Abstract
Increasing evidence suggests that the gut microbiota and the brain are closely connected via the so-called gut–brain axis. Small intestinal bacterial overgrowth (SIBO) is a gut dysbiosis in which the small intestine is abundantly colonized by bacteria that are typically found in the colon. Though not a disease, it may result in intestinal symptoms caused by the accumulation of microbial gases in the intestine. Intestinal inflammation, malabsorption and vitamin imbalances may also develop. SIBO can be eradicated by one or several courses of antibiotics but reappears if the predisposing condition persists. Parkinson’s disease (PD) is a common neurodegenerative proteinopathy for which disease modifying interventions are not available. Sporadic forms may start in the gut years before the development of clinical features. Increased gastrointestinal transit time is present in most people with PD early during the course of the disease, predisposing to gut dysbiosis, including SIBO. The role that gut dysbiosis may play in the etiopathogenesis of PD is not fully understood yet. Here, we discuss the possibility that SIBO could contribute to the progression of PD, by promoting or preventing neurodegeneration, thus being a potential target for treatments aiming at slowing down the progression of PD. The direct symptomatic impact of SIBO and its impact on symptomatic medication are also briefly discussed.
Keywords: gut dysbiosis, gut–brain axis, microbiota, Parkinson’s disease, small intestinal bacterial overgrowth, SIBO
1. Introduction
Parkinson’s disease (PD) is a prevalent and highly disabling neurodegenerative disorder affecting more than 6 million people worldwide [1]. In addition to typical parkinsonism, which is considered the core clinical feature, PD has a variety of nonmotor manifestations, including hyposmia, dysautonomia (e.g., constipation, orthostatic hypotension, etc.), neurocognitive impairment and sleep disturbances, that significantly contribute to the overall disease burden [1,2,3,4,5]. Currently there are no therapeutic interventions that can prevent, delay, stop or reverse the progression of PD, but only symptomatic treatments, with net benefits that diminish as the disease advances (i.e., for parkinsonism mainly levodopa with a decarboxylase inhibitor, dopamine receptor agonists, catechol-O methyltransferase inhibitors, and monoamine oxidase inhibitors) [1,6].
Sporadic forms of PD are thought to result from complex interactions between different genetic and environmental factors [6,7,8,9]. From a pathological perspective, PD is mainly characterized by alpha-synuclein phosphorylation and misfolding resulting in amyloid formation with intraneuronal accumulation of insoluble aggregates (i.e., Lewy bodies and neuritis) and subsequent neurodegeneration, accompanied by neuroinflammatory changes [10,11]. Increasing evidence suggests that in most cases the pathology begins in the gut and/or in the olfactory mucosa, spreading to the brain in a prion-like fashion, along nerve trajectories [7,8,9,10,11,12,13,14,15,16]. Thus, the gut microbiota may play a potentially important role in the etiopathogenesis of sporadic PD [16,17,18,19,20,21,22].
The gut microbiota is a vast and complex ecosystem with high intra- and interindividual variability. It comprises of bacteria, archaea, fungi, viruses, and parasites (the latter completely eradicated in most people from developed countries) and has bidirectional connections with the brain, via the so-called gut–brain axis [23]. The crosstalk between the gut microbiota and the host is typically mutually beneficial; however, the structure and function of the microbiota is constantly changing, being susceptible to environmental factors, such as diet and antibiotic use, and alterations in its quantity or quality may promote the development of certain diseases, a state known as dysbiosis [23,24]. Gut dysbiosis is prevalent in PD but it is not clear if it precedes PD pathology or is a consequence of the gut dysmotility or other predisposing conditions related to PD [17,18,19,20,21,22]. Irrespective of the initial events, gut dysbiosis may lead to intestinal inflammation and barrier dysfunction (i.e., ‘leaky gut’), as well as blood–brain barrier alteration and an inflammatory shift of the brain milieu, the presence of these changes being increasingly documented in PD [25,26,27,28,29,30,31]. Additionally, gut dysbiosis might promote alpha-synuclein expression and aggregation in neurons of the enteric plexuses and other intestinal cells [32,33,34,35], may result in increased exposure to various microbial or nonmicrobial xenobiotics with direct or indirect proinflammatory or neurotoxic effects [23,24,36], and may cause nutritional imbalances (e.g., malabsorption, altered production of vitamins by the microbiota) [23,24], all these mechanisms potentially causing neuronal injury or interfering with the neuronal susceptibility to injury and therefore with the onset and progression of PD [37].
Small intestinal bacterial overgrowth (SIBO) is a gut dysbiosis in which the small intestine is excessively colonized by bacteria that are typically found in the large intestine [38]. It appears mainly in individuals that have predisposing conditions, such as decreased gastrointestinal motility / increased gastrointestinal transit time [38,39]. It may result in nonspecific symptoms, related mostly to intestinal distension caused by the gases produced by the excessive intestinal microbiota or by intestinal inflammation, but is not necessarily symptomatic and is not a disease [38,39]. SIBO-related symptoms typically disappear after one or several courses of antibiotics but recur if the predisposing condition persists [22,40,41]. Considering the potential immunomodulatory/proinflammatory effects of SIBO, its impact on intestinal barrier permeability and the potential effects on the levels of the microbial gases and other microbial products that are produced in the small intestine, the involvement of SIBO in the etiopathogeneses of neurodegenerative disorders seems plausible [42,43,44,45]. In this review we discuss the possibility that SIBO could contribute to the progression of PD, by promoting or preventing neurodegeneration, thus being a potential therapeutic target not only for symptomatic management (i.e., by improving gastrointestinal symptoms and increasing the efficacy of the oral medication used for the symptomatic treatment of PD) but also for delaying or stopping disease progression.
2. SIBO in Clinical Practice
The commonly accepted reference standard for defining SIBO is a bacterial count of at least 103 colony forming units (CFU) per mL of small intestine fluid, in either aerobic or anaerobic conditions [38,39,46,47,48,49,50]. There is no correlation between small intestine pathology and SIBO, and the recommended 103 UFC/mL cut-off was chosen because the concentration of plankton bacteria in the small intestine is typically lower than that [46,49,50,51,52,53,54]. Asymptomatic individuals may have small intestine bacterial counts as high as 105 UFC/mL, in the absence of any known predisposing conditions, therefore some authors suggest this would be a better cut-off [55,56]. Additionally, the reference range of the ‘healthy’ gut microbiota for each topographic niche is still under debate and the products of the microbiota (not only its count or composition) are also relevant for its impact on health, therefore the above definition for SIBO is not a true gold standard [38,46,57].
The collection of intestinal fluid required for the bacterial count is performed by endoscopy (an invasive procedure, albeit minimal, that is more resource-consuming), therefore carbohydrate breath tests are increasingly recommended and used in clinical practice for identifying SIBO in people with predisposing conditions and persistent symptoms of unknown cause [38,46]. These tests are useful for the evaluation of several other common gastrointestinal conditions, are non-invasive, relatively inexpensive, widely available, and safe [38,46,55]. Moreover, unlike the small intestine cultures, breath tests offer both a quantitative and a qualitative assessment of the small intestine microbiota, the microbial gases predominantly produced in the context of SIBO having potentially different implications in health and disease [38,46]. The rationale behind performing breath tests for SIBO (see Table 1) is that bacteria, archaea, and fungi residing in the digestive tract metabolize simple carbohydrates to gases which are not produced by human cells; these gases easily pass into the blood stream and are subsequently exhaled. The most useful substrates for identifying SIBO are glucose, metabolized by the intestinal microbiota (mostly bacteria) to hydrogen (H2), and lactulose, metabolized to H2 and methane [46,47]. The recommended cut-off value for H2 is a rise of at least 20 ppm by 90 min following 75 g glucose or 10 g lactulose, administered orally, à jeun [46,55]. The cut-off level for methane is a level of at least 10 ppm, any time during the test (i.e., at baseline, à jeun, or up to 2 h following the ingestion of 10 g lactulose) [46,55]. Using 103 CFU/mL as reference, the positive and negative predictive values for the above tests is around 70%, increasing when both H2 and methane are tested [38,46,55].
Table 1.
Carbohydrate breath tests for SIBO.
| Breath Test | Interpretation |
|---|---|
|
Glucose test * (oral glucose, 75 g) |
SIBO (H2-predominant) = raise in H2 levels of ≥20 ppm by 90 min |
|
Lactulose test (oral lactulose, 10 g) |
H2-predominant SIBO = raise in H2 levels of ≥20 ppm by 90 min Methane-predominant SIBO = methane level of ≥10 ppm, anytime for up to 2 h or at baseline H2-/methane-predominant SIBO = both of the above |
Although it is not a disease, SIBO can cause intestinal symptoms related to the accumulation of microbial gases in the small intestine, triggered or exacerbated several minutes to hours after a meal (e.g., bloating, belching, flatulence, abdominal pain or discomfort, etc.), as well as changes in the intestinal transit times (i.e., diarrhea or, more rarely, constipation), and altered characteristics of the stool (i.e., consistency, shape, color, smell) [38,39,58,59]. Severe SIBO can also lead to macronutrient malabsorption (carbohydrate, fat, proteins) resulting in weight loss, as well as to vitamin imbalances (i.e., deficiency or excess, especially in relation to vitamins produced by the microbiota and lipid-soluble vitamins, such as vitamin D), while in rare instances it may also cause intestinal inflammation (e.g., ileitis) resulting in abdominal pain, decreased intestinal transit time, bloody stools, etc. [38,39,60,61]. Thus, SIBO can be suspected in all individuals with predisposing conditions, especially if they have bloating and/or other unexplained intestinal symptoms, unexplained weight loss, macronutrient malabsorption and/or vitamin imbalances [38,58,59].
Several patterns of H2 and methane production have been described in SIBO, mainly: hydrogen-predominant, methane-predominant, and mixed H2/methane bacterial overgrowth, the first two being the most studied [38,46,47]. The patients with either pattern may share similar symptoms, but those with excessive methane production are about five times more likely to experience constipation, its severity directly correlating with the methane levels (i.e., evidence suggests that methane may decrease the motility of the colon); moreover, the efficiency of different antibiotic regimes seems to differ between these groups, making the distinction clinically relevant [38,46,47,55].
3. SIBO and PD
SIBO is relatively common in people with PD, including those with recent onset of motor symptoms, around half of the patients with PD testing positive for SIBO, compared with only up to a quarter in the general population [42,43,44,45]. Notably, however, the prevalence may be as low as 14%, or as high as 67%, depending on the demographic and clinical characteristic of the population included in the study, as well as on the testing method that has been used (i.e., small intestine fluid bacterial count, H2 glucose breath testing, or H2 and methane lactulose breath testing, the highest percentages being reported in studies using both glucose and lactulose H2 and methane breath testing and the lowest in studies using H2 glucose testing alone) [43,62,63,64,65,66]. Additional conditions and medications interfering with the gut microbiota also need to be excluded for a reliable account of the direct association between SIBO and PD [45,62]. Despite this variability, a recently published meta-analysis found a strong association between SIBO and PD when compared to healthy controls, with a pooled prevalence of SIBO in patients with PD of 47% (95% confidence interval 36–56), higher in Western countries (i.e., 52%, compared to 33% in Eastern countries), and an overall odds ratio of SIBO in patients with PD of 5.22 (95% confidence interval 3.33–8.19, p < 0.00001) [45].
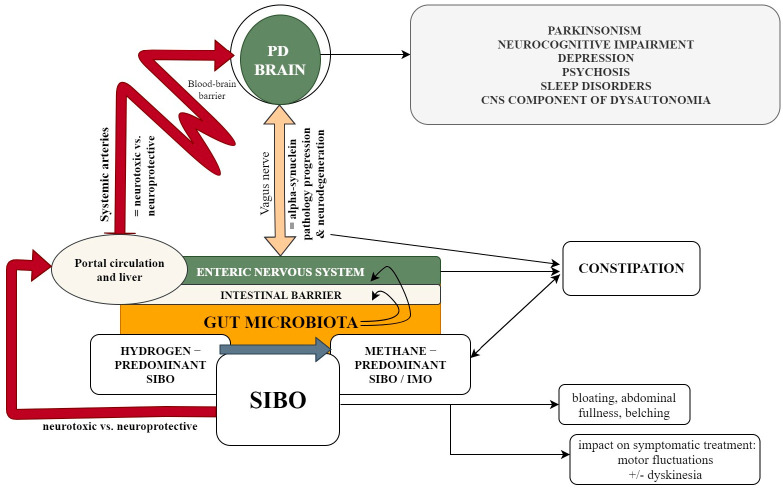
The relation between SIBO and specific characteristics of sporadic PD is not straightforward, but it is biologically plausible that SIBO might influence the etiopathogenesis, clinical phenotype and progression of sporadic PD, with potentially different effects of H2-predominant versus methane-predominant SIBO [17,25,26,27,28,29,30,37,62,65]. Furthermore, it is plausible that SIBO may interfere with the bioavailability and absorption of enterically administered medication used for the symptomatic treatment of PD, and increase or decrease gut motility, thus potentially exacerbating or alleviating PD-related intestinal symptoms [44,67,68,69]. In respect to its involvement in the etiopathogenesis of PD, SIBO might induce a local inflammatory response that disrupts the intestinal barrier integrity by affecting tight junctions and subsequently increases intestinal permeability, which may favor exposure of the intestinal mucosa to bacterial exotoxins, such as lipopolysaccharide (LPS) [37,70,71]. LPS and other products of the gut microbiota ascribed to local SIBO-related inflammatory changes may trigger and/or enhance alpha-synuclein amyloidogenesis and along with other xenobiotic compounds may increase the neuronal susceptibility to neurodegeneration by direct or indirect mechanisms [17,27,28,29,30,37]. Additionally, SIBO could contribute to nutritional imbalances that may increase neuronal susceptibility to injury [25,26,27,28,29,37,62,65]. On the other hand, the pathophysiology of SIBO in PD probably entails the early impairment of gastrointestinal motility that occurs in people with PD, due to the involvement of the enteric nervous system as well as of the autonomic nervous system, especially the vagus nerve nuclei and vagus nerve, which innervates the stomach, the small and large intestines, and the appendix [10]. All these are summarized in Figure 1.
Figure 1.
The relation between SIBO and specific characteristics of sporadic PD. The figure illustrates the potential relations between SIBO and specific clinical characteristics of sporadic PD, highlighting the potential pathogenic mechanisms that underlies these relations. SIBO may result in increased intestinal production of H2 and/or methane, with potential neuroprotective and neurotoxic consequences; H2 may also modulate the composition and function of the microbiota, for example by being substrate for hydrogenotrophic methanogenic archaea (see the main text), which may have potential indirect consequences on PD progression, both beneficial and deleterious. Gut dysbiosis, including SIBO, may alter the integrity of the intestinal barrier, thus exposing the central nervous system, via the neuronal and humoral pathways of the gut–brain axis, to microbial and non-microbial xenobiotic compounds that may have amyloidogenic or neurotoxic effects. On a clinical level SIBO may exacerbate (or alleviate) gastrointestinal symptoms related to PD, a potential connection existing between methane overproduction and the presence of constipation. SIBO may also interfere with drug bioavailability, interfering with their overall effect (i.e., symptomatic improvement, occurrence of side effects); this may be of particular importance in patients with advanced PD, that have motor fluctuations and dyskinesia.
There have been inconsistent findings regarding the association of SIBO with more advanced PD or longer disease duration [42,43,44,45]. The above-mentioned meta-analysis failed to identify any statistically significant differences of SIBO prevalence in people with PD and constipation, bloating, diarrhea, or longer disease duration [45]. However, H2-predominant versus methane-predominant SIBO may have different gastrointestinal effects, canceling out statistical significance when SIBO is analyzed overall, and people with PD typically already have increased orocecal transit times and constipation which could be improved to a certain degree by SIBO in some individuals [43]. Different studies found that the presence of SIBO in people with PD is associated with the severity of parkinsonism but not necessarily with the severity of levodopa-related motor complications (i.e., motor fluctuations and dyskinesia), while others found that people with SIBO and PD have worse motor fluctuations, more specifically longer daily off times and increased frequency of delayed on [43,45]. Moreover, improvement of motor fluctuations has been achieved after SIBO eradication [43,63,72], indirect evidence suggesting that Enterococcus species that overpopulate the small intestine in people with SIBO and express decarboxylases could metabolize levodopa prior to its absorption [68,69]. Concerning the severity of the intestinal symptoms, the findings are also inconclusive, but milder intestinal symptoms, especially constipation, have been reported in patients with PD and SIBO [44]. Though weight loss is a common occurrence in advanced PD, no correlation between SIBO and body weight or weight loss was found [62].
Available evidence suggests that medication used for the symptomatic improvement of parkinsonism in people with PD, especially catechol-O methyltransferase inhibitors, may also interfere with the composition of the gut microbiota [19,73]. Similarly, the PD medication could potentially interfere with the risk of developing SIBO, for example by increasing the gastrointestinal transit times or by facilitating bacterial colonization (i.e., some bacteria produce and use dopamine) [73,74]. In this respect, the available studies found no correlation between PD medication and SIBO, and the eradication of SIBO did not affect the pharmacokinetics of levodopa, despite improving motor fluctuations [43]. However, the available epidemiological data are scarce, and effect of levodopa-based products (as well as that of other drugs) might be difficult to assess, since almost all the patients included in the studies are treated. It is plausible to consider that the risk of SIBO could be higher in people with advanced PD treated with levodopa-based products administered by continuous intestinal infusion (e.g., levodopa/carbidopa intestinal gel), but data are lacking.
4. Molecular Hydrogen and PD
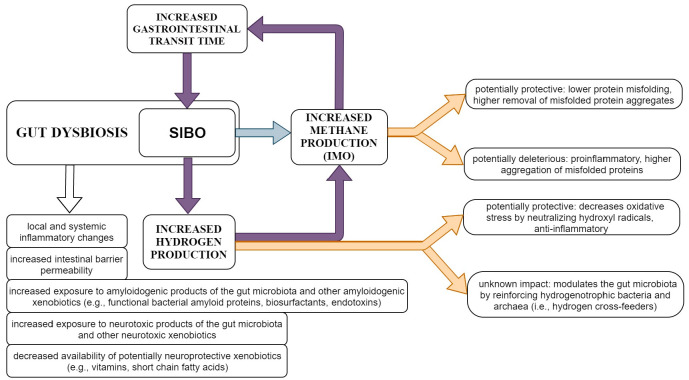
H2 is a bioactive gas utilized both by human and microbial cells [75,76]. In humans, H2 is exclusively produced by the microbiota (e.g., Blautia spp., Clostridium spp., etc.), mainly within the gut, via food fermentation—i.e., carbohydrate breakdown, more specifically glycolysis and acetate formation; small amounts are also produced by bacteria outside the gut [43,63,75,77]. H2 has a key role in many microbial metabolic pathways and is used as nutrient by certain bacteria and archaea that are called hydrogenotrophs or H2 cross-feeders, namely sulfate-reducing bacteria, acetogenic bacteria, and methanogenic archaea, that convert H2 into hydrogen sulfide, acetate, and methane, respectively [75]. Therefore, H2 modulates the composition and functionality of the microbiota, mainly by reinforcing hydrogen sulfide- and acetate-producing bacteria, and methane-producing archaea [75]. The relation between SIBO-related H2 and methane (over)production as well as the general mechanisms by which SIBO could contribute to the progression of sporadic PD are summarized in Figure 2.
Figure 2.
Main mechanisms underlying the relation between SIBO-related H2 and methane (over)production and the progression of sporadic PD. The figure illustrates the specific mechanisms that may link the intestinal production of H2 and methane with disease progression in people with sporadic PD and SIBO. General mechanisms related to gut dysbiosis, including SIBO, are also summarized. Depending on the functional characteristics of the SIBO microbiota (as well as particularities of the host) H2 and methane may have both beneficial and deleterious effects, requiring further investigation. Better understanding of these relations could offer means for personalized interventions and precision medicine in PD.
Oxidative stress represents the imbalance between the production and elimination of reactive oxygen species, leading to excessive oxidation reactions, with increased excitotoxicity and mitochondrial dysfunction, resulting in neuronal damage, even apoptosis [78]. This makes oxidative stress one of the main pathogenetic players in neurodegenerative disorders, including PD [78,79]. A recent experimental study by Musgrove et al. [79] found that oxidative stress increases the production of oxidatively-modified alpha-synuclein (including nitrated alpha-synuclein), promotes its pathological aggregation and exacerbates neuronal loss in the dorsal motor nucleus of the vagus nerve [79]. Furthermore, the study found that nitrated forms of alpha-synuclein are highly transferable, excessive oxidative stress enhancing the interneuronal alfa-synuclein transfer, therefore facilitating the spread of alpha-synuclein pathology [79]. At cellular levels, H2 acts as an electron sink, that can accept a new bond or a lone pair of electrons, being part of many chemical reactions, mainly mitigating oxidative stress by neutralizing hydroxyl radicals [43,63,75,76,77]. Considering the involvement of oxidative stress in the pathogenesis of PD and the antioxidant properties of H2, the presence of lower amounts of H2 may have a negative impact on PD progression [63,76,77,78].
Increasing evidence suggests that the gut microbiota of people with PD produces lower net levels of H2 [63,80,81]. However, data on the prevalence of methane-predominant versus H2-predominant SIBO in people with PD is not available yet [43,45,63]. The amount of H2 produced by the microbiota depends on its composition and metabolism [81]. The greatest H2 producers are Firmicutes, a phylum that has been shown to be altered both in terms of relative abundance and composition in people with PD [20,21,75,81]. A simulation of the gut H2 production based on the previously reported microbiota composition found a more than 2-fold reduction in the amount of produced H2 in patients with PD compared with controls [80]. Interestingly, a recent study on 20 patients with PD and 20 healthy controls found that the gut microbiota of PD had significantly higher levels of Desulfovibrio, a hydrogen sulfide producing hydrogenotrophic bacteria, the authors speculating that the LPS, hydrogen sulfide and magnetite produced by the strains of the Desulfovibrio bacteria could trigger alpha-synuclein conformational changes and aggregation [82]. Concurrently, hydrogen sulfide was shown to have both beneficial (i.e., neuroprotection, increase in neurogenesis) and deleterious effects in animal models of PD [82,83]. The potential impact of methanogen archaea is discussed below.
Several PD animal model studies found evidence that enterally administered H2 may be neuroprotective, downregulating peripheral inflammation, neutralizing toxic hydroxyl radicals, reducing oxidative stress within the brain, and preserving cerebral vascular reactivity [43,63,77,84,85]. In this respect, Fu Y. et al. [77] found a neuroprotective effect of H2-enriched drinking water in a mouse model of PD, with a reduction of dopamine neurons loss by 16% compared to the control group [77]. Another hemi-parkinsonism mouse model study found beneficial effects of a Si-based agent that generates large amounts of H2, probably by reducing oxidative stress [86].
The evidence on the potential effect of H2 in people with PD is scarce, but a few trials have been conducted. A 48-week pilot randomized placebo-controlled double-blind trial in humans found that H2-enhanced water is safe and significantly improves motor scores in patients with PD treated with levodopa [87]. However, a subsequent multicenter randomized placebo-controlled double-blind clinical trial failed to identify any beneficial effects in patients with PD [88]. Another more recent 16-week randomized placebo-controlled double-blind study found that H2 gas inhalation is safe but has no beneficial effects in people with PD [89].
5. Gut Methanogenesis and Possible Links to PD
Methanogens are anaerobic microorganisms that produce methane as a by-product of the metabolization of simple substrates such as H2 and carbon dioxide [75,81,90]. During the last five decades, different methanogen species have been identified in a variety of human biological specimens, such as periodontal, intestinal, colonic, or vaginal samples, feces, and even brain abscesses [90]. Despite initially being considered anaerobic bacteria, all currently known methanogens are Archaea species, minor and less-known constituents of the human microbiome, which are obligate bacteria cross-feeders and interact with bacteria in syntrophic ways [81,90]. A methanogenic archaeome is present in protists, plants and animals, where it interacts with the hosts in a mutually beneficial fashion (i.e., symbiosis) [90]. The most prevalent species reported in humans are Methanobrevibacter smithii [91] and Ca. Mmc. intestinalis [92], hydrogenotrophs found in up to 95% of the samples [75,81]. The archaeoma present in the gastrointestinal system of mammals differs consistently from the environmental one, probably because of alteration by horizontal gene transfer over time [93].
Considering that archaea are unable to degrade sugar and are obligate bacteria cross-feeders, the quantity, composition, and function of the methanogenic archaeoma is subjected to change in the context of bacterial dysbiosis, such as SIBO [55,81]. On the other hand, methane is produced by archaea, which may have distinct clinical implications, and its excessive production seems to occur throughout the intestinal tract in individuals with small intestine overproduction, therefore the latest SIBO Guideline of the American College of Gastroenterology proposed a more accurate delineation between SIBO and methane overproduction, defining a new entity called intestinal methanogen overgrowth (IMO)—a terminology that will probably replace the current one, i.e., methane-predominant SIBO [55].
The impact of methanogens on human health and disease is still largely unknown. Based on the current knowledge, methanogens are thought to have local and remote modulatory effects on the immune system and barrier role against pathogenic microorganisms in the digestive tract [90]. They seem to be able to interact with the immune system at the gastrointestinal system level, since exposure of dendritic cells to M. stadtmanae and M. smithii leads to release of proinflammatory cytokines, up-regulation of the cell-surface receptors CD86 and CD197 and alteration of antimicrobial peptides gene expression [94]. Moreover, M. stadtmanae was reported to activate innate immune receptors, its recognition being mediated by TLR7 and TLR8 receptors with subsequent NLRP3 inflammasome activation [95]. Although there are conflicting data regarding their pathogenic role, methanogenic archaea are widely considered possible contributors to a large array of disorders, such as inflammatory bowel disease, colon cancer, diabetes, and obesity (reviewed in [96]).
As already mentioned, sporadic PD is a multifactorial disorder, characterized at a molecular level by abnormal aggregation of proteins into Lewy bodies in specific neuronal populations, with a central nervous system ascending temporal pattern in most cases, according to the Braak scenario [7,10,11]. The onset of the pathology of most PD cases seems to start in the gut, more precisely in the neuroenteric plexus, which suggests that sporadic PD might have a gastrointestinal trigger [78]. A few reports link the methanogenic archaea to pathogenic gastrointestinal mechanisms of PD. For instance, the archaeal 20S proteasome seems to be able to effectively proteolyze aggregated misfolded proteins, such as alpha-synuclein, tau, or mutant superoxide-dismutase 1 [97]. Furthermore, experimental expression of archaea proteasome-activating nucleotidase in rod photoreceptors of mice leads to effective counteraction of misfolding retinopathy in Gγ1 knock-out mice, implying rescue from a protein-misfolding neurodegenerative disease [98]. Concurrently, the molecular chaperone prefoldin found in archaea can increase the generation of amyloid beta oligomers and decrease amyloid beta fibrils, meaning that it increases toxicity in an experimental setting [99].
6. SIBO Treatment as Therapeutic Opportunity for Interfering with PD Progression
It is generally agreed that symptomatic SIBO should be treated with one or several courses of antibiotics, accompanied by interventions aiming to eliminate the predisposing factors (e.g., using prokinetics in people with increased gastrointestinal transit times), the latter being a sine qua non condition in order to prevent recurrence [38,40,46]. The clinical relevance of asymptomatic SIBO remains unknown, but similar to other gut dysbiosis states, accumulating evidence suggests that it may have negative health consequences, e.g., by contributing to an overall proinflammatory state, neurotoxicity, neurodegeneration, etc. [38,100].
As previously discussed, increased intestinal transit time is a common occurrence in early PD, appearing years before the onset of motor symptoms, and presumably explaining the higher prevalence of SIBO in this population [42,43,44,62,63]. Local inflammatory responses induced by SIBO or other gut dysbiosis may activate the immune system and disrupt the intestinal barrier permeability, allowing for a close interaction between alpha-synuclein and various bacterial products that may have amyloidogenic effects, triggering or enhancing alpha-synuclein misfolding and accumulation, key features of PD pathology [22,27,53,69]. Furthermore, an altered intestinal barrier grants access to the gut–brain axis for potentially proinflammatory and neurotoxic microbial and nonmicrobial compounds, that may increase neuronal susceptibility to neurodegeneration [22,27,70,71].
Among all microbial products, gases pass most easily through membranes and may interfere with cellular functions throughout the body. The composition of the gas produced by the small intestine microbiota in people with SIBO (which, as discussed, can be assessed in respect to its H2 and methane content after oral carbohydrate challenge with glucose and lactulose—see above) mainly depends on the concentration and the types of colonizing bacteria, both the structure and the function of the microbiota being important [38,39,46,47,48,49,50]. These gases may interfere directly with PD progression, both in positive and negative ways, H2 having potentially neuroprotective effects by diminishing inflammation and oxidative stress [43,63,77,84], and methane having either neuroprotective or neurodegeneration-promoting effects, by counteracting protein misfolding and removing aggregates of misfolded proteins, respectively, by increasing the aggregation of misfolded proteins [97,98,99]—see Table 2. Considering the above, specific SIBO-related mechanisms could serve as potential therapeutic interventions or targets for PD progression—see Figure 3. On the contrary, considering that SIBO may result in proinflammatory changes, both locally and within the brain, and may increase the permeability of the intestinal and blood–brain barriers, exposing neurons to potentially amyloidogenic and neurotoxic compounds and accelerating the progression of PD, its eradication should be considered in people with PD even in the absence of SIBO-related symptoms.
Table 2.
Possible roles of SIBO subtype in PD progression.
| SIBO Subtype * | Presumptive Roles in PD Progression |
|---|---|
| H2-predominant SIBO | Protective: anti-inflammatory, decreases oxidative stress.Uncertain: modulates the gut microbiota (reinforces hydrogenotrophic bacteria and archaea); increased production of hydrogen sulfide. |
| Methane-predominant SIBO | Either protective or deleterious: may counteract protein misfolding and may effectively remove aggregates of misfolded proteins; proinflammatory and may increase aggregation of misfolded proteins. |
* As per carbohydrate (i.e., lactulose) breath test.
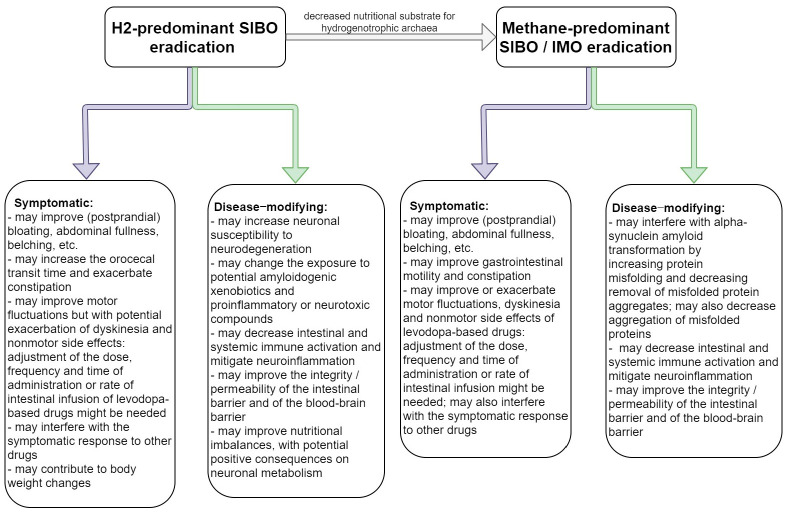
Figure 3.
Expected effects of SIBO eradication in people with sporadic PD. The figure illustrates the potential symptomatic and disease-modifying consequences of SIBO eradication in people with sporadic PD. Noteworthy, interventions done for SIBO eradication would also interfere with the gastric and colonic gut microbiota. The effects of SIBO eradication and modulation require further investigation.
The management of symptomatic SIBO is centered on the use of oral antibiotics that successfully eradicate the bacteria overpopulating the small intestine; the recommended antibiotic regimens usually consist of rifaximin (which has the advantage of very limited systemic absorption), metronidazole, ciprofloxacin, doxycycline, neomycin or amoxicillin-clavulanate; the typical treatment duration is 7 to 14 days [22,40,41]. Evidence concerning the short- and long-term efficacy of one antibiotic over another is modest, so the antibiotic is chosen based on individual safety concerns, individual preferences and prior SIBO history [22,40,41]. Since treating the predisposing condition is essential for the long-term remission of SIBO, and SIBO in PD seems to be closely related with the increase in gastrointestinal transit times, administering antibiotics that also have prokinetic effects, such as azithromycin and erythromycin, in people with SIBO and PD is tempting—nevertheless safety and efficacy trials for SIBO in people with PD are lacking and their use in clinical practice is limited by theoretical safety concerns related to potential cardiovascular side effects [40]. Dietary changes and other symptomatic interventions can also be considered. The use of probiotics (i.e., live bacteria), especially Lactobacillus strands, may be useful in the management of SIBO, helping to repopulate the intestinal flora with health-promoting bacteria [22,40,41]. Prebiotics are another potentially useful intervention for SIBO, however evidence on their efficacy very limited [22,41].
Small studies found that SIBO eradication is safe and achievable on the short-term (i.e., up to 6 months) in people with PD, and that it may help improve motor fluctuations and possibly gastrointestinal symptoms [43,45]. Gut microbiota manipulation by probiotic use or fecal transplantation in people with PD may result in clinical improvement, especially concerning gastrointestinal symptoms [41,101]; however, the effect of this interventions in SIBO is unknown. Clinical trials regarding a potential disease modifying effect of these types of interventions in PD are lacking and the clinical relevance of the SIBO-related mechanisms that were shown to interfere with the progression of PD pathology in experimental settings is currently unknown. Since these types of interventions do not pose major safety concerns, further studies are warranted. Depending on the results of these studies, screening for SIBO and eradicating methane-predominant (or H2-predominant) SIBO in people with sporadic PD might become a cheap, safe, and accessible intervention that could be used in everyday clinical practice to help mitigate PD progression, especially in the early stages; SIBO eradication might also prove potentially useful in people with advanced PD, either by eliminating the direct impact of SIBO on motor and nonmotor symptoms or by changing the intestinal bioavailability of symptomatic medications. Moreover, if methane-predominant or H2-predominant SIBO prove to be risk factors for developing sporadic PD, SIBO screening and eradication could become the first intervention for preventing or delaying sporadic PD in people at risk. Concurrently, modulation of SIBO-related mechanisms, for example by administering H2-enhanced water [87,88], could help develop neuroprotective interventions aimed at preventing PD or slowing down its progression.
7. Conclusions
Up to this point, an important array of data regarding the human gut microbiota (archaeome included) has emerged, with possible implications for the pathogenesis of neurodegenerative disorders and their therapeutic targets. This line of development might be of particular relevance for sporadic PD, since the first pathological lesions seem to appear in the neurenteric plexuses, possibly as a consequence of local gastrointestinal processes, and then spread to the brain by a prion-like mechanism.
People with PD have higher incidence of SIBO, and SIBO may interfere with the progression of PD, both in negative and positive manners, depending on the levels of H2 and/or methane produced and possibly on other functional characteristics of the microorganisms colonizing the small intestine. The levels of H2 and methane produced by the intestinal microbiota can be estimated by measuring them in the expiratory air after a carbohydrate challenge, and could serve as variables in clinical trials that aim to assess the impact of SIBO in PD progression. Considering the above, SIBO eradication and other modulation/manipulation of the small intestine microbiota require further investigation as potential disease modifying interventions aiming at slowing down the progression of PD.
Author Contributions
Conceptualization, A.D. and B.O.P.; writing—original draft preparation, A.D., A.L., D.T. and L.D.; writing—review and editing L.D. and B.O.P.; supervision, B.O.P.; funding acquisition, B.O.P. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was funded by the Ministry of Research and Innovation in Romania (currently the Ministry of Education and Research), PN 19.29.02.01, Program 1—Improvement of the National System of Research and Development, Subprogram 1.2—Institutional Excellence—Projects of Excellence Funding in RDI, and by CNFISFDI-2021-0300, RDI capabilities consolidation at Institutional level of the multidisciplinary research teams involved in the sustainability of UMFCD priority research directions.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rocca W.A. The burden of Parkinson’s disease: A worldwide perspective. Lancet Neurol. 2018;17:928–929. doi: 10.1016/S1474-4422(18)30355-7. [DOI] [PubMed] [Google Scholar]
- 2.Postuma R.B., Berg D., Stern M., Poewe W., Olanow C.W., Oertel W., Obeso J., Marek K., Litvan I., Lang A.E., et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 3.Berg D., Postuma R.B., Adler C.H., Bloem B.R., Chan P., Dubois B., Gasser T., Goetz C.G., Halliday G., Joseph L., et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 4.Heinzel S., Berg D., Gasser T., Chen H., Yao C., Postuma R.B., The MDS Task Force on the Definition of Parkinson’s Disease Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2019;34:1464–1470. doi: 10.1002/mds.27802. [DOI] [PubMed] [Google Scholar]
- 5.Berg D., Adler C.H., Bloem B.R., Chan P., Gasser T., Goetz C.G., Halliday G., Lang A.E., Lewis S., Li Y., et al. Movement disorder society criteria for clinically established early Parkinson’s disease. Mov. Disord. 2018;33:1643–1646. doi: 10.1002/mds.27431. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J., Tan E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91:795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 7.Braak H., Rub U., Gai W.P., Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 8.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: The dual hit theory revisited. Ann. N. Y. Acad. Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 10.Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 11.Halliday G.M., Del Tredici K., Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson’s disease. J. Neural. Transm. Suppl. 2006:99–103. doi: 10.1007/978-3-211-45295-0_16. [DOI] [PubMed] [Google Scholar]
- 12.Masuda-Suzukake M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D.M., Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rietdijk C.D., Perez-Pardo P., Garssen J., van Wezel R.J., Kraneveld A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017;8:37. doi: 10.3389/fneur.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H., de Vos R.A., Bohl J., Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Hilton D., Stephens M., Kirk L., Edwards P., Potter R., Zajicek J., Broughton E., Hagan H., Carroll C. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta. Neuropathol. 2014;127:235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 16.Svensson E., Horvath-Puho E., Thomsen R.W., Djurhuus J.C., Pedersen L., Borghammer P., Sorensen H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 17.Romano S., Savva G.M., Bedarf J.R., Charles I.G., Hildebrand F., Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7:27. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minato T., Maeda T., Fujisawa Y., Tsuji H., Nomoto K., Ohno K., Hirayama M. Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS ONE. 2017;12:e0187307. doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheperjans F., Aho V., Pereira P.A., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt S., Mohajeri M.H. Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients. 2018;10:708. doi: 10.3390/nu10060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q., Luo Y., Chaudhuri K.R., Reynolds R., Tan E.K., Pettersson S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights andtherapeutic options. Brain. 2021;144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 23.Margolis K.G., Cryan J.F., Mayer E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuwahara A., Matsuda K., Kuwahara Y., Asano S., Inui T., Marunaka Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020;41:199–216. doi: 10.2220/biomedres.41.199. [DOI] [PubMed] [Google Scholar]
- 25.Devos D., Lebouvier T., Lardeux B., Biraud M., Rouaud T., Pouclet H., Coron E., Bruley des Varannes S., Naveilhan P., Nguyen J.M., et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Becker A., Fassbender K., Oertel W.H., Unger M.M. A punch in the gut—Intestinal inflammation links environmental factors to neurodegeneration in Parkinson’s disease. Parkinsonism Relat. Disord. 2019;60:43–45. doi: 10.1016/j.parkreldis.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Dumitrescu L., Marta D., Danau A., Lefter A., Tulba D., Cozma L., Manole E., Gherghiceanu M., Ceafalan L.C., Popescu B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021;15:689723. doi: 10.3389/fnins.2021.689723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwiertz A., Spiegel J., Dillmann U., Grundmann D., Burmann J., Fassbender K., Schafer K.H., Unger M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat. Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Mulak A., Koszewicz M., Panek-Jeziorna M., Koziorowska-Gawron E., Budrewicz S. Fecal Calprotectin as a Marker of the Gut Immune System Activation Is Elevated in Parkinson’s Disease. Front. Neurosci. 2019;13:992. doi: 10.3389/fnins.2019.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houser M.C., Chang J., Factor S.A., Molho E.S., Zabetian C.P., Hill-Burns E.M., Payami H., Hertzberg V.S., Tansey M.G. Stool Immune Profiles Evince Gastrointestinal Inflammation in Parkinson’s Disease. Mov. Disord. 2018;33:793–804. doi: 10.1002/mds.27326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutta S.K., Verma S., Jain V., Surapaneni B.K., Vinayek R., Phillips L., Nair P.P. Parkinson’s Disease: The Emerging Role of Gut Dysbiosis, Antibiotics, Probiotics, and Fecal Microbiota Transplantation. J. Neurogastroenterol. Motil. 2019;25:363–376. doi: 10.5056/jnm19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S.G., Stribinskis V., Rane M.J., Demuth D.R., Gozal E., Roberts A.M., Jagadapillai R., Liu R., Choe K., Shivakumar B., et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016;6:34477. doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampson T.R., Challis C., Jain N., Moiseyenko A., Ladinsky M.S., Shastri G.G., Thron T., Needham B.D., Horvath I., Debelius J.W., et al. A gut bacterial amyloid promotes alpha-synuclein aggregation and motor impairment in mice. eLife. 2020;9 doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen K.K., Vad B.S., Kjaer L., Tolker-Nielsen T., Christiansen G., Otzen D.E. Pseudomonas aeruginosa rhamnolipid induces fibrillation of human alpha-synuclein and modulates its effect on biofilm formation. FEBS Lett. 2018;592:1484–1496. doi: 10.1002/1873-3468.13038. [DOI] [PubMed] [Google Scholar]
- 35.Deng I., Corrigan F., Zhai G., Zhou X.F., Bobrovskaya L. Lipopolysaccharide animal models of Parkinson’s disease: Recent progress and relevance to clinical disease. Brain Behav. Immun. Health. 2020;4:100060. doi: 10.1016/j.bbih.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manole E., Dumitrescu L., Niculite C., Popescu B.O., Ceafalan L.C. Potential roles of functional bacterial amyloid proteins, bacterial biosurfactants and other putative gut microbiota products in the etiopathogeny of Parkinson’s Disease. Biocell. 2021;45:1–16. doi: 10.32604/biocell.2021.013452. [DOI] [Google Scholar]
- 38.Rezaie A., Pimentel M., Rao S.S. How to Test and Treat Small Intestinal Bacterial Overgrowth: An Evidence-Based Approach. Curr. Gastroenterol. Rep. 2016;18:8. doi: 10.1007/s11894-015-0482-9. [DOI] [PubMed] [Google Scholar]
- 39.Lappinga P.J., Abraham S.C., Murray J.A., Vetter E.A., Patel R., Wu T.T. Small intestinal bacterial overgrowth: Histopathologic features and clinical correlates in an underrecognized entity. Arch. Pathol. Lab. Med. 2010;134:264–270. doi: 10.5858/134.2.264. [DOI] [PubMed] [Google Scholar]
- 40.Barboza J.L., Okun M.S., Moshiree B. The treatment of gastroparesis, constipation and small intestinal bacterial overgrowth syndrome in patients with Parkinson’s disease. Expert. Opin. Pharmacother. 2015;16:2449–2464. doi: 10.1517/14656566.2015.1086747. [DOI] [PubMed] [Google Scholar]
- 41.Cassani E., Privitera G., Pezzoli G., Pusani C., Madio C., Iorio L., Barichella M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011;57:117–121. [PubMed] [Google Scholar]
- 42.Gabrielli M., Bonazzi P., Scarpellini E., Bendia E., Lauritano E.C., Fasano A., Ceravolo M.G., Capecci M., Rita Bentivoglio A., Provinciali L., et al. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2011;26:889–892. doi: 10.1002/mds.23566. [DOI] [PubMed] [Google Scholar]
- 43.Fasano A., Bove F., Gabrielli M., Petracca M., Zocco M.A., Ragazzoni E., Barbaro F., Piano C., Fortuna S., Tortora A., et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013;28:1241–1249. doi: 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]
- 44.Tan A.H., Mahadeva S., Thalha A.M., Gibson P.R., Kiew C.K., Yeat C.M., Ng S.W., Ang S.P., Chow S.K., Tan C.T., et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Li X., Feng X., Jiang Z., Jiang Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: A systematic review and meta-analysis. Gut. Pathog. 2021;13:25. doi: 10.1186/s13099-021-00420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezaie A., Buresi M., Lembo A., Lin H., McCallum R., Rao S., Schmulson M., Valdovinos M., Zakko S., Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoshini R., Dai S.C., Lezcano S., Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig. Dis. Sci. 2008;53:1443–1454. doi: 10.1007/s10620-007-0065-1. [DOI] [PubMed] [Google Scholar]
- 48.Corazza G.R., Menozzi M.G., Strocchi A., Rasciti L., Vaira D., Lecchini R., Avanzini P., Chezzi C., Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–309. doi: 10.1016/0016-5085(90)90818-L. [DOI] [PubMed] [Google Scholar]
- 49.Jacobs C., Coss Adame E., Attaluri A., Valestin J., Rao S.S. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment. Pharmacol. Ther. 2013;37:1103–1111. doi: 10.1111/apt.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erdogan A., Rao S.S., Gulley D., Jacobs C., Lee Y.Y., Badger C. Small intestinal bacterial overgrowth: Duodenal aspiration vs glucose breath test. Neurogastroenterol. Motil. 2015;27:481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- 51.Miazga A., Osinski M., Cichy W., Zaba R. Current views on the etiopathogenesis, clinical manifestation, diagnostics, treatment and correlation with other nosological entities of SIBO. Adv. Med. Sci. 2015;60:118–124. doi: 10.1016/j.advms.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Saltzman J.R., Kowdley K.V., Pedrosa M.C., Sepe T., Golner B., Perrone G., Russell R.M. Bacterial overgrowth without clinical malabsorption in elderly hypochlorhydric subjects. Gastroenterology. 1994;106:615–623. doi: 10.1016/0016-5085(94)90693-9. [DOI] [PubMed] [Google Scholar]
- 53.Bures J., Cyrany J., Kohoutova D., Forstl M., Rejchrt S., Kvetina J., Vorisek V., Kopacova M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010;16:2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pyleris E., Giamarellos-Bourboulis E.J., Tzivras D., Koussoulas V., Barbatzas C., Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: Relationship with irritable bowel syndrome. Dig. Dis. Sci. 2012;57:1321–1329. doi: 10.1007/s10620-012-2033-7. [DOI] [PubMed] [Google Scholar]
- 55.Pimentel M., Saad R.J., Long M.D., Rao S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020;115:165–178. doi: 10.14309/ajg.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 56.Gasbarrini A., Lauritano E.C., Gabrielli M., Scarpellini E., Lupascu A., Ojetti V., Gasbarrini G. Small intestinal bacterial overgrowth: Diagnosis and treatment. Dig. Dis. 2007;25:237–240. doi: 10.1159/000103892. [DOI] [PubMed] [Google Scholar]
- 57.Arasaradnam R.P., Brown S., Forbes A., Fox M.R., Hungin P., Kelman L., Major G., O’Connor M., Sanders D.S., Sinha R., et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd edition. Gut. 2018;67:1380–1399. doi: 10.1136/gutjnl-2017-315909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choung R.S., Ruff K.C., Malhotra A., Herrick L., Locke G.R., III, Harmsen W.S., Zinsmeister A.R., Talley N.J., Saito Y.A. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment. Pharmacol. Ther. 2011;33:1059–1067. doi: 10.1111/j.1365-2036.2011.04625.x. [DOI] [PubMed] [Google Scholar]
- 59.Lewis S.J., Potts L.F., Malhotra R., Mountford R. Small bowel bacterial overgrowth in subjects living in residential care homes. Age Ageing. 1999;28:181–185. doi: 10.1093/ageing/28.2.181. [DOI] [PubMed] [Google Scholar]
- 60.van den Bos F., Speelman A.D., van Nimwegen M., van der Schouw Y.T., Backx F.J., Bloem B.R., Munneke M., Verhaar H.J. Bone mineral density and vitamin D status in Parkinson’s disease patients. J. Neurol. 2013;260:754–760. doi: 10.1007/s00415-012-6697-x. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y., Shen L., Ji H.F. Osteoporosis risk and bone mineral density levels in patients with Parkinson’s disease: A meta-analysis. Bone. 2013;52:498–505. doi: 10.1016/j.bone.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 62.DiBaise J.K., Crowell M.D., Driver-Dunckley E., Mehta S.H., Hoffman-Snyder C., Lin T., Adler C.H. Weight Loss in Parkinson’s Disease: No Evidence for Role of Small Intestinal Bacterial Overgrowth. J. Parkinsons. Dis. 2018;8:571–581. doi: 10.3233/JPD-181386. [DOI] [PubMed] [Google Scholar]
- 63.Ostojic S.M. Inadequate Production of H2 by Gut Microbiota and Parkinson Disease. Trends Endocrinol. Metab. 2018;29:286–288. doi: 10.1016/j.tem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Su A., Gandhy R., Barlow C., Triadafilopoulos G. Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastroenterol. 2017;4:e000132. doi: 10.1136/bmjgast-2017-000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobbs R.J., Charlett A., Dobbs S.M., Weller C., MA A.I., Iguodala O., Smee C., Plant J.M., Lawson A.J., Taylor D., et al. Leukocyte-subset counts in idiopathic parkinsonism provide clues to a pathogenic pathway involving small intestinal bacterial overgrowth. A surveillance study. Gut Pathog. 2012;4:12. doi: 10.1186/1757-4749-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies K.N., King D., Billington D., Barrett J.A. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad. Med. J. 1996;72:164–167. doi: 10.1136/pgmj.72.845.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gibson P.R., Barrett J.S. The concept of small intestinal bacterial overgrowth in relation to functional gastrointestinal disorders. Nutrition. 2010;26:1038–1043. doi: 10.1016/j.nut.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 68.van Kessel S.P., Frye A.K., El-Gendy A.O., Castejon M., Keshavarzian A., van Dijk G., El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019;10:310. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maini Rekdal V., Bess E.N., Bisanz J.E., Turnbaugh P.J., Balskus E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364:6323. doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhattacharyya D., Bhunia A. Gut-Brain axis in Parkinson’s disease etiology: The role of lipopolysaccharide. Chem. Phys. Lipids. 2021;235:105029. doi: 10.1016/j.chemphyslip.2020.105029. [DOI] [PubMed] [Google Scholar]
- 71.Keita A.V., Soderholm J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 72.Quigley E.M.M., Murray J.A., Pimentel M. AGA Clinical Practice Update on Small Intestinal Bacterial Overgrowth: Expert Review. Gastroenterology. 2020;159:1526–1532. doi: 10.1053/j.gastro.2020.06.090. [DOI] [PubMed] [Google Scholar]
- 73.Hill-Burns E.M., Debelius J.W., Morton J.T., Wissemann W.T., Lewis M.R., Wallen Z.D., Peddada S.D., Factor S.A., Molho E., Zabetian C.P., et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Kessel S.P., El Aidy S. Contributions of Gut Bacteria and Diet to Drug Pharmacokinetics in the Treatment of Parkinson’s Disease. Front. Neurol. 2019;10:1087. doi: 10.3389/fneur.2019.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith N.W., Shorten P.R., Altermann E.H., Roy N.C., McNabb W.C. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes. 2019;10:270–288. doi: 10.1080/19490976.2018.1546522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Javorac D., Stajer V., Ratgeber L., Olah A., Betlehem J., Acs P., Vukomanovic B., Ostojic S.M. Hydrotherapy with hydrogen-rich water compared with RICE protocol following acute ankle sprain in professional athletes: A randomized non-inferiority pilot trial. Res. Sports Med. 2020;29:517–525. doi: 10.1080/15438627.2020.1868468. [DOI] [PubMed] [Google Scholar]
- 77.Fu Y., Ito M., Fujita Y., Ito M., Ichihara M., Masuda A., Suzuki Y., Maesawa S., Kajita Y., Hirayama M., et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Dumitrescu L., Popescu-Olaru I., Cozma L., Tulba D., Hinescu M.E., Ceafalan L.C., Gherghiceanu M., Popescu B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid Med. Cell Longev. 2018;2018:2406594. doi: 10.1155/2018/2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musgrove R.E., Helwig M., Bae E.J., Aboutalebi H., Lee S.J., Ulusoy A., Di Monte D.A. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular alpha-synuclein transfer. J. Clin. Investig. 2019;129:3738–3753. doi: 10.1172/JCI127330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki A., Ito M., Hamaguchi T., Mori H., Takeda Y., Baba R., Watanabe T., Kurokawa K., Asakawa S., Hirayama M., et al. Quantification of hydrogen production by intestinal bacteria that are specifically dysregulated in Parkinson’s disease. PLoS ONE. 2018;13:e0208313. doi: 10.1371/journal.pone.0208313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carbonero F., Benefiel A.C., Gaskins H.R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012;9:504–518. doi: 10.1038/nrgastro.2012.85. [DOI] [PubMed] [Google Scholar]
- 82.Murros K.E., Huynh V.A., Takala T.M., Saris P.E.J. Desulfovibrio Bacteria Are Associated with Parkinson’s Disease. Front. Cell Infect Microbiol. 2021;11:652617. doi: 10.3389/fcimb.2021.652617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang M., Tang J.J., Wang L.X., Yu J., Zhang L., Qiao C. Hydrogen sulfide enhances adult neurogenesis in a mouse model of Parkinson’s disease. Neural. Regen. Res. 2021;16:1353–1358. doi: 10.4103/1673-5374.301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta. 2012;1820:586–594. doi: 10.1016/j.bbagen.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 85.Fujita K., Seike T., Yutsudo N., Ohno M., Yamada H., Yamaguchi H., Sakumi K., Yamakawa Y., Kido M.A., Takaki A., et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS ONE. 2009;4:e7247. doi: 10.1371/journal.pone.0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi Y., Imamura R., Koyama Y., Kondo M., Kobayashi H., Nonomura N., Shimada S. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci. Rep. 2020;10:5859. doi: 10.1038/s41598-020-62755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoritaka A., Takanashi M., Hirayama M., Nakahara T., Ohta S., Hattori N. Pilot study of H(2) therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov. Disord. 2013;28:836–839. doi: 10.1002/mds.25375. [DOI] [PubMed] [Google Scholar]
- 88.Yoritaka A., Ohtsuka C., Maeda T., Hirayama M., Abe T., Watanabe H., Saiki H., Oyama G., Fukae J., Shimo Y., et al. Randomized, double-blind, multicenter trial of hydrogen water for Parkinson’s disease. Mov. Disord. 2018;33:1505–1507. doi: 10.1002/mds.27472. [DOI] [PubMed] [Google Scholar]
- 89.Yoritaka A., Kobayashi Y., Hayashi T., Saiki S., Hattori N. Randomized double-blind placebo-controlled trial of hydrogen inhalation for Parkinson’s disease: A pilot study. Neurol. Sci. 2021;42:4767–4770. doi: 10.1007/s10072-021-05489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Djemai K., Drancourt M., Tidjani Alou M. Bacteria and Methanogens in the Human Microbiome: A Review of Syntrophic Interactions. Microb. Ecol. 2021:1–19. doi: 10.1007/s00248-021-01796-7. [DOI] [PubMed] [Google Scholar]
- 91.Dridi B., Henry M., El Khechine A., Raoult D., Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS ONE. 2009;4:e7063. doi: 10.1371/journal.pone.0007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borrel G., McCann A., Deane J., Neto M.C., Lynch D.B., Brugere J.F., O’Toole P.W. Genomics and metagenomics of trimethylamine-utilizing Archaea in the human gut microbiome. ISME J. 2017;11:2059–2074. doi: 10.1038/ismej.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansen E.E., Lozupone C.A., Rey F.E., Wu M., Guruge J.L., Narra A., Goodfellow J., Zaneveld J.R., McDonald D.T., Goodrich J.A., et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc. Natl. Acad. Sci. USA. 2011;108((Suppl. 1)):4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bang C., Weidenbach K., Gutsmann T., Heine H., Schmitz R.A. The intestinal archaea Methanosphaera stadtmanae and Methanobrevibacter smithii activate human dendritic cells. PLoS ONE. 2014;9:e99411. doi: 10.1371/journal.pone.0099411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vierbuchen T., Bang C., Rosigkeit H., Schmitz R.A., Heine H. The Human-Associated Archaeon Methanosphaera stadtmanae Is Recognized through Its RNA and Induces TLR8-Dependent NLRP3 Inflammasome Activation. Front. Immunol. 2017;8:1535. doi: 10.3389/fimmu.2017.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borrel G., Brugere J.F., Gribaldo S., Schmitz R.A., Moissl-Eichinger C. The host-associated archaeome. Nat. Rev. Microbiol. 2020;18:622–636. doi: 10.1038/s41579-020-0407-y. [DOI] [PubMed] [Google Scholar]
- 97.Yamada S., Niwa J., Ishigaki S., Takahashi M., Ito T., Sone J., Doyu M., Sobue G. Archaeal proteasomes effectively degrade aggregation-prone proteins and reduce cellular toxicities in mammalian cells. J. Biol. Chem. 2006;281:23842–23851. doi: 10.1074/jbc.M601274200. [DOI] [PubMed] [Google Scholar]
- 98.Brooks C., Snoberger A., Belcastro M., Murphy J., Kisselev O.G., Smith D.M., Sokolov M. Archaeal Unfoldase Counteracts Protein Misfolding Retinopathy in Mice. J. Neurosci. 2018;38:7248–7254. doi: 10.1523/JNEUROSCI.0905-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sakono M., Zako T., Ueda H., Yohda M., Maeda M. Formation of highly toxic soluble amyloid beta oligomers by the molecular chaperone prefoldin. FEBS J. 2008;275:5982–5993. doi: 10.1111/j.1742-4658.2008.06727.x. [DOI] [PubMed] [Google Scholar]
- 100.Riordan S.M., McIver C.J., Wakefield D., Bolin T.D., Duncombe V.M., Thomas M.C. Small intestinal bacterial overgrowth in the symptomatic elderly. Am. J. Gastroenterol. 1997;92:47–51. [PubMed] [Google Scholar]
- 101.Xu M.Q., Cao H.L., Wang W.Q., Wang S., Cao X.C., Yan F., Wang B.M. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J. Gastroenterol. 2015;21:102–111. doi: 10.3748/wjg.v21.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.