Abstract
In the present prospective study, a dot immunobinding assay (Dot-Iba) was standardized to measure the circulating mycobacterial antigen in cerebrospinal fluid (CSF) specimens for the laboratory diagnosis of tuberculous meningitis (TBM). Immunoglobulin G antibody specific for Mycobacterium tuberculosis in a CSF specimen from a patient with culture-proven TBM was isolated and was coupled with activated cyanogen bromide-Sepharose 4B. By immunosorbent affinity chromatography, a 14-kDa antigen was isolated from the culture filtrate of M. tuberculosis. Antibody to the 14-kDa mycobacterial antigen was raised in rabbits. The Dot-Iba in this study gave no false-positive results with CSF specimens from patients with nontuberculous neurological diseases. The assay gave positive results for all five patients with culture-proven TBM. The Dot-Iba described in the present report is simple, rapid, sensitive, specific, and, more importantly, suitable for routine application in laboratories in developing countries.
Tuberculous meningitis (TBM) is one of the common clinical manifestations of extrapulmonary tuberculosis. The incidence of TBM in developing countries like India during the past two decades has shown an upward trend. Autopsy studies from the Indian subcontinent revealed tuberculosis in 9.8% of 3,646 patients with TBM. A total of 65% of patients with tuberculosis had central nervous system (CNS) involvement (10). It was reported that 20% of pediatric patients who died from active tuberculosis were found at autopsy to have meningeal or CNS involvement (11). Early confirmative laboratory diagnosis and institution of effective antituberculosis chemotherapy (ATT) are essential in reducing the mortality rate and the neurological sequelae associated with TBM. Absolute criteria, i.e., the “gold standard,” for establishing the laboratory diagnosis of TBM depend upon the demonstration of Mycobacterium tuberculosis in cerebrospinal fluid (CSF) specimens from patients with TBM. The conventional bacteriological method not only is time-consuming but also lacks sensitivity (6). The immunological (1, 3, 5, 8, 9) and molecular biological (2) techniques that have been developed over the past decade as alternative methods for the laboratory diagnosis of TBM have evoked considerable interest among clinicians and laboratory investigators.
In this report we describe a rapid dot immunobinding assay (Dot-Iba) for the detection of mycobacterial antigen in CSF specimens from patients with TBM. The sensitivity of the Dot-Iba has been assessed with specimens from a small number of patients with culture-proven TBM, and the specificity has been evaluated with specimens from nontuberculous subjects. We recommended use of this technique by laboratories with limited resources and limited technical expertise.
MATERIALS AND METHODS
CSF specimens were collected from 40 patients with a clinical diagnosis of TBM admitted to the neurology unit of the Sree Chitra Triurnal Institute for Medical Sciences and Technology, a tertiary-care referral center for neurological diseases. At the time of admission, none of the patients had associated diseases like diabetes, human immunodeficiency virus infection, or immunodeficiency. With the exception of 2 patients, none of the 40 patients had earlier clinical manifestations of pulmonary tuberculosis or neurotuberculosis or had received chemotherapy for tuberculosis in the recent past. The diagnosis of TBM in all 40 patients was based on clinical features such as neck rigidity, positive Kernig's sign, and compatible CSF biochemical parameters such as elevated protein levels (60 to 400 mg%; mean, 98 mg%), low glucose concentrations (8 to 30 mg%; mean, 23 mg%), and pleocytosis (30 to 700 cells/cm3) in their CSF specimens. The CSF specimens were collected from all patients under aseptic conditions and were centrifuged at 5,000 × g for 30 min. The deposits were examined microscopically for acid-fast bacilli (Ziehl-Neelsen staining) and were inoculated onto a Lowenstein-Jensen slant for culture for M. tuberculosis. The supernatants from the centrifuged CSF specimens were coded and were used for the Dot-Iba. After 6 weeks the culture results showed the presence of M. tuberculosis in the CSF specimens from five patients, and these patients were regarded as having “confirmed” TBM. For the remaining 35 patients, repeated bacteriological investigations were negative for M. tuberculosis, fungi (Aspergillus), and bacteria (pnemococci, meningococci, and Haemophilus). India ink preparations of the CSF specimens were also negative for Cryptococcus neoformans. Routine skiagrams (roentgenograms) of the chest did not demonstrate any active pulmonary tuberculous lesions. However, magnetic resonance imaging scans for all patients showed various degrees of exudates in the base of the brain; since the clinical and neuroradiological features were suggestive of TBM, these 35 patients were categorized as having “probable” TBM. Patients with confirmed or probable TBM after studies of their CSF were given antituberculosis chemotherapy (rifampin at 450 mg, isoniazid at 300 mg, streptomycin at 0 to 7 g, and ethambutol at 80 mg) daily for 6 weeks during their hospital stays. These patients were advised to continue the antituberculosis chemotherapy for 3 months. All the patients were monitored in infectious disease clinics for assessment of their clinical response to chemotherapy. CSF specimens from 40 patients with nontuberculous neurological diseases were selected for use as controls. The diagnoses for the control group of patients were (i) bacterial meningitis due to Haemophilus influenzae (n = 3) and Neisseria meningitidis (n = 2) and (ii) partially treated pyogenic meningitis (n = 15), cryptococcal meningitis (n = 2), Japanese B virus encephalitis (n = 8), and CNS tumors (n = 10).
Human IgG to M. tuberculosis in CSF.
A total of 10 to 15 ml of cisternal CSF from a patient with culture-proven TBM as a positive control and 10 ml of cisternal CSF from a patient with rheumatic heart disease as a negative control were collected at autopsy. The immunoglobulin G (IgG) fractions in positive and negative control CSF specimens were eluted by passing the CSF through protein A-Sepharose 4B columns. The eluate was repeatedly dialyzed and concentrated with an ultrafiltration unit (Amicon-GmbH, Witten, Germany). The protein content was estimated, and the eluate was reconstituted to 3 mg/ml and stored in aliquots at −20°C.
Immunoabsorbent affinity chromatography.
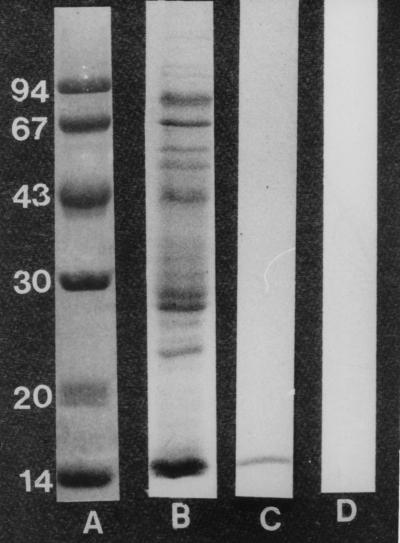
The technical procedure for immunoabsorbent affinity column chromatography was performed in accordance with the procedures described in previous reports (7). Briefly, 1 g of cyanogen bromide-Sepharose 4B (Sigma Chemical Co., St. Louis, Mo.) was reconstituted to 3.5 ml in distilled water and was washed with large volumes (20 times the original gel volume) of cold 0.1 M sodium bicarbonate buffer (pH 9). This was resuspended as a slurry of 50% (wt/vol) by the addition of 0.1 M sodium bicarbonate buffer. Human CSF IgG (3 mg/ml) to M. tuberculosis was added in an equal volume to the activated cyanogen bromide-Sepharose 4B, and the immunoabsorbent was incubated for 16 h at 4°C. The immunoabsorbent was washed five times with large volumes of 0.1 M sodium borate buffer (pH 9) alternating with 0.1 M sodium acetate buffer (pH 5), suspended in 0.1 M phosphate-buffered saline (PBS), poured into a glass chromatographic column (diameter, 1 cm), and equilibrated with 0.15 M PBS. The column was washed three times with 4 M urea in 0.15 M sodium bicarbonate buffer (pH 9), alternating with 0.15 M PBS to minimize the leaching out of IgG from the immunoabsorbent column. One milliliter (5 mg/ml) of culture filtrate of M. tuberculosis H37Ra was added, and the column was run with 0.15 M PBS. Every 10 min, a 1-ml fraction was collected until a blank reading at 280 nm was obtained. The specific mycobacterial antigen that bound to the immunoabsorbent column was eluted with 4 M urea in 0.15 M sodium bicarbonate buffer, and the absorbences of fractions at 280 nm were recorded. Fractions with absorbances of >0.05 were pooled and dialyzed against PBS. The protein content of the dialyzed material was estimated by the method of Lowry et al. (4) before it was stored in aliquots (100 μg/ml) at −20°C. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the eluate gave a single band, and the molecular mass was found to be 14 kDa (Fig. 1). The IgG from control CSF did not bind to the 14-kDa antigen. Antibody to the 14-kDa mycobacterial antigen was raised in rabbits. The IgG fraction in the immune rabbit serum was recovered by protein A-Sepharose 4B column chromatography, dialyzed, concentrated, and stored in aliquots (1 mg/ml) at −20°C.
FIG. 1.
SDS-polyacrylamide gel showing molecular mass standard (lane A), culture filtrate antigen (lane B), 14-kDa antigen isolated from culture filtrate antigen by specific IgG to M. tuberculosis (lane C), and control CSF IgG with no binding to 14-kDa antigen (lane D).
Standardization of Dot-Iba.
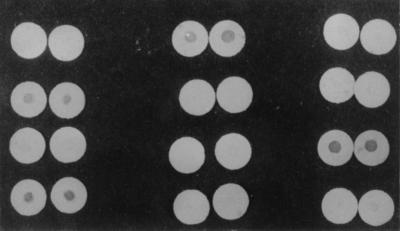
Prior to patient sampling, the Dot-Iba was standardized with different concentrations (5 to 500 ng/ml) of 14-kDa mycobacterial antigen in a nitrocellulose membrane (NCM). Circular NCM discs (diameter, 1 cm) were placed in each well of a (flat-bottom) microtiter plate. Five microliters of the 14-kDa mycobacterial antigen at each concentration was spotted onto the NCM discs, and the discs were incubated at 4°C for 12 h, after which the NCM discs were washed repeatedly with PBS-Tween 20 (PBS-T). The unbound sites in the NCM discs were quenched with 3% bovine serum albumin–PBS-T. Subsequently, the NCM discs were treated with rabbit IgG to the 14-kDa antigen (1:1,000 dilution) and were washed three times with PBS-T. The NCM discs were subsequently incubated with (i) anti-rabbit IgG–biotin conjugate (1:4,000 dilution) and (ii) Extr-avidin alkaline phosphatase (1:900) for 1 h each and were washed repeatedly with PBS-T. The NCM discs were then immersed in a substrate containing o-dianisidine tetrazotized (0.25 mg/ml), β-naphthyl acid phosphate (0.25 mg/ml), and magnesium sulfate (6 mg/ml) in 0.6 M sodium borate buffer (pH 9.7) for 10 min. The reaction was stopped by pouring off the substrate, followed by thorough washing in PBS-T. The NCM discs were fixed in a solution containing methanol, acetic acid, and distilled water in a ratio of 5:1:5. A positive reaction was indicated by the development of insoluble purple color in NCM discs (Fig. 2). The standardized Dot-Iba gave positive results for those NCM discs that contained 100 ng of the 14-kDa antigen per ml and above. The assay gave negative results for NCM discs that contained the 14-kDa antigen at less than 100 ng/ml.
FIG. 2.
Dot-Iba showing positive and negative reactions.
CSF samples from patients with confirmed cases of TBM and control groups were assayed for mycobacterial antigen as described above for the standardized Dot-Iba. All CSF samples were tested on two different occasions to evaluate the reliability as well as the reproducibility of the assay. The assay was performed with batches of 10 CSF specimens along with a positive control antigen standard (100 ng/ml in PBS) and a negative control consisting of PBS alone.
RESULTS
In contrast to conventional bacteriological methods, the Dot-Iba required only 6 h to perform in the laboratory. Standardization of the Dot-Iba indicated that the sensitivity of the assay is 100 ng of antigen/ml. NCM discs containing the 14-kDa (100-ng/ml) mycobacterial antigen were used as a positive control whenever a batch of CSF specimens was assayed. The NCM discs containing the 14-kDa antigen standard can be routinely stored at 4°C for at least 6 months. Dot-Iba gave positive results for all five culture-positive patients with TBM. Thus, the sensitivity of the assay for patients with confirmed cases of TBM was 100%. Ten of 35 CSF specimens from patients with probable TBM were positive by the Dot-Iba. For 25 of 35 patients with probable TBM, the Dot-Iba consistently gave negative results. In order to confirm the diagnosis for these 25 patients with probable TBM, PCR was performed, and the results were negative. Serological and PCR tests for pneumococci and fungi were also negative. Therefore, a microbiological diagnosis for these 25 patients with probable TBM could not be established. The repeat studies with the same CSF patients continued to show elevated protein levels and leukocyte counts and negative Dot-Iba results. For the 40 CSF samples from patients with nontuberculous neurological diseases, the assay gave consistently negative reactions. Thus, the specificity of the assay with the samples from the subgroups studied was 100%. The results for the patients with TBM and the control groups of patients were reproducible when the assay was performed on different occasions, and there was no variation in the results for any one of the CSF specimens from patients in the TBM and control groups.
DISCUSSION
Earlier immunoassays described in the literature for the detection of mycobacterial antigen in CSF from patients with TBM include a latex particle agglutination test with antiplasma membrane antibody (3), a sandwich enzyme-linked immunosorbent assay (ELISA) with anti-M. bovis BCG antibody (9), an inhibition ELISA with polyvalent antibody against M. tuberculosis (1), and a competitive ELISA with anti-M. bovis BCG antibody (8). Mastrioanni et al. (5) applied a dot blot method in their study of 38 patients with TBM. They used anti-M. bovis BCG antibody in their study, and their assay detected mycobacterial antigen at a concentration of 100 ng/ml. The assay detected the presence of antigen in all the culture-positive patients with TBM. Two of the 25 patients with nontuberculous meningitis gave false-positive results in their study (5). In that study, neither the biochemical nor the immunological properties of the antigen detected in the CSF specimens were highlighted. It would be worthwhile to note that in all the studies mentioned above, the antibodies used in the assay to detect mycobacterial antigen either were commercial products or were induced in other species. However, in our study, we have used specific human CSF IgG to M. tuberculosis to isolate a mycobacterial antigen from the culture filtratrate of M. tuberculosis. Immunoabsorbent affinity chromatography was applied to isolate a specific mycobacterial antigen, and this antigen showed a single band by SDS-PAGE and had a molecular mass of 14 kDa. The CSF specimens from all five patients with culture-proven TBM gave positive results in our assay. The results of Dot-Iba for these five patients were known within 6 h of receipt of CSF specimens in the laboratory. On the basis of the Dot-Iba results, these five patients received ATT, and they had positive clinical responses within 2 weeks of the commencement of therapy. Ten of 35 patients with probable TBM had demonstrable antigen in their CSF specimens. These 10 patients also had positive clinical responses to ATT. Dot-Iba gave negative results for the remaining 25 patients with probable TBM, and these patients did not show any clinical improvement following treatment. The precise etiologic agent for the occurrence of meningitis in these 25 patients remained undetermined, and they were reclassified as having chronic meningitis. Thus, we consider that a positive Dot-Iba result has definite diagnostic value and that a negative Dot-Iba result would suggest that ATT should not be continued in patients with probable TBM. The assay did not reveal false-positive results for any of the 40 patients with nontuberculous neurological diseases.
The Dot-Iba established in our laboratory is rapid and specific for the detection of mycobacterial antigen in CSF. More importantly, it can be readily performed in a routine clinical laboratory and does not require sophisticated equipment, and the results can easily be interpreted by visual examination of the NCM discs. The entire procedure requires only 6 h after the receipt of the CSF specimen in the laboratory. Although recent molecular biological techniques have emphasized the usefulness of PCR for the diagnosis of tuberculosis (2), the precise application of PCR for the routine laboratory diagnosis of TBM still remains to be determined. The technical aspects of the Dot-Iba described in this report can be performed very simply and a large number of CSF specimens can easily be handled by the staff of a single laboratory. The reagents used in the assay have shelf lives of more than 6 months, and more importantly, the assay is reproducible. We therefore consider this approach to be most suited to laboratories in developing countries where TBM is still prevalent. We are undertaking multicenter trials in India to evaluate the routine application of Dot-Iba for the laboratory diagnosis of TBM in outlying laboratories.
ACKNOWLEDGMENTS
We gratefully acknowledge the Department of Science and Technology, New Delhi, India, for the financial support to undertake the study (DST project SP-SO/B-33).
We thank the director of our institute for kind permission to publish this work. We are indebted to S. Vijayalekshmi for secretarial assistance.
REFERENCES
- 1.Bal V, Kamat R S, Kamath J, Kandoth P. Enzyme-linked immunosorbent assay for mycobacterial antigens. Indian J Med Res. 1983;78:477–483. [PubMed] [Google Scholar]
- 2.Kox F F, Kuijper S, Kolk H J. Early diagnosis of tuberculous meningitis by polymerase chain reaction. Neurology. 1995;45:2228–2232. doi: 10.1212/wnl.45.12.2228. [DOI] [PubMed] [Google Scholar]
- 3.Krambovitis E, McIllmurray M B, Lock P E, Hendrickse W, Hozel H. Rapid diagnosis of tuberculous meningitis by latex particle agglutination. Lancet. 1984;ii:1229–1231. doi: 10.1016/s0140-6736(84)92792-2. [DOI] [PubMed] [Google Scholar]
- 4.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 5.Mastroianni C M, Vullo V, Paoletti F, Massetti A P, Sorice F, Delia S. Detection of mycobacterial antigen by dot-blot assay in the cerebrospinal fluid of patients with TBM. J Infect. 1991;22:106–107. doi: 10.1016/0163-4453(91)91374-7. [DOI] [PubMed] [Google Scholar]
- 6.Mathai A, Radhakrishnan V V, Vehgal S. Diagnosis of tuberculous meningitis by enzyme-linked immunosorbent assay to detect mycobacteria antigen and antibody in cerebrospinal fluid. Med Microbiol Immunol. 1990;179:281–288. doi: 10.1007/BF00192466. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan V V, Mathai A, Vehgal S. Correlation between culture of Mycobacterium tuberculosis and IgG antibody to Mycobacterium tuberculosis antigen in the cerebrospinal fluid of patients with tuberculous meningitis. J Infect. 1990;21:271–277. doi: 10.1016/0163-4453(90)93957-t. [DOI] [PubMed] [Google Scholar]
- 8.Ramkisson A, Coovadia Y M, Coovadia H M. A competition ELISA for the detection of mycobacterial antigen in tuberculous exudate. Tubercle. 1988;69:209–212. doi: 10.1016/0041-3879(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 9.Sada E, Riuz-Palacios G M, Lopez-Vidal Y, Ponce de Leon S. Detection of mycobacterial antigens in cerebrospinal fluid of patients with tuberculous meningitis by enzyme-linked immunosorbent assay. Lancet. 1983;ii:651–652. doi: 10.1016/s0140-6736(83)92532-1. [DOI] [PubMed] [Google Scholar]
- 10.Udani P M, Parekh V, Compilation V C, Dastur D K. Tuberculosis of the central nervous system, incidence and classification. Indian Pediatr. 1973;10:647. [PubMed] [Google Scholar]
- 11.Udani P M, Dastur D K. Tuberculous encephalopathy with and without meningitis. J Neurol Sci. 1970;10:541–561. doi: 10.1016/0022-510x(70)90187-5. [DOI] [PubMed] [Google Scholar]