Purpose:
Cxbladder (Cxb) tests combine genomic biomarkers in urine with phenotypic and clinical data to classify hematuria patients into those at low/high probability of urothelial carcinoma (UC). Cxbladder Resolve (CxbR) is designed for use after Cxb Triage (CxbT) and Detect (CxbD), where CxbT-positive tests reflex to CxbD and CxbD-positive to CxbR to identify patients at high probability of high-impact tumors (HIT; high grade Ta, Tis or T1–T3). This study validated the diagnostic performance of CxbR in identifying HIT, and validated the algorithm of Cxb tests to segregate high-impact from low-impact tumors.
Materials and Methods:
CxbR was developed in 863 hematuria patients in 3 studies in United States, Australia and New Zealand. CxbR, separately and combined with other Cxb tests, was validated in a prospective, observational U.S. study in 548 hematuria patients. All UC diagnoses were confirmed by histopathology.
Results:
In the development data set, CxbR sensitivity was 92.4% (95% CI 83.3–96.7) and specificity 93.8% (95% CI 86.8–97.2) for identifying HIT within the high priority category. During external validation, sequential Cxb tests correctly ruled out 87.6% of patients from further workup (negative predictive value 99.4%); 100% of HIT were correctly identified (specificity 96.3%), and 3 low-grade tumors were missed. In both studies, all patients with HIT were correctly assigned to prioritized evaluation.
Conclusions:
CxbR has high sensitivity and specificity, correctly identifying all HIT. Sequential Cxb tests accurately segregate patients with a low vs high probability of HIT, focusing resources on those patients, with a diagnostic yield 4.8-fold higher than American Urological Association guideline stratification.
Key Words: biomarkers, hematuria, predictive value of tests, sensitivity and specificity, urinary bladder neoplasms
Abbreviations and Acronyms
- AUA
American Urological Association
- Cxb
Cxbladder
- CxbD
Cxbladder Detect
- CxbR
Cxbladder Resolve
- CxbT
Cxbladder Triage
- DDS
development data set
- HIT
high impact tumors
- KP
Kaiser Permanente
- LIT
low impact tumors
- NPV
negative predictive value
- PDP
physician-directed protocol
- UC
urothelial carcinoma
Hematuria is a common clinical presentation and key symptom of urothelial carcinoma (UC).1,2 American Urological Association (AUA) guidelines recommend clinical workup for patients with hematuria,3 but only 0.5%–5% of patients with asymptomatic microhematuria and 10%–15% with gross hematuria have confirmed UC.4,5 Most patients referred for evaluation are intermediate or high-risk for whom cystoscopy is recommended,3 but some of the investigated patients will return an inconclusive result, such as atypical cytology or equivocal cystoscopy.6 Conversely, many patients with microhematuria are not referred for evaluation even if they are at high risk.4,7
There is, therefore, an unmet need for an accurate noninvasive urinary biomarker test that can rule out patients without cancer and identify patients at greatest risk of UC. Such a test would have high clinical utility by reducing the diagnostic burden on individuals without UC and prioritizing evaluation for those at risk of more advanced UC.
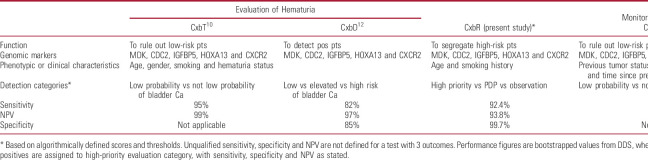
The family of Cxbladder (Cxb) tests (Pacific Edge, Dunedin, New Zealand) uses multiplexed mRNA biomarkers to guide the management of UC from diagnosis through to surveillance for disease recurrence (table 1).8–12 Specifically, Cxbladder Triage (CxbT) is a rule-out test with high sensitivity and high negative predictive value (NPV) to identify patients with a low likelihood of UC who may avoid unnecessary invasive investigations. Cxbladder Detect (CxbD) has high sensitivity and specificity for the identification of patients who require further workup for UC.12 Cxbladder Resolve (CxbR) was developed for use after both CxbT and CxbD to accurately segregate test-positive patients likely to have high impact tumors (HIT). Further details on Cxb tests are provided in the supplementary material (https://www.jurology.com).
Table 1.
Summary of published performance of all Cxb tests and clinical applications
The current study aimed to validate, firstly, the diagnostic performance of CxbR to identify HIT and, secondly, CxbR in combination with CxbT and CxbD using a single urine sample at the time of initial investigation as part of a proposed new algorithm, where CxbT-positive tests reflex to CxbD and CxbD-positive to CxbR (fig. 1).
Figure 1.
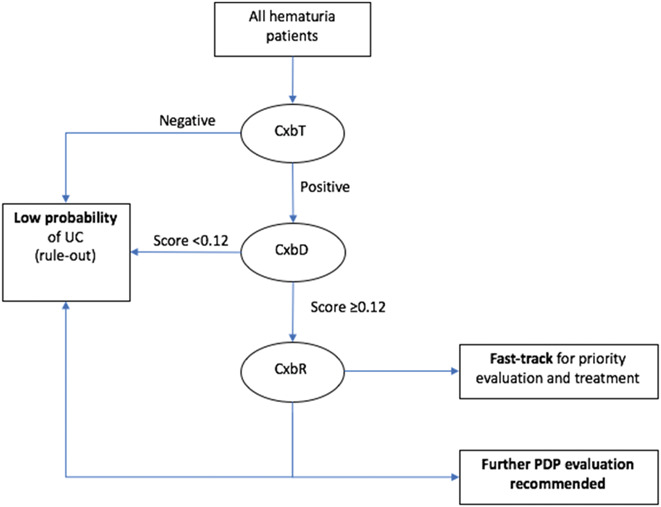
Proposed clinical algorithm for concatenated use of Cxb tests in assessment of patients with hematuria.
Patients and Methods
Design
CxbR was developed and internally validated in a cohort of patients (863) with hematuria undergoing urological investigation for UC in 3 separate prospective studies in the United States, Australia and New Zealand (supplementary methods and supplementary table, https://www.jurology.com). External validation of CxbR and validation of the algorithm (CxbT reflexing to CxbD reflexing to CxbR) was undertaken on an independent cohort from a prospective, observational study (548) at Kaiser Permanente (KP) in Southern California (supplementary methods, https://www.jurology.com).
The study protocols were approved by institutional review boards at participating centers (KP Independent Review Board No. 10477). All studies were performed in accordance with the principles of the Declaration of Helsinki, International Conference on Harmonization and Good Clinical Practice. Informed consent was obtained from all patients prior to enrollment.
Patients
For the development data set (DDS), all consecutive hematuria patients were screened for eligibility. Patients were aged ≥18 years in 2 studies, ≥45 years in another, and were scheduled to undergo cystoscopy for possible UC because of gross or microscopic hematuria (see supplementary table, https://www.jurology.com for full selection criteria). All patients underwent the standard workup.
The external validation cohort included hematuria patients undergoing routine workup at a KP center (supplementary methods, https://www.jurology.com). Patients were excluded for a prior history of UC, symptoms indicative of a current urinary tract infection, a confirmed diagnosis of prostate carcinoma or pregnancy (see supplementary table, https://www.jurology.com). Clinicodemographic characteristics were collected as determined by the routine clinical workup. Patients with incomplete data for Cxb tests or histopathological diagnosis were excluded from this analysis.
Cxb Testing
A single midstream urine sample was collected from each consenting patient and analyzed as a commercial Cxb test.10,12 Each Cxb test uses quantitative reverse transcription polymerase chain reaction to measure the expression of a common backbone of 5 genotypic biomarkers (MDK, CDK1, IGFBP5, HOXA13 and CXCR2) and combines these with specific phenotypic biomarkers and clinical characteristics to derive a test score.8 Thresholds based on these scores have been previously defined and validated for CxbT and CxbD (table 1 and supplementary methods, https://www.jurology.com).9,10,12 Samples from the patients testing positive on CxbT and CxbD underwent the CxbR test, which integrates the expression of the genotypic biomarkers with age and smoking history in a novel algorithm to identify patients with a high likelihood of HIT requiring priority workup, and segregate other patients into those who can be managed by observation and those can be worked up according to their physician’s specification (ie physician-directed protocol [PDP]). Ongoing followup in these patients was based on clinical indication/need (eg persistence of hematuria, suspicion of other urological diagnoses).
All patients underwent a standard workup to determine clinical outcome for UC based on histopathology-validated cystoscopic examination. Disease stage was classified using tumor-node-metastasis criteria.13 Grade was classified according to local pathology practice, World Health Organization (WHO) grading criteria (1973)14 or WHO/International Society of Urological Pathology consensus classification (1998).15 HIT included high-grade Ta, T1-T3 and Tis. Low impact tumors (LIT) included low-grade noninvasive papillary carcinoma (Ta) and papillary urothelial neoplasm of low malignant potential. Performers/readers of the Cxb tests were blinded to cystoscopic/histopathological results and physicians had no access to Cxb results.
Sequential use of Cxb tests was investigated using the algorithm in figure 1. CxbT and CxbD maximize safe rule-out of patients at low probability of disease (based on high sensitivity and NPV), and CxbR segregates patients at high probability of HIT (based on high sensitivity and specificity).
Statistical Analysis
Development and internal validation of CxbR were undertaken on the DDS (patients in 3 studies from the United States, Australia and New Zealand). As CxbR has 3 possible outcomes (high priority, PDP, or observation), 2 parameters of sensitivity and specificity were assessed: 1) sensitivity/specificity (HIT), based on the proportion of HIT in the samples assigned to the “high priority” category; 2) sensitivity/specificity (UC), based on the proportion of any UCs assigned to “high priority” or “PDP”. Other performance parameters were calculated using standard methods.16,17 Data analysis used R-3.5.1 software.18 Bootstrap methodology was used to correct for bias in the observed values and calculate confidence intervals.19 The external validation of CxbR performance (sensitivity, specificity and NPV) was undertaken in the patients recruited by KP. This data set was also used to evaluate the Cxb test algorithm described in figure 1 and supplementary methods (https://www.jurology.com).
Results
CxbR Development and Internal Validation
Patients
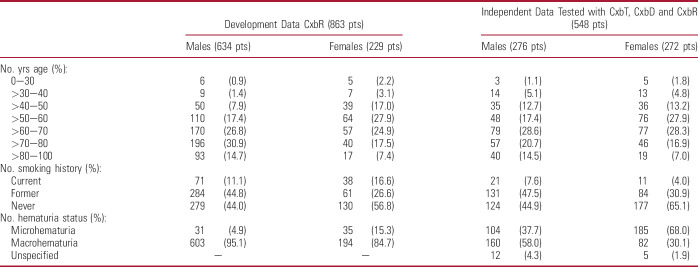
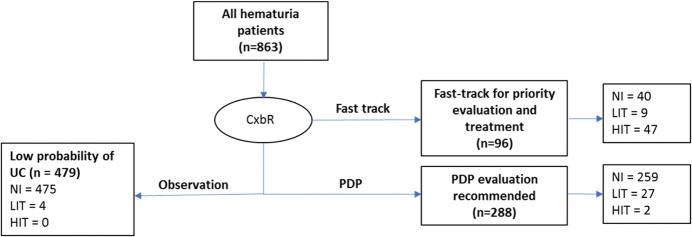
The DDS (863) included hematuria evaluation patients from Australia, New Zealand (620)10,12 and United States (243; table 2 and supplementary figure 1, https://www.jurology.com). Forty patients were diagnosed with LIT and 49 with HIT (table 3 and fig. 2).
Table 2.
Demographic characteristics of patients in DDS for CxbR, and those in external validation data set for CxbR who also underwent testing with concatenation of CxbT, CxbD and CxbR
Table 3.
Tumor stage and impact in DDS and independent validation data set
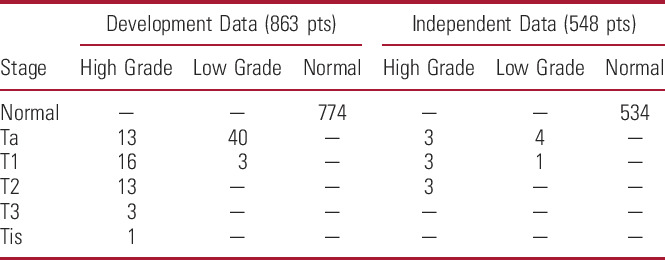
Figure 2.
Number of CxbR tests performed, results obtained and number of patients with low or high-impact UC diagnosed within each category in development/internal validation data set. HIT=high-grade Ta, Tis or T1–T3, or concurrent Tis; LIT=low-grade Ta or papillary urothelial neoplasm of low malignant potential. NI, no cancer present.
Diagnostic Performance
CxbR assigned 96 patients to the “high priority” category, including 56 tumor-positive patients, 47 with HIT and 9 with LIT (fig. 2). In the remaining 767 patients, CxbR assigned 288 to PDP workup and 479 to observation. In the PDP group, 29 patients had UC, of which 2 were HIT and 27 were LIT, while none of the patients assigned to observation had HIT and 4 had LIT (fig. 2).
An additional 5 patients had a confirmed diagnosis of upper tract UC; all 5 patients had been correctly assigned to the high priority CxbR category.
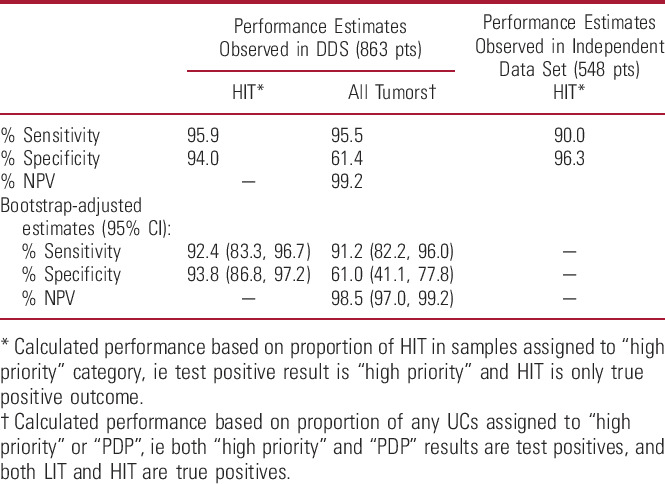
CxbR had a sensitivity (UC) of 95.5% and sensitivity (HIT) of 95.9%, with a NPV of 99.2% for any tumor (table 4). Bootstrap estimates gave a bias-corrected sensitivity (HIT) of 92.4% and specificity (HIT) of 93.8%; bias-corrected sensitivity (UC) was 91.2%, with NPV (UC) of 98.5% (table 4).
Table 4.
Diagnostic performance characteristics of CxbR
External Validation of CxbR Alone and Performance of Algorithm
Patients
Of the 641 consenting KP patients, 559 provided a urine sample, and 548 patients were included in the final analysis (11 patients excluded due to prior urinary cancer diagnosis; table 2 and supplementary fig. 2, https://www.jurology.com). Overall, 242 (44.2%) patients had macrohematuria, 289 (52.7%) had microhematuria, and 17 (3.1%) were not classified. Tumor characteristics are provided in table 3.
External Validation of CxbR Alone
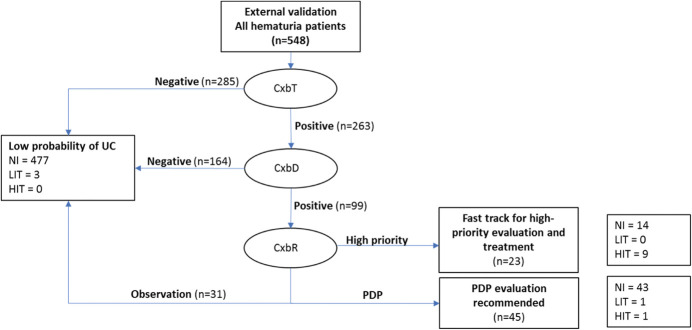
Validating CxbR alone, 29 patients were classified for “high priority” workup (supplementary fig. 3, https://www.jurology.com), including 9 tumor-positive patients, all with HIT. In the other 519 patients, CxbR identified 212 needing PDP and 307 for observation. Three patients in the PDP group had UC (2 LIT and 1 HIT), and 2 in the observation group had UC (both LIT; none had HIT). CxbR alone had sensitivity (HIT) of 90.0%, specificity (HIT) of 96.3%, and NPV (HIT) of 99.8% (table 4). All patients with HIT were classified by CxbR as needing either PDP (1) or high-priority workup (9; fig. 3). No patients in the validation data set had a diagnosis of upper tract UC.
Figure 3.
Results of using CxbT, CxbD and CxbR in succession to rule out patients unlikely to have UC, and to identify those who need further workup, in external validation (KP) data set. HIT=high-grade Ta, or T1–T3 or concurrent Tis; LIT=low-grade Ta or papillary urothelial neoplasm of low malignant potential. NI, no cancer present.
Algorithm Performance
In the same data set, we evaluated the performance of the algorithm shown in figure 1. All samples (548) underwent CxbT; 285/548 (52%) had negative results, including 1 patient with a low-grade Ta tumor (fig. 3 and supplementary figs. 3 to 5, https://www.jurology.com). CxbD was performed on the remaining 263/548 CxbT-positive samples (48%); 164/548 samples (30%) returned a CxbD result indicating a low probability of UC and were ruled out, including 1 patient with a low-grade Ta tumor (fig. 3 and supplementary figs. 3 to 5, https://www.jurology.com). Therefore, CxbT followed by CxbD ruled out 449/548 patients (81.9%), but included 2 false-negative results, both low-grade, small Ta tumors. No patients with HIT were incorrectly ruled out.
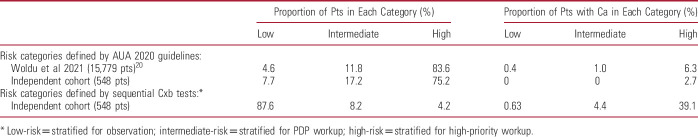
CxbR was performed on the 99/548 samples (18.1%) not ruled out by CxbT and CxbD, and classified 31/99 patients for observation, 45/99 for PDP workup, and 23/99 for high-priority workup (fig. 3 and supplementary figs. 3 to 5, https://www.jurology.com). The concatenated use of CxbT, CxbD and CxbR identified 68 patients (12.4%) as needing either PDP or high-priority workup. When the new AUA guideline risk classification3 was applied to patients in the external validation population (548), 7.7% were classified low-risk, 17.2% as intermediate and 75.2% as high-risk (table 5). Using the AUA guidelines,3 412/548 high-risk patients would be worked up and all 14 patients (3.4%) with tumors would be correctly identified. Using the Cxb algorithm to identify high-risk patients, 68/548 patients were assigned to workup capturing 11/14 (78.6%) tumors, including all HIT (the remaining 3 being low grade Tas).
Table 5.
Proportion of patients and incidence of cancer in low, intermediate and high-risk categories according to AUA 2020 Guideline and use of 3 sequential Cxb tests
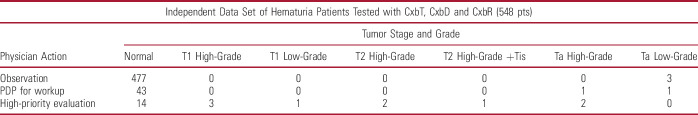
Of the 23 patients classified for high-priority evaluation, 9 had HIT. Of the 45 patients classified for PDP evaluation, 1 had LIT, 1 had HIT (high-grade Ta tumor) and 41 did not have UC (table 6). One patient in the observation category also had LIT (fig. 3 and supplementary figs. 3 to 5, https://www.jurology.com). The proposed algorithm of Cxb tests accurately ruled out 87% of patients who did not have UC and correctly identified 100% of HIT patients for further investigation (fig. 3 and supplementary figs. 4 and 5, https://www.jurology.com).
Table 6.
Tumors identified in hematuria patients in independent validation set according to segregation into categories using sequence of Cxb tests
Discussion
Bias-corrected estimates in the DDS demonstrated that CxbR alone is highly effective for assigning patients who have HIT to high-priority workup, with 92.4% sensitivity and 93.8% specificity. CxbR has a sensitivity of 91.2% for detecting any tumor and a 98.5% NPV. Data from the KP cohort validated CxbR performance with sensitivity of 90.0% and specificity of 96.3% for HIT. In both the DDS and external CxbR validation studies, all patients with HIT were correctly assigned to PDP or high-priority evaluation.
The reflex algorithm using 3 Cxb tests accurately identified 480/548 (87.6%) patients with low probability of UC to undergo observation (sensitivity 85.7% NPV 99.8%). All patients with positive CxbT and positive CxbD results reflexed to CxbR and all HIT were correctly identified. Three low-grade Ta tumors were missed in this cohort of 480 patients. Therefore, using the 3 tests together on a single urine sample at the initial workup of hematuria provides significant clinical utility to patients, physicians and payers. The tests accurately segregate out patients who need no further workup for bladder cancer from those who have LIT and require PDP, and those who have HIT and should be prioritized for further evaluation.
When the new AUA guideline risk classification3 was applied to patients in the external validation population, 7.7% were classified as low-risk, 17.2% as intermediate and 75.2% as high-risk (table 5), similar to the distribution reported by Woldu et al in a retrospective validation of guideline performance in 15,779 patients.20 Using AUA guidelines to segregate high-risk patients produced a diagnostic yield of 14/412 (3.4%) whereas Cxb had a diagnostic yield of 11/68 (16.9%), which is 4.8-fold higher. Significant advantages accrue if Cxb is used in the 75.2% patients classified as AUA high-risk since most of these would be reclassified as low-risk using Cxb, enabling physicians to accurately identify those with HIT from this much smaller patient cohort and surveil all others. This approach is consistent with a recent model, which showed that all patients with a positive urine biomarker test should be reclassified as high-risk, irrespective of AUA risk category, and those with a negative result considered as intermediate or low-risk.21
Our findings support previous studies showing highly repeatable performance of CxbT and CxbD. All Cxb tests have high sensitivity and NPV,9–12 and the combination of CxbT and CxbD has high specificity, sensitivity and NPV.6 Most hematuria patients do not have UC and therefore may receive unnecessary expensive and invasive tests, incurring extra costs and time delays.22–24 A real-world study showed that using CxbT in the clinical pathway for UC reduced the ordering of cystoscopy by 44% and computerized tomography by 20%.25 CxbT is now routinely combined with imaging at the time of hematuria evaluation in the clinical algorithm used by public health care providers in New Zealand.26 A recently published lookback on the real-world use of that algorithm reported sensitivity of 98.7%, NPV of 99.9%, and specificity of 39%, ruling out the need for further workup in 53% of hematuria patients.27
The current study extends these findings to the utility of consecutively using 3 Cxb tests on hematuria patients. Our results suggest that sequential use of CxbT, CxbD and CxbR on a single urine sample in the initial workup of hematuria correctly identifies all patients with HIT, including all HIT in AUA guideline-defined high-risk patients. Negative results spared 87.6% of individuals, unlikely to have UC, from the need for cystoscopy and imaging. A positive result prioritizes patients for workup, potentially obviating the need for flexible cystoscopy and fast-tracking patients for transurethral resection of the bladder tumor.
For various reasons, many patients who present to primary care with hematuria are not referred for workup. However, the availability of a highly reliable urine test that enriches the diagnostic yield in the hematuria population improves the proportion of at-risk patients being evaluated, resulting in a higher rate of early UC detection in the patients who are referred for workup. Cxb’s high level of clinical resolution to rule out patients without UC and prioritize others is likely to reduce referral time and enable specialist resources to be focused on patients most likely to have UC, providing a clinically meaningful benefit and reducing the cost of care.
Potential limitations of this study are the small proportion of patients with tumors in the validation (KP) data set, with a high proportion of patients with microhematuria selected to represent a more real world cohort than the development data. The development and external validation cohorts had differing proportions of macro and microhematuria and different gender ratios. Further research in larger and more diverse patient groups is needed to verify the current findings. The reflex algorithm missed 3 low-grade Ta tumors; however, as noted by Davidson et al, combining Cxb with imaging increased the NPV to 99.9%.26,27
Conclusions
CxbR, a noninvasive test with high sensitivity and specificity used on a single urine sample taken at initial investigation, correctly identified all patients with HIT, who can be prioritized for workup. The sequential use of CxbR in combination with CxbT and CxbD was validated to accurately segregate patients with no UC from those with LIT and HIT, allowing those who do not have UC to avoid further workup and focusing resources on HIT patients, with a diagnostic yield 4.8-fold higher than AUA guideline stratification.
Acknowledgments
We would like to thank Catherine Rees of Springer Healthcare Communications who wrote the outline and first draft of the manuscript. This medical writing assistance was funded by Pacific Edge Ltd.
Footnotes
Conflicts of interest/financial disclosures: Dr. Jay Raman has been a study investigator for trials sponsored by MDxHealth and Urogen Pharma LLC, and has provided advisory services to Urogen Pharma LLC. Dr. Yair Lotan has been a study investigator for Vessi Medical, CAPs Medical, Abbott, Cepheid, Pacific Edge, FKD, MDxHealth, Biocancell, GenomeDx Biosciences Inc., Storz, Photocure, Radera, Synergo, BMS, Urogen and Zymo, and has provided advisory services to C2I genomics, Photocure, AstraZeneca, Merck, Fergene, Abbvie, Cleveland Diagnostics, Nucleix, Amby, Seattle Genetics, Ferring Research, Hitachi, and Verity Pharmaceuticals. Dr. Badrinath Konety is a consultant to Ferring, Boston Scientific, Convergent Genomics and a clinical investigator for Merck, BMS, Pacific Edge and Photocure. Dr. Siamak Daneshmand has been study investigator for trials sponsored by Photocure, Janssen, Ferring, Taris, Pacific Edge, Johnson & Johnson, BMS, and QED and has provided advisory services to Janssen, Ferring, Photocure, Taris, Spectrum, Pacific Edge, QED, Abbvie, Johnson & Johnson, Seattle Genetics, Nucleix, Aduro, and BMS. Dr. Sima Porten is on the advisory board of ProTara, Kdx, has consulted for Fergene and Photocure and has provided research support to Genome Dx Biosciences.
Contributor Information
Jay D. Raman, Email: jraman@pennstatehealth.psu.edu.
Badrinath Konety, Email: badrinath_konety@rush.edu.
Sima Porten, Email: sima.porten@ucsf.edu.
Siamak Daneshmand, Email: daneshma@med.usc.edu.
Yair Lotan, Email: yair.lotan@UTSouthwestern.edu.
Ronald Loo, Email: ronald.k.loo@kp.org.
REFERENCES
- 1.Bruyninckx R, Buntinx F, Aertgeerts B, et al. : The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. Br J Gen Pract 2003; 53: 31. [PMC free article] [PubMed] [Google Scholar]
- 2.Sharp VJ Barnes KT and Erickson BA: Assessment of asymptomatic microscopic hematuria in adults. Am Fam Physician 2013; 88: 747. [PubMed] [Google Scholar]
- 3.Barocas DA, Boorjian S, Alvarez R, et al. : Microhematuria: AUA/SUFU Guideline. J Urol 204; 778: 2020. [DOI] [PubMed] [Google Scholar]
- 4.Loo RK, Lieberman SF, Slezak JM, et al. : Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria. Mayo Clinic Proc 2013; 88: 129. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen M and Qaseem A: Hematuria as a marker of occult urinary tract cancer: advice for high-value care from the American College of Physicians. Ann Intern Med 2016; 164: 488. [DOI] [PubMed] [Google Scholar]
- 6.Konety B, Shore N, Kader AK, et al. : Evaluation of Cxbladder and adjudication of atypical cytology and equivocal cystoscopy. Eur Urol 2019; 76: 238. [DOI] [PubMed] [Google Scholar]
- 7.Elias K, Svatek RS, Gupta S, et al. : High-risk patients with hematuria are not evaluated according to guideline recommendations. Cancer 2010; 116: 2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holyoake A, O'Sullivan P, Pollock R, et al. : Development of a multiplex RNA urine test for the detection and stratification of transitional cell carcinoma of the bladder. Clin Cancer Res 2008; 14: 742. [DOI] [PubMed] [Google Scholar]
- 9.Kavalieris L, O'Sullivan P, Frampton C, et al. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J Urol 2017; 197: 1419. [DOI] [PubMed] [Google Scholar]
- 10.Kavalieris L, O'Sullivan PJ, Suttie JM, et al. A segregation index combining phenotypic (clinical characteristics) and genotypic (gene expression) biomarkers from a urine sample to triage out patients presenting with hematuria who have a low probability of urothelial carcinoma. BMC Urol 2015; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotan Y, O'Sullivan P, Raman JD, et al. : Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol Oncol 2017; 35: 531 e15. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan P, Sharples K, Dalphin M, et al. : A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol 2012; 188: 741. [DOI] [PubMed] [Google Scholar]
- 13.Sobin LH Gospodarowicz MK and Wittekind C: TNM Classification of Malignant Tumours. 7th ed. Chichester, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 14.Mostofi FK Sobin LH and Torloni H. Histological Type of Urinary Bladder Tumours. Geneva, Switzerland: World Health Organization; 1973. [Google Scholar]
- 15.Epstein JI, Amin MB, Reuter VR, et al. : The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998; 22: 1435. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG and Bland JM: Diagnostic tests 2: predictive values. BMJ 1994; 309: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman DG and Bland JM: Diagnostic tests 1: sensitivity and specificity. BMJ 1994; 308: 1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 19.Harrell FE: Regression Modelling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 7th ed. Cham, Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 20.Woldu SL, Ng CK, Loo RK, et al. : Evaluation of the new American Urological Association guidelines risk classification for hematuria. J Urol 2021; 205: 1387. [DOI] [PubMed] [Google Scholar]
- 21.Woldu SL, Souter L, Boorjian SA, et al. : Urinary-based tumor markers enhance microhematuria risk stratification according to baseline bladder cancer prevalence. Urol Oncol 2021. doi: 10.1016/j.urolonc.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Burke DM Shackley DC and O'Reilly PH: The community-based morbidity of flexible cystoscopy. BJU Int 2002; 89: 347. [DOI] [PubMed] [Google Scholar]
- 23.King K and Steggall M: Haematuria: from identification to treatment. Br J Nurs 2014; 23: S28. [DOI] [PubMed] [Google Scholar]
- 24.Paul C, Carey M, Anderson A, et al. : Cancer patients' concerns regarding access to cancer care: perceived impact of waiting times along the diagnosis and treatment journey. Eur J Cancer Care 2012; 21: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darling D, Luxmanan C, O'Sullivan P, et al. : Clinical utility of Cxbladder for the diagnosis of urothelial carcinoma. Adv Ther 2017; 34: 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson PJ McGeoch G and Shand B: Inclusion of a molecular marker of bladder cancer in a clinical pathway for investigation of haematuria may reduce the need for cystoscopy. N Z Med J 2019; 132: 55. [PubMed] [Google Scholar]
- 27.Davidson PJ McGeoch G and Shand B: Assessment of a clinical pathway for investigation of haematuria that reduces the need for cystoscopy. N Z Med J 2020; 133: 71. [PubMed] [Google Scholar]