INTRODUCTION
Hereditary cancer syndromes result from germline genetic alterations predisposing individuals to higher lifetime cancer risk than the general population. Hereditary cancer syndromes account for 5%-10% of all malignancies and are characterized by early-onset, aggressive tumors affecting multiple tissue types.1 For example, patients with hereditary breast and ovarian cancer syndrome (HBOC) have high lifetime risk of breast (41%-90%) and ovarian (8%-62%) cancers and elevated risk for several other cancers.2 Similarly, patients with Lynch syndrome (LS) have high lifetime risk of colorectal (12%-52%) and endometrial (0%-57%) cancers, as well as other cancers.3 Reduced cost of genetic technologies4 and new guidance on collecting family history in primary care5 have enabled better detection of these syndromes, providing opportunities to reduce cancer risk.
CONTEXT
Key Objective
Individuals with hereditary cancer syndromes are predisposed to higher lifetime cancer risk than the general population. Early diagnosis of these syndromes can lead to downstream care that greatly reduces cancer morbidity and mortality. In this narrative review, we explore the factors affecting uptake of and adherence to hereditary cancer risk reduction strategies and how these factors interact with systemic barriers to care in medically marginalized populations.
Knowledge Generated
We identified factors at the health system, clinician, and patient levels that interact to affect downstream hereditary cancer care and have implications for health equity. We found that several interventions designed to improve downstream care utilization did not address systemic barriers affecting marginalized populations and suggest strategies to address these barriers in future intervention design.
Relevance
Our review creates a template for the design of more equitable interventions to improve downstream hereditary cancer care.
Multiple studies have shown that regular surveillance, chemoprophylaxis, and prophylactic surgery can improve survival in patients with hereditary cancer syndromes, demonstrating clinical utility of genetic testing for these conditions.2,3,6,7 However, this clinical utility can only be realized if patients have access to and take advantage of risk-reducing care. Unfortunately, receipt of recommended care is far from perfect. Even in HBOC and LS populations, where risk-reducing intervention effectiveness is well-studied and numerous professional guidelines recommend interventions,2,3,8-11 uptake of and adherence to risk-reducing strategies is relatively low (surveillance rates of 52%-85% depending on surveillance type and surgery uptake rates between 9% and 65%).12-21 As genetic testing rates improve, it is critical to address barriers to downstream care, particularly when they disproportionately affect patients from underserved groups.
Here, we provide a narrative review of factors affecting uptake and adherence to guideline-recommended risk-reducing interventions among individuals with hereditary cancer syndromes. We examine the implications of these factors for clinical care, specifically for individuals from populations that are medically underserved (eg, racial or ethnic minority, low-income, uninsured or underinsured, low-education, low-literacy, non–English-speaking, or rural populations); explore the promise and pitfalls of available interventions to improve access to and uptake of risk-reducing interventions; and critically evaluate future research needed in this arena.
OVERVIEW OF THE NARRATIVE REVIEW PROCESS
We conducted a literature review in March 2019 to identify (1) predictors of uptake of (ie, initiation of surveillance and/or having risk-reducing surgery) and adherence (ie, receiving surveillance at recommended intervals or adhering to pharmacotherapy regimens) to risk-reducing interventions, with particular attention to sociodemographic predictors associated with underserved populations; (2) barriers to care that may disproportionately affect individuals from underserved populations; and (3) behavioral and health care delivery interventions that have been successful at improving care uptake and adherence.
We used two search strategies. The first strategy focused on hereditary cancer syndrome care uptake and adherence in medically underserved populations, encompassing all literature before the search date. This strategy used keywords and MeSH terms to represent concepts of underserved populations, hereditary cancer, risk-reducing or early detection-based care, and uptake or adherence. Because of limited results from this search and the need to evaluate barriers to uptake or adherence, a second strategy was designed to include publications from January 2009 to the search date including the same keywords and MeSH terms but excluding keywords focused on underserved populations. One reviewer (KM) screened the results of these searches (N = 1,113) by title and abstract, reviewed full-text articles available in English, and developed an outline for the three narrative review questions above (predictors, barriers, and interventions, N = 160). After review, the team narrowed the scope of review to focus on the most well-studied conditions, HBOC and LS, only including publications focused on other hereditary cancer syndromes if they included concepts not studied in HBOC or LS. When previous reviews had already synthesized findings, primary literature was not included; when multiple studies were available to illustrate a concept, a representative selection was chosen; studies using data from medical records to verify adherence rates were given precedence over studies relying on patient self-report. Small exploratory and qualitative studies were included when quantitative data in larger cohorts were not available or when they provided novel insight. A small number of additional studies were added through May 2021 and identified via coauthor recommendation, search alerts created from the original search strategies, and bibliographies of included literature. Additional references were incorporated on the basis of reviewer suggestions.
FACTORS AFFECTING UPTAKE OF AND ADHERENCE TO RISK-REDUCING INTERVENTIONS FOR HEREDITARY CANCER SYNDROMES AND IMPLICATIONS FOR MEDICALLY UNDERSERVED POPULATIONS
Interacting Care System–Level and Clinician-Level Factors
Inconsistent institutional or clinician practice.
Patients show higher adherence to risk-reducing interventions recommended by a clinician.22 However, guidelines vary in both care recommended and recommendation strength, on the basis of different evaluations of evidence strength. Interclinician and interinstitutional variability in guideline choice likely affects patient uptake. Evidence suggests that clinician-recommended interventions vary by country, institution, clinician, and cancer type.23-25 A survey of representatives from 63 major research and clinical institutions engaged in LS care found that although 98% recommended colonoscopy every 1-2 years, other surveillance and risk-reducing intervention recommendations varied widely: 56% recommended esophagogastroduodenoscopy surveillance and 64% recommended prophylactic hysterectomy or oophorectomy.23 Another study found that although Canada has adopted National Comprehensive Cancer Network (NCCN) guidelines, many Canadian clinicians did not provide certain NCCN recommendations, such as prophylactic mastectomy, to patients with HBOC.24 Clinician guideline adherence rates may be even more variable—especially in nonacademic settings—in the United States, which does not uniformly adopt professional guidelines. This barrier to care would disproportionately affect patients from underserved populations, who are more likely to get care in safety net settings.26 Finally, although data are emerging about best practices for men with HBOC, this group faces unique barriers related to lack of clear guidelines and resulting high levels of inconsistency in recommendations.27
Clinician specialty.
Clinician knowledge and behavior also contribute to guideline adherence, and these may vary considerably between medical specialties in the United States. One survey of 225 physicians in Texas found that only 16% provided recommendations following NCCN guidelines for a hypothetical patient with a genetic diagnosis of HBOC.28 Although most physicians recommended breast magnetic resonance imaging, there was substantial heterogeneity between specialties, with only 62% of clinicians in family medicine recommending magnetic resonance imaging compared with 89% in obstetrics and gynecology.28 Family medicine physicians were also least likely to recommend mastectomy (32%), and internal medicine physicians were least likely to recommend oophorectomy (38%).28 Because patients who are underinsured and uninsured and publicly insured patients typically have less access to specialists, they may be less likely to receive appropriate recommendations.29
Responsibility for care coordination.
Exploratory studies have reported that both patients and clinicians experience confusion about responsibility for care coordination, and clinicians often do not agree regarding who is responsible for patient management.30-33 In one qualitative study of 10 clinicians (nine primary care providers and one specialist), eight clinicians reported heavy reliance on patients and/or specialists for expertise on LS patient management. The same study found that 10 of 12 patients with LS were very familiar with specific LS care recommendations, whereas only three of 10 clinicians could list specific recommendations. One third of interviewed patients reported taking sole responsibility for tracking recommended care and surveillance, rather than receiving reminders through their care system or provider.31 A small survey of gastroenterologists and general surgeons (N = 36) found most viewed coordination of hereditary cancer syndrome management as the responsibility of the patient (22%), primary care clinician (11%), or both (22%).33 Another small qualitative study found that both patients (n = 13) and primary care clinicians (n = 6) reported minimal discussion of LS diagnosis with one another. Gastroenterologists (n = 18) in the same study reported gaps in communication with gynecologists serving patients with LS,32 which could potentially contribute to low uptake of and adherence to gynecologic cancer interventions.20 In another exploratory study, two of nine female patients with LS could not recall receiving recommendations related to LS-associated gynecologic cancers.34 This is troubling, as knowledge of endometrial cancer risk was one of the strongest predictors of gynecologic screening adherence in women with LS in a large mixed methods study.35
Interacting Clinician- and Patient-Level Factors
Clinician-patient communication.
Even when clinicians make appropriate recommendations, recommendation framing can affect uptake. Austria, which has mandated nondirective genetic counseling to prevent clinicians from influencing patient decisions, has lower uptake of prophylactic surgery for hereditary cancer syndromes compared with other countries.36 This is consistent with qualitative patient reports of feeling helpless or disoriented when primary care clinicians do not offer direction for reducing cancer risk.30 Furthermore, a study of simulated genetic counseling sessions suggested that when clinicians frame recommendations in terms of risk increase if the participant does not follow the recommendation, participants have higher recommendation uptake intent than when the clinician frames the recommendation in terms of risk reduction.37 Longer counseling sessions for genetic results disclosure are also associated with higher uptake of risk-reducing interventions in patients with a pathogenic variant, although this may partially reflect variation in patient engagement.38 Finally, if clinicians do not account for health literacy when making recommendations, they may ineffectively communicate a patient's risk status and needed follow-up actions: a real-time observation study of 170 genetic counseling sessions about cancer risk with 49 follow-up patient interviews found that for a diverse group of underserved patients, vague discussions of screening and prevention recommendations contributed to ineffective communication.39 Patient communication also affects care. Despite knowing their LS status, only about one fourth of patients in one study (N = 74) reported explicitly sharing this status with their primary care provider; others conveyed their cancer risk only through family history, presumably leading to less appropriate care recommendations and screenings.20
Education and information access.
Several studies have found an association between greater educational attainment and more proactive attitudes toward or uptake of hereditary cancer syndrome risk-reducing actions.40-42 Similarly, more genetics knowledge is associated with greater uptake and adherence to risk-management behaviors among carriers of BRCA1/2 pathogenic variants.43 Many patients report needing more access to information about risk-reducing options, as well as their cancer-specific risk, to optimize risk reduction.44 These findings again indicate that current models of cancer genetics services can be inaccessible to patients from underserved backgrounds – particularly those with lower educational attainment or health literacy.
Interacting Patient-Level and Societal Factors
Financial and geographical barriers.
Several studies have reported that cost is a primary barrier to surveillance adherence for patients with BRCA1/2 pathogenic variants.45 In one small (N = 12) qualitative study, patients with these variants reported that the most rigid unanticipated barriers to risk-reducing surgery were insurance and financial concerns and these barriers affected timing and other factors related to uptake of surgery.46 Geographical barriers may also influence uptake; insured individuals with HBOC in rural areas were less likely to receive recommended surveillance than those in urban areas, although rates of mastectomy were similar.47 Distance from care may be an even more insurmountable challenge for patients also facing insurance or financial barriers.
Structural racism and medical system trust.
Although a long history of barriers to genetic diagnosis in marginalized racial or ethnic groups has likely contributed to a dearth of data on uptake of hereditary cancer risk management in these populations, the data that do exist suggest differences in uptake and adherence by racial and ethnic background. These differences may stem from access inequities or other barriers to receiving desired care. In one qualitative study of women at high risk of breast cancer, Black American women had considerably less access to specialists and knowledge about risk reduction options and more pressing health concerns that warranted their immediate attention and resources than their White counterparts,48 reflecting the broadly recognized pattern of systemic disadvantage and structural racism in health care faced by people of color in America.49-56 Accordingly, it is not surprising that Black women with BRCA1/2 pathogenic variants in the United States have lower rates of uptake of and adherence to cancer risk-reducing interventions than women from other racial groups.43,57 Although we did not find any studies evaluating trust or mistrust in underserved patients with hereditary cancer syndromes, data suggest that health system mistrust is an important factor in uptake of genetic testing and other types of cancer care in individuals from marginalized racial or ethnic groups; as such, it is likely that the trustworthiness of the health system will affect downstream care uptake and adherence in these patient groups.58
Personal and cultural values.
There has been significant research on the interplay between individual psychosocial characteristics and hereditary cancer syndrome management behaviors. Some studies have demonstrated that higher levels of cancer-specific distress or negative psychologic impact of a positive genetic test are linked to greater uptake of risk-reducing care.43,59,60 Patients who have a greater perception of vulnerability related to their hereditary cancer syndrome, who have a personal cancer history, or who have family history of cancer-related death are also more likely to adhere to surveillance.16,46,60-65 Having children is also associated with greater uptake of risk-reducing surgeries in patients with HBOC; this may be due to a desire to preserve fertility among those without children and/or greater perceived importance of staying healthy among those with children.30,60,63 Actual or potential desire to have children before undertaking risk-reducing surgeries can result in delay of procedures that affect child-bearing potential.40,66 Concerns about side effects (eg, surgical menopause), impacts on sexual health, and pain or discomfort from invasive procedures also adversely affect uptake.34,66-68 Patient perceptions of prophylactic surgeries' risks and benefits, decisional conflict, and ambiguity aversion are associated with surgery intentions.34,40,66-69 In some cases, observed racial or ethnic uptake differences may be due to personal or cultural preferences. Sociocultural attitudes and values about hereditary cancer syndrome interventions may vary by region or by ethnic or racial subpopulation in a given area—especially when it comes to care that affects reproductive organs or secondary sex organs.70-72 This can be the result of cultural stigma surrounding cancer or cultural values around childbearing or the affected organs.70,72-74 Additional stigma surrounding breast cancer risk exists for men, which may affect their adherence to screening recommendations.75 The cultural value of traditional medicine may also affect uptake and adherence to Western medical interventions in some populations.72
INTERVENTIONS TO IMPROVE ADHERENCE TO RISK-REDUCING INTERVENTIONS FOR HEREDITARY CANCER SYNDROMES AND IMPLICATIONS FOR UNDERSERVED POPULATIONS
Improving Guideline-Adherent Provision of Recommendations
One study successfully improved provision of guideline-concordant downstream care recommendations for patients with hereditary breast cancer at a high-risk breast cancer clinic.76 This was achieved through auditing recommendations, iterative education of clinicians, and templated procedures and notes. Unfortunately, patients still had low rates of recommendation adherence, indicating care gaps for ongoing management. Furthermore, a significantly higher proportion of patients at this clinic were White than those identified as high risk in the surrounding community, demonstrating racial inequities in care.76
Enhancing Care Coordination and Improving Access to Surveillance
To simplify the process of tracking and coordinating the numerous recommended surveillance and surgical procedures, some studies have explored the use of one-stop-shop multidisciplinary clinics, where patients can visit multiple specialists and undergo multiple surveillance procedures on the same day in a single location.77,78 Such models have shown improved adherence to guideline-recommended care and high patient uptake.77,78 At least one of these clinics has shown preliminary success in a community-based practice setting and with patients of predominantly Hispanic ethnicity, with 80% of patients (28 of 35) attending their first visit and receiving consultations with genetics clinicians, social workers, and/or relevant surgeons.77 Multidisciplinary clinics may also help patients with limited resources overcome scheduling and transportation barriers by streamlining care.
Another option for reaching rural patients is a mobile surveillance unit that travels to patients and performs care on-site, eliminating transportation barriers. Mobile colonoscopy units have been successful for patients with LS in low-income areas of South Africa, although adherence to recommended surveillance waned over time.79 Further evaluations of this model are needed to assess the financial feasibility of widespread implementation.
Reducing Pain
Combining colorectal and gynecologic cancer surveillance has the potential to improve adherance.80 Endometrial biopsy can be painful and, unlike colonoscopy, is not typically performed with conscious sedation. Women with LS who receive combined colonoscopy and endometrial biopsy have relatively high adherence and report lower pain, higher satisfaction, and greater convenience than those receiving endometrial biopsy alone.81,82
Increasing Patient Access to and Opportunity to Understand Information
Patients with HBOC have identified a need for ongoing decision support because factors affecting risk reduction decisions change over time.83 However, we identified just one decision aid aimed at promoting adherence to HBOC-related surveillance after an HBOC diagnosis.84 This prototype decision aid provided information on surveillance, pharmacologic, and surgical options and provided specific probabilities of risk of cancer after surgery or pharmaceutical intervention and risks associated with having surgery. The aid also provided a values assessment and a decision guide worksheet. The aid was rated by end users as useful, clear, and relevant to their needs.84 We identified a second iPhone-only application developed with patient input with some informational elements to support decision-making, including information on guidelines and recommended care; this application asked users about which recommended care they were interested in and allowed users to elect calendar reminders to schedule guideline-concordant care and reminders to have decision support conversations with their clinician.45 Preliminary data from predominantly White, college-educated users suggest that this application was acceptable and was primarily used to get reminders about upcoming procedures. The application's impact on patient uptake of and adherence to risk-reducing interventions has not yet been evaluated.85 Furthermore, this tool has not yet been evaluated for accessibility and acceptability in low-literacy, low-resource, or non-White populations.
DISCUSSION: THE FUTURE OF EQUITABLE CARE DELIVERY IN HEREDITARY CANCER SYNDROMES
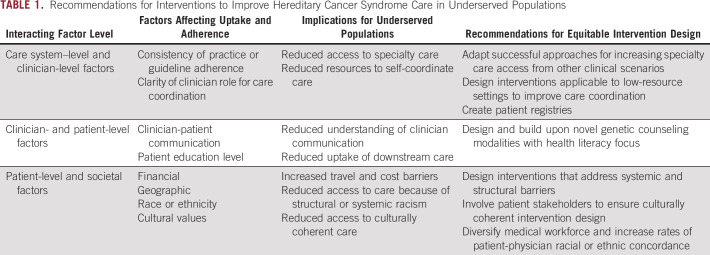
Current translational genetics research focuses primarily on utilization of and inequities in genetic testing.86,87 However, success does not end with hereditary cancer syndrome diagnosis, and interventions are needed to improve downstream care uptake and adherence, especially in medically underserved populations. We identified health system-level, clinician-level, and patient-level barriers to receiving downstream care, many of which disproportionately affect individuals from underserved populations. Below, we summarize important implications from our review for future research aimed at reducing hereditary cancer care inequities (Table 1).
TABLE 1.
Recommendations for Interventions to Improve Hereditary Cancer Syndrome Care in Underserved Populations
Interventions that interact with patient-level factors or do not address systemic and structural barriers may have unintended consequences of increasing inequities. Several interventions outlined in this review place substantial travel and logistical burden on the patient to be successful or fail to resolve barriers to care receipt, including financial burden. For example, interventions that place logistical burden on the patient by providing scheduling reminders or patient education45,84,85 will fail to help many patients who already face significant socioeconomic difficulties that affect cognitive load, increasing inequities for these populations.88 Furthermore, interventions using smartphone technology to engage patients are not accessible to patients without these resources. Interventions that interact with clinician-level factors, such as ongoing clinician education, are less likely to reach medically underserved patients, who have less access to specialty clinicians and academic medical centers, where these interventions are most often implemented. Similarly, although multidisciplinary clinics can improve care coordination,77,78 they are resource-intensive and require significant patient volume to justify. Therefore, they may not be feasible in low-resource settings or rural settings with a low number of affected individuals.
For equitable intervention design, it is critical to shift intervention focus to removing and reducing barriers. Interventions that remove or reduce structural and systemic barriers have greater potential to benefit the whole population of individuals with hereditary cancer syndromes, including underserved patients. On the basis of factors affecting uptake and adherence outlined in this review, several intervention avenues should be explored. Because multidisciplinary clinics improve patient uptake and adherence to recommendations, researchers should focus on whether this or other interventions to improve care coordination can be effectively implemented in low-resource settings and rural areas. Further evaluation of mobile surveillance units, which have had some success at increasing access in rural settings for patients with travel barriers, is warranted.79 Research should explore novel ways to leverage electronic medical records to support clinicians. Universal standards for structured genomics data have recently allowed genomics modules to be integrated into the electronic medical record.89 These data standards can facilitate automated patient identification for follow-up, automated clinical decision support, and automated reminders to clinicians about needed follow-up care.19,90,91 These standards could also facilitate creation of automated referrals to appropriate subspecialties, clinical role clarification notices, and centralization of clinic notes related to the genetic diagnosis.
Because patients do not always share their genetic status with primary care providers, alternate genetic counseling communication modalities—especially those with a health literacy focus and/or targeted at patients with limited English proficiency—may help to close this gap by improving patient understanding of the impact of their genetic diagnosis on their medical care.92 Patient registries may also decrease the burden on the patient to initiate conversations about their diagnosis with their clinicians.93 Because medical system trust plays a role in cancer care engagement,58 it is important to consider interventions to increase comfort and trust for marginalized populations. Research shows that patient-provider racial or ethnic concordance can improve patient-provider communication and increase patient health care engagement,94,95 highlighting the importance of ongoing interventions to increase research and medical workforce diversity and the need to increase patient-provider racial or ethnic concordance in genetics care.96-99 Additional interventions might include (1) specialized case managers to streamline care and address patient questions, a model that has been successful in the diagnostic pathway,100 and (2) leveraging celebrity hereditary cancer syndrome diagnosis disclosure, which has correlated with enhanced genetic testing usage and genetics knowledge seeking in the past, to create accessible and relatable interventions aimed at reducing stigma and increasing information access for lower health literacy populations.
Because cost is a key structural barrier to adherence,45,46 particularly for patients from underserved communities, and inequities in insurance coverage for risk-reducing care after hereditary cancer syndrome recognition may exacerbate care inequities,101 researchers should design these interventions with cost and coverage in mind. Researchers should also plan to partner effectively with safety net clinics during intervention research and implementation, to improve health equity in intervention design and implementation.102
Although few interventions have targeted uptake and adherence of risk-reducing care in patients with hereditary cancer syndromes, insights can be drawn from interventions to improve adherence to cancer risk-reducing treatment in other high-risk populations, such as individuals with a first-degree relative with colorectal cancer (CRC). Some promising strategies, including patient navigation103 and judicious use of fecal immunochemical tests (FITs) rather than colonoscopy,104 warrant exploration as options for hereditary cancer syndromes. Although FIT would not be an appropriate alternative for syndromes where polyp excision is a cornerstone of risk reduction, FIT or FIT-DNA could be examined for LS-related CRC detection as an adjunct to colonoscopy screenings and for patients unable to undergo regular colonoscopy.
Interventions for patients with hereditary cancer syndromes could also be informed by research on improving access to recommended general population cancer screening for underserved populations.105 For example, the National Breast and Cervical Cancer Early Detection Program provides a successful comprehensive model for free and low-cost breast cancer screening,106 and culturally targeted cancer literacy tools have improved screening intentions for breast and cervical cancers among Black, Hispanic, and Arab women, suggesting that such tools could increase equity in screening access.107
Since decisions to engage in downstream care are influenced by sociocultural beliefs, it is important to develop interventions in collaboration with patient stakeholders that improve genetic counselor and clinician competence in acknowledging and incorporating patient beliefs into care plans.72 Future research should budget time and finances to allow for an iterative design process in collaboration with stakeholders representing underserved populations of interest, including budgeting for culturally tailored translation to other languages.108 Patient stakeholder perspectives can provide insight into condition- or treatment-specific considerations, and population- or individual-specific barriers, that are relevant to intervention design. For example, simultaneously undergoing endometrial biopsy and colonoscopy was suggested by LS patient stakeholders80 and has proven to be successful.81,82 Inclusion of patient stakeholders may also help ensure that interventions are culturally competent and thus effective at influencing risk-reducing behavior.70,71 Such stakeholder approaches have been successful in designing tailored screening interventions for sporadic CRC in Hispanic populations and hold promise for improving equity of hereditary cancer prevention.109,110
In conclusion, to narrow health care inequities in genomic medicine, it is critical to reduce barriers to cancer risk-reducing interventions, especially in underserved populations. Our review identified care system and clinician factors, systemic and structural barriers, and some patient-level factors that affect uptake and adherence to risk-reducing interventions among those with hereditary cancer syndromes. There are potential avenues for intervention: improving care coordination, clarifying clinician roles and responsibilities, and reducing structural and systemic barriers to care. As low-resource patients and clinics may be less able to take on individual burden, it is critical to design interventions that focus on removing system and structural barriers to address inequities. Stakeholder input will be a critical component of effective intervention design.
ACKNOWLEDGMENT
The authors acknowledge Kevin Lutz, Dr Neon Brooks, Ana Reyes, and Angela Paolucci for editing and administrative assistance. The authors thank Dr Elizabeth Liles for valuable comments on early and late drafts.
Kathleen F. Mittendorf
Research Funding: GE Healthcare (Inst)
Heather Spencer Feigelson
Research Funding: Guardant Health
Sapna Syngal
Consulting or Advisory Role: Myriad Genetics, DC Health
Patents, Royalties, Other Intellectual Property: Dana-Farber Cancer Institute has a registered service mark for the PREMM5 model and holds copyrights for the PREMM questionnaires; Myriad Genetics (through Dana-Farber Cancer Institute) paid an inventor share of the IP (license issue fee)
No other potential conflicts of interest were reported.
See accompanying article doi: 10.1200/PO.21.00231
SUPPORT
This work was funded as part of the Clinical Sequencing Evidence-Generating Research (CSER) consortium funded by the National Human Genome Research Institute with cofunding from the National Institute on Minority Health and Health Disparities (NIMHD) and the National Cancer Institute (NCI). The CSER consortium represents a diverse collection of projects investigating the application of genome-scale sequencing in different clinical settings including pediatric and adult subspecialties, germline diagnostic testing and tumor sequencing, and specialty and primary care. This work was supported by a grant from the National Human Genome Research Institute (U01HG007292; MPIs: Wilfond, Goddard), with additional support from U24HG007307 (Coordinating Center).
AUTHOR CONTRIBUTIONS
Conception and design: Kathleen F. Mittendorf, Katherine P. Anderson, Heather Spencer Feigelson, Jessica Ezzell Hunter, Benjamin S. Wilfond, Katrina A. B. Goddard
Financial support: Benjamin S. Wilfond, Katrina A. B. Goddard
Administrative support: Tia L. Kauffman, Katrina A. B. Goddard
Collection and assembly of data: Kathleen F. Mittendorf, Tia L. Kauffman, Nangel M. Lindberg, Katherine P. Anderson, Heather Spencer Feigelson, Marian J. Gilmore, Stephanie A. Kraft, Jamilyn M. Zepp
Data analysis and interpretation: Kathleen F. Mittendorf, Sarah Knerr, Tia L. Kauffman, Nangel M. Lindberg, Katherine P. Anderson, Heather Spencer Feigelson, Jessica Ezzell Hunter, Galen Joseph, Stephanie A. Kraft, Sapna Syngal, Benjamin S. Wilfond
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kathleen F. Mittendorf
Research Funding: GE Healthcare (Inst)
Heather Spencer Feigelson
Research Funding: Guardant Health
Sapna Syngal
Consulting or Advisory Role: Myriad Genetics, DC Health
Patents, Royalties, Other Intellectual Property: Dana-Farber Cancer Institute has a registered service mark for the PREMM5 model and holds copyrights for the PREMM questionnaires; Myriad Genetics (through Dana-Farber Cancer Institute) paid an inventor share of the IP (license issue fee)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Garber JE, Offit K: Hereditary cancer predisposition syndromes. J Clin Oncol 23:276-292, 2005 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) : Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic V1.2020. NCCN, 2019 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) : Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal V3.2019. NCCN, 2019 [Google Scholar]
- 4.Hall MJ, Forman AD, Pilarski R, et al. : Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw 12:1339-1346, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Moyer VA: US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med 160:271-281, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Nelson HD, Pappas M, Zakher B, et al. : Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: A systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med 160:255-266, 2014 [DOI] [PubMed] [Google Scholar]
- 7.National Collaborating Centre for Cancer: National Institute for Health and Clinical Excellence : Guidance, Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. Cardiff, United Kingdom, National Collaborating Centre for Cancer, 2013 [PubMed] [Google Scholar]
- 8.Berliner JL, Fay AM: Risk assessment and genetic counseling for hereditary breast and ovarian cancer: Recommendations of the National Society of Genetic Counselors. J Genet Couns 16:241-260, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Saslow D, Boetes C, Burke W, et al. : American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75-89, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Stoffel EM, Mangu PB, Gruber SB, et al. : Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology clinical practice guidelines. J Clin Oncol 33:209-217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syngal S, Brand RE, Church JM, et al. : ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110:223-262, 2015; quiz 263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padamsee TJ, Wills CE, Yee LD, et al. : Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Res 19:34, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider KI, Schmidtke J: Patient compliance based on genetic medicine: A literature review. J Community Genet 5:31-48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton K, Green K, Lalloo F, et al. : Colonoscopy screening compliance and outcomes in patients with Lynch syndrome. Colorectal Dis 17:38-46, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Pylvanainen K, Kairaluoma M, Mecklin JP: Compliance and satisfaction with long-term surveillance in Finnish HNPCC families. Fam Cancer 5:175-178, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Stoffel EM, Mercado RC, Kohlmann W, et al. : Prevalence and predictors of appropriate colorectal cancer surveillance in Lynch syndrome. Am J Gastroenterol 105:1851-1860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadley DW, Jenkins JF, Dimond E, et al. : Colon cancer screening practices after genetic counseling and testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol 22:39-44, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Yurgelun MB, Mercado R, Rosenblatt M, et al. : Impact of genetic testing on endometrial cancer risk-reducing practices in women at risk for Lynch syndrome. Gynecol Oncol 127:544-551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittendorf KF, Hunter JE, Schneider JL, et al. : Recommended care and care adherence following a diagnosis of lynch syndrome: A mixed-methods study. Hered Cancer Clin Pract 17:31, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton-Chase AM, Hovick SR, Sun CC, et al. : Gynecologic cancer screening and communication with health care providers in women with Lynch syndrome. Clin Genet 86:185-189, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassem N, Stout LA, Hunter C, et al. : Precision prevention: The current state and future of genomically guided cancer prevention. JCO Precis Oncol 4:96-108, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Tinley ST, Houfek J, Watson P, et al. : Screening adherence in BRCA1/2 families is associated with primary physicians' behavior. Am J Med Genet A 125A:5-11, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Pan JY, Haile RW, Templeton A, et al. : Worldwide practice patterns in lynch syndrome diagnosis and management, based on data from the International Mismatch Repair Consortium. Clin Gastroenterol Hepatol 16:1901-1910.e11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe KA, Kim-Sing C, Ghadirian P, et al. : Health care provider recommendations for reducing cancer risks among women with a BRCA1 or BRCA2 mutation. Clin Genet 85:21-30, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Jain A, Shafer L, Rothenmund H, et al. : Suboptimal adherence in clinical practice to guidelines recommendation to screen for lynch syndrome. Dig Dis Sci 64:3489-3501, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Shin P, Rosenbaum SJ, Paradice J: Community health centers: The challenge of growing to meet the need for primary care in medically underserved communities, 2012, http://hsrc.himmelfarb.gwu.edu/sphhs_policy_ggrchn/49 [Google Scholar]
- 27.Gaddam S, Heller SL, Babb JS, et al. : Male breast cancer risk assessment and screening recommendations in high-risk men who undergo genetic counseling and multigene panel testing. Clin Breast Cancer 21:e74-e79, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Dhar SU, Cooper HP, Wang T, et al. : Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat 129:221-227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timbie JW, Kranz AM, Mahmud A, et al. : Specialty care access for Medicaid enrollees in expansion states. Am J Manag Care 25:e83-e87, 2019 [PMC free article] [PubMed] [Google Scholar]
- 30.Caiata-Zufferey M, Pagani O, Cina V, et al. : Challenges in managing genetic cancer risk: A long-term qualitative study of unaffected women carrying BRCA1/BRCA2 mutations. Genet Med 17:726-732, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Schneider JL, Goddard KAB, Muessig KR, et al. : Patient and provider perspectives on adherence to and care coordination of lynch syndrome surveillance recommendations: Findings from qualitative interviews. Hered Cancer Clin Pract 16:11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douma KFL, Bleeker FE, Medendorp NM, et al. : Information exchange between patients with lynch syndrome and their genetic and non-genetic health professionals: Whose responsibility? J Community Genet 10:237-247, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maceachern J, Mathews M, Green J, et al. : Specialists' perceptions of hereditary colorectal cancer screening in Newfoundland and Labrador. Curr Oncol 19:e123-8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steel E, Robbins A, Jenkins M, et al. : How does genetic risk information for Lynch syndrome translate to risk management behaviours? Hered Cancer Clin Pract 15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketabi Z, Mosgaard BJ, Gerdes AM, et al. : Awareness of endometrial cancer risk and compliance with screening in hereditary nonpolyposis colorectal cancer. Obstet Gynecol 120:1005-1012, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Singer CF, Tan YY, Rappaport C: Identification and management of familial breast cancer in Austria. Horm Mol Biol Clin Investig 32:pii: /j/hmbci.2017.32.issue-2/hmbci-2017-0025/hmbci-2017-0025.xml, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Taber JM, Aspinwall LG: Framing recommendations to promote prevention behaviors among people at high risk: A simulation study of responses to melanoma genetic test reporting. J Genet Couns 24:771-782, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Pal T, Lee JH, Besharat A, et al. : Modes of delivery of genetic testing services and the uptake of cancer risk management strategies in BRCA1 and BRCA2 carriers. Clin Genet 85:49-53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph G, Pasick RJ, Schillinger D, et al. : Information mismatch: Cancer risk counseling with diverse underserved patients. J Genet Couns 26:1090-1104, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisel SF, Rahman B, Side L, et al. : Genetic testing and personalized ovarian cancer screening: A survey of public attitudes. BMC Womens Health 16:46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong A, Kelly S, Nusbaum R, et al. : Intentions for risk-reducing surgery among high-risk women referred for BRCA1/BRCA2 genetic counseling. Psychooncology 24:33-39, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henry DA, Lee MC, Almanza D, et al. : Trends in use of bilateral prophylactic mastectomy vs high-risk surveillance in unaffected carriers of inherited breast cancer syndromes in the Inherited Cancer Registry (ICARE). Breast Cancer Res Treat 174:39-45, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchanan AH, Voils CI, Schildkraut JM, et al. : Adherence to recommended risk management among unaffected women with a BRCA mutation. J Genet Couns 26:79-92, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bannon SA, Mork M, Vilar E, et al. : Patient-reported disease knowledge and educational needs in lynch syndrome: Findings of an interactive multidisciplinary patient conference. Hered Cancer Clin Pract 12:1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scherr CL, Feuston JL, Nixon DM, et al. : A two-phase Approach to developing SNAP: An iPhone application to support appointment scheduling and management for women with a BRCA mutation. J Genet Couns 27:439-445, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Werner-Lin A, Ersig AL, Mueller R, et al. : Catalysts towards cancer risk management action: A longitudinal study of reproductive-aged women with BRCA1/2 mutations. J Psychosoc Oncol 36:529-544, 2018 [DOI] [PubMed] [Google Scholar]
- 47.Kolor K, Chen Z, Grosse SD, et al. : BRCA genetic testing and receipt of preventive interventions among women aged 18-64 years with employer-sponsored health insurance in nonmetropolitan and metropolitan areas—United States, 2009-2014. MMWR Surveill Summ 66:1-11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padamsee TJ, Meadows R, Hils M: Layers of information: Interacting constraints on breast cancer risk-management by high-risk African American women. Ethn Health 26:787-810, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardeman RR, Medina EM, Kozhimannil KB: Structural racism and supporting black lives—The role of health professionals. N Engl J Med 375:2113-2115, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansell DA, McDonald EK: Bias, black lives, and academic medicine. N Engl J Med 372:1087-1089, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Amutah C, Greenidge K, Mante A, et al. : Misrepresenting race—The role of medical schools in propagating physician bias. N Engl J Med 384:872-878, 2021 [DOI] [PubMed] [Google Scholar]
- 52.Bailey ZD, Feldman JM, Bassett MT: How structural racism works—Racist policies as a root cause of U.S. racial health inequities. N Engl J Med 384:768-773, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardeman RR, Murphy KA, Karbeah J, et al. : Naming institutionalized racism in the public health literature: A systematic literature review. Public Health Rep 133:240-249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams DR, Mohammed SA: Racism and health II: A needed research agenda for effective interventions. Am Behav Sci 57:1200-1226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bailey ZD, Krieger N, Agénor M, et al. : Structural racism and health inequities in the USA: Evidence and interventions. Lancet 389:1453-1463, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Agency for Healthcare Research and Quality : 2019 National Healthcare Quality and Disparities Report, 2019, https://www.ahrq.gov/research/findings/nhqrdr/nhqdr19/index.html
- 57.Cragun D, Weidner A, Lewis C, et al. : Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 123:2497-2505, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mouslim MC, Johnson RM, Dean LT: Healthcare system distrust and the breast cancer continuum of care. Breast Cancer Res Treat 180:33-44, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz MD, Isaacs C, Graves KD, et al. : Long-term outcomes of BRCA1/BRCA2 testing: Risk reduction and surveillance. Cancer 118:510-517, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julian-Reynier C, Bouhnik AD, Mouret-Fourme E, et al. : Time to prophylactic surgery in BRCA1/2 carriers depends on psychological and other characteristics. Genet Med 12:801-807, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Johns D, Agarwal J, Anderson L, et al. : Breast cancer risk reduction decisions of the BRCA-positive patient: An observational study at a single institution. J Womens Health (Larchmt) 26:702-706, 2017 [DOI] [PubMed] [Google Scholar]
- 62.van Driel CM, Eltahir Y, de Vries J, et al. : Risk-reducing mastectomy in BRCA1/2 mutation carriers: Factors influencing uptake and timing. Maturitas 77:180-184, 2014 [DOI] [PubMed] [Google Scholar]
- 63.Singh K, Lester J, Karlan B, et al. : Impact of family history on choosing risk-reducing surgery among BRCA mutation carriers. Am J Obstet Gynecol 208:329.e1-329.e6, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Manchanda R, Burnell M, Abdelraheim A, et al. : Factors influencing uptake and timing of risk reducing salpingo-oophorectomy in women at risk of familial ovarian cancer: A competing risk time to event analysis. BJOG 119:527-536, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Eriksson LE, Fritzell K, Rixon L, et al. : The role of illness perceptions in adherence to surveillance in patients with familial adenomatous polyposis (FAP). Psychooncology 25:699-706, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Hickey M, Rio I, Trainer A, et al. : Exploring factors that impact uptake of risk-reducing bilateral salpingo-oophorectomy (RRBSO) in high-risk women. Menopause 27:26-32, 2020 [DOI] [PubMed] [Google Scholar]
- 67.Douma KF, Bleiker EM, Aaronson NK, et al. : Long-term compliance with endoscopic surveillance for familial adenomatous polyposis. Colorectal Dis 12:1198-1207, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Skandarajah AR, Thomas S, Shackleton K, et al. : Patient and medical barriers preclude uptake of tamoxifen preventative therapy in women with a strong family history. Breast 32:93-97, 2017 [DOI] [PubMed] [Google Scholar]
- 69.Ladd MK, Peshkin BN, Senter L, et al. : Predictors of risk-reducing surgery intentions following genetic counseling for hereditary breast and ovarian cancer. Transl Behav Med 10:337-346, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hann KEJ, Ali N, Gessler S, et al. : Attitudes towards a programme of risk assessment and stratified management for ovarian cancer: A focus group study of UK South Asians' perspectives. BMJ Open 8:e021782, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwong A, Wong CH, Shea C, et al. : Choice of management of southern Chinese BRCA mutation carriers. World J Surg 34:1416-1426, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Shaw T, Ishak D, Lie D, et al. : The influence of Malay cultural beliefs on breast cancer screening and genetic testing: A focus group study. Psychooncology 27:2855-2861, 2018 [DOI] [PubMed] [Google Scholar]
- 73.Mellon S, Gauthier J, Cichon M, et al. : Knowledge, attitudes, and beliefs of Arab-American women regarding inherited cancer risk. J Genet Couns 22:268-276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marlow L, Vrinten C, Waller J: Cancer Stigma Among Ethnic Minority Women. National Cancer Research Institute Cancer Conference; 2016, https://abstracts.ncri.org.uk/abstract/cancer-stigma-among-ethnic-minority-women/ [Google Scholar]
- 75.Gao Y, Heller SL, Moy L: Male breast cancer in the age of genetic testing: An opportunity for early detection, tailored therapy, and surveillance. Radiographics 38:1289-1311, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laws A, Mulvey TM: Implementation of a high-risk breast clinic for comprehensive care of women with elevated breast cancer risk identified by risk assessment models in the community. JCO Oncol Pract 17:e217-e225, 2021 [DOI] [PubMed] [Google Scholar]
- 77.O'Leary MP, Goldner BS, Abboy S, et al. : A single visit multidisciplinary model for managing patients with mutations in moderate and high-risk genes in a community practice setting. Fam Cancer 17:175-178, 2018 [DOI] [PubMed] [Google Scholar]
- 78.Pichert G, Jacobs C, Jacobs I, et al. : Novel one-stop multidisciplinary follow-up clinic significantly improves cancer risk management in BRCA1/2 carriers. Fam Cancer 9:313-319, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Bruwer Z, Futter M, Ramesar R: A mobile colonoscopic unit for lynch syndrome: Trends in surveillance uptake and patient experiences of screening in a developing country. J Genet Couns 22:125-137, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Sun CC, Meyer LA, Daniels MS, et al. : Women's preferences for cancer risk management strategies in Lynch syndrome. Gynecol Oncol 152:514-521, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nebgen DR, Lu KH, Rimes S, et al. : Combined colonoscopy and endometrial biopsy cancer screening results in women with Lynch syndrome. Gynecol Oncol 135:85-89, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang M, Sun C, Boyd-Rogers S, et al. : Prospective study of combined colon and endometrial cancer screening in women with lynch syndrome: A patient-centered approach. JCO Oncol Pract 7:43-47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Underhill ML, Crotser CB: Seeking balance: Decision support needs of women without cancer and a deleterious BRCA1 or BRCA2 mutation. J Genet Couns 23:350-362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jabaley T, Underhill-Blazey ML, Berry DL: Development and testing of a decision aid for unaffected women with a BRCA1 or BRCA2 mutation. J Cancer Educ 35:339-344, 2020 [DOI] [PubMed] [Google Scholar]
- 85.Cohen SA, Scherr CL, Nixon DM: An iPhone application intervention to promote surveillance among women with a BRCA mutation: Pre-intervention data. J Genet Couns 27:446-456, 2018 [DOI] [PubMed] [Google Scholar]
- 86.Roberts MC, Clyne M, Kennedy AE, et al. : The current state of funded NIH grants in implementation science in genomic medicine: A portfolio analysis. Genet Med 21:1218-1223, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McBride CM, Birmingham WC, Kinney AY: Health psychology and translational genomic research: Bringing innovation to cancer-related behavioral interventions. Am Psychol 70:91-104, 2015 [DOI] [PubMed] [Google Scholar]
- 88.Mani A, Mullainathan S, Shafir E, et al. : Poverty impedes cognitive function. Science 341:976-980, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Alterovitz G, Warner J, Zhang P, et al. : SMART on FHIR genomics: Facilitating standardized clinico-genomic apps. J Am Med Inform Assoc 22:1173-1178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dolin RH, Boxwala A, Shalaby J: A pharmacogenomics clinical decision support service based on FHIR and CDS Hooks. Methods Inf Med 57:e115-e123, 2018 [DOI] [PubMed] [Google Scholar]
- 91.Watkins M, Eilbeck K: FHIR lab reports: Using SMART on FHIR and CDS Hooks to increase the clinical utility of pharmacogenomic laboratory test results. AMIA Jt Summits Transl Sci Proc 2020:683-692, 2020 [PMC free article] [PubMed] [Google Scholar]
- 92.Joseph G, Lee R, Pasick RJ, et al. : Effective communication in the era of precision medicine: A pilot intervention with low health literacy patients to improve genetic counseling communication. Eur J Med Genet 62:357-367, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoque DME, Kumari V, Hoque M, et al. : Impact of clinical registries on quality of patient care and clinical outcomes: A systematic review. PLoS One 12:e0183667, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jetty A, Jabbarpour Y, Pollack J, et al. : Patient-Physician racial concordance associated with improved healthcare use and lower healthcare expenditures in minority populations. J Racial Ethn Health Disparities 10.1007/s40615-020-00930-4 [epub ahead of print on January 5, 2021] [DOI] [PubMed] [Google Scholar]
- 95.Shen MJ, Peterson EB, Costas-Muñiz R, et al. : The effects of race and racial concordance on patient-physician communication: A systematic review of the literature. J Racial Ethn Health Disparities 5:117-140, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aibana O, Swails JL, Flores RJ, et al. : Bridging the gap: Holistic review to increase diversity in graduate medical education. Acad Med 94:1137-1141, 2019 [DOI] [PubMed] [Google Scholar]
- 97.Pierce AE, Moreno-Walton L, Boatright D, et al. : Advancing diversity and inclusion: An organized approach through a medical specialty academy. AEM Educ Train 4:S40-s46, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glazer G, Tobias B, Mentzel T: Increasing healthcare workforce diversity: Urban universities as catalysts for change. J Prof Nurs 34:239-244, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Estape ES, Quarshie A, Segarra B, et al. : Promoting diversity in the clinical and translational research workforce. J Natl Med Assoc 110:598-605, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miesfeldt S, Feero WG, Lucas FL, et al. : Association of patient navigation with care coordination in an Lynch syndrome screening program. Transl Behav Med 8:450-455, 2018 [DOI] [PubMed] [Google Scholar]
- 101.Prince AE: Prevention for those who can pay: Insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci 2:365-395, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brandt HM, Young VM, Campbell DA, et al. : Federally qualified health centers' capacity and readiness for research collaborations: Implications for clinical-academic-community partnerships. Clin Transl Sci 8:391-393, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paskett ED, Bernardo BM, Young GS, et al. : Comparative effectiveness of two interventions to increase colorectal cancer screening for those at increased risk based on family history: Results of a randomized trial. Cancer Epidemiol Biomarkers Prev 29:3-9, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quintero E, Carrillo M, Gimeno-Garcia AZ, et al. : Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 147:1021-1030.e1, 2014; quiz e16-e17 [DOI] [PubMed] [Google Scholar]
- 105.Shah SK, Nakagawa M, Lieblong BJ: Examining aspects of successful community-based programs promoting cancer screening uptake to reduce cancer health disparity: A systematic review. Prev Med 141:106242, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lantz PM, Mullen J: The National Breast and Cervical Cancer Early Detection Program: 25 Years of public health service to low-income women. Cancer Causes control 26:653-656, 2015 [DOI] [PubMed] [Google Scholar]
- 107.Nolan TS, Tan A, Williams KP: The ties that bind: Cancer history, communication, and screening intention associations among diverse families. J Med Screen 28:108-113, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindberg NM, Gutierrez AM, Mittendorf KF, et al. : Creating accessible Spanish language materials for clinical sequencing evidence-generating research consortium genomic projects: Challenges and lessons learned. Per Med 18:441-454, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coronado GD, Thompson JH, Petrik AF, et al. : Patient-refined messaging for a mailed colorectal cancer screening program: Findings from the PROMPT study. J Am Board Fam Med 32:318-328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thompson JH, Davis MM, Michaels L, et al. : Developing patient-refined messaging for a mailed colorectal cancer screening program in a Latino-based community health center. J Am Board Fam Med 32:307-317, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]