Abstract
To investigate the dissemination of vancomycin-resistant Enterococcus faecium (VREF) in a 728-bed tertiary-care hospital, all clinical VREF isolates recovered from June 1992 to June 1997 were typed by pulsed-field gel electrophoresis, and the transfer histories of the patients were documented. A total of 413 VREF isolates from urine (52%), wounds (16%), blood (11%), catheter tips (6%), and other sites (15%) were studied. VREF specimens mostly came from patients on wards (66%) but 34% came from patients in an intensive care unit. The number of VREF isolates progressively increased over time, with higher rates of isolation during the winter months and lower rates in the late summer months. Four distinct banding patterns were detected by pulsed-field gel electrophoresis among 316 samples (76%). Strain A (122 samples; 30%) appeared in June 1992 as the first VREF strain and was found until December 1994 throughout the entire hospital. Type B (92 samples; 22%) was initially detected in January 1994 and disappeared in November 1996. Strain C (10 samples; 2%) was limited to late 1996 and early 1997. Strain D (92 samples; 22%) showed two major peaks during March 1996 to August 1996 and January 1997 to February 1997. Unrelated strains (97 samples; 24%) appeared 1 year after the appearance of the first VREF isolate, and the numbers increased slightly over the years. Nosocomial acquisition (i.e., no known detection prior to admission and first isolation from cultures performed with samples retrieved ≥2 days after hospitalization) was found for 316 (91%) of 347 patients. Despite the implementation of Centers for Disease Control and Prevention guidelines, the proportion of related strains and high number of nosocomial cases of infection indicate a high transmission rate inside the hospital. The results imply an urgent need for stringent enforcement of more effective infection control measures.
Enterococci are the second leading cause of nosocomial urinary tract infections and the third leading cause of nosocomial wound and bloodstream infections (19). Resistance to environmental conditions such as heat or desiccation allow prolonged survival, and poor compliance with hand-washing procedures by health care workers results in the rapid spread of enterococci in hospitals (26, 42, 45, 66). Moreover, strains of enterococci have acquired resistance to essentially all antimicrobial agents over the past three decades (16, 65). The prevalence of vancomycin resistance in enterococci has dramatically increased in the last few years. In 1989, less than 0.4% of enterococci were resistant to vancomycin in general wards and intensive care units (ICUs). Eight years later, 15% of enterococcal isolates in wards and 23% in ICUs were resistant to vancomycin (36). Therefore, the increasing rate of spread of vancomycin-resistant enterococci (VRE) within hospitals in the United States and the especially high rates in ICUs indicate that VRE are some of the most important pathogens today and illustrate the need for effective infection control measures (28, 52).
The present study was initiated to investigate the relatedness of clinical VRE isolates collected in a 728-bed tertiary-care hospital from June 1992 to June 1997 by molecular biotyping methods and the hospital transfer histories of the patients.
(This study was presented at the 8th Annual Meeting of the Society for Healthcare Epidemiology of America, April 1998, Orlando, Fla. [abstr. S 44, p. 48].)
MATERIALS AND METHODS
Setting.
The study was performed at the Medical College of Virginia Hospitals, a 728-bed tertiary-care, teaching facility located in the city of Richmond, Va. The hospital houses nine specialized ICUs with a total of 104 beds.
Study design.
The present study comprised a retrospective analysis of all new clinical, vancomycin-resistant Enterococcus faecium (VREF) isolates collected between June 1992 and June 1997.
Sample preparation.
Bacterial isolates were stored at −70°C in medium containing 15% glycerol. For the present study, 411 VREF isolates were thawed, and the species identification was confirmed with the RAPID ID 32STREP system (bioMérieux sa, Marcy l'Etoile, France). Susceptibility to ampicillin, gentamicin (Ratiopharm GmbH&Co, Ulm, Germany), streptomycin (Merck AG, Darmstadt, Germany), vancomycin, teicoplanin, and the experimental antibiotic LY 333328 (Eli Lilly & Co, Indianapolis, Ind.) was determined by the broth microdilution method.
Genotype analysis.
Pulsed-field gel electrophoresis (PFGE) (CHEF DR III; Bio-Rad Laboratories, Richmond, Calif.) was performed in a 1% agarose gel for all isolates after digestion of total DNA with SmaI (GIBCO BRL, Grand Island, N.Y.), as described previously (37). The electrophoresis lasted 22 h, with the pulse time ramped from 1 to 35 s. The restriction profiles in ethidium bromide-stained gels were visually analyzed by one observer and were photographed. The images of the gels were also digitized for further investigation. For evaluation of epidemic isolates, the restriction patterns were compared according to previously published guidelines (62): isolates were considered indistinguishable if there was no band difference or closely related if they differed by two to three banding patterns, which indicated changes consistent with a single genetic event. Two independent genetic events indicated that isolates were possibly related to the outbreak strain. An isolate was defined as unrelated to an outbreak strain when its PFGE pattern differed from those of the outbreak strains by three or more independent genetic events.
Genotype determination.
The isolation of bacterial DNA and the amplification of a 377-bp fragment of the vanA gene by PCR were performed as described previously (31). For amplification of a vanB gene fragment (289 bp), the same PCR solutions used for vanA were used, but the cycles were modified (cycle 1 was 94°C for 6 min, 56°C for 1 min, and 72°C for 1 min and cycles 2 to 31 were 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min; cycle 32 was 72°C for 2 min) and the following primers were used: vanB-I (5′-CAT CGC CGT CCC CGA ATT TCA AA-3′) and vanB-II (5′-GAT GCG GAA GAT ACC GTG GCT-3′). The PCR products were detected in a 1.4% agarose gel (type II) (Medium EEO; Sigma Chemicals, St. Louis, Mo.).
Infection control measures.
In March 1994 an “antibiotic resistant precautions” infection control policy was introduced. This policy required the use of gloves and gowns upon entering the room of a patient colonized or infected with VRE. The gown requirement was modified in September 1994 to the use of gowns only if close contact or potential contamination of clothing was anticipated. No interventions were introduced to influence health care worker compliance with infection control policies.
Definition of community-acquired and nosocomial infections.
For each patient, admission and discharge dates from each hospital unit were documented. To estimate the annual rate of occurrence of VREF isolates in our hospital, the total number of new clinical isolates was divided by the total number of patient admissions. The definition of nosocomial acquisition was no known colonization or infection prior to admission and first isolation of VREF from cultures performed with samples retrieved at least 2 days after hospitalization or previous hospitalizations within the prior 2 months and cultures positive for VREF within 48 h after the current admission. Patients who were infected or colonized with VREF and who did not fulfill these criteria were defined as having community-acquired cases of infection.
RESULTS
Patients and materials.
A total of 413 patients colonized or infected with VREF were studied. From 1992 to 1997 the rate of isolation of VREF increased from 1.1 per 1,000 to 3.3 per 1,000 patient admissions. Clinical isolates were obtained mostly from urine (52%), followed by wounds (16%), blood (11%), catheter tips (6%), and other sites (15%).
PFGE results.
PFGE of 413 isolates revealed four distinct banding patterns (patterns A, B, C, and D) for 316 (77%) specimens (Fig. 1; Table 1). Ninety-seven (24%) isolates were not related to these strain types or to each other. Strains with the predominant pattern, pattern A, were found in 122 (30%) samples, strains with pattern B were found in 92 (22%) samples, strains with pattern C were found in 10 (2%) samples, and strains with pattern D were found in 92 (22%), respectively.
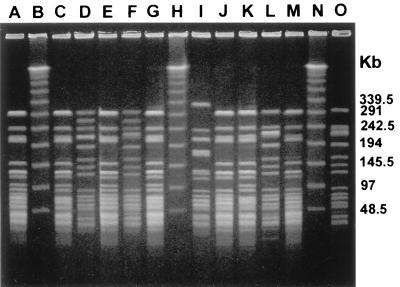
FIG. 1.
PFGE of SmaI-digested genomic DNA from 12 vanA-containing E. faecium strains. Lanes: B, H, and N, bacteriophage lambda ladder molecular mass standard; A, C, E, G, J, K, and M, outbreak isolates of pattern D; D, F, L, and O, outbreak isolates of pattern C; I, unrelated isolate.
TABLE 1.
Frequency of VREF strains, van genotypes, ampicillin and aminoglycoside susceptibilities, and detection periods
| Pattern | No. of isolates | Genotype | MIC (μg/ml)
|
Detection period | ||
|---|---|---|---|---|---|---|
| Ampicillin | Gentamicin | Streptomycin | ||||
| A | 122 | vanA | 16 | >1,024 | 32 | June 1992–December 1994 |
| B | 92 | vanB | 64 | >2,048 | >2,048 | January 1994–November 1996 |
| C | 10 | vanA | 128 | >2,048 | >2,048 | March 1995a and November 1996–February 1997 |
| D | 92 | vanA | 128 | >2,048 | >2,048 | November 1995–June 1997 |
Two isolates.
Resistance patterns of strains.
All isolates expressed high-level vancomycin resistance (MICs, ≥128 μg/ml; Table 1). Of the strains with the four patterns, only strains with pattern B were susceptible to teicoplanin (MICs, <1 μg/ml). Pattern B strains contained the vanB resistance gene, whereas the strains with the other three patterns contained vanA genes. No vanC strain was detected. High-level resistance to the aminoglycosides gentamicin (MICs, >1,024 μg/ml) and streptomycin (MICs, >2,048 μg/ml) was demonstrated in strains with patterns B, C, and D. Pattern A strains were resistant only to gentamicin (MICs, >1,024 μg/ml). Ampicillin resistance was noted in the isolates. The MIC of ampicillin was ≥16 μg/ml (MICs, 16 μg/ml [pattern A strains], 64 μg/ml [pattern B strains], and 128 μg/ml [pattern C and D strains]). Strains of all four patterns were susceptible to the investigational antibiotic LY 333328 (MICs, <0.125 μg/ml).
Dissemination of strains inside the hospital.
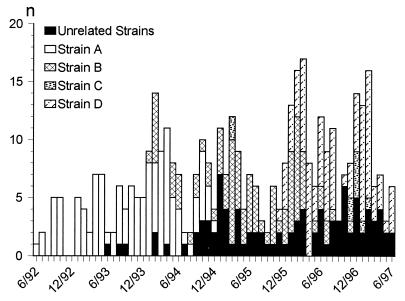
A pattern A strain appeared in June 1992 and was the first VREF isolate recovered from a patient on a ward. It was found throughout the hospital until December 1994 (Fig. 2). Eighty-six clinical isolates were obtained from patients on wards, whereas 36 isolates were obtained from patients in ICUs. Between February 1994 and April 1994 an increased rate of recovery of VREF isolates (up to eight isolates per month) was found in ICUs.
FIG. 2.
Frequency of isolation of VREF from incident clinical samples at the Medical College of Virginia Hospitals by strain type over time (June 1992 to June 1997).
A pattern B strain was initially detected in January 1994 in an ICU and disappeared in November 1996. The pattern B strains were detected in 38 samples from patients in ICUs and 54 samples from patients in wards.
The first two isolates with pattern C appeared in March 1995 in a patient in an ICU and a patient on a ward. Twenty months later, from December 1996 to February 1997, a second cluster of pattern C strains was detected. Most samples were found in patients on wards (seven isolates from patients on wards and three isolates from patients in ICUs).
The index pattern D strain was identified in November 1995 from a patient on a ward. Like pattern A strains, the pattern D strains were more concentrated in patients on wards (67 isolates) than in patients in ICUs (25 isolates). Two peaks appeared between March and August 1996 and the first 2 months of 1997 in patients on wards. Since the study ended in June 1997, no further data are available for evaluation of the further spread of this strain. Unrelated isolates were found 1 year after the first detection of a VREF strain. The detection rate increased slightly over the years.
Nosocomial cases of infection.
Sixty-nine percent of all VREF isolates (284 specimens) were from patients on wards, whereas 31% (129 specimens) were from patients in ICUs. Ninety-one percent (316 specimens) met the definition for nosocomial acquisition, including 251 of 266 (94%) related strains and 65 of 81 (80%) isolates of unrelated strain types. No data were available for 66 specimens (50 infected with related strains and 16 infected with unrelated strains).
DISCUSSION
Infection or colonization with VRE results in a wide spectrum of possible deleterious effects for patients in health care settings. Studies have demonstrated that VRE can cause serious infections, and the attributable mortality of bacteremia caused by VRE has been estimated to be nearly 40% (18, 43). Control of VRE may result in a reduced quality of life for patients, since isolation measures limit social exchange. In addition to possible increased length of hospitalization, VRE carrier status makes the integration of these patients more difficult, especially in rehabilitation or long-term health care settings (14, 27, 32, 69). Overall, problems related to therapy and isolation for patients colonized or infected with VRE lead to increased health care costs (61).
First detected in Europe in 1986 (33), VRE have emerged as important pathogens in the United States. Regarding the origin of VRE, European studies demonstrated that the carriage of VRE in the community was probably caused by the use of antibiotics as growth promoters in animals (1, 13). In the United States, however, Coque et al. (11) and Silverman et al. (57) could not isolate any VRE from community volunteers or consecutively admitted patients. These findings are reinforced by the wide variety of PFGE patterns in European studies compared to the that in the United States (3, 5). Van den Braak et al. (67) hypothesized that dissemination of resistance genes might be more important than strain transfer, explaining the clonal discrepancy of strains of VRE between animals and humans in Europe. Only recently, VRE have been isolated from animal feed in a small study in the United States (56). In our study, the number of outbreak strains was limited to four. Other investigators have observed similar results (9, 11, 15, 25, 40). Only Bonten et al. (7) found polyclonality of isolates of VRE in a U.S. ICU. Therefore, the origin of VRE in the United States is still unclear, but their distribution appears to be concentrated in health care settings.
VRE are usually transmitted by contact. Noskin et al. (44) found that VRE could survive for at least 1 h on gloved and ungloved fingertips and for 5 to 7 days on environmental surfaces. Other investigators have shown that VRE can exist in stool specimens of a carrier for up to 2 years, providing a source for environmental spread (34, 35, 39, 47, 54). These characteristics can result in the wide dissemination of VRE, as demonstrated in our study. Beginning with an index strain in June 1992, one VREF strain (pattern A) circulated throughout the entire hospital for a 2-year period. Unrelated strains appeared 12 months after the appearance of the first VREF strain, indicating that there was no additional outside source for VREF at the beginning of the observation period. Rapid dissemination of VRE has also been found by other investigators (9, 11, 25, 46). Investigations of different health care facilities in the same region by Moreno et al. (40) and Fridkin et al. (22) revealed that the spread of VRE strains is not limited to one hospital.
The majority of published studies of VRE have focused on ICUs. However, our investigation revealed that 66% of all VREF were found in ward areas. The VREF situation in our hospital evolved from a locally limited outbreak to a situation of endemicity affecting all units in a short time period. Therefore, hospitalwide approaches are vital to control the dissemination of VRE even at an early stage.
An interesting aspect of the dissemination of VRE in our study is the sudden disappearance of one outbreak strain followed by the appearance of another strain. Reasons for the strain shift remain unknown. Isolation and contact precaution measures cannot be considered, since another prominent strain emerged by a similar transmission route. Therefore, further studies should focus on the microbial mechanisms favoring one VRE strain over another as a cause of hospitalwide outbreaks (22).
In the last decade several approaches to preventing and limiting the spread of VRE were developed and integrated into national guidelines for hospitals (23, 29). These recommendations are based on the origin of VRE, the mechanism of transmission in the hospital environment, and the risk factors for acquisition.
Preventive measures taken to avoid selection of VRE include the restricted use of antibiotics, especially vancomycin (2, 8, 10, 12, 21, 24, 25, 30, 35, 40, 46, 48–51, 55, 58, 60, 63, 68). The Hospital Infection Control Practices Advisory Committee (HICPAC) of the Centers for Disease Control and Prevention published guidelines for the appropriate use of vancomycin in 1995 (29). These recommendations were partially implemented in our hospital. Evans and Kortas (21) reported that only 35% of 101 orders for vancomycin were consistent with the HICPAC guidelines in a university hospital. Administration of vancomycin or other antibiotics, such as cephalosporins, metronidazole, or clindamycin, can also increase the rate of skin colonization with VRE, enhancing possible transmission from patient to patient (2). Restriction of antibiotic use to an acceptable level may reduce the selection for VRE, but studies are still needed to prove the benefit of this measure.
Standard precautions should be followed for the handling of patients in the daily routine. Even if the patient is not known to be infected or colonized with a pathogen, these guidelines require the use of gloves for contact with blood, body fluids, secretions, excretions, and potentially contaminated items and hand washing after glove removal. However, measurement of compliance with hand washing by health care workers in our own facility has revealed unacceptably low hand-washing rates (4). The importance of this simple method is further strengthened by the large number of unrecognized, silent carriers of VRE (2, 6, 8, 12, 17, 25, 30, 35, 38, 40, 41, 46, 49, 53, 59, 63, 64, 66). If VRE are detected, isolation precautions should be implemented as described in Centers for Disease Control and Prevention and HICPAC guidelines (23, 29). In March 1994 “antibiotic resistant precautions” were introduced in our facility. These precautions require the use of gloves and gowns upon entering a patient's room. These interventions did not appear to have a significant impact on the spread of VREF. In summary, even if precautions are practical and useful, compliance by health care providers is a major factor for their success.
Because we collected only clinical isolates, we do not know how widespread colonization with VREF was and whether there were differences in rates of colonization with various strains. Furthermore, the outcome of infections caused by VREF depends highly on the severity of the underlying diseases. Therefore, the results of this study should be interpreted as a reflection of the potential risk for patients who acquire VREF and not as a predictive value of fatality.
In conclusion, the rapidly developing situation of endemicity in our hospital demonstrated the ability of VRE to spread, despite implementation of universal and specialized infection control measures. Control of VRE requires a multifaceted approach since existing measures seem to be inadequate and/or are not appropriately followed by health care providers.
ACKNOWLEDGMENTS
We are grateful to I. Klare, Robert Koch-Institute, Wernigerode, Germany, for determination of the van genotypes of the strain isolates.
W. E. Bischoff was supported by a grant from the Walter-Marget-Society, Freiburg, Germany.
The experimental antibiotic LY 333328 was provided by Eli Lilly & Co, Indianapolis, Ind.
REFERENCES
- 1.Bates J. Epidemiology of vancomycin-resistant enterococci in the community and the relevance of farm animals to human infection. J Hosp Infect. 1997;37:89–101. doi: 10.1016/s0195-6701(97)90179-1. [DOI] [PubMed] [Google Scholar]
- 2.Beezhold D W, Slaughter S, Hayden M K, Matuschek M, Nathan C, Trenhome G M, Weinstein R A. Skin colonization with vancomycin-resistant enterococci among hospitalized patients with bacteremia. Clin Infect Dis. 1997;24:704–706. doi: 10.1093/clind/24.4.704. [DOI] [PubMed] [Google Scholar]
- 3.Bingen E H, Denamur E, Lambert-Zechovsky N J, Elion J. Evidence for genetic unrelatedness of nosocomial outbreak of vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991;29:1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff W E, Reynolds T M, Sessler C N, Edmond M B, Wenzel R P. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Handwashing compliance by health care workers: the impact of an education and patient awareness program and the introduction of a new hand disinfectant, abstr. K-132; p. 540. [Google Scholar]
- 5.Boisivon A, Thibault M, Leclercq R. Colonization by vancomycin-resistant enterococci of the intestinal tract of patients in intensive care units from French general hospitals. Clin Microbiol Infect. 1997;3:175–179. doi: 10.1111/j.1469-0691.1997.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonilla H F, Zervos M A, Lyons M J, Bradley S F, Hedderwick S A, Ramsey M A, Paul L K, Kauffman C A. Colonization with vancomycin-resistant Enterococcus faecium: comparison of a long-term-care unit with an acute-care hospital. Infect Control Hosp Epidemiol. 1997;18:333–339. doi: 10.1086/647621. [DOI] [PubMed] [Google Scholar]
- 7.Bonten M J M, Hayden M K, Nathan C, van Voorhis J, Matushek M, Slaughter S, Rice T, Weinstein R A. Epidemiology of colonization of patient and environment with vancomycin-resistant enterococci. Lancet. 1996;348:1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 8.Boyce J M, Mermel L A, Zervos M J, Rice L B, Potter-Bynoe G, Giorgio C, Medeiros A A. Controlling vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 1995;16:634–637. doi: 10.1086/647028. [DOI] [PubMed] [Google Scholar]
- 9.Boyce J M, Opal S M, Chow J W, Zervos M J, Potter-Bynoe G, Sherman C B, Romulo R L C, Fortna S, Medeiros A A. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–1153. doi: 10.1128/jcm.32.5.1148-1153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle J F, Soumakis S A, Rendo A, Herrington J A, Gianarkis D G, Thurberg B E, Painter B G. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J Clin Microbiol. 1993;31:1280–1285. doi: 10.1128/jcm.31.5.1280-1285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coque T M, Tomayko J F, Ricke S C, Okhyusen P C, Murray B E. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–2609. doi: 10.1128/aac.40.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dembry L M, Uzokwe K, Zervos M J. Control of endemic glyocopeptide-resistant enterococci. Infect Control Hosp Epidemiol. 1996;17:286–292. doi: 10.1086/647297. [DOI] [PubMed] [Google Scholar]
- 13.Devriese L A, Ieven M, Goosens H, Vandamme P, Pot B, Hommez J, Haesebrouck F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duerden M E, Bergeron J, Baker R L, Braddon R L. Controlling the spread of vancomycin-resistant enterococci with a rehabilitation cohort unit. Arch Phys Med Rehabil. 1997;78:553–555. doi: 10.1016/s0003-9993(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 15.Dunne W M, Jr, Wang W. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J Clin Microbiol. 1997;35:388–392. doi: 10.1128/jcm.35.2.388-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edmond M B. Multidrug-resistant enterococci and the threat of vancomycin-resistant Staphyloccus aureus. In: Wenzel R P, editor. Prevention and control of nosocomial infections. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1997. pp. 339–355. [Google Scholar]
- 17.Edmond M B, Ober J F, Weinbaum D L, Pfaller M A, Hwang T, Sanford M D, Wenzel R P. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin Infect Dis. 1995;20:1126–1133. doi: 10.1093/clinids/20.5.1126. [DOI] [PubMed] [Google Scholar]
- 18.Edmond M B, Ober J F, Dawson J D, Weinbaum D, Wenzel R P. Vancomycin-resistant enterococcal bacteremia: natural history and attributable mortality. Clin Infect Dis. 1996;23:1234–1239. doi: 10.1093/clinids/23.6.1234. [DOI] [PubMed] [Google Scholar]
- 19.Emori T G, Gaynes R P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ena J, Dick R W, Jones R N, Wenzel R P. The epidemiology of vancomycin usage in a university hospital: a 10-year study. JAMA. 1993;269:598–602. [PubMed] [Google Scholar]
- 21.Evans M E, Kortas K J. Vancomycin use in a university medical center: comparison with Hospital Infection Control Practices Advisory Committee guidelines. Infect Control Hosp Epidemiol. 1996;17:356–359. doi: 10.1086/647316. [DOI] [PubMed] [Google Scholar]
- 22.Fridkin S K, Yokoe D S, Whitney C G, Onderdonk A, Hooper D C. Epidemiology of a dominant clonal strain of vancomycin-resistant Enterococcus faecium at separate hospitals in Boston, Massachusetts. J Clin Microbiol. 1998;36:965–970. doi: 10.1128/jcm.36.4.965-970.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garner J S. Hospital Infection Control Practices Advisory Committee. Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol. 1996;17:53–80. doi: 10.1086/647190. [DOI] [PubMed] [Google Scholar]
- 24.Gordts B, Van Landuyt H, Ieven M, Vandamme P, Goosens H. Vancomycin-resistant enterococci colonizing the intestinal tract of hospitalized patients. J Clin Microbiol. 1995;33:2842–2846. doi: 10.1128/jcm.33.11.2842-2846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Singh K V, Murray B E, Wolff J, Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 26.Hardie J M. Genus streptococcus. In: Sneath P H A, et al., editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1043–1047. [Google Scholar]
- 27.Henning K J, Delencastre H, Eagan J, Boone N, Brown A, Chung M, Wollner N, Armstrong D. Vancomycin-resistant Enterococcus faecium on a pediatric oncology ward: duration of stool shedding and incidence of clinical infection. Pediatr Infect Dis J. 1996;15:848–854. doi: 10.1097/00006454-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Herman D J, Gerding D N. Antimicrobial resistance among enterococci. Antimicrob Agents Chemother. 1991;35:1–4. doi: 10.1128/aac.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hospital Infection Control Practices Advisory Committee. Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–113. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 30.Karanfil L V, Murphy M, Josephson A, Gaynes R, Mandel L, Hill B C, Swenson J M. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992;13:195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- 31.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. VanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol Lett. 1995;125:165–172. doi: 10.1111/j.1574-6968.1995.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 32.Lai K K, Fontecchio S A, Kelley A L, Melvin Z S, Baker S. The epidemiology of fecal carriage of vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 1997;18:762–765. [PubMed] [Google Scholar]
- 33.Leclerq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 34.Linden P, Pasculle A W, Kramer D J, Kusne S, Manez R. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. The duration of fecal carriage on vancomycin-resistant Enterococcus faecium (VREF), abstr. J-150; p. 144. [Google Scholar]
- 35.Livornese L L, Dias S, Samel C, Romanowski B, Taylor S, May P, Pitsakis P, Woods G, Kaye D, Levison M E, Johnson C C. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992;117:112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- 36.Martone W J. Spread of vancomycin-resistant enterococci: why did it happen in the United States? Infect Control Hosp Epidemiol. 1998;19:539–545. doi: 10.1086/647870. [DOI] [PubMed] [Google Scholar]
- 37.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 38.McNeeley D F, Brown A E, Noel G J, Chung M, de Lencastre H. An investigation of vancomycin-resistant Enterococcus faecium within the pediatric service of a large urban medical center. Pediatr Infect Dis J. 1998;17:184–188. doi: 10.1097/00006454-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Montecalvo M A, de Lencastre H, Carraher M, Gedris C, Chung M, VanHorn K, Wormser G P. Natural history of colonization with vancomycin-resistant Enterococcus faecium. Infect Control Hosp Epidemiol. 1995;16:680–685. doi: 10.1086/647041. [DOI] [PubMed] [Google Scholar]
- 40.Moreno F, Grota P, Grisp C, Magnon K, Melcher G P, Jorgensen J H, Patterson J E. Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium during its emergence in a city in southern Texas. Clin Infect Dis. 1995;21:1234–1237. doi: 10.1093/clinids/21.5.1234. [DOI] [PubMed] [Google Scholar]
- 41.Morris J G, Shay D K, Hebden J N, McCarter R J, Perdue B E, Jarvis W, Johnson J A, Dowling T C, Polish L B, Schwalbe R S. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Ann Int Med. 1995;123:250–259. doi: 10.7326/0003-4819-123-4-199508150-00002. [DOI] [PubMed] [Google Scholar]
- 42.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newell K A, Millis J M, Arnow P M, Bruce D S, Woodle E S, Cronin D C, Loss G E, Grewal H, Lissoos T, Schiano T, Mead J, Thistlethwaite J R., Jr Incidence and outcome of infection by vancomycin-resistant Enterococcus following orthotopic liver transplantation. Transplantation. 1998;65:439–442. doi: 10.1097/00007890-199802150-00027. [DOI] [PubMed] [Google Scholar]
- 44.Noskin G A, Stosor V, Cooper I, Peterson L R. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 1995;16:577–581. doi: 10.1086/647011. [DOI] [PubMed] [Google Scholar]
- 45.Patterson J E, Sweeney A H, Simms M, Carley N, Mangi R, Sabetta J, Lyons R W. An analysis of 110 serious enterococcal infections: epidemiology, antibiotic susceptibility, and outcome. Medicine. 1995;74:191–200. doi: 10.1097/00005792-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Pegues D A, Pegues C F, Hibberd P L, Ford D S, Hooper D C. Emergence and dissemination of a highly vancomycin-resistant vanA strain of Enterococcus faecium in a large teaching hospital. J Clin Microbiol. 1997;35:1565–1570. doi: 10.1128/jcm.35.6.1565-1570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polish L, Hebden J, Shay D, Jarvis W, Schwalbe R, Morris J G., Jr . Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Duration of fecal carriage of vancomycin-resistant enterococci (VRE) in ICU patients, abstr. J-37; p. 263. [Google Scholar]
- 48.Porwancher R, Sheth A, Remphrey S, Taylor E, Hinkle C, Zervos M. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol. 1997;18:771–773. doi: 10.1086/647535. [DOI] [PubMed] [Google Scholar]
- 49.Quale J, Landman D, Saurina G, Atwood E, DiTore V, Patel K. Manipulation of a hospital antimicrobial formulary to control an outbreak of vancomycin-resistant enterococci. Clin Infect Dis. 1996;23:1020–1025. doi: 10.1093/clinids/23.5.1020. [DOI] [PubMed] [Google Scholar]
- 50.Rafferty M E, McCormick M I, Bopp L H, Baltch A L, George M, Smith R P, Rheal C, Ritz W, Schoonmaker D. Vancomycin-resistant enterococci in stool specimen submitted for Clostridium difficile cytotoxin assay. Infect Control Hosp Epidemiol. 1997;18:342–344. doi: 10.1086/647623. [DOI] [PubMed] [Google Scholar]
- 51.Rao G G, Ojo F, Kolokithas D. Vancomycin-resistant gram-positive cocci: risk factors for faecal carriage. J Hosp Infect. 1997;35:63–69. doi: 10.1016/s0195-6701(97)90169-9. [DOI] [PubMed] [Google Scholar]
- 52.Rice L B, Shlaes D M. Vancomycin resistance in the enterococcus: relevance in pediatrics. Pediatr Clin N Am. 1995;42:601–618. doi: 10.1016/s0031-3955(16)38981-7. [DOI] [PubMed] [Google Scholar]
- 53.Roghmann M C, McCarter R J, Brewrink J, Cross A S, Morris J G. Clostridium difficile infection is a risk factor for bacteremia due to vancomycin-resistant enterococci (VRE) in VRE-colonized patients with acute leukemia. Clin Infect Dis. 1997;25:1056–1059. doi: 10.1086/516112. [DOI] [PubMed] [Google Scholar]
- 54.Roghmann M C, Qaiyumi S, Johnson J A, Schwalbe R, Morris J G. Recurrent vancomycin-resistant Enterococcus faecium bacteremia in a leukemia patient who was persistently colonized with vancomycin-resistant enterococci for two years. Clin Infect Dis. 1997;24:514–515. doi: 10.1093/clinids/24.3.514. [DOI] [PubMed] [Google Scholar]
- 55.Rubin L G, Tucci V, Cercenado E, Eliopoulos G, Isenberg H D. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol. 1992;13:700–705. doi: 10.1086/648342. [DOI] [PubMed] [Google Scholar]
- 56.Schwalbe R S, McIntosh A C, Qaiyumi S, Johnson J A, Morris J G., Jr Isolation of vancomycin-resistant enterococci from animal feed in USA. Lancet. 1999;353:722. doi: 10.1016/s0140-6736(98)05441-5. [DOI] [PubMed] [Google Scholar]
- 57.Silverman J, Thal L A, Perri M B, Bostic G, Zervos M J. Epidemiologic evaluation of antimicrobial resistance in community-acquired enterococci. J Clin Microbiol. 1998;36:830–832. doi: 10.1128/jcm.36.3.830-832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer M V, Haft R, Barlam T, Aronson M, Shafer A, Sands K E. Vancomycin control measures at a tertiary-care hospital: impact of interventions on volume and patterns of use. Infect Control Hosp Epidemiol. 1998;19:248–253. doi: 10.1086/647803. [DOI] [PubMed] [Google Scholar]
- 59.Singh-Naz N, Sleemi A, Pikis A, Patel K M, Campos J M. Vancomycin-resistant Enterococcus faecium colonization in children. J Clin Microbiol. 1999;37:413–416. doi: 10.1128/jcm.37.2.413-416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slaughter S, Hayden M K, Nathan C, Hu T C, Rice T, Van Voorhis J V, Matushek M, Franklin C, Weinstein R A. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med. 1996;125:448–456. doi: 10.7326/0003-4819-125-6-199609150-00004. [DOI] [PubMed] [Google Scholar]
- 61.Stosor V, Peterson L R, Postelnick M, Noskin G A. Enterococcus faecium bacteremia: does vancomycin resistance make a difference? Arch Intern Med. 1998;158:522–527. doi: 10.1001/archinte.158.5.522. [DOI] [PubMed] [Google Scholar]
- 62.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tornieporth N G, Roberts R B, John J, Hafner A, Riley L. Risk factors associated with vancomycin-resistant Enterococcus faecium infection or colonization in 145 matched case patients and control patients. Clin Infect Dis. 1996;23:767–772. doi: 10.1093/clinids/23.4.767. [DOI] [PubMed] [Google Scholar]
- 64.Tucci V T, Haran M A, Isenberg H D. Epidemiology and control of vancomycin-resistant enterococci in an adult and children's hospital. Am J Infect Control. 1997;25:371–376. doi: 10.1016/s0196-6553(97)90080-8. [DOI] [PubMed] [Google Scholar]
- 65.Uttley A H C, Collins C H, Naidoo J, George R C. Vancomycin-resistant enterococci. Lancet. 1988;i:57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 66.Uttley A H C, George R C, Naidoo J, Woodford N, Johnson A P, Collins C H, Morrison D, Gilfillan A J, Fitch L E, Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989;103:173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van den Braak N, Van Belkum A, Van Keulen M, Vliegenthart J, Verburgh H A, Endtz H P. Molecular characterization of vancomycin-resistant enterococci from hospitalized patients and poultry products in The Netherlands. J Clin Microbiol. 1998;36:1927–1932. doi: 10.1128/jcm.36.7.1927-1932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Auwera P, Pensart N, Korten V, Murray B E, Leclercq R. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide-resistant enterococci. J Infect Dis. 1996;173:1129–1136. doi: 10.1093/infdis/173.5.1129. [DOI] [PubMed] [Google Scholar]
- 69.Wells C L, Juni B A, Cameron S B, Mason K R, Dunn D L, Ferrieri P, Rhame F S. Stool carriage, clinical isolation, and mortality during an outbreak of vancomycin-resistant enterococci in hospitalized medical and/or surgical patients. Clin Infect Dis. 1995;21:45–50. doi: 10.1093/clinids/21.1.45. [DOI] [PubMed] [Google Scholar]