 2017;45:1125–1134. doi: 10.1002/jmri.25415. [DOI] [PubMed] [Google Scholar]
- 105.Hlis R., Poh F., Xi Y., Chhabra A. Diffusion tensor imaging of diabetic amyotrophy. Skeletal Radiol. 2019;48:1705–1713. doi: 10.1007/s00256-019-03182-4. [DOI] [PubMed] [Google Scholar]
- 106.Wu C., Wang G., Zhao Y., Hao W., Zhao L., Zhang X., Cao J., Wang S., Chen W., Chan Q., et al. Assessment of tibial and common peroneal nerves in diabetic peripheral neuropathy by diffusion tensor imaging: A case control study. Eur. Radiol. 2017;27:3523–3531. doi: 10.1007/s00330-016-4698-3. [DOI] [PubMed] [Google Scholar]
- 107.Pham M., Oikonomou D., Hornung B., Weiler M., Heiland S., Bäumer P., Kollmer J., Nawroth P.P., Bendszus M. Magnetic resonance neurography detects diabetic neuropathy early and with Proximal Predominance. Ann. Neurol. 2015;78:939–948. doi: 10.1002/ana.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Newhart H., Patterson J., Gunasekaran A., Pandey T., Kumar M., Kazemi N. The Incremental Value of Magnetic Resonance Neurography for the Neurosurgeon: Review of the Literature. World Neurosurg. 2019;122:331–341. doi: 10.1016/j.wneu.2018.10.212. [DOI] [PubMed] [Google Scholar]
- 109.Diez A.I.G., Fuster D., Morata L., Torres F., Garcia R., Poggio D., Sotes S., Del Amo M., Isern-Kebschull J., Pomes J., et al. Comparison of the diagnostic accuracy of diffusion-weighted and dynamic contrast-enhanced MRI with 18F-FDG PET/CT to differentiate osteomyelitis from Charcot neuro-osteoarthropathy in diabetic foot. Eur. J. Radiol. 2020;132:109299. doi: 10.1016/j.ejrad.2020.109299. [DOI] [PubMed] [Google Scholar]
- 110.Ruiz-Bedoya C.A., Gordon O., Mota F., Abhishek S., Tucker E.W., Ordonez A.A., Jain S.K. Molecular imaging of diabetic foot infections: New tools for old questions. Int. J. Mol. Sci. 2019;20:5984. doi: 10.3390/ijms20235984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palestro C.J. Radionuclide imaging of osteomyelitis. Semin. Nucl. Med. 2015;45:32–46. doi: 10.1053/j.semnuclmed.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Rosales C., Lowell C.A., Schnoor M., Uribe-Querol E. Neutrophils: Their Role in Innate and Adaptive Immunity 2017. J. Immunol. Res. 2017;2017:9748345. doi: 10.1155/2017/9748345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Censullo A., Vijayan T. Using Nuclear Medicine Imaging Wisely in Diagnosing Infectious Diseases. Open Forum Infect. Dis. 2017;4:ofx011. doi: 10.1093/ofid/ofx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meller J., Köster G., Liersch T., Siefker U., Lehmann K., Meyer I., Schreiber K., Altenvoerde G., Becker W. Chronic bacterial osteomyelitis: Prospective comparison of 18F-FDG imaging with a dual-head coincidence camera and 111In-labelled autologous leucocyte scintigraphy. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:53–60. doi: 10.1007/s00259-001-0661-9. [DOI] [PubMed] [Google Scholar]
- 115.PubChem Compound Summary for CID 76957057. Technetium Tc-99M Sulfur Colloid. [(accessed on 1 February 2021)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Technetium-Tc-99M-sulfur-colloid.
- 116.Heiba S., Kolker D., Ong L., Sharma S., Travis A., Teodorescu V., Ellozy S., Kostakoglu L., Savitch I., Machac J. Dual-isotope SPECT/CT impact on hospitalized patients with suspected diabetic foot infection: Saving limbs, lives, and resources. Nucl. Med. Commun. 2013;34:877–884. doi: 10.1097/MNM.0b013e32836370a6. [DOI] [PubMed] [Google Scholar]
- 117.NDA 21-870: [18F] FDG Injection. [(accessed on 1 February 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021870lbl.pdf.
- 118.Ruotolo V., Di Pietro B., Giurato L., Masala S., Meloni M., Schillaci O., Bergamini A., Uccioli L. A new natural history of Charcot foot: Clinical evolution and final outcome of stage 0 Charcot neuroarthropathy in a tertiary referral diabetic foot clinic. Clin. Nucl. Med. 2013;38:506–509. doi: 10.1097/RLU.0b013e318292eecb. [DOI] [PubMed] [Google Scholar]
- 119.Kwee T.C., Kwee R.M., Alavi A. FDG-PET for diagnosing prosthetic joint infection: Systematic review and metaanalysis. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:2122–2132. doi: 10.1007/s00259-008-0887-x. [DOI] [PubMed] [Google Scholar]
- 120.Shemesh S., Kosashvili Y., Groshar D., Bernstine H., Sidon E., Cohen N., Luria T., Velkes S. The value of 18-FDG PET/CT in the diagnosis and management of implant-related infections of the tibia: A case series. Injury. 2015;46:1377–1382. doi: 10.1016/j.injury.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 121.Lemans J.V.C., Hobbelink M.G.G., IJpma F.F.A., Plate J.D.J., van den Kieboom J., Bosch P., Leenen L.P.H., Kruyt M.C., Glaudemans A.W.J.M., Govaert G.A.M. The diagnostic accuracy of 18F-FDG PET/CT in diagnosing fracture-related infections. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:999–1008. doi: 10.1007/s00259-018-4218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang H., Zhuang H., Rubello D., Alavi A. Mild-to-moderate hyperglycemia will not decrease the sensitivity of 18F-FDG PET imaging in the detection of pedal osteomyelitis in diabetic patients. Nucl. Med. Commun. 2016;37:259–262. doi: 10.1097/MNM.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 123.Kagna O., Kurash M., Ghanem-Zoubi N., Keidar Z., Israel O. Does Antibiotic Treatment Affect the Diagnostic Accuracy of 18F-FDG PET/CT Studies in Patients with Suspected Infectious Processes? J. Nucl. Med. 2017;58:1827–1830. doi: 10.2967/jnumed.117.192062. [DOI] [PubMed] [Google Scholar]
- 124.Vorster M., Maes A., van de Wiele C., Sathekge M. Gallium-68 PET: A Powerful Generator-based Alternative to Infection and Inflammation Imaging. Semin. Nucl. Med. 2016;46:436–447. doi: 10.1053/j.semnuclmed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 125.Palestro C.J. Radionuclide Imaging of Musculoskeletal Infection: A Review. J. Nucl. Med. 2016;57:1406–1412. doi: 10.2967/jnumed.115.157297. [DOI] [PubMed] [Google Scholar]
- 126.Jødal L., Jensen S.B., Nielsen O.L., Afzelius P., Borghammer P., Alstrup A.K.O., Hansen S.B. Kinetic Modelling of Infection Tracers [18F]FDG, [68Ga]Ga-Citrate, [11C]Methionine, and [11C]Donepezil in a Porcine Osteomyelitis Model. Contrast Media Mol. Imaging. 2017;2017:9256858. doi: 10.1155/2017/9256858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Velikyan I. Prospective of 68Ga Radionuclide Contribution to the Development of Imaging Agents for Infection and Inflammation. Contrast Media Mol. Imaging. 2018;2018:9713691. doi: 10.1155/2018/9713691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salomäki S.P., Kemppainen J., Hohenthal U., Luoto P., Eskola O., Nuutila P., Seppänen M., Pirilä L., Oksi J., Roivainen A. Head-to-Head Comparison of 68Ga-Citrate and 18F-FDG PET/CT for Detection of Infectious Foci in Patients with Staphylococcus aureus Bacteraemia. Contrast Media Mol. Imaging. 2017;2017:3179607. doi: 10.1155/2017/3179607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uçkay I., Aragón-Sánchez J., Lew D., Lipsky B.A. Diabetic foot infections: What have we learned in the last 30 years? Int. J. Infect. Dis. 2015;40:81–91. doi: 10.1016/j.ijid.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 130.Signore A., Artiko V., Conserva M., Ferro-Flores G., Welling M.M., Jain S.K., Hess S., Sathekge M. Imaging Bacteria with Radiolabelled Probes: Is It Feasible? J. Clin. Med. 2020;9:2372. doi: 10.3390/jcm9082372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ankrah A.O., Klein H.C., Elsinga P.H. New Imaging Tracers for the Infected Diabetic Foot (Nuclear and Optical Imaging) Curr. Pharm. Des. 2018;24:1287–1303. doi: 10.2174/1381612824666180227094454. [DOI] [PubMed] [Google Scholar]
- 132.Siaens R.H., Rennen H.J., Boerman O.C., Dierckx R., Slegers G. Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J. Nucl. Med. 2004;45:2088–2094. [PubMed] [Google Scholar]
- 133.Malamitsi J., Giamarellou H., Kanellakopoulou K., Dounis E., Grecka V., Christakopoulos J., Koratzanis G., Antoniadou A., Panoutsopoulos G., Batsakis C., et al. Infecton: A 99mTc-ciprofloxacin radiopharmaceutical for the detection of bone infection. Clin. Microbiol. Infect. 2003;9:101–109. doi: 10.1046/j.1469-0691.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 134.Britton K.E., Wareham D.W., Das S.S., Solanki K.K., Amaral H., Bhatnagar A., Katamihardja A.H.S., Malamitsi J., Moustafa H.M., Soroa V.E., et al. Imaging bacterial infection with (99m)Tc-ciprofloxacin (Infecton) J. Clin. Pathol. 2002;55:817–823. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sarda L., Saleh-Mghir A., Peker C., Meulemans A., Crémieux A.-C., Le Guludec D. Evaluation of (99m)Tc-ciprofloxacin scintigraphy in a rabbit model of Staphylococcus aureus prosthetic joint infection. J. Nucl. Med. 2002;43:239–245. [PubMed] [Google Scholar]
- 136.Dutta P., Bhansali A., Mittal B.R., Singh B., Masoodi S.R. Instant 99mTc-ciprofloxacin scintigraphy for the diagnosis of osteomyelitis in the diabetic foot. Foot Ankle Int. 2006;27:716–722. doi: 10.1177/107110070602700911. [DOI] [PubMed] [Google Scholar]
- 137.Vito A., Alarabi H., Czorny S., Beiraghi O., Kent J., Janzen N., Genady A.R., Al-Karmi S.A., Rathmann S., Naperstkow Z., et al. A 99mTc-Labelled Tetrazine for Bioorthogonal Chemistry. Synthesis and Biodistribution Studies with Small Molecule trans-Cyclooctene Derivatives. PLoS ONE. 2016;11:e0167425. doi: 10.1371/journal.pone.0167425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Oosten M., Schäfer T., Gazendam J.A.C., Ohlsen K., Tsompanidou E., de Goffau M.C., Harmsen H.J.M., Crane L.M.A., Lim E., Francis K.P., et al. Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nat. Commun. 2013;4:2584. doi: 10.1038/ncomms3584. [DOI] [PubMed] [Google Scholar]
- 139.Yang C., Ren C., Zhou J., Liu J., Zhang Y., Huang F., Ding D., Xu B., Liu J. Dual Fluorescent- and Isotopic-Labelled Self-Assembling Vancomycin for in vivo Imaging of Bacterial Infections. Angew. Chem. Int. Ed. Engl. 2017;56:2356–2360. doi: 10.1002/anie.201610926. [DOI] [PubMed] [Google Scholar]
- 140.Yao S., Xing H., Zhu W., Wu Z., Zhang Y., Ma Y., Liu Y., Huo L., Zhu Z., Li Z., et al. Infection Imaging With 18F-FDS and First-in-Human Evaluation. Nucl. Med. Biol. 2016;43:206–214. doi: 10.1016/j.nucmedbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 141.Ordonez A.A., Wintaco L.M., Mota F., Restrepo A.F., Ruiz-Bedoya C.A., Reyes C.F., Uribe L.G., Abhishek S., D’Alessio F.R., Holt D.P., et al. Imaging Enterobacterales infections in patients using pathogen-specific positron emission tomography. Sci. Transl. Med. 2021;13:1–10. doi: 10.1126/scitranslmed.abe9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martínez M.E., Kiyono Y., Noriki S., Inai K., Mandap K.S., Kobayashi M., Mori T., Tokunaga Y., Tiwari V.N., Okazawa H., et al. New radiosynthesis of 2-deoxy-2-[(18)F]fluoroacetamido-D-glucopyranose and its evaluation as a bacterial infections imaging agent. Nucl. Med. Biol. 2011;38:807–817. doi: 10.1016/j.nucmedbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 143.Ning X., Seo W., Lee S., Takemiya K., Rafi M., Feng X., Weiss D., Wang X., Williams L., Camp V.M., et al. PET imaging of bacterial infections with fluorine-18-labeled maltohexaose. Angew. Chem. Int. Ed. Engl. 2014;53:14096–14101. doi: 10.1002/anie.201408533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gowrishankar G., Hardy J., Wardak M., Namavari M., Reeves R.E., Neofytou E., Srinivasan A., Wu J.C., Contag C.H., Gambhir S.S. Specific Imaging of Bacterial Infection Using 6″-18F-Fluoromaltotriose: A Second-Generation PET Tracer Targeting the Maltodextrin Transporter in Bacteria. J. Nucl. Med. 2017;58:1679–1684. doi: 10.2967/jnumed.117.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gabr M.T., Haywood T., Gowrishankar G., Srinivasan A., Gambhir S.S. New synthesis of 6″-[18F]fluoromaltotriose for positron emission tomography imaging of bacterial infection. J. Label. Compd. Radiopharm. 2020;63:466–475. doi: 10.1002/jlcr.3868. [DOI] [PubMed] [Google Scholar]
- 146.Neumann K.D., Villanueva-Meyer J.E., Mutch C.A., Flavell R.R., Blecha J.E., Kwak T., Sriram R., VanBrocklin H.F., Rosenberg O.S., Ohliger M.A., et al. Imaging Active Infection in vivo Using D-Amino Acid Derived PET Radiotracers. Sci. Rep. 2017;7:7903. doi: 10.1038/s41598-017-08415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stewart M.N., Parker M.F.L., Jivan S., Luu J.M., Huynh T.L., Schulte B., Seo Y., Blecha J.E., Villanueva-Meyer J.E., Flavell R.R., et al. High Enantiomeric Excess In-Loop Synthesis of d-[methyl-11C]Methionine for Use as a Diagnostic Positron Emission Tomography Radiotracer in Bacterial Infection. ACS Infect. Dis. 2020;6:43–49. doi: 10.1021/acsinfecdis.9b00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Renick P.J., Mulgaonkar A., Co C.M., Wu C., Zhou N., Velazquez A., Pennington J., Sherwood A., Dong H., Castellino L., et al. Imaging of Actively Proliferating Bacterial Infections by Targeting the Bacterial Metabolic Footprint with d-[5-11C]-Glutamine. ACS Infect. Dis. 2021;7:347–361. doi: 10.1021/acsinfecdis.0c00617. [DOI] [PubMed] [Google Scholar]
- 149.Erba P.A., Cataldi A.G., Tascini C., Leonildi A., Manfredi C., Mariani G., Lazzeri E. 111In-DTPA-Biotin uptake by Staphylococcus aureus. Nucl. Med. Commun. 2010;31:994–997. doi: 10.1097/MNM.0b013e32833ce32c. [DOI] [PubMed] [Google Scholar]
- 150.Baldoni D., Waibel R., Bläuenstein P., Galli F., Iodice V., Signore A., Schibli R., Trampuz A. Evaluation of a Novel Tc-99m Labelled Vitamin B12 Derivative for Targeting Escherichia coli and Staphylococcus aureus In Vitro and in an Experimental Foreign-Body Infection Model. Mol. Imaging Biol. 2015;17:829–837. doi: 10.1007/s11307-015-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ordonez A.A., Weinstein E.A., Bambarger L.E., Saini V., Chang Y.S., DeMarco V.P., Klunk M.H., Urbanowski M.E., Moulton K.L., Murawski A.M., et al. A Systematic Approach for Developing Bacteria-Specific Imaging Tracers. J. Nucl. Med. 2017;58:144–150. doi: 10.2967/jnumed.116.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Diaz L.A., Foss C.A., Thornton K., Nimmagadda S., Endres C.J., Uzuner O., Seyler T.M., Ulrich S.D., Conway J., Bettegowda C., et al. Imaging of musculoskeletal bacterial infections by [124I]FIAU-PET/CT. PLoS ONE. 2007;2:e1007. doi: 10.1371/journal.pone.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X.M., Zhang H.H., McLeroth P., Berkowitz R.D., Mont M.A., Stabin M.G., Siegel B.A., Alavi A., Barnett T.M., Gelb J., et al. [124I]FIAU: Human dosimetry and infection imaging in patients with suspected prosthetic joint infection. Nucl. Med. Biol. 2016;43:273–279. doi: 10.1016/j.nucmedbio.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 154.Cho S.Y., Rowe S.P., Jain S.K., Schon L.C., Yung R.C., Nayfeh T.A., Bingham C.O., Foss C.A., Nimmagadda S., Pomper M.G. Evaluation of Musculoskeletal and Pulmonary Bacterial Infections With [124I]FIAU PET/CT. Mol. Imaging. 2020;19:1536012120936876. doi: 10.1177/1536012120936876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferreira I.M., de Sousa Lacerda C.M., Dos Santos S.R., de Barros A.L.B., Fernandes S.O., Cardoso V.N., de Andrade A.S.R. Detection of bacterial infection by a technetium-99m-labeled peptidoglycan aptamer. Biomed. Pharmacother. 2017;93:931–938. doi: 10.1016/j.biopha.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 156.Aznar A., Dellagi A. New insights into the role of siderophores as triggers of plant immunity: What can we learn from animals? J. Exp. Bot. 2015;66:3001–3010. doi: 10.1093/jxb/erv155. [DOI] [PubMed] [Google Scholar]
- 157.Hilty J., George Smulian A., Newman S.L. Histoplasma capsulatum utilizes siderophores for intracellular iron acquisition in macrophages. Med. Mycol. 2011;49:633–642. doi: 10.3109/13693786.2011.558930. [DOI] [PubMed] [Google Scholar]
- 158.Zhi H., Behnsen J., Aron A., Subramanian V., Liu J.Z., Gerner R.R., Petras D., Green K.D., Price S.L., Camacho J., et al. Siderophore-Mediated Zinc Acquisition Enhances Enterobacterial Colonization of the Inflamed Gut. [(accessed on 17 August 2021)]. Available online: https://www.biorxiv.org/content/10.1101/2020.07.20.212498v1.full. [DOI] [PMC free article] [PubMed]
- 159.Enyedy É.A., Pócsi I., Farkas E. Complexation of desferricoprogen with trivalent Fe, Al, Ga, In and divalent Fe, Ni, Cu, Zn metal ions: Effects of the linking chain structure on the metal binding ability of hydroxamate based siderophores. J. Inorg. Biochem. 2004;98:1957–1966. doi: 10.1016/j.jinorgbio.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 160.Petrik M., Zhai C., Haas H., Decristoforo C. Siderophores for molecular imaging applications. Clin. Transl. Imaging. 2017;5:15–27. doi: 10.1007/s40336-016-0211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pfister J., Lichius A., Summer D., Haas H., Kanagasundaram T., Kopka K., Decristoforo C. Live-cell imaging with Aspergillus fumigatus-specific fluorescent siderophore conjugates. Sci. Rep. 2020;10:15519. doi: 10.1038/s41598-020-72452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pfister J., Bata R., Hubmann I., Hörmann A.A., Gsaller F., Haas H., Decristoforo C. Siderophore Scaffold as Carrier for Antifungal Peptides in Therapy of Aspergillus fumigatus Infections. J. Fungi. 2020;6:367. doi: 10.3390/jof6040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Love C., Palestro C.J. Nuclear medicine imaging of bone infections. Clin. Radiol. 2016;71:632–646. doi: 10.1016/j.crad.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 164.Wiehr S., Warnke P., Rolle A.-M., Schütz M., Oberhettinger P., Kohlhofer U., Quintanilla-Martinez L., Maurer A., Thornton C., Boschetti F., et al. New pathogen-specific immunoPET/MR tracer for molecular imaging of a systemic bacterial infection. Oncotarget. 2016;7:10990–11001. doi: 10.18632/oncotarget.7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morad H.O.J., Wild A.-M., Wiehr S., Davies G., Maurer A., Pichler B.J., Thornton C.R. Pre-clinical Imaging of Invasive Candidiasis Using ImmunoPET/MR. Front. Microbiol. 2018;9:1996. doi: 10.3389/fmicb.2018.01996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pakos E.E., Koumoullis H.D., Koumoulis H.D., Fotopoulos A.D., Ioannidis J.P.A. Osteomyelitis: Antigranulocyte scintigraphy with 99mTC radiolabeled monoclonal antibodies for diagnosis- meta-analysis. Radiology. 2007;245:732–741. doi: 10.1148/radiol.2452061877. [DOI] [PubMed] [Google Scholar]
- 167.Sellmyer M.A., Lee I., Hou C., Weng C.-C., Li S., Lieberman B.P., Zeng C., Mankoff D.A., Mach R.H. Bacterial infection imaging with [18F]fluoropropyl-trimethoprim. Proc. Natl. Acad. Sci. USA. 2017;114:8372–8377. doi: 10.1073/pnas.1703109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee J.T., Zhang H., Moroz M.A., Likar Y., Shenker L., Sumzin N., Lobo J., Zurita J., Collins J., van Dam R.M., et al. Comparative Analysis of Human Nucleoside Kinase-Based Reporter Systems for PET Imaging. Mol. Imaging Biol. 2017;19:100–108. doi: 10.1007/s11307-016-0981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rajamani S., Kuszpit K., Scarff J.M., Lundh L., Khan M., Brown J., Stafford R., Cazares L.H., Panchal R.G., Bocan T. Bioengineering of bacterial pathogens for noninvasive imaging and in vivo evaluation of therapeutics. Sci. Rep. 2018;8:12618. doi: 10.1038/s41598-018-30806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meyr A.J., Seo K., Khurana J.S., Choksi R., Chakraborty B. Level of Agreement With a Multi-Test Approach to the Diagnosis of Diabetic Foot Osteomyelitis. J. Foot Ankle Surg. 2018;57:1137–1139. doi: 10.1053/j.jfas.2018.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.