Abstract
The ubiquitin-proteasome pathway (UPP) is involved in regulating several biological functions, including cell cycle control, apoptosis, DNA damage response, and apoptosis. It is widely known for its role in degrading abnormal protein substrates and maintaining physiological body functions via ubiquitinating enzymes (E1, E2, E3) and the proteasome. Therefore, aberrant expression in these enzymes results in an altered biological process, including transduction signaling for cell death and survival, resulting in cancer. In this review, an overview of profuse enzymes involved as a pro-oncogenic or progressive growth factor in tumors with their downstream signaling pathways has been discussed. A systematic literature review of PubMed, Medline, Bentham, Scopus, and EMBASE (Elsevier) databases was carried out to understand the nature of the extensive work done on modulation of ubiquitin-proteasome pathways in oncogenic signaling. Various in vitro, in vivo studies demonstrating the involvement of ubiquitin-proteasome systems in varied types of cancers and the downstream signaling pathways involved are also discussed in the current review. Several inhibitors of E1, E2, E3, deubiquitinase enzymes and proteasome have been applied for treating cancer. Some of these drugs have exhibited successful outcomes in in vivo studies on different cancer types, so clinical trials are going on for these inhibitors. This review mainly focuses on certain ubiquitin-proteasome enzymes involved in developing cancers and certain enzymes that can be targeted to treat cancer.
Keywords: cancer, ubiquitination, ubiquitin-proteasome system, deubiquitination, ubiquitin inhibitors
1. Introduction
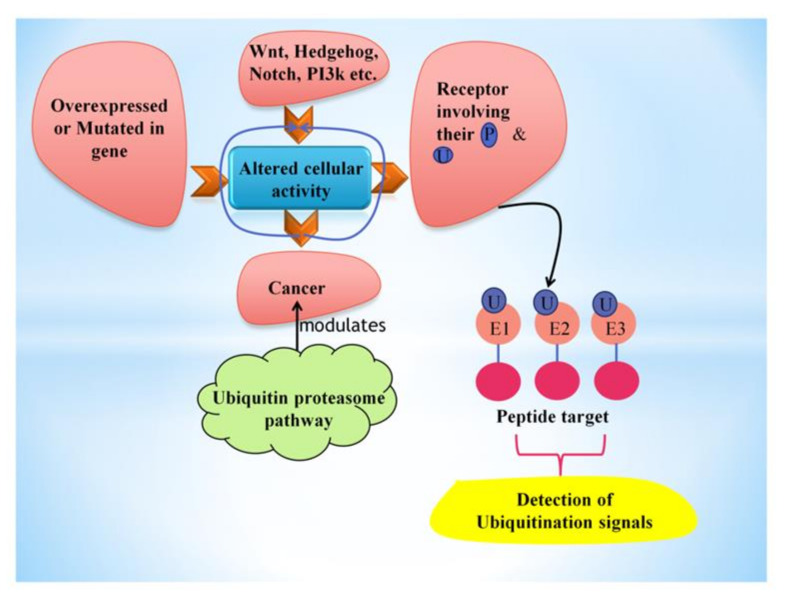
Cancer is one of the dreadful diseases increasingly affecting the population worldwide. There is an aberrant gene function in cancer cells, which controls protein synthesis, cell growth, differentiation, and cell death. These activities are regulated by various pathways interconnected with each other to form a complex network. Alterations in these signaling pathways alter cellular activity’s progress that might lead to the over-production of proteins and uncontrolled cell growth, resulting in cancer [1]. One of the reasons for altered cellular activity is mutations in their genes and the overexpression of these mutated genes (e.g., gene amplification), which diverts the action from normal cellular activity. Downstream nuclear targets of cellular pathways, e.g., chromatin remodelers (EZH2), transcriptional factors (Myc and NF-κB), and cell cycle effectors (cyclins), are upregulated in cancer and also act as pro-oncogenic in tumor onset (Figure 1). The presence of tumor suppressors like p53, PTEN, p16, etc., in the body regulates cell death; therefore, the mutated suppressor genes ultimately lead to uncontrolled cell growth [2]. The regulatory processes that allow specific, rapid, and usually irreversible differences in cell sensitivity to ligands have evolved towards regulating receptor degradation and downregulation.
Figure 1.
This figure shows the factors leading to the alteration of cellular activity, further causing cancer, which can be inhibited or influenced by the ubiquitin-proteasome pathway. Ubiquitin polymers attach covalently to peptide targets through a three-step (E1→E2→E3) conjugation cascade to detect particular ubiquitination signals.
These mechanisms are often controlled by post-translational modifications of receptors involving their phosphorylation and ubiquitination [3]. In eukaryotes, protein degradation is essential for removing excessive proteins, e.g., enzymes and transcriptional factors (that are no longer required) or exogenous peptides transported in the cells. Two larger involved protein degradation systems present in cells are the autophagy-lysosome and the ubiquitin-proteasome systems [4]. The ubiquitin-proteasome pathway degrades nuclear and cytosolic proteins through an ATP- and ubiquitin-dependent process focused on the multicatalytic proteinase complex known as the 26S proteasome [5]. Ubiquitin polymers are formed, attached covalently to peptide targets through a three-step (E1→E2→E3) conjugation cascade, detecting particular ubiquitination signals. Targets can exist in the cytoplasm, nucleus, even on cell or nuclear membrane surfaces, or from the endoplasmic reticulum (ER) after their retrograde transfer back to the cytoplasm [6]. Overall, ubiquitination-proteasome-deubiquitination is an essential regulatory activity that keeps equilibrium in responses to the surrounding in vivo [6]. Different enzymes involved in the ubiquitin-proteasome pathway have distinct cell roles that regulate cell growth and death via triggering or degrading intermediates of another signaling pathway.
Notably, the ubiquitin-proteasome pathway is responsible for degrading tumor suppressor components and can influence cell differentiation in cancerous cells. In brief, the ubiquitin-proteasome pathway and its components have both benefits and detriments depending on their nature towards cellular pathways, which have been explained below in this review.
Methodology
A systematic literature review of PubMed, Medline, Bentham, Scopus, and EMBASE (Elsevier) databases was carried out with the help of the keywords like “cancer; ubiquitination; ubiquitin-proteasome system; deubiquitination; ubiquitin inhibitors” till March 2021. The review was conducted using the above keywords to collect the latest articles and understand the nature of the extensive work done on the modulation of ubiquitin-proteasome pathways in oncogenic signaling (Figure 2).
Figure 2.
Flowchart of methodology.
2. Molecular Biology of the Components in Ubiquitin-Proteasome System
2.1. Ubiquitin
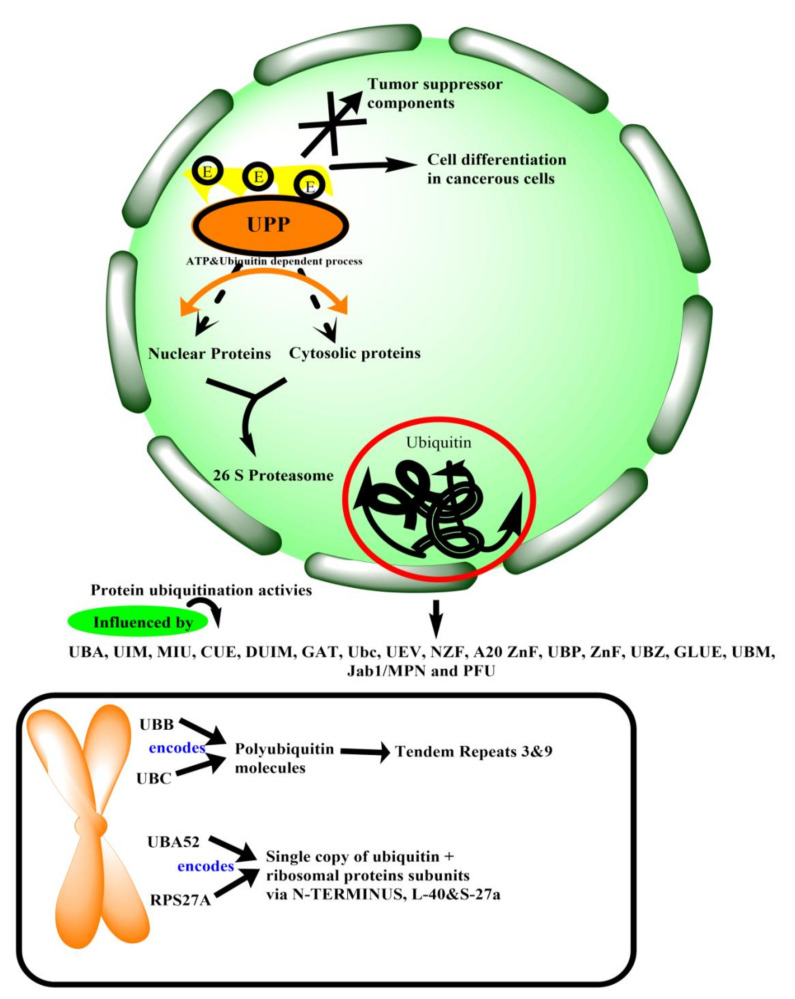
Ubiquitin comprises 3.5 turns α-helix, 5-stranded β-sheet, and a small 310 helix [7]. In the human genome, there are four genes, namely, UBB, UBC, UBA52, and RPS27A, that encode ubiquitin. Genes UBB and UBC encode for the polyubiquitin molecules that give tandem repeats 3 and 9, respectively. However, genes UBA52 and RPS27A encode for a single copy of ubiquitin, which is fused with the ribosomal protein subunits via N-terminus, L40 S27a, respectively [8]. Ubiquitin is a 76 amino acid protein having seven Lys residues, and all of them can be ubiquitinated to form isopeptide-linked ubiquitin chains [9]. As data indicates, ubiquitin is modified by post-translational modifications; six out of seven Lysine residues (Lys6, Lys11, Lys29, Lys27, Lys33, Lys48, Lys63) of ubiquitin can be acetylated [10]. Phosphorylating sites are also present on the surface of ubiquitin-Ser 57, Ser20, Ser65, Thr7, Thr12, Thr14, Tyr59 [10,11]. Spotting of ubiquitin and ubiquitinated proteins are perceived by ubiquitin-binding domains, which form (autonomous) folding units within ubiquitin receptor proteins [12]. More than 20 ubiquitin-binding domain families can read the ubiquitin code within ubiquitin-binding proteins, and ubiquitin receptors that interact with other ubiquitin surfaces through non-covalent bonds [13]. Various protein ubiquitination activities are influenced by ubiquitin-binding domains, which include at least 16 known domains: UBA, UIM, MIU, CUE, DUIM, GAT, Ubc, UEV, NZF, A20 ZnF, UBP ZnF, UBZ, GLUE, UBM, Jab1/MPN and PFU (ubiquitin associated domain; ubiquitin interacting motif; motif interacting with ubiquitin; coupling of ubiquitin; double-sided ubiquitin-interacting motif; GGA and Tom1; ubiquitin C; ubiquitin E2 variant; npl-4 Zinc finger; A20 zinc finger; ubiquitin-binding domain zinc-finger; ubiquitin-binding zinc finger; GRAM-like ubiquitin-binding in EAP45; ubiquitin-binding motif; and PLAA family ubiquitin, respectively) [14]. The ubiquitin-binding domain attachments have an essential influence on therapeutic strategy. For example, in a study, displacement of Ub from the Zn-finger ubiquitin-binding domains of HDAC6 (main cytoplasmic deacetylase in mammals) can be a useful target for multiple myeloma or other disease therapy [15].
2.2. Proteasome
Proteasome (also known as 26S proteasome) is a protein degradation machine in eukaryotic organisms. It is a 2.5 MDa complex that comprises approx. 33 different subunits arranged into two subcomplexes: a barrel-shaped proteolytic core particle (alias the 20S proteasome; CP) and one or two terminal(s) 19S regulatory particle(s) (alias PA700; RP) [16,17,18]. This holoenzyme’s proteolytic active sites are present within the core of the 20S core particle, consisting of four heptameric rings [18]. It forms a narrow axial pore, which does not allow folded protein or even unfolded large polypeptides to pass through, thus protecting normal body proteins from degrading (Figure 3). The regulatory particle(s) controls the opening of these pores [19,20], covering one or both ends of 20S core peptidase and transferring client protein into the degradation chamber [21]. The 19S RP seems to recognize ubiquitylated substrate proteins and is considered to serve a role in protein unfolding and translocating it into the interior of 20S CP. Catalytic threonine residues are present over the core particle’s surface, composed of two β-rings [22]. There are two subunits of the 20S- α-type [α1, α2, α3, α4, α5, α6, α7, α8] and β-type [β1, β2, β3, β4, β5, β6, β7, β1i, β2i, β5i, β5t] [18]. The 20S CP/20S proteasome (of 730kDa) is a well-arranged protein complex formed by four stacked hetero-heptameric rings, which comprises of 7 α-type subunits or seven β-type subunits in a symmetric configuration of α1−7/β1−7/β1−7/α1−7 C2 [23]. The 19S regulatory particle(s) of ~930 kDa is formed by at least 19 integral subunits of molecular masses ~10 to 110 kDa. It can be separated into two subcomplexes: the base and the lid [24]. The base is made with a ring of AAA-ATPases (Rpt1-6) with four non-ATPase subunits (Rpn1, 2, 10, 13) and contact with CP. The peripheral lid consists of Sem1 (alias Rpn15/Dss1) and an additional 10 ATPase subunits with different functions [25]. The contact between the base and lid is balanced by the subunit Rpn10 [22]. The scaffolding subunits (except for Rpn15) involves protein–protein interacting motifs called PCI [proteasome-CSN (COP9 signalosome)-eIF3 (eukaryotic translation initiation factor 3)] domains [26,27].
Figure 3.
Illustration of ubiquitin in cancerous cells and human genome encodings, i.e., UBB and UBC encode for polyubiquitin molecules and UBA52 and RPS27A encodes for single copy of ubiquitin and ribosomal proteins subunits. Ubiquitin-proteasome pathway degrades nuclear and cytosolic proteins through an ATP- and ubiquitin-dependent process. Ubiquitin polymers are formed by covalent attachment of E1, E2, E3 which involves different enzymes having distinct cell roles that regulate cell growth and death via triggering or degrading signaling pathways. The UPP is responsible for degrading tumor suppressor components and can influence cell differentiation in cancerous cells.
2.3. Deubiquitinase
DUBs are an enzyme that can reverse the activity of ubiquitination or ubiquitin-like modifications of substrate proteins, thereby protecting the protein from degrading [28,29]. DUB antagonizes protein ubiquitination similarly to phosphatases’ role in the kinase/phosphatase regulating pathway(s) [30]. Humans’ genomic system encodes for nearly 100 DUBs that are ubiquitin-specific and divided into five structurally unique DUB families. DUBs are categorized into five classes, including the ubiquitin-specific protease (USP) with 54 members, the ovarian-tumor proteases with 16 members, the ubiquitin C-terminal hydrolases with four members; the Josephin family with four members [31]. The fifth DUB family is MIU containing a novel DUB family (MINDY) with four members [32]. A Zn-dependent JAB1/MPN/MOV34 metalloprotease DUB family also exists with 16 members [33]. The ubiquitin removal step appears to be a highly regulated sequence of action connected with uncountable cellular functions. DUBs are implicated in maintaining cell cycle stages, double-stranded sliced repair, and the M/G 2 checkpoints, averting protein degradation and transcriptional activities. It is also involved in apoptosis, microbial pathogens, viral precursor protein, kinase activation [34].
2.4. Ubiquitination
Ubiquitination can be defined as a cascade of events by three enzymes performing their respective action to attach selected substrate proteins with ubiquitin for prior modifications. It is one of the essential protein modifications involved in cellular signaling and homeostasis control. The process initiates with enzyme E1 for activating ubiquitin. The activated ubiquitin is then transferred to the E2 enzyme, and ubiquitin linkage forms (mono-ubiquitination, polyubiquitination, branched ubiquitination). Detachment of ubiquitin from E2 can trigger attachment with substrate protein and directly approach for proteasome degradation [35]. Otherwise, the E2 enzyme serves to identify specific E3 ligases where it transfers the substrate protein. Then, ligase releases substrate protein to proteasome or are obstructed by deubiquitinase, leading to protein survival. The whole process can be called as ubiquitination of substrate protein. All the enzymes are explained in detail below:
2.4.1. E1 (Ubiquitin-Activating Enzyme)
Gathered literature suggests there are only two E1 enzymes in humans, i.e., a non-specific Uba 1 enzyme, which can activate ubiquitin for all ubiquitin-dependent reactions, and an organ-specific enzyme UBE1L2 [36,37,38]. Uba1-E1 comprises of four building blocks, in which first is an adenylation domain labeled ‘AAD’ (404–594) (for ATP-Ub binding) and ‘IAD’ (1–169) for active and inactive adenylation, respectively. Second is the catalytic cysteine half domain-containing E1 active site Cyt [CC (169–268) and CCD (594–860)] incorporated within each adenylation domain. The third block contains 4HB (helix bundle) (268–356) depicting the second insertion in IAD, and the fourth includes the C-terminal ubiquitin-fold domain, UFD (926–1024), which selects specific E2s for ubiquitin [39,40]. Recently found UBE1L2, i.e., ubiquitin activating enzyme E1-like protein 2, share its 40% identity with Ube1. There are two essential conserved domains of UBE1L2: the highly protective ATP-binding domain (amino acid 467–474; GXGXXGCE) and the putative active site domain (amino acid 623–631; PXCTXXXP) encompassing Cys-625, which can form thioester links with ubiquitin [40,41].
2.4.2. E2 (Ubiquitin-Conjugating Enzyme)
Humans have approximately 40 E2s that support the transfer of ubiquitin or ubiquitin-like proteins (e.g., NEDD8 and SUMO), and most of them are double the size of ubiquitin. The E2 enzymes are involved in tagged protein regulations for their localization, degradation, and other functions [42]. Some E2s have only a catalytic domain named ‘Class 1′ whereas some have either N- ‘Class 2′ or C- ‘Class 3′ terminal extensions or contain both ‘Class 4′ [42]. The E2 enzymes are “Ube2A, Ube2B, Ube2C, Ube2D1, Ube2D2, Ube2D3, Ube2D4, Ube2E1, Ube2E2, Ube2E3, Ube2G1, Ube2G2, Ube2H, Ube2J1, Ube2J2, Ube2K, Ube2L3, Ube2N, Ube2NL, Ube2O, Ube2Q1, Ube2Q2, Ube2QL, Ube2R1, UbE2R2, Ube2S, Ube2T, Ube2U, Ube2V1 Ube2V2, Ube2W, BIRC6” [43].
2.4.3. E3 (Ubiquitin-Ligase Enzyme)
The ubiquitin attached with E2 (linear or chain) is transferred to ubiquitin ligase E3, and the detached ubiquitin binds with substrate protein and ligase enzyme. According to the mechanism followed for the transfer from an E2 enzyme to substrate, E3s are classified into:
-
(i)
Really interesting new gene [RING] finger domain-containing ligases (BIRC7, Brca1, Cb1-b, cIAP1, IDOL, mdm2, SIAH1, RAD18, RNF4, TRAF6): The RING E3 enzymes are differentiated due to their RING or Ubox (CHIP) fold catalytic-domain which encourages the direct linking of ubiquitin from E2 with the substrate [44,45,46]. There are more than 600 RING finger E3s encoded in the mammalian genome. Structurally, it is a zinc coordinating domain that consists of spaced cysteine and histidine residues which promote E2 dependent ubiquitylation [47].
-
(ii)
Homologous to E6-associated protein C terminus [HECT] domain-containing ligases (SMURF1, NEDD4.1, HUWE1, E6AP): It consists of 28 members in human and based on similarities in the N- terminus domains, 15 members out of 28 can be divided into two subfamilies. The most prominent and well-studied category is the NEDD4 subfamily consisting of nine members, which are characterized by the presence of C2 and WW domains. Another subfamily is the HERC E3 ligase enzyme consisting of six members and shares one commonality, i.e., one or more RCC-like domains (regulators of chromatin condensation 1-like domains) [48].
-
(iii)
RING between RING (RBR) domain-containing ligases: Structurally, the RBR module has three Zn2+ binding motifs, a RING1 domain which interacts with E2 then followed by IBR, which is in-between RING1 finger domain and the RING2 domain of which catalytic cysteine is involved. There are 14 RBRs in humans; among these, only three members that are well understood are Parkin, HHAR1, and HOIP [49,50].
3. Physiological and Pathological Role of UPS in Human Body
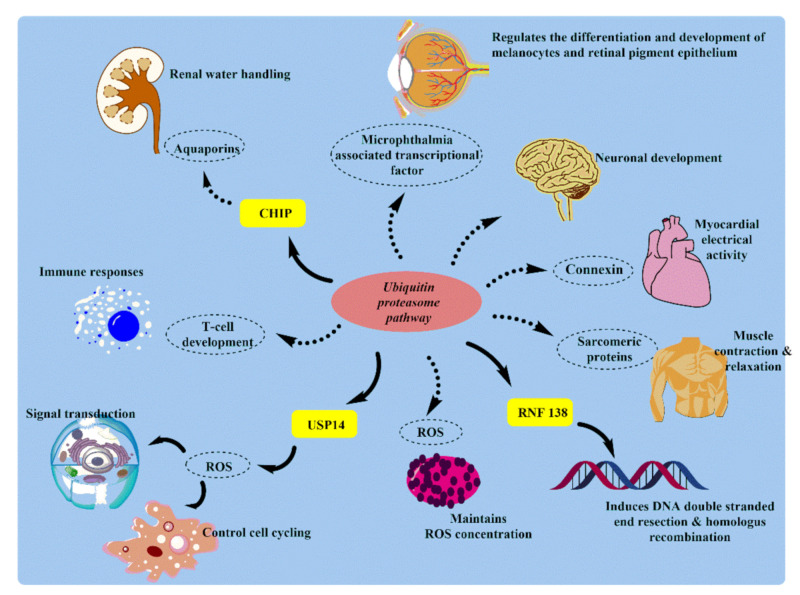
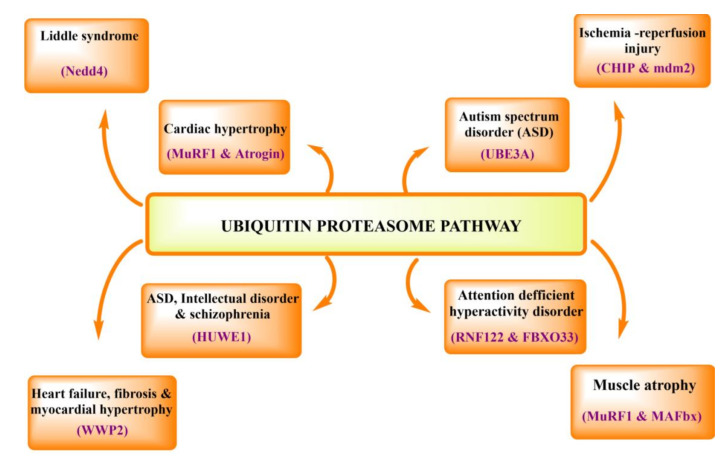
The ubiquitin-proteasome system plays a crucial role in protein quality control, cell cycle control, and signal transduction. Proteasome in the cytoplasm is mainly concentrated near the centrosome, which indicates its activity at the cellular level. Ubiquitin-proteasome pathway (UPP) components are seen in very specialized cells, including neurons of both pre-and post-synaptic knobs. In every physiological process, the ubiquitin-proteasome pathway exists, and system variation can lead to the onset or progression of human disease. These diseases include cancer, metabolic syndrome, neurodegenerative diseases, inflammatory disorders [51], infection [52], and muscle dystrophy [53] (Figure 4).
Figure 4.
This figure summarizes the pathophysiological role of UPP in various diseases and indicates the dysregulated enzyme in the respective condition.
In the nervous system, the ubiquitin-proteasome system exemplified its importance in regulating many aspects of synaptic activity such as spinogenesis, axon growth, pre-synaptic neurotransmission, synaptic scaling, long-term potentiation, apical dendrite outgrowth/polarization, synapse formation, dendritic arborization, and elimination [52,54,55].
In the cardiovascular system, like any other proteins within cells, cardiac myofibrillar proteins are also constantly being broken down and rebuilt. By investigating proteolytic systems’ role in skeletal muscle wasting and atrophy via the ubiquitin-proteasome system, it was found that the ubiquitin-proteasome system is essential for the degradation of the sarcomeric proteins [56,57,58]. In the muscular system, muscle atrophy/wasting is the main reason for the increase in protein degradation by autophagy, including the ubiquitin-proteasome system. During investigating the role of proteolytic systems in skeletal muscle wasting [59] and atrophy, it was found that not many enzymes are known yet. In several muscle wasting and atrophy cases, the ubiquitination system is activated and upregulated, increasing proteasome activity. For example, the highly expressed muscle-specific ubiquitin ligases (MuRF-1 and MAFbx/atrogin-1) recently came into view, which paves the way for the process of atrophy.
In spermatogenesis, protein’s selective degradation is reported in various data related to reproductive processes, especially spermatogenesis, fertilization, and testosterone biosynthesis [60]. Spermatogenesis can be represented as a complex succession of cell division and differentiation events resulting in spermatozoa’s continuous formation. In the kidney, microarray analysis showed that passive Heymann nephritis is associated with increased expression of genes which encodes for ubiquitin-conjugating enzymes and deubiquitinase enzymes. This analysis supports the UPS role in kidney functioning and disorders [61].
The ubiquitin-proteasome system’s importance extends from the kidney to the nephrons’ outline in glomerular cell identity and function, glucose reabsorption, erythropoiesis, and salt-water balance. The cortical collecting duct’s principal cells express various aquaporins on its surface, maintaining body water balance [62]. However, genomic stability maintenance in situations like DNA damage critically relies on quick recognition and healthy repair of damage [63,64].
4. Role of Ubiquitin-Proteasome Pathway in Cancer
Cancer is one of the leading causes of death globally for ages. There have been many types of research on cancer for its treatment and finding new targets, but no successful results have been found. Mortality data collected since 2016 by The National Center for Health Statistics and an estimate for 2019 indicates that approximately 626,000 cancer deaths and over 1,762,000 new cancer cases occur in the US [65]. Cancer can be defined as high cell proliferation and low cell death due to disturbance in the cell cycle, a mutation in existing cells, activation of tumor promoter genes, inactivation of the tumor suppressor, irregularities in a feedback mechanism, deregulation in cell cycle pathways, etc. [66]. The tumor suppressors are somewhat targeted by UPP-related enzymes and inhibit their role in cell death. Various studies mentioned below show the ubiquitin-proteasome pathway’s involvement via the above mediators/pathways, leading to cancer one way or another.
4.1. Colorectal Cancer
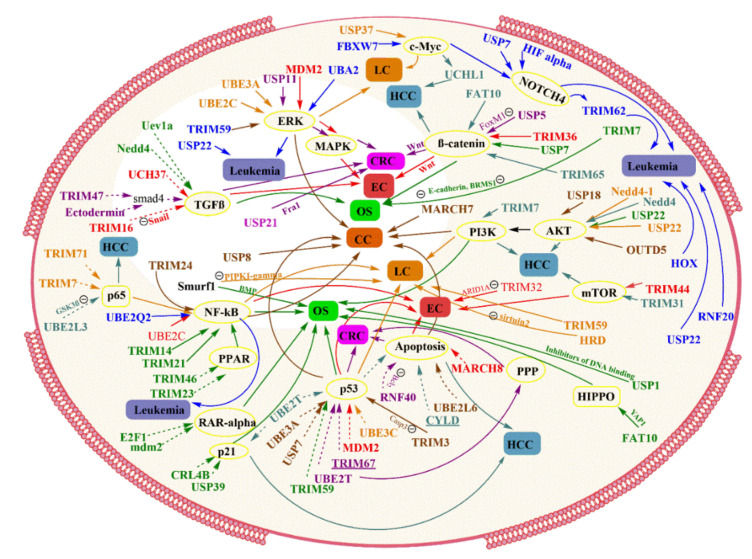
Colorectal cancer (CRC) is the third most prevalent cause of death from cancer in the US [65]. Lynch syndrome, serrated polyposis syndrome, MUTYH-associated polyposis, familial adenomatous polyposis, etc., are the commonly found hereditary colorectal cancer, and polyposis syndromes. Colorectal cancers can be associated with genetic inheritance, African-American ethnicity, inflammatory bowel disease, red meat/processed meat, abdominal radiation, cholecystectomy, also includes unhealthy lifestyle, etc. [67]. Mutations and dysregulation in the normal signaling pathways cause cancer. Pathway TGF-β involves a large family of proliferation and differentiation factors which include activin and inhibition. Transmission of signals occurs via Types I and II serine/threonine kinase receptors [68]. Ligand binding initiates and triggers the activation of Type I kinase by the Type II receptor kinase. Then, the signal is passed from the Type I receptor to an intracellular mediator of TGF-β, Smads, which initiates Smad signaling for nuclear translocation and activation of specific gene expression. Nearly 13% of colorectal carcinomas are caused due to replication error (or microsatellite instability) phenotype on inactivation and restoration of receptor Type II, resulting in low tumorigenicity. Among Smads, Smad 6 and 7 act as an inhibitor of Smad, which blocks TGF-β signaling by competitively associating with Type I or directing it towards Ub-mediated degradation [69], increasing cellular growth (Table 1). Many ligases like Smurf1, Smurf2, Nedd4-2, arkadia, etc., are involved in TGF-β signaling, but E3 ligase ectodermin is an enzyme whose altered expression can lead to tumor formation via inhibiting Smad4, thereby blocking the TGF-β pathway [70]. Aberrant activation of pathway Wnt/β-catenin in the colorectal region can cause uncontrolled tumor growth, invasion, and angiogenesis (Table 1). In normal human cell regulation, a ligase enzyme FBXW7 prevents this oncogenic pathway from causing this problem. FBXW7 ubiquitinates a novel component of Wnt-signaling, FoxM1, after phosphorylated by glycogen synthase kinase 3. But in cancer, the situation is reversed by enzyme USP5 that deubiquitinate and prevents the degradation of FoxM1 [71,72]. USP11 offers a relevant role in cancer progression through multiple pathways. This enzyme proliferated and promoted metastasis of colorectal cancer in vitro and in vivo through PPP1CA-ERK/MAPK pathway [73]. An activator protein-1 (AP-1), precisely its member Fos-related-antigen-1 (Fras 1), has shown a tumorigenic role in many cancers. Usually, the AP-1 is mediated through the ERK pathway because Ras-ERK signals to constrain the proteasomal degradation of member the Fra-1. The Ras-ERK function can be achieved through an enzyme called deubiquitinase which can change the fate of protein from degrading, and in this case, UPS21 protects Fra-1, which promotes cellular growth in colorectal cancer [74] (Figure 5).
Table 1.
The table summarizes various physiological roles of UPP and the enzymes/proteins involved in a cancer type.
| S. No. | Type of Enzyme | Enzymes Involved | Modulation of Pathways Involved |
Cancer Type | References |
|---|---|---|---|---|---|
| 1. | E3 ligase | Ectodermin, TRIM 47 | ↓ Smad 4 in TGF-β signaling | Promotes colorectal cancer | [75] |
| 2. | E3 ligase | FBXW7 | ↓ Wnt/β-catenin | Inhibits colorectal cancer | [71] |
| 3. | Deubiquitinating enzymes | USP5 | ↑ Wnt/β-catenin | Promotes colorectal cancer | [72] |
| 4. | Deubiquitinating enzymes | USP11, USP21 | ↑ERK/MAPK | Promotes colorectal cancer | [73,74] |
| 5. | Deubiquitinating enzymes | UBE2T | ↓ p53 pathway, ↑pentose phosphate pathway, etc., | Promotes colorectal cancer | [76] |
| 6. | E3 ligase | TRIM 67 | ↓ p53 degradation | Inhibits colorectal cancer | [75] |
| 7. | Ubiquitin-conjugating enzyme E2C | UBE2C | Pro-apoptotic | Inhibits esophageal cancer | [77] |
| 8. | E3 ligase | TRIM36 | ↑ Wnt/β-catenin | Promotes esophageal cancer | [78] |
| 9. | E3 ligase | TRIM44 | ↑ mTOR | Promotes esophageal cancer | [79] |
| 10. | E3 ligase | TRIM16 | ↑ TGF β/Snail | Promotes esophageal cancer | [80] |
| 11. | E3 ligase | RNF113A | ---- | Promotes esophageal cancer | [81] |
| 12. | E3 ligase | MARCH 8 | ---- | Promotes esophageal cancer | [82] |
| 13. | E3 ligase | Gankyrin | ↑ p53 degradation | Promotes esophageal cancer | [83] |
| 14. | Deubiquitinating enzymes | Ubiquitin carboxyl-terminal hydrolase 37 | ↑ TGF-β signaling | Promotes esophageal cancer | [84] |
| 15. | E2 ligase | Uev1A | bone morphogenetic protein signaling | Inhibits osteosarcoma | [85] |
| 16. | E3 ligase | Nedd4 | ↑ TGF-β signaling | Promotes osteosarcoma | [86] |
| 17. | E3 ligase | USP7 | ↑ Wnt/β-catenin | Promotes osteosarcoma | [87] |
| 18. | Deubiquitinating enzymes | USP1 | stabilize “inhibitors of DNA binding.” | Promotes osteosarcoma | [88] |
| 19. | E3 ligase | Deltex1 | ↓ NOTCH/HES1 | Inhibits osteosarcoma | [89] |
| 20. | E2 ligase | FAT10 | ↓ Hippo/YAP1 | Inhibits osteosarcoma | [90] |
| 21. | Deubiquitinating enzymes | USP39 | ↑ p21 | Inhibits osteosarcoma | [91] |
| 22. | E3 ligases | TRIM46, TRIM21, TRIM14, and TRIM23 | ↑NF-κB | Promotes osteosarcoma | [92] |
| 23. | E3 ligases | TRIM59 and TRIM7 | ↓ p53 and E-Cadherin | Promotes osteosarcoma | [93,94] |
| 24. | Deubiquitinating enzymes and E2 ligases | USP22 and UBE2T | ↑ PI3K/AKT | Promotes osteosarcoma | [95,96] |
| 25. | E3 ligases | Smurf1 | ↑ TGF-β signaling | Promotes lung cancer | [70] |
| 26. | E3 ligases | NEDD4-1 | ↑ PI3K/PTEN | Promotes lung cancer | [97] |
| 27. | E3 ligases | NEDD4 | EGFR mutation | Promotes lung cancer | [98] |
| 28. | E3 ligases | UBE3A | ↓ p16INK4a | Promotes lung cancer | [99] |
| 29. | E3 ligases | HRD1 | ↓ Sirtuin 2 | Promotes lung cancer | [100] |
| 30. | Deubiquitinating enzymes | USP37 | ↑ c-Myc | Promotes lung cancer | [101] |
| 31. | E3 ligases | TRIM7, TRIM71 | ↑ NF-κB | Promotes lung cancer | [102,103] |
| 32. | E2C ligases | UBE2C | ↑ ERK | Promotes Lung cancer | [104] |
| 33. | E3 ligases | UBE3C, TRIM59 | ↓ p53 | Promotes Lung cancer | [105,106] |
| 34. | Deubiquitinating enzymes | USP22 | ↑ ERK/AKT | Promotes lung cancer | [107] |
| 35. | E3 ligases | Prickle-1 | ↓ Wnt/β-catenin | Inhibits liver cancer | [108] |
| 36. | E3 ligases | TRIM31 | ↑ mTOR | Promotes liver cancer | [109] |
| 37. | E3 ligases | TRIM7 | ↓ PI3K | Inhibits liver cancer | [110] |
| 38. | E3 ligases | TRIM32 | ↑ mutated p53 | Promotes liver cancer | [111] |
| 39. | E3 ligases | TRIM65 | ↑ β-catenin | Promotes liver cancer | [112] |
| 40. | E2 ligases | UBE2L3 | ↓ p65 | Promotes liver cancer | [113] |
| 41. | E2 ligases | UBE2T | ↓ p53, p21, and noxa | Promotes liver cancer | [114] |
| 42. | Deubiquitinating enzymes | CYLD | ↓ NF-κB | Inhibits liver cancer | [115] |
| 43. | Deubiquitinating enzymes | UCHL1 | Apoptotic resistance | Promotes liver cancer | [116,117] |
| 44. | E3 ligases | NEDD4 | ↑ PTEN/PI3K/AKT | Promotes liver cancer | [118] |
| 45. | E3 ligases | FAT10 | ----- | Promotes liver cancer | [119] |
| 46. | E3 ligases | USP7 | Facilitates DNA repair by stabilizing MDC1 | Promotes cervical cancer | [120] |
| 47. | E2 ligases | E2-EPF | ↑ pVHL-HIF | Promotes cervical cancer | [121] |
| 48. | E3 ligases | MARCH 7 | ↑ VAV1/RAC1/CDC42 | Promotes cervical cancer | [122] |
| 49. | Deubiquitinating enzymes | Ovarian-tumor proteases deubiquitinase 5 | ↑ PI3K-AKT | Promotes cervical cancer | [123] |
| 50. | E3 ligases and E2 ligases | UHRF1, UBE2L6 | Promotes hypermethylation | Promotes cervical cancer | [124] |
| 51. | Deubiquitinating enzymes | USP18 | ↑ PI3K/AKT | Promotes cervical cancer | [125] |
| 52. | E3 ligases | UBE3A | ↓ ERK | Inhibits cervical cancer | [126] |
| 53. | Deubiquitinating enzymes | USP8 | Stabilizes FLIPL and EGFR signaling | Promotes cervical cancer | [127] |
| 54. | E3 ligases | TRIM 24 | ↑NF-κB/AKT | Promotes cervical cancer | [128] |
| 55. | E3 ligases | TRIM59 | ↓ p53 pathway, ↑ Ras/Rad, ↑ ERK | Promotes cervical cancer | [129] |
| 56. | E3 ligases | TRIM3 | ↑ p53 pathway, ↑ Caspase 3 | Inhibits cervical cancer | [130] |
| 57. | E1 ligases | UBA2 | ↑ ERK1/2, STAT3, and STAT5 | Promotes leukemia | [131] |
| 58. | E2 ligases and E2R1 | UBE2Q2 and CDC34 | ↓ IκB | Promotes leukemia | [132] |
| 59. | E2 ligases | UBE2E1 | ↓ HOX gene (HOXA9 and HOXA10) | Inhibits leukemia | [133] |
| 60. | E3 ligase | Fbxw7 | ↑ c-Myc, Notch1 | Promotes leukemia | [134] |
| 61. | E3 ligases | Triad1 | ↓HOX genes | Inhibits leukemia | [135] |
| 62. | E3 ligases | RNF20 | Interacts with histone H3 lys79 (H3K79) methyltransferase DOT1L | Promotes leukemia | [136] |
| 63. | E3 ligase | USP7 | ↑ NOTCH1 | Promotes leukemia | [137] |
| 64. | Deubiquitinating enzymes | USP22 | Stabilize BMI1 | Promotes leukemia | [138] |
| 65. | E3 ligases | TRIM62 | ↑ NOTCH and β-catenin signaling | Promotes leukemia | [139] |
Figure 5.
The figure represents various physiological roles of UPP and the enzymes/proteins involved. CRC; colorectal cancer, EC; esophageal cancer OS; osteosarcoma, LC; lung cancer, HCC; hepatocarcinoma cells, CC; cervical cancer, leukemia.
The ubiquitin-proteasome system participates in the DNA damage and repair pathway through UBE2T, a member of the ubiquitin-conjugating enzyme. In colorectal cancer, UBE2T role is defined as a promotor of tumor progression and metastasis. The exact pathway is still to be found because more than one pathway, “p53 pathway”, “pentose phosphate pathway,” etc., was noticed to be involved in cell line culture. Generally, cell homeostasis is maintained by p53 expression, and p53 activation can activate or deactivate other genes that regulate cell cycle arrest, apoptosis, metastasis, angiogenesis, and DNA repair [76]. As p53 degradation occurs through ligase mdm2, therefore the presence of ligase mdm2, which will increase cell growth and decrease cell apoptosis, eventually give rise to colorectal cancer malignancies. A ubiquitin ligase, tripartite motif-containing protein (TRIM) 67, links with the C-terminal of p53 and inhibits p53 degradation by mdm2; therefore, TRIM 67 is silenced in colorectal cancer. TRIM 47 is also seen to increase the ubiquitination and degradation of Smad4, blocking its inhibitory action on colorectal cancer. This activity occurs through C-C motif chemokine ligand 15 and C-C motif chemokine receptor 1 (CCL15-CCR1), promoting tumor growth and cell progression [75]. H2B ubiquitin ligase RING finger protein 40 showed to be a tumor promoter in various human colorectal cancer cell lines. Knocking down of RING finger protein 40 triggered apoptosis in cells through downregulating anti-apoptotic factor Bcl-2. Ligase enzyme RING finger protein 40 actively mediates the monoubiquitination of H2B and may exert pro-tumorigenic function in cell activity [140].
4.2. Esophageal Cancer
Esophageal cancer is the eighth most common cancer worldwide and the 6th leading cause of cancer-associated death in 2012. Histologically, there are two subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma [141,142]. Mutated genes responsible for esophageal squamous cell carcinoma are MLL2, NFE2L2, NOTCH1, TGFBR2, and ZNF750, whereas esophageal adenocarcinoma is ARID1A, CDKN2A, ERBB2, SMAD4, and TP53 [143]. UBE2C (conjugating enzyme) promotes the ubiquitination of mitotic checkpoint genes; therefore, its overexpression emphasizes removing the inhibitory signal of mitotic spindle checkpoint in cells [77]. UBE2C normally functions as pro-apoptotic and anti-proliferative, but its expression is decreased by ECRG4 (esophageal cancer-related gene 4) in esophageal squamous cell carcinoma via NF-κB signaling. However, the mechanism in detail is yet to be explored [144].
Main overexpressed E3 enzymes found in esophageal squamous cell carcinoma are mdm2 (RING-type E3), TRIMs (RING-type E3), CUL3 (cullin subunit of the CRL3 E3), SKP2 (F-box protein, substrate receptor of CRL1 E3 (SCF complex)), CDC20 (substrate adaptor of the APC/C E3 complex (early mitosis)), KEAP1 (substrate receptor of the CRL3 E3) [145]. TRIMs are a specific type of E3 ligases characterized by their domain structure RING finger, and their activity seems to be regulated by β-catenin. However, TRIM36 is recently found to have relevance in esophageal cancer through stabilization of β-catenin, where the signal for β-catenin accumulation is transmitted via the Wnt signaling pathway [78]. Another TRIM categorized ligase that gave oncogene effect in esophageal cancer is TRIM44. Research shows it exhibit its role in esophaga-gastric cancer, where the mechanistic function responsible was suspected to be mTOR. Although the responsible pathway for the tumor progression and metastasis of overexpressed TRIM44 has not been identified, mTOR can be explored to start with [79].
Similarly, the proliferation, invasion, and migration are promoted by TRIM16 via TGF β and Snail pathway, which regulates epithelial-mesenchymal transition in esophageal cancer cell lines [80]. Smurf2 acts as a ubiquitin ligase for Smad, and its expression is higher in tumor cells, mainly at the tumor front, where the proliferation in esophageal squamous cell carcinoma is higher [146]. Most of the Wnt signaling factors involved in the post-translational modification are modulated through ubiquitination and deubiquitination.
Usually, the ubiquitination of target proteins leads to proteasomal degradation of Wnt-signalling factors like β-catenin, glycogen synthase kinase 3, Axin, and Dvl. Fz-7 is one of the Wnt receptors seen to be upregulated in esophageal squamous cell carcinoma patients. Its overexpression induces the activity of β-catenin, mesenchymal markers, and epithelial markers [147]. Binding of Wnt/Fzd activates dishevelled (Dvl) that avert the phosphorylation and ubiquitination of β-catenin. This leads to β-catenin stabilization and accumulation in the cytoplasm, further activating transcriptional activity for cellular growth [148]. In cell lines, analysis of RNF113A showed its overexpressed feature in esophageal squamous carcinoma cells, but its mechanistic approach is not yet found. Research has not been done on RNF113A, but previous studies suggest the involvement of epithelial-mesenchymal transition property in the process [81]. A ubiquitin ligase (MARCH 8) has been observed to be overexpressed in preneoplastic and neoplastic esophageal tissues. The MARCH 8 silencing activated the cell death process in the cell cycle by increasing sub-Gο and G2/M presence and lowering the S-phase population, which induces apoptosis [82]. Mdm2 act as ligase for p53, which inhibits its activity and result in tumor growth. There are other factors as well which are responsible for affecting p53 activity even in the absence or presence of mdm2. An ankyrin repeat-containing protein, gankyrin, associates with the subunit of ATPase of 26S proteasome, increasing the association between ubiquitinated p53 and mdm2 with the proteasome [83]. A deubiquitinating enzyme “ubiquitin carboxyl-terminal hydrolase 37” is involved in the activated TGF-β receptor type-I’s deubiquitination. Thereby preventing it from degradation via the proteasome, which results in TGF-β dependent gene over-expression. Hence ubiquitin carboxyl-terminal hydrolase 37 plays an oncogenic factor in esophageal cancer [84].
4.3. Osteosarcoma
Osteosarcoma is an autosomal dominant form of bone cancer with intrinsic osteoid production. Though the exact cause of osteosarcoma is unknown, the disease can be multifactorial with genetic and environmental factors [149]. The inactivation of the tumor suppressor gene p53, which regulates cell cycle progression in the presence of DNA damage, may result in osteosarcoma. Other genes involved in the p53 pathway like mdm2, p14ART, and CDK4 may develop osteosarcoma in a person [150]. The ubiquitin-proteasome system mainly regulates proteins that manage bone cells, osteoclast, and osteoblast (responsible for aged bone resorption and new bone formation, respectively) [151]. The critical factors controlling osteoblast differentiation from mesenchymal stem cells are bone morphogenetic protein-2, Wnt/β-catenin pathway, and transcriptional factors- Runx2, activating transcription factor 4, and JunB. The ubiquitin ligases substrates that affect bone metabolism are Smurf1 (Smad1, BMP-2, Runx2, Jun B, Traf6), β-TrCP (β-catenin, Smad4, ATF4, CYLD, NRF2, GHR), Fbx112 (p57), Chip (Smad1), Keap-1-Cul3-Rbx1 (Bcl-2, IKK, Nrf2), c-Cbl (EGFR/FGFR, α5 Integrin, Lyn/Fyn) [152,153].
The ubiquitin-conjugating enzyme E2 variants (Uevs) are seen to be involved in the bone morphogenetic protein signaling pathway, and its inactivation will negatively affect the functioning of bone cells. Uev1A is a novel OS regulator linked with (E2-E3 complex) UbcH5B-Smurf1 and further enables the ubiquitination and degradation of osteosarcoma promoting factor Smad1. Uev1A has also shown its serious role in preventing osteosarcoma by promoting osteoblast differentiation; therefore, inactivation of Uev1A might result in osteosarcoma [85]. Nedd4 is an essential modulator of p-Smad1 (phosphorylated-Smad1) in both bone morphogenetic protein-1 and TGFβ1 (Table 1). Nedd4 overexpression suppressed the bone morphogenetic protein-induced trans-differentiation process in cells and promoted TGFβ1 induced p-Smad1 degradation by polyubiquitination. Also, the suppressed expression of nedd4 speeds up the osteoblast differentiation process in osteosarcoma cells [86]. In cancer, the Wnt/β-catenin signaling pathway can promote epithelial-mesenchymal transition in cells via the canonical Wnt pathway responsible for β-catenin accumulation in the cytoplasm. Besides, β-catenin is translocated into the nucleus, where different interaction of β-catenin regulates the downstream gene expression. β-catenin also recruits transcriptional factor, Snail, that functions as a key promoter in epithelial-mesenchymal transition, downregulates epithelial gene, and upregulates mesenchymal genes. USP7 acts as a DUB for β-catenin and prevents its degradation by ubiquitin-proteasome in osteosarcoma cells [87,154,155].
The basic “helix-loop-helix” transcriptional factors inhibit differentiation, thereby sustaining stem cell fate, and are opposed by the ‘inhibitors of DNA binding.’ The ubiquitination and degradation of DNA binding inhibitors occur in differentiated tissues but appear to escape degradation in many neoplasms. A DUB enzyme, USP1, and the stability of stem cell-like property in osteosarcoma promote the protein stability of “inhibitors of DNA binding” by binding & deubiquitinating it [88]. In cancers, the function of NOTCH signaling may vary according to cell context as it can act as both a tumor suppressor and oncogenic. It works by reciprocal inhibition of two NOTCH downstream effectors: Deltex1 (a RING finger E3 ligase) and a NOTCH’s primary target, HES1 (a helix-loop-helix repressor family). Deltex1 shows an inhibitory effect on the NOTCH/HES1 pathway through binding with NOTCH intracellular domain, leading to ubiquitination and degradation of NOTCH receptors.
On the contrary, HES1 directly binds with the promotor of Deltex1 and causes transcriptional inhibition of Deltex1 [89]. HES1 promotes invasiveness and metastasis in vivo; therefore, an over-expression of HES1 and under-expression of Deltex1 are seen in osteosarcoma conditions. The overexpression of a highly regulated pathway like Hippo/YAP1 is closely related to tumorigenesis, regulated through the ubiquitin-proteasome pathway.
Ubiquitin-like protein, FAT10, initially acts as deubiquitin and protects the degradation of component YAP1 in the pathway, promoting aggressive growth of tumors in osteosarcomas [90]. The discovery of mdm2 acting like ligases has revealed regulations of many oncogene proteins in tumors. Its involvement has been seen in retinoic acid receptor alpha (RARα), which mediates all-trans retinoic acid biological effects (ATRA). Mdm2 leads to the degradation of RARα, thereby impairs ATRA-induced osteogenic differentiation in osteosarcoma cells [156]. USP39 is known to be a critical eukaryotic gene expression and classified under deubiquitinase. In research, USP39 knockdown has positively influenced cell apoptosis by arresting cell division at the G2/M phase via the p21 pathway. Due to its involvement in maintaining spindle checkpoints and supporting cytokinesis, its overexpression led to cells’ continuous growth [91]. Ligase TRIMs correlations are seen in osteosarcoma and TRIM46, TRIM21, TRIM14, and TRIM23 following the NF-κB pathway [92]. Other components concerned with osteosarcoma are reducing p53 and E-Cadherin, which are followed by TRIM59 and TRIM7 [93,94]. One of the important promoters of tumor growth in osteosarcoma is the PI3K/AKT pathway, and a lot of enzymes are known, involved in various cancer forms. USP22 and UBE2T are related to PI3K/AKT pathway and promote cell progression and invasiveness in osteosarcoma [95,96].
4.4. Lung Cancer
Lung cancer is the second most common cancer, with an estimated 1.6 million mortality rate each year. The cause of lung cancer may differ across the world, reflecting the geographical differences in air quality and tobacco use [157]. Most of the patients (approx. 85%) have histological subtype NSCLC (non-small cell lung cancer cells), among which lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) are the most common [158]. Zhao, X.C. et al., studied the ubiquitylation pathway’s involvement in modulating non-small cell lung cancer cell growth and found cell division cycle 34 (ubiquitin conjugating enzyme) essential candidates for cell. Cell division cycle 34 is increased in nearly 66% of non-small cell lung cancer cells in smoker patient tumor tissues than non-smoker patients [159]. TGF-β (which serves as a negative growth regulator), when modulated by inhibitory-Smads (Smad6 and Smad7), interrupts the receptor-mediated phosphorylation of Smad2/3 and represses the signaling pathway. Smad6 is seen to be overexpressed in tumor cells of lung cancers [160]. Smad ubiquitination regulatory factor 1 (Smurf1) is an E3 ubiquitin ligase that contains the WW domain, C2 domain, and HECT domain. In the study, it was observed that the Smurf1 positive non-small cell lung cancer patients have better chances at survival; therefore, the negative regulation of Smurf1 results in lung carcinogenic due to interruption in TGF-β signaling [161].
A PTEN gene, tumor suppressor, encodes a lipid phosphatase that dephosphorylates the secondary messenger phosphatidylinositol 3,4,5-triphosphate and opposes the action of PI3K (phosphatidylinositol 3-kinase). Hence, the increase and activating mutations in the PI3K gene or loss of PTEN gene leading to PI3K activation have been observed in non-small cell lung cancer cells. In lung cancer, NEDD4-1 as E3 ligase handles PTEN stability and gives a mechanism that contributes to the inactivation of the PTEN gene [97]. NEDD4 has also proved its oncogenic activity via EGFR in lung cancer cell lines [98]. The p16INK4a is a tumor suppressor, in which one protein is encoded by one of its locus INK4/ARF and is absent in various cancer, including non-small cell lung cancer cells. One of the mechanisms which silence the INK4/ARF is E3 ubiquitin ligase and transcriptional cofactor E6AP (UBE3A). E6AP induces the expression at the transcriptional level by restraining CDC6 transcription, a gene that encodes an essential repressor of the INK4/ARF locus [99]. E3 ligase HMG-CoA reductase degradation protein 1 (HRD1) deficiency induces Sirtuin2 upregulation. Sirtuin protein is responsible for the regulation of mitotic cell exits and, therefore, cell cycle maintenance. In lung cancer, the sirtuin2 expression is downregulated due to a negative correlation with HRD1 expression [100]. A deubiquitinating enzyme stabilizes the oncoprotein c-Myc (DUB) USP37 through direct binding and ceases its degradation. Clinically, USP 37 regulates cell proliferation and is upregulated in human lung cancer tissues, which are positively correlated with c-Myc protein expression [101]. Various evidence states that several TRIM (E3 ligase) act as regulators of NF-κB and are involved in ubiquitinating proteins at different NF-κB pathway steps. Usually, the canonical activation of NF-κB depends on IκB degradation, but some cases suggest that p65 protein degradation is required for the termination of NF-κB transduction; therefore, p65 may as well play a role in NF-κB signaling.
In non-small cell lung cancer cells, TRIM7 interacts with p65, thereby promoting its ubiquitin-mediated degradation, and therefore TRIM7 regulates NF-κB signaling [102]. Another TRIM acting like ligase which promotes proliferation through NF-κB is TRIM71. It is highly expressed in the non-small cell lung cancer cell line due to its involvement in reducing IκB [103]. In non-small cell lung cancer cells, an oncogenic role of the UBE2C [162] conjugating enzyme is observed. UBE2C can regulate gene expressions associated with apoptosis, angiogenesis, and tumor growth via the ERK pathway, according to array analysis. This enzyme was examined in a culture, and researchers discovered that it helps trigger apoptosis in cells by directly regulating phospho-ERK1/2 [104]. The conjugating enzyme has a specific role in p53 activation for oncogene triggering. It appears that overexpression of the enzyme UBE3C reduces the presence and function of AHNAK. Mechanistically, UBE3C interacts with AHNAK and disturbs its complex with p53, which blocks its inhibitory action on tumor growth, resulting in enhanced stemness [105] (Table 1). USP22 has been implicated in early-stage non-small cell lung cancer, but its mechanistic approach has not been identified yet. However, previous studies on other cancers suggest its role in the AKT pathway [107]. Many TRIMs have shown their oncogenic function in lung cancer involving TRIM 59. In the cell line culture of lung cancer, the absence of TRIM59 triggered cell cycle arrest at the G2 phase, which shows its tendency to increase cell cycle-related proteins in cancer. Earlier studies confirm that its proto-oncogenic role can be through multiple pathways other than p53 [106].
4.5. Liver Cancer
The sixth most frequently detected cancer worldwide is Liver cancer. Most liver cancers (approx. 75–-90%) are primarily hepatocellular carcinomas, malignant tumors in the liver’s parenchymal cells. The incidence of hepatocellular carcinomas is associated with chronic hepatitis B virus & hepatitis C virus infection and other factors like alcohol-related cirrhosis, smoking, etc. The other liver cancer type is intrahepatic cholangiocarcinoma, a tumor in cells lining bile ducts and can occur due to the high prevalence of chronic liver fluke infestation [163]. In hepatocellular carcinomas, the aberrant Wnt signaling and abnormal increases in β-catenin regulated by disheveled (Dsh/Dvl) mediators are responsible for tumor growth. Recently, Prickle-1 as a Dvl-associated protein has been identified in human hepatocellular carcinomas cells. A novel mechanism associated with Prickle-1 and Dvl3 in the Wnt/β-catenin pathway shows that Prickle-1 facilitates the degradation of Dvl3 via the ubiquitination pathway, thereby suppressing β-catenin activity and cell growth. Therefore, the under expressed Prickle-1 results in the accumulation of Dvl3 and β-catenin and larger tumor growth [108]. TRIM proteins act as an E3 ubiquitin ligase; also, the role of TRIM31 has been demonstrated lately. Its upstream expression is responsible for malignant behavior in hepatocellular carcinoma cells via the nTORC1 pathway. TRIM31 interacts with Tuberous sclerosis complex1 and Tuberous sclerosis complex 2 complex (an upstream suppressor for mTORC1 pathway) and mediates E3 ligase linked ubiquitination and degradation of the interacted complex [109].
Similarly, TRIM7 has shown its correlation with proto-oncogene Src, the nonreceptor tyrosine kinase family in a clinical specimen. The activation of Src triggers multiple cellular cascades PI3K and protein kinase B (PKB), mitogen-activated protein kinase, and signal transducer and activator of transcription 3, which are vital for cell survival. Therefore, TRIM7 and Src negative interrelation affect the course of action regarding hepatocarcinoma cell progression [110]. TRIM32 acts as an oncogene by downregulating the activity of apoptosis, cell cycle arrest, and senescence occurring due to stress in the first place. TRIM32 overexpression mediates p53-dependent activity to seize and promotes oncogenic transformation in p53-linked responses [111]. Yang, Y.F. et al., reported the oncogenic activity of TRIM65, which is regulated by HMGA1. Collectively, it exerts ubiquitylation of Axin1, which in turn activates and accumulates β-catenin in the cell [112]. In hepatocellular carcinoma samples, the upregulation of UBE2L3 is also suspected to be the reason for tumor progression. The raised UBE2L3 mediates the glyco3β degradation via proteasome-degradation, which blocks the activation of p65 in cellular signaling. The upregulated UBE2L3 activity is seen as a critical pro-tumorigenic factor in liver cancer [113]. UBE2T is generally aimed by miR-543, which is, however, low in hepatocellular carcinoma conditions. The UBE2T ectopic expression results in lowering p53, p21, and noxa facilitated by the ubiquitination and degradation of p53, overall, suggesting the role of UBE2T as a prognostic factor in hepatocellular carcinomas [114].
Some of the deubiquitinases play a novel role in average cell growth, and their irregulating factors lead to cell masses. A K-63 linkage-specific deubiquitinase, CYLD, is a necessary modifier of NF-κB signaling and controls the ubiquitination state of NF-κB activation factor NEMO. NF-κB is known to be a critical controlling molecule for apoptosis. As CYLD functions as a tumor suppressor, its downregulation leads to the degradation of IκB and activation of NF-κB, resulting in apoptotic resistance in tumor cells [115]. Deubiquitinase enzyme “UCHL1” presented a contradicting action in tumor cells, having oncogenic and suppressor effects. In hepatocellular carcinoma, the UCHL1 gene is related to the apoptosis feature in tumor cells. However, the overexpressed c-myc in tumor cells was observed to have aggressive tumor growth due to UCHL1 that caused apoptotic resistance in hepatocarcinoma cells [116,117]. A very common ligase, NEDD4 overexpression, is associated with tumor onset and hepatocellular carcinoma progression. It is suggested that NEDD4 oncogenic activity is due to its influence on the activation of the PTEN/PI3K/AKT signaling pathway as NEDD4 depletion affects the phosphorylation of AKT (Figure 6) [118]. A member of the ubiquitin-like modifier family, deubiquitin FAT10, was identified as the most upregulated gene in lung cancer. Yuan, R., et al., have reported the upregulation of FAT10 expression in 90% of hepatocellular carcinoma patients, though the exact mechanism is yet to be identified [119].
Figure 6.
This figure depicts the complex interconnection between various enzymes and the pathway enzymes follow. The enzymes modulate one or more oncogenic pathways through components actively functional in the process.
4.6. Cervical Cancer
Cervical cancer is the fourth most known female malignancy in the world, and approximately 90% of cervical cancer occur in low-income and middle-income countries where organized screening and HPV vaccination programs are lagging [164]. Tobacco smoking was found to be a paramount causative factor for cervical precancer and cancer in a cohort study on more than 300,000 women [165]. The most common histological subtypes are squamous cell carcinoma and adenocarcinoma that account for approximately 70% and 25% of all cervical cancers, respectively [164]. DNA damage due to internal and external factors results in DNA double-stranded break can give rise to an imbalanced cell process. The key to recognition, signaling, and repair of DNA (double-stranded break) is the MRE11-RAD50-NBS1 (MRN) complex and mediator of MDC1 (DNA damage checkpoint protein 1). USP7 interacts with the MRN-MDC1 complex and stabilizes MDC1. The accumulated complex leads to the recruitment of ub p53 binding protein 1 and BRCA1 (breast cancer protein 1) to DNA double-stranded break [120]. E2-EPF is a member of ubiquitin-conjugating enzymes and is overexpressed in cervical squamous cancer through its effect on the pVHL-HIF pathway. Hypoxia predisposes to high tumor metastasis by inducing hypoxia-inducible factors (HIF-1).
Studies have suggested E2-EPF role in stabilizing HIF-1α via selectively targeting pVHL in the normoxic situation, and the forced expression of E2-EPF speeds up tumor proliferation, metastasis, and invasion [121]. E3 ligase MARCH 7 acts as a tumor-promoting gene in human cervix cancer via interacting with VAV2, triggering the activation of CDC42 and RAC1, i.e., VAV1/RAC1/CDC42 pathway [122]. The low expression of “ovarian-tumor proteases deubiquitinase 5” is connected with metastatic nodes, tumor stages, and tumor subtypes, such as those associated with PI3K-AKT signaling, epithelial-mesenchymal transition, and hormones. The tumor activity of “ovarian-tumor proteases deubiquitinase 5” is also observed in cervical cancer and can be related to the same signaling pathways, one or all, as mentioned above [123]. Ubiquitin-like, containing PHD and RING finger domains 1, UHRF1 regulates the UBE2L6 (conjugating enzyme) gene, and the normal UHRF1 function is to restore the UbcH8-induced apoptosis. There is an increased level of UHRF1, which regulates UBE2L6 by promoting hypermethylation in cervix cancer cells [124]. In a study, the enzyme USP18 is observed to act as a promoter of cell proliferation and inhibitor of apoptosis in cervical cancer cells. It might have pro-proliferative and anti-apoptotic factors by regulating PI3K/AKT pathway as USP18 knockdown suppressed AKT phosphorylation in tumor cells [125]. Mechanistically, UBE3A function has been demonstrated in in vitro cells through its binding members, HPV18 E6 and E6 target protein p53, and the loss of either of them blocks the effect of UBE3A. The reduction in UBE3A increased ERK pathway signaling and a decrease in growth factor-mediated ERK activation. Therefore, UBE3A negates the activated p53 consequences on ERK signaling pathway [126]. The USP8 protein level is higher in cervical squamous carcinoma cells. It is believed that USP8 directly deubiquitylates and stabilizes the long isoform of FLICE like inhibitory protein (FLIPL) in the cervical cancer cell line. The final effect of FLIPL can be stated as a cell death receptor mediating cell apoptosis. So, one can conclude that the USP8-overexpressing cells have suppressed the apoptotic pathway. However, previous studies on USP8 suggest its tumor effect via stabilizing EGFR signaling pathway [127].
TRIM 24 has a positive role in the progression of growth in tumor cells of various cancers. The accumulated TRIM24 activates the NF-κB/AKT pathway and thereby regulates cyclin D activity in the cell cycle in cervical cancer cell lines [128]. Cell cycle proteins are also regulated by TRIM59 ligase leading to tumor progression and growth. However, studies depict its inculcating nature in the p53 pathway and the activation of Ras/Rad, which triggers an oncogenic protein ERK signaling pathway. Knockdown of TRIM59 in cells inhibited cell progression by causing cell cycle arrest at phase S, and both the mentioned pathways are associated with the cell cycle arrest [129]. Exceptionally, a few TRIM members like TRIM3 show opposite activity towards growth property in tumor cells. TRIM3 actively enhances Caspase 3 and p53 activity and negatively affects the p38 pathway, responsible for cervical cancer [130].
4.7. Leukemia
Leukemia is a malignancy (cancer) of blood cells, and there are two types—acute and chronic; further distinguished based on cells. Acute myeloid leukemia is characterized by an increase in myeloid cells in the marrow and maturation stage arrest. The US’s annual incidence is approximately 2.4 per 100,000, which increases progressively with age having 5 years life expectancy of less than 15% [166]. Acute lymphoblastic leukemia encompasses a group of lymphoid neoplasms that morphologically and immunophenotypically are similar to B-lineage and T-lineage precursor cells representing 75% of acute leukemias [167]. Chronic myeloid leukemia is associated with a specific genetic lesion, the Philadelphia chromosome, which harbors BCR-ABL oncogene [168]. In leukemia cells, the increased mass of ubiquitinated protein is certainly not due to imbalanced degradation of ubiquitinated protein but because of elevated activity in the ubiquitination pathway [169]. The UBA2-WTIP fusion gene is suggested to be an oncogenic fusion gene that contains the N-terminus E1 enzyme member, VAE ubiquitin-like domains of UBA2, and the C-terminus LIM domains of WTIP. Mechanistically, the UBA2-WTIP fusion induces phosphorylation of ERK1/2, STAT3, and STAT5 and repels WTIP-induced mammalian processing body formation [131].
UBE2Q2 and CDC34 are E2 enzymes; the involvement of the components of the ubiquitin-proteasome pathway in leukemia has been known in studies. UBE2Q2 activity is significantly upregulated in acute lymphoblastic leukemia compared to normal tissue. Additionally, part of the pathway remains unknown [132]. CDC34 is also overexpressed in pediatric acute lymphoblastic leukemia. CDC34 is known to be a cell cycle regulator by its complex form with SCF and degrades IκB protein; therefore, hypothetically, this can cause CDC34 activity [170]. The UBE2E1 expression is adversely related to acute myeloid leukemia lifespan, though its role is unclear. UBE2E1 links with HOX gene regulation for its prognostic role in acute myeloid leukemia because HOX gene (HOXA9 and HOXA10) promotes acute myeloid leukemia leukemogenesis [133]. Expression of Fbxw7, a subunit of SCF ubiquitin ligase complex, is essential for regulating the threshold of c-Myc in favor of leukemia-initiating cells in chronic myeloid leukemia. The mutated Fbxw7 can activate c-Myc, a protein thought to be connected with Notch1, which, in turn, activates a group of genes necessary for the transformation to leukemia [134]. ARIH2 gene encodes an anti-proliferative E3 ubiquitin ligase, Triad1, which has a role in leukemogenesis, is induced by M11-E11 (MLL1 fusion protein) in acute myeloid leukemia. The presence of HOX genes affects the expression of Triad1 in M11-E11+ cells; therefore, Triad1 activity is regulated by HOX genes [135]. The histone H2B E3 ligase RNF20 is an additional chromatin regulator necessary for mixed-lineage leukemia fusion mediated leukemogenesis. When the transcriptional regulators are disrupted, mixed-lineage leukemia-fusion proteins modify gene expression in hematopoietic cells via interacting with histone H3 lys79 (H3K79) methyltransferase DOT1L. To balance the local levels of H3K79 methylation by DOT1L, RNF20 is required [136]. A role of deubiquitinase, USP7, is observed to be upregulated in human T-acute lymphoblastic leukemia cell lines and patient samples. USP7 can stabilize NOTCH1 protein level in in vitro and in vivo records, and the interaction is supported by the ubiquitin-like and MATH domains of USP7 [137].
An in vivo study was conducted for an enzyme USP22 to identify its response in the leukemia model. It was observed that USP22 promotes glioma tumorigenesis through deubiquitinating BMI1, which serves to trigger its oncogenic action in cells [138]. NOTCH and β-catenin signaling, additionally with an increase in glycogen synthase kinase 3β pathway, can lower the expression of ligase enzyme TRIM62. Loss of TRIM62 showed progression in tumorigenesis in cells due to various oncogenic proteins in cells. A low level of p53 and a high level of mdm2 also affect the functioning of TRIM62 in cells [139]. Multiple enzymes in different types of cancer are described above in the review. However, it is not right to say if the ubiquitin-proteasome pathway only exhibits an oncogenic effect in the cell. These enzymes are present in abundance in every part of the body and have numerous effects. They act upon multiple pathways, but these enzymes’ central pathway to give an oncogenic effect is considered the mode of action. These enzymes regulate the proper functioning of cellular activity via targeting and degrading abnormal proteins in normal conditions. The modified transcriptional factors in these enzymes give rise to their altered expression, and the above collected data reveals how these modifications progress into cancer. Exploring ubiquitin-proteasome enzymes have a pursuit towards developmental in cancer studies.
5. Preclinical Studies
5.1. In Vivo Studies
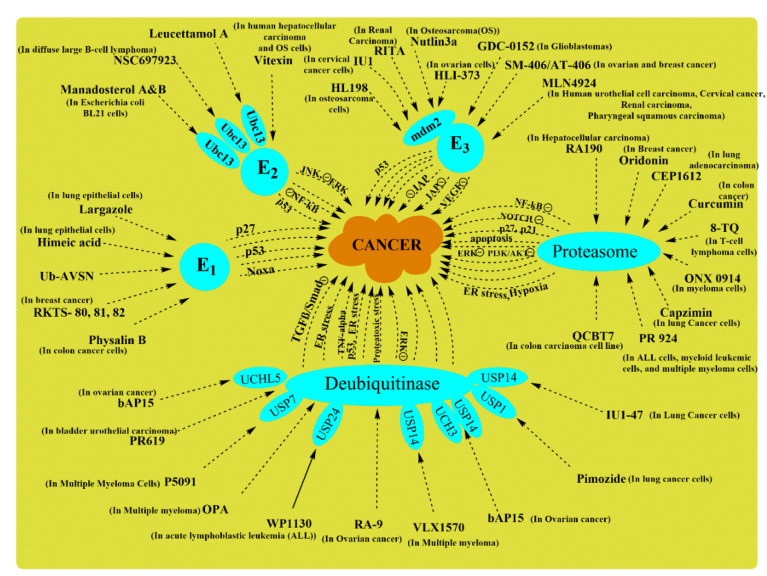
Various studies have been done on ubiquitin-proteasome inhibitors, which support the suppression of tumor factors in different signaling pathways. In the given table, preclinical in vitro and in vivo studies of various inhibitors in different cancers are discussed (Table 2).
Table 2.
In vivo studies of the ubiquitin inhibitors supporting the suppression of tumors.
| S. No. | Drug | Cancer | Signaling Pathway | Animal Models | Reference |
|---|---|---|---|---|---|
| 1. | RNF152 | Colorectal cancer | It is inactivating mTORC1 to induce autophagy and apoptotic cell death. | Immunodeficient nude mice | [171] |
| 2. | RITA (2,5-bis[5-hydroxymethyl2-thienyl] furan, NSC 652287) | Renal carcinoma | Block TP53–mdm2 complex and reactivation of p53 and Induction of Tumor cell Apoptosis | Mouse xenograft model | [172] |
| 3. | RA-9 | Ovarian cancer | Apoptosis and proteotoxic stress | Mice xenograft model | [173] |
| 4. | WP1130 | T-cell acute lymphoblastic leukemia | Induces apoptosis by accelerating the collapse of mitochondrial transmembrane potential via USP24-Mcl-1 axis | Tumor xenografts in NOD-SCID mice | [174] |
| 5. | The bis-benzylidine piperidone RA190 | Hepatocellular carcinoma | Nuclear factor κB (NF-κB) signaling | Male nude mice CAnN.Cg-Foxn1nu/CrlNarl | [175] |
| 6. | O-phenanthroline (OPA) | Multiple myeloma | Caspase cascade and endoplasmic stress response signaling | Murine xenograft model of human MM | [176] |
| 7. | Nutlin-3a | Osteosarcoma | Competitively binds the mdm2-p53 interacting site, activates p53 pathway | Human xenograft OS animal model with SAOS-2, U2OS, MG63 cell lines in SCID mice | [177] |
| 8. | GDC-0152 | Glioblastomas | Antagonists of the inhibitor of IAPs Postponed tumor formation and slowed down tumor growth |
U87MG- iRFP cell grafted mice | [178] |
| 9. | SM-406/AT-406 | Human cancer cell (ovarian and breast cancer) | Antagonizes XIAP BIR3 induces rapid degradation of cellular cIAP1 protein | SCID mice bearing MDA-MB-231 xenograft tumors | [179] |
| 10. | Oridonin | Breast cancer | Tumor suppressive effect via inhibiting Notch receptors expression | Male BALB/C athymic nude mice | [180] |
| 11. | MLN4924 | Human urothelial cell carcinoma, cervical cancer, renal carcinoma, pharyngeal squamous carcinoma |
Inhibits cell viability and induced apoptosis in HUVECs (human umbilical vascular endothelial cells) | Xenograft SCID mice | [181] |
| 12. | P5091 | Colorectal cancer | Elevated mRNA level of IFN-γ and TNF-α | Female BALB/c mice (CT26 xenograft model) | [182] |
| 13. | bAP15 | Ovarian cancer | Regulating TGF-β signaling, dephosphorylating Smad2, inducing apoptosis | Mice xenograft models of SKOV3 | [183] |
| 14. | PR-619 | Bladder urothelial carcinoma (UC) | Suppression of the Bcl-2 level | Nude mice Xenograft Matrigel culture | [184] |
| 15. | CEP1612 [phthalimide-(CH2)8CH-(cyclopentyl) CO-Arg(NO2)-Leu-H] | Human lung adenocarcinoma | Accumulation of p21WAF1 and p27KIP1, inducing apoptosis | A-549 tumor-bearing nude mice | [185] |
| 16. | Curcumin | Human colon cancer | Inhibit the proteasome and induce apoptosis | HCT-116 tumor-bearing ICR SCID mice | [186] |
| 17. | P5091 | Multiple Myeloma Cells | Inhibited USP7 activity, decreased HDM2, and increased p21 levels, induces apoptosis | Human plasmacytoma xenograft and SCID-hu mouse models | [155] |
| 18. | ECRG4 | Esophageal cancer | Inhibits NF-κB expression and nuclear translocation, attenuates NF-κB target gene COX-2 expression | BALB/c nude mice | [187] |
| 19. | 8-(tosylamino) quinoline (8-TQ) | Human cancer cells | Inhibition of molecular signaling machineries composed of phosphoinositide 3-kinase (PI3K)/phosphoinositide-dependent kinase-1 (PDK1)/Akt and extracellular-signal-regulated kinase (ERK) | murine T-cell lymphoma RMA cells in mice | [188] |
| 20. | VLX1570 | Multiple myeloma | Decrease in ERK phosphorylation; USP14 inhibitor | Xenograft model in immunocompromised mice | [189] |
| 21. | b-AP15 | Large B cell lymphoma | Inhibits Wnt/β-catenin and TGFβ/Smad pathways; USP14 and UCHL5 deubiquitinases | Mouse xenograft models of SU-DHL-4 and SU-DHL-2 cells | [190] |
5.2. In Vitro Studies
Preclinical studies start with understanding the impact of the drug on cell lines. Due to these cell line cultures, most animal lives are spared to understand its toxicity on body cells (Table 3).
Table 3.
In vitro studies of the ubiquitin inhibitors supporting the suppression of tumors.
| S. No. | Drugs | Category | Cell Lines | Reference |
|---|---|---|---|---|
| 1. | Largazole | Ubiquitin activating enzyme (UAE) inhibitor | Kip16, a GFP-p27 expressing Cell Line | [191] |
| 2. | Himeic acid A | UAE inhibitor | Western blotting with anti-Flag antibody | [192,193] |
| 3. | Ub-vinylsulfonamide (Ub-AVSN) | UAE inhibitor | N597A variant and the WT assay | [194,195] |
| 4. | Pimozide or GW7647 | USP1/UAF1 inhibitor | H596 and H460 cell lines | [196] |
| 5. | Leucettamol A | Ubc13-Uev1A inhibitor | Escherichia coli BL21 cells | [197] |
| 6. | NSC697923 | Ubc13-Uev1A inhibitor | ABC (activated B cell-like)-DLBCL cells and GCB (germinal center B cell-like)-DLBCL cells | [198] |
| 7. | Manadosterols A and B | Ubc13-Uev1A inhibitor | Escherichia coli BL21 cells | [199] |
| 8. | Vitexin | ubiquitin-conjugating enzyme E2-25K inhibitor | Rat pheochromocytoma PC12 cells, HepG2 (human hepatocellular carcinoma), and HOS (human osteosarcoma) cells | [200] |
| 9. | HLI98 family (C, D, E) | Ubiquitin ligase enzyme inhibitor | SAOS cells | [201] |
| 10. | RKTS-80, -81, -82 | E1 inhibitors | human breast cancer MCF-7 cells | [202] |
| 11. | Physalin B | proteasome inhibitors | human DLD-1 colon cancer cells | [203] |
| 12. | HLI-373 | E3 ligase inhibitor | ovarian SKOV3 cells | [204] |
| 13. | ONX-0914 | Immunoproteasome inhibitors | KMS-11 cells | [205] |
| 14. | PR-924 | Immunoproteasome inhibitor | Human T-cell ALL CCRF-CEM cells, human myeloid leukemic THP1 cells, and human multiple myeloma RPMI-8226 cells | [206] |
| 15. | Capzimin | Proteasome inhibitor | HCT116 cell lines | [207] |
| 16. | QCBT7 | Proteasome inhibitor | colon carcinoma cell line HCT 116. | [208] |
| 17. | IU1-47 | USP14 inhibitor | A549 and H1299 cell lines | [209] |
| 18. | IU1 | USP14 selective inhibitor | HeLa and SiHa cells (cervical cancer cells) | [210] |
6. Clinical Trial Drugs with Ongoing Stage and Category
There are several drugs that are conducted in a better way to prevent cancer. Lists of such drugs namely TAK-243, Disulfiram and Cooper, KPG-818, Vorinostat and Bortizomib, MLN4924, Bortezomib + Doxorubicin, NPI-0052, NPI-0052 + Vorinostat, JNJ-26854165, Bbortezomib (PS-341), TAK-981 + Pembrolizumab, Oprozomib, Carfilzomib, MLN9708, MLN9708 + Vorinostat, GSK2110183, Trastuzumab and PS-341, Finasteride have been summarized here in this Table 4.
Table 4.
Clinical status of the drugs.
| S. No. | Drug in Clinical Trials | Category | Cancer | Phase | NCT Number |
|---|---|---|---|---|---|
| 1. | TAK-243 (formerly known as MLN7243) | UAE (ubiquitin-activating enzyme) inhibitor | Advanced Malignant Solid tumors | Phase I (terminated) | NCT02045095 |
| 2. | Disulfiram and Cooper | Zinc fingers and RING-finger ubiquitin E3 ligases inhibitors | Breast Neoplasm Female, Metastatic Breast Cancer | Phase 2 | NCT03323346 |
| 3. | KPG-818 | Ubiquitin ligase modulator | Selected hematological malignancies (multiple myeloma, mantle cell lymphoma, follicular lymphoma, diffuse large B-cell lymphoma, indolent lymphoma, adult T-cell leukemia-lymphoma, or chronic lymphocytic leukemia | Phase 1 | NCT04283097 |
| 4. | Vorinostat (MK-0683) + Bortezomib | HDAC (Histone deacetylases) inhibitors + proteasome inhibitor | Multiple Myeloma | Phase 3 | NCT00773747 |
| 5. | MLN4924 | Nedd8 activating enzyme inhibitor | lymphoma or multiple myeloma | Phase 1 (completed) | NCT00722488 |
| 6. | Bortezomib + Doxorubicin | Proteasome inhibitor | Advanced, Recurrent, or Metastatic Adenoid Cystic Carcinoma of the Head and Neck | Phase 2 (completed) | NCT00077428 |
| 7. | NPI-0052 | Proteasome inhibitor | Solid tumors, lymphomas, leukemias, and multiple myeloma. | Phase 1 (completed) | NCT00629473 |
| 8. | Marizomib(NPI-0052) + Vorinostat | Proteasome inhibitor + HDAC (Histone deacetylases) inhibitors | Non-Small Cell Lung cancer, Pancreatic cancer, Melanoma, Lymphoma Multiple Myeloma | Phase 1 (completed) | NCT00667082 |
| 9. | JNJ-26854165 | E3 ligase inhibitors | Neoplasms | Phase 1 (completed) | NCT00676910 |
| 10. | Bortezomib (PS-341) | proteasome inhibitor | Squamous cell carcinomas of the head and neck (SCCHN) | Phase 1 (completed) | NCT00011778 |
| 11. | TAK-981 + Pembrolizumab | SUMOylation inhibitor + immunosuppressant | Advanced or Metastatic Solid tumors | Phase 1 Phase 2 |
NCT04381650 |
| 12. | Oprozomib | Proteasome inhibitor | Advanced Refractory or Recurrent Solid tumors | Phase 1 (completed) | NCT01129349 |
| 13. | Carfilzomib | Proteasome inhibitor | Neuroendocrine cancer | Phase 2 | NCT02318784 |
| 14. | MLN9708 | Proteasome inhibitor | Advanced non-hematologic malignancies | Phase 1 (completed) | NCT00830869 |
| 15. | MLN9708 + Vorinostat | Proteasome inhibitor + HDAC inhibitor | Advanced p53 Mutant malignancies | Phase 1 | NCT02042989 |
| 16. | GSK2110183 | Proteasome inhibitor | Multiple myeloma | Phase 1 (completed) | NCT01445587 |
| 17. | Trastuzumab + PS-341 | Proteasome inhibitor | Breast cancer, Stage 4 | Phase 1 (completed) | NCT00199212 |
| 18. | Finasteride | Ubiquitin-conjugating enzyme inhibitor | Adenocarcinoma of the ProstateStage II Prostate cancer | Phase 2 | NCT00438464 |
7. Cancer Therapeutic Strategy via Targeting UPS
Instability in signaling pathways and their components can cause dysregulation in many intracellular processes that result in malignancies since ubiquitin-proteasome systems are crucial to almost every physiological function in an organism. A breakdown in their signaling results in serious diseases [211]. Ubiquitin proteasome system regulates the cell cycle, and abnormalities in its activity lead to oncogenesis as overexpression or down-regulation of ubiquitin-proteasome system components can lead to critical cellular phenotypes. Various studies on ubiquitin-proteasome systems have recently been published, suggesting its involvement in cellular function and the possibility of targeting ubiquitin-proteasome system components for novel anti-cancer agents [212]. Many discoveries have come across drugs showing inhibitory action on a particular enzyme/proteasome for a potential neoplastic agent. Substantial evidence on the relationship between enzymes with their signaling pathway has been proved. Therefore, it will ultimately suppress its role in the pathway if the enzyme is inhibited, let it be oncogenic or non-oncogenic. In this section of the review, drugs with inhibitory action on the ubiquitin-proteasome system have been discussed with their effect on the connected signaling pathway.
7.1. E1 Ubiquitin-Activating Enzyme Inhibitors
Ubiquitination is an essential modification system for various cellular proteins. The first step in the ubiquitin conjugation system is ubiquitin’s activation to high energy intermediate, catalyzed by E1 (the ubiquitin-activating enzyme). The core of the ubiquitin-proteasome system will halt by simply inhibiting the E1 enzyme. Yang, Y. et al., discovered a drug with the chemical formula “4[4-(5-nitro-furan-2- yl-methylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester” and named it PYR-41 (Figure 7). It was examined in vitro to know its focus on the particular enzyme when it suppressed ubiquitination function and reported it as a first cell-permeable E1 inhibitor. The pyrazone derivative PYR-41 weakens the activation of NF-κB activation through enhancing IκB activation. PYR-41 also prevents the degradation of p53, which gives a transcriptional signal for cell death.
Figure 7.
This figure represents the UPP and their respective enzymes i.e., Nedd4, MuRF1& Atrogin, UBE3A, CHIP & mdm2, HUWE1, RNF122 & FBXO33, WWWP2, MuRF1 & MAFbx IN Different diseases.
Moreover, this drug has the potential to kill mutated p53-expressing cells as well [213]. The research reported Largazole and its analog selectively inhibit E1 activity in vitro by blocking the adenylation-activation step without disturbing ubiquitin transfer from E1 to E2. Largazole in cell culture affects multiple pathways other than inhibiting activating enzymes as it also inhibits proteasome activity. It is involved in preventing the degradation of an essential anti-oncogenic factor, p53, and inhibits cdk activity, enhancing p53 efforts in cell death [191].
In cell lines and primary samples of acute myeloid leukemia, TAK-243 can trigger cell death and downregulate clonogenic growth. Barghout, S.H. et al., evaluated a first-in-class UBA1 inhibitor TAK-243 in acute myeloid leukemia preclinical models and also supported TAK-243 for a clinical trial in their patients. In acute myeloid leukemia cells, TAK-243 was seen to attach preferentially with UBA1 over the similar enzymes, UBA2, UBA3, and UBA6 [214]. TAK-243 decreases the level of ubiquitin-protein conjugates and stabilizes short-lived proteins such as p53, myeloid cell leukemia-1, c-Myc, etc. Notably, TAK-243 has overcome resistance to conventional drugs, including bortezomib and carfilzomib resistance, in cell-line models. It also showed effectiveness against primary cells from refractory/relapsed myeloma patients [215]. TAK-243 is also known under names pevonedistat, MLN7243, and MLN4924 in Phase I/II clinical trials [216]. Another Uba1 inhibitor, PYZD-4409, is structurally similar to PYR-41 and induces cell death predominantly in primary patient samples and hematological cell lines over normal hematopoietic cells. PYZD induced cell death is associated with endoplasmic reticulum stress and stabilization of cyclin D3 along with p53. It is important to note that the intraperitoneal injection of PYZD-4409 in leukemic mouse decreased the tumor weight efficiently while comparing its untoward side effects [169,217,218]. Panepophenanthrin has been claimed in research that it is the first inhibitor of the ubiquitination pathway extracted from mushroom strain IFO8994 [219]. It belongs to an epoxyquinoid class of natural products; its unique structure shows its potential for oxygen functionalization. Future research might provide more information on its mechanistic approach in showing anti-oncogenic effects [220]. A new strain was found while investigating panepophenanthrin for its biological derivatives responsible for its impact on ubiquitination mechanism in vivo and in vitro. Matsuzawa, M., 2006 discovered new derivatives, RKTS-80, -81, and -82, as E1 inhibitors when tested on human breast cancer MCF-7 cells which efficiently in a dose-dependent manner blocked the cell growth. Unfortunately, other than being cell-permeable, no other property for these new derivatives is known [202].
7.2. E2 Ubiquitin-Conjugating Enzyme Inhibitors
After the ATP-dependent activation of ubiquitin via the ubiquitin-activating enzyme, ubiquitin is next transferred to a specific cysteine residue on any of the various ubiquitin-conjugating enzymes called E2 proteins. Therefore, if this component of the ubiquitin-proteasome system is inhibited, it will ultimately obstruct further process steps. Previously, a drug Bay 11-7082 was reported to have an inhibitory activity on NF-κB and induces apoptosis in human T-cell lymphotropic virus-induced negative T-cells by downregulating the antiapoptotic gene (Bcl-xL) expression [221]. But in recent studies, it has been reported that Bay 11-7082 does not affect NF-κB; instead, it inhibits Ubc 12 & UbcH7 and suppresses the activation of LPS (lipopolysaccharide)-stimulated RAW macrophages, with another inflammatory pathway [222]. As lipopolysaccharides induce activation of the noncanonical NF-κB signaling pathway, Bay 11-7082 can show an anti-tumor role in cancer via inhibiting Ubc 12 (have a role in ovarian cancer) and UbcH7 (DSB regulator). NSC697923 is a UBE2N inhibitor that can exhibit a cytotoxic effect on neuroblastoma cell lines, evidenced by its ability to induce p53-induced apoptosis. Even in mutant p53 cells, NSC697923 has shown anti-tumor response via activating the JNK pathway [223]. In diffuse large B-cell lymphoma, NSC697923 impeded the activity of Ubc13-Uev1A as it suppresses NF-κB signaling activity inactivated and germinal center B-cell-like large diffuse B-cell lymphoma cells [198].
CC0651 is a reversible small-molecule inhibitor of Ube2R1, and molecular studies revealed its binding with allosteric mode (away from the active site). Some E2 families use its backside (surface opposite to catalytic site) interaction to enhance, inhibit or modulate catalytic activity [224]. CC0651 has been identified as an inhibitor of the human CDC34 ubiquitin-conjugating enzyme. The inhibitory activity of CC0651 is concerned with its interference in the step “removal of Ub” to acceptor Lys residues; however, it plays no part in interaction with E1 or E3 or in Ub thioester bond formation [225,226]. TZ9 (Twelve triazines) and 4-amino- N ′-phenyl-6-(arylamino)-1,3,5-triazine-2-carbohydrazides are reported to be Rad6B- inhibitor as diaminotriazinylmethyl benzoate anti-cancer agents. Similar to these, series of N′-phenyl-4,6- bis(arylamino)-1,3,5-triazine-2-carbohydrazides (6a–e) and 4-amino-6-(arylamino)-1,3,5-triazine-2-carbohydrazides (3a–e) have been synthesized and abbreviated as ‘new triazines.’ In the absence of E3 ligases, Rad6B can ubiquitinate not only histones but also ubiquitinate β-catenin. A ubiquitinated β-catenin is insensitive to 26S proteasome, indicating that Rad6B is essential for β-catenin stabilization and activation in breast cancer [227]. These new triazines inhibit Rad6B ubiquitin conjugation and have shown anticancer action against many human cancer cell lines [228].
7.3. E3 Ubiquitin Ligase Inhibitors
When the polyubiquitinated chain is formed in the ubiquitination process, the chain is attached with substrate protein with E3 ligase. E3 ubiquitin ligase transfers substrate protein on the ubiquitin chain for the attachment, which takes it to the proteasome; thus, E3 enzyme inhibitors may protect the protein from degradation. Gene p53 acts as a tumor suppressor, but its interaction with ligases mdm2 suppresses p53 activity, resulting in tumor formation. This interaction between mdm2 with p53 can be targeted for a therapeutic approach, and one of the selective E3 ligase targeting molecules is called Nutlins. This cis-imidazoline analog interacts with the p53 binding site instead of mdm2, leading to p53 stabilization, cell cycle arrest, and ultimately apoptosis in cancer cells [229]. A highly potent drug, RG7112, is a small molecule inhibitor that selectively targets structures found within the p53 binding site of mdm2 [230]. Cullin-RING ubiquitin ligases (CRLs) are the most prominent family of E3 ligases, and it requires cullin neddylation for their activation.
Ligase NEDD activity has been observed in various cancers mentioned above. The NEDD8- activating enzyme is inhibited by the MLN4924 drug, which blocks cullin neddylation and, therefore, inactivates CRLs and promotes apoptosis. In ovarian cell lines, reducing CRL4 components (Roc1/2, DDB1, and Cul4a) has also shown an inhibitory effect on tumor cells, similar to the MLN4924 effect. Therefore, the tumor-suppressing effect of CRL4 is suggested to contribute to the chemotherapeutic effect of MLN4924 in ovarian tumor cells [231]. The mdm2 protein contains a C-terminal RING domain which coordinates two Zn atoms responsible for p53 nuclear export and degradation via proteasome machinery. In colon and breast cancer cells, the critical role of Zn was exhibited in the reactivation of p53 when MI-219 ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)) was used. However, the activity of mdm2 inhibitor (apoptosis and colony formation), namely, MI-219, was enhanced under the influence of ZnCl2 [232]. A small-molecule TAME (tosyl-L-arginine methyl ester), belonging to a microtubule inhibitor, induces mitotic arrest and inhibits the ubiquitin ligase function of APC, i.e., This anaphase-promoting complex is usually required for mitotic exit. The mitotic arrest and anaphase-promoting complex inhibition are a response to the activation of the spindle assembly checkpoint. It is given in the form of a cell-permeable derivative- proTAME. The antagonism action of both the factors, i.e., anaphase-promoting complex, and spindle assembly checkpoint, is responsible for amplifying TAME’s influence on cells [233]. Mdm2 ligase activity is also down-regulated by treating a small molecule inhibitor drug (JNJ-26854164) as the influence of mdm2 is reversed on p53, which induces apoptosis in cells [234]. Other than p53 mediated antitumor activity of mdm2 also influences pathways like TGF-β.
In ovarian cells, E3 ligase inhibitor (HLI-373) targets the C-terminus of mdm2 and impairs TGF-β promoted epithelial-mesenchymal transition via β-smad-snail/slug pathway [204,235]. Thalidomide was previously used in morning sickness and is nowadays used for treating multiple myelomas. The primary target of thalidomide is cereblon which forms an E3 ligase complex with disrupted DNA-binding protein 1 and cullin-4A. The drug selectively binds with cereblon, thereby, inhibit its associated ubiquitin ligase activity [236,237]. Another mdm2 antagonist, MI-312 with cisplatin combined treatment, act synergistically, thereby suppressing cell growth and inducing apoptosis. The combination activates pathways that are downstream of p73 involving the necessary cell cycle modulator p21WAF1, showing its p53-independent mode of action [238].
8. Proteasome Inhibitor
Ubiquitination is found to target proteins for proteasomal degradation explained above with sequence of action and its composition. The proteasome is believed to have different subunits in its 20S core particle: caspase-like, trypsin-like, chymotrypsin-like [239]. Bortezomib which is widely used for treating multiple myeloma is known to have proteasome inhibiting activity. Other commonly used proteasome inhibitors are ixazomib and carfilzomib. Recent reports suggest that their concentration highly influences their activity at low concentrations. These inhibitors affect the chymotrypsin-like proteasome activity, inhibiting caspase-like activity at high concentrations [240,241]. Through extensive research with their known properties, they can be used against various cancer growths. In in vitro research on the cytotoxic effects of epoxomicin, it was found that it was a potent inhibitor of the proteasome’s chymotrypsin-like activity. Previously, it was suggested to have an inhibitory effect on NF-κB activation in cancerous cell line culture [242]. However, in amelanotic melanoma cells, it exhibits anti-apoptotic activity in the mitochondrial pathway by accumulating the noxa proapoptotic factor and anti-apoptotic Mcl-1. Epoxomicin inactivates caspase, which causes an increase in cdk inhibitors, i.e., p21Cip1/Waf1 and p27Kip [243].
The upregulated levels of cdk inhibitors are also seen in leukemia cell lines when treated with a second-generation drug, carfilzomib, for hematological malignancies. Carfilzomib is a tetrapeptide epoxyketone proteasome inhibitor, and extensive research has shown that it induces cell cycle arrest in the G2/M phase by inhibiting cyclin-dependent kinase 1 [244]. A proteasome inhibitor delanzomib (otherwise called CEP-18770), has shown promising anti-myeloma property in preclinical studies. It is a reversible P2 threonine boronic acid inhibitor, binds with β5/β1 proteasome subunits inhibiting chymotrypsin-like (β5) and caspase-like (β1) activity of proteasome [245]. A new proteasome inhibitor, QCBT7 (aquinolin-chlorobenzothioate 7), showed its cytotoxic effect in multiple cancer cell lines. It is a stable derivative of quinoline-8-thiol, which attacks the regulatory subunit instead of targeting the proteasome’s catalytic subunit. Overall, its anti-tumor activity is manifested by inducing proteasome inhibition, endoplasmic reticulum stress, hypoxic response, and glycolysis, resulting in cell death. A potential biomarker of proteasome inhibitor was discovered in the same study named PFKPB4 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4) can be used for monitoring drugs therapeutic response in pancreatic cancer [208]. A novel proteasome inhibitor, physalin B, was isolated as interfering in the ubiquitin-proteasome pathway. However, when more information was obtained, physalin B impairs proteasome function in the ubiquitin-proteasome pathway. However, it was found to impair proteasome function in the ubiquitin-proteasome pathway when more information was obtained. It promotes apoptosis in a cell by inducing pro-apoptotic NOXA, a feature of the ubiquitin-proteasome pathway [203].
9. Conclusions and Future Perspective
Ubiquitin proteasome pathway regulates uncountable body functioning through its network of enzymes spread all over the body. It is connected like a thread sewed in a cloth with many transduction process pathways, including cellular signalling pathways for cell differentiation and division. Therefore, UPP’s role in diseases like cancers is explained in various researches, and the disrupted expression of specific enzymes of the pathway responsible for cancer needs immense study. For example, the enzyme UBE2T in colorectal cancer has shown its oncogenic role in cells. It has shown its relation with p53, pentose phosphate pathway, and other pathways, but its exact role in the pathway is still unknown. However, if this enzyme is studied in other cancer types, it might indicate its pathways. Molecular mechanisms associated with metabolic reprogramming in cancer have been studied extensively over the last few decades. The roles of ubiquitination and deubiquitination as cancer modulators are highlighted in this review.
As discussed in the review, deubiquitinase enzymes also have a characteristic property of preventing protein degradation by proteasome via attaching with the substrate protein and blocking its link with E3 ligase. In the process, it might save a pro-oncogenic protein like UCHL1, which has been observed to be over-expressed in the hepatocarcinoma state. The connection between these enzymes and cancer can be used as a therapeutic strategy. Some inhibitors of enzymes like ligase inhibitors, deubiquitinase inhibitors, proteasome inhibitors are already under clinical trial. It might result in offering a suitable drug for curing this dreadful disease. In clinical trials, combination treatments are being encouraged like bortezomib + doxorubicin for a successful synergistic approach against cancer cells. Overall, due to the vast availability and interlink of these UPP enzymes involved in oncogenic activity, they can be targeted for a favorable outcome to treat cancer. To summarise, if the mechanism of UPP dysfunction and the precise function of enzymes are fully explored, it may pave the way for treating a variety of diseases other than cancer. Drugs like vitexin (a platelet aggregation inhibitor) efficiency have been checked on multiple cancer cultures but have no details about its effect on animals.
Abbreviations
| BMP-2 | Bone morphogenetic protein-2 |
| BTRC | β-transducin repeat containing |
| Cdc20 | Cell division cycle 20 |
| DDR | DNA damage response |
| DSBs | DNA double-strand breaks |
| FBA | F-box associated region |
| FBW7 | F box/WD repeat-containing protein 7 |
| FOXN | A member of the Forkhead box transcription factors |
| MARCH8 | Membrane-associated RING-CH |
| NAE | Neddylation activation enzyme |
| NAE1 | NEDD8 activating enzyme E1 |
| NEDD4L | Neural precursor cell expressed, developmentally down-regulated 4-like |
| OTUB | Ovarian tumor domain-containing ubiquitin aldehyde binding protein |
| Pirh2 | p53-Induced ring-H2 protein |
| PPAR | Peroxisome proliferator-activated receptors |
| TSC | Tuberous sclerosis complex |
| UBCH | Ubiquitin-conjugating enzyme H |
| Ube | Ubiquitin-conjugating enzyme |
| UCH | Ubiquitin carboxyl-terminal hydrolase |
| UCP | Ubiquitin carrier protein |
| UHRF | Ubiquitin-like with plant homeodomain and ring finger domains |
| UPP | Ubiquitin proteasome pathway |
| UPS | Ubiquitin proteasome system |
| USP | Ubiquitin specific peptidase |
| VHL | Von Hippel-Lindau |
| β-Trcp | β-transducin repeat-containing protein |
Author Contributions
Conceived and designed the experiments: T.G.S. Analyzed the data: A.K.G. and H.K. Wrote the manuscript: A.S., H.K., M.K. and A.E.A. Editing of the Manuscript: A.K.G., A.N. and M.K.-R., M.M.A.-D. Critically reviewed the article: T.G.S. Supervision: T.G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Taif University Researchers Supporting Program (Project number: TURSP-2020/93), Taif University, Saudi Arabia.
Data Availability Statement
Not applicable, the study did not report any data.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martin G.S. Cell signaling and cancer. Cancer Cell. 2003;4:167–174. doi: 10.1016/S1535-6108(03)00216-2. [DOI] [PubMed] [Google Scholar]
- 2.Sever R., Brugge J.S. Signaling in cancer. Cold Spring Harb. Perspect. Med. 2014;5:a006098. doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huangfu W.C., Fuchs S.Y. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1:725–734. doi: 10.1177/1947601910382901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nepal S., Shrestha A., Park P.H. Ubiquitin specific protease 2 acts as a key modulator for the regulation of cell cycle by adiponectin and leptin in cancer cells. Mol. Cell. Endocrinol. 2015;412:44–55. doi: 10.1016/j.mce.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Spataro V., Norbury C., Harris A.L. The ubiquitin-proteasome pathway in cancer. Br. J. Cancer. 1998;77:448–455. doi: 10.1038/bjc.1998.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smalle J., Vierstra R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 7.Dikic I., Wakatsuki S., Walters K.J. Ubiquitin-binding domains—from structures to functions. Nat. Rev. Mol. Cell Biol. 2009;10:659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng L., Meng T., Chen L., Wei W., Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct. Target. Ther. 2020;5:11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swatek K.N., Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., Olsen J.V., Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 11.Weinert B.T., Schölz C., Wagner S.A., Iesmantavicius V., Su D., Daniel J.A., Choudhary C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4:842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira de Freitas R., Harding R.J., Franzoni I., Ravichandran M., Mann M.K., Ouyang H., Lautens M., Santhakumar V., Arrowsmith C.H., Schapira M. Identification and structure–activity relationship of HDAC6 zinc-finger ubiquitin binding domain inhibitors. J. Med. Chem. 2018;61:4517–4527. doi: 10.1021/acs.jmedchem.8b00258. [DOI] [PubMed] [Google Scholar]
- 13.Scott D., Oldham N.J., Strachan J., Searle M.S., Layfield R. Ubiquitin-binding domains: Mechanisms of ubiquitin recognition and use as tools to investigate ubiquitin-modified proteomes. Proteomics. 2015;15:844–861. doi: 10.1002/pmic.201400341. [DOI] [PubMed] [Google Scholar]
- 14.Randles L., Walters K.J. Ubiquitin and its binding domains. Front. Biosci. 2012;17:2140. doi: 10.2741/4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley J.H., Lee S., Prag G. Ubiquitin-binding domains. Biochem. J. 2006;399:361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumeister W., Walz J., Zühl F., Seemüller E. The proteasome: Paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/S0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 17.DeMartino G.N., Gillette T.G. Proteasomes: Machines for all reasons. Cell. 2007;129:659–662. doi: 10.1016/j.cell.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Saeki Y., Tanaka K. Assembly and function of the proteasome. Ubiquitin Fam. Modif. Proteasome. 2012;832:315–337. doi: 10.1007/978-1-61779-474-2_22. [DOI] [PubMed] [Google Scholar]
- 19.Tian G., Park S., Lee M.J., Huck B., McAllister F., Hill C.P., Gygi S.P., Finley D. An asymmetric interface between the regulatory and core particles of the proteasome. Nat. Struct. Mol. Biol. 2011;18:1259–1267. doi: 10.1038/nsmb.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillette T.G., Kumar B., Thompson D., Slaughter C.A., DeMartino G.N. Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and activation of the 26 S proteasome. J. Biol. Chem. 2008;283:31813–31822. doi: 10.1074/jbc.M805935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bard J.A., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B. 2009;85:12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall R.S., Vierstra R.D. Dynamic regulation of the 26S proteasome: From synthesis to degradation. Front. Mol. Biosci. 2019;6:40. doi: 10.3389/fmolb.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glickman M.H., Rubin D.M., Coux O., Wefes I., Pfeifer G., Cjeka Z., Baumeister W., Fried V.A., Finley D. A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell. 1998;94:615–623. doi: 10.1016/S0092-8674(00)81603-7. [DOI] [PubMed] [Google Scholar]
- 26.Scheel H., Hofmann K. Prediction of a common structural scaffold for proteasome lid, COP9-signalosome and eIF3 complexes. BMC Bioinform. 2005;6:71. doi: 10.1186/1471-2105-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya S., Yu H., Mim C., Matouschek A. Regulated protein turnover: Snapshots of the proteasome in action. Nat. Rev. Mol. Cell Biol. 2014;15:122–133. doi: 10.1038/nrm3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijman S.M., Luna-Vargas M.P., Velds A., Brummelkamp T.R., Dirac A.M., Sixma T.K., Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Reyes-Turcu F.E., Ventii K.H., Wilkinson K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clague M.J., Barsukov I., Coulson J.M., Liu H., Rigden D.J., Urbé S. Deubiquitylases from genes to organism. Physiological. Rev. 2013;93:1289–1315. doi: 10.1152/physrev.00002.2013. [DOI] [PubMed] [Google Scholar]
- 31.Komander D., Clague M.J., Urbé S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 32.Rehman S.A., Kristariyanto Y.A., Choi S.Y., Nkosi P.J., Weidlich S., Labib K., Hofmann K., Kulathu Y. MINDY-1 is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol. Cell. 2016;63:146–155. doi: 10.1016/j.molcel.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mevissen T.E., Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 2017;86:159–192. doi: 10.1146/annurev-biochem-061516-044916. [DOI] [PubMed] [Google Scholar]
- 34.Ramakrishna S., Suresh B., Baek K.H. The role of deubiquitinating enzymes in apoptosis. Cell. Mol. Life Sci. 2011;68:15–26. doi: 10.1007/s00018-010-0504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermanns T., Hofmann K. Bacterial DUBs: Deubiquitination beyond the seven classes. Biochem. Soc. Trans. 2019;47:1857–1866. doi: 10.1042/BST20190526. [DOI] [PubMed] [Google Scholar]
- 36.Tsakiri E.N., Trougakos I.P. The amazing ubiquitin-proteasome system: Structural components and implication in aging. Int. Rev. Cell Mol. Biol. 2015;314:171–237. doi: 10.1016/bs.ircmb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Pelzer C., Kassner I., Matentzoglu K., Singh R.K., Wollscheid H.P., Scheffner M., Schmidtke G., Groettrup M. UBE1L2, a Novel E1 Enzyme Specific for Ubiquitin. J. Biol. Chem. 2007;282:23010–23014. doi: 10.1074/jbc.C700111200. [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni M., Smith H.E. E1 ubiquitin-activating enzyme UBA-1 plays multiple roles throughout C. elegans development. PLoS Genet. 2008;4:e1000131. doi: 10.1371/journal.pgen.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee I., Schindelin H. Structural insights into E1-catalyzed ubiquitin activation and transfer to conjugating enzymes. Cell. 2008;134:268–278. doi: 10.1016/j.cell.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 40.Allan D.C., Phillips J.C. Evolution of the ubiquitin-activating enzyme Uba1 (E1) Phys. A Stat. Mech. Appl. 2017;483:456–461. doi: 10.1016/j.physa.2017.04.144. [DOI] [Google Scholar]
- 41.Zhou M.J., Chen F.Z., Chen H.C. Ubiquitination involved enzymes and cancer. Med. Oncol. 2014;31:93. doi: 10.1007/s12032-014-0093-6. [DOI] [PubMed] [Google Scholar]
- 42.Van Wijk S.J., Timmers H.M. The family of ubiquitin-conjugating enzymes (E2s): Deciding between life and death of proteins. FASEB J. 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- 43.Stewart M.D., Ritterhoff T., Klevit R.E., Brzovic P.S. E2 enzymes: More than just middle men. Cell Res. 2016;26:423–440. doi: 10.1038/cr.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deshaies R.J., Joazeiro C.A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 45.Zheng N., Shabek N. Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 2017;86:129–157. doi: 10.1146/annurev-biochem-060815-014922. [DOI] [PubMed] [Google Scholar]
- 46.Balaji V., Hoppe T. Regulation of E3 ubiquitin ligases by homotypic and heterotypic assembly. F1000Research. 2020;9:88. doi: 10.12688/f1000research.21253.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metzger M.B., Hristova V.A., Weissman A.M. HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 2012;125:531–537. doi: 10.1242/jcs.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sluimer J., Distel B. Regulating the human HECT E3 ligases. Cell. Mol. Life Sci. 2018;75:3121–3141. doi: 10.1007/s00018-018-2848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martino L., Brown N.R., Masino L., Esposito D., Rittinger K. Determinants of E2-ubiquitin conjugate recognition by RBR E3 ligases. Sci. Rep. 2018;8:68. doi: 10.1038/s41598-017-18513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walden H., Rittinger K. RBR ligase–mediated ubiquitin transfer: A tale with many twists and turns. Nat. Struct. Mol. Biol. 2018;25:440–445. doi: 10.1038/s41594-018-0063-3. [DOI] [PubMed] [Google Scholar]
- 51.Khan H., Gupta A., Singh T.G., Kaur A. Mechanistic insight on the role of leukotriene receptors in ischemic–reperfusion injury. Pharmacol. Rep. 2021;73:1240–1254. doi: 10.1007/s43440-021-00258-8. [DOI] [PubMed] [Google Scholar]
- 52.Popovic D., Vucic D., Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 53.Rehni A.K., Singh T.G., Behl N., Arora S. Possible involvement of ubiquitin proteasome system and other proteases in acute and delayed aspects of ischemic preconditioning of brain in mice. Biol. Pharm. Bull. 2010;33:1953–1957. doi: 10.1248/bpb.33.1953. [DOI] [PubMed] [Google Scholar]
- 54.Caldeira M.V., Salazar I.L., Curcio M., Canzoniero L.M., Duarte C.B. Role of the ubiquitin–proteasome system in brain ischemia: Friend or foe? Prog. Neurobiol. 2014;112:50–69. doi: 10.1016/j.pneurobio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Q., Huang T., Zhang L., Zhou Y., Luo H., Xu H., Wang X. Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Front. Aging Neurosci. 2016;8:303. doi: 10.3389/fnagi.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kostin S., Pool L., Elsässer A., Hein S., Drexler H.C., Arnon E., Hayakawa Y., Zimmermann R., Bauer E., Klövekorn W.P., et al. Myocytes die by multiple mechanisms in failing human hearts. Circ. Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 57.Eefting F., Rensing B., Wigman J., Pannekoek W.J., Liu W.M., Cramer M.J., Lips D.J., Doevendans P.A. Role of apoptosis in reperfusion injury. Cardiovasc. Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 58.Shukla S.K., Rafiq K. Proteasome biology and therapeutics in cardiac diseases. Transl. Res. 2019;205:64–76. doi: 10.1016/j.trsl.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Attaix D., Ventadour S., Codran A., Béchet D., Taillandier D., Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–186. doi: 10.1042/bse0410173. [DOI] [PubMed] [Google Scholar]
- 60.Lv C., Wang X., Guo Y., Yuan S. Role of Selective Autophagy in Spermatogenesis and Male Fertility. Cells. 2020;9:2523. doi: 10.3390/cells9112523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauser P.V., Perco P., Mühlberger I., Pippin J., Blonski M., Mayer B., Alpers C.E., Oberbauer R., Shankland S.J. Microarray and bioinformatics analysis of gene expression in experimental membranous nephropathy. Nephron Exp. Nephrol. 2009;112:e43–e58. doi: 10.1159/000213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kortenoeven M.L., Fenton R.A. Renal aquaporins and water balance disorders. Biochim. Biophys. Acta Gen. Subj. 2014;1840:1533–1549. doi: 10.1016/j.bbagen.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Sharma V., Kaur A., Singh T.G. Counteracting role of nuclear factor erythroid 2-related factor 2 pathway in Alzheimer’s disease. Biomed. Pharmacother. 2020;129:110373. doi: 10.1016/j.biopha.2020.110373. [DOI] [PubMed] [Google Scholar]
- 64.Gao C., Huang W., Kanasaki K., Xu Y. The role of ubiquitination and sumoylation in diabetic nephropathy. BioMed Res. Int. 2014;2014:160692. doi: 10.1155/2014/160692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 66.Williams G.H., Stoeber K. The cell cycle and cancer. J. Pathol. 2012;226:352–364. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 67.Thanikachalam K., Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11:164. doi: 10.3390/nu11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sporn M.B. TGF-β: 20 years and counting. Microbes Infect. 1999;1:1251–1253. doi: 10.1016/S1286-4579(99)00260-9. [DOI] [PubMed] [Google Scholar]
- 69.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 70.Glasgow E., Mishra L. Transforming growth factor-β signaling and ubiquitinators in cancer. Endocr. Relat. Cancer. 2008;15:59–72. doi: 10.1677/ERC-07-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stolz A., Neufeld K., Ertych N., Bastians H. Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Rep. 2015;16:490–499. doi: 10.15252/embr.201439410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y., Li Y., Xue J., Gong A., Yu G., Zhou A., Lin K., Zhang S., Zhang N., Gottardi C.J., et al. Wnt-induced deubiquitination FoxM1 ensures nucleus β-catenin transactivation. EMBO J. 2016;35:668–684. doi: 10.15252/embj.201592810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun H., Ou B., Zhao S., Liu X., Song L., Liu X., Wang R., Peng Z. USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine. 2019;48:236–247. doi: 10.1016/j.ebiom.2019.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yun S.I., Hong H.K., Yeo S.Y., Kim S.H., Cho Y.B., Kim K.K. Ubiquitin-specific protease 21 promotes colorectal cancer metastasis by acting as a Fra-1 deubiquitinase. Cancers. 2020;12:207. doi: 10.3390/cancers12010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang Q., Tang C., Tang M., Zhang Q., Gao Y., Ge Z. TRIM47 is up-regulated in colorectal cancer, promoting ubiquitination and degradation of SMAD4. J. Exp. Clin. Cancer Res. 2019;38:159. doi: 10.1186/s13046-019-1143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X., Liu G., Liu R., He J., Wang G., Zhang H., Liu T., Bai J., Cheng N., Qiu J. Expression of ubiquitin conjugating enzyme E2T in colorectal cancers and clinical implications. Oncol. Lett. 2020;20:275. doi: 10.3892/ol.2020.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hao Z., Zhang H., Cowell J. Ubiquitin-conjugating enzyme UBE2C: Molecular biology, role in tumorigenesis, and potential as a biomarker. Tumor Biol. 2012;33:723–730. doi: 10.1007/s13277-011-0291-1. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H., Sun W., Qiao G., Zhao B., Liu X., Zhu F. The Expression of Tripartite Motif Protein 36 and β-Catenin Correlates with the Prognosis of Esophageal Cancer. Gastroenterol. Res. Pract. 2020;2020:7641761. doi: 10.1155/2020/7641761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawaguchi T., Komatsu S., Ichikawa D., Hirajima S., Nishimura Y., Konishi H., Shiozaki A., Fujiwara H., Okamoto K., Tsuda H., et al. Overexpression of TRIM44 is related to invasive potential and malignant outcomes in esophageal squamous cell carcinoma. Tumor Biol. 2017;39:1010428317700409. doi: 10.1177/1010428317700409. [DOI] [PubMed] [Google Scholar]
- 80.Zheng L., Zhao Z., Rong L., Xue L., Song Y. RASSF6-TRIM16 axis promotes cell proliferation, migration and invasion in esophageal squamous cell carcinoma. J. Genet. Genom. 2019;46:477–488. doi: 10.1016/j.jgg.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Wang L., Hou Z., Hasim A., Abuduerheman A., Zhang H., Niyaz M., Awut I., Upur H., Sheyhidin I. RNF113A promotes the proliferation, migration and invasion, and is associated with a poor prognosis of esophageal squamous cell carcinoma. Int. J. Oncol. 2018;52:861–871. doi: 10.3892/ijo.2018.4253. [DOI] [PubMed] [Google Scholar]
- 82.Singh S., Saraya A., Das P., Sharma R. Increased expression of MARCH8, an E3 ubiquitin ligase, is associated with growth of esophageal tumor. Cancer Cell Int. 2017;17:116. doi: 10.1186/s12935-017-0490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta A., Shah K., Oza M.J., Behl T. Reactivation of p53 gene by MDM2 inhibitors: A novel therapy for cancer treatment. Biomed. Pharmacother. 2019;109:484–492. doi: 10.1016/j.biopha.2018.10.155. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y., Fu D., Xi J., Ji Z., Liu T., Ma Y., Zhao Y., Dong L., Wang Q., Shen X. Expression and clinical significance of UCH37 in human esophageal squamous cell carcinoma. Dig. Dis. Sci. 2012;57:2310–2317. doi: 10.1007/s10620-012-2181-9. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W., Zhuang Y., Zhang Y., Yang X., Zhang H., Wang G., Yin W., Wang R., Zhang Z., Xiao W. Uev1A facilitates osteosarcoma differentiation by promoting Smurf1-mediated Smad1 ubiquitination and degradation. Cell Death Dis. 2017;8:e2974. doi: 10.1038/cddis.2017.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim B.G., Lee J.H., Yasuda J., Ryoo H.M., Cho J.Y. Phospho-Smad1 modulation by nedd4 e3 ligase in BMP/TGF-β signaling. J. Bone Miner. Res. 2011;26:1411–1424. doi: 10.1002/jbmr.348. [DOI] [PubMed] [Google Scholar]
- 87.Georges A., Marcon E., Greenblatt J., Frappier L. Identification and characterization of USP7 targets in cancer cells. Sci. Rep. 2018;8:15833. doi: 10.1038/s41598-018-34197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams S.A., Maecker H.L., French D.M., Liu J., Gregg A., Silverstein L.B., Cao T.C., Carano R.A., Dixit V.M. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146:918–930. doi: 10.1016/j.cell.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 89.Zhang P., Yang Y., Nolo R., Zweidler-McKay P.A., Hughes D.P. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene. 2010;29:2916–2926. doi: 10.1038/onc.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi X., Deng X., Zhao Y., Deng B., Deng J., Fan H., Du Y., Hao L. Ubiquitin-like protein FAT10 promotes osteosarcoma growth by modifying the ubiquitination and degradation of YAP1. Exp. Cell Res. 2020;387:111804. doi: 10.1016/j.yexcr.2019.111804. [DOI] [PubMed] [Google Scholar]
- 91.Gan Z., Han K., Lin S., Hu H., Shen Z., Min D. Knockdown of ubiquitin-specific peptidase 39 inhibited the growth of osteosarcoma cells and induced apoptosis in vitro. Biol. Res. 2017;50:15. doi: 10.1186/s40659-017-0121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang W., Cai X., Xu T., Liu K., Yang D., Fan L., Li G., Yu X. Tripartite Motif-Containing 46 Promotes Viability and Inhibits Apoptosis of Osteosarcoma Cells by Activating NF-κB Signaling Through Ubiquitination of PPARα. Oncol. Res. 2020;28:409. doi: 10.3727/096504020X15868639303417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang J., Xing D., Li Z., Shen J., Zhao H., Li S. TRIM59 is upregulated and promotes cell proliferation and migration in human osteosarcoma. Mol. Med. Rep. 2016;13:5200–5206. doi: 10.3892/mmr.2016.5183. [DOI] [PubMed] [Google Scholar]
- 94.Zhou C., Zhang Z., Zhu X., Qian G., Zhou Y., Sun Y., Yu W., Wang J., Lu H., Lin F., et al. N6-Methyladenosine modification of the TRIM7 positively regulates tumorigenesis and chemoresistance in osteosarcoma through ubiquitination of BRMS1. EBioMedicine. 2020;59:102955. doi: 10.1016/j.ebiom.2020.102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang D., Jiang F., Wang X., Li G. Downregulation of Ubiquitin-Specific Protease 22 Inhibits Proliferation, Invasion, and Epithelial–Mesenchymal Transition in Osteosarcoma Cells. Oncol. Res. 2017;25:743. doi: 10.3727/096504016X14772395226335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., Leng H., Chen H., Wang L., Jiang N., Huo X., Yu B. Knockdown of UBE2T inhibits osteosarcoma cell proliferation, migration, and invasion by suppressing the PI3K/Akt signaling pathway. Oncol. Res. 2016;24:361. doi: 10.3727/096504016X14685034103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khan H., Singh A., Thapa K., Garg N., Grewal A.K., Singh T.G. Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res. 2021;1761:147399. doi: 10.1016/j.brainres.2021.147399. [DOI] [PubMed] [Google Scholar]
- 98.Shao G., Wang R., Sun A., Wei J., Peng K., Dai Q., Yang W., Lin Q. The E3 ubiquitin ligase NEDD4 mediates cell migration signaling of EGFR in lung cancer cells. Mol. Cancer. 2018;17:24. doi: 10.1186/s12943-018-0784-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gamell C., Gulati T., Levav-Cohen Y., Young R.J., Do H., Pilling P., Takano E., Watkins N., Fox S.B., Russell P., et al. Reduced abundance of the E3 ubiquitin ligase E6AP contributes to decreased expression of the INK4/ARF locus in non–small cell lung cancer. Sci. Signal. 2017;10:eaaf8223. doi: 10.1126/scisignal.aaf8223. [DOI] [PubMed] [Google Scholar]
- 100.Liu L., Yu L., Zeng C., Long H., Duan G., Yin G., Dai X., Lin Z. E3 ubiquitin ligase HRD1 promotes lung tumorigenesis by promoting sirtuin 2 ubiquitination and degradation. Mol. Cell. Biol. 2020;40:e00257-19. doi: 10.1128/MCB.00257-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan J., Deng Q., Jiang C., Wang X., Niu T., Li H., Chen T., Jin J., Pan W., Cai X., et al. USP37 directly deubiquitinates and stabilizes c-Myc in lung cancer. Oncogene. 2015;34:3957–3967. doi: 10.1038/onc.2014.327. [DOI] [PubMed] [Google Scholar]
- 102.Singh S., Singh T.G. Role of nuclear factor kappa b (Nf-κb) signalling in neurodegenerative diseases: An mechanistic approach. Curr. Neuropharmacol. 2020;18:918–935. doi: 10.2174/1570159X18666200207120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren H., Xu Y., Wang Q., Jiang J. E3 ubiquitin ligase tripartite motif-containing 71 promotes the proliferation of non-small cell lung cancer through the inhibitor of kappaB-α/nuclear factor kappaB pathway. Oncotarget. 2018;9:10880. doi: 10.18632/oncotarget.19075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Z., Liu P., Wang J., Gong T., Zhang F., Ma J., Han N. Ubiquitin-conjugating enzyme E2C regulates apoptosis-dependent tumor progression of non-small cell lung cancer via ERK pathway. Med. Oncol. 2015;32:149. doi: 10.1007/s12032-015-0609-8. [DOI] [PubMed] [Google Scholar]
- 105.Gu J., Mao W., Ren W., Xu F., Zhu Q., Lu C., Lin Z., Zhang Z., Chu Y., Liu R., et al. Ubiquitin-protein ligase E3C maintains non-small-cell lung cancer stemness by targeting AHNAK-p53 complex. Cancer Lett. 2019;443:125–134. doi: 10.1016/j.canlet.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 106.Zhan W., Han T., Zhang C., Xie C., Gan M., Deng K., Fu M., Wang J.B. TRIM59 promotes the proliferation and migration of non-small cell lung cancer cells by upregulating cell cycle related proteins. PLoS ONE. 2015;10:e0142596. doi: 10.1371/journal.pone.0142596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ning J., Zhang J., Liu W., Lang Y., Xue Y., Xu S. Overexpression of ubiquitin-specific protease 22 predicts poor survival in patients with early-stage non-small cell lung cancer. Eur. J. Histochem. 2012;56:e46. doi: 10.4081/ejh.2012.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan D.W., Chan C.Y., Yam J.W., Ching Y.P., Ng I.O. Prickle-1 negatively regulates Wnt/β-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 2006;131:1218–1227. doi: 10.1053/j.gastro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 109.Guo P., Ma X., Zhao W., Huai W., Li T., Qiu Y., Zhang Y., Han L. TRIM31 is upregulated in hepatocellular carcinoma and promotes disease progression by inducing ubiquitination of TSC1–TSC2 complex. Oncogene. 2018;37:478–488. doi: 10.1038/onc.2017.349. [DOI] [PubMed] [Google Scholar]
- 110.Zhu L., Qin C., Li T., Ma X., Qiu Y., Lin Y., Ma D., Qin Z., Sun C., Shen X., et al. The E3 ubiquitin ligase TRIM7 suppressed hepatocellular carcinoma progression by directly targeting Src protein. Cell Death Differ. 2020;27:1819–1831. doi: 10.1038/s41418-019-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J., Zhang C., Wang X.L., Ly P., Belyi V., Xu-Monette Z.Y., Young K.H., Hu W., Feng Z. E3 ubiquitin ligase TRIM32 negatively regulates tumor suppressor p53 to promote tumorigenesis. Cell Death Differ. 2014;21:1792–1804. doi: 10.1038/cdd.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang Y.F., Zhang M.F., Tian Q.H., Zhang C.Z. TRIM65 triggers β-catenin signaling via ubiquitylation of Axin1 to promote hepatocellular carcinoma. J. Cell Sci. 2017;130:3108–3115. doi: 10.1242/jcs.206623. [DOI] [PubMed] [Google Scholar]
- 113.Tao N.N., Zhang Z.Z., Ren J.H., Zhang J., Zhou Y.J., Wong V.K., Law B.Y., Cheng S.T., Zhou H.Z., Chen W.X., et al. Overexpression of ubiquitin-conjugating enzyme E2 L3 in hepatocellular carcinoma potentiates apoptosis evasion by inhibiting the GSK3β/p65 pathway. Cancer Lett. 2020;481:1–4. doi: 10.1016/j.canlet.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 114.Liu L.P., Yang M., Peng Q.Z., Li M.Y., Zhang Y.S., Guo Y.H., Chen Y., Bao S.Y. UBE2T promotes hepatocellular carcinoma cell growth via ubiquitination of p53Biochem. Biophys. Res. Commun. 2017;493:20–27. doi: 10.1016/j.bbrc.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 115.Urbanik T., Köhler B.C., Boger R.J., Woerns M.A., Heeger S., Otto G., Hövelmeyer N., Galle P.R., Schuchmann M., Waisman A., et al. Down-regulation of CYLD as a trigger for NF-κB activation and a mechanism of apoptotic resistance in hepatocellular carcinoma cells. Int. J. Oncol. 2011;38:121–131. [PubMed] [Google Scholar]
- 116.Seliger B., Fedorushchenko A., Brenner W., Ackermann A., Atkins D., Hanash S., Lichtenfels R. Ubiquitin COOH-Terminal Hydrolase 1: A Biomarker of Renal Cell Carcinoma Associated with Enhanced Tumor Cell Proliferation and Migration. Clin. Cancer Res. 2007;13:27–37. doi: 10.1158/1078-0432.CCR-06-0824. [DOI] [PubMed] [Google Scholar]
- 117.Nanok C., Jearanaikoon P., Proungvitaya S., Limpaiboon T. Aberrant methylation of HTATIP2 and UCHL1 as a predictive biomarker for cholangiocarcinoma. Mol. Med. Rep. 2018;17:4145–4153. doi: 10.3892/mmr.2017.8319. [DOI] [PubMed] [Google Scholar]
- 118.Huang Z.J., Zhu J.J., Yang X.Y., Biskup E. NEDD4 promotes cell growth and migration via PTEN/PI3K/AKT signaling in hepatocellular carcinoma. Oncol. Lett. 2017;14:2649–2656. doi: 10.3892/ol.2017.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yuan R., Wang K., Hu J., Yan C., Li M., Yu X., Liu X., Lei J., Guo W., Wu L., et al. Ubiquitin-like protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying β-catenin degradation. Cancer Res. 2014;74:5287–5300. doi: 10.1158/0008-5472.CAN-14-0284. [DOI] [PubMed] [Google Scholar]
- 120.Su D., Ma S., Shan L., Wang Y., Wang Y., Cao C., Liu B., Yang C., Wang L., Tian S., et al. Ubiquitin-specific protease 7 sustains DNA damage response and promotes cervical carcinogenesis. J. Clin. Investig. 2018;128:4280–4296. doi: 10.1172/JCI120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liang J., Nishi H., Bian M.L., Higuma C., Sasaki T., Ito H., Isaka K. The ubiquitin-conjugating enzyme E2-EPF is overexpressed in cervical cancer and associates with tumor growth. Oncol. Rep. 2012;28:1519–1525. doi: 10.3892/or.2012.1949. [DOI] [PubMed] [Google Scholar]
- 122.Hu J., Meng Y., Zeng J., Zeng B., Jiang X. Ubiquitin E3 Ligase MARCH7 promotes proliferation and invasion of cervical cancer cells through VAV2-RAC1-CDC42 pathway. Oncol. Lett. 2018;16:2312–2318. doi: 10.3892/ol.2018.8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bai M., Che Y., Lu K., Fu L. Analysis of deubiquitinase OTUD5 as a biomarker and therapeutic target for cervical cancer by bioinformatic analysis. PeerJ. 2020;8:e9146. doi: 10.7717/peerj.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q., Qiao L., Wang X., Ding C., Chen J.J. UHRF1 epigenetically down-regulates UbcH8 to inhibit apoptosis in cervical cancer cells. Cell Cycle. 2018;17:300–308. doi: 10.1080/15384101.2017.1403686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diao W., Guo Q., Zhu C., Song Y., Feng H., Cao Y., Du M., Chen H. USP18 promotes cell proliferation and suppressed apoptosis in cervical cancer cells via activating AKT signaling pathway. BMC Cancer. 2020;20:741. doi: 10.1186/s12885-020-07241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aguilar-Martinez E., Morrisroe C., Sharrocks A.D. The ubiquitin ligase UBE3A dampens ERK pathway signalling in HPV E6 transformed HeLa cells. PLoS ONE. 2015;10:e0119366. doi: 10.1371/journal.pone.0119366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan M., Zhao C., Wei N., Wu X., Cui J., Xing Y. High expression of ubiquitin-specific protease 8 (USP8) is associated with poor prognosis in patients with cervical squamous cell carcinoma. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:4934. doi: 10.12659/MSM.909235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin L., Zhao W., Sun B., Wang X., Liu Q. Overexpression of TRIM24 is correlated with the progression of human cervical cancer. Am. J. Transl. Res. 2017;9:620. [PMC free article] [PubMed] [Google Scholar]
- 129.Aierken G., Seyiti A., Alifu M., Kuerban G. Knockdown of tripartite-59 (TRIM59) inhibits cellular proliferation and migration in human cervical cancer cells. Oncol. Res. 2017;25:381. doi: 10.3727/096504016X14741511303522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song Y., Guo Q., Gao S., Hua K. Tripartite motif-containing protein 3 plays a role of tumor inhibitor in cervical cancer. Biochem. Biophys. Res. Commun. 2018;498:686–692. doi: 10.1016/j.bbrc.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 131.Lu X., Zhuang H., Yu Q., Zhang X., Wu Z., Zhang L., Xu Y., Wu B., Yang L., Ma A., et al. Identification of the UBA2-WTIP fusion gene in acute myeloid leukemia. Exp. Cell Res. 2018;371:409–416. doi: 10.1016/j.yexcr.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 132.Seghatoleslam A., Monabbati A., Bozorg-Ghalati F., Nikseresht M., Bordbar M.R., Rahvar M., Owji A.A. Expression of UBE2Q2, a putative member of the ubiquitin-conjugating enzyme family in pediatric acute lymphoblastic leukemia. Arch. Iran. Med. 2012;15:352–355. [PubMed] [Google Scholar]
- 133.Luo H., Qin Y., Reu F., Ye S., Dai Y., Huang J., Wang F., Zhang D., Pan L., Zhu H., et al. Microarray-based analysis and clinical validation identify ubiquitin-conjugating enzyme E2E1 (UBE2E1) as a prognostic factor in acute myeloid leukemia. J. Hematol. Oncol. 2016;9:125. doi: 10.1186/s13045-016-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.King B., Trimarchi T., Reavie L., Xu L., Mullenders J., Ntziachristos P., Aranda-Orgilles B., Perez-Garcia A., Shi J., Vakoc C., et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell. 2013;153:1552–1566. doi: 10.1016/j.cell.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang H., Bei L., Shah C.A., Huang W., Platanias L.C., Eklund E.A. The E3 ubiquitin ligase Triad1 influences development of Mll-Ell-induced acute myeloid leukemia. Oncogene. 2018;37:2532–2544. doi: 10.1038/s41388-018-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang E., Kawaoka S., Yu M., Shi J., Ni T., Yang W., Zhu J., Roeder R.G., Vakoc C.R. Histone H2B ubiquitin ligase RNF20 is required for MLL-rearranged leukemia. Proc. Natl. Acad. Sci. USA. 2013;110:3901–3906. doi: 10.1073/pnas.1301045110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shan H., Li X., Xiao X., Dai Y., Huang J., Song J., Liu M., Yang L., Lei H., Tong Y., et al. USP7 deubiquitinates and stabilizes NOTCH1 in T-cell acute lymphoblastic leukemia. Signal. Transduct. Target Ther. 2018;3:29. doi: 10.1038/s41392-018-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qiu G.Z., Mao X.Y., Ma Y., Gao X.C., Wang Z., Jin M.Z., Sun W., Zou Y.X., Lin J., Fu H.L., et al. Ubiquitin-specific protease 22 acts as an oncoprotein to maintain glioma malignancy through deubiquitinating B cell-specific Moloney murine leukemia virus integration site 1 for stabilization. Cancer Sci. 2018;109:2199–2210. doi: 10.1111/cas.13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quintás-Cardama A., Zhang N., Qiu Y.H., Post S., Creighton C.J., Cortes J., Coombes K.R., Kornblau S.M. Loss of TRIM62 expression is an independent adverse prognostic factor in acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2015;15:115–127. doi: 10.1016/j.clml.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schneider D., Chua R.L., Molitor N., Hamdan F.H., Rettenmeier E.M., Prokakis E., Mishra V.K., Kari V., Wegwitz F., Johnsen S.A., et al. The E3 ubiquitin ligase RNF40 suppresses apoptosis in colorectal cancer cells. Clin. Epigenet. 2019;11:98. doi: 10.1186/s13148-019-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thrift A.P. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol. 2016;41:88–95. doi: 10.1016/j.canep.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 142.Smyth E.C., Lagergren J., Fitzgerald R.C., Lordick F., Shah M.A., Lagergren P., Cunningham D. Oesophageal cancer. Nat. Rev. Dis Primers. 2017;3:17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lagergren J., Smyth E., Cunningham D., Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396. doi: 10.1016/S0140-6736(17)31462-9. [DOI] [PubMed] [Google Scholar]
- 144.Li L., Li X., Wang W., Gao T., Shi Z. UBE2C is involved in the functions of ECRG4 on esophageal squamous cell carcinoma. Biomed. Pharmacother. 2018;98:201–206. doi: 10.1016/j.biopha.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 145.Gatti V., Bernassola F., Talora C., Melino G., Peschiaroli A. The impact of the ubiquitin system in the pathogenesis of squamous cell carcinomas. Cancers. 2020;12:1595. doi: 10.3390/cancers12061595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuwano H., Saeki H., Kawaguchi H., Sonoda K., Kitamura K., Nakashima H., Toh Y., Sugimachi K. Proliferative activity of cancer cells in front and center areas of carcinoma in situ and invasive sites of esophageal squamous-cell carcinoma. Int. J. Cancer. 1998;78:149–152. doi: 10.1002/(SICI)1097-0215(19981005)78:2<149::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 147.Park H.B., Kim J.W., Baek K.H. Regulation of Wnt signaling through ubiquitination and deubiquitination in cancers. Int. J. Mol. Sci. 2020;21:3904. doi: 10.3390/ijms21113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moghbeli M., Abbaszadegan M.R., Golmakani E., Forghanifard M.M. Correlation of Wnt and NOTCH pathways in esophageal squamous cell carcinoma. Cell Commun. Signal. 2016;10:129–135. doi: 10.1007/s12079-016-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moore D.D., Luu H.H. Osteosarcoma. Orthop. Oncol. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 150.Kansara M., Thomas D.M. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26:959248. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- 151.Ohata Y., Ozono K. Bone and stem cells. The mechanism of osteogenic differentiation from mesenchymal stem cell. Clin. Calcium. 2014;24:501–508. [PubMed] [Google Scholar]
- 152.Vriend J., Reiter R.J. Melatonin, bone regulation and the ubiquitin-proteasome connection: A review. Life Sci. 2016;145:152–160. doi: 10.1016/j.lfs.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 153.Franceschi R.T., Ge C., Xiao G., Roca H., Jiang D. Transcriptional regulation of osteoblasts. Cells Tissues Organs. 2009;189:144–152. doi: 10.1159/000151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z., Kang W., You Y., Pang J., Ren H., Suo Z., Liu H., Zheng Y. USP7: Novel drug target in cancer therapy. Front. Pharmacol. 2019;10:427. doi: 10.3389/fphar.2019.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chauhan D., Tian Z., Nicholson B., Kumar K.S., Zhou B., Carrasco R., McDermott J.L., Leach C.A., Fulcinniti M., Kodrasov M.P., et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–358. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ying M., Zhang L., Zhou Q., Shao X., Cao J., Zhang N., Li W., Zhu H., Yang B., He Q. The E3 ubiquitin protein ligase MDM2 dictates all-trans retinoic acid-induced osteoblastic differentiation of osteosarcoma cells by modulating the degradation of RARα. Oncogene. 2016;35:4358–4367. doi: 10.1038/onc.2015.503. [DOI] [PubMed] [Google Scholar]
- 157.De Groot P.M., Wu C.C., Carter B.W., Munden R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018;7:220. doi: 10.21037/tlcr.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X.C., Wang G.Z., Wen Z.S., Zhou Y.C., Hu Q., Zhang B., Qu L.W., Gao S.H., Liu J., Ma L., et al. Systematic identification of CDC34 that functions to stabilize EGFR and promote lung carcinogenesis. EBioMedicine. 2020;53:102689. doi: 10.1016/j.ebiom.2020.102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jeon H.S., Jen J. TGF-2 Signaling and the Role of Inhibitory Smads in Non-small Cell Lung Cancer. J. Thorac. Oncol. 2010;5:417–419. doi: 10.1097/JTO.0b013e3181ce3afd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu H., Li D., Zhou P., Li W. Smurf1-positive expression indicates favorable survival for resected non-small cell lung cancer patients. Int. J. Clin. Exp. Pathol. 2018;11:399. [PMC free article] [PubMed] [Google Scholar]
- 162.Xie C., Powell C., Yao M., Wu J., Dong Q. Ubiquitin-conjugating enzyme E2C: A potential cancer biomarker. Int. J. Biochem. Cell Biol. 2014;47:113–117. doi: 10.1016/j.biocel.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 163.Gupta A., Khan H., Kaur A., Singh T.G. Novel Targets Explored in the Treatment of Alcohol Withdrawal Syndrome. CNS Neurol. Disord. Drug Targets. 2020;20:158–173. doi: 10.2174/1871527319999201118155721. [DOI] [PubMed] [Google Scholar]
- 164.Cohen P.A., Jhingran A., Oaknin A., Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 165.Roura E., Castellsagué X., Pawlita M., Travier N., Waterboer T., Margall N., Bosch F.X., De Sanjosé S., Dillner J., Gram I.T., et al. Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. Int. J. Cancer. 2014;135:453–466. doi: 10.1002/ijc.28666. [DOI] [PubMed] [Google Scholar]
- 166.Döhner H., Weisdorf D.J., Bloomfield C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 167.Pui C.H., Relling M., Downing J.R. Acute lymphoblastic leukemia. N. Engl. J. Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 168.Melo J.V., Hughes T.P., Apperley J.F. Chronic myeloid leukemia. ASH Educ. Program Book. 2003;1:132–152. doi: 10.1182/asheducation-2003.1.132. [DOI] [PubMed] [Google Scholar]
- 169.Xu G.W., Ali M., Wood T.E., Wong D., Maclean N., Wang X., Gronda M., Skrtic M., Li X., Hurren R., et al. The ubiquitin-activating enzyme E1 as a therapeutic target for the treatment of leukemia and multiple myeloma. Blood Am Soc Hemat. 2010;115:2251–2259. doi: 10.1182/blood-2009-07-231191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eliseeva E., Pati D., Diccinanni M.B., Yu A.L., Mohsin S.K., Margolin J.F., Plon S.E. Expression and localization of the CDC34 ubiquitin-conjugating enzyme in pediatric acute lymphoblastic leukemia. Cell Growth Differ. Publ. Am. J. Cancer Res. 2001;12:427–434. [PubMed] [Google Scholar]
- 171.Cui X., Shen W., Wang G., Huang Z., Wen D., Yang Y., Liu Y., Cui L. Ring finger protein 152 inhibits colorectal cancer cell growth and is a novel prognostic biomarker. Am. J. Transl. Res. 2018;10:3701. [PMC free article] [PubMed] [Google Scholar]
- 172.Peyser B.D., Hermone A., Salamoun J.M., Burnett J.C., Hollingshead M.G., McGrath C.F., Gussio R., Wipf P. Specific RITA modification produces hyperselective cytotoxicity while maintaining in vivo antitumor efficacy. Mol. Cancer Ther. 2019;18:1765–1774. doi: 10.1158/1535-7163.MCT-19-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Coughlin K., Anchoori R., Iizuka Y., Meints J., MacNeill L., Vogel R.I., Orlowski R.Z., Lee M.K., Roden R.B., Bazzaro M. Small-molecule RA-9 inhibits proteasome-associated DUBs and ovarian cancer in vitro and in vivo via exacerbating unfolded protein responses. Clin. Cancer Res. 2014;20:3174–3186. doi: 10.1158/1078-0432.CCR-13-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Luo H., Jing B., Xia Y., Zhang Y., Hu M., Cai H., Tong Y., Zhou L., Yang L., Yang J., et al. WP1130 reveals USP24 as a novel target in T-cell acute lymphoblastic leukemia. Cancer Cell Int. 2019;19:56. doi: 10.1186/s12935-019-0773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Soong R.S., Anchoori R.K., Roden R.B., Cho R.L., Chen Y.C., Tseng S.C., Huang Y.L., Liao P.C., Shyu Y.C. Bis-benzylidine Piperidone RA190 treatment of hepatocellular carcinoma via binding RPN13 and inhibiting NF-κB signaling. BMC Cancer. 2020;20:386. doi: 10.1186/s12885-020-06896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song Y., Li S., Ray A., Das D.S., Qi J., Samur M.K., Tai Y.T., Munshi N., Carrasco R.D., Chauhan D., et al. Blockade of deubiquitylating enzyme Rpn11 triggers apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Oncogene. 2017;36:5631–5638. doi: 10.1038/onc.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang B., Fang L., Zhao H., Xiang T., Wang D. MDM2 inhibitor Nutlin-3a suppresses proliferation and promotes apoptosis in osteosarcoma cells. Acta Biochim. Biophys. Sin. 2012;44:685–691. doi: 10.1093/abbs/gms053. [DOI] [PubMed] [Google Scholar]
- 178.Tchoghandjian A., Soubéran A., Tabouret E., Colin C., Denicolaï E., Jiguet-Jiglaire C., El-Battari A., Villard C., Baeza-Kallee N., Figarella-Branger D. Inhibitor of apoptosis protein expression in glioblastomas and their in vitro and in vivo targeting by SMAC mimetic GDC-0152. Cell Death Dis. 2016;7:e2325. doi: 10.1038/cddis.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cai Q., Sun H., Peng Y., Lu J., Nikolovska-Coleska Z., McEachern D., Liu L., Qiu S., Yang C.Y., Miller R., et al. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J. Med. Chem. 2011;54:2714–2726. doi: 10.1021/jm101505d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xia S., Zhang X., Li C., Guan H. Oridonin inhibits breast cancer growth and metastasis through blocking the Notch signaling. Saudi Pharm. J. 2017;25:638–643. doi: 10.1016/j.jsps.2017.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shi C.S., Kuo K.L., Lin W.C., Chen M.S., Liu S.H., Liao S.M., Hsu C.H., Chang Y.W., Chang H.C., Huang K.H. Neddylation inhibitor, MLN4924 suppresses angiogenesis in huvecs and solid cancers: In vitro and in vivo study. Am. J. Cancer Res. 2020;10:953. [PMC free article] [PubMed] [Google Scholar]
- 182.Fu C., Zhu X., Xu P., Li Y. Pharmacological inhibition of USP7 promotes antitumor immunity and contributes to colon cancer therapy. OncoTargets Ther. 2019;12:609. doi: 10.2147/OTT.S182806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fukui S., Nagasaka K., Miyagawa Y., Kikuchi-Koike R., Kawata Y., Kanda R., Ichinose T., Sugihara T., Hiraike H., Wada-Hiraike O., et al. The proteasome deubiquitinase inhibitor bAP15 downregulates TGF-β/Smad signaling and induces apoptosis via UCHL5 inhibition in ovarian cancer. Oncotarget. 2019;10:5932. doi: 10.18632/oncotarget.27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuo K.L., Liu S.H., Lin W.C., Chow P.M., Chang Y.W., Yang S.P., Shi C.S., Hsu C.H., Liao S.M., Chang H.C., et al. The deubiquitinating enzyme inhibitor PR-619 enhances the cytotoxicity of cisplatin via the suppression of anti-apoptotic bcl-2 protein: In vitro and in vivo study. Cells. 2019;8:1268. doi: 10.3390/cells8101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sun J., Nam S., Lee C.S., Li B., Coppola D., Hamilton A.D., Dou Q.P., Sebti S.M. CEP1612, a dipeptidyl proteasome inhibitor, induces p21WAF1 and p27KIP1 expression and apoptosis and inhibits the growth of the human lung adenocarcinoma A-549 in nude mice. Cancer Res. 2001;61:1280–1284. [PubMed] [Google Scholar]
- 186.Milacic V., Banerjee S., Landis-Piwowar K.R., Sarkar F.H., Majumdar A.P., Dou Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li L.W., Yu X.Y., Yang Y., Zhang C.P., Guo L.P., Lu S.H. Expression of esophageal cancer related gene 4 (ECRG4), a novel tumor suppressor gene, in esophageal cancer and its inhibitory effect on the tumor growth in vitro and in vivo. Int. J. Cancer Res. 2009;125:1505–1513. doi: 10.1002/ijc.24513. [DOI] [PubMed] [Google Scholar]
- 188.Jung Y., Yi Y.S., Yoo D.S., Kim J.H., Yang W.S., Lee J., Park K.W., Kweon D.H., Hong S., Cho J.Y. 8-(Tosylamino) quinoline inhibits tumour progression through targeting phosphoinositide-3-kinase/Akt pathway. Pharm. Int. J. Pharm. Sci. 2013;68:146–152. [PubMed] [Google Scholar]
- 189.Wang X., Mazurkiewicz M., Hillert E.K., Olofsson M.H., Pierrou S., Hillertz P., Gullbo J., Selvaraju K., Paulus A., Akhtar S., et al. The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci. Rep. 2016;6:26979. doi: 10.1038/srep26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang L., Sun Y., Wang J., He Q., Chen X., Lan X., Chen J., Dou Q.P., Shi X., Liu J. Proteasomal cysteine deubiquitinase inhibitor b-AP15 suppresses migration and induces apoptosis in diffuse large B cell lymphoma. J. Exp. Clin. Cancer Res. 2019;38:453. doi: 10.1186/s13046-019-1446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ungermannova D., Parker S.J., Nasveschuk C.G., Wang W., Quade B., Zhang G., Kuchta R.D., Phillips A.J., Liu X. Largazole and its derivatives selectively inhibit ubiquitin activating enzyme (E1) PLoS ONE. 2012;7:e29208. doi: 10.1371/journal.pone.0029208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tsukamoto S., Hirota H., Imachi M., Fujimuro M., Onuki H., Ohta T., Yokosawa H. Himeic acid A: A new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp. Bioorg. Med. Chem. Lett. 2005;15:191–194. doi: 10.1016/j.bmcl.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 193.Hashimoto M., Kato H., Katsuki A., Tsukamoto S., Fujii I. Identification of the Biosynthetic Gene Cluster for Himeic Acid A: A Ubiquitin-Activating Enzyme (E1) Inhibitor in Aspergillus japonicus MF275. ChemBioChem. 2018;19:535–539. doi: 10.1002/cbic.201700584. [DOI] [PubMed] [Google Scholar]
- 194.Hann Z.S., Ji C., Olsen S.K., Lu X., Lux M.C., Tan D.S., Lima C.D. Structural basis for adenylation and thioester bond formation in the ubiquitin E1. Proc. Natl. Acad. Sci. USA. 2019;116:15475–15484. doi: 10.1073/pnas.1905488116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Itoh Y., Suzuki M. Design, synthesis, and biological evaluation of novel ubiquitin-activating enzyme inhibitors. Bioorg. Med. Chem. Lett. 2018;28:2723–2727. doi: 10.1016/j.bmcl.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 196.Chen J., Dexheimer T.S., Ai Y., Liang Q., Villamil M.A., Inglese J., Maloney D.J., Jadhav A., Simeonov A., Zhuang Z. Selective and cell-active inhibitors of the USP1/UAF1 deubiquitinase complex reverse cisplatin resistance in non-small cell lung cancer cells. Chem. Biol. 2011;18:1390–1400. doi: 10.1016/j.chembiol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tsukamoto S., Takeuchi T., Rotinsulu H., Mangindaan R.E., van Soest R.W., Ukai K., Kobayashi H., Namikoshi M., Ohta T., Yokosawa H. Leucettamol A: A new inhibitor of Ubc13-Uev1A interaction isolated from a marine sponge, Leucetta aff. microrhaphis. Bioorg. Med. Chem. Lett. 2008;18:6319–6320. doi: 10.1016/j.bmcl.2008.10.110. [DOI] [PubMed] [Google Scholar]
- 198.Pulvino M., Liang Y., Oleksyn D., DeRan M., Van Pelt E., Shapiro J., Sanz I., Chen L., Zhao J. Inhibition of proliferation and survival of diffuse large B-cell lymphoma cells by a small-molecule inhibitor of the ubiquitin-conjugating enzyme Ubc13-Uev1A. Blood Am. Soc. Hemat. 2012;120:1668–1677. doi: 10.1182/blood-2012-02-406074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ushiyama S., Umaoka H., Kato H., Suwa Y., Morioka H., Rotinsulu H., Losung F., Mangindaan R.E., de Voogd N.J., Yokosawa H., et al. Manadosterols A and B, sulfonated sterol dimers inhibiting the Ubc13–Uev1A interaction, isolated from the marine sponge Lissodendryx fibrosa. J. Nat. Prod. 2012;75:1495–1499. doi: 10.1021/np300352u. [DOI] [PubMed] [Google Scholar]
- 200.Choi H.J., Eun J.S., Kim B.G., Kim S.Y., Jeon H., Soh Y. Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells. Mol. Cells. 2006;22:291–299. [PubMed] [Google Scholar]
- 201.Yang Y., Ludwig R.L., Jensen J.P., Pierre S.A., Medaglia M.V., Davydov I.V., Safiran Y.J., Oberoi P., Kenten J.H., Phillips A.C., et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547–559. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 202.Matsuzawa M., Kakeya H., Yamaguchi J., Shoji M., Onose R., Osada H., Hayashi Y. Enantio-and Diastereoselective Total Synthesis of (+)-Panepophenanthrin, a Ubiquitin-Activating Enzyme Inhibitor, and Biological Properties of Its New Derivatives. Chem. Asian J. 2006;1:845–851. doi: 10.1002/asia.200600199. [DOI] [PubMed] [Google Scholar]
- 203.Vandenberghe I., Créancier L., Vispé S., Annereau J.P., Barret J.M., Pouny I., Samson A., Aussagues Y., Massiot G., Ausseil F., et al. Physalin B, a novel inhibitor of the ubiquitin-proteasome pathway, triggers NOXA-associated apoptosis. Biochem. Pharmacol. 2008;76:453–462. doi: 10.1016/j.bcp.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 204.Chen Y., Wang D.D., Wu Y.P., Su D., Zhou T.Y., Gai R.H., Fu Y.Y., Zheng L., He Q.J., Zhu H., et al. MDM2 promotes epithelial–mesenchymal transition and metastasis of ovarian cancer SKOV3 cells. Br. J. Cancer. 2017;117:1192–1201. doi: 10.1038/bjc.2017.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Downey-Kopyscinski S., Daily E.W., Gautier M., Bhatt A., Florea B.I., Mitsiades C.S., Richardson P.G., Driessen C., Overkleeft H.S., Kisselev A.F. An inhibitor of proteasome β2 sites sensitizes myeloma cells to immunoproteasome inhibitors. Blood Adv. 2018;2:2443–2451. doi: 10.1182/bloodadvances.2018016360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Niewerth D., van Meerloo J., Jansen G., Assaraf Y.G., Hendrickx T.C., Kirk C.J., Anderl J.L., Zweegman S., Kaspers G.J., Cloos J. Anti-leukemic activity and mechanisms underlying resistance to the novel immunoproteasome inhibitor PR-924. Biochem. Pharmacol. 2014;89:43–51. doi: 10.1016/j.bcp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 207.Li J., Yakushi T., Parlati F., Mackinnon A.L., Perez C., Ma Y., Carter K.P., Colayco S., Magnuson G., Brown B., et al. Capzimin is a potent and specific inhibitor of proteasome isopeptidase Rpn11. Nat. Chem. Bio. 2017;13:486–493. doi: 10.1038/nchembio.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hu S., Jin Y., Liu Y., Ljungman M., Neamati N. Synthesis and mechanistic studies of quinolin-chlorobenzothioate derivatives with proteasome inhibitory activity in pancreatic cancer cell lines. Eur. J. Med. Chem. 2018;158:884–895. doi: 10.1016/j.ejmech.2018.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Han K.H., Kwak M., Lee T.H., Park M.S., Jeong I.H., Kim M.J., Jin J.O., Lee P.C. USP14 inhibition regulates tumorigenesis by inducing autophagy in lung cancer in vitro. Int. J. Mol. Sci. 2019;20:5300. doi: 10.3390/ijms20215300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xu L., Wang J., Yuan X., Yang S., Xu X., Li K., He Y., Wei L., Zhang J., Tian Y. IU1 suppresses proliferation of cervical cancer cells through MDM2 degradation. Int. J. Mol. Sci. 2020;16:2951. doi: 10.7150/ijbs.47999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reinstein E., Ciechanover A. Narrative review: Protein degradation and human diseases: The ubiquitin connection. Ann. Intern. Med. 2006;145:676–684. doi: 10.7326/0003-4819-145-9-200611070-00010. [DOI] [PubMed] [Google Scholar]
- 212.Micel L.N., Tentler J.J., Smith P.G., Eckhardt G.S. Role of ubiquitin ligases and the proteasome in oncogenesis: Novel targets for anticancer therapies. J. Clin. Oncol. 2013;31:1231. doi: 10.1200/JCO.2012.44.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang Y., Kitagaki J., Dai R.M., Tsai Y.C., Lorick K.L., Ludwig R.L., Pierre S.A., Jensen J.P., Davydov I.V., Oberoi P., et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 2007;67:9472–9481. doi: 10.1158/0008-5472.CAN-07-0568. [DOI] [PubMed] [Google Scholar]
- 214.Barghout S.H., Patel P.S., Wang X., Xu G.W., Kavanagh S., Halgas O., Zarabi S.F., Gronda M., Hurren R., Jeyaraju D.V., et al. Preclinical evaluation of the selective small-molecule UBA1 inhibitor, TAK-243, in acute myeloid leukemia. Leukemia. 2019;33:37–51. doi: 10.1038/s41375-018-0167-0. [DOI] [PubMed] [Google Scholar]
- 215.Zhuang J., Shirazi F., Singh R.K., Kuiatse I., Wang H., Lee H.C., Berkova Z., Berger A., Hyer M., Chattopadhyay N., et al. Ubiquitin-activating enzyme inhibition induces an unfolded protein response and overcomes drug resistance in myeloma. Blood Am. Soc. Hematol. 2019;133:1572–1584. doi: 10.1182/blood-2018-06-859686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hyer M.L., Milhollen M.A., Ciavarri J., Fleming P., Traore T., Sappal D., Huck J., Shi J., Gavin J., Brownell J., et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 2018;24:186–193. doi: 10.1038/nm.4474. [DOI] [PubMed] [Google Scholar]
- 217.Best S., Hashiguchi T., Kittai A., Bruss N., Paiva C., Okada C., Liu T., Berger A., Danilov A.V. Targeting ubiquitin-activating enzyme induces ER stress–mediated apoptosis in B-cell lymphoma cells. Blood Adv. 2019;3:51–62. doi: 10.1182/bloodadvances.2018026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shen M., Schmitt S., Buac D., Dou Q.P. Targeting the ubiquitin–proteasome system for cancer therapy. Expert Opin. Ther. Targets. 2013;17:1091–1108. doi: 10.1517/14728222.2013.815728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sekizawa R., Ikeno S., Nakamura H., Naganawa H., Matsui S., Iinuma H., Takeuchi T. Panepophenanthrin, from a mushroom strain, a novel inhibitor of the ubiquitin-activating enzyme. J. Nat. Prod. 2002;65:1491–1493. doi: 10.1021/np020098q. [DOI] [PubMed] [Google Scholar]
- 220.Mehta G., Ramesh S.S. Enantioselective total synthesis of (+)-panepophenanthrin, a novel inhibitor of the ubiquitin-activating enzyme. Tetrahedron Lett. 2004;45:1985–1987. doi: 10.1016/j.tetlet.2003.12.149. [DOI] [Google Scholar]
- 221.Mori N., Yamada Y., Ikeda S., Yamasaki Y., Tsukasaki K., Tanaka Y., Tomonaga M., Yamamoto N., Fujii M. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-I–infected T-cell lines and primary adult T-cell leukemia cells. Blood Am. Soc. Hematol. 2002;100:1828–1834. doi: 10.1182/blood-2002-01-0151. [DOI] [PubMed] [Google Scholar]
- 222.Strickson S., Campbell D.G., Emmerich C.H., Knebel A., Plater L., Ritorto M.S., Shpiro N., Cohen P. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem. J. 2013;451:427–437. doi: 10.1042/BJ20121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cheng J., Fan Y.H., Xu X., Zhang H., Dou J., Tang Y., Zhong X., Rojas Y., Yu Y., Zhao Y., et al. A small-molecule inhibitor of UBE2N induces neuroblastoma cell death via activation of p53 and JNK pathways. Cell Death Dis. 2014;5:e1079. doi: 10.1038/cddis.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Garg P., Ceccarelli D.F., Keszei A.F., Kurinov I., Sicheri F., Sidhu S.S. Structural and functional analysis of ubiquitin-based inhibitors that target the backsides of E2 enzymes. J. Mol. Biol. 2020;432:952–966. doi: 10.1016/j.jmb.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ceccarelli D.F., Tang X., Pelletier B., Orlicky S., Xie W., Plantevin V., Neculai D., Chou Y.C., Ogunjimi A., Al-Hakim A., et al. An allosteric inhibitor of the human Cdc34 ubiquitin-conjugating enzyme. Cell. 2011;145:1075–1087. doi: 10.1016/j.cell.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 226.Zhang S., Sun Y. Targeting CDC34 E2 ubiquitin conjugating enzyme for lung cancer therapy. EBioMedicine. 2020;54:102718. doi: 10.1016/j.ebiom.2020.102718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sanders M.A., Brahemi G., Nangia-Makker P., Balan V., Morelli M., Kothayer H., Westwell A.D., Shekhar M.P. Novel inhibitors of Rad6 ubiquitin conjugating enzyme: Design, synthesis, identification, and functional characterization. Mol. Cancer Ther. 2013;12:373–383. doi: 10.1158/1535-7163.MCT-12-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kothayer H., Spencer S.M., Tripathi K., Westwell A.D., Palle K. Synthesis and in vitro anticancer evaluation of some 4, 6-diamino-1, 3, 5-triazine-2-carbohydrazides as Rad6 ubiquitin conjugating enzyme inhibitors. Bioorg. Med. Chem. Lett. 2016;26:2030–2034. doi: 10.1016/j.bmcl.2016.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sharma P., Nag A. CUL4A ubiquitin ligase: A promising drug target for cancer and other human diseases. Open Biol. 2014;4:130217. doi: 10.1098/rsob.130217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Al-Ghabkari A., Narendran A. In vitro characterization of a potent p53-MDM2 inhibitor, RG7112 in neuroblastoma cancer cell lines. Cancer Biother. Radiopharm. 2019;34:252–257. doi: 10.1089/cbr.2018.2732. [DOI] [PubMed] [Google Scholar]
- 231.Pan W.W., Zhou J.J., Yu C., Xu Y., Guo L.J., Zhang H.Y., Zhou D., Song F.Z., Fan H.Y. Ubiquitin E3 ligase CRL4CDT2/DCAF2 as a potential chemotherapeutic target for ovarian surface epithelial cancer. J. Biol. Chem. 2013;288:29680–29691. doi: 10.1074/jbc.M113.495069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Azmi A.S., Philip P.A., Beck F.W., Wang Z., Banerjee S., Wang S., Yang D., Sarkar F.H., Mohammad R.M. MI-219-zinc combination: A new paradigm in MDM2 inhibitor-based therapy. Oncogene. 2011;30:117–126. doi: 10.1038/onc.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zeng X., Sigoillot F., Gaur S., Choi S., Pfaff K.L., Oh D.C., Hathaway N., Dimova N., Cuny G.D., King R.W. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382–395. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.You L., Liu H., Huang J., Xie W., Wei J., Ye X., Qian W. The novel anticancer agent JNJ-26854165 is active in chronic myeloid leukemic cells with unmutated BCR/ABL and T315I mutant BCR/ABL through promoting proteosomal degradation of BCR/ABL proteins. Oncotarget. 2017;8:7777. doi: 10.18632/oncotarget.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Makii C., Oda K., Ikeda Y., Sone K., Hasegawa K., Uehara Y., Nishijima A., Asada K., Koso T., Fukuda T., et al. MDM2 is a potential therapeutic target and prognostic factor for ovarian clear cell carcinomas with wild type TP53. Oncotarget. 2016;7:75328. doi: 10.18632/oncotarget.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 237.Asatsuma-Okumura T., Ito T., Handa H. Molecular mechanisms of cereblon-based drugs. Pharmacol. Ther. 2019;202:132–139. doi: 10.1016/j.pharmthera.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 238.Azmi A.S., Aboukameel A., Banerjee S., Wang Z., Mohammad M., Wu J., Wang S., Yang D., Philip P.A., Sarkar F.H., et al. MDM2 inhibitor MI-319 in combination with cisplatin is an effective treatment for pancreatic cancer independent of p53 function. Eur. J. Cancer. 2010;46:1122–1131. doi: 10.1016/j.ejca.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kisselev A.F., Callard A., Goldberg A.L. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J. Biol. Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 240.Lawasut P., Chauhan D., Laubach J., Hayes C., Fabre C., Maglio M., Mitsiades C., Hideshima T., Anderson K.C., Richardson P.G. New proteasome inhibitors in myeloma. Curr. Hematol. Malig. Rep. 2012;7:258–266. doi: 10.1007/s11899-012-0141-2. [DOI] [PubMed] [Google Scholar]
- 241.McHugh A., Fernandes K., South A.P., Mellerio J.E., Salas-Alanís J.C., Proby C.M., Leigh I.M., Saville M.K. Preclinical comparison of proteasome and ubiquitin E1 enzyme inhibitors in cutaneous squamous cell carcinoma: The identification of mechanisms of differential sensitivity. Oncotarget. 2018;9:20265. doi: 10.18632/oncotarget.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Meng L., Mohan R., Kwok B.H., Elofsson M., Sin N., Crews C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl. Acad. Sci. USA. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sidor-Kaczmarek J., Cichorek M., Spodnik J.H., Wójcik S., Moryś J. Proteasome inhibitors against amelanotic melanoma. Cell Biol. Toxicol. 2017;33:557–573. doi: 10.1007/s10565-017-9390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhou Y., Wang K., Zhen S., Wang R., Luo W. Carfilzomib induces G2/M cell cycle arrest in human endometrial cancer cells via upregulation of p21Waf1/Cip1 and p27Kip1. Taiwan J. Obstet. Gynecol. 2016;55:847–851. doi: 10.1016/j.tjog.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 245.Vogl D.T., Martin T.G., Vij R., Hari P., Mikhael J.R., Siegel D., Wu K.L., Delforge M., Gasparetto C. Phase I/II study of the novel proteasome inhibitor delanzomib (CEP-18770) for relapsed and refractory multiple myeloma. Leuk. Lymphoma. 2017;58:1872–1879. doi: 10.1080/10428194.2016.1263842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable, the study did not report any data.