Abstract
Purpose: To evaluate the role of diabetes mellitus in the incidence, risk factors, and outcomes of AKI (acute kidney injury) in patients admitted with ACS (acute coronary syndrome). Methods: We performed a comparative evaluation of ACS patients with vs. without DM who developed AKI enrolled in the biennial ACS Israeli Surveys (ACSIS) between 2000 and 2018. AKI was defined as an absolute increase in serum creatinine (≥0.5 mg/dL) or above 1.5 mg/dL or new renal replacement therapy upon admission with ACS. Outcomes included 30-day major adverse cardiovascular events (MACE) and 1-year all-cause mortality. Results: The current study included a total of 16,879 patients, median age 64 (IQR 54–74), 77% males, 36% with DM. The incidence of AKI was significantly higher among patients with vs. without DM (8.4% vs. 4.7%, p < 0.001). The rates of 30-day MACE (40.8% vs. 13.4%, p < 0.001) and 1-year mortality (43.7% vs. 10%, p < 0.001) were significantly greater among diabetic patients who developed vs. those who did not develop AKI respectively, yet very similar among patients that developed AKI with vs. without DM (30-day MACE 40.8% vs. 40.3%, p = 0.9 1-year mortality 43.7 vs. 44.8%, p = 0.8, respectively). Multivariate analyses adjusted to potential confounders, showed similar independent predictors of AKI among patients with and without DM, comprising; older age, chronic kidney disease, congestive heart failure, and peripheral arterial disease. Conclusions: Although patients with DM are at much greater risk for AKI when admitted with ACS, the independent predictors of AKI and the worse patient outcomes when AKI occurs, are similar irrespective to DM status.
Keywords: acute kidney injury, diabetes mellitus, acute coronary syndrome
1. Introduction
The prevalence of diabetes mellitus (DM), a major cardiovascular risk factor, is increasing worldwide [1,2]. Among patients presenting with an acute coronary syndrome (ACS), those with DM are at increased risk of in-hospital morbidity and mortality compared with those without DM [3,4]. Acute kidney injury (AKI) is a common complication in patients presenting with acute coronary syndrome (ACS), particularly following percutaneous coronary intervention (PCI) [5,6,7,8,9]. Furthermore, when AKI occurs in patients with ACS, it is associated with significantly worse short- and long-term outcomes that include increased risk for renal replacement therapy, prolonged hospitalization, greater mortality and economic burden [5,6,7,8,9,10]. Patients with DM are considered to be at increased risk for AKI in the setting of ACS and PCI [10,11,12,13]. Moreover, the treatment for AKI in this scenario is rather preventive and consists mostly of supportive care or hemodialysis. However, data evaluating and directly comparing (from a single cohort) the incidence, outcomes, and the prognostic markers of AKI among patients, with and without DM, are sparse.
The aim of the current study was to evaluate the role of diabetes mellitus in the incidence, risk factors, and outcomes of AKI in patients admitted with ACS.
2. Methods
2.1. Study Design and Population
The current study included 16,879 consecutive patients from the ACS Israeli Surveys (ACSIS) between 2000 and 2018. Details of the registry have been previously reported [14,15]. In shortly, ACSIS is a biennial prospective national registry of all patients with ACS hospitalized in 26 coronary care units and cardiology departments in all general hospitals in Israel over a 2-month period (March to April). Data were recorded on prespecified forms for all admitted patients diagnosed with ACS. Admission and discharge diagnoses were recorded by the attending physicians. Patient management was at the discretion of the attending physicians. All patients signed an informed consent form for participating in the ACSIS registry at each medical center, and each institution received the approval of its institutional review board (ethics committee). AKI was defined as an absolute increase in serum creatinine (≥0.5 mg/dL) or above 1.5 mg/dL (when no prior level existed in patients without a diagnosis of chronic kidney disease) or new renal replacement therapy upon admission with ACS. It should be stated that a gold standard for the definition of AKI and the ideal margins of absolute and relative increases in serum creatinine used for the definition is controversial [16]. As our study included not only contrast-induced AKI (CI-AKI) but rather all AKI in the context of ACS, even fewer data exists about the optimal definition. An increase of 0.5 mg/dL is one of the previously reported and accepted definitions that have been shown to be quite accurate [16,17] and is not the most strict or lenient, hence, it was chosen by us for this study. Regarding patients without a recent previous creatinine value, who did not have a diagnosis of chronic kidney disease, we chose the definition of creatinine above 1.5 mg/dL based on the previously reported definition of the relative increase in serum creatinine to ≥1.5 times baseline [18] assuming the baseline to be 1 mg/dL (which is close to the upper limit of normal values in both sexes). We believe using this definition would be more accurate than excluding these patients (due to missing values) and causing a potentially significant selection bias.
Following comparison between patients with vs. without AKI, the study cohort was divided into four groups according to the DM and AKI to enable status as following:
no-AKI_no-DM: patients without DM that did not develop AKI.
no-AKI_DM: patients with DM that did not develop AKI.
AKI_no-DM: patients without DM that developed AKI.
AKI_DM: patients with DM that developed AKI.
These subgroups enabled the performance of analyses intended to better and more accurately separate and investigate the role of DM among patients with ACS who developed AKI.
2.2. Outcomes
The outcomes included 30-day major adverse cardiovascular events (MACE), comprised of: all-cause mortality, myocardial infarction (MI), stroke, unstable angina, stent thrombosis, and urgent revascularization. An additional outcome was 1-year all-cause mortality. Data regarding the outcomes were determined by hospital chart review, telephone contact, clinical follow-up, and by matching identification numbers of patients with the Israeli National Population Registry (for 30-day and 1-year mortality).
2.3. Statistical Analysis
Patients’ characteristics are presented as median (IQR) for continuous variables and as frequency (%) for categorical variables. Comparisons between the study groups were tested with chi-square for categorical variables and with Mann–Whitney–Wilcoxon test for non-normally distributed continuous variables. The normality of continuous variables was assessed using Shapiro–Wilk test. Survival curves were plotted, and the Kaplan–Meier log-rank test was used to test the variable of interest on survival.
Logistic regression models were used to assess the relationship between patients’ baseline characteristics and the outcome of developing AKI, and Cox proportional hazards regression models were used for the outcome of 1-year mortality among the whole cohort and among the subsets of diabetic and non-diabetic patients.
All tests were conducted at a two-sided overall 5% significance level (p = 0.05). Statistical analyses were performed using R software.
3. Results
The study cohort included a total of 16,879 patients, median age 64 (IQR 54–74), 77% males, 36% with DM. The overall incidence of AKI was 6% (1016 patients), significantly higher among patients with DM vs. without DM (8.4% vs. 4.7%, p < 0.001). Comparison of baseline characteristics according to AKI in the entire cohort are presented in the Supplementary Table S1. A multivariate analysis (Supplementary Table S2) showed that DM was independently associated with AKI (adjusted odds ratio (OR) 1.4, 95% confidence interval (CI) 1.2–1.6, p < 0.001). The baseline characteristics of the patients with and without DM by AKI are presented in Table 1. Compared to patients without AKI, those who developed AKI (diabetics and non-diabetic) were older, mostly females, with a higher rate of chronic kidney disease, hypertension, and congestive heart failure and presentation as STEMI, yet lower rate of smokers and positive family history. Furthermore, patients who developed AKI had greater rates of prior myocardial infarction and prior coronary artery bypass graft surgery, and higher rate of treatment with ACE inhibitors/ARBs, nitrates, and diuretics compared with patients that did not develop AKI.
Table 1.
Baseline characteristics by DM status and AKI.
| Overall | no-AKI_no_DM | no-AKI_DM | AKI_ no-DM | AKI_DM | p * | p ** | |
|---|---|---|---|---|---|---|---|
| n | 16,879 | 10,276 | 5587 | 503 | 513 | ||
| Baseline characteristics and demographics | |||||||
| Age, years (median [IQR) | 64 (54, 74) | 61 (52, 72) | 66 (57, 74) | 78 (69, 83) | 73 (65, 80) | <0.001 | <0.001 |
| Gender (male) | 13038 (77.2) | 8263 (80.4) | 4065 (72.8) | 363 (72.2) | 347 (67.6) | 0.13 | 0.015 |
| Higher education/ academic | 1455 (29.5) | 955 (32.9) | 438 (24.3) | 34 (35.4) | 28 (20.3) | 0.015 | 0.34 |
| Marital status: married | 6951 (78.4) | 4157 (79.2) | 2471 (79.1) | 135 (61.6) | 188 (68.6) | 0.13 | <0.001 |
| Dyslipidemia | 10968 (65.2) | 6053 (59.1) | 4304 (77.3) | 246 (49.1) | 365 (71.4) | <0.001 | 0.003 |
| Hypertension | 10035 (59.6) | 5087 (49.6) | 4190 (75.2) | 341 (67.8) | 417 (81.4) | <0.001 | 0.002 |
| Current smokers | 6303 (37.5) | 4384 (42.8) | 1704 (30.7) | 117 (23.4) | 98 (19.5) | 0.15 | <0.001 |
| Diabetes mellitus | 6100 (36.1) | 0 (0.0) | 5587 (100.0) | 0 (0.0) | 513 (100.0) | NA | NA |
| Family history of CAD | 4072 (26.0) | 2753 (28.3) | 1193 (23.7) | 54 (11.6) | 72 (16.1) | 0.062 | <0.001 |
| BMI (kg/m2), (median [IQR]) | 27 (25, 30) | 27 (24, 29) | 28 (25, 31) | 26 (24, 29) | 27 (25, 31) | <0.001 | 0.049 |
| Prior MI | 5275 (31.3) | 2646 (25.8) | 2224 (39.9) | 179 (35.7) | 226 (44.2) | 0.007 | 0.064 |
| Prior CABG | 1655 (9.8) | 724 (7.0) | 774 (13.9) | 61 (12.1) | 96 (18.8) | 0.005 | 0.003 |
| Prior PCI | 4765 (28.3) | 2363 (23.0) | 2115 (38.0) | 116 (23.1) | 171 (33.6) | <0.001 | 0.057 |
| Chronic renal failure | 1832 (10.9) | 559 (5.4) | 815 (14.6) | 207 (41.2) | 251 (49.0) | 0.014 | <0.001 |
| PVD | 1393 (8.3) | 513 (5.0) | 675 (12.1) | 83 (16.5) | 122 (23.9) | 0.004 | <0.001 |
| s/p CVA/TIA | 1373 (8.1) | 596 (5.8) | 631 (11.3) | 60 (11.9) | 86 (16.8) | 0.034 | <0.001 |
| History of CHF | 1363 (8.1) | 479 (4.7) | 651 (11.7) | 104 (20.7) | 129 (25.4) | 0.088 | <0.001 |
| Grace score > 140 | 1451 (15.0) | 576 (9.9) | 631 (19.0) | 116 (50.2) | 128 (44.3) | 0.2 | <0.001 |
| Earliest creatinine (mg/dL) (median [IQR]) | 1.00 (0.9, 1.2) | 1 (0.85, 1.1) | 1 (0.83, 1.3) | 1.7 (1.40, 2.3) | 1.8 (1.4, 2.6) | 0.12 | <0.001 |
| Medical therapy prior to admission | |||||||
| Aspirin | 7021 (47.8) | 3427 (38.4) | 3117 (63.5) | 196 (47.5) | 281 (64.6) | <0.001 | 0.7 |
| Clopidogrel | 1356 (9.4) | 591 (6.7) | 683 (14.2) | 31 (7.5) | 51 (11.8) | 0.05 | 0.19 |
| ACE-I | 3163 (31.3) | 1387 (23.0) | 1567 (44.2) | 81 (31.8) | 128 (43.5) | 0.006 | 0.87 |
| ARB | 1216 (12.5) | 501 (8.6) | 617 (18.1) | 23 (9.2) | 75 (26.0) | <0.001 | 0.001 |
| Beta blockers | 5335 (37.2) | 2625 (30.3) | 2322 (48.3) | 171 (42.3) | 217 (50.0) | 0.03 | 0.52 |
| Statins | 6601 (47.4) | 3245 (38.7) | 2956 (62.5) | 149 (38.1) | 251 (59.1) | <0.001 | 0.17 |
| Calcium channel blockers | 2989 (21.5) | 1380 (16.4) | 1338 (28.8) | 104 (26.1) | 167 (39.4) | <0.001 | <0.001 |
| Nitrates | 1531 (11.2) | 670 (8.0) | 685 (15.0) | 78 (19.6) | 98 (23.4) | 0.2 | <0.001 |
| Hypoglycemic agents | 3488 (23.1) | 34 (0.4) | 3192 (62.9) | 2 (0.5) | 260 (58.2) | <0.001 | 0.054 |
| Diuretics | 2066 (17.5) | 818 (11.5) | 987 (24.5) | 101 (28.9) | 160 (43.0) | <0.001 | <0.001 |
CHF: congestive heart failure; CAD: coronary artery disease; BMI: body mass index; MI: myocardial infarction; TIA: transient ischemic attack; CVA: cerebrovascular attack; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blockers, PVD: peripheral vascular (arterial) disease; PCI: percutaneous coronary interventional; CABG: coronary artery bypass graft surgery. * p for comparison between the following groups: AKI_ DM vs. AKI_no-DM (comparison between diabetic patients with AKI vs. non-diabetic patients that developed AKI). ** p for comparison between the following groups: AKI_DM vs. no-AKI_DM (comparison between diabetic patients with AKI vs. diabetic patients that did not develop AKI.).
As presented in Table 2, The clinical presentation and most in-hospital complications (e.g., pulmonary edema, cardiogenic shock, mechanical and arrhythmic complications) were more severe/prevalent among patients with AKI (both diabetics and non-diabetics) vs. patients who did not develop AKI. However, the rates of coronary angiography and PCI were lower among patients with AKI (among patients with and without DM).
Table 2.
In hospital complications, reperfusion therapy and treatment upon discharge by DM status and AKI.
| Overall | no-AKI_no_DM | no-AKI_DM | AKI_ no-DM | AKI_DM | p * | p ** | |
|---|---|---|---|---|---|---|---|
| In-hospital complications | |||||||
| CHF mild-moderate (Killip-2) | 1544 (9.2) | 720 (7.0) | 570 (10.2) | 106 (21.5) | 148 (29.1) | 0.007 | <0.001 |
| Pulmonary oedema (Killip-3) | 1099 (6.5) | 360 (3.5) | 419 (7.5) | 155 (30.9) | 165 (32.2) | 0.7 | <0.001 |
| Cardiogenic shock (Killip-4) | 569 (3.4) | 200 (1.9) | 124 (2.2) | 137 (27.3) | 108 (21.1) | 0.03 | <0.001 |
| Hemodynamically significant RVI | 88 (0.8) | 39 (0.6) | 22 (0.6) | 10 (4.0) | 17 (5.6) | 0.5 | <0.001 |
| Reinfarction | 223 (1.3) | 106 (1.0) | 70 (1.3) | 24 (4.8) | 23 (4.5) | 0.95 | <0.001 |
| Post MI angina | 793 (4.7) | 463 (4.5) | 246 (4.4) | 33 (6.6) | 51 (10.0) | 0.07 | <0.001 |
| Stent thrombosis (definite/probable/possible) | 79 (0.7) | 46 (0.7) | 28 (0.7) | 2 (0.8) | 3 (1.0) | 1 | 0.87 |
| Free wall rupture | 59 (0.3) | 36 (0.4) | 15 (0.3) | 6 (1.2) | 2 (0.4) | 0.27 | 0.95 |
| Tamponade | 43 (0.3) | 25 (0.2) | 12 (0.2) | 4 (0.8) | 2 (0.4) | 0.66 | 0.76 |
| VSD | 20 (0.1) | 8 (0.1) | 5 (0.1) | 2 (0.4) | 5 (1.0) | 0.47 | <0.001 |
| MR moderate—severe | 323 (1.9) | 125 (1.2) | 96 (1.7) | 48 (9.6) | 54 (10.6) | 0.69 | <0.001 |
| Pericarditis | 107 (0.6) | 72 (0.7) | 23 (0.4) | 4 (0.8) | 8 (1.6) | 0.4 | 0.002 |
| Sustained VT (>125 bpm) | 247 (1.5) | 132 (1.3) | 54 (1.0) | 30 (6.0) | 31 (6.0) | 1 | <0.001 |
| Primary VF | 311 (1.8) | 225 (2.2) | 55 (1.0) | 18 (3.6) | 13 (2.5) | 0.43 | 0.003 |
| Secondary VF | 126 (0.7) | 62 (0.6) | 28 (0.5) | 20 (4.0) | 16 (3.1) | 0.56 | <0.001 |
| New A. Fib. | 895 (5.3) | 400 (3.9) | 265 (4.7) | 123 (24.5) | 107 (20.9) | 0.2 | <0.001 |
| High degree (2–3 degree) AV block | 382 (2.3) | 197 (1.9) | 106 (1.9) | 37 (7.4) | 42 (8.2) | 0.7 | <0.001 |
| Asystole | 359 (2.1) | 138 (1.3) | 81 (1.4) | 67 (13.3) | 73 (14.3) | 0.74 | <0.001 |
| Stroke | 103 (0.6) | 42 (0.4) | 36 (0.6) | 11 (2.2) | 14 (2.7) | 0.73 | <0.001 |
| Bleeding | 238 (1.4) | 99 (1.0) | 72 (1.3) | 33 (6.6) | 34 (6.6) | 1 | <0.001 |
| Blood transfusions | 170 (3.1) | 57 (1.8) | 54 (2.6) | 23 (18.9) | 36 (22.4) | 0.57 | <0.001 |
| Coronary angiography and PCI | |||||||
| PCI | 10,308 (61.1) | 6631 (64.5) | 3196 (57.2) | 237 (47.1) | 244 (47.6) | 0.94 | <0.001 |
| STEMI | 7659 (45.4) | 5087 (49.5) | 2114 (37.9) | 243 (48.3) | 215 (41.9) | 0.1 | <0.001 |
| Coronary angiography | 11,428 (87.2) | 7068 (89.7) | 3874 (85.7) | 230 (70.6) | 256 (68.4) | 0.6 | <0.001 |
| Medical therapy upon discharge | |||||||
| Aspirin | 15,557 (94.4) | 9665 (95.5) | 5163 (94.4) | 357 (80.2) | 372 (83.2) | 0.28 | <0.001 |
| P2Y12 | 11,926 (72.9) | 7504 (74.6) | 3923 (72.3) | 227 (51.6) | 272 (60.7) | 0.008 | <0.001 |
| Statins | 13,946 (85.2) | 8612 (85.6) | 4777 (88.0) | 256 (58.3) | 301 (67.3) | 0.007 | <0.001 |
| ACE-I/ARB | 12,029 (74.2) | 7139 (71.9) | 4412 (82.2) | 237 (51.9) | 241 (52.9) | 0.8 | <0.001 |
| Beta blockers | 12,873 (79.9) | 7910 (80.1) | 4416 (82.6) | 255 (57.4) | 292 (65.0) | 0.002 | <0.001 |
CHF: congestive heart failure; CAD: coronary artery disease; BMI: body mass index; MI: myocardial infarction; TIA: transient ischemic attack; CVA: cerebrovascular attack; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blockers, PVD: peripheral vascular (arterial) disease; PCI: percutaneous coronary interventional; CABG: coronary artery bypass graft surgery; VT: ventricular tachycardia; VF: ventricular fibrillation; RVI: right ventricular infarction. * p for comparison between the following groups: AKI_ DM vs. AKI_no-DM (comparison between diabetic patients with AKI vs. non-diabetic patients that developed AKI). ** p for comparison between the following groups: AKI_DM vs. no-AKI_DM (comparison between diabetic patients with AKI vs. diabetic patients that did not develop AKI.).
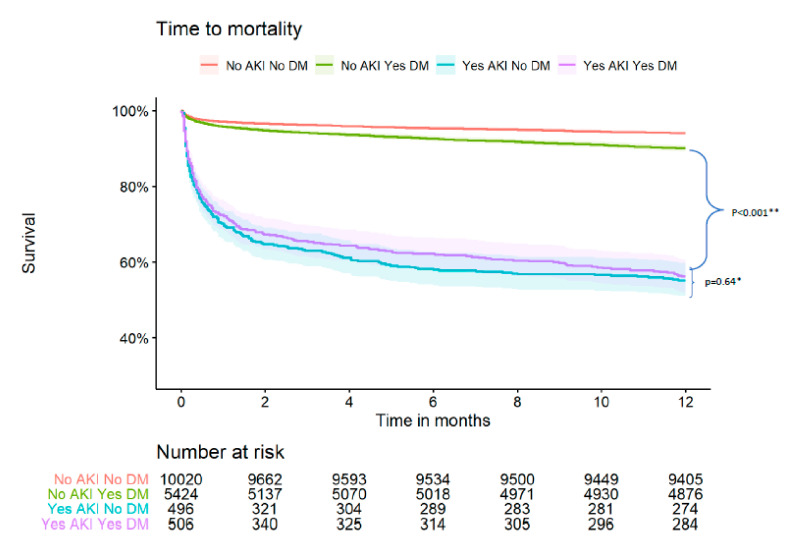
Table 3 and Figure 1 present the 30-day MACE and 1-year mortality according to DM status and the occurrence of AKI. The rates of 30-day MACE and nearly all its components, as well as the rates of 1-year mortality, were significantly greater among patients who developed AKI. However, the rates of the latter outcome were similar among patients with AKI irrespective of their DM status (diabetics = 43.7%, non-diabetics 44.8%). Additionally, multivariable analysis adjusted to potential confounders (Supplementary Table S3) shows no statistically significant interaction between DM and AKI in the prediction of 1-year mortality (p = 0.1).
Table 3.
Patient outcomes by DM status and AKI.
| Overall | no-AKI_no-DM | no-AKI_DM | AKI_ no-DM | AKI_DM | p * | p ** | |
| 30-day outcomes | |||||||
| Major adverse cardiac events | 2376 (14.1) | 1221 (11.9) | 744 (13.4) | 202 (40.3) | 209 (40.8) | 0.92 | <0.001 |
| mortality | 805 (4.8) | 290 (2.8) | 227 (4.1) | 148 (29.7) | 140 (27.4) | 0.45 | <0.001 |
| re-hospitalization | 2868 (18.9) | 1697 (18.1) | 1001 (19.9) | 83 (23.4) | 87 (23.8) | 0.99 | 0.09 |
| reinfarction | 286 (1.9) | 150 (1.7) | 92 (1.8) | 21 (5.0) | 23 (5.2) | 1 | <0.001 |
| Angina | 322 (4.0) | 189 (3.9) | 123 (4.3) | 5 (3.6) | 5 (2.8) | 0.91 | 0.4 |
| CABG | 1397 (8.4) | 785 (7.8) | 556 (10.1) | 30 (6.0) | 26 (5.2) | 0.69 | <0.001 |
| 1-year outcomes | |||||||
| mortality | 1580 (9.6) | 597 (6.0) | 540 (10.0) | 222 (44.8) | 221 (43.7) | 0.78 | <0.001 |
CABG: coronary artery bypass graft surgery. * p for comparison between the following groups: AKI_ DM vs. AKI_no-DM (comparison between diabetic patients with AKI vs. non-diabetic patients that developed AKI). ** p for comparison between the following groups: AKI_DM vs. no-AKI_DM (comparison between diabetic patients with AKI vs. diabetic patients that did not develop AKI.).
Figure 1.
Kaplan Meier survival curve by DM status and AKI. * p for comparison between the following groups: AKI_ DM vs. AKI_no-DM (comparison between diabetic patients with AKI vs. non-diabetic patients that developed AKI). ** p for comparison between the following groups: AKI_DM vs. no-AKI_DM (comparison between diabetic patients with AKI vs. diabetic patients that did not develop AKI).
Additionally, a comparison of the baseline characteristics between patients with and without DM is presented in Supplementary Table S4.
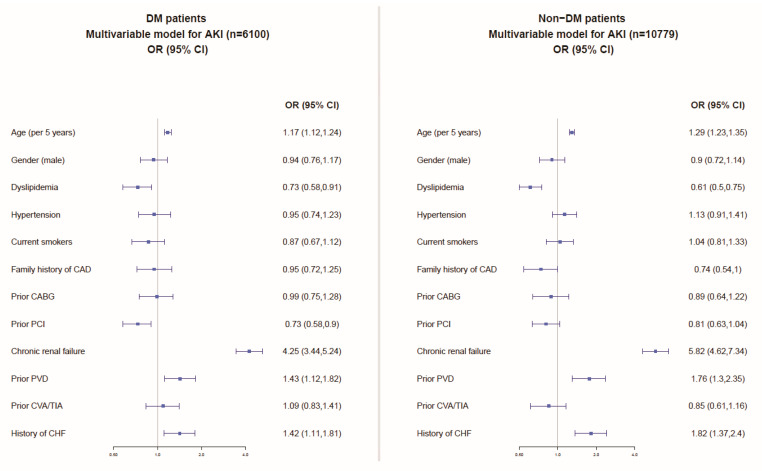
Multivariable analyses, including the patients’ baseline characteristics (Figure 2), showed similar independent predictors of AKI among patients with and without DM comprising older age, chronic kidney disease, peripheral vascular (arterial) disease, and congestive heart failure.
Figure 2.
Multivariable model for the prediction of AKI among patients with and without DM. CHF: congestive heart failure; CAD: coronary artery disease; MI: myocardial infarction; TIA: transient ischemic attack; CVA: cerebrovascular attack; PVD: peripheral vascular (arterial) disease; PCI: percutaneous coronary interventional; CABG: coronary artery bypass graft surgery.
Furthermore, a multivariable analysis with 1-year mortality as the outcome (Supplementary Figure S1) showed similar predictors among patients with and without DM, specifically AKI was the strongest independent predictor in both groups (with DM: OR = 3.55, 95% CI: 2.99–4.21, p < 0.001, without DM: HR = 3.69, 95% CI: 3.09–4.4, p < 0.001) with similar HR in both.
4. Discussion
In the current study, we have shown that although patients with DM have a greater risk for developing AKI when admitted with ACS, other independent predictors of AKI and the worse outcomes associated with AKI are similar among diabetics and non-diabetic patients.
The observed prevalence of DM in our cohort (36%) is overall similar to previous reports that included ACS patients [3,4,19]. The incidence of AKI among patients presenting with AMI has been reported between 8–37% [5,6,7,8,9], yet lower rates, similar to those found in our study, were reported following coronary angiography (not in the setting of AMI) and when preventive measures such as the use of low amounts of contrast agents and routine hydration were implemented [9,12,20]. The increased risk for the development of AKI in this setting among patients with DM is consistent with previous reports [12,13,21,22,23].
The etiologies attributed to the development of AKI in the setting of ACS are diverse and include contrast-induced AKI (CI-AKI) following coronary angiography and especially PCI, hemodynamic instability with compromised renal perfusion (i.e., cardiogenic shock, mechanical complication, or arrhythmias), volume status changes, medications (e.g., nitroglycerine, diuretics, ACE I/ARBs), atheroembolism during PCI, ischemia driven alteration in the function and structure of epithelial cells, non-cardiovascular complications such as infections or inflammatory states as well as patient-associated comorbidity and predisposing risk factors (prior chronic kidney disease, diabetes, increased age, etc.) [24,25,26,27,28,29,30,31]. CI-AKI is a prominent and widely investigated mechanism for AKI in ACS patients following coronary angiography/PCI. In the current study, the rate of PCI was lower among patients that developed AKI, possibly due to differences in presentation and comorbidity (higher rate of chronic kidney disease among patients with AKI). Patients with DM and high glucose levels were reported to be particularly sensitive to these mechanisms [23,32]. DM has been associated with increased reactive oxygen species (ROS) and increased oxidative stress, mediated by increased mitochondrial superoxide and enhanced activity of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [23]. Furthermore, DM is associated with increased renal oxygen consumption through the enhancement of the load of several ion pumps (e.g., Na+-glucose transporter in renal tubular epithelial cells and Na+-K+-ATPase activity in the medulla) [33]. Additionally, stronger renal vessel constriction and dysfunction of vasoactive substances were reported [34,35,36]. Moreover, various immunological alterations (e.g., infiltration of immune cells into the kidney and release of proinflammatory factors and upregulation of cytokines such as IL-1 (interleukin-1), IL-18, IL-6, IL-33, TGF-β (tumor growth factor-beta), IFN-c (interferon-gamma), and TNF-α (tumor necrosis factor-alpha)) among patients with DM, could directly or indirectly facilitate AKI (particularly CI-AKI) [23]. Different signaling pathways control the pathological changes of renal cells in AKI. Those that were reported to be related to DM are PKB/FoxO, PKB/mTOR/p70S6, p38 MAPK, JNK, and NF-kappa B [23].
Considering the abovementioned mechanisms, the increased incidence of AKI among patients with DM, as found here and elsewhere [12,13,21,22,23], is quite clear. Nevertheless, the similar predictors and particularly the outcomes of AKI in the setting of ACS in patients with vs. without DM, as observed in the current study could be surprising. It seems that the consequences of AKI, when it occurs in the setting of ACS, are so significant that they overshadow other significant risk factors such as DM, which become much less substantial in this setting. This is further supported by AKI being the strongest predictor of mortality with nearly identical HR, both in patients with and without DM, and in the lack of interaction between DM and AKI in the prediction of mortality in the entire cohort adjusted to potential confounders. In addition, AKI could express or be a consequence of other severe complications that cause worse outcomes (e.g., cardiogenic shock, malignant arrhythmia, mechanical complications). Furthermore, it may imply similarity in the mechanisms of AKI between patients with and without DM. Moreover, the worse outcomes of DM patients with ACS could be mediated by the increased risk for AKI hence further emphasizing the great importance of preventive measures for AKI in this group of patients. The independent predictors of AKI in the current study are overall consistent with previously reported risk scores, with the most prominent parameters being CKD, older age and CHF [10,12,28].
The significantly increased mortality rates among patients with AKI versus patients without in the setting of ACS and following PCI are consistent with previous reports [5,6,7,8,9,10,11,25,37,38]. However, the mortality rates among ACS patients who developed AKI in our study are somewhat higher than those reported in most previous studies (up to 44%). This difference probably stems from the non-selective “all-comer” population, including patients with cardiogenic shock and post-resuscitation enrolled in our study. Furthermore, patients were enrolled from ICCUs, and only patients with ACS, while many other studies enrolled patients after PCI (including stable, often ambulatory patients without ACS). Our study also included patients treated two decades ago when outcomes of ACS were worse. Moreover, the definition of AKI used by us was not the most lenient one, as previously mentioned, hence probably excluding the easiest cases with best outcomes.
5. Limitations
The current study was retrospective and observational (although patients were enrolled prospectively), and thus shares the limitations of such a design. However, the registry reflects real-life data of all-comers (rather than that of a highly selective population from a clinical trial) which increases the robustness and representativity of our findings. Several unaccounted confounders such as contrast volume (as a cause of contrast-induced AKI), prevention measures, the management of AKI or of predisposing factors (e.g., fluid challenge, mechanical circulatory support), and adherence with treatment recommendations were not reported in the registry. The occurrence of AKI was defined retrospectively by the ACSIS CRF and was not screened prospectively, which might introduce some bias. Furthermore, it was not based on one uniform definition, and rather, a mixture of definitions with some adjustments was used based on existing data and in order to minimize other types of bias which might have introduced some inaccuracies and limited comparability with other studies. Finally, data regarding mortality causes are not reported in the ACSIS registry.
6. Conclusions
Patients with DM are at much greater risk for AKI when admitted with ACS. However, the outcomes of AKI, once developed in the setting of ACS, are similar between diabetics and none-diabetics, and the independent predictors of AKI among patients with and without DM are virtually identical. These findings help clinicians in risk stratification as well as making therapeutic decisions specifically regarding measures that affect the risk of AKI according to the diabetes status in order to implement intervention for prevention of AKI (e.g., amount of contrast material, hydration, maintaining adequate perfusion, potentially nephrotoxic medications, etc.).
Acknowledgments
The authors wish to thank Robert Klempfner, for his support and Nir Shlomo for assisting with the statistical analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10214931/s1, Table S1: Baseline characteristics by AKI, Table S2: multivariate model for prediction of AKI among ACS patients, Table S3: multivariate model for prediction of 1-year all-cause mortality following ACS, Table S4: Baseline characteristics by DM, Figure S1: Multivariable analysis for prediction of 1-year mortality according to DM status.
Author Contributions
Conceptualization, A.S. (Arthur Shiyovich), K.S. and K.O.; methodology, A.S. (Arthur Shiyovich), K.S., T.S., K.O.; software, T.O.; validation, A.S., K.O. and T.O.; formal analysis, T.O.; investiga-tion, A.S. (Arthur Shiyovich), K.O.; resources, K.O., R.K., R.B.; data cura-tion, A.S. (Arthur Shiyo-vich), K.S., S.G., T.O.; writing—original draft preparation, A.S. (Arthur Shiyovich); writing—review and editing, all authors.; visualization, K.O.; supervision, K.O., A.S. (Arthur Shiyovich), R.K., R.B.; project administration, T.O., A.S. (Arthur Shiyovich), K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (approval number RMC 06222-17).
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The data that support the findings of this study are available from the ACSIS registry organization, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the ACSIS registry organization.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ezzati M., Obermeyer Z., Tzoulaki I., Mayosi B.M., Elliott P., Leon D.A. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat. Rev. Cardiol. 2015;12:508–530. doi: 10.1038/nrcardio.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G.A., Singh G.M., Lu Y., Danaei G., Lin J.K., Finucane M.M., Bahalim A.N., McIntire R.K., Gutierrez H.R., Cowan M., et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmberg K., Yusuf S., Gerstein H.C., Brown J., Zhao F., Hunt D., Piegas L., Calvin J., Keltai M., Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–1019. doi: 10.1161/01.CIR.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 4.Franklin K., Goldberg R.J., Spencer F., Klein W., Budaj A., Brieger D., Marre M., Steg P.G., Gowda N., Gore J.M. Implications of diabetes in patients with acute coronary syndromes. The Global Registry of Acute Coronary Events. Arch. Intern. Med. 2004;164:1457–1463. doi: 10.1001/archinte.164.13.1457. [DOI] [PubMed] [Google Scholar]
- 5.Xu F.B., Cheng H., Yue T., Ye N., Zhang H.J., Chen Y.P. Derivation and validation of a prediction score for acute kidney injury secondary to acute myocardial infarction in Chinese patients. BMC Nephrol. 2019;20:195. doi: 10.1186/s12882-019-1379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox C.S., Muntner P., Chen A.Y., Alexander K.P., Roe M.T., Wiviott S.D. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: A report from the national cardiovascular data registry. Circulation. 2012;125:497–504. doi: 10.1161/CIRCULATIONAHA.111.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruetto R.G., Rodrigues F.B., Torres U.S., Otaviano A.P., Zanetta D.M., Burdmann E.A. Renal function at hospital admission and mortality due to acute kidney injury after myocardial infarction. PLoS ONE. 2012;7:e35496. doi: 10.1371/journal.pone.0035496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M.J., Choi H.S., Oh S.H., Lee H.C., Kim C.S., Choi J.S., Park J.W., Bae E.H., Ma S.K., Kim N.H., et al. Impact of acute kidney injury on clinical outcomes after ST elevation acute myocardial infarction. Yonsei Med. J. 2011;52:603–609. doi: 10.3349/ymj.2011.52.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skalsky K., Shiyovich A., Bental T., Vaknin-Assa H., Assali A., Gal T.B., Avraham B.B., Eisen A., Steinmetz T., Kornowski R., et al. Temporal trends of acute kidney injury in patients undergoing percutaneous coronary intervention over a span of 12 years. Int. J. Cardiol. 2021;326:44–48. doi: 10.1016/j.ijcard.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Yao Z.F., Shen H., Tang M.N., Yan Y., Ge J.B. A novel risk assessment model of contrast-induced nephropathy after percutaneous coronary intervention in patients with diabetes. Basic Clin. Pharmacol. Toxicol. 2021;128:305–314. doi: 10.1111/bcpt.13501. [DOI] [PubMed] [Google Scholar]
- 11.Tsai T.T., Patel U.D., Chang T.I., Kennedy K.F., Masoudi F.A., Matheny M.E., Kosiborod M., Amin A.P., Messenger J.C., Rumsfeld J.S., et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI registry. JACC Cardiovasc. Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng J.F., Chen S.Q., Ye J.F., Chen Y., Lei L., Liu X.Q., Liu Y., Wang Y., Lin J.J., Chen J.Y. A simple risk score model for predicting contrast-induced nephropathy after coronary angiography in patients with diabetes. Clin. Exp. Nephrol. 2019;23:969–981. doi: 10.1007/s10157-019-01739-0. [DOI] [PubMed] [Google Scholar]
- 13.Mehran R., Aymong E.D., Nikolsky E., Lasic Z., Iakovou I., Fahy M., Mintz G.S., Lansky A.J., Moses J.W., Stone G.W., et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 14.Hammer Y., Eisen A., Hasdai D., Goldenberg I., Shlomo N., Cohen T., Beigel R., Kornowski R., Iakobishvili Z. Comparison of Outcomes in Patients With Acute Coronary Syndrome Presenting With Typical Versus Atypical Symptoms. Am. J. Cardiol. 2019;124:1851–1856. doi: 10.1016/j.amjcard.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Shiyovich A., Shlomo N., Cohen T., Iakobishvili Z., Kornowski R., Eisen A. Temporal trends of patients with acute coronary syndrome and multi-vessel coronary artery disease—From the ACSIS registry. Int. J. Cardiol. 2020;304:8–13. doi: 10.1016/j.ijcard.2020.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Skalsky K., Levi A., Bental T., Vaknin-Assa H., Assali A., Steinmetz T., Kornowski R., Perl L. The Definition of "Acute Kidney Injury" Following Percutaneous Coronary Intervention and Cardiovascular Outcomes. Am. J. Cardiol. 2021;156:39–43. doi: 10.1016/j.amjcard.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Keaney J.J., Hannon C.M., Murray P.T. Contrast-induced acute kidney injury: How much contrast is safe? Nephrol. Dial. Transplant. 2013;28:1376–1383. doi: 10.1093/ndt/gfs602. [DOI] [PubMed] [Google Scholar]
- 18.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 19.Rousan T.A., Pappy R.M., Chen A.Y., Roe M.T., Saucedo J.F. Impact of diabetes mellitus on clinical characteristics, management, and in-hospital outcomes in patients with acute myocardial infarction (from the NCDR) Am. J. Cardiol. 2014;114:1136–1144. doi: 10.1016/j.amjcard.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Ranucci M., Castelvecchio S., Menicanti L., Frigiola A., Pelissero G. Risk of assessing mortality risk in elective cardiac operations: Age, creatinine, ejection fraction, and the law of parsimony. Circulation. 2009;119:3053–3061. doi: 10.1161/CIRCULATIONAHA.108.842393. [DOI] [PubMed] [Google Scholar]
- 21.Maioli M., Toso A., Gallopin M., Leoncini M., Tedeschi D., Micheletti C., Bellandi F. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J. Cardiovasc. Med. 2010;11:444–449. doi: 10.2459/JCM.0b013e328335227c. [DOI] [PubMed] [Google Scholar]
- 22.Brown J.R., DeVries J.T., Piper W.D., Robb J.F., Hearne M.J., Ver Lee P.M., Kellet M.A., Watkins M.W., Ryan T.J., Silver M.T., et al. Serious renal dysfunction after percutaneous coronary interventions can be predicted. Am. Heart J. 2008;155:260–266. doi: 10.1016/j.ahj.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Ren K. The Mechanism of Contrast-Induced Acute Kidney Injury and Its Association with Diabetes Mellitus. Contrast Media Mol. Imaging. 2020;2020:3295176. doi: 10.1155/2020/3295176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C., Pei Y.Y., Ma Y.H., Ma X.L., Liu Z.W., Zhu J.H., Li C.S. Risk factors for acute kidney injury in patients with acute myocardial infarction. Chin. Med. J. 2019;132:1660–1665. doi: 10.1097/CM9.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y.B., Liu B.C., Zou Y., Pan J.R., Tao Y., Yang M. Risk factors of acute kidney injury after acute myocardial infarction. Ren. Fail. 2016;38:1353–1358. doi: 10.3109/0886022X.2016.1148558. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y.B., Tao Y., Yang M. Assessing the influence of acute kidney injury on the mortality in patients with acute myocardial infarction: A clinical trail. Ren. Fail. 2018;40:75–84. doi: 10.1080/0886022X.2017.1419969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giacoppo D., Madhavan M.V., Baber U., Warren J., Bansilal S., Witzenbichler B., Dangas G.D., Kirtane A.J., Xu K., Kornowski R., et al. Impact of Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention on Short- and Long-Term Outcomes: Pooled Analysis From the HORIZONS-AMI and ACUITY Trials. Circ. Cardiovasc. Interv. 2015;8:e002475. doi: 10.1161/CIRCINTERVENTIONS.114.002475. [DOI] [PubMed] [Google Scholar]
- 28.Abusaada K., Yuan C., Sabzwari R., Butt K., Maqsood A. Development of a novel score to predict the risk of acute kidney injury in patient with acute myocardial infarction. J. Nephrol. 2017;30:419–425. doi: 10.1007/s40620-016-0326-1. [DOI] [PubMed] [Google Scholar]
- 29.Shacham Y., Leshem-Rubinow E., Gal-Oz A., Arbel Y., Keren G., Roth A., Steinvil A. Acute Cardio-Renal Syndrome as a Cause for Renal Deterioration Among Myocardial Infarction Patients Treated With Primary Percutaneous Intervention. Can. J. Cardiol. 2015;31:1240–1244. doi: 10.1016/j.cjca.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Shacham Y., Steinvil A., Arbel Y. Acute kidney injury among ST elevation myocardial infarction patients treated by primary percutaneous coronary intervention: A multifactorial entity. J. Nephrol. 2016;29:169–174. doi: 10.1007/s40620-015-0255-4. [DOI] [PubMed] [Google Scholar]
- 31.Plakht Y., Gad Saad S.N., Gilutz H., Shiyovich A. Potassium levels as a marker of imminent acute kidney injury among patients admitted with acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) Project. Int. J. Cardiol. 2021;322:214–219. doi: 10.1016/j.ijcard.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 32.Heyman S.N., Rosenberger C., Rosen S., Khamaisi M. Why is diabetes mellitus a risk factor for contrast-induced nephropathy? BioMed Res. Int. 2013;2013:123589. doi: 10.1155/2013/123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark B.A., Kim D., Epstein F.H. Endothelin and atrial natriuretic peptide levels following radiocontrast exposure in humans. Am. J. Kidney Dis. 1997;30:82–86. doi: 10.1016/S0272-6386(97)90568-0. [DOI] [PubMed] [Google Scholar]
- 35.Khamaisi M., Raz I., Shilo V., Shina A., Rosenberger C., Dahan R., Abassi Z., Meidan R., Lecht S., Heyman S.N. Diabetes and radiocontrast media increase endothelin converting enzyme-1 in the kidney. Kidney Int. 2008;74:91–100. doi: 10.1038/ki.2008.112. [DOI] [PubMed] [Google Scholar]
- 36.Nechemia-Arbely Y., Khamaisi M., Rosenberger C., Koesters R., Shina A., Geva C., Shriki A., Klaus S., Rosen S., Rose-John S., et al. In vivo evidence suggesting reciprocal renal hypoxia-inducible factor-1 upregulation and signal transducer and activator of transcription 3 activation in response to hypoxic and non-hypoxic stimuli. Clin. Exp. Pharmacol. Physiol. 2013;40:262–272. doi: 10.1111/1440-1681.12064. [DOI] [PubMed] [Google Scholar]
- 37.Carande E.J., Brown K., Jackson D., Maskell N., Kouzaris L., Greene G., Mikhail A., Obaid D.R. Acute Kidney Injury Following Percutaneous Coronary Intervention for Acute Coronary Syndrome: Incidence, Aetiology, Risk Factors and Outcomes. Angiology. 2021;28:00033197211040375. doi: 10.1177/00033197211040375. [DOI] [PubMed] [Google Scholar]
- 38.Gupta R., Gurm H.S., Bhatt D.L., Chew D.P., Ellis S.G. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter. Cardiovasc. Interv. 2005;64:442–448. doi: 10.1002/ccd.20316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the ACSIS registry organization, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the ACSIS registry organization.