Abstract
Uterine adenomyosis is a commonly encountered estrogen-dependent disease in reproductive-age women, causing heavy menstrual bleeding, intense pelvic pain, and infertility. Although adenomyosis was previously considered a disease of multiparous women, it is becoming increasingly evident that it also affects younger nulliparous women and may compromise their fertility potential. It is clear that hysterectomy, the standard approach to definitively manage the disease, is not an option for patients wishing to preserve their fertility, so there is an urgent need to develop novel conservative strategies. We searched the current literature for available methods for conservative management of adenomyosis, including both pharmacological and surgical approaches. There is no existing drug that can cure adenomyosis at present, but some off-label treatment options may be used to tackle disease symptoms and improve fertility outcomes. Adenomyosis in patients wishing to conceive can be ‘treated’ by conservative surgery, though these procedures require highly experienced surgeons and pose a considerable risk of uterine rupture during subsequent pregnancies. While currently available options for conservative management of adenomyosis do have some capacity for alleviating symptoms and enhancing patient fertility perspectives, more effective new options are needed, with gonadotropin-releasing hormone antagonists showing encouraging results in preliminary studies.
Keywords: uterine adenomyosis, infertility, heavy menstrual bleeding, dysmenorrhea, medical treatment, GnRH antagonist, conservative surgery
1. Introduction
Uterine adenomyosis is a chronic estrogen-dependent condition affecting approximately 20% of gynecology patients [1]. Its most common symptoms include heavy menstrual bleeding, intense pelvic pain, and infertility. Despite the high prevalence and severe symptoms of the disease, its pathogenesis is not yet fully understood [1,2]. A lack of critical information on the origin of the disease and the absence of a universal classification system create a kind of therapeutic anarchy, with approximately 82% of adenomyosis patients eventually resorting to hysterectomy, a somewhat extreme way to deal with the disease [3,4,5].
We searched the PubMed database (https://pubmed.ncbi.nlm.nih.gov/, accessed during 2020 and 2021 until 11 July 2021) for English-language articles focusing on adenomyosis, its main symptoms (menorrhagia/heavy menstrual bleeding, dysmenorrhea, pelvic pain, infertility), and treatment options. Key words and phrases included ‘medical’ or ‘pharmaceutical’ or ‘surgical treatment,’ as well as specific names of drug categories and procedures. Reference lists of studies of interest were also screened for potentially matching articles.
Our search revealed that there is as yet no drug specifically registered to treat adenomyosis, but various off-label treatments have emerged over the years, some of them with acceptable efficacy [6]. On the other hand, conservative surgical approaches have been developed, ranging from partial reduction surgery to complete adenomyosis excision, but these methods remain a source of controversy, primarily due to the risk of uterine rupture during subsequent pregnancies. The choice of suitable therapy depends on individual patient age, severity of symptoms, and reproductive status, and can vary from full-scale hysterectomy to medical management of clinical symptoms and infertility [6]. A number of small studies have been conducted, but are still insufficient to determine universal management guidelines. The specific medical and surgical approaches we identified and their associated outcomes based on published reports are presented below.
2. Medical Treatment of Adenomyosis
Currently available medical therapies for adenomyosis aim to alleviate local hyperestrogenism and tackle the most severe symptoms of adenomyosis, namely heavy menstrual bleeding, dysmenorrhea, and non-menstrual pelvic pain. Little is known about the exact causes underlying adenomyosis-related infertility, but pharmacological management protocols have been developed with favorable results, as discussed further in this review. Here, we explore the most popular medical treatments used to manage the disease. A summary of the advantages and drawbacks of each of these medications can be found in Table 1.
Table 1.
Advantages and drawbacks of the main drug groups used against adenomyosis symptoms.
| Medication | Advantages | Drawbacks |
|---|---|---|
| NSAIDs | Effective against mild pain | Extensive use linked to side effects |
| Non-hormonal composition | Inability to treat underlying causes of pain | |
| Safe for women wishing to conceive | Questionable effectiveness against HMB | |
| COCs | Relatively effective in relieving pain | Limited efficacy in reducing HMB and uterine volume |
| Fewer side effects than other drugs | Risk of thromboembolic events | |
| Progestins | Alleviate local hyperestrogenism | Ineffective in about one-third of patients |
| Relieve pain symptoms | Frequently cause metrorrhagia at varying severity | |
| Possibility of long-term symptom management | Doubtful efficacy in diminishing uterine volume | |
| Ample evidence in favor of LNG-IUD use | ||
| UPA | Lower serum estradiol levels | Numerous reports of symptom and imaging feature exacerbation |
| May reduce HMB | Limited to restricted indications by the European Medicines Agency | |
| GnRH agonists | Alleviate pain | Flare-up effect |
| Induce amenorrhea | Severe hypoestrogenic side-effects (i.e., vasomotor syndrome, loss of BMD) | |
| Reduce uterine volume and JZ thickness | Long-term administration not indicated even with add-back therapy | |
| Beneficial as pre-treatment in infertile patients | ||
| GnRH antagonists | Easy and tolerable oral administration | Loss of BMD at high doses |
| Rapid action skipping initial flare-up | Less efficient when combined with add-back medication | |
| Effectively reduce HMB and pain symptoms | ||
| Dose-dependent estradiol suppression with less severe side-effects |
NSAIDs: non-steroidal anti-inflammatory drugs; HMB: heavy menstrual bleeding; COCs: combined oral contraceptives; LNG-IUD: levonorgestrel-releasing intrauterine device; UPA: ulipristal acetate; GnRH: gonadotropin-releasing hormone; JZ: junctional zone; BMD: bone mineral density.
2.1. Non-Steroidal Anti-Inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) are the most frequently prescribed class of drugs to treat dysmenorrhea, especially primary dysmenorrhea, showing acceptable efficiency but being prone to adverse side effects when used extensively [7,8]. NSAIDs block cyclooxygenase, suppress prostaglandin, and alleviate uterine hypercontractility, so they relieve pain without treating potential underlying causes, as in case of adenomyosis and endometriosis [8]. Regarding heavy menstrual bleeding, one systematic review concluded that NSAIDs are more effective at treating this particular symptom compared to placebo, but not as effective as other drug categories like progestins [9]. Despite their questionable effectiveness and inability to treat the underlying pathogenic mechanisms of adenomyosis, the non-hormonal nature of NSAIDs might render them an attractive option for women with mild symptoms seeking to conceive.
2.2. Combined Oral Contraceptives
Combined oral contraceptives (COCs) act by inhibiting follicle-stimulating hormone (FSH) and luteinizing hormone (LH), subsequently blocking follicle development and endometrial proliferation, which is why they were proposed to treat adenomyosis-related symptoms [10]. To the best of our knowledge, two studies have investigated their use in the clinical management of adenomyosis, comparing them with other available options [11,12]. According to Shaaban et al., a 6-month treatment with COCs did alleviate pain in adenomyosis patients, but both pain and uterine volume showed more pronounced decrease in the levonorgestrel-releasing intrauterine device (LNG-IUD)-treated group [11]. Consistent with these findings, Hassanin et al. found COCs to be effective at diminishing pain, while dienogest was superior at reducing bleeding and uterine volume, but at the cost of more side effects [12]. It should be noted, however, that extensive use of COCs has been linked to increased risk of thromboembolic events [13].
2.3. Progestins
There is ample evidence on the use of oral and systemic progestins for treatment of adenomyosis and endometriosis. Progestins exert a broad range of contraceptive effects, including on the endometrium, which they render non-receptive to embryos [14]. They have become popular in adenomyosis management thanks to their ability to suppress secretion of FSH and LH, ultimately inhibiting ovarian steroid formation and allaying adenomyosis-related hyperestrogenism [15].
Numerous clinical trials have focused on use of dienogest, an oral progestin, most of them with encouraging results. However, a pilot study in 15 patients receiving dienogest for 24 weeks failed to demonstrate significant reduction in either uterine or lesion size or the width of the junctional zone, though pain relief levels were acceptable [16]. Moreover, anemia due to metrorrhagia was observed in one-third of patients receiving the treatment. Indeed, a number of clinical trials on long-term use of dienogest in women with dysmenorrhea have reported metrorrhagia as the most common side effect, occurring in almost all subjects at varying severity [17,18]. A shorter course of dienogest therapy (16 weeks) was still associated with abnormal menstrual bleeding, but not severe anemia in most patients [19]. According to a small retrospective study, patients with intrinsic adenomyosis (based on the classification of Kishi et al. [20]) may be more prone to developing serious menorrhagia upon dienogest administration than other disease subtypes [21]. Nevertheless, current data are insufficient to determine whether the subtype of adenomyosis can predict the severity of side effects.
Similar results have been obtained with subcutaneous progestin (etonogestrel), particularly a clear reduction in pain symptoms, but vaginal bleeding caused several patients to have their implants removed [22,23,24]. Decreased uterine volume, an important parameter linked to menorrhagia, may or may not be achieved in adenomyosis patients treated with etonogestrel, depending on the study [23,24].
The LNG-IUD, an intrauterine version of progestin, has also been proposed to manage symptomatic adenomyosis, yielding satisfactory outcomes, but with some debate around appropriate treatment durations [11,25,26,27,28,29,30]. One clinical trial involving 47 patients reported pain relief from the sixth week after insertion, and a significant decrease in mean uterine volume by month 12 [28]. In the same study, however, it was noted that efficacy declined over time, with pain and uterine volume increasing two years after insertion. On the contrary, another trial demonstrated more long-term effects of the LNG-IUD against adenomyosis symptoms, as both dysmenorrhea and uterine volume remained significantly reduced after 3 years of follow-up of 94 enrolled patients [29]. A more recent retrospective study suggested that the beneficial impact of LNG-IUDs on heavy bleeding and dysmenorrhea, but not uterine volume reduction, may last up to six years post-insertion [30].
The major disadvantage of progestin use is that approximately one-third of patients do not respond to these drugs, most likely due to progesterone resistance [15,31,32]. As in endometriosis, progesterone receptors in adenomyotic uteri are either diminished or inactive, thus inhibiting the action of both local and synthetic progesterone forms [1,15,31,32]. As also described in our previous review article, other important limitations of progestins include doubtful efficacy in reducing uterine volume and possible links to venous or arterial embolism events [15]. Taken together, progestins constitute a common and acceptable option for managing adenomyosis symptoms, but the high percentage of non-responders highlights the need to develop new options.
2.4. Ulipristal Acetate
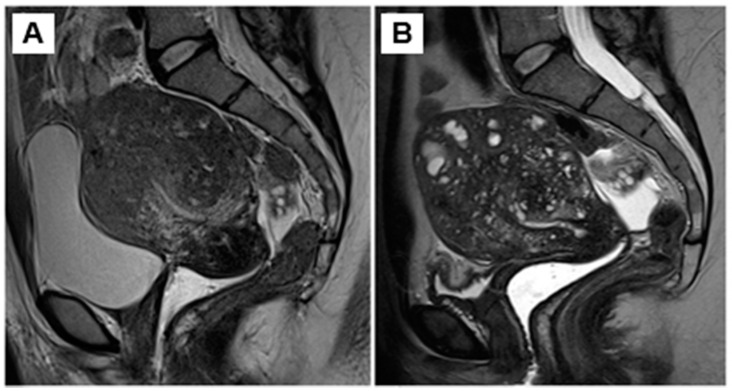
Ulipristal acetate (UPA) is a selective progesterone receptor modulator currently registered for specific clinical indications: emergency contraception and long-term management of symptomatic uterine fibroids or prior to myoma surgery [33,34]. It is capable of delaying ovulation and endometrial maturation by lowering serum estradiol levels [35]. Based on these properties, UPA has been tested in managing adenomyosis symptoms, but only limited clinical evidence supports its use. Two recent studies reported a diminution in blood loss after a 12-week treatment with UPA in 30 patients with adenomyosis and 41 patients with concurrent adenomyosis and leiomyoma [36,37]. Conversely, three studies have recorded worsening of symptoms and imaging features of adenomyosis, suggesting that it may be time to abandon this option [38,39,40]. Donnez and Donnez recently reported aggravation of symptoms and magnetic resonance imaging (MRI) features of adenomyosis after a 3-month course of UPA (Figure 1) [38]. Similarly, Conway et al. demonstrated exacerbation of ultrasound characteristics of adenomyosis in women inappropriately treated with UPA for leiomyomas, observing enlarged intramyometrial cysts and enhanced vascularization [39]. Most patients also experienced increased pain after treatment, including pelvic pain, dyspareunia, and bowel symptoms, leading the authors to emphasize the importance of accurate diagnosis of adenomyosis, and effectively dismissing UPA as a credible treatment for the disease [39]. Finally, Calderon et al. observed progression of adenomyosis by MRI as early as three months after UPA treatment initiation in patients with concurrent leiomyomas [40].
Figure 1.
(A) MRI showing an enlarged uterus with diffuse and disseminated adenomyosis. (B) Worsening of MRI features after therapy with UPA. Numerous spots typical of adenomyosis are visible: asymmetric heterogeneous myometrium with multiple myometrial cysts, related to dilated islets of ectopic endometrium.
2.5. Gonadotropin-Releasing Hormone (GnRH) Agonists
GnRH agonist competitively binds to the natural GnRH receptors, but has a longer life span than the endogenous peptide [41]. Its use in the context of adenomyosis relies on its anti-proliferative effects in the myometrium, following the suppression of gonadotropin secretion and the subsequent drastic decline in estradiol [6]. The efficacy of GnRH agonist at reducing uterine volume, inducing amenorrhea, and relieving pain was demonstrated by case reports as far back as three decades ago [42,43]. It was subsequently shown that a 6-month course of GnRH agonist was able to significantly reduce both uterine volume and JZ thickness in 18 adenomyosis patients, some of whom had concurrent endometriosis [44]. Its effect on chronic pelvic pain symptoms also appears considerable, curbing pain medication use and curtailing lost work productivity [45].
Despite these promising results, GnRH agonists are associated with severe hypoestrogenic side effects, especially a reduction in bone mineral density, so they cannot be used long-term, while symptoms tend to reappear upon treatment cessation [13,46]. Add-back therapy is helpful in decreasing this effect, but experts in the field have argued that long-term treatment of adenomyosis with GnRH agonist should be limited to cases not responding to other medications or where surgical intervention is not a safe option [6,47]. On the other hand, pre-treatment with GnRH agonist in infertile patients seeking undergoing assisted reproduction may indeed be a viable option, as discussed further in this review [48].
2.6. Oral GnRH Antagonists
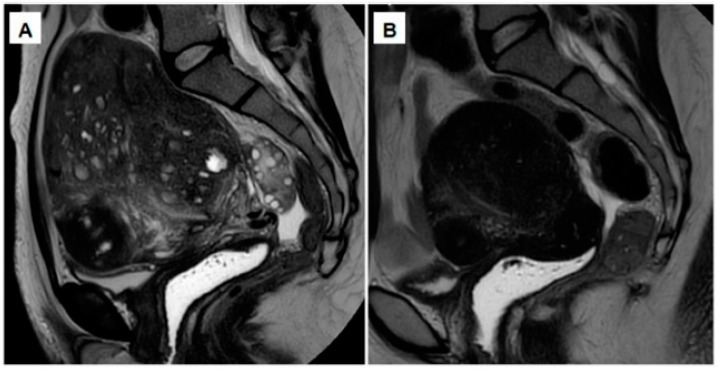
While not yet registered for clinical use against symptomatic adenomyosis, oral GnRH antagonists constitute an attractive option, increasingly gaining the interest of the medical community and yielding promising results in preliminary studies [49,50]. These molecules antagonize GnRH binding to its receptors, leading to dose-dependent suppression of FSH and LH and a subsequent decline in ovarian steroids [15,41]. The major advantages of these drugs over GnRH agonists are: (i) their water-soluble nature, allowing easy and tolerable oral administration; (ii) rapid action without the initial 1–2 weeks of the flare-up effect; (iii) dose-dependent and easily reversible modulation of the hypothalamic-pituitary-gonadal axis; and (iv) incomplete suppression of FSH and LH when administered at low doses, hence avoiding related side effects [15]. Early data indeed demonstrated their effectiveness in lesion regression, reduction of uterine volume, and alleviation of clinical symptoms in a patient with severe adenomyosis [38] (Figure 2). The results of a more recently published study [51] further provide evidence that linzagolix administered at a high dose (200 mg daily) for 12 weeks, followed by a lower maintenance dose (100 mg daily) for 12 weeks, substantially reduced uterine volume and adenomyotic lesions, as well as adenomyosis-related pain. Future studies will determine the advantages of continuing the intake of linzagolix 100 mg daily after week 24 to avoid regrowth of the uterus [51]. Moreover, Borini and Coticchio discussed the potential superiority of GnRH antagonist over GnRH agonist in patients wishing to preserve their fertility, primarily because it does not radically suppress circulating LH, and its impact on the hypothalamic-pituitary-gonadal axis can be easily reversed [52]. This is a major advantage in the context of infertility management and, as described in a recent review paper, would entail oocyte retrieval and vitrification followed by a 12-week course of treatment to reduce lesion size and uterine volume prior to frozen-thawed embryo transfer [53]. Ongoing and future clinical trials are expected to confirm the efficacy and safety of GnRH antagonists against symptomatic adenomyosis and its potential benefits for infertile patients.
Figure 2.
(A) MRI showing an enlarged uterus, consistent with severe full-thickness adenomyosis. (B) After a 12-week course of GnRH antagonist (200 mg linzagolix daily), a reduction can be observed in both uterine size and adenomyotic foci.
Despite limited data in the context of adenomyosis, oral GnRH antagonist-based elagolix and linzagolix have proved efficient in treating endometriosis-related pain and improving patient quality of life [54,55,56,57]. Moreover, although one trial showed loss of bone mineral density at higher doses, add-back therapy could be avoided with the 75 mg dose, while still maintaining the treatment’s beneficial effects [55]. Consistent with these findings, an ongoing clinical trial has reported that relugolix, another drug in the same category, can also successfully reduce endometriosis-associated pain when administered for up to 24 weeks at a dose of 40 mg [58]. Indeed, we have already stressed that adenomyosis and endometriosis, particularly deep endometriosis, frequently coexist and may as well share the same pathogenic origin, suggesting the potential value of extrapolating treatment results from one condition to the other [59,60].
2.7. Experimental Approaches and Preclinical Models
2.7.1. Bromocriptine
A variety of medical treatments for adenomyosis have been proposed primarily based on findings from preclinical models, but have served little or no clinical purpose so far. One such treatment is bromocriptine, a dopamine agonist frequently prescribed to manage hyperprolactinemia. Its use is based on the observation that prolactin is aberrantly expressed and may induce an adenomyosis-like phenotype in mouse models [61,62]. In studies by Andersson et al., a 6-month course of vaginal bromocriptine was found to significantly reduce adenomyosis-associated pain and JZ thickness in a small number of patients [63,64]. Uterine bleeding also declined post-treatment but remained high [63]. Indeed, high serum prolactin has been linked to endometriosis-related infertility, but a strong causal link between this protein and adenomyosis or endometriosis has not yet been identified [65]. The association of adenomyosis and aberrant prolactin signaling is almost exclusively corroborated by findings in a mouse model, so its mechanisms of action in human adenomyosis development and symptoms are unclear [65].
2.7.2. Aromatase Inhibitors
Aromatase inhibitors have also been theoretically proposed, but have not yet found widespread clinical application in the context of adenomyosis. In one case report, concomitant administration of an oral aromatase inhibitor (anastrozole) and a GnRH agonist (goserelin acetate) was successful in reducing uterine volume and inhibiting bleeding in a patient with endometriosis and severe adenomyosis, who was resistant to progestin [66]. To the best of our knowledge, only one prospective study involving 32 patients has analyzed the effectiveness of letrozole compared to GnRH agonist in treating adenomyosis [67]. A significant reduction in uterine volume was observed post-treatment in both study groups, with GnRH agonist emerging as more effective in the fourth and eighth weeks of treatment. It was also superior in relieving menorrhagia, dysmenorrhea, dyspareunia, and non-menstrual pelvic pain. All in all, data supporting use of aromatase inhibitors against adenomyosis symptoms remain limited and inconclusive, with endometrial and ectopic lesion production of aromatase still a matter of debate [68,69,70].
2.7.3. Anti-Platelet Therapy
One mouse model-based study suggested the potential utility of ozagrel, an anti-platelet drug, for treatment of adenomyosis, after observing enhanced platelet aggregation in mouse and human lesions [71,72]. More specifically, anti-platelet therapy appeared to lessen uterine contractility, hyperalgesia, and immune cell infiltration in an adenomyosis mouse model [71]. It should, however, be pointed out that the potential impact of such treatment on heavy menstrual bleeding cannot be investigated in non-menstruating mice. No data are yet available on the possible effect of ozagrel or other anti-platelet agents on human adenomyosis, while the actual role of platelet aggregation in its pathogenesis remains contentious [60,73].
2.7.4. MicroRNAs
Developing a novel drug targeting adenomyosis-related signaling pathways without affecting nearby tissues certainly sounds appealing. Indeed, microRNA therapeutics are increasingly attracting the attention of the scientific community thanks to their unique ability to specifically target and simultaneously silence multiple genes, including genes crucial to human disease development. In theory, restoring expression of selected dysregulated microRNAs would result in transcriptional regulation of their downstream pathways and, hence, disease treatment. It has been demonstrated that several microRNAs are dysregulated in endometrium from adenomyosis patients, raising hopes of developing a treatment by normalizing expression of these molecules [74]. According to subsequent studies, let-7a, miR-145-5p, miR-17, and miR-2861 have all been found to be differentially expressed in adenomyosis and may constitute targets for development of novel therapies against the disease [75,76,77,78]. Nevertheless, there is still a huge gap to bridge between in vitro studies and development of a drug for clinical application, with the first ever small RNA-based therapeutic (patisiran) only obtaining FDA approval as recently as 2018.
2.8. Medical Management of Infertility in Adenomyosis Patients
Adenomyosis has been repeatedly associated with both poor in vitro fertilization (IVF) outcomes and pregnancy complications [79,80]. The underlying mechanisms remain unclear, but a variety of factors have been suspected of contributing to adenomyosis-related infertility. Uterine hypercontractility, a hallmark of adenomyosis, may inhibit physiological sperm transport through the female reproductive tract, thus inhibiting oocyte fertilization [81]. Impaired stromal decidualization may constitute another reason, given the frequently encountered resistance to progesterone in adenomyosis patients, a hormone playing a pivotal role in the transition of endometrium to its decidualized state [1,15,82,83]. On the other hand, the chronic inflammatory endometrial environment may be the underlying cause of adenomyosis-related infertility, as it is hostile to embryos [84]. Treating adenomyosis-related hyperestrogenism, hypercontractility, and local inflammation, therefore, appears to be crucial to enhancing patient fertility.
A number of studies have suggested the possible utility of GnRH agonist pre-treatment in infertile adenomyosis patients undergoing IVF. In fact, according to a study of 45 subjects, triptorelin with add-back therapy may be beneficial to infertile patients irrespective of IVF, by reducing uterine size and enhancing elasticity, hence facilitating spontaneous pregnancy [85]. Several case reports have further demonstrated effectiveness in patients with severe adenomyosis suffering from secondary infertility [86,87,88]. One of these women later presented with severe pregnancy complications due to insufficiently decidualized endometrium, but it was not clear whether this event was related to the effect of GnRH agonist [88]. Niu et al. reported a significant improvement in rates of clinical pregnancy, successful implantation, and ongoing pregnancy in 339 infertile patients with adenomyosis undergoing treatment with GnRH agonist prior to frozen embryo transfer [48]. A more recent study, however, failed to identify a significant difference in clinical pregnancy rates between 241 treated and untreated infertile adenomyosis patients in this category [89]. Two studies concluded that conservative surgery combined with GnRH agonist, rather than GnRH agonist alone, is able to improve symptoms and boost reproductive potential in adenomyosis patients [90,91]. Finally, an ultra-long GnRH protocol before ovarian stimulation appears to yield better pregnancy outcomes than the corresponding long protocol [92,93]. More specifically, one study reported significantly higher pregnancy and live birth rates in patients with diffuse adenomyosis receiving a 3.75 mg dose of diphereline subcutaneously on a monthly basis for 2–4 months prior to stimulation compared to corresponding patients receiving a single 0.93–1.87 mg dose on the 18th–20th day [92]. Consistent with these findings, a second study reported a significant increase in rates of both clinical pregnancy and live births in adenomyosis patients pre-treated with triptorelin (3.75 mg) intramuscularly every 28 days for at least 3 months, compared with 0.1 mg/day dose for 10 days [93].
3. Surgical Management of Adenomyosis
The first conservative surgical therapy for adenomyosis in young women, namely adenomyomectomy, dates all the way back to 1952 [94]. In the 1970s, cytoreductive surgery in the form of partial excision of adenomyotic tissue became more popular [95]. Common approaches to conservative partial or complete adenomyosis excision are summarized below.
3.1. Partial Reduction Surgery
Wedge Resection
Wedge resection involves identifying the seromuscular layer where the adenomyoma is located, before removing parts of the affected serosa and uterine adenomyosis. In 1991, a modification to partial adenomyosis excision was reported, which entailed cutting the adenomyomatous tissue into thin slices using a microsurgical technique [95]. A transverse H incision was then made on the uterine fundus. After removal of the adenomyotic tissue, the myometrial edges were sutured to ensure hemostasis in one or two layers. Serosal flaps resulting from the vertical and transverse incisions were closed with interrupted subserosal sutures. In a study by Fujishita et al., 41 patients underwent the H incision technique and 31 attempted to conceive [96]. Twelve women became pregnant of whom 5 (16.1%) miscarried, leaving 7 (22.5%) reported live births.
3.2. Complete Adenomyosis Excision
3.2.1. The Triple-Flap Method
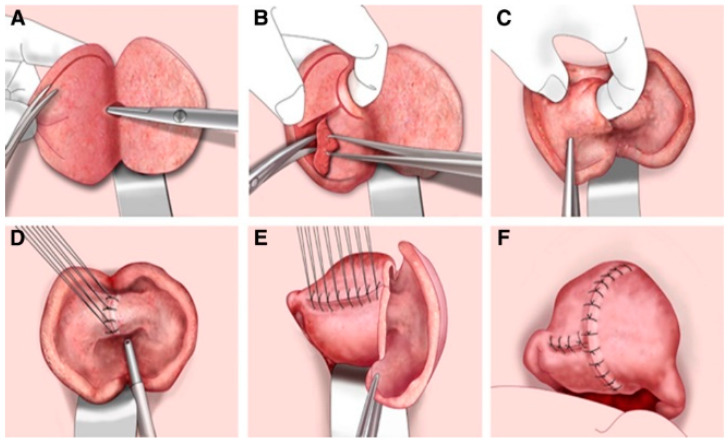
With a view to future pregnancy, the triple-flap method has been proposed as an innovative and safer technique for removal of adenomyosis and reconstruction of the uterine wall [95,97]. The approach involves complete extraction of the uterine adenomyosis, followed by reconstruction of the uterine cavity to allow it to sustain subsequent pregnancy, inside which an endometrial uterine muscle flap is created by metroplasty (Figure 3). Uterine muscle located on the serosal side is used to fill the large uterine wall muscle defect. This technique, therefore, repairs the uterine wall defect using normal uterine muscle and, according to Osada, is effective for both diffuse and nodular adenomyosis, potentially helping to prevent uterine rupture [95]. The triple-flap method is extremely difficult to perform by laparoscopic surgery, so open surgery is preferable. Indeed, removal of adenomyotic tissue is ideally carried out under hand palpation, with careful suture of the myometrial layers. In one study, blood flow in the operated area returned to normal within 6 months in 81.4% of women (n = 113) undergoing the triple-flap technique. Of 62 women who wished to conceive, 32 (51%) gave birth to a healthy baby by elective cesarean section [98].
Figure 3.
Schematic representation of the triple-flap method applied to the anterior and posterior uterine wall. The approach involves complete extraction of the uterine adenomyosis (A–C), followed by reconstruction of the uterine cavity (D–F). An endometrial uterine muscle flap is created by metroplasty and uterine muscle located on the serosal side is used to fill the large uterine wall muscle defect (E,F) (reprinted with permission from [95]).
3.2.2. Asymmetric Dissection
The asymmetric dissection method is an alternative approach that involves incision of the uterus longitudinally and dissection of the myometrium diagonally, before making a transverse incision to open the uterine cavity [95]. While inserting the index finger into the uterine cavity, the adenomyotic lesion is excised to >5 mm from the inner myometrium, and then excised to >5 mm from the serosal myometrium on the left uterine side. The uterine cavity is subsequently sutured and closed, followed by uterine reconstruction, with the left side covering the right. Uterine rupture was observed in 5 cases out of a series of 1349 patients undergoing this technique [99,100].
3.3. Important Remarks on the Surgical Approach
It should be noted that the boundary between adenomyotic tissue and normal myometrium is typically felt by palpation with fingers, so adenomyotic tissue dissection/resection often requires open surgery with all the risks it entails. However, while these experimental techniques are currently performed via an open approach, it is not inconceivable that these same techniques could be adopted via laparoscopy, especially with the benefits of a robotic surgical platform. On the other hand, due to the absence of a cleavage plane, adenomyotic tissue is sometimes overlooked and repair of the uterine wall becomes difficult. Osada pointed out that forced reconstruction of the uterine wall would strain the tissue abnormally and likely result in ruptured sutures and anastomotic leakage [95]. In the view of many gynecologists, diffuse lesions must be excised by laparotomy, which is also needed for the suture of myometrial tissue, and is far more challenging after adenomyotic resection than after myomectomy. Laparoscopy should be restricted to well delimited and focal adenomyomas.
Most importantly, we require clarification on the elevated risk of uterine rupture during pregnancy after adenomyosis surgery, which is provided in Osada’s review [95]. Uterine rupture is a serious obstetrical complication, sometimes even endangering the life of the patient, and its incidence is much higher after adenomyosis excision than after myomectomy. The surgeon should take care to avoid discoloration and heat denaturation of the incision edges, which would make the suture area more fragile and probably increase the risk of rupture. Giving patients and gyne-obstetricians accurate information and obtaining informed preoperative consent are absolutely vital.
4. Non-Surgical Alternatives
Uterine artery embolization (UAE) has been proposed as a less invasive alternative to hysterectomy to treat adenomyosis. During this angiographic procedure, an embolic agent is injected into the uterine arteries to induce ischaemic infarction of adenomyotic foci [101,102]. In an extensive review by de Bruyn et al. [101], it was showed that UAE improves clinical symptoms in 83% of patients, but great JZ thickness is associated with decreased effectiveness. The authors also concluded that there is a lack of strong clinical evidence regarding UAE and randomized controlled trials are essential to determine if this procedure is a valuable alternative to hysterectomy. Indeed, as clarified by the same authors, limitations are numerous and include the retrospective evaluation of data, small sample size, and vague description of methodology [101]. Moreover, no solid conclusion concerning uterine volume can be drawn, and fertility after UAE was not evaluated.
In a more recent study [102], women having undergone UAE for adenomyosis were contacted via SMS, email, or phone calls and invited to participate in the follow-up study on a voluntary basis. Improvement of symptoms was indeed observed in 80% of patients responding to the survey, but it should be stressed that recall bias may limit the accuracy of these findings. Unfortunately, data on uterine volume after UAE are missing from this study as well. Future randomized clinical trials should evaluate if UAE should be offered to women in whom conservative management for adenomyosis has failed as a less invasive alternative to hysterectomy.
5. Conclusions
Uterine adenomyosis remains an enigmatic disease and there are currently no widely accepted guidelines on its conservative management. The surgical approach is a source of concern, because of the risk of complications during subsequent pregnancy, pushing us to explore new options. Novel treatment with GnRH antagonist has already yielded encouraging results [14,39,49]. However, appropriate therapy for each patient needs to be carefully determined, taking into account lesion size, severity of symptoms, potential wish to conceive, and presence or absence of infertility. Medical treatment is the option of choice to improve clinical symptoms and partially reverse infertility, especially in milder cases. Conservative surgery may be required when lesions persist, bearing in mind all the associated risks.
Acknowledgments
The authors thank Mira Hryniuk for reviewing the English language of the manuscript.
Author Contributions
Conceptualization, C.A.S. and J.D.; methodology, investigation, data curation, writing—original draft preparation, C.A.S. and J.D.; writing—review and editing, C.A.S., J.D. and M.-M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fonds National de la Recherche Scientifique de Belgique (grant FC39041 awarded to C.A.S. and grant 5/4/150/5 awarded to M.-M.D.), PregLem via the Fondation Saint-Luc (grant awarded to C.A.S.), and donations from the Ferrero family.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Jacques Donnez is a member of the Scientific Advisory Board of Obseva and PregLem. Christina Anna Stratopoulou and Marie-Madeleine Dolmans declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stratopoulou C.A., Donnez J., Dolmans M.M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021;28:2087–2097. doi: 10.1007/s43032-020-00361-w. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J., Donnez O., Dolmans M.M. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil. Steril. 2018;109:369–370. doi: 10.1016/j.fertnstert.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Garry R. The endometriosis syndromes: A clinical classification in the presence of aetiological confusion and therapeutic anarchy. Hum. Reprod. 2004;19:760–768. doi: 10.1093/humrep/deh147. [DOI] [PubMed] [Google Scholar]
- 4.Habiba M., Gordts S., Bazot M., Brosens I., Benagiano G. Exploring the challenges for a new classification of adenomyosis. Reprod. Biomed. Online. 2020;40:569–581. doi: 10.1016/j.rbmo.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Yu O., Schulze-Rath R., Grafton J., Hansen K., Scholes D., Reed S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020;223:94.e1–94.e10. doi: 10.1016/j.ajog.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Vannuccini S., Luisi S., Tosti C., Sorbi F., Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil. Steril. 2018;109:398–405. doi: 10.1016/j.fertnstert.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Marjoribanks J., Ayeleke R.O., Farquhar C., Proctor M. Nonsteroidal anti-inflammatory drugs for primary dysmenorrhoea. Cochrane Database Syst. Rev. 2015;2015:CD001751. doi: 10.1002/14651858.CD001751. [DOI] [PubMed] [Google Scholar]
- 8.Iacovides S., Avidon I., Baker F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update. 2015;21:762–778. doi: 10.1093/humupd/dmv039. [DOI] [PubMed] [Google Scholar]
- 9.Bofill Rodriguez M., Lethaby A., Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2019;9:CD000400. doi: 10.1002/14651858.CD000400.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera R., Yacobson I., Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 1999;181:1263–1269. doi: 10.1016/S0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- 11.Shaaban O.M., Ali M.K., Sabra A.M., Abd El Aal D.E. Levonorgestrel-releasing intrauterine system versus a low-dose combined oral contraceptive for treatment of adenomyotic uteri. A randomized clinical trial. Contraception. 2015;92:301–307. doi: 10.1016/j.contraception.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Hassanin A.I., Youssef A.A., Yousef A.M., Ali M.K. Comparison of dienogest versus combined oral contraceptive pills in the treatment of women with adenomyosis. A randomized clinical trial. Int. J. Gynaecol. Obstet. 2021;154:263–269. doi: 10.1002/ijgo.13600. [DOI] [PubMed] [Google Scholar]
- 13.Berlanda N., Somigliana E., Viganò P., Vercellini P. Safety of medical treatments for endometriosis. Expert Opin. Drug Saf. 2016;15:21–30. doi: 10.1517/14740338.2016.1121991. [DOI] [PubMed] [Google Scholar]
- 14.Levy D., Christin-Maitre S., Leroy I., Bergeron C., Garcia E., Freitas S., Coelingh-Bennink H.J., Bouchard P. The endometrial approach in contraception. Ann. N. Y. Acad. Sci. 1997;828:59–83. doi: 10.1111/j.1749-6632.1997.tb48524.x. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J., Dolmans M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists. A Review. J. Clin. Med. 2021;10:1085. doi: 10.3390/jcm10051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata T., Izumi G., Takamura M., Saito A., Nakazawa A., Harada M., Hirota Y., Koga K., Fujii T., Osuga Y. Efficacy of dienogest in the treatment of symptomatic adenomyosis: A pilot study. Gynecol. Endocrinol. 2014;30:726–729. doi: 10.3109/09513590.2014.926882. [DOI] [PubMed] [Google Scholar]
- 17.Osuga Y., Hayashi K., Kanda S. Long-term use of dienogest for the treatment of primary and secondary dysmenorrhea. J. Obstet. Gynaecol. Res. 2020;46:606–617. doi: 10.1111/jog.14209. [DOI] [PubMed] [Google Scholar]
- 18.Osuga Y., Watanabe M., Hagino A. Long-term use of dienogest in the treatment of painful symptoms in adenomyosis. J. Obstet. Gynaecol. Res. 2017;43:1441–1448. doi: 10.1111/jog.13406. [DOI] [PubMed] [Google Scholar]
- 19.Osuga Y., Fujimoto-Okabe H., Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: A randomized, double-blind, multicenter, placebo-controlled study. Fertil. Steril. 2017;108:673–678. doi: 10.1016/j.fertnstert.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Kishi Y., Suginami H., Kuramori R., Yabuta M., Suginami R., Taniguchi F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012;207:111–117. doi: 10.1016/j.ajog.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara S., Kawaguchi R., Akinishi M., Nagayasu M., Iwai K., Niiro E., Yamada Y., Tanase Y., Kobayashi H. Subtype I (intrinsic) adenomyosis is an independent risk factor for dienogest-related serious unpredictable bleeding in patients with symptomatic adenomyosis. Sci. Rep. 2019;9:17654. doi: 10.1038/s41598-019-54096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu X., Luo Q., Wang C., Zhu L., Huang L. Effects of Etonogestrel implants on pelvic pain and menstrual flow in women suffering from adenomyosis or endometriosis: Results from a prospective, observational study. Medicine. 2021;100:e24597. doi: 10.1097/MD.0000000000024597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J., Huang Y., Chen L., Hu J., Zou Y. Treatment of Adenomyosis with Subcutaneous Etonogestrel Implants. A Clinical Observational Study in 17 Patients. Med. Sci. Monit. 2018;24:6085–6092. doi: 10.12659/MSM.908979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nie L., Zou H., Ma X., Cheng L., Jiao J., Wang F., Liang W., Zhang P. A clinical observational study on the efficacy of subcutaneous etonogestrel implants for adenomyosis in 20 patients. Gynecol. Endocrinol. 2021;37:735–739. doi: 10.1080/09513590.2021.1922886. [DOI] [PubMed] [Google Scholar]
- 25.Ozdegirmenci O., Kayikcioglu F., Akgul M.A., Kaplan M., Karcaaltincaba M., Haberal A., Akyol M. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil. Steril. 2011;95:497–502. doi: 10.1016/j.fertnstert.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Ekin M., Cengiz H., Ayağ M.E., Kaya C., Yasar L., Savan K. Effects of the levonorgestrel-releasing intrauterine system on urinary symptoms in patients with adenomyosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170:517–520. doi: 10.1016/j.ejogrb.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Bragheto A.M., Caserta N., Bahamondes L., Petta C.A. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195–199. doi: 10.1016/j.contraception.2007.05.091. [DOI] [PubMed] [Google Scholar]
- 28.Cho S., Nam A., Kim H., Chay D., Park K., Cho D.J., Park Y., Lee B. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am. J. Obstet. Gynecol. 2008;198:371–377. doi: 10.1016/j.ajog.2007.10.798. [DOI] [PubMed] [Google Scholar]
- 29.Sheng J., Zhang W.Y., Zhang J.P., Lu D. The LNG-IUS study on adenomyosis. A 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189–193. doi: 10.1016/j.contraception.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Song S.Y., Lee S.Y., Kim H.Y., Park D.B., Kim D.E., Lee K.H., Lee S., Yang J.B., Yoo H.J. Long-term efficacy and feasibility of levonorgestrel-releasing intrauterine device use in patients with adenomyosis. Medicine. 2020;99:e20421. doi: 10.1097/MD.0000000000020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vercellini P., Buggio L., Berlanda N., Barbara G., Somigliana E., Bosari S. Estrogen-progestins and progestins for the management of endometriosis. Fertil. Steril. 2016;106:1552. doi: 10.1016/j.fertnstert.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Flores V.A., Vanhie A., Dang T., Taylor H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018;103:4561–4568. doi: 10.1210/jc.2018-01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnez J., Tatarchuk T.F., Bouchard P., Puscasiu L., Zakharenko N.F., Ivanova T., Ugocsai G., Mara M., Jilla M.P., Bestel E., et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 34.De Milliano I., Van Hattum D., Ket J.C.F., Huirne J.A.F., Hehenkamp W.J.K. Endometrial changes during ulipristal acetate use: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017;214:56–64. doi: 10.1016/j.ejogrb.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Stratton P., Hartog B., Hajizadeh N., Piquion J., Sutherland D., Merino M., Lee Y.J., Nieman L.K. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum. Reprod. 2000;15:1092–1099. doi: 10.1093/humrep/15.5.1092. [DOI] [PubMed] [Google Scholar]
- 36.Capmas P., Brun J.L., Legendre G., Koskas M., Merviel P., Fernandez H. Ulipristal acetate use in adenomyosis: A randomized controlled trial. J. Gynecol. Obstet. Hum. Reprod. 2021;50:101978. doi: 10.1016/j.jogoh.2020.101978. [DOI] [PubMed] [Google Scholar]
- 37.Gracia M., Alcalà M., Ferreri J., Rius M., Ros C., Saco M.A., Martínez-Zamora M., Carmona F. Ulipristal Acetate Improves Clinical Symptoms in Women with Adenomyosis and Uterine Myomas. J. Minim. Invasive Gynecol. 2018;25:1274–1280. doi: 10.1016/j.jmig.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Donnez O., Donnez J. Gonadotropin-releasing hormone antagonist (linzagolix): A new therapy for uterine adenomyosis. Fertil. Steril. 2020;114:640–645. doi: 10.1016/j.fertnstert.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Conway F., Morosetti G., Camilli S., Martire F.G., Sorrenti G., Piccione E., Zupi E., Exacoustos C. Ulipristal acetate therapy increases ultrasound features of adenomyosis: A good treatment given in an erroneous diagnosis of uterine fibroids. Gynecol. Endocrinol. 2019;35:207–210. doi: 10.1080/09513590.2018.1505846. [DOI] [PubMed] [Google Scholar]
- 40.Calderon L., Netter A., Grob-Vaillant A., Mancini J., Siles P., Vidal V., Agostini A. Progression of adenomyosis magnetic resonance imaging features under ulipristal acetate for symptomatic fibroids. Reprod. Biomed. Online. 2021;42:661–668. doi: 10.1016/j.rbmo.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Maggi R., Cariboni A.M., Marelli M.M., Moretti R.M., Andrè V., Marzagalli M., Limonta P. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update. 2016;22:358–381. doi: 10.1093/humupd/dmv059. [DOI] [PubMed] [Google Scholar]
- 42.Grow D.R., Filer R.B. Treatment of adenomyosis with long-term GnRH analogues: A case report. Obstet. Gynecol. 1991;78:538–539. [PubMed] [Google Scholar]
- 43.Nelson J.R., Corson S.L. Long-term management of adenomyosis with a gonadotropin-releasing hormone agonist: A case report. Fertil. Steril. 1993;59:441–443. doi: 10.1016/S0015-0282(16)55704-5. [DOI] [PubMed] [Google Scholar]
- 44.Imaoka I., Ascher S.M., Sugimura K., Takahashi K., Li H., Cuomo F., Simon J., Arnold L.L. MR imaging of diffuse adenomyosis changes after GnRH analog therapy. J. Magn. Reason. Imaging. 2002;15:285–290. doi: 10.1002/jmri.10060. [DOI] [PubMed] [Google Scholar]
- 45.Morelli M., Rocca M.L., Venturella R., Mocciaro R., Zullo F. Improvement in chronic pelvic pain after gonadotropin releasing hormone analogue (GnRH-a) administration in premenopausal women suffering from adenomyosis or endometriosis. A retrospective study. Gynecol. Endocrinol. 2013;29:305–308. doi: 10.3109/09513590.2012.743017. [DOI] [PubMed] [Google Scholar]
- 46.Sauerbrun-Cutler M.T., Alvero R. Short- and long-term impact of gonadotropin-releasing hormone analogue treatment on bone loss and fracture. Fertil. Steril. 2019;112:799–803. doi: 10.1016/j.fertnstert.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 47.Surrey E.S. Add-back therapy and gonadotropin-releasing hormone agonists in the treatment of patients with endometriosis: Can a consensus be reached? Add-Back Consensus Working Group. Fertil. Steril. 1999;71:420–424. doi: 10.1016/S0015-0282(98)00500-7. [DOI] [PubMed] [Google Scholar]
- 48.Niu Z., Chen Q., Sun Y., Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol. Endocrinol. 2013;29:1026–1030. doi: 10.3109/09513590.2013.824960. [DOI] [PubMed] [Google Scholar]
- 49.Donnez O., Donnez J. P-299 Efficacy and safety of linzagolix for the treatment of severe adenomyosis. Initial results from a pilot study. Hum. Reprod. 2021;36:deab130.298. doi: 10.1093/humrep/deab130.298. [DOI] [Google Scholar]
- 50.Donnez J., Stratopoulou C.A., Dolmans M.-M. Uterine Adenomyosis: From Disease Pathogenesis to a New Medical Approach Using GnRH Antagonists. Int. J. Environ. Res. Public Health. 2021;18:9941. doi: 10.3390/ijerph18199941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donnez J., Donnez O., Brethous M., Bestel E., Garner E., Charpentier S., Humberstone A., Loumaye E. Treatment of symptomatic uterine adenomyosis with linzagolix, an oral gonadotropin-releasing hormone receptor antagonist: A pilot study. Reprod. Biomed. Online. 2021 doi: 10.1016/j.rbmo.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Borini A., Coticchio G. Gonadotropin-releasing hormone antagonist linzagolix: Possible treatment for assisted reproduction patients presenting with adenomyosis and endometriosis? Fertil. Steril. 2020;114:517–518. doi: 10.1016/j.fertnstert.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Donnez J., Dolmans M.M. Hormone therapy for intramural myoma-related infertility from ulipristal acetate to GnRH antagonist: A review. Reprod. Biomed. Online. 2020;41:431–442. doi: 10.1016/j.rbmo.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Taylor H.S., Giudice L.C., Lessey B.A., Abrao M.S., Kotarski J., Archer D.F., Diamond M.P., Surrey E., Johnson N.P., Watts N.B., et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017;377:28–40. doi: 10.1056/NEJMoa1700089. [DOI] [PubMed] [Google Scholar]
- 55.Abrao M.S., Surrey E., Gordon K., Snabes M.C., Wang H., Ijacu H., Taylor H.S. Reductions in endometriosis-associated pain among women treated with elagolix are consistent across a range of baseline characteristics reflective of real-world patients. BMC Women’s Health. 2021;21:246. doi: 10.1186/s12905-021-01385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnez J., Taylor H.S., Taylor R.N., Akin M.D., Tatarchuk T.F., Wilk K., Gotteland J.P., Lecomte V., Bestel E. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: A randomized clinical trial. Fertil. Steril. 2020;114:44–55. doi: 10.1016/j.fertnstert.2020.02.114. [DOI] [PubMed] [Google Scholar]
- 57.Diamond M.P., Carr B., Dmowski W.P., Koltun W., O’Brien C., Jiang P., Burke J., Jimenez R., Garner E., Chwalisz K. Elagolix treatment for endometriosis-associated pain: Results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod. Sci. 2014;21:363–371. doi: 10.1177/1933719113497292. [DOI] [PubMed] [Google Scholar]
- 58.Osuga Y., Seki Y., Tanimoto M., Kusumoto T., Kudou K., Terakawa N. Relugolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, in women with endometriosis-associated pain: Phase 2 safety and efficacy 24-week results. BMC Women’s Health. 2021;21:250. doi: 10.1186/s12905-021-01393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donnez J., Dolmans M.M., Fellah L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019;111:454–456. doi: 10.1016/j.fertnstert.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Stratopoulou C.A., Camboni A., Donnez J., Dolmans M.-M. Identifying Common Pathogenic Features in Deep Endometriotic Nodules and Uterine Adenomyosis. J. Clin. Med. 2021;10:4585. doi: 10.3390/jcm10194585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singtripop T., Mori T., Park M.K., Sakamoto S., Kawashima S. Development of uterine adenomyosis after treatment with dopamine antagonists in mice. Life Sci. 1991;49:201–206. doi: 10.1016/0024-3205(91)90004-U. [DOI] [PubMed] [Google Scholar]
- 62.Yamashita M., Matsuda M., Mori T. Increased expression of prolactin receptor mRNA in adenomyotic uterus in mice. Life Sci. 1997;60:1437–1446. doi: 10.1016/S0024-3205(96)00649-2. [DOI] [PubMed] [Google Scholar]
- 63.Andersson J.K., Khan Z., Weaver A.L., Vaughan L.E., Gemzell-Danielsson K., Stewart E.A. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: A pilot study. Acta Obstet. Gynecol. Scand. 2019;98:1341–1350. doi: 10.1111/aogs.13632. [DOI] [PubMed] [Google Scholar]
- 64.Andersson J.K., Pozzi Mucelli R., Epstein E., Stewart E.A., Gemzell-Danielsson K. Vaginal bromocriptine for treatment of adenomyosis. Impact on magnetic resonance imaging and transvaginal ultrasound. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;254:38–43. doi: 10.1016/j.ejogrb.2020.08.040. [DOI] [PubMed] [Google Scholar]
- 65.Auriemma R.S., Del Vecchio G., Scairati R., Pirchio R., Liccardi A., Verde N., de Angelis C., Menafra D., Pivonello C., Conforti A., et al. The Interplay Between Prolactin and Reproductive System: Focus on Uterine Pathophysiology. Front. Endocrinol. 2020;11:594370. doi: 10.3389/fendo.2020.594370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura F., Takahashi K., Takebayashi K., Fujiwara M., Kita N., Noda Y., Harada N. Concomitant treatment of severe uterine adenomyosis in a premenopausal woman with an aromatase inhibitor and a gonadotropin-releasing hormone agonist. Fertil. Steril. 2007;87:1468.e9–1468.e12. doi: 10.1016/j.fertnstert.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Badawy A.M., Elnashar A.M., Mosbah A.A. Aromatase inhibitors or gonadotropin-releasing hormone agonists for the management of uterine adenomyosis: A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2012;91:489–495. doi: 10.1111/j.1600-0412.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- 68.Colette S., Donnez J. Endometriosis. N. Engl. J. Med. 2009;360:1911–1912. doi: 10.1056/NEJMc090328. [DOI] [PubMed] [Google Scholar]
- 69.Colette S., Lousse J.C., Defrère S., Curaba M., Heilier J.F. Van Langendonckt, A.; Mestdagt, M.; Foidart, J.M.; Loumaye, E.; Donnez, J. Absence of aromatase protein and mRNA expression in endometriosis. Hum. Reprod. 2009;24:2133–2141. doi: 10.1093/humrep/dep199. [DOI] [PubMed] [Google Scholar]
- 70.Kitawaki J., Noguchi T., Amatsu T., Maeda K., Tsukamoto K., Yamamoto T., Fushiki S., Osawa Y., Honjo H. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol. Reprod. 1997;57:514–519. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 71.Zhu B., Chen Y., Shen X., Liu X., Guo S.W. Anti-platelet therapy holds promises in treating adenomyosis: Experimental evidence. Reprod. Biol. Endocrinol. 2016;14:66. doi: 10.1186/s12958-016-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X., Shen M., Qi Q., Zhang H., Guo S.W. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum. Reprod. 2016;31:734–749. doi: 10.1093/humrep/dew018. [DOI] [PubMed] [Google Scholar]
- 73.Mosele S., Stratopoulou C.A., Camboni A., Donnez J., Dolmans M.M. Investigation of the role of platelets in the aetiopathogenesis of adenomyosis. Reprod. Biomed. Online. 2021;42:826–834. doi: 10.1016/j.rbmo.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Herndon C.N., Aghajanova L., Balayan S., Erikson D., Barragan F., Goldfien G., Vo K.C., Hawkins S., Giudice L.C. Global Transcriptome Abnormalities of the Eutopic Endometrium From Women With Adenomyosis. Reprod. Sci. 2016;23:1289–1303. doi: 10.1177/1933719116650758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J.H., Duan H., Wang S., Wang Y.Y., Lv C.X. Upregulated microRNA let-7a accelerates apoptosis and inhibits proliferation in uterine junctional zone smooth muscle cells in adenomyosis under conditions of a normal activated hippo-YAP1 axis. Reprod. Biol. Endocrinol. 2021;19:81. doi: 10.1186/s12958-021-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y.Y., Duan H., Wang S., Quan Y.J., Huang J.H., Guo Z.C. Talin1 Induces Epithelial-Mesenchymal Transition to Facilitate Endometrial Cell Migration and Invasion in Adenomyosis Under the Regulation of microRNA-145-5p. Reprod. Sci. 2021;28:1523–1539. doi: 10.1007/s43032-020-00444-8. [DOI] [PubMed] [Google Scholar]
- 77.Hu H., Li H., He Y. MicroRNA-17 downregulates expression of the PTEN gene to promote the occurrence and development of adenomyosis. Exp. Ther. Med. 2017;14:3805–3811. doi: 10.3892/etm.2017.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu K., Cui S., Xue T. LncRNA MIR22HG is downregulated in adenomyosis and upregulates miR-2861 through demethylation to inhibit endometrial cell proliferation. J. Obstet. Gynaecol. Res. 2021;47:1837–1845. doi: 10.1111/jog.14665. [DOI] [PubMed] [Google Scholar]
- 79.Younes G., Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: A meta-analysis. Fertil. Steril. 2017;108:483. doi: 10.1016/j.fertnstert.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 80.Horton J., Sterrenburg M., Lane S., Maheshwari A., Li T.C., Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update. 2019;25:592–632. doi: 10.1093/humupd/dmz012. [DOI] [PubMed] [Google Scholar]
- 81.Kissler S., Zangos S., Wiegratz I., Kohl J., Rody A., Gaetje R., Doebert N., Wildt L., Kunz G., Leyendecker G., et al. Utero-tubal sperm transport and its impairment in endometriosis and adenomyosis. Ann. N. Y. Acad. Sci. 2007;1101:38–48. doi: 10.1196/annals.1389.036. [DOI] [PubMed] [Google Scholar]
- 82.Nisolle M., Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997;68:585–596. doi: 10.1016/S0015-0282(97)00191-X. [DOI] [PubMed] [Google Scholar]
- 83.Large M.J., DeMayo F.J. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol. Cell Endocrinol. 2012;358:155–165. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tremellen K.P., Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: Adenomyosis and macrophages. J. Reprod. Immunol. 2012;93:58–63. doi: 10.1016/j.jri.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Xie M., Yu H., Zhang X., Wang W., Ren Y. Elasticity of adenomyosis is increased after GnRHa therapy and is associated with spontaneous pregnancy in infertile patents. J. Gynecol. Obstet. Hum. Reprod. 2019;48:849–853. doi: 10.1016/j.jogoh.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Hirata J.D., Moghissi K.S., Ginsburg K.A. Pregnancy after medical therapy of adenomyosis with a gonadotropin-releasing hormone agonist. Fertil. Steril. 1993;59:444–445. doi: 10.1016/S0015-0282(16)55703-3. [DOI] [PubMed] [Google Scholar]
- 87.Silva P.D., Perkins H.E., Schauberger C.W. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil. Steril. 1994;61:171–172. doi: 10.1016/S0015-0282(16)56471-1. [DOI] [PubMed] [Google Scholar]
- 88.Agrawala S., Patil J., Campbell S., Woodard T.L. A rare case of extensive placenta accreta in twin pregnancy after GnRH agonist treatment of adenomyosis. Fertil. Res. Pract. 2021;7:5. doi: 10.1186/s40738-021-00097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park C.W., Choi M.H., Yang K.M., Song I.O. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin. Exp. Reprod. Med. 2016;43:169–173. doi: 10.5653/cerm.2016.43.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Al Jama F.E. Management of adenomyosis in subfertile women and pregnancy outcome. Oman. Med. J. 2011;26:178–181. doi: 10.5001/omj.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang B.S., Seow K.M., Tsui K.H., Huang C.Y., Lu Y.F., Wang P.H. Fertility outcome of infertile women with adenomyosis treated with the combination of a conservative microsurgical technique and GnRH agonist: Long-term follow-up in a series of nine patients. Taiwan J. Obstet. Gynecol. 2012;51:212–216. doi: 10.1016/j.tjog.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 92.Lan J., Wu Y., Wu Z., Wu Y., Yang R., Liu Y., Lin H., Jiao X., Zhang Q. Ultra-Long GnRH Agonist Protocol During IVF/ICSI Improves Pregnancy Outcomes in Women With Adenomyosis: A Retrospective Cohort Study. Front. Endocrinol. 2021;12:609771. doi: 10.3389/fendo.2021.609771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou X., Xing J., Shan H., Mei J., Sun Y., Yan G., Sun H., Wang J. The effect of adenomyosis on IVF after long or ultra-long GnRH agonist treatment. Reprod. Biomed. Online. 2020;41:845–853. doi: 10.1016/j.rbmo.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 94.Hyams L.L. Adenomyosis; its conservative surgical treatment (hysteroplasty) in young women. N. Y. State J. Med. 1952;52:2778–2784. [PubMed] [Google Scholar]
- 95.Osada H. Uterine adenomyosis and adenomyoma: The surgical approach. Fertil. Steril. 2018;109:406–417. doi: 10.1016/j.fertnstert.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 96.Fujishita A.H.K., Kitajima M., Matsumoto Y., Satoh H., Masuzaki H. Shikyusenkinsho to shikyu no onzon-chiryo. Uterine adenomyosis and uterine preservation treatment. J. Obstet. Gynecol. Pract. 2010;59:769–776. [Google Scholar]
- 97.Osada H., Silber S., Kakinuma T., Nagaishi M., Kato K., Kato O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod. Biomed. Online. 2011;22:94–99. doi: 10.1016/j.rbmo.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 98.Osada H., Teramoto S. Shikyukin furappuho niyoru shikyu-senkinsho tekishutsujutsu: Rinshoteki choki-yogo oyobi shikyuharetsu-yobokoka no kento. Adenomyomectomy by uterine muscle flap method: Clinical outcome and investigation of the preventive effect on uterine rupture. Obstet. Gynecol. 2017;84:1303–1315. [Google Scholar]
- 99.Nishida M., Takano K., Arai Y., Ozone H., Ichikawa R. Conservative surgical management for diffuse uterine adenomyosis. Fertil. Steril. 2010;94:715–719. doi: 10.1016/j.fertnstert.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 100.Nishida M.O.Y., Ichikawa Y., Arai Y., Sakanaka S. Shikyusenkinsho-kakushutsujutsu-go ninshin-ji no shikyuharetsu-yobo ni tsuite (Prevention of uterine rupture during pregnancy after adenomyomectomy) Obstet. Gynecol. Surg. 2016;27:69–76. [Google Scholar]
- 101.De Bruijn A.M., Smink M., Lohle P.N.M., Huirne J.A.F., Twisk J.W.R., Wong C., Schoonmade L., Hehenkamp W.J.K. Uterine Artery Embolization for the Treatment of Adenomyosis: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2017;28:1629. doi: 10.1016/j.jvir.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 102.Ma J., Brown B., Liang E. Long-term durability of uterine artery embolisation for treatment of symptomatic adenomyosis. Aust. N. Z. J. Obstet. Gynaecol. 2021;61:290–296. doi: 10.1111/ajo.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.